Abstract
Borrelia burgdorferi, the agent of Lyme borreliosis, is genetically more heterogeneous than previously thought. In Europe five genospecies have been described from the original B. burgdorferi sensu lato (sl): B. burgdorferi sensu stricto (ss), B. garinii, B. afzelii, B. lusitaniae, and B. valaisiana. In the United States, B. burgdorferi ss as well as B. bissettii in California and B. andersonii on the East Coast were differentiated. In Asia, B. japonica has been identified along, with B. garinii, B. afzelii, and B. valaisiana. In order to evaluate sensitivity and specificity of four species-specific monoclonal antibodies, we analyzed 210 B. burgdorferi sl isolates belonging to eight genospecies by immunoblot and confirmed genospecies by restriction fragment length polymorphism (RFLP) of rrf (5S)-rrl (23S) intergenic spacer amplicon. Monoclonal antibody H3TS had 100% sensitivity for 55 B. burgdorferi ss isolates but showed reactivity with all four isolates belonging to B. bissetii. Monoclonal antibody I 17.3 showed 100% specificity and sensitivity for 45 B. afzelii isolates. Monoclonal antibody D6 was 100% specific for B. garinii but missed 1 of 64 isolates (98.5% sensitivity). Monoclonal antibody A116k was 100% specific for B. valaisiana but was unreactive with 4 of 24 isolates (83.5% sensitivity). Genetic analysis correlated well with results of reactivity and confirmed efficacy of the phenotypic typing of these antibodies. Some isolates showed atypical RFLP. Therefore, both phenotypic and genotypic analyses are needed to characterize new Borrelia isolates.
Borrelia burgdorferi, the agent of Lyme borreliosis, has been found to be genetically more heterogeneous than previously thought. In Europe, five genospecies have been described from the original B. burgdorferi, now called B. burgdorferi sensu lato: B. burgdorferi sensu stricto, B. garinii, B. afzelii, B. lusitaniae, and B. valaisiana (3, 9, 19, 32). Barbour et al. (4) and Wilske et al. (35) first provided early evidence of the heterogeneity among European isolates of B. burgdorferi, whereas U.S. isolates appeared to be a homogeneous group except for a few variants observed in California (6, 25) and on the East Coast from Ixodes dentatus (B. andersonii). In Asia, some new species have been differentiated as B. japonica and B. turdae. B. garinii, B. afzelii, and B. valaisiana also appear to be endemic species (11; T. Masuzawa, Y. Imai, and Y. Yanagihara, Abstr. VIII. Int. Conf. Lyme Borreliosis Other Tick-Borne Dis., 1999, abstr. O5, p. 5).
The European borreliae apparently have distinct reservoir hosts. Apodemus mice and Clethrionomys glareolus voles appear to be the main reservoirs for B. afzelii (14, 16). Similarly, birds of the genus Turdus are reservoirs for B. garinii and B. valaisiana (15), and the red squirrel Sciurus vulgaris might be a reservoir for B. burgdorferi sensu stricto and B. afzelii (13). Although not absolute, associations of particular clinical manifestations in humans with distinct species of B. burgdorferi sensu lato have been documented. Acrodermatitis chronica atrophicans is clearly associated with infection due to B. afzelii (9, 10). Patients with Lyme arthritis are more often infected with B. burgdorferi sensu stricto (18) or show higher serological reactions with this particular Borrelia species, and patients with neuroborreliosis are more frequently infected with B. garinii (8) or present serological reactions in accordance with this association (1, 2, 24, 29). We have previously reported serological evidence for a pathogenic potential of B. valaisiana in humans (29). Sera from three patients with neuroborreliosis and from one patient with Lyme arthritis showed higher reactivity with this Borrelia species.
Genetic analysis based on 16S rDNA, restriction fragment length polymorphism (RFLP), arbitrarily primed PCR, and other methods for phylogenetic study of bacterial population, such as multilocus enzyme electrophoresis, all confirmed the subdivision of B. burgdorferi sensu lato into different species worldwide.
The serotyping method developed by Wilske et al. (34) and classification based on protein profiles provided similar data. Monoclonal antibodies specific to some of these species have been described (3, 9, 22), and a new monoclonal antibody to B. valaisiana has been produced in our laboratory. In the present study, we evaluated the specificity and sensitivity of four species-specific monoclonal antibodies based on the analysis of 210 isolates of B. burgdorferi sensu lato.
MATERIALS AND METHODS
Culture of Borrelia isolates.
All isolates (Table 1) were cultured in BSK II medium at 34°C, and spirochetes were harvested during the late log phase by centrifugation at 10,000 × g for 10 min. The pellet was washed twice in phosphate-buffered saline with 5 mM MgCl2 and finally resuspended in distilled water. Protein concentration was adjusted to 1 mg/ml. The preparation was frozen at −20°C until use.
TABLE 1.
B. burgdorferi sensu lato isolates evaluated in this studya
| Strain | Country of isolation | Biological origin | RFLP pattern | Source |
|---|---|---|---|---|
| B. afzelii | ||||
| 934U | Korea | Apodemus agrarius | D | K.-J. Hwang |
| A26S | The Netherlands | Human (skin) | D | A. J. van Dam |
| A38S | The Netherlands | Human (skin) | D | A. J. van Dam |
| A39S | The Netherlands | Human (skin) | D | A. J. van Dam |
| A40S | The Netherlands | Human (skin) | D | A. J. van Dam |
| A42S | The Netherlands | Human (skin) | D | A. J. van Dam |
| A45aS | The Netherlands | Human (skin) | D | A. J. van Dam |
| A51T | The Netherlands | I. ricinus | D | A. J. van Dam |
| A58T | The Netherlands | I. ricinus | D | A. J. van Dam |
| A59T | The Netherlands | I. ricinus | D | A. J. van Dam |
| A76S | The Netherlands | Human (skin) | D | A. J. van Dam |
| A100S | The Netherlands | Human (skin) | D | A. J. van Dam |
| ACA1 | Sweden | Human (skin) | D | S. Bergström |
| BO23 | Germany | Human (skin) | D | B. Wilske and V. Preac-Mursic |
| DK3 | Denmark | Human (skin) | D | T. Balmelli |
| DK8 | Denmark | Human (skin) | D | T. Balmelli |
| F1 | Sweden | I. ricinus | D | S. Bergström |
| IP3 | CIS | I. persulcatus | D | S. Bergström |
| Iper | Japan | I. persulcatus | D | D. Postic |
| M7 | China | I. persulcatus | D | D. Postic |
| M55 | The Netherlands | I. ricinus | D | A. van den Bogaard |
| NE28 | Switzerland | C. glareolus | D | P. F. Humair |
| NE29 | Switzerland | C. glareolus | D | P. F. Humair |
| NE30 | Switzerland | C. glareolus | D | P. F. Humair |
| NE35 | Switzerland | Apodemus flavicollis | nd | P. F. Humair |
| NE36 | Switzerland | C. glareolus | D | P. F. Humair |
| NE39 | Switzerland | C. glareolus | D | P. F. Humair |
| NE42 | Switzerland | C. glareolus | D | P. F. Humair |
| NE43 | Switzerland | C. glareolus | D | P. F. Humair |
| NE44 | Switzerland | C. glareolus | D | P. F. Humair |
| NE45 | Switzerland | C. glareolus | D | P. F. Humair |
| NE53 | Switzerland | I. ricinus | D | P. F. Humair |
| P/Sto | Germany | Human (skin) | D | B. Wilske and V. Preac-Mursic |
| PGau | Germany | Human (skin) | D | B. Wilske and V. Preac-Mursic |
| PKo 2-85 | Germany | Human (skin) | D | B. Wilske and V. Preac-Mursic |
| Pspe | Germany | Human (CSF) | D | B. Wilske and V. Preac-Mursic |
| Pwud I | Germany | Human (skin) | D | B. Wilske and V. Preac-Mursic |
| SMS1 | Sweden | Apodemus flavicollis | D | D. Postic |
| SO2 | United Kingdom | I. ricinus | D | S. J. Cutler |
| UM01 | Sweden | Human (skin) | D | S. Bergström |
| VS18 | Switzerland | I. ricinus | D | OL |
| VS25R-Or | Switzerland | Apodemus flavicollis | D | OL |
| VS42 | Switzerland | I. ricinus | D | OL |
| VS42R-R | Switzerland | Apodemus sylvaticus | D | OL |
| VS461 | Switzerland | I. ricinus | D | OL |
| B. andersonii | ||||
| 19952 | United States | I. dentatus | L | G. Baranton |
| 21123 | United States | I. dentatus | L | G. Baranton |
| B. bissettii | ||||
| 25015 | United States | I. scapularis | K | G. Baranton |
| CA-128 | United States | I. neotomae | J | D. Postic |
| CA-55 | United States | I. neotomae | J | D. Postic |
| DN127 | United States | I. pacificus | I | G. Baranton |
| B. burgdorferi sensu stricto | ||||
| 297 | United States | Human (CSF) | A | D. Postic |
| 13062 | Yugoslavia | I. ricinus | A | J. Wilhelm |
| 13063 | Yugoslavia | I. ricinus | A | J. Wilhelm |
| 20006 | France | I. ricinus | A | G. Baranton |
| A44S | The Netherlands | Human (skin) | A1 | A. J. van Dam |
| ATCC35211 | Switzerland | I. ricinus | A | A. Barbour |
| B31 | United States | I. scapularis | A | W. Burgdorfer |
| BE1 | Switzerland | Human (synovial fluid) | A | J. Schmidli |
| CA-2-87 | United States | I. pacificus | A1 | D. Postic |
| CA-5 | United States | I. pacificus | A | D. Postic |
| Charlie Tick | United States | I. scapularis | A1 | G. Baranton |
| Geho | Germany | Human (skin) | A | B. Wilske and V. Preac-Mursic |
| HUM3336 | United States | I. pacificus | A | D. Postic |
| IP1 | France | Human (CSF) | A | G. Baranton |
| IP2 | France | Human (CSF) | A | G. Baranton |
| IP3 | France | Human (CSF) | A | G. Baranton |
| IRS | Switzerland | I. ricinus | A | A. Barbour |
| IXD | United States | I. scapularis | A | W. Burgdorfer |
| M14 | The Netherlands | I. ricinus | A | A. van den Bogaard |
| MAC3EMCNY86 | United States | Human (skin) | A | W. Burgdorfer |
| NE48 | Switzerland | I. ricinus | A | P. F. Humair |
| NE49 | Switzerland | I. ricinus | A2 | L. Gern |
| NE50 | Switzerland | I. ricinus | A1 | P. F. Humair |
| NE56 | Switzerland | I. ricinus | A | P. F. Humair |
| PBre | Germany | Human (skin) | A | B. Wilske and V. Preac-Mursic |
| PKA | Germany | Human (skin) | A | B. Wilske and V. Preac-Mursic |
| SON328 | United States | I. pacificus | A | D. Postic |
| VS2 | United States | I. scapularis | A | OL |
| VS14 | Switzerland | I. ricinus | A | OL |
| VS44 | Switzerland | I. ricinus | A | OL |
| VS73 | Switzerland | I. ricinus | A1 | OL |
| VS82 | Switzerland | I. ricinus | A1 | OL |
| VS106 | Switzerland | I. ricinus | A1 | OL |
| VS108 | Switzerland | I. ricinus | A1 | OL |
| VS109 | Switzerland | I. ricinus | A | OL |
| VS115 | Switzerland | I. ricinus | A1 | OL |
| VS123 | Switzerland | I. ricinus | A | OL |
| VS130 | Switzerland | I. ricinus | A | OL |
| VS134 | Switzerland | I. ricinus | A1 | OL |
| VS137 | Switzerland | I. ricinus | A1 | OL |
| VS139 | Switzerland | I. ricinus | A1 | OL |
| VS146 | Switzerland | I. ricinus | A1 | OL |
| VS149 | Switzerland | I. ricinus | A1 | OL |
| VS161 | Switzerland | I. ricinus | A1 | OL |
| VS206 | Switzerland | I. ricinus | A | OL |
| VS215 | Switzerland | I. ricinus | A | OL |
| VS219 | Switzerland | I. ricinus | A | OL |
| VS293 | Switzerland | I. ricinus | A | OL |
| VS393 | Switzerland | I. ricinus | A | OL |
| VS396 | Switzerland | I. ricinus | A | OL |
| VS405 | Switzerland | I. ricinus | A | OL |
| VS423 | Switzerland | I. ricinus | A | OL |
| VS619 | Switzerland | I. ricinus | A1 | OL |
| VS623 | Switzerland | I. ricinus | A1 | OL |
| VS753 | Switzerland | I. ricinus | A | OL |
| B. garinii | ||||
| 387 | Germany | Human (CSF) | B | G. Baranton |
| 20047 | France | I. ricinus | B | G. Baranton |
| 935T | Korea | I. persulcatus | B1 | K.-J. Hwang |
| A19S | The Netherlands | Human (skin) | B2 | A. J. van Dam |
| A77C | The Netherlands | Human (CSF) | B | A. J. van Dam |
| AR-1 | The Netherlands | I. ricinus | B | S. Rijpkema |
| BB153 | France | I. ricinus | B | D. Postic |
| BITS | Italy | I. ricinus | B | V. Sambri |
| FAR01 | Denmark | I. uriae | B | A. Gylfe |
| FAR02 | Denmark | I. uriae | B | A. Gylfe |
| FIS01 | Iceland | I. uriae | B | A. Gylfe |
| G25 | Sweden | I. ricinus | B | D. Postic |
| HP3 | Japan | I. persulcatus | B | D. Postic |
| Ip89 | CIS | I. persulcatus | C | G. Baranton |
| Ip90 | CIS | I. persulcatus | B | S. Bergström |
| IUB18 | Sweden | I. uriae | B1 | A. Gylfe |
| M50 | The Netherlands | I. ricinus | B | A. van den Bogaard |
| M63 | The Netherlands | I. ricinus | B | A. van den Bogaard |
| N34 | Germany | I. ricinus | B | G. Baranton |
| NBS16 | Sweden | I. ricinus | B1 | S. Bergström |
| NBS23a | Sweden | I. ricinus | B | S. Bergström |
| NE2 | Switzerland | I. ricinus | nd | L. Gern |
| NE11H | Switzerland | I. ricinus | B | L. Gern |
| NE47 | Switzerland | I. ricinus | B | P. F. Humair |
| NE51 | Switzerland | I. ricinus | B | P. F. Humair |
| NE52 | Switzerland | I. ricinus | B | P. F. Humair |
| NE58 | Switzerland | I. ricinus | B | L. Gern |
| NE60 | Switzerland | I. ricinus | B | L. Gern |
| NE83 | Switzerland | I. ricinus | B | L. Gern |
| NE84 | Switzerland | I. ricinus | B | L. Gern |
| NT29 | Japan | I. persulcatus | C | G. Baranton |
| P/Bi | Germany | Human (CSF) | B | B. Wilske and V. Preac-Mursic |
| P/Br | Germany | Human (CSF) | B | B. Wilske and V. Preac-Mursic |
| PD89 | China | Human (blood) | B | D. Postic |
| Pwud II | Germany | I. ricinus | B | B. Wilske and V. Preac-Mursic |
| SIKA1 | Japan | I. ovatus | B | D. Postic |
| SIKA2 | Japan | I. persulcatus | B | D. Postic |
| SO1 | United Kingdom | I. ricinus | B | S. J. Cutler |
| T25 | Germany | I. ricinus | B | D. Postic |
| TN | Germany | I. ricinus | B | D. Postic |
| VS3 | Switzerland | I. ricinus | B | OL |
| VS100 | Switzerland | I. ricinus | B | OL |
| VS102 | Switzerland | I. ricinus | B | OL |
| VS156 | Switzerland | I. ricinus | B | OL |
| VS185 | Switzerland | I. ricinus | B | OL |
| VS244 | Switzerland | I. ricinus | B | OL |
| VS277 | Switzerland | I. ricinus | B | OL |
| VS286 | Switzerland | I. ricinus | B | OL |
| VS290 | Switzerland | I. ricinus | B | OL |
| VS307 | Switzerland | I. ricinus | B | OL |
| VS416 | Switzerland | I. ricinus | B | OL |
| VS421 | Switzerland | I. ricinus | B | OL |
| VS464 | Switzerland | I. ricinus | B | OL |
| VS468 | Switzerland | I. ricinus | B | OL |
| VS488 | Switzerland | I. ricinus | B1 | OL |
| VS492 | Switzerland | I. ricinus | B1 | OL |
| VS518 | Switzerland | I. ricinus | B | OL |
| VS600 | Switzerland | I. ricinus | B | OL |
| VS641 | Switzerland | I. ricinus | B | OL |
| VS704 | Switzerland | I. ricinus | B | OL |
| VS711 | Switzerland | I. ricinus | B1 | OL |
| VSBM | Switzerland | Human (CSF) | B | OL |
| VSBP | Switzerland | Human (CSF) | B | OL |
| VSDA | Switzerland | Human (CSF) | B1 | OL |
| B. japonica | ||||
| COW611a | Japan | I. ovatus | E | G. Baranton |
| COW611c | Japan | I. ovatus | E | G. Baranton |
| F63B | Japan | I. ovatus | E | G. Baranton |
| Fi340 | Japan | I. ovatus | E | T. Masuzawa |
| FiAE2 | Japan | Apodemus speciosus | E | T. Masuzawa |
| FiEE2 | Japan | Eothenomys smithi | E | T. Masuzawa |
| FsAE4 | Japan | Apodemus argenteus | E | T. Masuzawa |
| HO14 | Japan | I. ovatus | E | D. Postic |
| IKA2 | Japan | I. ovatus | — | D. Postic |
| O612 | Japan | I. ovatus | E | G. Baranton |
| B. lusitaniae | ||||
| BR41 | Czech Republic | I. ricinus | G | D. Postic |
| IR345 | Belorussia | I. ricinus | G | D. Postic |
| POTIB1 | Portugal | I. ricinus | G | S. Nuncio |
| POTIB2 | Portugal | I. ricinus | G | S. Nuncio |
| POTIB3 | Portugal | I. ricinus | H | S. Nuncio |
| B. valaisiana | ||||
| AG1 | Switzerland | I. ricinus | F | I. Heinzer |
| AR-2 | The Netherlands | I. ricinus | F | S. Rijpkema |
| F10.8.94 | Germany | I. ricinus | F | M. M. Wittenbrink |
| Frank | Germany | I. ricinus | F | M. M. Wittenbrink |
| M7 | The Netherlands | I. ricinus | F | A. P. van Dam |
| M19 | The Netherlands | I. ricinus | F | A. van den Bogaard |
| M52 | The Netherlands | I. ricinus | F | A. P. van Dam |
| M53 | The Netherlands | I. ricinus | F | A. P. van Dam |
| M57 | The Netherlands | I. ricinus | F | A. van den Bogaard |
| NE168 | Switzerland | I. ricinus | F | P. F. Humair |
| NE218 | Switzerland | Turdus merula | F | P. F. Humair |
| NE223 | Switzerland | Turdus merula | F | P. F. Humair |
| NE224 | Switzerland | Turdus merula | F | P. F. Humair |
| NE225 | Switzerland | Turdus merula | F | P. F. Humair |
| NE226 | Switzerland | I. ricinus | F | P. F. Humair |
| NE229 | Switzerland | Turdus merula | F | P. F. Humair |
| NE230 | Switzerland | Turdus merula | F | P. F. Humair |
| NE231 | Switzerland | Turdus merula | F | P. F. Humair |
| NE248 | Switzerland | I. ricinus | F | P. F. Humair |
| NE253 | Switzerland | Turdus merula | F | P. F. Humair |
| UK | United Kingdom | I. ricinus | F | S. J. Cutler |
| VS116 | Switzerland | I. ricinus | F | OL |
| VS732 | Switzerland | I. ricinus | F | OL |
| Z6.11.93 | Germany | I. ricinus | F | M. M. Wittenbrink |
| H11 | Italy | Human (blood) | B | V. Sambri |
| B. anserina | United States | Ornithodorus anserina | nd | T. Schwan |
| B. coriaceae | United States | Ornithodorus coriaceae | nd | T. Schwan |
| B. hermsii | United States | Ornithodorus hermsii | nd | T. Schwan |
| B. parkeri | United States | Ornithodorus parkeri | nd | T. Schwan |
| B. turicata | United States | Ornithodorus turicata | nd | T. Schwan |
Abbreviations: CIS, Commonwealth of Independent States; OL, our laboratory; nd, not determined. —, no specific amplification.
T. Balmelli, G. Baranton, A. G. Barbour, S. Bergström, A. van den Bogaard, W. Burgdorfer, S. J. Cutler, A. J. van Dam, L. Gern, A. Gylfe, I. Heinzer, P. F. Humair, K.-J. Hwang, T. Masuzawa, S. Nuncio, D. Postic, V. Preac-Mursic, S. Rijpkema, V. Sambri, J. Schmidli, T. Schwan, J. Wilhelm, M. M. Wittenbrink, and B. Wilske kindly provided us with various isolates.
Phenotypic typing of B. burgdorferi sensu lato.
Electrophoresis and immunoblots were performed as previously described (23). Briefly, a suspension of washed borreliae (protein concentration, 1 mg/ml) was dissolved (1:1) in sample buffer with 0.6% sodium dodecyl sulfate (final concentration) and 50 mM dithiothreitol as a reducing agent. The samples were boiled for 5 min before undergoing electrophoresis (constant voltage, 170 V) on a polyacrylamide gel at 12.5% for the separating gel. Standards (Bio-Rad low-range protein molecular weight standards) were used as a reference for the calculation of relative molecular masses. After electrophoresis, proteins were transferred by Western blot to polyvinylidene difluoride (Immobilon; Millipore, Bedford, Mass.) membranes.
After transfer, the membrane was stained with Coomassie blue. The membrane was then cut at the level of OspA and OspB as well as below the 14.4-kDa marker, and these two pieces were destained in a bath of pure methanol for a few seconds. They were saturated with 5% gelatin in a Tris-NaCl buffer (pH 7.5) for 1 h at 37°C and washed three times for 5 min each in a Tris-Tween 20 (0.05%) buffer containing 0.1% gelatin. The pieces containing OspA and OspB were incubated for 2 h at room temperature with monoclonal antibodies H3TS (Symbicon, Stockholm, Sweden) or A116k (K. Ryffel, unpublished data) and I17.3 (kindly provided by G. Baranton) (9) diluted 1:500, 1:1,000 and 1:500,000, respectively, in the same buffer with 1% gelatin. The piece below 14.4 kDa was incubated as described above with monoclonal antibody D6 (22) diluted 1:100. After washing, monoclonal antibodies fixed specifically on the antigens were demonstrated by a second goat anti-mouse immunoglobulin for H3TS, A116k, and I17.3 monoclonal antibodies or goat anti-mouse immunoglobulin M (μ-chain specific) for D6 monoclonal antibody conjugated to alkaline phosphatase, followed by three washes and the addition of 5-bromo-4-chloro-3-indolyl p-toluidine phosphate and p-nitroblue tetrazolium chloride substrate (Kirkegaard and Perry Laboratories, Gaithersburg, Md.).
At least one isolate each of B. burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana were run in each blot as positive controls for reactivity with the monoclonal antibodies.
Genotypic typing.
The method described by Postic (25) was used for typing, using the restriction pattern of amplicons in the rrf (5S)-rrl (23S) intergenic spacer region.
In short, 50 μl of reaction mixture containing 5 μl of bacterial thermolysate (95°C for 10 min) with 2 U of Extra-Pol II DNA polymerase (Eurobio, Les Ulis, France) and 200 μmole of deoxynucleoside triphosphate mix were subjected to 40 cycles (94°C for 1 min, 55°C for 1 min, 72°C for 1 min) in a Perkin-Elmer GeneAmp PCR System 9600.
Several negative controls were included in each run as well as reference strains. Amplified products were electrophoresed on 2% agarose gels stained with ethidium bromide at 0.5 μg/ml.
A total of 210 amplicons were digested overnight at 37°C with restriction endonucleases MseI (2.5 U/10 μl of PCR product), and if needed (43 amplicons) with DraI (10 U/10 μl of PCR product) (Gibco-BRL, Life Technologies, Paisley, United Kingdom). The digested products were electrophoresed on 16% acrylamide-bisacrylamide (19:1) (Bio-Rad, Hercules, Calif.) for 3 h at 100 V.
To resolve particular RFLP, amplicons were purified using a Qiaquick PCR purification kit (Qiagen, Hilden, Germany). DNA sequencing was performed on a Li-Cor 4000 automated sequencer, using IRD800-labeled primers (Lincoln, Neb.) and Thermo-sequenase (Nycomed, Amersham, United Kingdom).
RESULTS
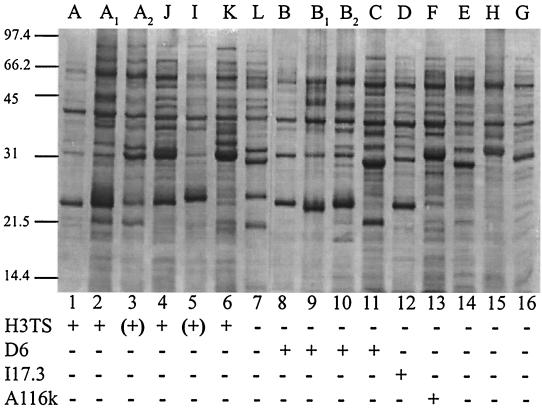
Protein profiles based on OspA and OspB allowed us to easily recognize three of the five European groups. Two of these showed both OspA and OspB with distinct electrophoretic mobility (Fig. 1). B. burgdorferi sensu stricto had an OspA of 31 kDa and an OspB of 34 kDa, and B. afzelii had an OspA of 32 kDa and an OspB of 35 kDa. The other groups presented a pattern with an OspA without an apparent OspB, also with distinct electrophoretic mobility, B. garinii of 32.5 kDa and B. valaisiana of 33 to 33.5 kDa. B. lusitaniae showed an OspA profile very similar to that of B. valaisiana, which did not provide sufficient characteristics to differentiate them. We used these distinct protein patterns to predict the reactivity of the isolates with species-specific monoclonal antibodies.
FIG. 1.
(Top) Protein profiles of B. burgdorferi sensu lato isolates after polyacrylamide gel electrophoresis and Western blot on polyvinylidene difluoride membrane stained with Coomassie blue. Representative isolates of each Borrelia genospecies are ordered according to their RFLP pattern. Sizes are shown in kilodaltons. Lanes 1 to 3, B. burgdorferi sensu stricto (VS215, VS619, and NE49). Lanes 4 to 6, B. bissettii (CA128, DN127, and 25015). Lane 7, B. andersonii (21123). Lanes 8 to 11, B. garinii (VS102, VSDA, AS19s, and NT29). Lane 12, B. afzelii (VS461). Lane 13, B. valaisiana (VS116). Lane 14, B. japonica (FiAE2). Lanes 15 and 16, B. lusitaniae (PotiB3 and IR345). (Bottom) Reactivity with monoclonal antibodies. (+), weak reactivity.
H3TS was confirmed to react specifically with OspA of all B. burgdorferi sensu stricto isolates expressing an OspA of 31 kDa and an OspB of 34 kDa. All 55 isolates originally classified as B. burgdorferi sensu stricto were reactive with H3TS. In addition, four B. bissettii evaluated in this study, two isolates from Ixodes neotomae (CA128 and CA55), one from Ixodes scapularis (25015), and one from Ixodes pacificus (DN 127) were also reactive (Table 2). This last isolate reacted very weakly. Isolate NE49, whose OspA and OspB profiles are typical of B. burgdorferi sensu stricto, showed at most very weak reactivity with H3TS. The 156 other isolates were not reactive with this monoclonal antibody.
TABLE 2.
Sensitivity and specificity of four monoclonal antibodies to 210 B. burgdorferi sensu lato isolates and five relapsing-fever Borrelia species
| Antibodya | No. of isolates unreactive/no. tested (% specificity) | No. of isolates detected/total (% sensitivity) | Species |
|---|---|---|---|
| H3TS | 156/160 (97.5) | 55/55 (100) | B. burgdorferi sensu stricto |
| D6 | 151/151 (100) | 63/64 (98.5) | B. garinii |
| I17.3 | 170/170 (100) | 45/45 (100) | B. afzelii |
| A116k | 87/87 (100) | 20/24 (83.5) | B. valaisiana |
H3TS showed cross-reactivity with four isolates of B. bissettii and weak reactivity with isolate NE49.
Among the 64 isolates classified as B. garinii with respect to their protein profile (OspA of 32.5 kDa), only strain 935T isolated from a Korean Ixodes persulcatus did not react with monoclonal antibody D6. We observed that a few isolates (NT29, IP89, and A19S) presented a protein reactive with monoclonal antibody D6 of lower molecular mass (<12 kDa) than the other B. garinii isolates (Table 2). Monoclonal antibody D6 did not react with any of 151 isolates belonging to other species. Similarly, only the 45 isolates of B. afzelii were all specifically recognized by monoclonal antibody I17.3. These isolates typically had an OspA of 32 kDa and an OspB of 35 kDa (Table 2). No other of 170 isolates were reactive with this monoclonal antibody.
The specificity and sensitivity of monoclonal antibody A116k were evaluated with 111 Borrelia isolates, including all the B. valaisiana isolates available (Table 2). This monoclonal antibody appears to be specific but was not reactive with 4 of the 24 B. valaisiana isolates.
The four monoclonal antibodies tested did not show any reaction with B. anserina, B. coriaceae, B. hermsii, B. parkeri, B. turicata, B. japonica, B. lusitaniae, or B. andersonii.
Monoclonal antibodies D6, I17.3, and A116k showed 100% specificity, whereas H3TS had a specificity of 92.5% (12 of 13 evaluated species) or 98% with respect to all isolates examined (211 of 215). Sensitivity of 100% was observed with monoclonal antibodies H3TS (55 of 55) and I17.3 (45 of 45), 98.5% (63 of 64) with monoclonal antibody D6, and 83.5% (20 of 24) with monoclonal antibody A116k (Table 2).
One isolate (H11) was received as a mixture of two Borrelia species (B. burgdorferi sensu stricto and B. garinii). In the first passages, H11 was confirmed to be B. burgdorferi sensu stricto, and after two to four additional passages in our BSK II medium, a mixed population of B. garinii and B. burgdorferi sensu stricto was observed. In further passages, only the B. garinii population of spirochetes persisted. Similar results were obtained from several aliquots of an original culture that we have received. We could clearly observe the appearance of the mixed populations with the Osp profiles on the Coomassie blue staining as well as with the reactivity with monoclonal antibodies H3TS and D6.
Genotypic typing.
Confirmation of the phenotypic determination was made at the genetic level. Amplicons generated by PCR in the rrf (5S)-rrl (23S) intergenic spacer region had sizes of about 250 bp (226 to 266 bp). The isolate IKA2 generated a nonspecific fragment of about 700 bp.
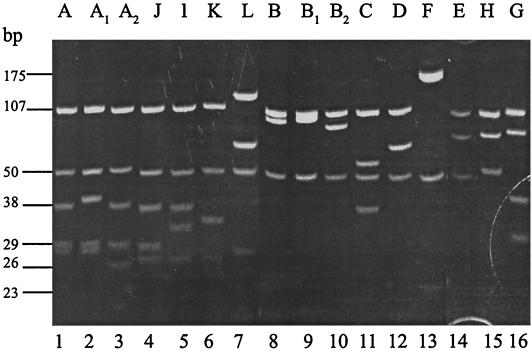
Several restriction patterns were observed for B. burgdorferi sensu stricto, B. garinii, and B. lusitaniae, including some hitherto undescribed patterns (Fig. 2 and Table 3).
FIG. 2.
Msel restriction patterns of rrf-rrl intergenic spacer amplicons of B. burgdorferi sensu lato. Representative isolates of each Borrelia genospecies are ordered according to their RFLP pattern. Lanes 1 to 3, B. burgdorferi sensu stricto (VS219, VS619, and NE49). Lanes 4 to 6, B. bissettii (CA128, DN127, and 25015). Lane 7, B. andersonii (21123). Lanes 8 to 11: B. garinii (VS618, VSDA, AS19s, and IP89). Lane 12, B. afzelii (VS18). Lane 13, B. valaisiana (AG1). Lane 14, B. japonica (FiAE2). Lanes 15 and 16, B. lusitaniae (PotiB3 and IR345).
TABLE 3.
MseI and DraI restriction fragments of rrf-rrl intergenic spacer ampliconsa
| Pattern | Sizes (bp) of fragments produced by MseI | No. of isolates | Sizes (bp) of fragments produced by DraI | No. of isolates |
|---|---|---|---|---|
| A | 107, 52, 38, 29, 28 | 37 | 144, 53, 29, 28 | 2 |
| A1 | 107, 52, 40, 29, <28 | 17b | 146, 53, 29, 28 | 10 |
| A2 | 107, 52, 38, 29, 26 | 1b | 144, 80, 29 | 1 |
| B | 107, 95, 50 | 54 | 204, 49 | 4 |
| B1 | 107, 98, 50 | 7b | 204, 49 | 7 |
| B2 | 107, (93–94), 50 | 1 | 204, 49 | 1 |
| C | 107, 57, 50, 38 | 2 | nd | |
| D | 107, 68, 50, 20 | 45 | nd | |
| E | 107, 78, 50 | 9 | 235 | 3 |
| F | 175, 50, (23), (7) | 24 | 206, 49 | 1 |
| G | 107, 81, 39, 29 | 4 | nd | |
| H | 107, 79, 52, (16) | 1 | nd | |
| I | 107, 52, 38, 33, 27 | 1 | 144, 53, 33, 27 | 1 |
| J | 107, 52, 38, 29, 27 | 2 | 144, 53, 29, 27 | 2 |
| K | 107, 52, 34, 27, (17), (12), (4) | 1 | 174, 53, 27 | 1 |
| L | 120, 67, 52, 28 | 2 | nd |
DraI fragments were defined by Postic et al. (25–27). Parentheses indicate that the fragment was not always detected in gels or of the estimated size. nd, not done.
Sequence from this study and Péter et al. (24).
The majority of B. burgdorferi sensu stricto isolates (37 of 55, 67%) showed the typical MseI pattern A, whereas 17 isolates (31%), including 13 isolates from our region (Valais, Switzerland), revealed a pattern referred to as A1 (Table 3). The isolates belonging to pattern A1 possessed a particular fragment of 40 bp instead of the 38-bp fragment. One isolate, NE49, presented a unique pattern, with a fragment of 26 bp instead of 28 bp. After DraI digestion, 11 isolates with the A or A1 RFLP exhibited identical patterns, but NE49 (A2) showed three fragments of 144, 80, and 29 bp instead of four fragments of 144, 53, 29, and 28 bp.
Among the 64 B. garinii isolates analyzed, 54 (84%) had RFLP pattern B, identical to reference strain 20047 after digestion with MseI. Two isolates showed pattern C, and seven other isolates had a particular pattern called B1, with a fragment of 98 bp instead of the expected 95-bp fragment. In one isolate from the Netherlands, A19S, a fragment of 93 to 94 bp was observed (not sequenced). This pattern was called B2. DraI digestion of 12 amplicons with RFLP patterns B, B1, and B2 resulted in the same RFLP, with fragments of 204 and 49 bp, respectively.
All 45 B. afzelii isolates analyzed showed pattern D after digestion of amplicons with MseI, although the 20-bp fragment was rarely observed in our gels. The 24 B. valaisiana amplicons (pattern F) consistentely showed the typical fragment of 175 bp after MseI digestion, along with the other fragments, though the 7-bp fragment was never detected. B. andersonii isolates (pattern L) were also easily identified by their 120-bp fragment, characteristic of this Borrelia species, after MseI digestion. The B. lusitaniae isolates presented two patterns, named G and H. Since the expected 16-bp fragment was not visible on our gel, pattern H was almost indistinguishable from pattern E described for B. japonica. The four B. bissettii fell within three different patterns named I, J, and K, with pattern J being close to pattern A of B. burgdorferi sensu stricto.
All the isolates classified by phenotypic characteristics were confirmed by genetic analysis. By RFLP analysis, we were also able to show the presence of two Borrelia species (B. burgdorferi sensu stricto and B. garinii) in the H11 isolate, as detected by phenotypic analysis as well.
DISCUSSION
Comparison of both phenotypic and genotypic analyses revealed an excellent agreement for isolates belonging to B. burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana, each specifically recognized by species-specific monoclonal antibodies. Monoclonal antibody H3TS had an estimated sensitivity of 100% to B. burgdorferi sensu stricto, but was also reactive with some B. bissettii isolates. Monoclonal antibody I17.3 recognized all and only the B. afzelii isolates, revealing both a sensitivity and a specificity of 100%. Monoclonal antibody D6 was reactive to all B. garinii isolates except one isolate from Korea. Specificity was 100%, and estimated sensitivity was 98.5% (63 of 64).
The specificity of monoclonal antibody A116k was 100%, and the sensitivity was about 83.5%. The OspA to which this monoclonal antibody is reactive appears to be quite heterogeneous in B. valaisiana isolates, showing different electrophoretic mobilities, as reported previously (32). Based on both the electrophoretic mobility of the OspA and OspB (22) and the reactivity of monoclonal antibodies H3TS, I17.3, D6, and A116k, the phenotypic characterization of new European Borrelia isolates is efficient. At the regional level, we were able to define the Borrelia species merely from the electrophoretic profile of OspA and OspB. For example, of 50 local isolates, 48 were classified correctly, and two isolates belonging to B. valaisiana would not have been differentiated from B. lusitaniae isolates. We realize that our observation still needs confirmation by specific methods with monoclonal antibodies and further genetic analysis.
The genetic analysis by RFLP with MseI, as described by Postic et al. (25, 26), is an excellent and powerful typing method for B. burgdorferi sensu lato isolates. Isolate NE49 was readily identified as a new subgroup in B. burgdorferi sensu stricto. This observation was confirmed by Postic et al. (27). All the other RFLP groups were previously described by Postic et al. (25). However, about 10% of all isolates needed a second step of digestion with the enzyme DraI. Some RFLP patterns were not easily resolved, above all when a difference of ±2 bp was observed on one fragment (i.e., groups A, A1, A2, and J or groups E and H). In order to interpret this, the sequence of such amplicons will have to be determined. One additional problem arose with short restriction fragments (<20 bp). These fragments were usually not visible in our gels and thus were not informative. Only full sequencing of the amplicons allowed us to determine the original size of such small fragments.
Among all the isolates investigated, we noticed that different B. burgdorferi sensu lato isolates have identical names, such as M7, one isolated from Ixodes ricinus in the Netherlands (B. valaisiana), and one isolated from I. persulcatus in China (B. afzelii), or IP3, isolated from human cerebrospinal fluid (CSF) in France (B. burgdorferi sensu stricto) and one isolated from I. persulcatus in the Commonwealth of Independent States (B. afzelii). One B. garinii referred to in this study as isolate M50 (isolated from I. ricinus in the Netherlands) was typed as B. valaisiana in a previous report (32). Similarly, isolate A76S was clearly identified as B. afzelii in this study but was previously described as B. garinii (31). We do not know whether these isolates were not initially pure and if further passages in culture medium in the two laboratories have selected two different isolates, or if these isolates were incorrectly labeled. In our hands, these isolates were never found to be a mixture of two Borrelia species.
Other genetic methods often used, such as DNA-DNA hybridization (3), pulsed-field electrophoresis (5), multilocus enzyme electrophoresis (7), arbitrarily primed PCR (33), specific typing of 16S rDNA (20), and species-specific hybridization (28), allow us to type B. burgdorferi sensu lato isolates. All these methods have confirmed the presence of these different species among B. burgdorferi sensu lato, with some particular geographical distribution (30). There is no doubt that the description of these different Borrelia species allowed us to better understand the complex natural history of Lyme borreliosis in Europe. For example, different reservoir hosts were described for some Borrelia species (13–15). Similarly, several reports have suggested an association of particular clinical symptoms in human with some Borrelia species (1, 2, 10, 24, 29). The typing of B. burgdorferi sensu lato isolates is essential to clarify this specific point and consequently to understand the physiopathology of each Borrelia species (12, 17, 21).
Our results have shown that some B. burgdorferi sensu lato isolates cannot be easily typed by genetic methods and need cumbersome techniques. In this respect, monoclonal antibodies may greatly help to type closely related isolates at one particular point of the genome. Therefore, phenotypic and genotypic methods appear to be complementary. Phenotypic methods, particularly monoclonal antibodies, are helpful epidemiological tools that may be essential for laboratories which lack facility in genetic methods.
ACKNOWLEDGMENTS
We are grateful to all the colleagues who provided us with Borrelia isolates as well as with monoclonal antibodies.
This work was supported by the Institut Central des Hôpitaux Valaisans and the Fonds National Suisse de la Recherche Scientifique (grant 32-52739.97).
REFERENCES
- 1.Anthonissen F M, De Kessel M, Hoet P P, Bigaignon G H. Evidence for the involvement of different genospecies of Borrelia in the clinical outcome of Lyme disease in Belgium. Res Microbiol. 1994;145:327–331. doi: 10.1016/0923-2508(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 2.Assous M V, Postic D, Paul G, Nevot P, Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borrelia strains used as antigens. Eur J Clin Microbiol Infect Dis. 1993;12:261–268. doi: 10.1007/BF01967256. [DOI] [PubMed] [Google Scholar]
- 3.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 4.Barbour A G, Heiland R A, Howe T R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985;152:478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- 5.Belfaiza J, Postic D, Bellenger E, Baranton G, Girons I S. Genomic fingerprinting of Borrelia burgdorferi sensu lato by pulsed- field gel electrophoresis. J Clin Microbiol. 1993;31:2873–2877. doi: 10.1128/jcm.31.11.2873-2877.1993. . (Erratum, 32:2040, 1994.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bissett M L, Hill W. Characterization of Borrelia burgdorferi strains isolated from Ixodes pacificus ticks in California. J Clin Microbiol. 1987;25:2296–2301. doi: 10.1128/jcm.25.12.2296-2301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boerlin P, Péter O, Bretz A G, Postic D, Baranton G, Piffaretti J C. Population genetic analysis of Borrelia burgdorferi isolates by multilocus enzyme electrophoresis. Infect Immun. 1992;60:1677–1683. doi: 10.1128/iai.60.4.1677-1683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busch U, Hizo-Teufel C, Boehmer R, Fingerle V, Nitschko H, Wilske B, Preac-Mursic V. Three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B. afzelii, and B. garinii) identified from cerebrospinal fluid isolates by pulsed-field gel electrophoresis and PCR. J Clin Microbiol. 1996;34:1072–1078. doi: 10.1128/jcm.34.5.1072-1078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canica M M, Nato F, du Merie L, Mazie J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzelii sp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 10.Dunand V, Bretz A G, Suard A, Praz G, Dayer E, Péter O. Acrodermatitis chronica atrophicans and serologic confirmation of infection due to Borrelia afzelii and/or Borrelia garinii by immunoblot. Clin Microbiol Infect. 1998;4:159–163. doi: 10.1111/j.1469-0691.1998.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 11.Fukunaga M, Hamase A, Okada K, Nakao M. Borrelia tanukii sp. nov. and Borrelia turdae sp. nov. found from ixodid ticks in Japan: rapid species identification by 16S rRNA gene-targeted PCR analysis. Microbiol Immunol. 1996;40:877–881. doi: 10.1111/j.1348-0421.1996.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Monco J C, Fernandez-Villar B, Rogers R C, Szczepanski A, Wheeler C M, Benach J L. Borrelia burgdorferi and other related spirochetes bind to galactocerebroside. Neurology. 1992;42:1341–1348. doi: 10.1212/wnl.42.7.1341. [DOI] [PubMed] [Google Scholar]
- 13.Humair P F, Gern L. Relationship between Borrelia burgdorferi sensu lato species, red squirrels (Sciurus vulgaris) and Ixodes ricinus in enzootic areas in Switzerland. Acta Trop. 1998;69:213–227. doi: 10.1016/s0001-706x(97)00126-5. [DOI] [PubMed] [Google Scholar]
- 14.Humair P F, Péter O, Wallich R, Gern L. Strain variation of Lyme disease spirochetes isolated from Ixodes ricinus ticks and rodents collected in two endemic areas in Switzerland. J Med Entomol. 1995;32:433–438. doi: 10.1093/jmedent/32.4.433. [DOI] [PubMed] [Google Scholar]
- 15.Humair P F, Postic D, Wallich R, Gern L. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zentralbl Bakteriol. 1998;287:521–538. [PubMed] [Google Scholar]
- 16.Humair P F, Rais O, Gern L. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology. 1999;118:33–42. doi: 10.1017/s0031182098003564. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs R. Borrelia burgdorferi bind to epithelial cell proteoglycan. J Clin Investig. 1994;93:809–819. doi: 10.1172/JCI117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaulhac B, Chary-Valckenaere I, Sibilia J, Javier R M, Piemont Y, Kuntz J L, Monteil H, Pourel J. Detection of Borrelia burgdorferi by DNA amplification in synovial tissue samples from patients with Lyme arthritis. Arthritis Rheum. 1996;39:736–745. doi: 10.1002/art.1780390505. [DOI] [PubMed] [Google Scholar]
- 19.Le Fleche A, Postic D, Girardet K, Péter O, Baranton G. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int J Syst Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- 20.Marconi R T, Garon C F. Identification of a third genomic group of Borrelia burgdorferi through signature nucleotide analysis and 16S rRNA sequence determination. J Gen Microbiol. 1992;138:533–536. doi: 10.1099/00221287-138-3-533. [DOI] [PubMed] [Google Scholar]
- 21.Parveen N, Robbins D, Leong J M. Strain variation in glycosaminoglycan recognition influences cell type-specific binding by Lyme disease spirochetes. Infect Immun. 1999;67:1743–1749. doi: 10.1128/iai.67.4.1743-1749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Péter O, Bretz A G. Polymorphism of outer surface proteins of Borrelia burgdorferi as a tool for classification. Zentralbl Bakteriol. 1992;277:28–33. doi: 10.1016/s0934-8840(11)80867-4. [DOI] [PubMed] [Google Scholar]
- 23.Péter O, Bretz A G, Bee D. Occurrence of different genospecies of Borrelia burgdorferi sensu lato in ixodid ticks of Valais, Switzerland. Eur J Epidemiol. 1995;11:463–467. doi: 10.1007/BF01721234. [DOI] [PubMed] [Google Scholar]
- 24.Péter O, Bretz A G, Postic D, Dayer E. Association of distinct species of Borrelia burgdorferi sensu lato with neuroborreliosis in Switzerland. Clin Microbiol Infect. 1997;3:423–431. doi: 10.1111/j.1469-0691.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 25.Postic D, Assous M V, Grimont P A, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 26.Postic D, Ras N M, Lane R S, Hendson M, Baranton G. Expanded diversity among Californian borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127) J Clin Microbiol. 1998;36:3497–3504. doi: 10.1128/jcm.36.12.3497-3504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postic D, Ras N M, Lane R S, Humair P, Wittenbrink M M, Baranton G. Common ancestry of Borrelia burgdorferi sensu lato strains from North America and Europe. J Clin Microbiol. 1999;37:3010–3012. doi: 10.1128/jcm.37.9.3010-3012.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rijpkema S G, Molkenboer M J, Schouls L M, Jongejan F, Schellekens J F. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J Clin Microbiol. 1995;33:3091–3095. doi: 10.1128/jcm.33.12.3091-3095.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryffel K, Péter O, Rutti B, Suard A, Dayer E. Scored antibody reactivity determined by immunoblotting shows an association between clinical manifestations and presence of Borrelia burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana in humans. J Clin Microbiol. 1999;37:4086–4092. doi: 10.1128/jcm.37.12.4086-4092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saint Girons I, Gern L, Gray J S, Guy E C, Korenberg E, Nuttall P A, Rijpkema S G, Schonberg A, Stanek G, Postic D. Identification of Borrelia burgdorferi sensu lato species in Europe. Zentralbl Bakteriol. 1998;287:190–195. doi: 10.1016/s0934-8840(98)80120-5. [DOI] [PubMed] [Google Scholar]
- 31.van Dam A P, Kuiper H, Vos K, Widjojokusumo A, de Jongh B M, Spanjaard L, Ramselaar A C, Kramer M D, Dankert J. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 32.Wang G, van Dam A P, Le Fleche A, Postic D, Péter O, Baranton G, de Boer R, Spanjaard L, Dankert J. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borrelia genomic groups VS116 and M19) Int J Syst Bacteriol. 1997;47:926–932. doi: 10.1099/00207713-47-4-926. [DOI] [PubMed] [Google Scholar]
- 33.Welsh J, Pretzman C, Postic D, Saint Girons I, Baranton G, McClelland M. Genomic fingerprinting by arbitrarily primed polymerase chain reaction resolves Borrelia burgdorferi into three distinct phyletic groups. Int J Syst Bacteriol. 1992;42:370–377. doi: 10.1099/00207713-42-3-370. [DOI] [PubMed] [Google Scholar]
- 34.Wilske B, Preac-Mursic V, Gobel U B, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilske B, Preac-Mursic V, Schierz G, Kuhbeck R, Barbour A G, Kramer M. Antigenic variability of Borrelia burgdorferi. Ann NY Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]