Abstract
Background
We investigated the proportion of patients in an initial good clinical condition who developed devastating DCI, and aimed to characterize these patients by aneurysm location, blood pressure instability prior to DCI, and the extent of cerebral ischemia.
Methods
We included aSAH patients admitted between 2010 and 2021 with a Glasgow Coma Scale of 11 or higher 24 h after aneurysm treatment, who developed devastating DCI, defined as DCI leading to coma for at least 48 h with cerebral infarction on the subsequent scan. Blood pressure instability was defined as nimodipine-induced blood pressure drops, dosage adjustments, or the use of blood pressure drugs before onset of DCI. Descriptive statistics were used to summarize the data.
Results
Out of 1,211 consecutive aSAH patients, 617 patients had a good clinical condition after aneurysm treatment of whom 16 (3%) patients [14 (88%) women] were included in this study. Thirteen (81%) patients had an aneurysm in the anterior circulation. Thirteen patients (81%) had blood pressure instability: twelve (75%) had nimodipine-induced blood pressure drops, eleven (69%) received antihypertensive drugs, and 7 (44%) received hypertension induction before onset of DCI. Thirteen (81%) patients had bilateral ischemia, mainly in the anterior circulation (56%).
Conclusions
The proportion of aSAH patients with a good clinical condition after aneurysm treatment who develop devastating DCI is small. The vast majority of these patients had blood pressure instability. Future studies are needed to investigate if a reduction in the number and extent of blood pressure fluctuations decreases the incidence of devastating DCI.
Keywords: subarachnoid hemorrhage, delayed cerebral ischemia, blood pressure instability, coma, nimodipine
Introduction
Delayed cerebral ischemia (DCI) is an important determinant of poor functional outcome after aneurysmal subarachnoid hemorrhage (aSAH). Approximately 30% of aSAH patients develops DCI (1). The clinical course of DCI is highly variable. Symptoms may spontaneously improve or can be devastating with progression to coma and finally death. In aSAH patients in an initial good clinical condition there may be a window of opportunity to avert devastating DCI. We aimed to characterize devastating DCI in aSAH patients who were in good clinical condition after aneurysm treatment by aneurysm location, blood pressure instability prior to DCI, and extent of cerebral ischemia.
Methods
Study population
The Institutional Review Board of the University Medical Center Utrecht (UMC Utrecht) waived individual patient consent and formal ethics approval for this study, since we used data available from routine patient care. We performed a retrospective analysis of consecutive aSAH patients admitted to the UMC Utrecht between January 1, 2010 and April 5, 2021. Inclusion criteria were: (1) aneurysm treatment ≤72 h after ictus; (2) a Glasgow Coma Scale (GCS) score of 11 or higher 24 h after aneurysm treatment; (3) development of devastating DCI, which was defined as DCI leading to coma (GCS ≤8) lasting for at least 48 h with confirmation of cerebral infarction on subsequent head computed tomography (CT) or magnetic resonance imaging (MRI) (2); and (4) onset of devastating DCI within 14 days after ictus. Intubated patients were included if 24 h after aneurysm treatment eye opening was spontaneous or in response to verbal command, in combination with obeying commands. Patients in the intervention arm of the HIMALAIA trial (induced hypertension for DCI) were excluded (3). Our local treatment protocol for prevention and treatment of DCI is described in the Supplementary material.
Data collection
The following characteristics were retrieved: age, sex, loss of consciousness at onset, GCS score on admission and 24 h after aneurysm treatment, Prognosis on Admission of Aneurysmal Subarachnoid Hemorrhage (PAASH) score (4), Hijdra et al. (5) sum score for the amount of extravasated blood on the initial head CT, history of hypertension, smoking status, location of the ruptured aneurysm, aneurysm treatment modality, complications ≤14 days after ictus, nimodipine-induced blood pressure drops and dosage adjustments, prescription of other blood pressure drugs (antihypertensive drugs and hypertension induction), and modified Rankin Scale (mRS) score 3 months after ictus (definitions are described in the Supplementary material). Blood pressure instability was defined as nimodipine-induced blood pressure drops, nimodipine dosage adjustments, and the use of blood pressure drugs and evaluated until the onset of devastating DCI.
Statistical analysis
Data were summarized using descriptive statistics.
Results
Of the 1,211 aSAH patients, 617 patients had a good clinical condition after aneurysm treatment of whom 17 (3%) developed devastating DCI (illustrative case Figure 1; Supplementary Figure 1). One patient was excluded due to participation in another study (hypertension induction for DCI) (3). Patient characteristics are shown in Tables 1, 2. Thirteen (81%) patients had an aneurysm in the anterior circulation. Thirteen patients (81%) had blood pressure instability. Twelve (75%) patients had nimodipine-induced blood pressure drops and in 9 (56%) patients nimodipine dosing was adjusted, reduced or temporarily halted. Eleven (69%) patients received antihypertensive drugs, while 7 (44%) received hypertension induction at any moment before neurological deterioration. Five (31%) patients received both antihypertensive drugs and hypertension induction (not related to DCI) during the clinical course, but not simultaneously. Thirteen (81%) patients developed bilateral cerebral infarction and three (19%) patients unilateral cerebral infarction, mainly in the anterior circulation (69 and 67%). Three months after ictus, 13 (81%) patients had died, 2 (13%) patients were severely disabled, and 1 (6%) patient was moderately severe disabled.
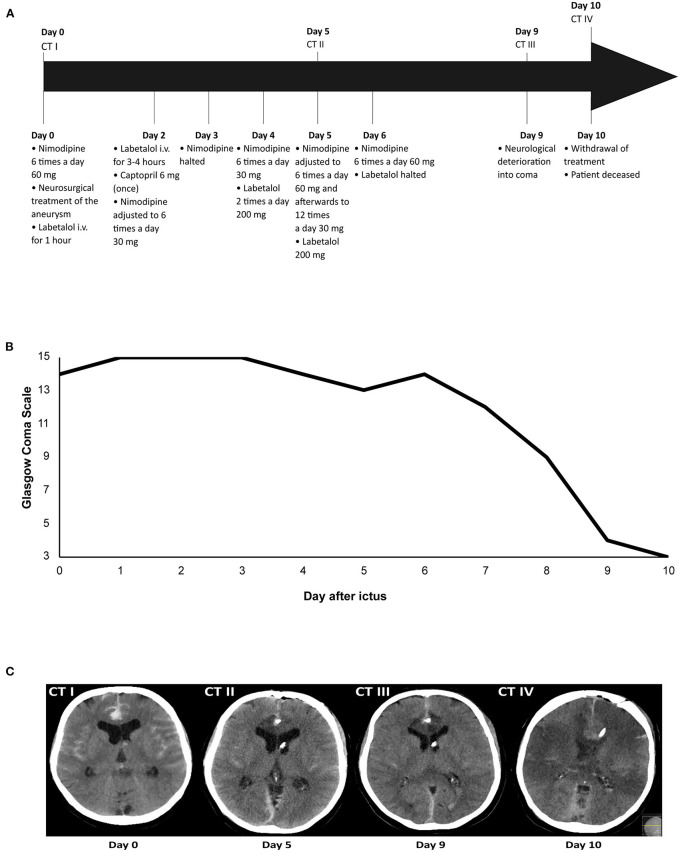
Figure 1.
Illustrative case of a patient with devastating DCI. Time overview of: (A) Blood pressure medication adjustments and computed tomography (CT) scans; (B) Glasgow Coma Scale score; and (C) Ischemic lesions due to delayed cerebral ischemia on CT. The patient was admitted on day 0 and received nimodipine 6 times a day 60 mg. After neurosurgical treatment of the aneurysm on day 0, labetalol i.v. was administered for 1 h due to a high blood pressure, after which it was halted. Administration of nimodipine 6 times a day 60 mg continued. On day 2, labetalol i.v. was restarted because of a high blood pressure, but after 3–4 h labetalol was tapered and halted. Captopril 6 mg was administered once to avert a rise in blood pressure after labetalol was halted. Administration of nimodipine 60 mg resulted in a diastolic blood pressure drop of ≥10 mmHg, after which nimodipine dosing was lowered to 6 times a day 30 mg. On day 3, nimodipine administration was halted because of a drop in diastolic blood pressure ≥10 mmHg. After nimodipine administration was halted for 12 h, it was restarted again on day 4 at a dosage of 6 times a day 30 mg. Because of persistent systolic blood pressures >200 mmHg on day 4, the patient received labetalol 2 times a day 200 mg. On day 5, nimodipine dosing was increased to 6 times a day 60 mg, after which it was adjusted to 12 times a day 30 mg due to a drop in diastolic blood pressure ≥10 mmHg. The patient also received labetalol 200 mg. On day 6, nimodipine dosing was increased again to 6 times a day 60 mg and labetalol was halted as it was decided to accept systolic blood pressures >200 mm Hg. On day 9, the patient deteriorated into coma due to delayed cerebral ischemia. The patient was intubated and transferred to the ICU. On day 10, the decision was made to withdraw treatment, after which the patient was extubated. The patient died on day 10 after ictus.
Table 1.
Patient characteristics.
| All patients N = 16 (%) | |
|---|---|
| Female sex | 14 (88) |
| Mean age, years [SD] | 55 [13] |
| PAASH score on admission* | |
| 1 (GCS 15) | 4 (25) |
| 2 (GCS 11–14) | 6 (38) |
| 3 (GCS 8–10) | 2 (13) |
| 4 (GCS 4–7) | 2 (13) |
| 5 (GCS 3) | 0 (0) |
| Loss of consciousness at onset | 10 (62) |
| Median Hijdra sum score [IQR] | 29 [24–31] |
| History of hypertension | 5 (31) |
| Smoking status | |
| Current smoker | 8 (50) |
| Past smoker | 3 (19) |
| Never smoker | 3 (19) |
| Unknown | 2 (13) |
| Anterior circulation aneurysm | 13 (81) |
| Aneurysm treatment modality | |
| Endovascular coiling | 5 (31) |
| Neurosurgical clipping | 11 (69) |
| Occurrence of complications ≤ 14 days after ictus | 14 (88) |
| Rebleeding | 4 (25) |
| Hydrocephalus | 9 (56) |
| Infection | 7 (44) |
| Nimodipine-induced blood pressure drops† | 12 (75) |
| Nimodipine dosing adjusted, reduced or temporarily discontinued | 9 (56) |
| Use of antihypertensive drugs other than nimodipine | 11 (69) |
| IV antihypertensive drugs | 11 (69) |
| Oral antihypertensive drugs | 8 (50) |
| Use of hypertension induction not related to DCI | 7 (44) |
| Noradrenaline | 6 (38) |
| Phenylephrine | 3 (19) |
| Subsequent use of antihypertensive drugs (other than nimodipine) and hypertension induction | 5 (31) |
| Extent of cerebral infarction | |
| Unilateral | 3 (19) |
| Anterior circulation | 2 (67) |
| Anterior and posterior circulation | 1 (33) |
| Bilateral | 13 (81) |
| Anterior circulation | 9 (69) |
| Anterior and posterior circulation | 4 (31) |
| mRS score 3 months after ictus | |
| Moderately severe disability | 1 (6) |
| Severe disability | 2 (13) |
| Death | 13 (81) |
*PAASH score could not be determined in two intubated patients;†Defined as: (1) a drop ≥20% in the MAP after nimodipine administration; or (2) a diastolic blood pressure drop ≥10 mmHg within 2 h of nimodipine administration. SD, standard deviation; GCS, glasgow coma scale; IQR, interquartile range; IV, intravenous; and Mrs, modified rankin scale.
Table 2.
Individual patient characteristics.
| No. | Sex | Age | Aneurysm location | Type of aneurysm treatment | Blood pressure instability | Day of DCI onset* | Day of death** | WOC | Cause of death | Infections | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nimodipine-induced blood pressure drops | Nimodipine dosage adjustments | Anti-hypertensive drugs | Specification | Hypertension induction not related to DCI | Specification | ||||||||||
| 1 | F | 46 | Right MCA | Neurosurgical clipping | + | + | - | + | 4 days noradrenaline | 11 | 79 | No | Unknown (transfer to other hospital) | ||
| 2 | F | 73 | ACOM | Endovascular coiling | + | + | + | 1 day furosemide | + | 6 days noradrenaline | 9 | n/a | Yes | n/a | Pneumonia |
| 3 | F | 41 | ACOM | Neurosurgical clipping | - | - | - | - | 8 | 14 | Yes | DCI | |||
| 4 | F | 47 | ACOM | Neurosurgical clipping | + | - | + | 2 days labetalol IV 1 day clonidine IV |
- | 9 | 10 | Yes | DCI | Pneumonia | |
| 5 | F | 66 | Left pericallosal artery | Neurosurgical clipping | - | - | - | - | 11 | 16 | Yes | DCI | |||
| 6 | F | 57 | Basilar artery | Endovascular coiling | + | - | + | 1 day labetalol IV 6 days perindopril |
- | 7 | n/a | No | n/a | ||
| 7 | M | 47 | ACOM | Neurosurgical clipping | + | + | + | 1 day labetalol IV 3 days a combination of perindopril, amlodipine, metoprolol and clonidine |
+ | 2 days noradrenaline | 10 | 11 | Yes | DCI | |
| 8 | F | 53 | Right vertebral artery | Neurosurgical clipping | + | + | + | 1 day labetalol IV 1 day clonidine |
+ | 1 day phenylephrine | 5 | 12 | Yes | DCI | |
| 9 | F | 31 | Basilar artery | Endovascular coiling | - | - | + | 1 day labetalol IV | - | 7 | 9 | Yes | DCI | Meningitis (not culture-proven) | |
| 10 | F | 61 | Left MCA | Neurosurgical clipping | + | + | + | 3 days labetalol IV | + | 1 day noradrenaline 1 day phenylephrine |
10 | 39 | Yes | DCI | Urinary tract infection Meningitis (not culture-proven) |
| 11 | M | 81 | ACOM | Neurosurgical clipping | + | + | + | 2 days metoprolol | + | 2 days noradrenaline 2 days phenylephrine |
8 | 10 | Yes | DCI | |
| 12 | F | 38 | ACOM | Neurosurgical clipping | + | + | + | 1 day labetalol IV | - | 8 | 10 | Yes | DCI | ||
| 13 | F | 52 | ACOM | Endovascular coiling | - | - | - | - | 10 | n/a | No | n/a | S. aureus bacteremia and endocarditis | ||
| 14 | F | 62 | ACOM | Neurosurgical clipping | + | + | - | + | 1 day noradrenaline | 9 | 25 | Yes | DCI | Pneumonia | |
| 15 | F | 55 | ACOM | Endovascular coiling | + | - | + | 4 days labetalol IV | - | 3 | 5 | Yes | DCI | Pneumonia | |
| 16 | F | 65 | Left pericallosal artery | Neurosurgical clipping | + | + | + | 4 days labetalol IV | - | 9 | 10 | Yes | DCI | ||
*In days (ictus = day 0). **In days (ictus = day 0) within 3 months after ictus. F, female; M, male; MCA, middle cerebral artery; ACOM, anterior communicating artery; DCI, delayed cerebral ischemia; WOC, withdrawal of care.
Discussion
The proportion of aSAH patients with a good clinical condition after aneurysm treatment who develop devastating DCI is small. Most of these patients had blood pressure instability during hospital stay before onset of devastating DCI.
Several previous studies investigated the relationship between nimodipine, blood pressure, and DCI (6–9). In one study, all patients who developed DCI had an impaired cerebral autoregulation (6). In other studies, DCI was associated with increased systolic blood pressures (SBP) during the first days, and a larger fall in SBP after nimodipine administration compared to patients without DCI (7, 8). Nimodipine dosage reductions or discontinuations occurred in half of the patients with cerebral infarction, which is in line with the observations in our study (9).
Several hypotheses have been postulated to explain the pathogenesis of DCI, including macrovascular spasm, microvascular spasm, microthrombosis, impaired cerebral autoregulation, inflammation, and cortical spreading ischemia. The blood pressure instability we observed in our patients prior to the onset of DCI support the hypothesis of an impaired cerebral autoregulation. However, whether such an impaired cerebral autoregulation may lead to repetitive ischemic insults to the brain or whether it is a manifestation of DCI remains to be investigated.
Some limitations need to be addressed. First, this was a retrospective analysis of clinical data from prospectively identified patients. Data were therefore not documented in a standardized way. As a result, there were missing data on the extent of blood pressure drops after the administration of nimodipine or antihypertensive drugs. Second, administration of analgesics may have occurred simultaneously with nimodipine administration and therefore influenced blood pressure values. However, most nimodipine-induced blood pressure drops occurred multiple times during admission, making it less likely that these drops were caused by analgesics. Third, this study is an exploratory study without a comparator group. Results should therefore be validated in a case-control study.
In conclusion, most patients with devastating DCI had blood pressure instability. Future studies are needed to investigate if a reduction in the number and extent of blood pressure fluctuations decreases the incidence of devastating DCI.
Data availability statement
Data will be shared upon reasonable request to the corresponding author after signing a Data Transfer Agreement.
Ethics statement
The Institutional Review Board of the University Medical Center Utrecht (UMC Utrecht) waived individual patient consent and formal ethics approval for this study, since we used data available from routine patient care.
Author contributions
IK and PW contributed to the study concept/design, data collection, data- and statistical analysis, interpretation of data, and drafting of the manuscript. MV and GR contributed to the study concept and design, data collection, interpretation of data, manuscript revision, and provided study supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by a combined grant from the Netherlands Organization for Health Research and Development and the Dutch Brain Foundation (Grant Number: 95105015). MV was financially supported by a personal grant from the Dutch Heart Foundation (Clinical Established Investigator grant 2018T076).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1016111/full#supplementary-material
References
- 1.Mees SMD, Kerr RS, Rinkel GJE, Algra A, Molyneux AJ. Occurrence and impact of delayed cerebral ischemia after coiling and after clipping in the international subarachnoid aneurysm trial (ISAT). J Neurol. (2012) 259:679. 10.1007/s00415-011-6243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EFM, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. (2010) 41:2391–5. 10.1161/STROKEAHA.110.589275 [DOI] [PubMed] [Google Scholar]
- 3.Gathier CS, van den Bergh WM, van der Jagt M, Verweij BH, Dankbaar JW, Müller MC, et al. Induced hypertension for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: a randomized clinical trial. Stroke. (2018) 49:76–83. 10.1161/STROKEAHA.117.017956 [DOI] [PubMed] [Google Scholar]
- 4.Takagi K, Tamura A, Nakagomi T, Nakayama H, Gotoh O, Kawai K, et al. How should a subarachnoid hemorrhage grading scale be determined? a combinatorial approach based solely on the glasgow coma scale. J Neurosurg. (1999) 90:680–7. 10.3171/jns.1999.90.4.0680 [DOI] [PubMed] [Google Scholar]
- 5.Hijdra A, Brouwers PJ, Vermeulen M, Van Gijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. (1990) 21:1156–61. 10.1161/01.STR.21.8.1156 [DOI] [PubMed] [Google Scholar]
- 6.Santos GA, Petersen N, Zamani AA, Du R, LaRose S, Monk A, et al. Pathophysiologic differences in cerebral autoregulation after subarachnoid hemorrhage. Neurology. (2016) 86:1950–6. 10.1212/WNL.0000000000002696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teping F, Albanna W, Clusmann H, Schulze-Steinen H, Mueller M, Hoellig A, et al. Spontaneous elevation of blood pressure after SAH: an epiphenomenon of disease severity and demand, but not a surrogate for outcome? Neurocrit Care. (2018) 29:214–24. 10.1007/s12028-018-0528-6 [DOI] [PubMed] [Google Scholar]
- 8.Fischer C, Goldberg J, Vulcu S, Wagner F, Schöni D, Söll N, et al. Nimodipine-induced blood pressure changes can predict delayed cerebral ischemia. Front Neurol. (2019) 10:1–7. 10.3389/fneur.2019.01161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandow N, Diesing D, Sarrafzadeh A, Vajkoczy P, Wolf S. Nimodipine dose reductions in the treatment of patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2016) 25:29–39. 10.1007/s12028-015-0230-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared upon reasonable request to the corresponding author after signing a Data Transfer Agreement.