Abstract
In order to confirm the efficiency of an experimental RB51-based complement fixation (CF) test in identifying cattle vaccinated with Brucella abortus strain RB51, 831 sera from 110 vaccinated and 48 unvaccinated Hereford heifers of Iowa, collected for studies conducted in different years, were sent to Italy without coding to be tested in a CF test using RB51 as antigen. Most of the calves, aged from 3 to 10 months, were vaccinated subcutaneously with the recommended dosage of 1010 CFU of RB51 commercial vaccine, while only six calves received 109 CFU of the same vaccine. Serum samples for serologic testing, collected until 16 postinoculation weeks (PIW), were also tested by routine surveillance tests for brucellosis such as rose bengal plate and CF tests performed with B. abortus smooth strain 99 as control antigen. RB51 CF test results obtained by testing sera from cattle vaccinated in 1999 indicate that the sensitivity of the reaction is 97% at 2 to 3 PIW and 90% until 8 PIW and decreases to 65% at 12 PIW, the specificity remaining at 100%. Collectively, the results of this study confirm that serologic standard tests fail to detect antibodies to RB51 while the RB51-based CF test is able to monitor antibody responses to RB51 until 15 to 16 PIW with a specificity of 100%. In addition, unlike the RB51-based dot blot assay, which is the only test currently used to monitor antibody responses to RB51, the CF test also detected specific responses following vaccination with 109 CFU of RB51, although seroconversion was only 50% at 8 PIW. In conclusion, because of high specificity and sensitivity, the CF test described here can be used to efficaciously monitor serologic responses following RB51 vaccination in cattle and could also be employed to detect RB51 infection in humans exposed to this strain.
Brucellosis is a potential human health hazard. Because cattle and small ruminants are the major sources of human brucella infection in most countries, programs to eradicate the disease have been aimed largely at these animals, serologic detection of antibodies being the mainstay of bovine, ovine, and caprine brucellosis control and eradication plans. The complement fixation (CF) test is among the most useful tests in this respect because of its high level of agreement with the results of microbiological examinations (3,7) and is currently used as a confirmatory test. Strain RB51 is a lipopolysaccharide O-antigen-deficient mutant of Brucella abortus virulent strain 2308 (10). Due to the lack of O side chain, RB51 does not induce in cattle antibodies that can be detected by routine brucellosis surveillance tests, which identify antibodies against lipopolysaccharide (4, 5, 12). In addition, detection of accidental human infection with RB51 vaccine is complicated by the unavailability of serologic tests (6). In order to measure serologic responses of RB51-vaccinated cattle, a dot blot assay has been developed using gamma-irradiated strain RB51, which is currently the only test used to monitor seroconversion to RB51 in cattle (9). The purpose of this study was to evaluate, under field conditions, the sensitivity and specificity of an experimental CF test performed with B. abortus RB51 (previously deprived of the anticomplementary activity due to the rough phenotype [1, 2]) as antigen by testing sera from RB51-vaccinated and unvaccinated cattle of Iowa, where RB51 is currently used as a calfhood vaccine in the brucellosis eradication campaign.
MATERIALS AND METHODS
RB51 vaccination and serum collection.
A total of 831 serum samples from 158 Hereford heifers aged 3 to 10 months and belonging to four brucellosis-free herds of Iowa were tested. Of these sera, 491 (59.1%) were from 110 vaccinated heifers and 340 (40.9%) were from 48 unvaccinated controls which received 0.15 M NaCl solution. Vaccination of heifers was performed in different years, from 1991 to 1999, in separate studies investigating the efficacy of the RB51 vaccine. For these studies, most of the heifers were vaccinated subcutaneously with 1010 CFU of a commercial RB51 vaccine (Colorado Serum Co., Denver), while six heifers received 109 CFU of the same vaccine. The vaccine was administered once. Blood samples were collected by venipuncture from vaccinated and unvaccinated calves before vaccination (time zero) and at 1, 2, 3, 4, 7, 8, 11, 12, 15, and 16 postinoculation weeks (PIW). Calves vaccinated in 1999 were bled at time zero and at 1, 2, 4, 8, and 12 PIW. Sera were stored at −70°C. All serum samples, without coding, were sent to Italy for serologic testing.
Preparation of RB51 antigen for the CF test.
Unlike smooth Brucella strains, B. abortus RB51 shows a considerable anticomplementary activity due to its rough phenotype that inhibits its use in the CF test. As described in previous studies (1, 2), to overcome this limitation, a B. abortus RB51 suspension in Veronal buffer (BioMérieux, Marcy l'Etoile, France) containing 109 CFU per ml (A650 = 0.2) was incubated overnight at room temperature with an equal volume of heat-inactivated (58°C, 30 min), fetal bovine serum (GIBCO BRL). To minimize the risks for laboratory technicians, 1% phenol was added to the fetal bovine serum prior to incubation with RB51 suspension. Before use in the experiment, the dilution of RB51 antigen which showed the highest sensitivity and specificity in the CF test was determined by titrating the antigen against bovine anti-B. abortus RB51 and negative serum. The antigen was also tested for anticomplementary activity against 2, 1, and 0.5 U of complement, as described previously (2).
Serologic tests.
Uncoded serum samples were tested for the presence of antibodies against Brucella by conventional CF and rose bengal plate (RBP) tests using the official S-type B. abortus strain 99 as antigen. The RBP test was performed according to the method of Alton et al. (3). All sera were also tested with the CF test using the homologous B. abortus RB51 antigen which had been previously deprived of anticomplementary activity as described above. The CF test was performed according to Decreto Ministeriale of 27 August 1994, no. 65. Briefly, in microtiter plates of 96 wells with round bottoms, 25 μl of each serum was serially diluted in Veronal buffer (BioMérieux) from 1:2 to 1:128, and 25 μl of previously titrated antigen was added to each well followed by 25 μl of complement (BioMérieux) containing 2 CF units giving 50% hemolysis. After incubation in a shaker for 30 min at 37°C, 25 μl of sensitized erythrocytes was added to each well and plates were incubated as described above. Serum titers are reported as the endpoint dilutions that still give positive reactions by using a dilution of 1:4 showing 50% hemolysis as the threshold of the reaction (2). On each CF assay, serum samples used as controls included a previously titrated anti-RB51 serum collected from experimentally vaccinated cattle (1), the International Standard anti-B. abortus serum from Veterinary Laboratories of Weybridge, England, and a pool of sera from brucellosis-free cattle as negative serum.
Statistical analysis.
The RB51 CF test results were compared with the data on the vaccination status of calves, and the specificity, sensitivity, concordance, prevalence, and positive predictive value of the RB51 CF test were determined as described by Martin (8) and Smith (11).
RESULTS
All sera tested in the blind assay had negative responses in the RBP and CF tests performed using B. abortus strain 99 as antigen. In the CF test performed with RB51 as antigen, using a dilution of 1:4 showing 50% hemolysis as the threshold of the reaction, 344 sera (41.4%) reacted positively while 487 (58.6%) were negative. Analysis of the RB51 CF test data, performed after receiving the coding of samples, showed that when serum samples of calves vaccinated with 1010 CFU of RB51 were tested, the concordance with the vaccination status ranged from 71% for sera collected in the period from 1991 to 1996 to 94% for sera collected in 1999. The sensitivity ranged from 57 to 89% within the same time frame, while the specificity ranged from 95 to 100%. For the overall time period, concordance, sensitivity, and specificity were 82, 68, and 98%, respectively (Table 1). When only the samples collected from 1998 to 99 were evaluated with respect to the time elapsed between vaccination and sampling, sensitivity and concordance peaked at 2 to 3 PIW (97 and 98%, respectively) and significantly decreased to 38 and 55% at 15 to 16 PIW (Table 2). Since prevalence levels range from 55 to 63% (Table 1) and from 65 to 72% (Table 2), statistical analysis of data can be accurately performed.
TABLE 1.
Evaluation of RB51-based CF test by analysis of CF results of all sera examined
| Attribute | Value (%) for CF test:
|
|||
|---|---|---|---|---|
| For indicated yr of serum sample collection
|
For overall period | |||
| 1991–1996 | 1998 | 1999 | ||
| Concordance | 71 | 80 | 94 | 82 |
| Sensitivity | 57 | 65 | 89 | 68 |
| Specificity | 95 | 100 | 100 | 98 |
| Prevalence | 63 | 58 | 55 | 59 |
| Positive predictive value | 95 | 100 | 100 | 98 |
TABLE 2.
Evaluation of RB51-based CF test measured by testing serum samples collected from 1998 to 1999 at different PIW
| Attribute | Value (%) for CF test:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| At indicated PIW
|
For overall period | |||||||
| 0 | 1 | 2–3 | 4 | 7–8 | 11–12 | 15–16 | ||
| Concordance | 100 | 96 | 98 | 92 | 92 | 76 | 55 | 89 |
| Sensitivity | NDa | 94 | 97 | 87 | 88 | 64 | 38 | 81 |
| Specificity | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Prevalence | ND | 65 | 70 | 65 | 68 | 67 | 72 | 68 |
| Positive predictive value | ND | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
ND, not determined.
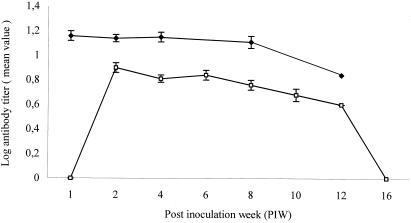
A decrease of titers of antibody to RB51 was observed when sera from calves vaccinated in 1995 were tested and compared with sera from calves vaccinated with the same dosage in 1999 (Fig. 1), with the highest titers reaching 1:16 and 1:64, respectively (data not shown).
FIG. 1.
Serum antibody responses measured by RB51-based CF test at different PIW of 32 calves vaccinated in 1999. (⧫) and 15 calves vaccinated in 1995 (□) with 1010 CFU of RB51. The results are expressed as means ± standard errors.
Among the bovine sera collected in 1995 from heifers vaccinated at 3 months of age with 109 CFU of RB51, 50% were found by the RB51 CF test to be seroconverted at 8 PIW (Table 3).
TABLE 3.
RB51 CF titers obtained by testing bovine sera collected in 1995 from calves vaccinated at 3 months of age with 109 CFU of RB51
| PIW | No. of animals clustered for CF titer
|
No. positive/no. vaccinated (%) | ||||
|---|---|---|---|---|---|---|
| 1:4a | 1:8 | 1:16 | 1:32 | 1:64 | ||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1 | 0 | 0 | 0 | 0 | 1/6 (16.6) |
| 4 | 2 | 0 | 0 | 0 | 0 | 2/6 (33.3) |
| 6 | 2 | 1 | 0 | 0 | 0 | 3/6 (50.0) |
| 8 | 1 | 2 | 0 | 0 | 0 | 3/6 (50.0) |
| 10 | 2 | 0 | 0 | 0 | 0 | 2/6 (33.3) |
| 12 | 2 | 0 | 0 | 0 | 0 | 2/6 (33.3) |
| 16 | 2 | 0 | 0 | 0 | 0 | 2/6 (33.3) |
Titer of 1:4 showing 50% hemolysis was used as the threshold of the CF test.
DISCUSSION
The results of this study confirm that vaccination of cattle with strain RB51 does not induce antibodies which are detectable by RBP and CF serologic surveillance tests performed with B. abortus strain 99. In contrast, as reported in preliminary studies (1), the RB51 CF test is able to specifically monitor antibody responses to RB51 in cattle.
Since a decrease of titers of antibody to RB51 was observed in sera subjected to a long storage period (Fig. 1), RB51 CF data from sera collected from 1998 to 1999 were analyzed separately. These data indicate that the RB51-based CF test was able to identify heifers vaccinated with 1010 CFU of RB51 up to 15 to 16 weeks after vaccination, when the sensitivity decreased to 38%, with the specificity remaining at 100%.
Positive predictive values of 100% indicate that CF-positive samples are from RB51-vaccinated cattle. Antibody responses measured in calves vaccinated with 109 CFU of RB51 confirm that specific serologic responses are significantly lower than those obtained from cattle vaccinated at the same age with the recommended calfhood dosage (9). However, the RB51 CF test also detected responses following vaccination with 109 CFU of RB51, although seroconversion was only 50% at 8 PIW. In spite of the low number of animals tested, these data confirmed the results obtained in previous studies conducted on sheep vaccinated with 5 × 109 CFU of RB51 (2).
The dot blot assay, the only test used to monitor serologic responses of cattle following RB51 vaccination, is highly specific in determining antibody titers during the first 8 weeks after vaccination with 1010 CFU of RB51, but it does not differentiate responses induced in cattle vaccinated with 109 CFU of RB51 from those of unvaccinated controls (9). In addition, the dot blot assay is subjective and unlikely to be acceptable for use in diagnostic laboratories for large numbers of samples.
In conclusion, our data suggest that the RB51 CF test can detect antibody responses of cattle following RB51 vaccination with high specificity and sensitivity. In countries in which vaccination against animal brucellosis is prohibited, according to the European Community Council Decision dated 21 May 1991, this test permits serologic identification of animals illegally vaccinated with RB51 that elude official surveillance tests for brucellosis. This test could also be used as a diagnostic tool for humans for detection of infection with RB51 following accidental exposure.
REFERENCES
- 1.Adone R, Ciuchini F. Uso della reazione di fissazione del complemento per il rilievo della risposta anticorpale in bovini vaccinati con il ceppo Brucella abortus RB51 in fase rugosa. Selezione Vet. 1999;3:149–155. [Google Scholar]
- 2.Adone R, Ciuchini F. Complement fixation test to assess humoral immunity in cattle and sheep vaccinated with Brucella abortus RB51. Clin Diagn Lab Immunol. 1999;6:787–790. doi: 10.1128/cdli.6.6.787-790.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alton G G, Jones L M, Angus R D, Verger J M. Techniques for the brucellosis laboratory. Paris, France: Institut National de la Recherche Agronomique; 1988. [Google Scholar]
- 4.Cheville N F, Jensen A E, Halling S M, Tatum F M, Morfitt D C, Hennager S G, Frerichs W M, Schurig G G. Bacterial survival, lymph node changes and immunologic responses of cattle vaccinated with standard and mutant strains of Brucella abortus. Am J Vet Res. 1992;53:1881–1888. [PubMed] [Google Scholar]
- 5.Cheville N F, Stevens M G, Jensen E, Tatum F M, Halling S M. Immune responses and protection against infection and abortion in cattle experimentally vaccinated with mutant strains of Brucella abortus. Am J Vet Res. 1993;54:1591–1597. [PubMed] [Google Scholar]
- 6.Karhel S C. Brucella abortus strain RB51: its advantages and risks. Am J Vet Med Assoc. 1998;213:12–22. [PubMed] [Google Scholar]
- 7.MacMillan A. Conventional serological tests. In: Nilsen K, Duncan J R, editors. Animal brucellosis. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 153–197. [Google Scholar]
- 8.Martin S W. The evaluation of tests. Can J Comp Med. 1977;41:19–25. [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen S C, Stevens M G, Cheville N F, Schurig G G. Experimental use of a dot-blot assay to measure serologic responses of cattle vaccinated with Brucella abortus strain RB51. J Vet Diagn Investig. 1997;9:363–367. doi: 10.1177/104063879700900404. [DOI] [PubMed] [Google Scholar]
- 10.Schurig G G, Roop II R M, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991;28:171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- 11.Smith R D. Veterinary clinical epidemiology. Woburn, Mass: Butterworth-Heinemann; 1991. p. 234. [Google Scholar]
- 12.Stevens M G, Hennager S G, Olsen S C, Cheville N F. Serologic responses in diagnostic tests for brucellosis in cattle vaccinated with Brucella abortus 19 or RB51. J Clin Microbiol. 1994;32:1065–1066. doi: 10.1128/jcm.32.4.1065-1066.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]