Abstract
Primary vaginal melanoma is rare compared to cutaneous melanoma, and it has a high rate of invasion and metastasis. The prognosis of tumors is poor and the quality of life of patients is low. In addition to traditional surgical and non-surgical treatment, combined treatments are more effective for patients with distant metastatic melanoma. Due to the high immunogenicity of vaginal malignant melanoma, immunotherapy has become an effective treatment strategy for distant metastatic vaginal melanoma, and with the application of immune checkpoint inhibitors (ICIs), outstanding achievements have been made in the treatment of the disease. Studies have shown that ICIs combined with radiotherapy (RT) or anti-angiogenic therapy can have a synergistic effect in certain tumor treatments, normalizing blood vessels and enhancing immune responses. However, whether ICIs combined with RT and anti-angiogenic therapy can benefit patients with distant metastatic vaginal melanoma remains unclear. The present study reports the clinical case of a patient with distant metastatic vaginal melanoma treated with combined RT, anti-angiogenic therapy and ICIs. The patient's primary tumor and distant metastases improved after combination therapy. The combination therapy successfully improved the patient's prognosis and prolonged the patient's overall survival. This provides a reference for combining all three treatment modalities in the treatment of distant metastatic vaginal melanoma.
Keywords: primary vaginal malignant melanoma, abscopal effect, radiation therapy, immune checkpoint inhibitors, anti-angiogenic therapy, combination therapy
Introduction
Vaginal malignant melanoma (VMM), a type of primary malignant melanoma (MM), is an aggressive and rare gynecological malignancy, accounting for 3% of all vaginal malignancies (1) and 0.3-0.8% of all MMs (2). VMM has been shown to arise from the melanocytes present in the basal layer of the vaginal epithelium (3). Although MM might arise anywhere in the vagina, the anterior wall of the lower third portion is the most common site (4). The most common symptom of VMM is vaginal bleeding (5). Despite multiple treatment options, the prognosis is poor, with a 5-year survival rate of 5–25% (1). One reason for this poor prognosis is that patients are often at an advanced stage when first diagnosed.
VMM is usually treated by means of radiotherapy (RT) combined with chemotherapy after surgery. The surgical strategies include radical local resection of the primary tumor and lymph node dissection. For cases of advanced disease, especially those with distant metastases, the prognosis is poor (6). Since the disease is rare, there is a lack of systematic and effective guidelines. The present study reports a case in which a patient with advanced stage primary VMM benefitted from RT combined with anti-angiogenic therapy and immune checkpoint inhibitors (ICIs). This reported case, together with other successful cases reported in the literature, provide useful references for the application of combination therapy to treat MM.
Case report
A 55-year-old female patient was admitted to Affiliated Hospital of Weifang Medical University (Weifang, China) in June 2016 with a mass on the left side of the vaginal opening. For personal reasons, the surgery for complete resection was postponed to September 2016 and performed at another hospital. Data from another hospital showed that the postoperative pathology was MM with BRAF V600F gene mutation. For 45 months after the operation, the patient was treated in other hospitals. The patient received high-dose interferon α-2b (20 MIU/m2; 5 times a week) for 3 months. Then a low-dose interferon α-2b (10 MIU/m2; 3 times a week) maintenance therapy was performed for 1 month. After 1 month of low-dose interferon maintenance therapy, the bilateral inguinal lymph nodes developed metastases. The patient experienced recurrence of the primary tumor in September 2017, May 2018 and October 2018. Both the December 2018 and February 2019 follow-up showed that the vulvar mass was enlarged. The tumor invaded the vagina, cervix and adjacent muscles in September 2019. In January 2020, the patient's re-examination revealed left adrenal metastases. When the above condition progressed, the patient received irregular treatments, such as surgery, interferon therapy, chemotherapy and targeted therapy in other hospitals. The specific treatment plans used by the other hospitals are unknown. The last tumor assessment in other hospitals was stable disease (SD).
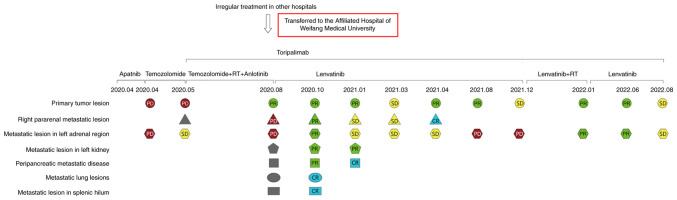
Until April 2020, the patient was admitted to Affiliated Hospital of Weifang Medical University (Weifang, China) again due to local tumor recurrence and progression. A physical examination revealed structural changes in the vulva and swelling of the left vulva. The pudendal mass was ~8×5 cm in size, with convex growth, ulceration and bleeding. The mass was hard and poorly mobile. The patient was treated with apatinib for 15 days [500 mg every day (qd)]. After taking Apatinib, the tumor was slightly smaller than before, but typical symptoms of nephrotic syndrome occurred, so the drug was discontinued. For personal reasons, the patient underwent blood genetic testing at another hospital (Weifang People's Hospital, Weifang, China). Blood genetic tests 1 week after presentation revealed BRAF (V600 wild-type). The patient was resistant to dabrafenib and vemurafenib. After 8 days, the whole abdomen was imaged. This was compared with the examination report from the visit to the other hospitals 3 months before. The local lesions and metastases were enlarged. The overall tumor assessment indicated progressive disease (PD). The next day, the patient started four cycles of intravenous chemotherapy with temozolomide [200 mg/m2 on days 1–5, every 4 weeks (q4w)]. After 1 month, the magnetic resonance imaging was reviewed and the primary tumor was found to be larger than before (Fig. 1A and B), with new metastases. The tumor status was assessed as PD (Fig. 1). The patient was administered primary tumor RT (2 GY each fraction for 35 fractions) combined with 4 cycles of temozolomide (200 mg/m2 on days 1–5, q4w), 6 cycles of anlotinib (8 mg on days 1–14, q3w), and 8 cycles of toripalimab (240 mg q2w) (Fig. 2 shows the usage time of various drugs). Over 3 months later, magnetic resonance imaging showed that the primary tumor was smaller than before (Fig. 3A and B); however, the left adrenal metastases were larger than before (Fig. 3C and D) and new metastases had appeared in multiple places (Fig. 2). The tumor status was assessed as PD (Fig. 3). The antitumor regimen was changed to lenvatinib (12 mg qd) combined with toripalimab (240 mg q2w). The patient was followed up after 4 cycles of combination therapy, and partial response (PR) was found in tumor evaluation (Fig. 4). Both the primary tumor (Fig. 4A and B) and metastases (Fig. 4C and D) were smaller than before. Afterwards, the patient was regularly reviewed every 3 months, and SD was assessed for each tumor status. However, tumor status was assessed as PR at a review in August 2021. In December 2021, the patient was re-examined (Fig. 5) and the left adrenal metastases were significantly larger than before (Fig. 5C and D). The tumor status was evaluated as PD. From mid-December 2021 to mid-January 2022, RT was added for the left adrenal metastatic tumor area (2.8 GY each fraction for 15 fractions). After RT, the patient underwent blood sample analysis at another hospital (Weifang People's Hospital, Weifang, China), which showed elevated CD4/CD8 values in lymphocytes (2.24; normal range, 0.98-1.94). The patient's symptoms improved and the tumor status was evaluated as PR. The patient was scheduled to receive lenvatinib (12 mg qd) in combination with 10 cycles of toripalimab (240 mg q2w) for 5 months. At 6 months post-RT, both the primary tumor (Fig. 6A and B) and the left adrenal metastases (Fig. 6C and D) were smaller than before on magnetic resonance imaging assessment. The tumor status was assessed as PR (Fig. 6). In August 2022, the patient was readmitted to Affiliated Hospital of Weifang Medical University (Weifang, China) for review. The patient's condition was stable, and the tumor status was evaluated as SD (Fig. 2). The patient had no obvious discomfort and had a good prognosis. The patient was asked to have a follow up every 3 months thereafter.
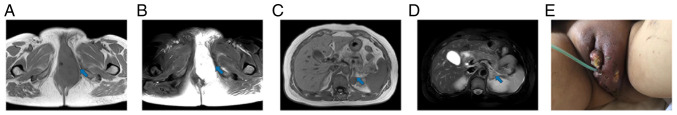
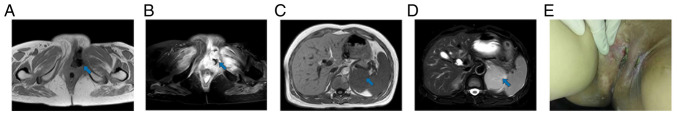
Figure 1.
Image before first combination therapy (May 2020). The blue arrow points to the lesion to be evaluated. (A and B) Magnetic resonance imaging showed tumor recurrence in the perineum. The size of the mass was ~9.0×7.2 cm. (C and D) Magnetic resonance imaging revealed multiple metastases in the left adrenal region. The largest was ~2.0×1.3 cm. (E) The gross appearance of the tumor.
Figure 2.
Diagnosis and treatment process. Red, PD; yellow, SD; green, PR; blue, CR; grey, new metastatic lesions. RT, radiotherapy; PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response.
Figure 3.
Stable condition of the patient after first combination therapy (August 2020). The blue arrow points to the lesion to be evaluated. (A and B) Magnetic resonance imaging showed that the left perineal mass was significantly smaller than before. The size of the mass was ~3.3×1.4 cm. (C and D) Magnetic resonance imaging showed that the metastases in the left adrenal region were larger than before. The largest was ~2.8×1.2 cm. (E) The gross appearance of the tumor.
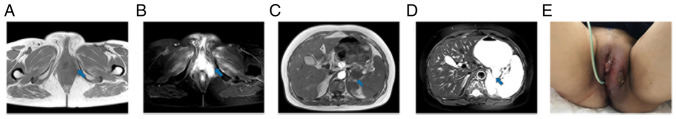
Figure 4.
At 2 months after the first combination therapy, the tumor status was partial response (October 2020). The blue arrow points to the lesion to be evaluated. (A and B) Magnetic resonance imaging showed that the left perineal mass was smaller than before. The size of the mass was ~2.7×1.1 cm. (C and D) Magnetic resonance imaging showed that the largest metastasis in the left adrenal gland was ~1.5×1.0 cm, which was smaller than before. (E) The gross appearance of the tumor.
Figure 5.
Tumors showed PD at 18 months after the first combination therapy (December 2021). The blue arrow points to the lesion to be evaluated. (A and B) Magnetic resonance imaging showed a left perineal mass, and muscle and soft tissue edema. The size of the mass was ~1.8×0.9 cm. (C and D) Magnetic resonance imaging showed that the largest metastasis in the left adrenal gland was ~5.5×5.2 cm, which was significantly larger than before. (E) The gross appearance of the tumor.
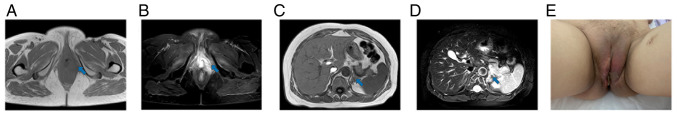
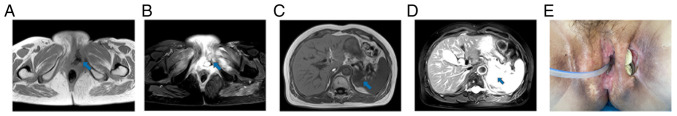
Figure 6.
Tumor status was PR at 6 months after the second combination therapy (June 2022). The blue arrow points to the lesion to be evaluated. (A and B) Magnetic resonance imaging showed that the left perineal mass was smaller than before, and the soft tissue edema around the perineum was reduced. The size of the mass was ~1.3×0.9 cm. (C and D) Magnetic resonance imaging showed that the largest metastasis in the left adrenal gland was ~3.8×3.7 cm, which was smaller than before. (E) The gross appearance of the tumor.
Discussion
VMM is an aggressive tumor, with a 5-year survival rate of 5–25% and a high recurrence rate; it easily spreads to the local lymph nodes and develops distant metastasis (7). At present, there are a variety of treatments for VMM (2). Early VMM can be treated by complete surgical resection. However, distant metastatic vaginal melanoma has a poor prognosis. For distant metastatic vaginal melanoma, more emphasis is placed on combination therapy.
Due to the highly immunogenic nature of MM, immunotherapy is an effective treatment strategy for primary VMM (8). Studies have shown that RT and anti-angiogenic therapy combined with immunotherapy can enhance the immune efficacy in melanoma (9–12). In addition to normalizing blood vessels and improving the hypoxic environment of tumors, anti-angiogenic drugs have been shown to promote tumor infiltration of CD8+ T lymphocytes and enhance cancer immunotherapy (12). In this way, the combination of targeted drugs and ICIs has a synergistic effect. Anti-angiogenic agents in combination with programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) antibodies have now become the standard first-line therapy for non-small cell lung cancer (NSCLC), renal cell carcinoma, endometrial cancer and hepatocellular carcinoma (13).
Clinical studies have shown that RT can promote and activate the immune system, showing that immunotherapy combined with RT can obtain better clinical efficacy. RT and immunotherapy synergistically inhibit tumor growth and exceed the additive effect of the two, which is beneficial to patients (14). Both previous studies (15,16) and the present report found that after local RT, the tumor at the RT site was reduced, and multiple metastases at the non-RT site were also reduced, which was considered to be the abscopal effect of tumor treatment-RT to one site resulting in regression of distant non-irradiated metastatic cancer (17). Targeted ionizing radiation has long been known to lead to direct local cell death. However, it is increasingly recognized that radiation is able to induce tumor regression at distant tumor sites that are not irradiated. For example, Stamell et al (18) reported that patients with metastatic melanoma who received primary palliative RT also experienced regression of non-irradiated metastases. Despite a growing number of trials and cases reporting the absolute efficacy of RT alone, the overall incidence of the abscopal effect remains relatively low (19,20). Considering that immunotherapy decreases the immunotolerance of the host to tumors, combining RT with immunotherapy may enhance the antitumor immune response, thus increasing the chance of an abscopal effect (21–23). In a study using a mouse model of melanoma, administration of radiation prior to or concurrent with cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blockade delayed tumor growth and prolonged survival time compared with that in mice treated with anti-CTLA-4 alone (24). Another important rationale for combining RT and immunotherapy is that RT has the potential to overcome resistance to immune checkpoint blockade. Early evidence suggests the clinical feasibility of combining RT and immunotherapy in NSCLC. Yuan et al (25) described the case of a patient with PD-L1-negative metastatic scaly NSCLC refractory to nivolumab. After palliative RT, the patient showed near total regression of the irradiated primary tumor and marked regression of the metastases in the RT field, consistent with an abscopal effect. Komatsu et al (26) reported a case of enlarged liver metastases in a patient with metastatic NSCLC after treatment with nivolumab. The patient subsequently underwent RT, which resulted in shrinkage of both irradiated liver metastases and non-irradiated lung metastases. The lung metastases shrank without RT, in line with the abstract effect. These case reports point to a potential role for RT in overcoming immune resistance mechanisms in patients with cancer when RT is performed after the initial failure of immunotherapy. However, a recent retrospective study of 225 patients did not find a prolonged survival benefit of combined ICIs and RT in patients with advanced mucosal melanoma, although it could improve the local symptoms at the irradiation site and improve the quality of life of affected patients (27).
The abscopal effect of ipilimumab in combination with stereotactic RT in patients with melanoma was reported by Chicas-Sett et al (28). Another study reported the more favorable benefit-risk ratio of anti-PD-1 therapy compared with that of other adjuvant therapies, and indicated that the treatment should be used preferentially (29). A further study reported that anti-PD-1 therapy exhibited improved outcomes compared with immunotherapy in the treatment of female lower genital tract melanoma (30). Therefore, promising clinical immunological agents, such as PD-1/PD-L1 blockers, should also be explored in combination with RT (31). In the study by Deng et al (32), PD-L1 expression was upregulated in the tumor microenvironment after RT, and anti-PD-L1 enhanced the efficacy of RT through cytotoxic T cells. It was demonstrated that RT and anti-PD-L1 treatment synergistically promoted antitumor immunity (32). A prospective study demonstrated that immune checkpoint blockade produces a response even in the absence of PD-L1 expression (2). This suggests that more people may benefit from ICIs (2).
ICIs cause the release of T cells to attack tumor cells, while also regulating the tumor microenvironment by the normalization of the tumor blood vessels. This normalization enhances the infiltration of lymphocytes, and increased numbers and activation of CD4+ T lymphocytes in turn promote the normalization of the blood vessels, in a cycle of mutual regulation. Tumor blood vessel normalization by ICIs not only creates a feedback loop, reprograms the tumor immune microenvironment and enhances the immunotherapeutic effect, it also improves tumor hypoxia, decreases HIF-1 expression, decreases reactive oxygen species inactivation and sensitizes tumors to radiation. Most anti-angiogenic drugs also increase radiosensitivity and are combined with RT (33). In the present report, a patient with advanced primary VMM underwent surgery, interferon therapy, chemotherapy and targeted therapy, and the patient's disease progressed. The patient was transferred to Affiliated Hospital of Weifang Medical University (Weifang, China) for RT combined with anti-angiogenesis therapy and ICIs, and their condition improved. After 19 months of anti-angiogenic therapy combined with ICI maintenance therapy, the patient's tumor progressed. After the patient underwent RT combined with anti-angiogenic therapy and ICIs again, the tumor status was evaluated as PR (Fig. 7).
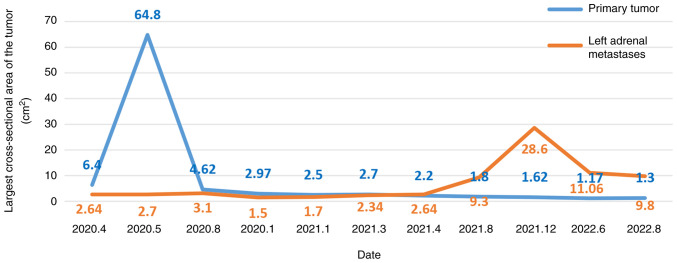
Figure 7.
Time course showing the changes in primary and metastatic tumor size. The first and second combinations of immune checkpoint inhibitors, radiotherapy and antiangiogenic therapy were administered in May 2020 and December 2021, respectively. The maximum cross-sectional area of the primary tumor and left adrenal metastases decreased after two combined treatments.
In conclusion, primary VMM is an aggressive and rare malignancy that is usually diagnosed at an advanced stage, has a high rate of recurrence and metastasis, and has no consistent guidelines in terms of treatment. Based on aforementioned interactions and synergy, as well as the efficacy in the patient reported in the present case, a trimodal approach combining RT with anti-angiogenic therapy and immunotherapy is a promising treatment strategy. To the best of our knowledge, no clinical trials combining all three treatment modalities have been published. RT with either anti-angiogenic therapy or immunotherapy appears feasible, but presents clinicians and researchers with numerous challenges.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
PY, XLM, YFZ, YS, YTW and ZL contributed to the study conception and design. Material preparation, and data collection and analysis were performed by ZL, XLM, YFZ, YS and YTW. The first draft of the manuscript was written by PY and all authors commented on previous versions of the manuscript. ZL and XLM confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study involving human participants was reviewed and approved by the Affiliated Hospital of Weifang Medical University Ethics Committee (approval no. wyfy-2021-ky-072). Informed consent was obtained from all individual participants included in the study.
Patient consent for publication
The patient provided written informed consent for the publication of the study and the associated images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Puri S, Asotra S. Primary vaginal malignant melanoma: A rare entity with review of literature. J Cancer Res Ther. 2019;15:1392–1394. doi: 10.4103/jcrt.JCRT_893_15. [DOI] [PubMed] [Google Scholar]
- 2.Wang HY, Wu XY, Zhang X, Yang XH, Long YK, Feng YF, Wang F. Prevalence of NRAS mutation, PD-L1 expression and amplification, and overall survival analysis in 36 primary vaginal melanomas. Oncologist. 2020;25:e291–e301. doi: 10.1634/theoncologist.2019-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta D, Malpica A, Deavers MT, Silva EG. Vaginal melanoma: A clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 2002;26:1450–1457. doi: 10.1097/00000478-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Piura B. Management of primary melanoma of the female urogenital tract. Lancet Oncol. 2008;9:973–981. doi: 10.1016/S1470-2045(08)70254-7. [DOI] [PubMed] [Google Scholar]
- 5.Terzakis E, Androutsopoulos G, Adonakis G, Zygouris D, Grigoriadis C, Decavalas G. Vaginal primary malignant melanoma: Report of four cases and review of the literature. Eur J Gynaecol Oncol. 2011;32:122–124. [PubMed] [Google Scholar]
- 6.Guo N, Zhang J. Primary vaginal malignant melanoma: A rare case report of successful treatment with nivolumab. Medicine (Baltimore) 2021;100:e25691. doi: 10.1097/MD.0000000000025691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frumovitz M, Etchepareborda M, Sun CC, Soliman PT, Eifel PJ, Levenback CF, Ramirez PT. Primary malignant melanoma of the vagina. Obstet Gynecol. 2010;116:1358–1365. doi: 10.1097/AOG.0b013e3181fb8045. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi J, Nagasawa S. Immunostimulatory effects of radiotherapy for local and systemic control of melanoma: A review. Int J Mol Sci. 2020;21:9324. doi: 10.3390/ijms21239324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Chen Z, Zhong Q, Chen Y, Shangguan W, Xie W. Efficacy and safety of immunological checkpoint inhibitors combined with anti-angiogenic drugs in first-line treatment of metastatic renal cell carcinoma: A systematic review and meta-analysis. Transl Androl Urol. 2021;10:300–309. doi: 10.21037/tau-20-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52:1475–1485. doi: 10.1038/s12276-020-00500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goedegebuure RSA, de Klerk LK, Bass AJ, Derks S, Thijssen VLJL. Combining radiotherapy with anti-angiogenic therapy and immunotherapy; a therapeutic triad for cancer? Front Immunol. 2019;9:3107. doi: 10.3389/fimmu.2018.03107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: Challenges and opportunities. Front Immunol. 2020;11:598877. doi: 10.3389/fimmu.2020.598877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang B, Yan X, Sheng X, Si L, Cui C, Kong Y, Mao L, Lian B, Bai X, Wang X, et al. Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients. J Hematol Oncol. 2019;12:7. doi: 10.1186/s13045-018-0693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11:104. doi: 10.1186/s13045-018-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao X, Shao C. Radiotherapy-mediated immunomodulation and anti-tumor abscopal effect combining immune checkpoint blockade. Cancers (Basel) 2020;12:2762. doi: 10.3390/cancers12102762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313–322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys. 2013;85:293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers SJ, Puric E, Eberle B, Datta NR, Bodis SB. Radiotherapy for melanoma: More than DNA damage. Dermatol Res Pract. 2019;2019:9435389. doi: 10.1155/2019/9435389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrickx A, Cozzio A, Plasswilm L, Panje CM. Radiotherapy for lentigo maligna and lentigo maligna melanoma-a systematic review. Radiat Oncol. 2020;15:174. doi: 10.1186/s13014-020-01615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm. 2012;27:12–22. doi: 10.1089/cbr.2012.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. doi: 10.1158/1078-0432.728.11.2. [DOI] [PubMed] [Google Scholar]
- 23.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan Z, Fromm A, Ahmed KA, Grass GD, Yang GQ, Oliver DE, Dilling TJ, Antonia SJ, Perez BA. Radiotherapy rescue of a nivolumab-refractory immune response in a patient with PD-L1-negative metastatic squamous cell carcinoma of the lung. J Thorac Oncol. 2017;12:e135–e136. doi: 10.1016/j.jtho.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Komatsu T, Nakamura K, Kawase A. Abscopal effect of nivolumab in a patient with primary lung cancer. J Thorac Oncol. 2017;12:e143–e144. doi: 10.1016/j.jtho.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Umeda Y, Yoshikawa S, Kiniwa Y, Maekawa T, Yamasaki O, Isei T, Matsushita S, Nomura M, Nakai Y, Fukushima S, et al. Real-world efficacy of anti-PD-1 antibody or combined anti-PD-1 plus anti-CTLA-4 antibodies, with or without radiotherapy, in advanced mucosal melanoma patients: A retrospective, multicenter study. Eur J Cancer. 2021;157:361–372. doi: 10.1016/j.ejca.2021.08.034. [DOI] [PubMed] [Google Scholar]
- 28.Chicas-Sett R, Morales-Orue I, Rodriguez-Abreu D, Lara-Jimenez P. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: A systematic review. Clin Transl Radiat Oncol. 2017;9:5–11. doi: 10.1016/j.ctro.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moya-Plana A, Herrera Gómez RG, Rossoni C, Dercle L, Ammari S, Girault I, Roy S, Scoazec JY, Vagner S, Janot F, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171–1178. doi: 10.1007/s00262-019-02351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Indini A, Di Guardo L, Cimminiello C, Lorusso D, Raspagliesi F, Del Vecchio M. Investigating the role of immunotherapy in advanced/recurrent female genital tract melanoma: A preliminary experience. J Gynecol Oncol. 2019;30:e94. doi: 10.3802/jgo.2019.30.e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41:503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Zhao Q, Zheng Z, Liu S, Meng L, Dong L, Jiang X. Vascular normalization in immunotherapy: A promising mechanisms combined with radiotherapy. Biomed Pharmacother. 2021;139:111607. doi: 10.1016/j.biopha.2021.111607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.