Abstract
Background/purpose
With improved cancer survivorship, non-cancer events, especially heart disease (HD), have become the underlying cause of death in cancer patients, but the risk of HD mortality in sarcoma patients remains poorly characterized. Therefore, our purpose was to: (1) identify sarcoma patients at the highest risk of fatal HD compared with the general population, (2) identify patients and sarcoma characteristics associated with a higher risk of HD death, and (3) determine if chemotherapy increased the risk of HD death in sarcoma patients.
Methods
From 1975 to 2016, we identified patients diagnosed with bone and soft tissue sarcoma from the Surveillance, Epidemiology, and End Results (SEER) database in the US. Standardized mortality ratios (SMRs) were evaluated using mortality data from the general population collected by the National Center for Health Statistics. This was the largest retrospective cohort study of fatal HD in individuals with sarcoma.
Results
In 80,905 sarcoma patients observed for 530,290 person-years, 3,350 deaths from HD were identified with a mortality of 631.7/100,000 person-years. The SMR of death from HD was 1.38 (95% CI: 1.33–1.42). The highest risks of death from HD were observed in patients with Ewing sarcoma (SMR = 5.44; 95% CI: 3.38–8.75) and osteosarcoma (SMR = 1.92; 95% CI: 1.55–2.38). Patients diagnosed at < 19 years old had the highest SMR in all age subgroups, and a higher risk of fatal HD relative to the general population was observed in sarcoma survivors diagnosed at < 85 years old. In patients diagnosed at < 19 years old, HD plurality occurred in those with Ewing sarcoma (29.4%) and osteosarcoma (32.4%) and at > 35 years old, HD plurality occurred in those diagnosed with liposarcoma (19.0%) and malignant fibro histiocytoma (MFH) (23.6%). For sarcoma survivors, HD mortality risks were highest within the first year after diagnosis (SMR = 1.31; 95% CI: 1.21–1.41), and this risk remained elevated throughout follow-up compared with the general population. Subgroup analyses indicated that chemotherapy significantly increased the risk of fatal HD in patients with localized osteosarcoma (Hazard ratio (HR) = 3.18; 95% CI: 1.24–8.13; P = 0.016), but not in patients with other histological sarcoma subtypes and clinical stages.
Conclusion
The risk of death from HD mainly varied in patients with different histological sarcoma subtypes and clinical stages. Chemotherapy increased the risk of fatal HD in patients with localized osteosarcoma. To lower the risk of fatal HD in patients with sarcoma, we call for enhanced multidisciplinary cooperation, including cardiologists and orthopedic surgeons.
Keywords: sarcoma, chemotherapy, osteosarcoma, heart diseases, standardized mortality ratio
Introduction
Over several decades, heart diseases (HD) have become the first leading cause of death globally, and in 2019, killed approximately nine million individuals, accounting for 9% of all deaths (1). Cancer and HD may occur separately in patients, or cancer may cause HD via non-bacterial thrombotic endocarditis and chemoradiation therapy (2–4). As survival rate of cancer patients improve, medical officers have gradually realized that the risk of death from other non-cancer diseases in cancer patients was higher when compared with the general population (5–7), furthermore, HD as the first leading cause of non-cancer death among cancer patients have attracted more and more attention from clinicians (7–9).
Bone and soft tissue sarcoma comprise several rare malignant tumors which arise from mesenchymal tissue (10), they are responsible for more deaths than testicular cancer, Hodgkin’s disease, and thyroid cancer combined due to their more recurrent and metastatic nature (11). The standard therapy for patients with sarcoma is neoadjuvant chemotherapy, surgical resection, and adjuvant chemotherapy (12). Anthracyclines are commonly used chemotherapy drugs, but may cause HD, reduce the quality of life in patients, and increase mortality in sarcoma patients (3). Previous studies reporting the risk of HD in sarcoma patients were limited by small sample sizes and data collected primarily at single institutions (13–16). Currently, limited guidelines are available on fatal HD prevention, identification, or management, specifically in sarcoma patients. One strategy aimed at preventing fatal HD in this group is to identify and target subgroups at greatest HD risk, therefore, retrospective cohort studies such as ours could be used by clinicians to generate survivorship programs and mitigate HD risks in these patients.
In our study, we had the following objectives: (1) to identify sarcoma patients at highest risk of fatal HD compared with the general population, (2) to identify patients and sarcoma characteristics associated with a higher risk of HD death, and (3) to determine if chemotherapy increased the risk of HD death in sarcoma patients.
Materials and methods
In this retrospective cohort study, we used the Surveillance, Epidemiology, and End Results (SEER) database at the National Cancer Institute, which comprised 18 registries and covered approximately 28% of the US general population (17). Given that anthracyclines or cisplatin were discovered in the 1980s, we included patients diagnosed with sarcoma between 1975 and 2016 (18). As a comparison, general population mortality data, spanning 1969–2016 from the National Center for Health Statistics, were used (5, 17, 19). Using exclusion criteria (Figure 1), we identified the final study cohort. Patients whose information was obtained solely from death certificates or autopsies were excluded due to no survival time data (<1.5% of patients). Patients diagnosed with bone and soft tissue sarcoma without a definite socioeconomic status were also excluded (11). Sarcomas were classified into ten histological subtypes according to the International Classification of Disease for Oncology third revision (ICD-O-3), and included chondrosarcoma, osteosarcoma, Ewing sarcoma, liposarcoma, malignant fibro histiocytoma (MFH), leiomyosarcoma, fibrosarcoma, synovial sarcoma, Malignant Peripheral Nerve Sheath Tumor (MPNST), and others (ICD-O-3 codes are shown in Table 1) (11).
FIGURE 1.
Flowchart describing initial dataset and exclusions leading to final study cohorts.
TABLE 1.
Death from HD among patients with bone and soft tissue sarcomas by demographic and tumor characteristics.
| Characteristic | Patients with cancer in SEER |
HD death | Person-years accrued | Mortality1 | SMR2 | 95% CI | ||
|
|
|
|||||||
| No. | % | No. | % | |||||
| Sex | ||||||||
| Female | 36283 | 45 | 1353 | 40 | 247636 | 546.4 | 1.45 | 1.37–1.53 |
| Male | 44622 | 55 | 1997 | 60 | 282655 | 706.5 | 1.33 | 1.28–1.39 |
| Race3 | ||||||||
| White | 65618 | 81 | 2876 | 84 | 433078 | 664.1 | 1.35 | 1.30–1.40 |
| Black | 8530 | 11 | 303 | 9 | 54297 | 558.0 | 1.48 | 1.32–1.66 |
| Other | 6757 | 8 | 171 | 5 | 42915 | 398.5 | 1.70 | 1.47–1.98 |
| American Indian/Alaska Native | 553 | 1 | 12 | 1 | 3762 | 318.9 | 2.13 | 1.21–3.75 |
| Asian or Pacific Islander | 5430 | 7 | 149 | 4 | 33934 | 439.1 | 1.70 | 1.45–2.00 |
| Unknown | 774 | 1 | 10 | 1 | 5218 | 191.6 | 1.42 | 0.77–2.64 |
| Year of diagnosis | ||||||||
| 1975–1977 | 1667 | 2 | 194 | 6 | 22941 | 845.6 | 1.75 | 1.52–2.02 |
| 1978–1980 | 1797 | 2 | 183 | 5 | 25057 | 730.3 | 1.86 | 1.61–2.15 |
| 1981–1983 | 1879 | 2 | 184 | 5 | 25039 | 734.8 | 2.00 | 1.73–2.31 |
| 1984–1986 | 1986 | 2 | 180 | 5 | 25146 | 715.8 | 1.73 | 1.50–2.00 |
| 1987–1989 | 2055 | 3 | 180 | 5 | 26061 | 690.7 | 1.61 | 1.39–1.86 |
| 1990–1992 | 2683 | 3 | 206 | 6 | 31502 | 653.9 | 1.65 | 1.44–1.89 |
| 1993–1995 | 3593 | 4 | 237 | 7 | 38861 | 609.9 | 1.43 | 1.26–1.62 |
| 1996–1998 | 3984 | 5 | 251 | 7 | 40156 | 625.1 | 1.37 | 1.21–1.55 |
| 1999–2001 | 7253 | 9 | 376 | 11 | 63231 | 594.6 | 1.37 | 1.24–1.52 |
| 2002–2004 | 9463 | 12 | 372 | 11 | 71767 | 518.3 | 1.09 | 0.98–1.20 |
| 2005–2007 | 9997 | 12 | 347 | 10 | 60811 | 570.6 | 1.14 | 1.03–1.27 |
| 2008–2011 | 14594 | 18 | 387 | 12 | 64184 | 603.0 | 1.19 | 1.08–1.32 |
| 2012–2016 | 19954 | 25 | 253 | 8 | 35529 | 712.1 | 1.29 | 1.14–1.46 |
| Marital status | ||||||||
| Married | 38650 | 48 | 1690 | 50 | 257737 | 655.7 | 1.24 | 1.18–1.30 |
| Unknown | 4312 | 5 | 233 | 7 | 26155 | 890.8 | 1.57 | 1.38–1.78 |
| Unmarried | 37943 | 47 | 1427 | 43 | 246397 | 579.1 | 1.54 | 1.47–1.63 |
| Education4 | ||||||||
| High | 26221 | 32 | 888 | 27 | 137715 | 644.8 | 1.39 | 1.30–1.49 |
| Median | 27786 | 34 | 1227 | 37 | 190351 | 644.6 | 1.38 | 1.30–1.45 |
| Low | 26898 | 33 | 1235 | 37 | 202224 | 610.7 | 1.37 | 1.29–1.44 |
| Income4 | ||||||||
| High | 26657 | 33 | 1159 | 35 | 191456 | 605.4 | 1.32 | 1.24–1.39 |
| Median | 23844 | 29 | 910 | 27 | 156387 | 581.9 | 1.28 | 1.20–1.37 |
| Low | 30404 | 38 | 1281 | 38 | 182447 | 702.1 | 1.52 | 1.44–1.61 |
| Insurance | ||||||||
| Uninsured | 1259 | 2 | 13 | 1 | 3741 | 347.5 | 2.25 | 1.31–3.88 |
| Any Medicaid | 5668 | 7 | 73 | 2 | 15481 | 471.5 | 2.13 | 1.69–2.68 |
| Insured | 29498 | 36 | 621 | 19 | 95223 | 652.1 | 1.13 | 1.04–1.22 |
| Unknown | 44480 | 55 | 2643 | 78 | 415844 | 635.6 | 1.44 | 1.38–1.49 |
| Histology5 | ||||||||
| Ewing sarcoma | 3055 | 4 | 17 | 1 | 21430 | 79.3 | 5.44 | 3.38–8.75 |
| Osteosarcoma | 6504 | 8 | 83 | 2 | 46552 | 178.3 | 1.92 | 1.55–2.38 |
| Synovial sarcoma | 3171 | 4 | 42 | 1 | 23181 | 181.2 | 1.55 | 1.14–2.10 |
| MPNST | 2116 | 3 | 64 | 2 | 11374 | 562.7 | 1.57 | 1.23–2.00 |
| Chondrosarcoma | 5941 | 7 | 232 | 7 | 50229 | 461.9 | 1.37 | 1.20–1.55 |
| MFH | 8492 | 10 | 775 | 23 | 66089 | 1172.7 | 1.34 | 1.25–1.44 |
| Fibrosarcoma | 4102 | 5 | 185 | 6 | 29838 | 620.0 | 1.27 | 1.10–1.47 |
| Liposarcoma | 10819 | 13 | 623 | 19 | 81894 | 760.7 | 1.26 | 1.16–1.36 |
| Leiomyosarcoma | 7532 | 9 | 338 | 10 | 41938 | 805.9 | 1.18 | 1.06–1.31 |
| Chordoma | 1605 | 2 | 55 | 2 | 10147 | 542.0 | 1.05 | 0.81–1.37 |
| Others | 27568 | 34 | 936 | 28 | 147614 | 634.1 | 1.59 | 1.49–1.69 |
| Grade at presentation6 | ||||||||
| Grade I | 9595 | 12 | 485 | 14 | 84323 | 575.2 | 1.22 | 1.11–1.33 |
| Grade II | 10074 | 12 | 438 | 13 | 80079 | 547.0 | 1.23 | 1.12–1.35 |
| Grade III | 11701 | 14 | 429 | 13 | 58801 | 729.6 | 1.39 | 1.27–1.53 |
| Grade IV | 17159 | 21 | 559 | 17 | 76839 | 727.5 | 1.34 | 1.24–1.46 |
| Unknown | 32376 | 40 | 1439 | 43 | 230247 | 625.0 | 1.51 | 1.43–1.59 |
| Stage at presentation | ||||||||
| Localized | 42042 | 52 | 2152 | 64 | 332076 | 648.0 | 1.33 | 1.27–1.38 |
| Regional | 19671 | 24 | 706 | 21 | 126953 | 556.1 | 1.32 | 1.23–1.42 |
| Distant | 12414 | 15 | 199 | 6 | 30812 | 645.8 | 2.11 | 1.84–2.42 |
| Unknown | 6778 | 8 | 293 | 9 | 40448 | 724.4 | 1.60 | 1.42–1.79 |
| Chemotherapy | ||||||||
| No/Unknown | 57208 | 71 | 3059 | 91 | 395330 | 773.8 | 1.35 | 1.31–1.40 |
| Yes | 23697 | 29 | 291 | 9 | 134960 | 215.6 | 1.69 | 1.51–1.90 |
| Radiotherapy | ||||||||
| No/Unknown | 52386 | 65 | 2314 | 69 | 355007 | 651.8 | 1.49 | 1.43–1.56 |
| Yes | 28519 | 35 | 1036 | 31 | 175283 | 591.0 | 1.17 | 1.10–1.25 |
| Surgery | ||||||||
| None | 14481 | 18 | 427 | 13 | 40693 | 1049.3 | 2.16 | 1.96–2.37 |
| Yes | 64412 | 80 | 2826 | 84 | 476861 | 592.6 | 1.29 | 1.24–1.34 |
| Unknown | 2012 | 2 | 97 | 3 | 12736 | 761.6 | 2.04 | 1.67–2.49 |
| All sarcoma patients | 80905 | 100 | 3350 | 100 | 530290 | 631.7 | 1.38 | 1.33–1.42 |
HD, heart diseases; SEER, Surveillance, Epidemiology, and End Results; SMR, standardized mortality ratio; MFH, malignant fibrohistiocytoma; MPNST, Malignant Peripheral Nerve Sheath Tumor; NOS, not of specific.
1Per 100,000 person-years.
2SMRs were estimated as the ratios of observed to expected number of deaths. Observed number of HD death represent the total number of deaths from HD among patients with sarcoma recorded during the study period. Expected death represent the number of individuals who died of HD in the general population with a similar distribution of age at diagnosis (5-year intervals), sex, race (white, black, and other), and calendar year of diagnosis (3-year intervals). The calculation for SMRs were adjusted to the age, sex, race/ethnicity, and calendar year distributions between sarcoma patients and the general population.
3Others included American Indian/AK Native, Asian/Pacific Islander and unknown race.
4Education status and income level were categorized into tertiles.
5ICD-O-3: Chondrosarcoma, 9220–9243; Osteosarcoma, 9180–9200; Ewing sarcoma, 9260; Liposarcoma, 8850–8858; MFH, 8830; Leiomyosarcoma, 8890–8891 and 8896; Synovial sarcoma, 9040–9044; MPNST, 9540 and 9561; Chordoma, 9370–9372.
6Grade I: Well differentiated; Grade II: Moderately differentiated; Grade III: Poorly differentiated; Grade IV: Undifferentiated; anaplastic.
We extracted demographic data, including age at diagnosis, sex (female, male), race (white, black, and others), calendar year of diagnosis (1975–2016), marital status at diagnosis (married, unmarried, and unknown), insurance status (insured, Medicaid, uninsured, unknown), and socioeconomic indicators (income and educational status). Income (median family income) and educational level (percentage of individuals > 25 years of age with at least a high school degree) from county-level data were calculated by referring to 2000 US census data and categorizing data into tertiles (high, median, low). Tumor-related characteristics included histological subtype, grade (I–IV), clinical sarcoma stage (localized, regional, distant, and unknown), and treatment information (chemotherapy, radiotherapy, and surgery status). Time of follow-up and cause of death were also available and collected. Patients were considered to have committed HD death if the cause of death variable was coded as “Diseases of Heart (50060).”
For objective 1, we calculated standardized mortality ratios (SMRs), which provided the relative risk of HD death for sarcoma patients when compared with all US residents, stratified by histological subgroup. SMRs and 95% confidence intervals (CIs) were calculated as previously described (19, 20). Briefly, SMRs were estimated as the ratio of observed to expected numbers of deaths. The observed number of HD deaths represented the total number of deaths from heart events in sarcoma patients during the follow-up period; the expected number of deaths represented the number of deaths from heart events in the general population with a similar age at diagnosis, sex, race, and calendar year distribution. Five-year age categories and three-year calendar categories were used for standardization (21). Mortality from fatal HD was calculated as the number of deaths from HD divided by person-years at risk (20).
For objective 2, considering other competitive risk events, we plotted cumulative incidence curves and compared them using the Gray test. Furthermore, we constructed logistic regression and multivariate Cox proportional hazards models to identify risk factors associated with a higher risk of HD death in sarcoma patients.
For objective 3, we conducted subgroup analysis by histological subtype and clinical stage to determine if chemotherapy increased the risk of fatal HD in sarcoma patients. The survival time was from sarcoma diagnosis until fatal HD; values recorded as 0 months in the SEER database were converted to one-half of a month according to accepted epidemiological practices (19). Statistical significance was accepted at P < 0.05 (two-sided). Analyses were performed using SEER*Stat software version 8.3.6 and R version 3.51 statistical software.
Results
In total, 3,350 HD deaths were identified in 80,905 patients with bone and soft tissue sarcoma over 530,291 person-years, and provided an age-, sex-, race-, and year-adjusted HD mortality of 631.7/100,000 person-years. The corresponding HD mortality in the general US population was 16.7/100,000 person-years. This generated an SMR = 1.38 (95% CI: 1.33–1.42). The survival time range was 0–39.25 years, with a mean survival time of 7.34 years for sarcoma patients dying from HD.
Sarcoma patient risk of fatal heart disease versus the general population
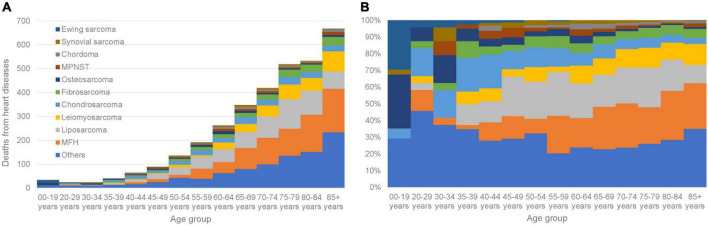
The characteristics of sarcoma patients and those who died of HD vs. all cancer patients are shown (Table 1). Higher SMRs for fatal HD in patients with sarcoma were associated with the female sex (546.4/100,000 person-years; SMR = 1.45; 95% CI: 1.37–1.53), the American Indian/Alaskan Native race (318.9/100,000 person-years; SMR = 2.13; 95% CI: 1.21–3.75), and an unmarried status (579.1/100,000 person-years; SMR = 1.54; 95% CI: 1.47–1.63). For patients as a whole, the risk of fatal HD was higher when compared with the general population over the 40 years covered in the SEER data, except for those diagnosed between 2002 and 2004 (518.3/100,000 person-years; SMR = 1.09; 95% CI: 0.98–1.20). Patients with high and low educational levels were equal likely to die from HD: 32% vs. 33%. Patients with low income levels (SMR = 1.52; 95% CI: 1.44–1.61) and an uninsured status (SMR = 2.25; 95% CI: 1.31–3.88) had a higher SMR for fatal HD when compared with those with a high income level (SMR = 1.32; 95% CI: 1.24–1.39) and an insured status (SMR = 1.13; 95% CI: 1.04–1.22). Patients diagnosed at a younger age had a higher SMR for HD, and SMRs gradually declined as patients were diagnosed at later ages (Table 2); patients < 19 years old had an SMR = 9.46 (95% CI: 6.76–13.24, 32.7/100,000 person-years) vs. >85 years old who had an SMR = 1.04 (95% CI: 0.96–1.12, 7,295.3/100,000 person-years). Fatal HD in sarcoma patients as a function of age group is shown (Figure 2). In patients diagnosed at <19 years old, HD plurality occurred in those with Ewing sarcoma (29.4%) and osteosarcoma (32.4%). In contrast, in patients diagnosed at >35 years old, HD plurality occurred in those diagnosed with liposarcoma (19.0%) and MFH (23.6%).
TABLE 2.
Risk of fatal HD among patients with sarcoma by age at diagnosis.
| Age at diagnosis (years) | Mortality in general population1 |
Mortality in patients with sarcoma2 |
Person-years accrued |
No. of deaths |
SMR3 |
95% CI |
| 00–19 | 2.6 | 32.7 | 103,970 | 34 | 9.46 | 6.76–13.24 |
| 20–29 | 4.5 | 42.7 | 56,215 | 24 | 7.24 | 4.86–10.81 |
| 30–34 | 7.7 | 76.9 | 31,216 | 24 | 5.59 | 3.75–8.34 |
| 35–39 | 14.5 | 113.7 | 35,173 | 40 | 4.11 | 3.01–5.60 |
| 40–44 | 29.2 | 170.1 | 37,626 | 64 | 3.09 | 2.42–3.95 |
| 45–49 | 58.8 | 217.6 | 40,893 | 89 | 2.17 | 1.77–2.68 |
| 50–54 | 109.5 | 330.0 | 41,213 | 136 | 1.96 | 1.65–2.32 |
| 55–59 | 188.7 | 481.2 | 39,694 | 191 | 1.71 | 1.49–1.97 |
| 60–64 | 310.4 | 709.5 | 36,925 | 262 | 1.60 | 1.41–1.80 |
| 65–69 | 508.0 | 1,050.7 | 33,121 | 348 | 1.55 | 1.40–1.72 |
| 70–74 | 804.0 | 1,501.6 | 27,903 | 419 | 1.37 | 1.24–1.51 |
| 75–79 | 1308.2 | 2,303.0 | 22,535 | 519 | 1.31 | 1.20–1.43 |
| 80–84 | 2133.1 | 3,636.1 | 14,658 | 533 | 1.22 | 1.12–1.33 |
| 85+ | 3586.2 | 7,295.3 | 9,142 | 667 | 1.04 | 0.96–1.12 |
HD, heart diseases; SMR, standardized mortality ratio.
1Per 100,000 person-years. Reference population: general US population, 1969–2016.
2Among persons with sarcoma in the populations served by the SEER program.
3Adjusted to the race and sex distributions between patients and the general population.
FIGURE 2.
Fatal HD among sarcoma patients as a function of age group. (A) The y-axis depicts the absolute number of HD deaths and the x-axis depicts the age group at time of diagnosis. The colors depict the histological subtypes of sarcoma. (B) The y-axis depicts the relative number of HD deaths and the x-axis depicts the age group at time of diagnosis. The colors depict the histological subtypes of sarcoma.
Histological sarcoma subtype is associated with a higher risk of fatal heart disease
The risk of fatal HD in patients with most sarcoma subtypes was higher when compared with the general US population, except for chordoma (542.0/100,000 person-years; SMR = 1.05; 95% CI: 0.81–1.37) (Table 1). The highest SMR was observed in patients with Ewing sarcoma (79.3/100,000 person-years; SMR = 5.44; 95% CI: 3.38–5.75), followed by osteosarcoma (178.3/100,000 person-years; SMR = 1.92; 95% CI: 1.55–2.38), MPNST (562.7/100,000 person-years; SMR = 1.57; 95% CI: 1.23–2.00), and synovial sarcoma (181.2/100,000 person-years; SMR = 1.55; 95% CI: 1.14–2.10). Also, patients with MFH had the highest mortality for fatal HD across all patients (1172.7/100,000 person-years; SMR = 1.34; 95% CI: 1.25–1.44). In patients with Ewing sarcoma, osteosarcoma, MPNST, and MFH with chemotherapy a higher relative risk of fatal HD was observed when compared with the general population (Table 3). However, patients with synovial sarcoma (SMR = 1.69; 95% CI: 0.88–3.52), chondrosarcoma (SMR = 1.10; 95% CI: 0.52–2.30), fibrosarcoma (SMR = 1.28; 95% CI: 0.64–2.56), liposarcoma (SMR = 1.25; 95% CI: 0.83–1.88), leiomyosarcoma (SMR = 1.30; 95% CI: 0.87–1.94), and chordoma (SMR = 0.66; 95% CI: 0.09–4.67), and receiving chemotherapy had an equal risk when compared with the general population.
TABLE 3.
Standardized mortality ratios (SMRs) of fatal HD among patients with sarcoma by histology and chemotherapy status.
|
Histology |
Patients without chemotherapy |
Patients with chemotherapy |
||||||||
| No. of deaths | No. of patients | Person-years | Mortality1 | SMR (95% CI) | No. of deaths | No. of patients | Person-years | Mortality1 | SMR (95% CI) | |
| Ewing sarcoma | 3 | 241 | 1,796 | 167.0 | 5.12 (1.65–15.87) | 14 | 2,814 | 19,634 | 71.3 | 5.52 (3.27–9.31) |
| Osteosarcoma | 45 | 1,683 | 11,899 | 378.2 | 1.49 (1.11–1.99) | 38 | 4,821 | 34,654 | 109.7 | 2.93 (2.13–4.02) |
| Synovial sarcoma | 33 | 1,797 | 14,677 | 224.8 | 1.51 (1.08–2.13) | 9 | 1,374 | 8,504 | 105.8 | 1.69 (0.88–3.25) |
| MPNST | 57 | 1,591 | 9,338 | 610.4 | 1.47 (1.33–1.90) | 7 | 525 | 2,037 | 343.7 | 3.59 (1.71–7.52) |
| Chondrosarcoma | 225 | 5,352 | 47,285 | 475.8 | 1.38 (1.21–1.57) | 7 | 589 | 2,944 | 237.8 | 1.10 (0.52–2.30) |
| MFH | 716 | 6,973 | 56,245 | 1,273.0 | 1.33 (1.24–1.43) | 59 | 1,519 | 9,845 | 599.3 | 1.41 (1.09–1.82) |
| Fibrosarcoma | 177 | 3,613 | 27,338 | 647.4 | 1.27 (1.10–1.48) | 8 | 489 | 2,500 | 320.0 | 1.28 (0.64–2.56) |
| Liposarcoma | 600 | 9,735 | 75,766 | 791.9 | 1.26 (1.16–1.36) | 23 | 1,084 | 6,129 | 375.3 | 1.25 (0.83–1.88) |
| Leiomyosarcoma | 314 | 5,997 | 36,725 | 855.0 | 1.17 (1.05–1.31) | 24 | 1,535 | 5,214 | 460.3 | 1.30 (0.87–1.94) |
| Chordoma | 54 | 1,532 | 9,782 | 552.0 | 1.06 (0.81–1.39) | 1 | 73 | 366 | 273.3 | 0.66 (0.09–4.67) |
| Others | 835 | 18,694 | 104,478 | 799.2 | 1.56 (1.46–1.67) | 101 | 8,874 | 43,136 | 234.1 | 1.80 (1.48–2.18) |
SMR, standardized mortality ratio; HD, heart diseases; MFH, Malignant fibrohistiocytoma; MPNST, Malignant Peripheral Nerve Sheath Tumor.
1Per 100,000 person-years.
Risk of heart disease death over time after diagnosis
For all sarcoma subtypes, the risk of death from HD was higher relative to the general population in the first year after a diagnosis, but decreased gradually from 1–5 years, but then increased after this (Table 4). The relative risk of fatal HD in sarcoma patients when compared with the general population was highest in the 10 years after a sarcoma diagnosis (SMR = 2.85; 95% CI: 2.68–3.04). For most sarcoma types, the risk of death from HD was equal to the general population in the first year of diagnosis. For patients with osteosarcoma, the relative risk was higher when compared with the general population in the first year after a sarcoma diagnosis (SMR = 1.73; 95% CI: 1.12–2.69) and after 10 years (SMR = 4.75; 95% CI: 3.43–6.59).
TABLE 4.
Fatal HD among patients with sarcoma by histological subtypes and years since diagnosis.
|
Histology |
Time since diagnosis |
|||
| 0–1 Year | 1–5 Years | 5–10 Years | >10 Years | |
| All | ||||
| No. of deaths | 675 | 1016 | 720 | 939 |
| Person-years | 69,322 | 181,220 | 130,829 | 154,069 |
| SMR1 | 1.31 | 0.94 | 1.32 | 2.85 |
| 95%CI | 1.21–1.41 | 0.89–1.00 | 1.22–1.42 | 2.68–3.04 |
| Chondrosarcoma | ||||
| No. of deaths | 27 | 73 | 38 | 94 |
| Person-years | 5,388 | 16,056 | 12,997 | 16,202 |
| SMR | 0.9 | 1.05 | 0.92 | 2.98 |
| 95%CI | 0.62–1.31 | 0.84–1.33 | 0.67–1.27 | 2.44–3.65 |
| Chordoma | ||||
| No. of deaths | 9 | 16 | 17 | 13 |
| Person-years | 1,458 | 4,184 | 2,643 | 1,973 |
| SMR | 0.84 | 0.61 | 1.48 | 2.77 |
| 95%CI | 0.44–1.62 | 0.37–0.99 | 0.92–2.38 | 1.61–4.78 |
| Ewing sarcoma | ||||
| No. of deaths | 1 | 4 | 3 | 9 |
| Person-years | 2,804 | 7,044 | 4,947 | 6,844 |
| SMR | 1.28 | 3.42 | 4.81 | 15.16 |
| 95%CI | 0.18–9.09 | 1.28–9.11 | 1.55–14.91 | 7.89–29.13 |
| Fibrosarcoma | ||||
| No. of deaths | 33 | 62 | 38 | 52 |
| Person-years | 3,685 | 10,331 | 7,218 | 8,885 |
| SMR | 1.1 | 0.91 | 1.17 | 3.12 |
| 95%CI | 0.78–1.54 | 0.71–1.17 | 0.85–1.61 | 2.38–4.1 |
| Leiomyosarcoma | ||||
| No. of deaths | 72 | 115 | 77 | 74 |
| Person-years | 6,450 | 16,327 | 10,387 | 9,252 |
| SMR | 1.13 | 0.86 | 1.25 | 2.33 |
| 95%CI | 0.89–1.42 | 0.72–1.03 | 1.00–1.57 | 1.86–2.93 |
| Liposarcoma | ||||
| No. of deaths | 72 | 153 | 162 | 236 |
| Person-years | 9,812 | 28,926 | 21,418 | 22,498 |
| SMR | 0.88 | 0.73 | 1.28 | 2.89 |
| 95%CI | 0.70–1.11 | 0.62–0.85 | 1.10–1.49 | 2.54–3.28 |
| MFH | ||||
| No. of deaths | 120 | 250 | 184 | 221 |
| Person-years | 7,472 | 20,681 | 16,734 | 21,766 |
| SMR | 1.06 | 0.99 | 1.35 | 2.60 |
| 95%CI | 0.89–1.27 | 0.87–1.12 | 1.17–1.56 | 2.28–2.97 |
| MPNST | ||||
| No. of deaths | 14 | 19 | 12 | 19 |
| Person-years | 1,805 | 4,148 | 2,872 | 2,677 |
| SMR | 1.62 | 1.02 | 1.32 | 3.8 |
| 95%CI | 0.96–2.74 | 0.65–1.60 | 0.75–2.32 | 2.43–5.96 |
| Osteosarcoma | ||||
| No. of deaths | 20 | 15 | 12 | 36 |
| Person-years | 5,743 | 14,378 | 10,884 | 15,972 |
| SMR | 1.73 | 0.96 | 1.33 | 4.75 |
| 95%CI | 1.12–2.69 | 0.58–1.59 | 0.76–2.35 | 3.43–6.59 |
| Synovial sarcoma | ||||
| No. of deaths | 8 | 10 | 7 | 17 |
| Person-years | 2,872 | 7,842 | 5,681 | 7,005 |
| SMR | 1.46 | 0.93 | 1.12 | 3.35 |
| 95%CI | 0.73–2.91 | 0.50–1.74 | 0.53–2.35 | 2.08–5.39 |
| Others | ||||
| No. of deaths | 299 | 299 | 170 | 168 |
| Person-years | 21,827 | 51,298 | 35,044 | 40,989 |
| SMR | 1.87 | 1.12 | 1.51 | 2.82 |
| 95%CI | 1.67–2.09 | 1.00–1.25 | 1.30–1.76 | 2.43–3.28 |
SMR, standardized mortality ratio; MFH, Malignant fibrohistiocytoma; MPNST, Malignant Peripheral Nerve Sheath Tumor; NOS, not of specific.
1Reference population: general US population, 1969 to 2016. Adjusted to the age, sex and race distributions between patients and the general population.
Characteristics associated with a higher risk of fatal heart disease
From multivariable logistic regression (Table 5) of sarcoma patients, older age at diagnosis [odds ratio (OR) = 1.06; 95% CI: 1.05–1.07; P < 0.001], male sex (OR = 1.44; 95% CI: 1.33–1.56; P < 0.001), black race (OR = 1.28; 95% CI: 1.12–1.45; P < 0.001), unmarried status (OR = 1.22; 95% CI: 1.13–1.33; P < 0.001), and a low income (OR = 1.16; 95% CI: 1.05–1.29; P = 0.004) were associated with significantly greater odds of dying from HD. Neither educational level nor insurance status were associated with a risk of dying from HD in the multivariable model. Moreover, sarcoma patients with distant metastases of sarcoma had a lower OR of fatal HD than patients with localized sarcoma (OR = 0.36; 95% CI: 0.3–0.43; P < 0.001). Receiving chemotherapy was associated with marginally lower odds of fatal HD (vs. without chemotherapy; OR = 0.77; 95% CI: 0.67–0.88; P < 0.001). As shown (Table 5 – Cox proportional hazards model in the right panel), the HRs of patients who died of HD are stratified by subgroup. In the Cox regression model, receiving radiotherapy (vs. without radiotherapy; HR = 0.80; 95% CI: 0.74–0.87; P < 0.001) and surgery (vs. without surgery; HR = 0.56; 95% CI: 0.50–0.63; P < 0.001) was still associated with a lower odds of fatal HD.
TABLE 5.
Odds ratios and hazard ratios of fatal HD among sarcoma patients.
| Logistic regression model |
Cox proportional hazards model |
|||||
| Odds ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| Age at diagnosis1 | 1.06 | 1.05–1.07 | <0.001 | 1.10 | 1.09–1.12 | <0.001 |
| Sex | ||||||
| Female | Reference | Reference | ||||
| Male | 1.44 | 1.33–1.56 | <0.001 | 1.63 | 1.51–1.75 | <0.001 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 1.27 | 1.11–1.45 | <0.001 | 1.33 | 1.18–1.50 | <0.001 |
| Other | 0.83 | 0.70–0.97 | 0.025 | 0.79 | 0.68–0.93 | 0.003 |
| Year of diagnosis1 | 0.94 | 0.92–0.95 | <0.001 | 0.97 | 0.96–0.98 | <0.001 |
| Marital status | ||||||
| Married | Reference | Reference | ||||
| Unmarried | 1.22 | 1.13–1.33 | <0.001 | 1.48 | 1.37–1.60 | <0.001 |
| Unknown | 1.33 | 1.14–1.55 | <0.001 | 1.27 | 1.11–1.47 | 0.001 |
| Income | ||||||
| High | Reference | Reference | ||||
| Medium | 1.00 | 0.91–1.09 | 0.922 | 1.02 | 0.94–1.12 | 0.592 |
| Low | 1.16 | 1.05–1.29 | 0.004 | 1.22 | 1.11–1.34 | <0.001 |
| Education | ||||||
| High | Reference | Reference | ||||
| Medium | 0.96 | 0.86–1.07 | 0.428 | 0.97 | 0.88–1.07 | 0.542 |
| Low | 0.99 | 0.88–1.11 | 0.829 | 0.99 | 0.89–1.11 | 0.894 |
| Insurance | ||||||
| Uninsured | Reference | Reference | ||||
| Any Medicaid | 1.03 | 0.58–1.96 | 0.923 | 0.94 | 0.70–2.14 | 0.831 |
| Insured | 0.85 | 0.51–1.56 | 0.566 | 0.59 | 0.34–1.02 | 0.060 |
| Unknown | 1.27 | 0.76–2.34 | 0.399 | 0.59 | 0.34–1.03 | 0.062 |
| Grade at presentation | ||||||
| Grade I | Reference | Reference | ||||
| Grade II | 0.90 | 0.78–1.03 | 0.127 | 1.07 | 0.94–1.22 | 0.312 |
| Grade III | 0.83 | 0.72–0.96 | 0.010 | 1.41 | 1.23–1.61 | <0.001 |
| Grade IV | 0.78 | 0.68–0.89 | <0.001 | 1.35 | 1.19–1.54 | <0.001 |
| Other | 0.84 | 0.75–0.94 | 0.003 | 1.21 | 1.09–1.35 | <0.001 |
| Stage at presentation | ||||||
| Localized | Reference | Reference | ||||
| Regional | 0.76 | 0.69–0.84 | <0.001 | 1.02 | 0.94–1.12 | 0.589 |
| Distant | 0.36 | 0.31–0.43 | <0.001 | 1.31 | 1.12–1.53 | <0.001 |
| Unknown | 0.70 | 0.61–0.81 | <0.001 | 0.91 | 0.80–1.04 | 0.156 |
| Radiotherapy | ||||||
| No/Unknown | Reference | Reference | ||||
| Yes | 0.84 | 0.77–0.91 | <0.001 | 0.80 | 0.74–0.87 | <0.001 |
| Chemotherapy | ||||||
| No/Unknown | Reference | Reference | ||||
| Yes | 0.77 | 0.67–0.88 | <0.001 | 0.97 | 0.85–1.11 | 0.659 |
| Surgery | ||||||
| None | Reference | Reference | ||||
| Yes | 1.18 | 1.04–1.33 | 0.009 | 0.56 | 0.50–0.63 | <0.001 |
| Unknown | 0.53 | 0.41–0.67 | <0.001 | 0.86 | 0.63–1.00 | 0.050 |
HD, heart diseases; NOS, not of specific.
1Increase 1 year.
Subgroup analysis of the risk of fatal heart disease
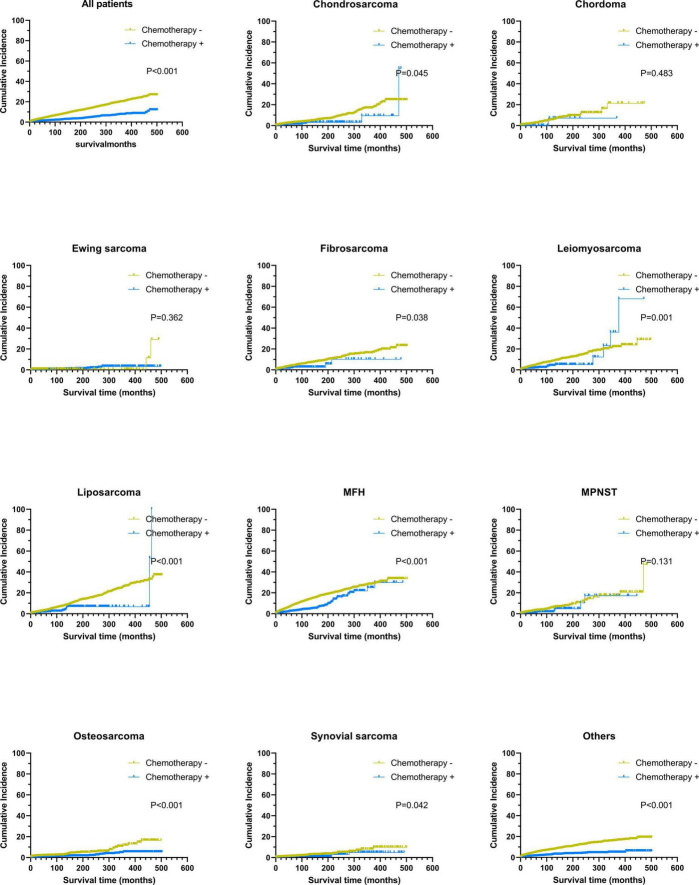
We conducted subgroup analysis of the study cohort based on histological sarcoma subtypes. Cumulative incidence curves are shown (Figure 3). Survival analysis indicated that all patients with sarcoma benefited from chemotherapy except those with chordoma (P = 0.280) and Ewing sarcoma (P = 0.555). To further determine if chemotherapy increased the risk of fatal HD in patients with sarcoma, we conducted subgroup analysis by clinical stage. In the subgroup analysis, chemotherapy could protect patients with regional Ewing sarcoma from dying from HD (Table 6). Moreover, we found that chemotherapy could increase significantly the risk of fatal HD among patients with localized osteosarcoma (HR = 3.18; 95% CI: 1.24–8.13; P = 0.016), but not those with regional (HR = 0.63; 95% CI: 0.28–1.40; P = 0.259) or advanced osteosarcoma (HR = 0.25; 95% CI: 0.06–1.03; P = 0.055). For other subgroups with sarcoma at different clinical stages, chemotherapy did not have any effect on the risk of fatal HD.
FIGURE 3.
Subgroup analysis: the graph showed cumulative incidence curves of death resulting from heart events. MFH, malignant fibro histiocytoma; MPNST, malignant peripheral nerve sheath tumors.
TABLE 6.
Subgroup analysis of fatal HD among patients with sarcoma by histology and clinical stage.
| Histology | Clinical stage | HR1 | 95% CI | P-value |
| Ewing sarcoma | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | Inf | 0.00 – Inf | 0.999 | |
| Regional | 0.04 | 0.01–0.29 | 0.001 | |
| Distant | 0.00 | 0.00 – Inf | 1.000 | |
| Osteosarcoma | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | 3.18 | 1.24–8.13 | 0.016 | |
| Regional | 0.63 | 0.28–1.40 | 0.259 | |
| Distant | 0.25 | 0.06–1.03 | 0.055 | |
| Synovial sarcoma | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | 0.40 | 0.06–2.86 | 0.363 | |
| Regional | 3.06 | 0.85–11.11 | 0.088 | |
| Distant | Inf | 0.00–Inf | 1.000 | |
| MPNST | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | 4.59 | 0.85–24.67 | 0.076 | |
| Regional | 0.71 | 0.05–10.57 | 0.804 | |
| Distant | 0.87 | 0.18–4.24 | 0.867 | |
| Chondrosarcoma | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | 0.44 | 0.10–1.87 | 0.268 | |
| Regional | 1.05 | 0.30–3.60 | 0.949 | |
| Distant | 0.52 | 0.10–2.81 | 0.449 | |
| MFH | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | 1.17 | 0.83–1.65 | 0.375 | |
| Regional | 0.89 | 0.51–1.53 | 0.667 | |
| Distant | 0.28 | 0.07–1.16 | 0.079 | |
| Fibrosarcoma | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | 0.94 | 0.36–2.47 | 0.902 | |
| Regional | 1.76 | 0.38–8.23 | 0.474 | |
| Distant | 1.06 | 0.06–18.89 | 0.968 | |
| Liposarcoma | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | 0.84 | 0.48–1.45 | 0.523 | |
| Regional | 1.33 | 0.54–3.23 | 0.534 | |
| Distant | 0.49 | 0.06–3.94 | 0.504 | |
| Leiomyosarcoma | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | 0.83 | 0.39–1.77 | 0.635 | |
| Regional | 1.31 | 0.57–2.97 | 0.524 | |
| Distant | 0.44 | 0.14–1.39 | 0.162 | |
| Chordoma | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | 1.66 | 0.16–17.36 | 0.673 | |
| Regional | 0.00 | 0.00 – Inf | 0.998 | |
| Distant | 0.00 | 0.00 – Inf | 1.000 | |
| Others | ||||
| All | 0.97 | 0.85–1.11 | 0.659 | |
| Localized | 0.97 | 0.70–1.34 | 0.848 | |
| Regional | 0.76 | 0.46–1.24 | 0.273 | |
| Distant | 0.47 | 0.29–0.77 | 0.003 |
HD, heart diseases; HR, hazard ratio; MFH, malignant fibrohistiocytoma; MPNST, Malignant Peripheral Nerve Sheath Tumor.
1We performed a survival analysis using a Cox proportional hazards model to calculate hazard ratios (HRs), adjusting for demographics: age, sex, race, year of diagnosis, socioeconomic status, insurance status and tumor characteristics: clinical stage, grade at presentation, radiotherapy and surgery status.
Discussion
With the development of multimodality therapy, including advances in imaging techniques and neoadjuvant chemotherapy, sarcoma patient survival has improved significantly (22). In addition to prolonging survival rates, clinicians are now increasingly concerned about complications caused by sarcoma therapy (20, 23). Several small sample or single-center clinical cohort studies reported that antineoplastic agents such as anthracyclines increased the risk of dying from HD in sarcoma patients (14, 15). However, due to low sarcoma incidence rates and limitations with small sample or single center studies, no robust data are available to inform clinical practice. To address this, and to our knowledge, ours is the largest retrospective cohort study on the risk of fatal HD in patients with bone and soft tissue sarcoma.
We performed a contemporary analysis of the risk of fatal HD in >80,000 sarcoma patients and showed that HD risk varied as a function of age, histological subtype, clinical stage, and time after diagnosis. Earlier studies reported that cancers were associated with a high risk of death from HD (4, 6, 7). We first reported that sarcoma patients had a higher risk of fatal HD when compared with the general US population, which was potentially attributed to antineoplastic agents such as anthracycline (24). Anthracyclines are antibiotics discovered approximately 50 years ago, are used as antineoplastic agents, and are the most successful anticancer therapies ever developed for sarcoma (3). However, a worrisome adverse side effect is ventricular dysfunction and heart failure (25). Due to low sarcoma incidence rates, previous cardio-toxicity studies in sarcoma patients receiving chemotherapy were conducted in small sample or at single centers (13–15). In a recent comparative study of 95 patients with sarcoma, approximately 17% developed cardio-toxicity after receiving doxorubicin (26). Indeed, the death rate due to HD in patients receiving chemotherapy was seriously underreported due to inaccurate statistics about cause of death.
The systematic and standardized data collection procedures are used to ensure that the causes of death recoded in SEER are accurate (27); therefore, we believe our study presents a reliable picture of death rates from HD in patients with sarcoma in the US. Using the SEER database, we showed that the incidence rate of fatal HD in sarcoma patients was 631.7/100,000 person-years. In subgroup analyses, chemotherapy for sarcoma only increased the risk of fatal HD in patients with localized osteosarcoma, but not for other subtypes. These subgroups results may be attributed to the low incidence rates of Ewing sarcoma in the SEER database.
Apart from chemotherapy, surgery is the main treatment method for sarcoma as it aims to remove all sarcoma traces from patients (22). In 2005, an estimated 18,000 patients underwent amputation due to bone and soft tissue sarcoma, which was the third leading cause of amputation in the US (28). We showed that 79% of patients underwent sarcoma surgery in the study cohort. These patients typically lost a certain degree of physical activity, which is a known risk factor for cardiovascular events (24, 29). Moreover, earlier reports indicated that sarcoma patients presented a certain degree of anxiety and depression which were related to functional and appearance changes (30, 31). Physical inactivity and demoralization are risk factors for HD (32). In our study, the higher relative risk of fatal HD in sarcoma patients to general population in the first year was possibly attributed to chemotherapy, and the relative risk of fatal HD was highest during follow-up after 10 years, which was possibly attributed to combined physical inactivity and demoralization effects. Therefore, clinicians should orchestrate specific rehabilitation care strategies to help long-term sarcoma survivors improve their exercise regimens. Also, evidence now suggests that exercise may inhibit both early and late doxorubicin-induced cardiotoxicity (33). Likewise, psychological assessments such as the Hamilton Depression and Hamilton Anxiety Scales are necessary for long-term sarcoma survivors (20), and can help orthopedic surgeons identify psychological disorders in patients and allow psychologists commence interventions as early as possible.
Cardiovascular events have typically been regarded as age-related disorders, but in our study, younger patients with sarcoma had a higher relative risk of fatal HD when compared with older patients. In the general population aged < 19 years old, HD mortality was very low, at only 2.6/100,000 person-years. But in similar-aged sarcoma patients, this mortality was 32.7/100,000 person-years due to sarcoma and chemotherapy. Given the high risk of fatal HD among younger patients with sarcoma, clinicians should closely monitor cardiac functions in these younger patients.
Our study had several limitations, most of which were related to the SEER database. Firstly, the database program was limited in terms of detailed information on chemoradiotherapy such as doses and cycles. In a phase III ANNOUNCE trial evaluating cardiotoxicity in patients with soft-tissue sarcoma given doxorubicin, cardiac dysfunction occurred in 2% of patients receiving doses of <450 mg/m2, 3% at 450 – <600 mg/m2, and 1.1% at ≥600 mg/m2 (14). Considering the dose-dependent cardiotoxicity of doxorubicin (34, 35), direct associations must be assessed between chemotherapy dose or cycles and the risk of HD death in sarcoma patients. Future analyses can be augmented by the SEER-Medicare data set, which may elucidate direct relationships with chemotherapy doses and cycles. Secondly, in subgroup analyses, we observed no chemotherapy effects on the risk of fatal HD in other subgroups, such as patients with Ewing sarcoma, inconsistent with previous studies (14, 33, 36). A possible explanation could be the relatively low number of deaths due to cardiovascular events which in turn may have reduced the statistical power of our subgroup analyses, therefore, this requires further investigation. Despite these limitations, ours is the first large sample and population-based study with the longest follow-up times, to explore the risk of HD death in sarcoma patients. Our results are reliable and robust and may be used to guide clinical practice.
Conclusion
This is the first large population-based study on the risk of fatal HD in sarcoma patients, which up to now were rarely reported. Our results suggest that the risk of fatal HD in sarcoma patients is higher than that of the general population, and increases with longer follow-up times. The relative risk of HD to the general population varied in patients with different histological sarcoma subtypes and clinical stage. Subgroup analyses indicated that chemotherapy increased the risk of fatal HD in patients with localized osteosarcoma. To mitigate the risk of fatal HD in sarcoma patients, enhanced multidisciplinary cooperation is warranted, including cardiologists and orthopedic surgeons.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for this study in accordance with the local legislation and institutional requirements. Written informed consent from the participants was not required for this study in accordance with the local legislation and institutional requirements.
Author contributions
BC and JT: conception and design of study. BC and XZ: acquisition of data. BC, XZ, XL, JL, and JT: analysis and/or interpretation of data. BC, XL, JL, and JT: drafting the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81871577 by JT) and the Natural Science Foundation of Hunan Province China (2022JJ30001 by JL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.World Health Organization [WHO]. The Top 10 Causes of Death. (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed December 9, 2020). [Google Scholar]
- 2.Raposeiras Roubín S, Cordero A. The two-way relationship between cancer and atherosclerosis. Revista Española de Cardiol. (2019) 72:487–94. [DOI] [PubMed] [Google Scholar]
- 3.Mazevet M, Moulin M, Llach-Martinez A, Chargari C, Deutsch É, Gomez A-M, et al. Complications of chemotherapy, a basic science update. Presse Med. (2013) 42(9 Pt 2.):e352–61. [DOI] [PubMed] [Google Scholar]
- 4.Navi BB, Howard G, Howard VJ, Zhao H, Judd SE, Elkind MSV, et al. New diagnosis of cancer and the risk of subsequent cerebrovascular events. Neurology. (2018) 90:e2025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misono S, Weiss NS, Fann JR, Redman M, Yueh B. Incidence of suicide in persons with cancer. J Clin Oncol. (2008) 26:4731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. (2019) 40:3889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaorsky NG, Zhang Y, Tchelebi LT, Mackley HB, Chinchilli VM, Zacharia BE. Stroke among cancer patients. Nat Commun. (2019) 10:5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith GL, Smith BD, Buchholz TA, Giordano SH, Garden AS, Woodward WA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol. (2008) 26:5119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henson KE, Reulen RC, Winter DL, Bright CJ, Fidler MM, Frobisher C, et al. Cardiac mortality among 200000 five-year survivors of cancer diagnosed at 15 to 39 years of age: the teenage and young adult cancer survivor study. Circulation. (2016) 134:1519–31. 10.1161/CIRCULATIONAHA.116.022514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith GM, Johnson GD, Grimer RJ, Wilson S. Trends in presentation of bone and soft tissue sarcomas over 25 years: little evidence of earlier diagnosis. Ann R Coll Surg Engl. (2011) 93:542–7. 10.1308/147870811X13137608455055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu K, Chen Y, Song K, Xiong F, Tian Y, Guan H, et al. Impact of limb salvage on prognosis of patients diagnosed with extremity bone and soft tissue sarcomas. Front Oncol. (2022) 12:873323. 10.3389/fonc.2022.873323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argenziano M, Tortora C, Pota E, Di Paola A, Di Martino M, Di Leva C, et al. Osteosarcoma in children: not only chemotherapy. Pharmaceuticals. (2021) 14:923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Tine BA, Agulnik M, Olson RD, Walsh GM, Klausner A, Frank NE, et al. A phase II clinical study of 13-deoxy, 5-iminodoxorubicin (GPX-150) with metastatic and unresectable soft tissue sarcoma. Cancer Med. (2019) 8:2994–3003. 10.1002/cam4.2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones RL, Wagner AJ, Kawai A, Tamura K, Shahir A, Van Tine BA, et al. Prospective evaluation of doxorubicin cardiotoxicity in patients with advanced soft-tissue sarcoma treated in the announce phase III randomized trial. Clin Cancer Res. (2021) 27:3861–6. 10.1158/1078-0432.CCR-20-4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Tine BA, Hirbe AC, Oppelt P, Frith AE, Rathore R, Mitchell JD, et al. Interim analysis of the phase II study: noninferiority study of doxorubicin with upfront dexrazoxane plus olaratumab for advanced or metastatic soft-tissue sarcoma. Clin Cancer Res. (2021) 27:3854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vos M, Sleijfer S, Litiere S, Touati N, Duffand F, van der Graaf WT, et al. Association of pazopanib-induced toxicities with outcome of patients with advanced soft tissue sarcoma; a retrospective analysis based on the European Organisation for Research and Treatment of Cancer (EORTC) 62043 and 62072 clinical trials. Acta Oncol. (2019) 58:872–9. [DOI] [PubMed] [Google Scholar]
- 17.NIH National Cancer Institute. Overview of the SEER Program. (2018). Available online at: http://seer.cancer.gov/about/overview.html (accessed July 8, 2016). [Google Scholar]
- 18.Fung C, Fossa SD, Milano MT, Sahasrabudhe DM, Peterson DR, Travis LB. Cardiovascular disease mortality after chemotherapy or surgery for testicular nonseminoma: a population-based study. J Clin Oncol. (2015) 33:3105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koepsell TD, Weiss NS. Epidemiologic Methods: Studying the Occurrence of Illness. New York, NY: Oxford University Press; (2003). [Google Scholar]
- 20.Yu K, Wu B, Chen Y, Kang H, Song K, Dong Y, et al. Suicide and accidental deaths among patients with primary malignant bone tumors. J Bone Oncol. (2021) 27:100353. 10.1016/j.jbo.2021.100353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Yu K, Xiong J, Zhang J, Zhou S, Dai J, et al. Suicide and accidental death among women with primary ovarian cancer: a population-based study. Front Med. (2022) 9:833965. 10.3389/fmed.2022.833965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagarajan R, Neglia JP, Clohisy DR, Robison LL. Limb salvage and amputation in survivors of pediatric lower-extremity bone tumors: what are the long-term implications? J Clin Oncol. (2002) 20:4493–501. 10.1200/JCO.2002.09.006 [DOI] [PubMed] [Google Scholar]
- 23.Yu K, Chen Y, Tian Y, Kang H, Song K, Dong Y, et al. Characteristics, incidence, and risk factors for death from fatal pneumonia among patients with primary malignant bone tumors: a SEER-based observational study. Transl. Cancer Res. (2021) 10:3659–70. 10.21037/tcr-21-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seixas AA, Vallon J, Barnes-Grant A, Butler M, Langford AT, Grandner MA, et al. Mediating effects of body mass index, physical activity, and emotional distress on the relationship between short sleep and cardiovascular disease. Medicine. (2018) 97:e11939. 10.1097/MD.0000000000011939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oikonomou EK, Kokkinidis DG, Kampaktsis PN, Amir EA, Marwick TH, Gupta D, et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. (2019) 4:1007–18. 10.1001/jamacardio.2019.2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S-H, Cho I, You S-C, Cha M-J, Chang J-S, Kim WD, et al. Cancer therapy-related cardiac dysfunction in patients treated with a combination of an immune checkpoint inhibitor and doxorubicin. Cancers. (2022) 14:2320. 10.3390/cancers14092320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Overview of the surveillance, epidemiology, and end results database: evolution, data variables, and quality assurance. Curr Probl Cancer. (2012) 36:183–90. 10.1016/j.currproblcancer.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 28.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. (2008) 89:422–9. [DOI] [PubMed] [Google Scholar]
- 29.Mioc M-L, Prejbeanu R, Vermesan D, Haragus H, Niculescu M, Pop DL, et al. Deep vein thrombosis following the treatment of lower limb pathologic bone fractures - a comparative study. BMC Musculoskelet Disord. (2018) 19:213. 10.1186/s12891-018-2141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paredes T, Canavarro MC, Simões MR. Anxiety and depression in sarcoma patients: emotional adjustment and its determinants in the different phases of disease. Eur J Oncol Nurs. (2011) 15:73–9. 10.1016/j.ejon.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 31.Wiener L, Battles H, Bernstein D, Long L, Derdak J, Mackall CL, et al. Persistent psychological distress in long-term survivors of pediatric sarcoma: the experience at a single institution. Psychooncology. (2006) 15:898–910. 10.1002/pon.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raiè M. Depression and heart diseases: leading health problems. Psychiatr Danub. (2017) 29(Suppl 4.):770–7. [PubMed] [Google Scholar]
- 33.Wang F, Chandra J, Kleinerman ES. Exercise intervention decreases acute and late doxorubicin-induced cardiotoxicity. Cancer Med. (2021) 10:7572–84. 10.1002/cam4.4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Weng J, Sun S, Zhou J, Yang Q, Huang X, et al. Ononin alleviates endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity by activating SIRT3. Toxicol Appl Pharmacol. (2022) 452:116179. 10.1016/j.taap.2022.116179 [DOI] [PubMed] [Google Scholar]
- 35.Kim SW, Ahn B-Y, Tran TTV, Pyun J-H, Kang J-S, Leem Y-E. PRMT1 suppresses doxorubicin-induced cardiotoxicity by inhibiting endoplasmic reticulum stress. Cell Signal. (2022) 98:110412. 10.1016/j.cellsig.2022.110412 [DOI] [PubMed] [Google Scholar]
- 36.Vitfell-Rasmussen J, Krarup-Hansen A, Vaage-Nilsen M, Kümler T, Zerahn B. Real-life incidence of cardiotoxicity and associated risk factors in sarcoma patients receiving doxorubicin. Acta Oncol. (2022) 61:801–8. 10.1080/0284186X.2022.2082884 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.