Abstract
Since early 2020, the entire world has been facing a disastrous outbreak of the SARS-CoV-2 virus, with massive reporting of death and infections per day. Medical practitioners adopted certain measures such as convalescent plasma therapy, antibody treatment, and injecting vaccines to eradicate the pandemic. In this review, we have primarily focused on the neutralizing antibodies presently under pre-clinical and clinical trials, focusing on their structures, binding affinity, mechanism of neutralization, and advantages over other therapeutics. We have also enlisted all the nAbs against SARS-CoV-2 and its emerging variants in different phases of clinical trials (phase-1, phase-II, and phase-III). The efficacy of administering antibody cocktails over the normal antibodies and their efficacy for the mutant variants of the SARS-CoV-2 virus in minimizing viral virulence is discussed. The potent neutralizing antibodies have eliminated many of the common problems posed by several other therapeutics. A common mechanism of the antibodies and their relevant sources have also been listed in this review.
Keywords: neutralizing antibody, SARS-CoV-2, pre-clinical trials, clinical trials
1. Introduction
The current pandemic of Coronavirus disease (COVID-19) started in the Wuhan province of China in December 2019. During infection, the SARS-CoV-2 virus triggers various immune cascades. Effective, balanced immune components are required to control the pathogenesis of COVID-19 [1,2,3]. The neutralizing antibodies (nAb’s) have shown defense against infected or vaccinated individuals, and can be used as a promising therapeutics component in humans [4]. The nAb is a class of antibodies that neutralize the invading cells of the disease-causing pathogens, thus providing immunity. Such antibodies might be triggered by the use of vaccines or an earlier infection, which are retained inside the body for a longer time than the therapeutic ones. Therefore, neutralizing antibodies are employed for treating several critical pathogenic infections due to their enhanced specificity [5,6].
Before or after viral infection, nAbs can be transferred passively to patients to treat COVID-19 [6]. It has also been proven to be very effective for patients with clinically mild symptoms in the early onset of disease [7]. One of these nAb’s sources is the B cell, isolated from the convalescent plasma donors. An elucidative screening of these antibodies has shown that they can hinder viral entry and prevent SARS-CoV-2 infection [8,9,10]. Some of these nAbs can be derived from humanized mice or convalescent patients and follow the same action mechanism [11]. Antibody development events from the smallpox vaccination were a breakthrough, and this event has shown a new direction for the treatment of COVID-19 as an anti-SARS-CoV-2 mAb (Figure 1). Events that led to the development of antibodies are shown in Table 1. nABs have an effective therapeutic role in preventing SARS-CoV-2 infection. Considering the antigenic part of the spike (S)-protein, the nAbs are developed, which can be specifically bound to the RBD of S-glycoprotein [12,13,14]. The nAbs development depends not only on the structure but also on the alteration of the protein conformation. The nAb, with accurate structure and conformation, invades the host cells, and it is important for the functionality of the nAbs against the infection of the virus [6,15].
Figure 1.
Timelines show different breakthroughs in antibody development.
Table 1.
Events that led to the development of antibodies.
| Sl. No. | Year | Scientists Involved | Progress in the Development of Antibodies |
Reference |
|---|---|---|---|---|
| 1. | 1798 | Edward Jenner | The breakthrough of the smallpox vaccine | [16] |
| 2. | 1890 | Emil von Behring and Shibasabura Kitasato | The transfer of serum to cure diphtheria taken from immunized animal | [17] |
| 3. | 1900 | Paul Ehrlich | The advancements of several concepts such as antigen-antibody binding, side-chain theory, and complement activation | [18] |
| 4. | 1948 | Astrid Fagraeus | Elucidated the importance of B cells | [19] |
| 5. | 1959 | Gerald Edelman and Rodney R. Porter | The publication of the molecular tructures of various antibodies | [20] |
| 6. | 1973 | D Inbar, J Hochman, and D Givol | The publication of the molecular tructures of the antibodies fragment | [21] |
| 7. | 1990 | A Plückthun | Antibody engineering | [22] |
| 8. | 1997–2015 | - | Development of various antibodies such as CD20, HER2, CD52, VEGF-A, EGFR, VEGFR2, and IL17A | [23,24] |
| 9. | 2021 | - | Development of anti-SARS-CoV-2 antibodies such as Bamlanivimab plus etesevimab, Casirivimab plus imdevimab, and Sotrovimab | [25] |
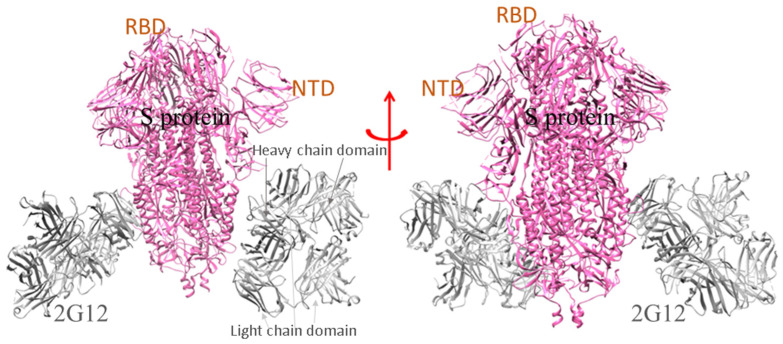
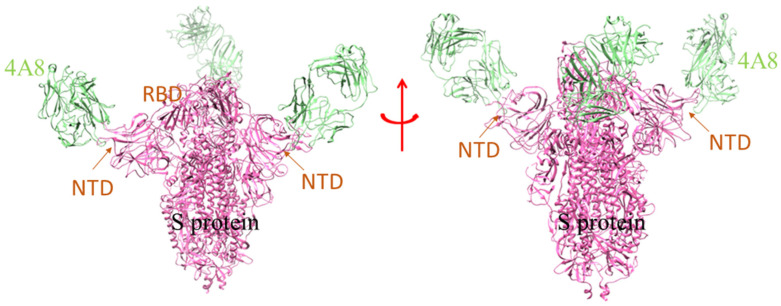
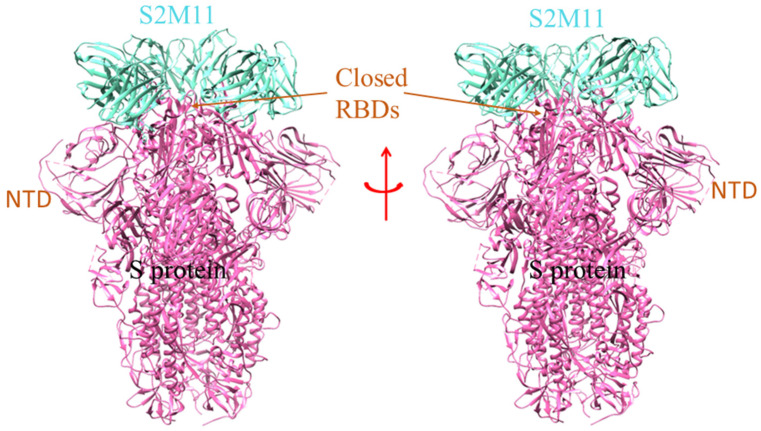
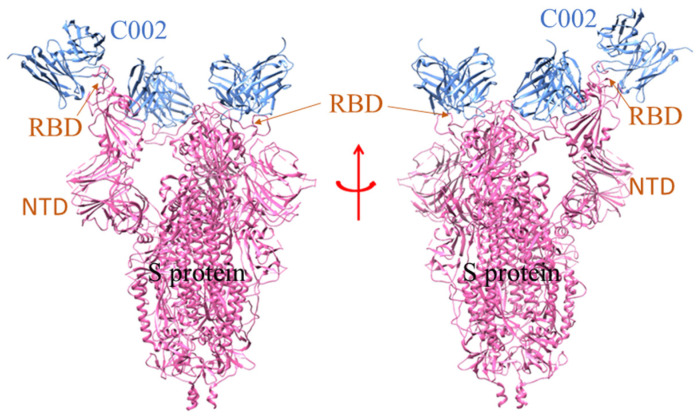
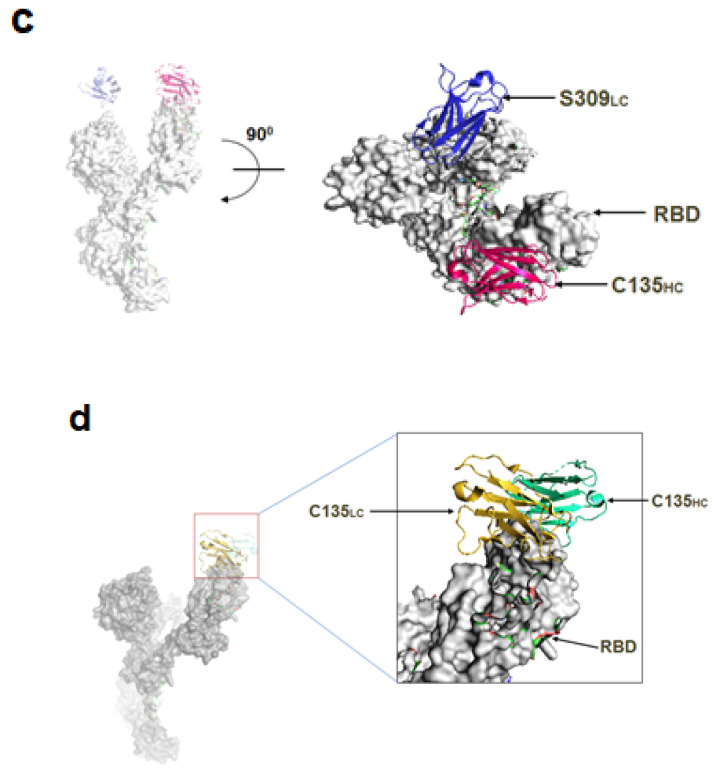
For the SARS-CoV-2 infection, a few nAbs are highly specific to the S-glycoprotein and prevent the binding of S-glycoprotein to the RBD-ACE2 complex in the host cell. A group of scientists isolated the B cells from the infected individuals and initiated the preparation of different types of nAbs, currently in the pre-clinical and clinical phases (P2C-1F11, BD-368-2, P2B-2F6, COV2-2196, COV2-2130, etc.) [6,26,27]. A model shows where the SARS-CoV-2 spike glycoprotein (S-protein) ectodomain is bound to two copies of domain-swapped natural antibody 2G12 (Figure 2). Likewise, SARS-CoV-2 S-protein in trimeric form also makes a complex with the human nAb (C002) Fab fragment (Figure 3). Many of the nAb isolated from human beings had proven to be effective in treating the SARS-CoV-2 infection in several animals, namely the transgenic mice, hamsters, etc. The nAb (S2M11) Fab part bind with the adjacent receptor-binding domains of S-glycoprotein present in a closed conformation (Figure 4). Another nAbs, named Vh–Fc ab8, targets the spike RBD, which is also very effective in treating the infection [17,18,19]. However, researchers developed some nAbs with a different target site other than the spike RBD. They also targeted other regions besides RBD in the S-protein, and are currently entering the pre-clinical stages [6,28]. It has been noted that nAbs targeting the spike RBD are more efficient than the nAb targeting the other regions of S-protein (other than RBD). At the same time, scientists have shown, through electron microscopy, that the nAb can also bind to the NTD of S-protein. One such example is mAb (4A8), which binds with the S-protein NTD part (Figure 5). Therefore, the NTD might be a potent target for therapeutic mAbs against the COVID-19. This target specificity of the spike RBD has not only proved to be effective for the wild-type strain but also for several emerging mutant variants. They have very minimal immune escape property [13,29]. Liu et al. stated that RBD is a highly conserved region, and researchers should develop more nAb targeting RBD to treat the infection [30].
Figure 2.
The ribbon model shows the SARS-CoV-2 2P S-protein ectodomain bound to two copies of domain-swapped antibody 2G12 [PDB id: 7L06]. The heavy chain domain of Ab(2G12) interacts with the NTD part of the S-protein.
Figure 3.
The model demonstrates the binding of human mAb (4A8) to the NTD of S-protein of SARS-CoV-2 [PDB id: 7C2L]. The chains of the mAb unit interact with the different NTD of the S-protein trimeric sub-unit domain.
Figure 4.
The model shows the human nAb (S2M11) binding with the adjacent part of RBD in closed conformation of S-protein [PDB id: 7K43]. The different domain of SARS-CoV-2 S-protein is marked, where the RBD region interacts with the single unit of nAb (S2M11).
Figure 5.
The structure shows the SARS-CoV-2 S 6P trimer in a complex with human nAb (C002) Fab fragment [PDB id: 7K8T]. The nAb (C002) Fab fragment partially interacts with the RBD and NTD regions of the S-protein of SARS-CoV-2.
It has been noted that various nAbs were employed to treat SARS-CoV infection previously, and these nAbs are also used for neutralizing the SARS-CoV-2 infection. However, some of the nAbs of the SARS-CoV virus fail to target the spike RBD region of the SARS-CoV-2 and are unable to neutralize the future viral infection [6]. Some mutational modifications in the SARS-CoV-2 variants may affect the viral infectivity, and also, the mutation-related S-protein configuration change might alter the nAbs binding affinity. On the other hand, it was also recorded that the protein modifications can make them efficient in targeting the conserved epitopes of spike RBD which could enhance the neutralizing capacity of nAbs [31,32,33]. The researcher also reported that the nAbs inhibit the interaction of spike protein with the ACE2 receptor preventing membrane fusion [34]. It is also noted that some of the RBD targeting nAb or the non-RBD targeting ones are incompatible with preventing spike protein interaction against the ACE2 receptor. These nAbs exhibit the viral neutralizing capacity and bind to other S-glycoprotein regions, preventing the entry of the SARS-CoV-2 virus [35]. Several scientists analyzed the detailed phenomena of incapability of nAb to prevent viral entry. They found that these nAbs interact with the Fcγ receptor and lead to the antibody-dependent enhancement (ADE) with the target cells. This ADE formation subsequently leads to the release of cytokines such as IL-6 [36]. However, there are no reports of ADE formation in patients with SARS-CoV-2 infection till today [6].
Given the therapeutic potential of nAbs in viral protection, here, we summarize the nAbs presently under pre-clinical and clinical trials for COVID-19 treatment with special attention to their structures, binding affinity, mechanism of neutralization, and advantages over other therapeutics. Subsequently, we also highlighted the efficacy of administering antibody cocktails over the normal ones and their efficacy against the significant mutant variants of the SARS-CoV-2 virus. The specific role of potent single domains antibodies is also discussed for therapeutics. A collective mechanism of the neutralizing antibodies and their sources are also listed in this review.
2. Structure of a Neutralizing Antibody
One of the common features of all the nAbs is the CDRH3 region (complementarity-determining region 3) in the heavy chain. The CDRH3 region comprises of a few gene segments with unique amino acid residues. The three genes present are V (variable), D (diversity), and J (joining). Several studies highlighted that the antibodies interact with the antigens and elicit the immune response, which solely depends on the CDRH3 region [37]. The researcher also studied the CDRH3 region of the antibodies released from the B cells elicited by the spike glycoprotein of SARS-CoV-2. They did not find any significant variation in the length of the CDRH3 region in antibodies compared to the normal population. However, they found that the average length of these isolated CDRH3 regions was nearly 20 amino acids long [37].
The nAbs isolated from the convalescent plasma donors are specific to the RBD epitopes. However, some of these nAbs might target overlapping epitopes [38]. These antibodies protect the virus from interacting with the ACE2 receptor preventing viral infection. Like other antibodies, the nAbs employed specifically for preventing the SARS-CoV-2 infection also comprise two chains: heavy and light. The heavy chain is segmented into smaller regions which are encoded by the VH3-53 or VH3-66 genes. In addition, it comprises three complementary determining regions of the heavy chain (CDRH), namely CDRH1, CDRH2, and CDRH3. The CDRH3 region is generally shorter in length than the other two regions [37]. One in vitro study isolated the SARS-CoV-2 nAb and found that it possesses a similar target and comprises VH3-30 genes in the CDRH3 region [37]. According to the structural and functional attributes, Barnes et al. have categorized the nAbs into four types which are:
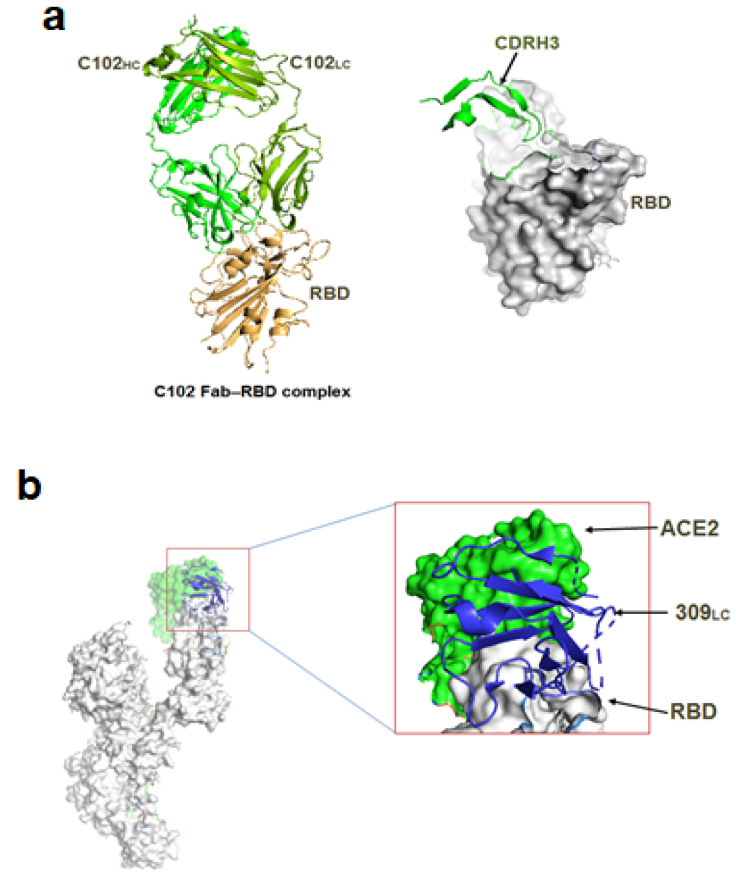
i. VH3-53 encoded gene that blocks the host ACE2 only in the ‘up’ conformation of the RBD. They exhibit a shorter CDRH3 region (Figure 6a).
Figure 6.
Different types of nAb based on structural and functional attributes. (a) Class I nAb (CDRH3) binds with open RBD (C102 Fab-RBD complex). (b) Class II nAb S309 bind with both ACE2 and RBD (309LC-ACE2 RBD complex). (c) Class III nAb C135 bind distinct RBD part (C135HC and S309LC with RBD). (d) Class IV nAb C135 bind with only up conformation of RBD (C135HC and C135LC with RBD). [PDB id: 6WPS, 7K8M].
ii. Another class of ACE2 blocking antibody is functional in both (up and down) RBD conformations and can even contact adjacent RBDs (Figure 6b).
iii. An additional class of nAb that hinders viral entry by occupying the outer surface of the ACE2; functional in both the up and down conformation of the RBD (Figure 6c).
iv. The fourth class does not interact with the ACE2 receptor and is functional in the ‘up’ conformation of the RBD (Figure 6d) [37].
3. Types of mAbs
Remarkably, the beneficial antibodies being cloned in a laboratory need not come from humans but can be from definite animals also. Accordingly, mAbs are of four extensive types.
i. Murine: made from mouse proteins; names of drugs based on this end in -omab.
ii. Chimeric: a combination of mouse and human proteins; names of drugs based on this end in -ximab.
iii. Humanized: here, small doses of mouse proteins are attached to human proteins; names of drugs based on this end in -zumab.
iv. Human: these are fully human proteins; names of drugs based on this end in -umab.
3.1. mAb in Treatment of COVID-19
The mAbs have been used to combat MERS, SARS, and other important infections caused by the corona family of viruses in the last decade. Since the COVID-19 pandemic broke out in early 2020, there has been an accelerated drive to use mAbs to fight the virus. The FDA performed a significant role in approving and regulating the use of mAbs to treat COVID-19. Moreover, it was in charge of the guidelines on which mAb-based drugs should be used.
Therefore, in the last two years, a number of drugs are already in use, such as Bamlanivimab and etesevimab, which are used when there is mild to moderate infection by SARS-CoV-2 infection. Casirivimab and imdevimab are used when there is mild to moderate infection and the patient is at risk of developing a severe infection, but the person does not need oxygen therapy. In some previously infected people with the SARS-CoV-2 virus, the immune system goes into overdrive, releasing bursts of proteins known as cytokine storms. While these may or may not fight this virus effectively, they definitely cause severe inflammation in the body, which can be life-threatening. In such a scenario, the antigens from these cytokines must also be suppressed. This is undertaken by a definite category of mAbs called anti-interleukin-6 receptor mABs. Levilimab and Tocilizumab (which are widely present) are examples of such drugs.
Anti-CD6 mAbs are similar to anti-interleukin-6 receptor mAbs, but the biochemical mechanism is slightly different. Itolizumab is an example of such a drug type.
3.2. Mechanism of Action of SARS-CoV-2 nAb
The main target of most of the SARS-CoV-2 nAb is the spike glycoprotein which is responsible for triggering a strong host immune response due to its high antigenicity [38,39,40]. Among the reported nAbs, more than 90% can bind with the RBD and block the viral interaction with the host ACE2. According to Jin D et al., the binding of the nAb to the ACE2 receptor can be subdivided into two regions, namely a/b. The binding affinity of the nAbs to these two sites is expected to inhibit a potent neutralizing effect in hindering the binding of the spike protein. Moreover, these antibodies are unable to bind with the RBD in the same manner. The variable binding of these antibodies helps segregate them [41]. Therapeutic monoclonal antibodies can interact with one or more epitopes of the RBD region [42]. This variation of neutralization in the case of nAb also varies with respect to diverse interaction sites of antigens. For instance, the mAb named S2H13 possesses neutralizing activity by identifying the conformation of the spike protein. However, it has been noted that the EY6A and ACE2 neutralization have different action mechanisms. It has been revealed that during neutralization, EY6A binds to the lower portions of the b region of ACE2 and thus is unable to interact with S1 and S2 junctions of S-glycoprotein. Therefore, these event makes the epitope incapable of binding with the ACE2 receptor (Figure 7). Usually, the nAbs that target a single epitope alter the conformation of the spike glycoprotein (in a down conformation). It helps to make it inaccessible to interact with the ACE2 receptor [43,44]. One of the major reasons enabling the neutralization of the SARS-CoV-2 virus is a creation of a stearic obstacle due to the orientation of the nAbs. This obstacle makes the spike protein incapable of interacting with the host receptor. S2A4 has a very strong viral neutralizing capacity compared to the other nAb’s. After binding with the spike RBD, this antibody causes the shedding of the S1 subunit, hindering the access to bind with the ACE2 receptor. Some nAbs use three spike epitopes to restrain the interaction with the ACE2 receptor. These antibodies surround the RBD in many ways. They bind to the edges and tip of the RBD and use either the heavy chain or light chain to interact with the viral epitopes [41].
Figure 7.
The SARS-CoV-2 S-protein in a complex with a nAb EY6A Fab [PDB id: 6ZDH].
Here, in the earlier section, we have discussed that the nAbs target the RBD and NTD (N-terminal domain). The exact mechanism of NTD attachment is not vividly elucidated to date. However, the structural analysis of the spike protein showed that these nAbs bind with the NTD and alter the RBD conformation (down conformation). This phenomenon creates a stearic obstacle coinciding with the antibody and its binding to the ACE2 receptor. Consequently, the spike protein is unable to interact with the ACE2 receptor due to nAb-ACE2 complex [31].
4. Advantages of nAb over Vaccines and Convalescent Plasma Therapy
During the pandemic, one of the alarming situations prevailing throughout the world is the evolving SARS-CoV-2 variants and their strategy to immune escape. In this regard, the application of nAbs might be more effective than vaccines. The administration of two or more antibodies (antibody cocktails) together has proven too efficient in the case of evolving variants [45]. Moreover, a more efficient approach must be taken to develop vaccines against the evolving variants to eradicate the pandemic.
Researchers aim to establish the COVID-19 treatment using several nAbs by replacing convalescent plasma therapy (CPT). The primary reason behind replacing CPT with nAbs is the elimination of some blood diseases which are generally the side-effects of CPT. The use of the nAbs helps in the faster development of epitope-specific antibodies. Moreover, a proper dosage of these nAbs forms a high titer of antibodies within a very short time compared to CPT. The high efficacy of nAbs has also proven to have superior results in cases of COVID-19 and certain other disease outbreaks [11].
It has been noted that the FDA approved the CPT to treat hospitalized COVID-19 patients; however, more accurate results are awaited from the undergoing clinical trials. Patients undergoing plasma transfusion should not be comorbid and should not have any chance of antibody-dependent enhancement (ADE). In this regard, nAbs are highly effective against the CPT treatment [46]. We have listed different nAbs in different phases of clinical trials (Table 2).
Table 2.
Different neutralizing antibodies which are in clinical trials.
| Sl. No. | nAb | Trial No. | Status | Recruitment | No. of Participants | Sponsor | Country | Allocation | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1. | JS016 | NCT04441918 | Phase II | Recruiting | 40 | Shanghai Junshi Bioscience Co., Ltd. | China | Randomized | A randomized, placebo-controlled study reporting its safety, pharmacokinetics, and immunogenicity administered in healthy subjects. |
| 2. | LY3832479 | NCT04441931 | Phase II | Completed | 26 | Eli Lilly and Company | United States | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics of the mAb in healthy adult volunteers. |
| 3. | LY-CoV016 | NCT04427501 | Phase II | Active but not recruiting | 3290 | Eli Lilly and Company | United States | Randomized | A randomized, placebo-controlled study reporting the tolerability, efficiency, and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. |
| 4. | TY027 | NCT04429529 | Phase III | Completed | 32 | Tychan Pte Ltd. | Singapore | Randomized Randomized |
A randomized, placebo-controlled, time-lagged study conducted in healthy subjects. |
| NCT04649515 | Recruiting | 1305 | A randomised, placebo controlled study of TY027 aimed for treating COVID-19 patients. | ||||||
| 5. | BRII-196 | NCT04479631 | Phase III | Completed | 16 | Brii Biosciences Limited | China | Randomized | A randomized, placebo-controlled study of BRIL-196 monoclonal antibodies reporting its safety, tolerability, and pharmacokinetics. |
| 6. | BRII-198 | NCT04479644 | Phase III | Completed | 17 | Brii Biosciences Limited | China | Randomized | A randomized, placebo-controlled study of BRIL-196 monoclonal antibodies reporting its safety, tolerability, and pharmacokinetics. |
| 7. | CT-P63 | NCT05017168 | Phase I pending | Not yet recruiting | 24 | Celltrion | Poland | Randomized | A randomized, placebo-controlled study reporting the tolerability, efficiency, and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. |
| 8. | XVR011 | NCT04884295 | Phase I | Recruiting | 279 | ExeVir Bio BV | Belgium and Italy | Randomized | A randomized, placebo-controlled study reporting the tolerability, efficiency, and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. |
| 9. | ABBV-47D11 | NCT04644120 | Phase I | Completed | 25 | AbbVie | United States | Randomized | A randomized, placebo-controlled study of ABBV-47D11 and ABBV-2B04 monoclonal antibodies reporting its safety, pharmacodynamics, and pharmacokinetics. |
| 10. | HFB30132A | NCT04590430 | Phase I | Active but not recruiting | 24 | HiFiBiO Therapeutics | United States | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics of the mAb in healthy adult volunteers. |
| 11. | ADM03820 | NCT04592549 | Phase I | Recruiting | 40 | Ology Bioservices | United States | Randomized | A randomized, placebo-controlled study reporting its safety, pharmacokinetics, and immunogenicity. |
| 12. | DXP604 | NCT04669262 | Phase I | Completed | 25 | BeiGene | Australia | Randomized | A randomized, placebo-controlled study reporting its safety, pharmacokinetics, and immunogenicity in healthy volunteers. |
| 13. | HLX70 | NCT04561076 | Phase I | Not yet | 24 | Hengenix Biotech Inc | United States | Randomized | A randomized, placebo-controlled study reporting its safety and pharmacokinetics. |
| 14. | COR-101 | NCT04674566 | Phase I and Phase II | Recruiting | 45 | Corat Therapeutics Gmbh | Germany | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics and immunogenicity of COR-101 in hospitalized COVID patients. |
| 15. | VIR-7832 | NCT04746183 | Phase I and Phase II | Recruiting | 600 | University of Liverpool | United Kingdom | Randomized | A randomized, placebo-controlled trial aimed to evaluate the efficacy of the drug in treating COVID-19 patients. |
| 16. | LY-CoV1404, LY3853113 | NCT04634409 | Phase II | Active but not recruiting | 1782 | Eli Lilly and Company | United States | Randomized | A randomized, placebo-controlled study reporting the tolerability, efficiency, and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. |
| 17. | COVI-AMG (STI-2020) | NCT04734860 | Phase II | Recruiting | 500 | Sorrento Therapeutics, Inc. | United States | Randomized | A randomized, placebo-controlled study aimed to evaluate the safety and efficacy of the nAb in patients having mild COVID-19 symptoms. |
| 18. | DXP593 | NCT04532294 | Phase II | Completed | 18 | BeiGene | Australia | Randomized | A randomized, placebo-controlled study reporting its safety, pharmacokinetics, and immunogenicity in healthy volunteers. |
| NCT04551898 | 181 | United States | A randomized, placebo-controlled study highlighting the neutralizing efficiency of the BGBDXP593 mAb in COVID-19 patients having mild and moderate symptoms. | ||||||
| 19. | MW33 | NCT04533048 | Phase II | Completed | 42 | Mabwell (Shanghai) Bioscience Co., Ltd. | China | Randomized | A clinical study to evaluate the safety, pharmacokinetics, and immunogenicity of MW33 in normal, healthy volunteers. |
| NCT04627584 | Recruiting | 150 | A randomized, placebo-controlled study reporting the efficiency and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. | ||||||
| 20. | MAD0004J08 | NCT04932850 | Phase II and Phase III | Active but not recruiting | 30 | Toscana Life Sciences Sviluppo s.r.l. | Italy | Randomized | A randomized study to evaluate the safety, pharmacokinetics, and immunogenicity of MAD0004J08 in normal, healthy volunteers. |
| NCT04952805 | Recruiting | 800 | A randomized, placebo-controlled study aimed to evaluate the safety and efficacy profile of the antibody in adult COVID-19 volunteers who were asymptomatic, or had moderately severe symptoms. | ||||||
| 21. | C144-LS and C-135-LS | NCT04700163 | Phase II | Active but not recruiting | 23 | Rockefeller University | United States | Randomized | A randomized study to evaluate the safety, pharmacokinetics of two antibodies in normal, healthy volunteers. |
| 22. | SCTA01 | NCT04483375 | Phase II and Phase III | Completed | 33 | Sinocelltech Ltd. | China | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics of SCTA01 in healthy adult volunteers. |
| NCT04644185 | Recruiting | 795 | United States | A randomized, placebo-controlled study employed for examining the efficiency of SCTA01 in COVID-19 affected subjects having severe symptoms. | |||||
| 23. | ADG20 | NCT04805671 | Phase II and Phase III | Recruiting | 1084 | Adagio Therapeutics, Inc. | Germany, Greece, Brazil, Argentina, Poland, Ukraine | Randomized | A randomized, placebo-controlled study reporting the efficacy of the ADG20 mAb in healthcare workers having mild to moderate symptoms. |
| NCT04859517 | 6412 | United States | A randomized, placebo controlled trial for evaluating the safety profile of the antibody in preventing SARS-CoV-2 infection. | ||||||
| 24. | AZD7442 (AZD8895 + AZD1061) | NCT04507256 | Phase III | Active but not recruiting | 60 | AstraZeneca | United Kingdom | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics of AZD7442 in healthy adult volunteers. |
| NCT04625725 | 5197 | United States | A randomized, placebo-controlled study employed for evaluating the efficiency of AZD7442 in subjects who are not yet encountered by the SARS-CoV-2 virus. | ||||||
| NCT04625972 | 1121 | United States | A randomized, placebo-controlled study employed for evaluating the efficiency of AZD7442 in subjects who are already infected by the SARS-CoV-2 virus. | ||||||
| 25. | CT-P59 | NCT04525079 | EUA | Recruiting | 32 | Celltrion | Republic of Korea | Randomized | A randomized, placebo-controlled study reporting its safety and pharmacokinetics of CT-P59 in healthy volunteers. |
| NCT04593641 | Active but not recruiting | 18 | A randomized, Double-placebo-controlled, study reporting the viral nature, safety, and tolerability of the nAb in patients having mild symptoms. | ||||||
| NCT04602000 | Recruiting | 1020 | A randomized, placebo-controlled study employed for examining the efficiency of CT-P59 in COVID-19 affected subjects having severe symptoms. | ||||||
| 26. | VIR-7831 | NCT04545060 | EUA | Completed | 1057 | Vir Biotechnology, Inc. | United States | Randomized | A randomized, placebo-controlled study employed for evaluating the safety and efficiency of the nAb for treating COVID-19 patients who did not require any hospital support. |
| 27. | REGN-COV2 | NCT04425629 | EUA | Recruiting | 6420 | Regeneron Pharmaceuticals | United States | Randomized | A master protocol study reporting the safety and efficacy of the anti-spike mAbs in healthcare works affected with SARS-CoV-2 virus. |
| NCT04426695 | Completed | 2252 | A master protocol study reporting the safety and efficacy of the anti-spike mAbs in COVID-19 positive patients who required hospital support. | ||||||
| NCT04452318 | Active but not recruiting | 3750 | A randomized, placebo-controlled study evaluating the efficacy and the safety of the mAb in the household contacts to prevent SARS-CoV-2 infection. | ||||||
| 28. | LY-CoV555 (LY3819253); combination of LY-CoV555 with LY-CoV016 (LY3832479) |
NCT04411628 | Phase I | Completed | 24 | Eli Lilly and Company | United States | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics of LY3819253 in hospitalized COVID-19 patients. |
| NCT04427501 | Phase II | Recruiting | 3290 | A randomized, placebo-controlled study highlighting the neutralizing efficiency of the mAb in COVID-19 patients having mild and moderate symptoms. | |||||
| NCT04497987 | Phase III | Completed | 1374 | A randomized, placebo-controlled trial highlighting the safety and efficacy of the mAb alone and in combination to evaluate the immune response in nursing staffs to prevent SARS-CoV-2 infection. | |||||
| NCT04501978 | Phase III | Recruiting | 10,000 | University of Minnesota | A randomized, blinded controlled trial reporting the safety and efficacy of the COVID-19 positive patients who required hospital support. | ||||
| NCT04518410 | Phase II and Phase III | Recruiting | 8797 | National Institute of Allergy and Infectious Diseases (NIAID) | A randomized study evaluating the efficacy of LY3819253 in COVID-19 patients who did not require hospital support. | ||||
| 29. | Anti-SARS-CoV-2 mAb | NCT04748588 | Phase IV | Recruiting | 648 | University of Calgary | Canada | Randomized | A final trial aiming to evaluate the efficacy and safety of the antibody in the nosocomial COVID-19 patients in Canada. |
| 30. | BI 767551 | NCT04822701 | Phase II and Phase III | Active but not recruiting | 5 | Boehringer Ingelheim | United States | Randomized | A randomized, placebo-controlled study reporting the tolerability, efficiency, and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. |
| 31. | SAB- 185 |
NCT04469179 | Phase I | Active but not recruiting |
21 | SAb Biotherap eutics, Inc. |
United States | Randomized | A randomized study evaluating the efficacy of SAB-185 in COVID-19 patients who did not require hospital support. |
| 32. | Bamlanivimab | NCT04796402 | Phase IV | Active but not recruiting | 576 | Fraser Health | Canada | Randomized | A Phase IV study implicated for the emergency use of Bamlanivimab during the pandemic. |
5. Different nAbs Employed for Treating SARS-CoV-2 That Are in Clinical Trials
We have listed different nAbs that are currently in clinical trials (Table 2). The detailed account of these nAbs is also discussed in the following sections.
5.1. JS016
Etesevimab (also known as JS016) is a neutralizing monoclonal antibody possessing certain replacements in the amino acid residue (L234A, L235A) in the Fc region, which prevents the interaction of the S glycoprotein with the ACE2 receptor. This mAb also aims to prevent host–cell invasion and viral replication. It belongs to the class of IgG1 isotype and the LALA mutation in the Fc region that prevents various properties such as antibody-dependent cellular cytotoxicity and antibody-dependent enhancement. It mitigates the activation of macrophages to diminish the excessive cytokine storm observable in severely affected COVID-19 patients, proving the effectiveness of the antibody. An in vitro study conducted on a macaque model reported satisfactory results of JS016 in preventing SARS-CoV-2 infection [47].
5.2. MW33
The MW33 is a nAb of the type IgG1κ, highlighting several essential features for preventing COVID-19 disease. The rhesus monkey was the animal model where this particular antibody was administered. The MW33 antibody targets the spike RBD, but the conventional cytochrome P450 enzymes do not mediate its expulsion from the body. Instead, it is carried out by some non-specific proteolytic enzymes. The Phase I clinical trials of the MW33 have shown some decrement in the biochemical parameters. Later stages of the clinical trials will be performed to conclude more about the MW33 antibody to assess its safety, tolerability, and other important profiles [48].
5.3. CT-P59
The CT-P59 is a nAb obtained from patients who had convalescent plasma therapy for treating the SARS-CoV-2 infection. This antibody hinders the interaction of the spike RBD with the host ACE2 receptor, and this antibody inhibits the viral replication capacity, thus, reducing the viral load. The Phase I trial was conducted in two stages to establish the safety, tolerability, and pharmacokinetic profile of the CT-P59 antibody in healthy volunteers as well as patients with mild symptoms. It is also proved to be very efficient against the evolving variants. For example, the administration of CT-P59 proved to reduce the viral load in the respiratory tract (upper and lower) against the Beta variant. Further trials are also in the process that will elucidate more about the safety, tolerability, and pharmacokinetics profiling of the antibody [49].
5.4. REGEN-COV
According to the information obtained from the reports of the three clinical trial phases, REGEN-COV proved to be an efficient nAb for COVID-19 disease. The first two phases indicate that the administration of REGEN-COV has potentially lowered the rate of hospitalizations for COVID patients. In addition, it also hinders viral replication capacity, leading to a lower viral load. REGEN-COV was able to lower the rate of mortality as well as intensive care support, and it reduced the symptoms caused by the SARS-CoV-2 infection. The REGEN-COV also proved to be a very efficient mAb for several emerging SARS-CoV-2 variants, namely the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2) variants. In late 2020, due to the increased efficacy, REGEN-COV also achieved emergency approval from the FDA for administration in the SARS-CoV-2-infected patients with mild and moderate symptoms who did not require hospitalization support [50].
5.5. LY3819253/LY-CoV555
The administration of LY3819253 nAb was mainly on COVID-19 patients whose symptoms ranged from mild to moderate. The trial report indicated a reduction in the patients’ viral load administered with the antibody compared to the placebo. Moreover, the safety profile analysis for the antibody is very convincing, suggesting it to be an efficient treatment method for COVID patients in an emergency. Even it proved its superiority in the various zones having high-risk patients. The administration also reduced the rate of hospitalization, in turn reducing mortality. In addition, the administration of LY3819253 showed no adverse effects other than diarrhea and vomiting in a few volunteers. It also possesses special features responsible for viral clearance within a very short time [51].
5.6. VIR-7831
The VIR-7831 is similar to the S309 antibody isolated from the patients who recovered from SARS-CoV-2 infection. S309 has also shown quite a good result in neutralizing the SARS-CoV-2 virus. VIR-7831 is being modified to enhance its ability to recognize the SARS-CoV-2 virus. The main aim behind engineering VIR-7831 is to make it capable of recruiting cytotoxic T cells, killing the virally infected cells effectively. In addition, antibody engineering will strengthen the safety, tolerability, and pharmacokinetic profile of VIR-7831. However, this antibody administration results in certain adverse outcomes, notably the formation of anti-drug antibodies against VIR-7831. Further trials are still undergoing and are expected to overcome the flaws, and therefore, VIR-7831 will be an effective treatment against treating the pandemic [52].
5.7. BGB DXP593
The exact mechanism that enables the BGB-DXP593 antibody to inhibit the SARS-CoV-2 virus entry into the host cell is not completely known. However, by analyzing the structural similarity of the SARS-CoV and SARS-CoV-2 researchers expect that the antibody employed for neutralizing SARS-CoV could be potentially be useful in treating the SARS-CoV-2 infection. This antibody possesses a complementary region named CDR3H, which targets the spike RBD. BGB-DXP593 mainly inhibits viral entry by CDR3H. Currently, the Phase 2 trial of this antibody is elucidating more about its efficiency and safety profile in preventing SARS-CoV-2 infection. This is mainly applied to COVID-19 patients having mild to moderate symptoms [52].
5.8. SCTA01
Like Etesevimab, SCTA01 also possesses LALA modification in the Fc region and hinders the interaction between the spike protein and the ACE2 receptor. It is also known as HB27, and is a member of the IgG1 antibody isotype. It shares some common functionality with the Bamlanivimab. The amino acid residue (LALA) mutation is responsible for eliminating the antibody-dependent cellular cytotoxicity and antibody-dependent enhancement properties. The pre-clinical reports of SCTA01 elucidated the safety and antiviral characteristic of this mAb. All the potential effects seen in the volunteers were mild and did not require additional support to cure them. The in vitro study of this nAb on mice and rhesus monkeys also highlighted its ability to lower the viral load [53].
5.9. DZIF-10c
The DZIF-10c is one of the most potent antibodies employed for treating the SARS-CoV-2 infection. The evolving mutations in several VOCs and VOIs are proven to impart immune escape properties. Studies have shown that DZIF-10c effectively neutralizes the virus in 16 prevalent mutations. The neutralizing inability was only observed in the case of the K444Q mutation. DZIF-10c possesses a much greater antiviral characteristic than the other antibodies, suggesting a more efficient neutralization. This antibody’s safety and pharmacokinetic profiling highlighted its use for clinical purposes. In addition, the extended half-life and greater loads of neutralization titers make it more suitable for clinical usage. It is completely capable of neutralizing the SARS-CoV-2 infection by the alpha variant (B.1.1.7), and it also plays a pivotal role in neutralizing the infection by the beta variant (B.1.351) [54].
5.10. SAB-185
According to the Phase I clinical trial reports, SAB-185 has shown a convincing safety and tolerability profile for future use. It is a very potent, full-human polyclonal antibody capable of neutralizing most of the evolving mutations. The common immune escape property of S477N, D614G, N501Y, and E484K are eliminated by the administration of SAB-185. This antibody is isolated from the specially engineered bovines by hyperimmunization. This polyclonal antibody can recognize a series of epitopes in the spike antigen. As a result, single-point mutations cannot alter their neutralizing capacity. This antibody has provided a potent neutralization for SARS-CoV-2 and many viruses, namely Ebola, Haantan, MERS-CoV, etc. [55].
5.11. COR-101
COR-101, also known as STE90-C11, targets the ACE2-RBD complex, affecting viral entry. The neutralizing effect of this antibody notably brings no difference in the case of the mutations in the RBD. These antibodies share some common features with the human germline genes (VH3-66 family). The selection of COR-101 is better for SARS-CoV RBD because it does alter its conformation, and there is no problem of any stearic clash. COR-101 also interacts with the CB6 and B38 epitopes of RBD, such as the other antibodies. COR-101 has successfully treated patients with mild to moderate infection symptoms. Moreover, another advantage of COR-101 is its interaction with the 473 to 476 amino acid residues, a harbor for many evolving mutations. It has shown a greater tolerance for most of the evolving variants such as Kappa, Delta, etc. [56].
5.12. Bamlanivimab and Etesevimab
This antibody cocktail comprises two mAbs, Bamlanivimab and Etesevimab resulting in the spike protein binding with the Fc fragment of the two and making it more efficient. A comparison of the effects of these nAbs with the placebo group after 3 to 11 days significantly reduced the viral load, and a minimal amount of people infected with COVID-19 required hospitalization support. A report from the Phase III clinical trial also indicated the efficiency of this antibody cocktail in high-risk groups of people. This nAb cocktail reduced the hospitalization and death rate by up to 70% compared to the placebo group [11].
6. nAbs Employed for Treating SARS-CoV-2 and Are in Pre-Clinical Trial
The numbers of nAbs against SARS-CoV-2 infection in the pre-clinical trial are listed in Table 3.
Table 3.
Different neutralizing antibodies which are in the pre-clinical trial.
| Sl. No. | nAb | International Nonpropreitary Name (INN) | Source | Type |
|---|---|---|---|---|
| 1. | LY-CoV555 | Bamlanivimab | Human B cells | mAb human IgG1 |
| 2. | JS016 | Etesevimab + Bamlanivimab | Human B cells | mAb human, combination of 2 mAb |
| 3. | LY-CoV016 | Etesevimab + Bamlanivimab | Human B cells | mAb human, combination of 2 mAb |
| 4. | LY3832479 | Etesevimab + Bamlanivimab | Human B cells | mAb human, combination of 2 mAb |
| 5. | REGN-COV2 | Casirivimab + Imdevimab | Convalescent sources and immunization | mAb human |
| 6. | TY027 | - | - | mAb |
| 7. | BRII-196 | - | Human B cells | mAb human |
| 8. | BRII-198 | - | Human B cells | mAb human |
| 9. | CT-P59 | Regdanvimab | Human B cells | mAb human |
| 10. | SCTA01 | - | - | mAb humanized |
| 11. | SAB- 185 |
- | Immunization | Polyclonal recombinant human Ab |
| 12. | MW33 | - | - | mAb human |
| 13. | AZD7442 | Tixagevimab + Cilgavimab | Human B cells | mAb human |
| 14. | VIR-7831 | Sotrovimab | Human B cells | mAb human |
| 15. | DXP-593 | - | Human B cells | mAb |
| 16. | Anti-SARS-CoV-2 mAb | - | - | mAb, chicken IgY |
| 17. | ABBV-47D11 | - | Immunization | mAb human IgG1 |
| 18. | DXP604 | - | Human B cells | mAb |
| 19. | COVI-AMG (STI-2020) | - | In vitro libraries | mAb human |
| 20. | C144-LS and C-135-LS | - | - | Mixture of 2 mAb |
| 21. | ADG20 | - | Human B cells | mAb human |
| 22. | COR-101 | - | In vitro libraries and human B cells | mAb human |
6.1. AR-712
The antibody cocktail is a way of treating the present VOCs and VOIs. AR-712 is a cocktail that efficiently neutralizes the dominating Delta variant. This mAb cocktail, developed by Aridis Pharmaceuticals, was self-administered to COVID-19 patients and did not require any hospitalization. This cocktail was identified from the convalescent plasma of the SARS-CoV-2 infected patients. It consists of two IgGs isolated from the B-cells of these patients [57].
6.2. IMM-BCP-01
The IMM-BCP-01, developed by Immunome Inc., is an antibody cocktail with great potential in neutralizing the Delta variant of the SARS-CoV-2. According to the reports published in late July 2021, this nAb will enter the first phase of the clinical trial. It is a cocktail functional with three mAbs and targets nearly three non-overlapping epitopes of this virus. It will also collaborate with the US FDA to submit an Investigational New Drug Report before entering the clinical trial [57].
6.3. SPKM001
An anti-SARS-CoV-2 nAb developed by SpikImm and Institut Pasteur is the SPKM001. It is expected to enter the clinical trial in Europe, Brazil, and North America by early 2022. It has effectively neutralized most VOCs and VOIs such as Alpha, Delta, Beta, Gamma, and Delta Plus. It has a strong binding affinity towards the RBD of SARS-CoV-2, thus hindering the interaction of these variants with the host receptors [57].
7. New Emerging SARS-CoV-2 Variants and Possible Therapeutic Interventions
With the advent of time, several new mutations have accumulated in the SARS-CoV-2 viral genome. These mutations have altered the characteristics of the virus in terms of transmissibility and infectivity. On this basis, the WHO and CDC categorized emerging variants as VOC and the VOI. Several mutations in these variants confer the ability to escape the mAbs and vaccines [58,59].
7.1. B.1.1.7 (Alpha)
This variant was first detected in the UK. Several mAbs, including antibody cocktails, have been found to be very potent in neutralizing this variant. For instance, the COVOX-222 has an efficient adequate neutralizing system. It interferes with the amino acid substitution at the 417 positions and neutralizes this variant efficiently [59]. Due to the presence of the N501Y mutation, the interaction with ACE2 and RBD is strengthened, facilitating increased neutralization. Another unique antibody cocktail, LYCoV-555 (Bamlanivimab + Etesevimab), has a very strong binding affinity with the RBD in both possible conformations and is not afflicted by the substitution of Y501. According to Jiejie Geng and his colleagues, CD147 is very active in blocking viral entry into the host cells [60]. CD147 has a neutralization efficiency nearly equal to 69% at a certain concentration. It also prevents the building of cytokine storms in individuals infected with the virus. A combination of casirivimab and imdevimab has shown efficient neutralization efficiency in the case of the Alpha variant. This combination attaches to both sides of the RBD of the S-glycoprotein [60,61].
7.2. B.1.351 (Beta)
This lineage isolated from South Africa possesses several missense mutations and deletions. The neutralizing mAb MG1141A has been extremely proficient in neutralizing the B.1.351 variant. In addition to neutralization, MG1141A also plays a pivotal role in viral clearance by utilizing the immune cells’ property to undergo phagocytosis [62]. As previously stated, CD147 functions to prevent the entry of the Beta variant. In the case of B.1.351, the neutralization efficiency is nearly 75%. Another antibody cocktail, Tixagevimab and Cilgavimab, is very potent in dominating the B.1.351 variant [63]. It can significantly identify the non-conserved epitopes residing in the RBD of the S-glycoprotein and inhibit the viral entry into the host cells.
7.3. P.1 (Gamma)
The first evidence of the Gamma variant was made in Manaus, Brazil. It consists of several mutations, making it inevitable that the available antibodies will neutralize it. Many antibodies neutralizing the alpha and beta variants have also efficiently blocked P.1 [60]. The mAbs such as COVOX-222 and COVOX-253 follow a common mechanism of neutralization, i.e., they interact with the ACE2 receptor, making it difficult to bind with the RBD of the spike protein. However, casirivimab, a potent nAb, is inefficient at blocking the Gamma variant. It needs to be combined with imdevimab to accelerate the neutralization efficiency [64]. The most common antibody that can neutralize most of the evolving SARS-CoV-2 variants is CD147 or meplazumab. In the case of P.1, its neutralization efficiency stands at nearly 50% [59].
7.4. B.1.617.2 (Delta)
This VOC, which dominated the second wave of the COVID-19 pandemic, was isolated from India. It differs from the other VOCs to a greater extent, possessing a single mutation (D614G) common with the others. The exclusive mutations L452R and T478K make this variant extremely contagious with more virulence. According to the data highlighted in Table 4, it is evident that the commonly used antibodies for Alpha and Beta variants have shown significant neutralization efficacy for Delta. The mechanism of action of these neutralizing antibodies is also the same in this case, as discussed earlier.
Table 4.
Emerging variants of SARS-CoV-2 and nAb, which are in the pre-clinical and clinical stage.
| Sl. No. | Name of the Variant | Effective nAb against the SARS-CoV-2 Variants | Reference |
|---|---|---|---|
| 1. | B.1.1.7 (Alpha) | CD147 (Meplazumab), COVOX-222, COVOX-253, A23-58.1, MG1141A, Sotrovimab, Casirivimab + Imdevimab, Bamlanivimab + Etesevimab, Tixagevimab + Cilgavimeb | [59,60,61] |
| 2. | B.1.351 (Beta) | CD147 (Meplazumab), MG1141A, Casirivimab + Imdevimab, Sotrovimab, Tixagevimab + Cilgavimeb | [59,60,62] |
| 3. | P.1 (Gamma) | CD147 (Meplazumab), COVOX-222, COVOX-253, A23-58.1, Sotrovimab, Casirivimab + Imdevimab, MG1141A, Tixagevimab + Cilgavimeb | [59,60,62] |
| 4. | B.1.617.2 (Delta) | CD147 (Meplazumab), A23-58.1, Sotrovimab, Casirivimab + Imdevimab, Bamlanivimab + Etesevimab, Tixagevimab + Cilgavimeb | [60,62,67] |
| 5. | B.1.1.529 (Omicron) | Sotrovimab, Paxlovid, molnupiravir | [73,74] |
7.5. B.1.1.529 (Omicron)
According to Zeng et al., the Omicron variant confers a wider ability to escape antibodies compared to the other variants [65]. The structural modeling and sequence-based study also stated that the improved binding affinity of Omicron S-protein with the hACE2 receptor caused increased virulence [66]. The Omicron variant has more mutations than any other previously reported SARS-CoV-2 variant. It possesses 50 mutations, out of which 32 pertain to the spike protein, which is the target site for most vaccines to neutralize the virus. Many mutations are novel and not found in the previous viral variants. Specifically, the variant is characterized by 30 amino acid changes, three small deletions, and one small insertion in the spike protein compared with the original virus, of which 15 are located in the receptor-binding domain (residues 319–541) [67]. The nAbs Sotrovimab, Paxlovid, and molnupiravir have shown efficiency in the case of this variant. Due to a large number of residing mutations, the Omicron variant is highly resistant to antibody cocktails [68,69,70]. Many resistant mutations residing in the spike protein of the Omicron variant are responsible for lowering the Ab titers elicited by the vaccination [71]. Rather, a single antibody is more efficient in combating the SARS-CoV-2 infection. Sotrovimab is extremely efficient in binding to the conserved antigenic epitopes rather than the non-overlapping ones. However, the most efficient antibody that can combat the Omicron variant is molnupiravir. Upon entering the host cell, molnupiravir interferes with the viral replication of the B.1.1.529 variant, a unique property that is possessed by an antibody [72]. Thus, molnupiravir can neutralize this variant to a greater extent.
8. Heavy Chain Antibodies (HCAbs) against SARS-CoV-2
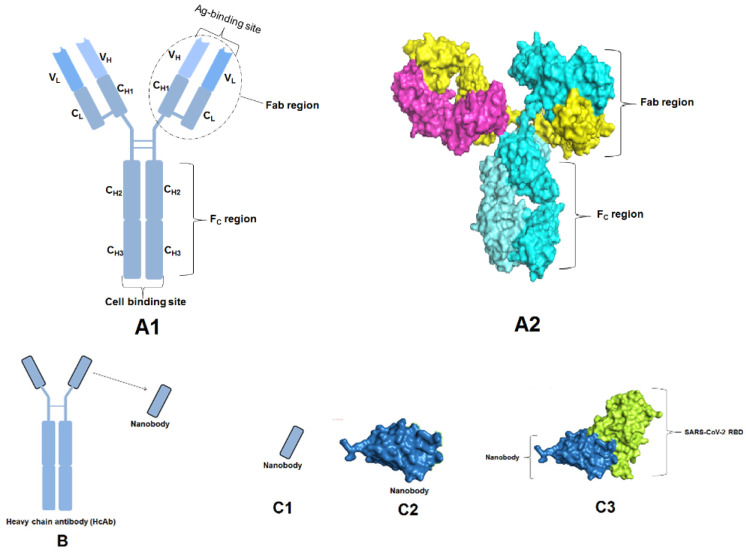
Heavy chain antibodies (HCAbs) are specialized active antibody fragments that are not associated with the light chains, and their VH (variable heavy) regions are also functional as a part of a single unit (Figure 8(A1,A2)) [75]. The VH regions serve as perfect building blocks for several antibody-based treatments as they permit the addition of molecules in sequence to construct multispecific antibodies. The HCAbs possess a unique paratope that interacts with the variable domain of the heavy chain without involving any light chain domains (Figure 8B). These classes of antibodies originated from the camelid species and were found to be extremely proficient in treating COVID-19. They are more immunogenic than conventional ones and possess some unique physical properties that help in the larger production of these antibodies. These HCAbs, however, have a lower affinity for binding with the antigenic epitope and are easily excreted by the kidney. The HCAbs (especially nanobodies) can even be used to detect the presence of the SARS-CoV-2 virus (Figure 8(C1–C3)). These nanobodies are capable of being inhaled by the patients, and, thus, can be used to prevent viral replication in the lungs. Mostly, the RBD of the spike protein is a potential target of HCAbs [76,77].
Figure 8.
Structure of heavy chain antibodies and its parts. (A1) Schematic structure depicted different parts. (A2) Surface structure shows Fc and Fab region. (B) Heavy chain antibodies along with the nanobody. (C) Nanobody complex with the SARS-CoV-2 RBD.
9. Single Domain Antibody against SARS-CoV-2
To establish an efficient therapeutic against the SARS-CoV-2, heavy chain single domain antibodies (sdAb) have shown promising results. Experiments suggest that the competitive binding of these sdAbs plays a pivotal role in hindering the interaction between the hACE2 receptor and the viral RBD [75,78]. Moreover, fusing the IgG1 Fc with these sdAbs accelerates their neutralizing efficiency to a greater extent. These antibodies, also called nanobodies, have the antigen-binding capacity to a greater extent, making them an effective tool for designing therapeutics to eradicate this global outbreak. These sdAbs are cost-effective and comparatively more stable than nAbs [75]. Considering the present scenario of COVID-19 treatment with the administration of certain vaccines and antibody therapy, the development of the sdAbs will be more susceptible to treating the infection. The administration of these antibody cocktails is believed to give a more durable protective response than the current therapeutics [78]. According to several pieces of research, sdAbs are extremely efficient in targeting the epitopes in the RBD of the SARS-CoV-2 variants [75]. These epitopes, in turn, are responsible for the extremities caused to human health upon the viral entry. The extraordinary feature of these antibodies makes them compatible with being used as a particle delivery system. sdAbs can be delivered into the lungs through nasal delivery as well as the gastrointestinal tract to prevent the interaction of the virus with the ACE2 receptor [79,80]. Studies also suggest that IgA is a better neutralizing tool than IgG; thus, the fusion of IgA with the sdAb’s will be extremely efficient in serving as a diagnostic tool to eradicate the pandemic.
10. Conclusions
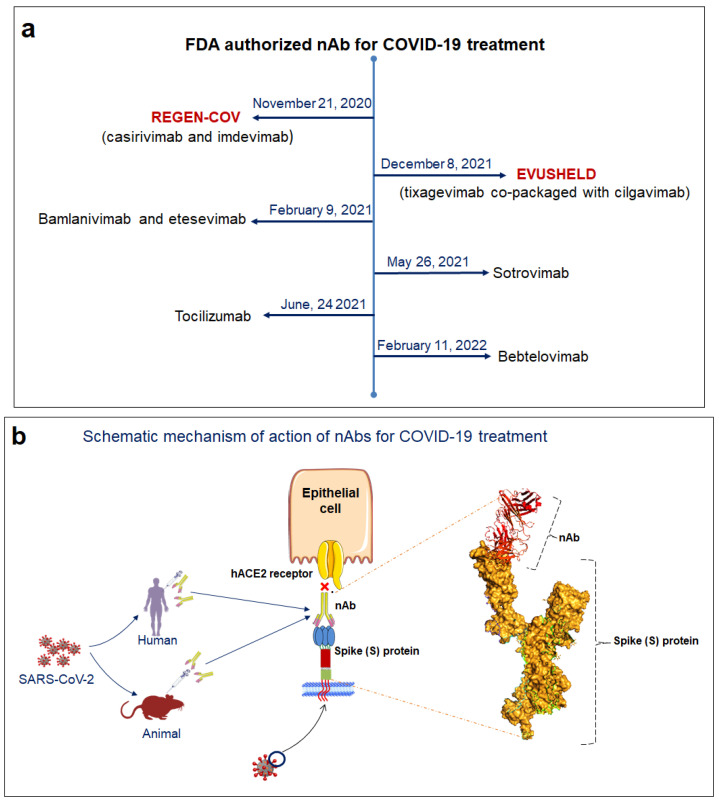
An efficient strategy to combat this pandemic is the administration of nAbs. The FDA has approved several mAbs for use against SARS-CoV-2. A brief timeline depicting the development of FDA approval for the mAbs against SARS-CoV-2 with their mechanism of neutralization is shown in the Figure 9.
Figure 9.
FDA approved mAbs against SARS-CoV-2 and their mode of action. (a) Timeline depicting the development of FDA approval for the mAbs against SARS-CoV-2. (b) The schematic diagram illustrated the mechanism of action of nAbs. It shows the neutralization of SARS-CoV-2 spike protein.
These nAbs have given promising results in minimizing the virulence of the SARS-CoV-2 virus. Several antibody treatments administered by following an appropriate dosage are considered prophylactic measures for treating severe patients. Before the emergence of vaccines, this therapeutic strategy provided a bit of relief to the world in controlling the havoc. Moreover, the administration of combined antibodies (known as the antibody cocktail) has been extraordinarily efficient for the evolving mutants, reducing the chance of escaping the immune system. Several subsets of nAbs isolated from SARS-CoV and MERS-CoV patients had also shown viral neutralization capacity in SARS-CoV-2 patients. Studies also highlight that the administration of these antibodies at an early stage will be more helpful for the population in preventing COVID-19. In turn, high-throughput engineering strategies can be used to construct more neutralizing antibodies with a very high binding affinity, thereby providing great relief for the entire world.
Acknowledgments
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF—2020R1C1C1008694).
Abbreviations
| nAb | Neutralizing Antibody |
| mAb | Monoclonal Antibody |
| RBD | Receptor binding domain |
| ACE2 | Angiotensin-converting enzyme 2 |
| S-protein | Spike glycoprotein |
| NTD | N-terminal domain |
| CPT | Convalescent plasma therapy |
| ADE | Antibody-dependent enhancement |
| HCAbs | Heavy chain antibodies |
| IL-6 | Interleukin 6 |
| CDRH | Complementarity-determining regions of heavy-chain |
| FDA | Food & Drug Administration |
| IgA | Immunoglobulin A |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| VH | Variable domain heavy chain |
| VOI | Variant of Interest |
| VOC | Variant of Concern |
Author Contributions
M.B.: Validation, Formal analysis, Investigation, Figure development, Writing—Original Draft. S.C.: Data curation, Resources, Investigation, Writing—Original Draft. B.M.: Validation; Figure development. A.R.S.: Investigation, Formal analysis, Validation, Writing—Review & Editing, Fund acquisition. C.C.: Conceptualization, Methodology, Project administration, Writing—Review & editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing interests.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhattacharya M., Sharma A.R., Mallick B., Sharma G., Lee S.-S., Chakraborty C. Immunoinformatics approach to understand molecular interaction between multi-epitopic regions of SARS-CoV-2 spike-protein with TLR4/MD-2 complex. Infect. Genet. Evol. 2020;85:104587. doi: 10.1016/j.meegid.2020.104587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty C., Sharma A.R., Bhattacharya M., Sharma G., Lee S.-S. Immunoinformatics approach for the identification and characterization of T cell and B cell epitopes towards the peptide-based vaccine against SARS-CoV-2. Arch. Med. Res. 2021;52:362–370. doi: 10.1016/j.arcmed.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriyama S., Adachi Y., Sato T., Tonouchi K., Sun L., Fukushi S., Yamada S., Kinoshita H., Nojima K., Kanno T., et al. Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity. 2021;54:1841–1852.e1844. doi: 10.1016/j.immuni.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheedarla N., Hanna L.E. Recent Developments in Applied Microbiology and Biochemistry. Elsevier; Amsterdam, The Netherlands: 2019. Functional and Protective Role of Neutralizing Antibodies (NAbs) Against Viral Infections; pp. 83–93. [Google Scholar]
- 6.Jiang S., Zhang X., Yang Y., Hotez P.J., Du L. Neutralizing antibodies for the treatment of COVID-19. Nat. Biomed. Eng. 2020;4:1134–1139. doi: 10.1038/s41551-020-00660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Heide V. Neutralizing antibody response in mild COVID-19. Nat. Rev. Immunol. 2020;20:352. doi: 10.1038/s41577-020-0325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muruato A.E., Fontes-Garfias C.R., Ren P., Garcia-Blanco M.A., Menachery V.D., Xie X., Shi P.-Y. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat. Commun. 2020;11:4059. doi: 10.1038/s41467-020-17892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suthar M.S., Zimmerman M.G., Kauffman R.C., Mantus G., Linderman S.L., Hudson W.H., Vanderheiden A., Nyhoff L., Davis C.W., Adekunle O. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep. Med. 2020;1:100040. doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodruff M.C., Ramonell R.P., Nguyen D.C., Cashman K.S., Saini A.S., Haddad N.S., Ley A.M., Kyu S., Howell J.C., Ozturk T. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020;21:1506–1516. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor P.C., Adams A.C., Hufford M.M., de la Torre I., Winthrop K., Gottlieb R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021;21:382–393. doi: 10.1038/s41577-021-00542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurt A.C., Wheatley A.K. Neutralizing Antibody Therapeutics for COVID-19. Viruses. 2021;13:628. doi: 10.3390/v13040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty C., Sharma A.R., Bhattacharya M., Lee S.-S. A Detailed Overview of Immune Escape, Antibody Escape, Partial Vaccine Escape of SARS-CoV-2 and Their Emerging Variants With Escape Mutations. Front. Immunol. 2022;13:801522. doi: 10.3389/fimmu.2022.801522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross C.P., Sepkowitz K.A. The myth of the medical breakthrough: Smallpox, vaccination, and Jenner reconsidered. Int. J. Infect. Dis. 1998;3:54–60. doi: 10.1016/S1201-9712(98)90096-0. [DOI] [PubMed] [Google Scholar]
- 15.Jiang S., Zhang X., Du L. Therapeutic antibodies and fusion inhibitors targeting the spike protein of SARS-CoV-2. Expert Opin. Ther. Targets. 2021;25:415–421. doi: 10.1080/14728222.2020.1820482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riedel S. Edward Jenner and the history of smallpox and vaccination. Bayl. Univ. Med. Cent. Proc. 2005;18:21–25. doi: 10.1080/08998280.2005.11928028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann S.H. Remembering Emil von Behring: From tetanus treatment to antibody cooperation with phagocytes. MBio. 2017;8:00117-17. doi: 10.1128/mBio.00117-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bordon Y. Milestone 2: The many sides of Paul Ehrlich. Nat. Milest. Antib. 2016;S:6. [Google Scholar]
- 19.Kugelberg E. Searching for the antibody producers. Nat. Immunol. 2016;17((Suppl. S1)):S7. doi: 10.1038/ni.3602. [DOI] [Google Scholar]
- 20.Ribatti D. Edelman’s view on the discovery of antibodies. Immunol. Lett. 2015;164:72–75. doi: 10.1016/j.imlet.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Inbar D., Hochman J., Givol D. Localization of antibody-combining sites within the variable portions of heavy and light chains. Proc. Natl. Acad. Sci. USA. 1972;69:2659–2662. doi: 10.1073/pnas.69.9.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plückthun A. Antibody engineering. Curr. Opin. Biotechnol. 1991;2:238–246. doi: 10.1016/0958-1669(91)90016-X. [DOI] [PubMed] [Google Scholar]
- 23.Demarest S.J., Hariharan K., Dong J. Emerging antibody combinations in oncology. MAbs. 2011;3:338–351. doi: 10.4161/mabs.3.4.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass T.R., Ohmura N., Saiki H., Sawadaishi K., Kataoka C., Takagi Y., Ohiwa T. Development and characterization of new monoclonal antibodies specific for coplanar polychlorinated biphenyls. Anal. Chim. Acta. 2004;517:161–168. doi: 10.1016/j.aca.2004.04.038. [DOI] [Google Scholar]
- 25.Qiu H., Yuan X.Y., Cabral T., Manguiat K., Robinson A., Wood H., Grant C., McQueen P., Westmacott G., Beniac D.R., et al. Development and characterization of SARS-CoV-2 variant-neutralizing monoclonal antibodies. Antivir. Res. 2021;196:105206. doi: 10.1016/j.antiviral.2021.105206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., Yu J., Shan S., Zhou B., Song S. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 27.Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Nkolola J.P., Schäfer A., Reidy J.X., Trivette A., Nargi R.S. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584:443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.-T., Limbo O., Smith C., Song G., Woehl J. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369:956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakraborty C., Bhattacharya M., Sharma A.R. Emerging mutations in the SARS-CoV-2 variants and their role in antibody escape to small molecule-based therapeutic resistance. Curr. Opin. Pharmacol. 2022;62:64–73. doi: 10.1016/j.coph.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L.D., Lian C., Yeap L.-S., Meng F.-L. The development of neutralizing antibodies against SARS-CoV-2 and their common features. J. Mol. Cell Biol. 2020;12:980–986. doi: 10.1093/jmcb/mjaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharya M., Chatterjee S., Sharma A.R., Agoramoorthy G., Chakraborty C. D614G mutation and SARS-CoV-2: Impact on S-protein structure, function, infectivity, and immunity. Appl. Microbiol. Biotechnol. 2021;105:9035–9045. doi: 10.1007/s00253-021-11676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mor M., Werbner M., Alter J., Safra M., Chomsky E., Lee J.C., Hada-Neeman S., Polonsky K., Nowell C.J., Clark A.E. Multi-clonal SARS-CoV-2 neutralization by antibodies isolated from severe COVID-19 convalescent donors. PLoS Pathog. 2021;17:e1009165. doi: 10.1371/journal.ppat.1009165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang Y., Nambulli S., Xiao Z., Liu H., Sang Z., Duprex W.P., Schneidman-Duhovny D., Zhang C., Shi Y. Versatile and multivalent nanobodies efficiently neutralize SARS-CoV-2. Science. 2020;370:1479–1484. doi: 10.1126/science.abe4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I.-C., Tiu C., Hu Z., Chen V.C.-W., Young B.E., Sia W.R. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 36.Boonnak K., Dambach K.M., Donofrio G.C., Tassaneetrithep B., Marovich M.A. Cell type specificity and host genetic polymorphisms influence antibody-dependent enhancement of dengue virus infection. J. Virol. 2011;85:1671–1683. doi: 10.1128/JVI.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes C.O., Jette C.A., Abernathy M.E., Dam K.-M.A., Esswein S.R., Gristick H.B., Malyutin A.G., Sharaf N.G., Huey-Tubman K.E., Lee Y.E. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharya M., Sharma A.R., Patra P., Ghosh P., Sharma G., Patra B.C., Lee S.S., Chakraborty C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J. Med. Virol. 2020;92:618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhattacharya M., Sharma A.R., Patra P., Ghosh P., Sharma G., Patra B.C., Saha R.P., Lee S.-S., Chakraborty C. A SARS-CoV-2 vaccine candidate: In-silico cloning and validation. Inform. Med. Unlocked. 2020;20:100394. doi: 10.1016/j.imu.2020.100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J., Gao K., Wang R., Nguyen D.D., Wei G.-W. Review of COVID-19 antibody therapies. Annu. Rev. Biophys. 2021;50:1–30. doi: 10.1146/annurev-biophys-062920-063711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin D., Wei J., Sun J. Analysis of the molecular mechanism of SARS-CoV-2 antibodies. Biochem. Biophys. Res. Commun. 2021;566:45–52. doi: 10.1016/j.bbrc.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussain A., Hasan A., Babadaei M.M.N., Bloukh S.H., Chowdhury M.E., Sharifi M., Haghighat S., Falahati M. Targeting SARS-CoV2 spike protein receptor binding domain by therapeutic antibodies. Biomed. Pharmacother. 2020;130:110559. doi: 10.1016/j.biopha.2020.110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavor E., Choong Y.K., Er S.Y., Sivaraman H., Sivaraman J. Structural basis of SARS-CoV-2 and SARS-CoV–antibody interactions. Trends Immunol. 2020;41:1006–1022. doi: 10.1016/j.it.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y., Wang F., Shen C., Peng W., Li D., Zhao C., Li Z., Li S., Bi Y., Yang Y. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368:1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ku Z., Xie X., Davidson E., Ye X., Su H., Menachery V.D., Li Y., Yuan Z., Zhang X., Muruato A.E. Molecular determinants and mechanism for antibody cocktail preventing SARS-CoV-2 escape. Nat. Commun. 2021;12:469. doi: 10.1038/s41467-020-20789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harvala H., Robb M.L., Watkins N., Ijaz S., Dicks S., Patel M., Supasa P., Wanwisa D., Liu C., Mongkolsapaya J. Convalescent plasma therapy for the treatment of patients with COVID-19: Assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transfus. Med. 2021;31:167–175. doi: 10.1111/tme.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu X., Li N., Wang G., Liu W., Yu J., Cao G., Wang J., Chen Y., Ma J., Wu J. Tolerability, Safety, Pharmacokinetics, and Immunogenicity of a Novel SARS-CoV-2 Neutralizing Antibody, Etesevimab in Chinese Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, First-In-Human Phase 1 Study. Antimicrob. Agents Chemother. 2021;65:e00350-21. doi: 10.1128/AAC.00350-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng X., Wang P., Xiong Y., Wu Y., Lin X., Lu S., Li R., Zhao B., Liu J., Zeng S. Safety, tolerability, pharmacokinetic characteristics, and immunogenicity of MW33: A Phase 1 clinical study of the SARS-CoV-2 RBD-targeting monoclonal antibody. Emerg. Microbes Infect. 2021;10:1638–1648. doi: 10.1080/22221751.2021.1960900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J.Y., Jang Y.R., Hong J.H., Jung J.G., Park J.-H., Streinu-Cercel A., Streinu-Cercel A., Săndulescu O., Lee S.J., Kim S.H. Safety, Virologic Efficacy, and Pharmacokinetics of CT-P59, a Neutralizing Monoclonal Antibody Against SARS-CoV-2 Spike Receptor-Binding Protein: Two Randomized, Placebo-Controlled, Phase I Studies in Healthy Individuals and Patients With Mild SARS-CoV-2 Infection. Clin. Ther. 2021;43:1706–1727. doi: 10.1016/j.clinthera.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Xiao J., Hooper A.T., Hamilton J.D., Musser B.J. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N. Engl. J. Med. 2021;385:e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tuccori M., Ferraro S., Convertino I., Cappello E., Valdiserra G., Blandizzi C., Maggi F., Focosi D. MAbs. Volume 12. Taylor & Francis; Abingdon, UK: 2020. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: Clinical pipeline; p. 1854149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y., Qi L., Bai H., Sun C., Xu S., Wang Y., Han C., Li Y., Liu L., Cheng X. Safety, tolerability, pharmacokinetics and immunogenicity of a monoclonal antibody (SCTA01) targeting SARS-CoV-2 in healthy adults: A randomized, double-blind, placebo-controlled, phase I study. Antimicrob. Agents Chemother. 2021;65:e01063-21. doi: 10.1128/AAC.01063-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halwe S., Kupke A., Vanshylla K., Liberta F., Gruell H., Zehner M., Rohde C., Krähling V., Gellhorn-Serra M., Kreer C. Intranasal administration of a monoclonal neutralizing antibody protects mice against SARS-CoV-2 infection. Viruses. 2021;13:1498. doi: 10.3390/v13081498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z., Wu H., Egland K.A., Gilliland T.C., Dunn M.D., Luke T.C., Sullivan E.J., Klimstra W.B., Bausch C.L., Whelan S.P. Human immunoglobulin from transchromosomic bovines hyperimmunized with SARS-CoV-2 spike antigen efficiently neutralizes viral variants. Hum. Vaccines Immunother. 2022;18:1940652. doi: 10.1080/21645515.2021.1940652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertoglio F., Fühner V., Ruschig M., Heine P.A., Abassi L., Klünemann T., Rand U., Meier D., Langreder N., Steinke S. A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients binds to the ACE2-RBD interface and is tolerant to most known RBD mutations. Cell Rep. 2021;36:109433. doi: 10.1016/j.celrep.2021.109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The Antibody Society COVID-19 Biologics Tracker. 2021. [(accessed on 13 July 2022)]. Available online: https://www.antibodysociety.org/covid-19-biologics-tracker/
- 58.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shrestha L.B., Tedla N., Bull R.A. Broadly-Neutralizing Antibodies Against Emerging SARS-CoV-2 Variants. Front. Immunol. 2021:4025. doi: 10.3389/fimmu.2021.752003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geng J., Chen L., Yuan Y., Wang K., Wang Y., Qin C., Wu G., Chen R., Zhang Z., Wei D. CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma. Signal Transduct. Target. Ther. 2021;6:347. doi: 10.1038/s41392-021-00760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abani O., Abbas A., Abbas F., Abbas M., Abbasi S., Abbass H., Abbott A., Abdallah N., Abdelaziz A., Abdelfattah M. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2022;399:665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee S., Jang S., Kang J., Park S.B., Han Y.W., Nam H., Kim M., Lee J., Cho K.J., Kim J. MG1141A as a Highly Potent Monoclonal Neutralizing Antibody Against SARS-CoV-2 Variants. Front. Immunol. 2021;12:778829. doi: 10.3389/fimmu.2021.778829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boschi C., Colson P., Bancod A., Moal V., La Scola B. Omicron variant escapes therapeutic mAbs including recently released Evusheld®, contrary to eight prior main VOC. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022;75:e534–e535. doi: 10.1093/cid/ciac143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falcone M., Tiseo G., Valoriani B., Barbieri C., Occhineri S., Mazzetti P., Vatteroni M.L., Suardi L.R., Riccardi N., Pistello M. Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe COVID-19 and role of variants of concern. Infect. Dis. Ther. 2021;10:2479–2488. doi: 10.1007/s40121-021-00525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng C., Evans J.P., Chakravarthy K., Qu P., Reisinger S., Song N.J., Rubinstein M.P., Shields P.G., Li Z., Liu S.L. COVID-19 mRNA booster vaccines elicit strong protection against SARS-CoV-2 Omicron variant in patients with cancer. Cancer Cell. 2022;40:117–119. doi: 10.1016/j.ccell.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar R., Murugan N.A., Srivastava V. Improved binding affinity of omicron’s spike protein for the human angiotensin-converting enzyme 2 receptor is the key behind its increased virulence. Int. J. Mol. Sci. 2022;23:3409. doi: 10.3390/ijms23063409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bayani F., Hashkavaei N.S., Uversky V.N., Mozaffari-Jovin S., Sefidbakht Y. Insights into the structural peculiarities of the N-terminal and receptor binding domains of the spike protein from the SARS-CoV-2 Omicron variant. Comput. Biol. Med. 2022;147:105735. doi: 10.1016/j.compbiomed.2022.105735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhattacharya M., Sharma A.R., Dhama K., Agoramoorthy G., Chakraborty C. Omicron variant (B. 1.1. 529) of SARS-CoV-2: Understanding mutations in the genome, S-glycoprotein, and antibody-binding regions. GeroScience. 2022;44:619–637. doi: 10.1007/s11357-022-00532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kozlov M. Omicron overpowers key COVID antibody treatments in early tests. Nature. 2021;10:20211221. doi: 10.1038/d41586-021-03829-0. [DOI] [PubMed] [Google Scholar]
- 70.Hoffmann M., Krüger N., Schulz S., Cossmann A., Rocha C., Kempf A., Nehlmeier I., Graichen L., Moldenhauer A.S., Winkler M.S., et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tada T., Zhou H., Dcosta B.M., Samanovic M.I., Chivukula V., Herati R.S., Hubbard S.R., Mulligan M.J., Landau N.R. Increased resistance of SARS-CoV-2 Omicron variant to neutralization by vaccine-elicited and therapeutic antibodies. EBioMedicine. 2022;78:103944. doi: 10.1016/j.ebiom.2022.103944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kannan S.R., Spratt A.N., Sharma K., Chand H.S., Byrareddy S.N., Singh K. Omicron SARS-CoV-2 variant: Unique features and their impact on pre-existing antibodies. J. Autoimmun. 2022;126:102779. doi: 10.1016/j.jaut.2021.102779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang D.T., McCreary E.K., Bariola J.R., Minnier T.E., Wadas R.J., Shovel J.A., Albin D., Marroquin O.C., Kip K.E., Collins K. Effectiveness of casirivimab and imdevimab, and sotrovimab during Delta variant surge: A prospective cohort study and comparative effectiveness randomized trial. medRxiv. 2021 doi: 10.1101/2021.12.23.21268244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shafer S.L., Simoneaux R. Omicron Therapeutics. ASA Monitor. 2022;86:21–37. doi: 10.1097/01.ASM.0000820396.70292.ef. [DOI] [Google Scholar]
- 75.Czajka T.F., Vance D.J., Mantis N.J. Slaying SARS-CoV-2 one (single-domain) antibody at a time. Trends Microbiol. 2021;29:195–203. doi: 10.1016/j.tim.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clarke S.C., Ma B., Trinklein N.D., Schellenberger U., Osborn M.J., Ouisse L.-H., Boudreau A., Davison L.M., Harris K.E., Ugamraj H.S. Multispecific antibody development platform based on human heavy chain antibodies. Front. Immunol. 2019;9:3037. doi: 10.3389/fimmu.2018.03037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zare H., Aghamollaei H., Hosseindokht M., Heiat M., Razei A., Bakherad H. Nanobodies, the potent agents to detect and treat the Coronavirus infections: A systematic review. Mol. Cell. Probes. 2021;55:101692. doi: 10.1016/j.mcp.2020.101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chi X., Liu X., Wang C., Zhang X., Li X., Hou J., Ren L., Jin Q., Wang J., Yang W. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting the spike receptor binding domain. Nat. Commun. 2020;11:4528. doi: 10.1038/s41467-020-18387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye Q., Wang B., Zhang T., Xu J., Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am. J. Physiol. -Gastrointest. Liver Physiol. 2020;319:G245–G252. doi: 10.1152/ajpgi.00148.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haga K., Takai-Todaka R., Matsumura Y., Song C., Takano T., Tojo T., Nagami A., Ishida Y., Masaki H., Tsuchiya M. Nasal delivery of single-domain antibody improves symptoms of SARS-CoV-2 infection in an animal model. PLoS Pathog. 2021;17:e1009542. doi: 10.1371/journal.ppat.1009542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.