Abstract
Twin studies suggest a considerable genetic contribution to the variability in 25-hydroxyvitamin D (25(OH)D) concentrations, reporting heritability estimates up to 80% in some studies. While genome-wide association studies (GWAS) suggest notably lower rates (13–16%), they have identified many independent variants that associate with serum 25(OH)D concentrations. These discoveries have provided some novel insight into the metabolic pathway, and in this review we outline findings from GWAS studies to date with a particular focus on 35 variants which have provided replicating evidence for an association with 25(OH)D across independent large-scale analyses. Some of the 25(OH)D associating variants are linked directly to the vitamin D metabolic pathway, while others may reflect differences in storage capacity, lipid metabolism, and pathways reflecting skin properties. By constructing a genetic score including these 25(OH)D associated variants we show that genetic differences in 25(OH)D concentrations persist across the seasons, and the odds of having low concentrations (<50 nmol/L) are about halved for individuals in the highest 20% of vitamin D genetic score compared to the lowest quintile, an impact which may have notable influences on retaining adequate levels. We also discuss recent studies on personalized approaches to vitamin D supplementation and show how Mendelian randomization studies can help inform public health strategies to reduce adverse health impacts of vitamin D deficiency.
Keywords: 25-hydroxyvitamin D, vitamin D, genetic risk, heritability, personalized supplementation, genome-wide association study, Mendelian randomization
1. Introduction
Interest in the genetic architecture of 25-hydroxyvitamin D (25(OH)D) has been active during the past couple of decades, promoted by heritability estimates from twin studies which suggest that up to 80% of the variability in 25(OH)D concentrations might be explained by genetic variation in some populations [1,2,3,4,5]. Also an evolutionary perspective provides strong cues about the importance of genetic variation for vitamin D metabolism [6], as differences in skin colour are believed to have evolved at least in part as an adaptation to ultraviolet B radiation exposure during migration to more northern latitudes, where reduction in skin pigmentation became critical to vitamin D synthesis. Important insights into the genetic architecture of 25(OH)D concentrations have been obtained from genome-wide association studies (GWASs) which have used information across thousands of genomes to find polymorphisms which are statistically associated with 25(OH)D. For this paper, we systematically looked through the GWAS literature for genes that influence serum 25(OH)D levels. We describe key variants for which evidence has been provided from several independent studies and address the public health importance and some of the uses of this information.
2. Materials and Methods
For the systematic search of GWASs, we searched the MEDLINE, Embase, Cochrane, CINAHL and NHGRI-EBI GWAS catalogue [7] databases for original studies and meta-analyses of studies performed in humans and published in English from inception to February, 2022. The search terms used were (“vitamin D” OR “calcidiol” OR “25-hydroxyvitamin D” OR “25(OH)D”) AND (“genome-wide association study” OR “genome-wide association scan” OR “genome-wide association analysis”) along with the expanded MeSH search terms (in titles, abstracts, or keywords). The search identified 791 publications in total. After excluding duplicate entries, 522 publications remained, of which 29 were relevant. These were further scrutinized to identify sample overlap. We also scrutinized references within the selected articles, and from studies otherwise known to the authors, with evidence on gene function queried using the gene ontology (GO) resource (http://geneontology.org/, accessed on 27 September 2022), KEGG (https://www.genome.jp/kegg/, accessed on 27 September 2022), ConsensusPathDB (http://cpdb.molgen.mpg.de/, accessed on 27 September 2022) and other NCBI (https://www.ncbi.nlm.nih.gov/guide/all/, accessed on 27 September 2022) databases.
3. Results
3.1. Genome-Wide Association Studies on 25(OH)D
Our literature search identified 29 published GWASs that looked for SNPs associated with 25(OH)D [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], although many of these analyses were conducted using overlapping samples. Most of the studies only include adult participants of white European ancestry (n = 6722 to 443,374) [8,10,14,16,19,20,22,24,26,29,30,32,33,34,36]. There were seven studies including data from transethnic analyses [15] and studies with small to modest sample sizes (n = 697 to 9823) which had been conducted using data from African Americans (n = 697 in the discovery sample) [25], African descent in the UK (n = 9354) [35], Hispanic (n = 1190) [9] and Asian populations (n = 1387 to 9823) [13,23,28,35]. There were also five small GWASs on children/toddlers/new-borns [11,12,18,21,27].
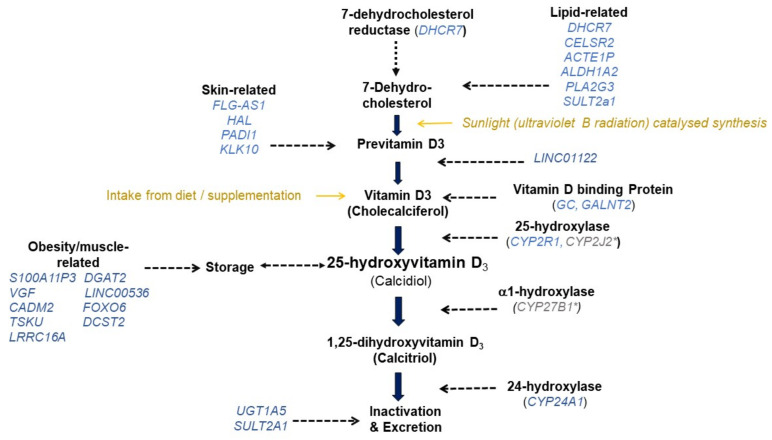
Three loci, including DHCR7, CYP2R1, and GC were consistently reported across European [8,10,14,16,19,20,22,24,26,34] and several non-European GWASs [11,13,15,18,21,23,25,27,28]. These loci were also confirmed in GWAS conducted in children/toddlers/new-borns [11,12,18,21,27]. These genes fit with existing evidence of the involvement of their corresponding proteins in the vitamin D metabolic pathway. The DHCR7 gene encodes the 7-dehydrocholesterol reductase, which is an enzyme that converts dehydrocholesterol to cholesterol in the skin, and affects the substrate availability for vitamin D3 synthesis, which is a precursor of 25(OH)D [37] (Figure 1). The CYP2R1 gene encodes the enzyme in the cytochrome P-450 family 2R1, and is the primary 25-hydroxylase in the liver, converting vitamin D to 25(OH)D. In the circulation, vitamin D metabolites including 25(OH)D are mainly found bound to vitamin D binding protein (encoded by GC), which in most of the GWASs to date, have come up with the strongest signal for 25(OH)D. GWAS on non-European cohorts have also reported some novel loci (e.g., FOXA2/SSTR4 [13], HSPG2 [25], TINK [25] and KIF4B [15]) which have not been identified in GWAS studies on white European ancestry. Independent replication is lacking with respect to most of these novel loci, and many have no clear link with the vitamin D metabolic pathway. One exception is CYP2J2, discovered in a multi-ethnic cohort of 942 pregnant women of Malay, Indian and Chinese ancestry [27]. CYP2J2 encodes an enzyme (Cytochrome P-450 family 2, subfamily J, polypeptide 2) shown in vitro to act as a vitamin D hydroxylase [38]. There is thus a strong biological basis for this association. It is notable that variants coding the α1-hydroxylase (CYP27B1) or the vitamin D receptor (VDR) have not been identified by the GWASs on 25(OH)D conducted to date. Concentrations of active 1,25(OH)2D in the circulation are ~1000 times lower than those of 25(OH)D. Therefore, it is possible that differences arising from the conversion of 25(OH)D to 1,25(OH)2D, or those reflecting VDR related differences in the ‘usage’ of 1,25(OH)2D, may simply be too small to detect.
Figure 1.
Possible role of selected replicated variants in the vitamin D pathway. * Indicates candidate genes with a confirmed role in vitamin D metabolism but which were not among the replicating variants. For the full list of single nucleotide polymorphism relating to each gene, please refer to Table 1.
Variants with Evidence for Replicated Association with 25(OH)D
In the largest GWAS published to date, identified variants for 25(OH)D concentrations were enriched in genes in the metabolic vitamin D pathway, lipid and lipoprotein pathways, and pathways related to skin properties. The liver, the brain and the skin were identified as the top three locations where 25(OH)D-associated loci may exert their actions [20]. In Table 1 we present more detailed information for the 35 common autosomal SNPs which were identified as top hits in the GWAS conducted in the UK Biobank [20], and which were associated in a consistent direction with serum 25(OH)D concentrations in the SUNLIGHT consortium meta-analyses [16,20,39]. In addition, there were 70 common variants that could not be replicated in the SUNLIGHT consortium meta-analyses. These included variants that are known to be pleiotropic (i.e., affect multiple traits) and/or affect cholesterol metabolism (e.g., PCSK9, LIPC, ABCA1, CETP, APOE, APOB, APOC1, LIPG and LDLR). It is possible that some of the differences between the UK Biobank findings and the SUNLIGHT consortium meta-analysis relates to the body mass index adjustment by the SUNLIGHT, which reduces the likelihood of adipose tissue related variants (reflecting differences in storage capacity) being detected. It should be noted, however, that replication does not imply causality, and the possible connection which we have identified with vitamin D metabolism/function is only based on literature available to date and may not fully describe the connection with 25(OH)D concentrations.
Table 1.
Genetic variants with replicating evidence for an association with 25-hydroxyvitamin D concentrations.
| Gene (SNP) | CHR | |
|---|---|---|
| PEX10 (rs6671730) | 1 | PEX10 encodes a protein involved in import of peroxisomal matrix proteins. Mutations in PEX10 gene have led to Zellweger syndrome [40] and osteopenia [41], for which vitamin D supplementation has been the treatment. |
| PADI1 (rs35408430) | 1 | PADI1 encodes an enzyme, which catalyses the post-translational deimination of proteins by converting arginine residues into citrullines in the presence of calcium ions [42]. Deimination by PADIs occurs during epidermal differentiation [43], with possible influence on skin properties [20]. |
| FOXO6 (rs7522116) | 1 | FOXO6 encodes a protein that has been predicted to enable DNA-binding transcription factor activity, and RNA polymerase related DNA binding activity [44]. FoxO6 expression is downregulated in the brain of dietary obese mice [45]. |
| CELSR2 (rs7528419) | 1 | CELSR2 encodes the cadherin EGF LAG seven-pass G-type receptor 2 that is involved in contact-mediated communication, with cadherin domains acting as homophilic binding regions and the EGF-like domains involved in cell adhesion and receptor-ligand interactions [46]. |
| FLG-AS1 (rs1933064) | 1 | FLG antisense RNA 1 (FLG-AS1) is an RNA Gene that is affiliated with the long non-coding RNA class. Skin pigmentation-related diseases such as Ichthyosis Vulgaris [47] and Peeling Skin Syndrome 6 [48] have been shown to be associated with FLG-AS1. |
| DCST2 (rs76798800) | 1 | DCST2 gene encodes the DC-STAMP domain containing 2 protein that has been shown to be an important regulator of osteoclast cell-fusion in bone homeostasis [49]. DCST2 gene is associated with early length and adult height [50]. |
| GALNT2 (rs6672758) | 1 | GALNT2 gene encodes the polypeptide N-acetylgalactosaminyltransferase 2 which is a member of the glycosyltransferase 2 protein family and which has been linked to post-translational modification of vitamin D-binding protein [51]. |
| LINC01122 (rs727857) | 2 | LINC01122 gene is an RNA gene that is affiliated with the lncRNA class [52]. LINC01122 was one of the 989 differentially expressed genes which was significantly enriched in vitamin D3 biosynthesis [53]. |
| CPS1 (rs1047891) | 2 | CPS1 gene encodes the carbamoyl-phosphate synthase 1 which is a mitochondrial enzyme that catalyses synthesis of carbamoyl phosphate from ammonia and bicarbonate [54]. |
| UGT1A5 * (rs2012736) | 2 | UGT1A5 gene encodes the UDP glucuronosyltransferase family 1 member A5 which has been shown to transform small lipophilic molecules, into water-soluble, excretable metabolites [55]. Related isoenzymes have been identified as catalysts for 25(OH)D3 glucuronidation in the human liver [56]. |
| CADM2 (rs6782190) | 3 | CADM2 gene encodes the cell adhesion molecule 2 which is a member of the synaptic cell adhesion molecule 1 family [57]. In animal studies, CADM2 is associated with metabolic traits [58], suggesting possible influence on vitamin D concentrations through its effect on obesity and storage capacity of 25(OH)D. |
| GC (rs705117, rs1352846) | 4 | GC gene encodes the vitamin D binding protein which binds to vitamin D and its plasma metabolites and transports them to target tissues [59]. |
| CARMIL1/LRRC16A (rs78151190) | 6 | CARMIL1 gene encodes the capping protein regulator and myosin 1 linker 1 with a role in actin filament network formation [60]. Approximately 10% of muscle tissue consists of actin, providing a possible link with 25(OH)D through storage capacity. |
| VGF (rs75741381) | 7 | VGF gene encodes a protein that is expressed in neuroendocrine cells and is upregulated by nerve growth factor [61]. VGF has been linked with appetite control [62], and diet-induced obesity [63], with a possible link through storage capacity. |
| LINC00536 (rs12056768) | 8 | LINC00536 gene interacts with Wnt3a/β-Catenin signalling [64]. Wnt/β -Catenin signalling is an important signalling pathway in regulating adipose tissue lipogenesis with a possible link with 25(O)D through storage capacity. |
| GRID1 (rs77532868) | 10 | GRID1 gene encodes the glutamate ionotropic receptor delta type subunit 1 which is a subunit of glutamate receptor channels that mediate the fast excitatory synaptic transmission in the central nervous system [65]. |
| CYP2R1 (rs12794714) | 11 | CYP2R1 gene encodes the cytochrome P450 family 2 subfamily R member 1 which acts as 25-hydroxylase of vitamin D [66]. |
| TMEM151A (rs61891388) | 11 | TMEM151A has been predicted to be an integral component of membrane and CD248 enables extracellular matrix binding activity and regulates endothelial cell apoptotic process. |
| AP002387.1/ACTE1P (rs1660839, rs12803256) | 11 | ACTE1P gene is an RNA gene. ACTE1P [67] and vitamin D [68] are both involved in adolescent idiopathic scoliosis (abnormal curvature of the spine), suggesting a possible role of ACTE1P in bone health. |
| S100A11P3 (rs12798050) | 11 | S100A11P3 gene encodes the S100 calcium binding protein A11 pseudogene 3. It has multiple roles in buffering calcium ion concentration, participating in energy metabolism, regulating cell proliferation and differentiation [69]. |
| DGAT2 (rs72997623) | 11 | DGAT2 encodes the diacylglycerol O-acyltransferase 2, catalysing the synthesis of triglycerides [70]. Affects adipose tissue formation [71] with possible link to 25(OH)D storage. |
| GUCY2EP/TSKU (rs1149605) | 11 | GUCY2EP gene encodes guanylate cyclase 2E that is involved in chemosensation and TSKU gene encodes tsukushi, small leucine rich proteoglycan that has been predicted to act upstream/within several processes, including negative regulation of Wnt signaling pathway. |
| HAL (rs10859995) | 12 | HAL gene is upregulated during the differentiation of keratinocytes [72]. HAL deaminates L-histidine to trans-uronic acid [73], which in the stratum corneum absorbs UVB [74] and reduce the production 25(OH)D [75]. |
| SEC23A (rs8018720) | 14 | SEC23A gene encodes the Sec23 homolog A, coat complex II component which plays a role in the ER-Golgi protein trafficking. |
| ALDH1A2 (rs261291) | 15 | ALDH1A2 gene encodes aldehyde dehydrogenase 1 family member A2 which catalyses the synthesis of retinoic acid (RA) from retinaldehyde [76]. |
| PDILT (rs77924615) | 16 | PDILT/PDIA7 gene encodes the protein disulphide isomerase like, testis expressed which catalyses protein folding and thiol-disulphide interchange reactions [77]. |
| SULT2A1 (rs212100) | 19 | SULT2A1 gene encodes a liver- and intestine-expressed sulpho-conjugating enzyme that is responsible for the inactivation by sulphonation of 25(OH)D [78,79]. |
| KLK10 (rs10426) | 19 | KLK10 gene encodes the kallikrein related peptidase 10 that has been shown to play a role in dermal integrity [80]. |
| CYP24A1 † | 20 | CYP24A1 gene encodes cytochrome P450 family 24 subfamily A member 1 which is an important candidate for vitamin D metabolic pathway given that it initiates the degradation of 1,25-dihydroxyvitamin D3 by hydroxylation of the side chain [81]. In addition, this enzyme also plays a role in calcium homeostasis and vitamin D endocrine system [82]. |
| PLA2G3 (rs2074735) | 22 | PLA2G3 gene encodes the phospholipase A2 group III which functions in lipid metabolism and catalyses the calcium-dependent hydrolysis of the sn-2 acyl bond of phospholipids to release arachidonic acid and lysophospholipids [83]. |
* UGT1A5, UGT1A6, UGT1A7, UGT1A8, UGT1A9, UGT1A10. † rs6123359, rs17216707, rs2585442, rs2762943.
For many of the variants which have a replicated association with 25(OH)D we found some evidence that was compatible with a role in the vitamin D pathway (Figure 1, Table 1). Several of the variants were related to lipid levels, while others had been linked with skin integrity, suggesting a possible link with substrate availability for conversion to the circulating 25(OH)D metabolite. Despite body mass index adjustment in the SUNLIGHT consortium meta-analyses, for several of the replicating variants we observed suggested links with adiposity or muscle mass, which may be because these tissues serve as storage sites for 25(OH)D. In addition to confirming the role of 24-hydroxylation in the inactivation and removal of vitamin D metabolites, GWAS identified variants in UGT1A5 and SULT2A1 as potentially relevant. This suggests that sulphonation and glucuronidation pathways, which conjugate sulphur and glucuronide with vitamin D metabolites, are relevant for 25(OH)D excretion and/or recycling.
3.2. Heritability and the Genetic Contribution to the Prevalence of Deficiency
25(OH)D is a commonly used indicator of ‘vitamin D status’ with much of the concentrations determined based on availability of sunlight induced skin synthesis, with contributions from supplement intake and diet. According to twin- and family-based studies, there is great variability in the heritability of 25(OH)D, with overall estimates ranging from 28% to 80% [1,2,3,4]. As ‘heritability’ merely reflects the proportion of total variance that can be explained by genetic factors, it will be the higher with lower environmental contributions (total variance = genetic variance + environmental variance). Indeed, the highest heritability rates are seen in populations measured during winter, when contributions from sunlight synthesis (and hence, the environmental effects) are at their lowest. This may explain why a study on 510 middle-aged male twins found heritability estimates to be ~70% when assessed in winter compared to negligible (~0%) during summer [84]. Also a twin study of Hispanics and African Americans, reported heritabilities of 23%, 28% and 41% for 25(OH)D levels from data taken from California, Texas, and Colorado (n = 1530 individuals from 130 families), respectively [85]. These differences were broadly reflective of geographical location, such that the higher the latitude (and the less sunlight exposure) the higher the estimated heritability. However, seasonal differences in heritability have not been consistently observed, and for example in the recent 25(OH)D GWAS [20], heritability was higher in summer than in winter (0.19 vs. 0.10, respectively). Indeed, another way to estimate heritability is to use information from unrelated individuals using GWAS data (i.e., SNP heritability) and in the large UK Biobank GWAS on white Europeans, SNP heritability for serum 25(OH)D concentrations was estimated to be 13–16% [19,20]. Of practical relevance is the extent to which these genetic variants affect the circulating 25(OH)D levels and the prevalence of vitamin D deficiency. In Table 2, we show the distribution of 25(OH)D concentrations based on data from the 35 replicating variants in the UK Biobank. The odds of low concentrations are about halved for individuals in the highest 20% of vitamin D genetic risk score compared to the lowest quintile. Again, these genetic associations appear to be slightly stronger during summer compared to winter, possibly reflecting genetic differences in vitamin D skin synthesis. The overall difference in the mean 25(OH)D between individuals in the highest vs. lowest quartile of the GRS is about 9 nmol/L [40], which is similar to the association seen with self-reported use of vitamin D supplementation in the UK Biobank during winter (9.7 nmol/L) [84]. This suggests that if the supply from sunlight or diet is limited, differences in 25(OH)D concentrations by higher genetic burden may be of clinical relevance.
Table 2.
Average 25-hydroxyvitamin D level and the odds of low concentrations by quintiles in vitamin D genetic risk score in the UK Biobank.
| Vitamin D Winter (n = 176,577) | Vitamin D Summer (n = 130,855) | |||||
|---|---|---|---|---|---|---|
| 25(OH)D Mean (SD) |
<25 nmol/L OR (95% CI) |
<50 nmol/L OR (95% CI) |
25(OH)D Mean (SD) |
<25 nmol/L OR (95% CI) |
<50 nmol/L OR (95% CI) |
|
| Quintile 1 (Lowest 20%) |
39.16 (17.41) | Reference | Reference | 52.13 (17.37) | Reference | Reference |
| Quintile 2 | 41.84 (18.47) | 0.79 (0.76–0.82) | 0.75 (0.73–0.78) | 56.16 (18.51) | 0.72 (0.66–0.79) | 0.68 (0.66–0.71) |
| Quintile 3 | 43.73 (19.36) | 0.68 (0.66–0.71) | 0.64 (0.61–0.66) | 58.50 (19.20) | 0.63 (0.57–0.69) | 0.56 (0.54–0.58) |
| Quintile 4 | 45.40 (20.14) | 0.60 (0.58–0.62) | 0.54 (0.53–0.56) | 60.65 (20.05) | 0.53 (0.48–0.58) | 0.49 (0.47–0.51) |
| Quintile 5 | 47.51 (21.25) | 0.52 (0.50–0.54) | 0.47 (0.45–0.48) | 64.05 (21.17) | 0.42 (0.38–0.47) | 0.39 (0.37–0.40) |
Genetic risk score calculated using 35 variants with replicated association with 25(OH)D concentrations. Adjusted for age, sex, month in which blood sample was taken, fasting time before blood sample was taken, sample aliquots for measurement, assessment centres, SNP array, and top 40 genetic principal components. Vitamin D winter classified as November to May and vitamin D summer as June to October, based on distribution of 25(OH)D concentrations in the UK biobank [86].
3.3. Genetic Differences in Response to Supplementation and the Need for Personalized Approaches
There were 25 independent loci which were suggestive of gene–environment (GxE) interaction in the recent GWAS [20], suggesting that the size of the genetic association with 25(OH)D can vary by environmental factors influencing serum 25(OH)D concentrations. For five loci, including CYP2R1 and SEC23A there was a genome-wide significant interaction with season [19,20], where the carriers of 25(OH)D-lowering alleles appeared to be less responsive to season compared to non-carriers. This could suggest that some individuals may be more prone to low serum 25(OH)D levels regardless of the season of measurement [19]. In an earlier genome-wide GxE analysis, carriers of 25(OH)D-lowering allele at the CYP2R1 locus were less responsive to dietary vitamin D intake [16]. A similar interaction with vitamin D lowering alleles has also been observed in the context of the GC locus and vitamin D supplementation [87], of vitamin D3-fortified bread and milk consumption [86,87] and UVB treatment [88,89].
There has been recent interest in genetic risk scores (GRS) that combine variants according to their vitamin D lowering alleles, and which look into whether individuals with genetically low 25(OH)D are less or more responsive to treatments for correcting low vitamin D status [87,88,89]. One study used a GRS combining variants in the CYP2R1 and GC loci, and reported a somewhat more modest (~23%) increase in serum 25(OH)D concentrations in response to UVB treatment for individuals carrying four risk alleles compared to the 54% increase for those carrying no risk alleles [88]. They also found that individuals with four risk alleles benefitted the least from the consumption of vitamin D3–fortified bread and milk during this 6-month study [88]. GRS for 25(OH)D has been suggested to be useful for guiding the screening and treatment for vitamin D deficiency. This was tested in a recent study [26], where participants with serum 25(OH)D < 50 nmol/L were recommended to take vitamin D supplements, adjusting the dosage according to their genetic risk. Again, this study used a simple GRS (two SNPs only, taken from GC and CYP2R1), and the individuals with three or four 25(OH)D-lowering alleles were instructed to take 50 µg (2000IU) per day, those with one to two risk alleles to take 20–30 µg/day and those with no risk alleles, 10–20 µg per day. In their study, recommendation to take 50 µg (2000IU) per day over 4 months was enough to reduce the gap between individuals carrying three or four risk alleles and those with no risk alleles both with respect to serum 25(OH)D concentration and the prevalence of 25(OH)D < 50 nmol/L. However, the prevalence of 25(OH)D < 50 nmol/L remained elevated for those with two risk alleles compared to no risk alleles. While these results are very interesting and even promising, they are tentative, as the higher vitamin D intakes were achieved by recommendations, and not by testing in a placebo controlled, and randomized context. This was also a relatively small study (n = 10 to n = 36 per treatment group), so further trials with appropriate controls and a larger sample are required to examine possible benefits and effective approaches for personalized vitamin D supplementation. Given more profound genetic adaptations to differences in vitamin D intakes (‘vitamin D scarcity’) are possible, more research is also needed to establish target levels reflecting ‘optimal’ 25(OH)D concentrations and supplementation approaches for specific population groups, including indigenous Arctic and Tropical peoples [90].
3.4. Mendelian Randomization to Establish Evidence for Causal Effects of 25(OH)D
With the identification of genetic variants associated with serum 25(OH)D concentrations, it has become possible to use Mendelian randomization (MR) to examine evidence for the causal effect of vitamin D on other traits. This method is sometimes called the “natures controlled trial”, as assuming random allocation of genetic variants during the gamete formation, individuals are randomized on different exposure groups based on the genetic variants they carry. Reliable causal inference based on MR studies is conditional to some key method assumptions, and where these hold, this method can help avoid bias due to confounding and reverse causation which more strongly affect other types of observational studies [91]. MR analyses on 25(OH)D have used several strategies, and many of the studies have restricted the variants used to those in the actual vitamin D pathway (including DHCR7, CYP2R1, GC and CYP24A1). With additional loci being discovered for serum 25(OH)D [19,20], MR studies now commonly incorporate these new loci into the analyses. While the inclusion of additional loci can improve statistical power, it is also important to keep in mind the potential for pleiotropic effects that these variants could bring into the models and which could bias the MR analysis [92].
MR studies on 25(OH)D have been conducted across a wide range of outcomes, with evidence supportive of a causal effect seen for multiple sclerosis [93], type 2 diabetes [94] and hypertension [95]. However, many of the newly discovered variants do not have clear or known function with respect to vitamin D metabolism, and some appear pleiotropic, with associations with other traits, such as BMI, and lipid measures. One approach to alleviate concerns relating to pleiotropy and residual genetic confounding affecting variant selection, is to restrict the analyses to variants which have consistent replicating association with 25(OH)D concentrations [39], as would be the case if we use the 35 SNPs described above. However, even there, pleiotropy is likely to remain a concern, and sensitivity analyses using different sets of variants and different analytical approaches will be required to help to assess the robustness of the findings. A multivariable MR approach, which directly accounts for pleiotropic effects by modelling the genetic effects on 25(OH)D simultaneously with pleiotropy related indicators, may also be helpful. However, to allow for the use of this approach, the relevant pleiotropic pathways will need to be hypothesized and relevant information must be available for the analyses. In the context of threshold effects, rigorously conducted MR studies that take into account non-linearity can increase the value of the genetic approach for vitamin D research, as evidence for an effect may only be seen at very low or high levels [96,97]. Recruiting people with vitamin D deficiency to supplementation trials is an important challenge, and often studies test the effects of supplementation in individuals who already have adequate concentrations, and who are typically allowed to take over-the-counter supplements [96,97]. Evidence for benefits with rectifying vitamin D deficiency with respect to outcomes such as mortality [98,99], cardiovascular disease [39] and dementia [100] has been obtained from recent studies using non-linear or stratified MR approaches. For dementia, evidence for a causal effect of vitamin D had already been provided by linear MR studies [101,102,103], while effects on mortality had been supported by RCT meta-analyses [104], but the non-linear studies in both contexts suggest that the benefits of increasing levels may be largely confined to the correction of clinical deficiency. These findings provide important insight into strategies that are likely to provide the greatest benefits, suggesting that large-dose supplementation is unlikely to be required, but population level strategies such as food fortification, which can ensure at least minimal intakes and eradicate severe deficiency across the range of population groups, is likely to work.
4. Conclusions
Vitamin D status is in part determined by genetic variation and GWAS studies have identified a large number of variants that are associated with circulating 25(OH)D concentrations. Some of them are linked to the actual vitamin D metabolic pathway, and others to lipid metabolism and skin properties. In terms of methodology, they may provide MR studies with the means to measure the various determinants of serum 25(OH)D concentrations. Further research is needed to understand how such genetic information may be used to personalize vitamin D supplementation and prevent vitamin D deficiency.
Author Contributions
E.H. conceptualized and wrote the paper. A.Z. conducted literature review, analysed data and wrote the paper. K.S.V. reviewed variants for gene function and prepared the figure with E.H. All authors approved the paper for submission. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. UK Biobank provided data for Table 2, with analyses conducted under project 20175. Ethical approval for the UK Biobank was granted by the National Information Governance Board for Health and Social Care and North West Multicentre Research Ethics Committee (11/NW/0382).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Original data for Table 2 is available from the UK Biobank upon application.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by National Health and Medical Research Council (Australia), GNT1123603.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hunter D., De Lange M., Snieder H., MacGregor A.J., Swaminathan R., Thakker R.V., Spector T.D. Genetic Contribution to Bone Metabolism, Calcium Excretion, and Vitamin D and Parathyroid Hormone Regulation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2001;16:371–378. doi: 10.1359/jbmr.2001.16.2.371. [DOI] [PubMed] [Google Scholar]
- 2.Orton S.-M., Morris A.P., Herrera B.M., Ramagopalan S.V., Lincoln M.R., Chao M.J., Vieth R., Sadovnick A.D., Ebers G.C. Evidence for Genetic Regulation of Vitamin D Status in Twins with Multiple Sclerosis. Am. J. Clin. Nutr. 2008;88:441–447. doi: 10.1093/ajcn/88.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shea M.K., Benjamin E.J., Dupuis J., Massaro J.M., Jacques P.F., D’Agostino R.B., Ordovas J.M., O’Donnell C.J., Dawson-Hughes B., Vasan R.S., et al. Genetic and Non-Genetic Correlates of Vitamins K and D. Eur. J. Clin. Nutr. 2009;63:458–464. doi: 10.1038/sj.ejcn.1602959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wjst M., Altmüller J., Braig C., Bahnweg M., André E. A Genome-Wide Linkage Scan for 25-OH-D(3) and 1,25-(OH)2-D3 Serum Levels in Asthma Families. J. Steroid Biochem. Mol. Biol. 2007;103:799–802. doi: 10.1016/j.jsbmb.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 5.Berry D., Hypponen E. Determinants of Vitamin D Status: Focus on Genetic Variations. Curr. Opin. Nephrol. Hypertens. 2011;20:331–336. doi: 10.1097/MNH.0b013e328346d6ba. [DOI] [PubMed] [Google Scholar]
- 6.Jablonski N.G., Chaplin G. The Roles of Vitamin D and Cutaneous Vitamin D Production in Human Evolution and Health. Int. J. Paleopathol. 2018;23:54–59. doi: 10.1016/j.ijpp.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Buniello A., MacArthur J.A.L., Cerezo M., Harris L.W., Hayhurst J., Malangone C., McMahon A., Morales J., Mountjoy E., Sollis E., et al. The NHGRI-EBI GWAS Catalog of Published Genome-Wide Association Studies, Targeted Arrays and Summary Statistics 2019. Nucleic Acids Res. 2019;47:D1005–D1012. doi: 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn J., Yu K., Stolzenberg-Solomon R., Simon K.C., McCullough M.L., Gallicchio L., Jacobs E.J., Ascherio A., Helzlsouer K., Jacobs K.B., et al. Genome-Wide Association Study of Circulating Vitamin D Levels. Hum. Mol. Genet. 2010;19:2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelman C.D., Meyers K.J., Ziegler J.T., Taylor K.D., Palmer N.D., Haffner S.M., Fingerlin T.E., Wagenknecht L.E., Rotter J.I., Bowden D.W., et al. Genome-Wide Association Study of Vitamin D Concentrations in Hispanic Americans: The IRAS Family Study. J. Steroid Biochem. Mol. Biol. 2010;122:186–192. doi: 10.1016/j.jsbmb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T.J., Zhang F., Richards J.B., Kestenbaum B., Van Meurs J.B., Berry D., Kiel D.P., Streeten E.A., Ohlsson C., Koller D.L., et al. Common Genetic Determinants of Vitamin D Insufficiency: A Genome-Wide Association Study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasky-Su J., Lange N., Brehm J.M., Damask A., Soto-Quiros M., Avila L., Celedon J.C., Canino G., Cloutier M.M., Hollis B.W., et al. Genome-Wide Association Analysis of Circulating Vitamin D Levels in Children with Asthma. Hum. Genet. 2012;131:1495–1505. doi: 10.1007/s00439-012-1185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson D., Holt B.J., Pennell C.E., Holt P.G., Hart P.H., Blackwell J.M. Genome-Wide Association Study of Vitamin D Levels in Children: Replication in the Western Australian Pregnancy Cohort (Raine) Study. Genes Immun. 2014;15:578–583. doi: 10.1038/gene.2014.52. [DOI] [PubMed] [Google Scholar]
- 13.Sapkota B.R., Hopkins R., Bjonnes A., Ralhan S., Wander G.S., Mehra N.K., Singh J.R., Blackett P.R., Saxena R., Sanghera D.K. Genome-Wide Association Study of 25(OH) Vitamin D Concentrations in Punjabi Sikhs: Results of the Asian Indian Diabetic Heart Study. J. Steroid Biochem. Mol. Biol. 2016;158:149–156. doi: 10.1016/j.jsbmb.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manousaki D., Dudding T., Haworth S., Hsu Y.-H., Liu C.-T., Medina-Gomez C., Voortman T., van der Velde N., Melhus H., Robinson-Cohen C., et al. Low-Frequency Synonymous Coding Variation in CYP2R1 Has Large Effects on Vitamin D Levels and Risk of Multiple Sclerosis. Am. J. Hum. Genet. 2017;101:227–238. doi: 10.1016/j.ajhg.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong J., Hatchell K.E., Bradfield J.P., Bjonnes A., Chesi A., Lai C.-Q., Langefeld C.D., Lu L., Lu Y., Lutsey P.L., et al. Transethnic Evaluation Identifies Low-Frequency Loci Associated with 25-Hydroxyvitamin D Concentrations. J. Clin. Endocrinol. Metab. 2018;103:1380–1392. doi: 10.1210/jc.2017-01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X., O’Reilly P.F., Aschard H., Hsu Y.-H., Richards J.B., Dupuis J., Ingelsson E., Karasik D., Pilz S., Berry D., et al. Genome-Wide Association Study in 79,366 European-Ancestry Individuals Informs the Genetic Architecture of 25-Hydroxyvitamin D Levels. Nat. Commun. 2018;9:260. doi: 10.1038/s41467-017-02662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien K.M., Shi M., Weinberg C.R., Sandler D.P., Harmon Q.E., Taylor J.A. Genome-Wide Association Study of Serum 25-Hydroxyvitamin D in US Women. Front. Genet. 2018;9:67. doi: 10.3389/fgene.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kampe A., Enlund-Cerullo M., Valkama S., Holmlund-Suila E., Rosendahl J., Hauta-Alus H., Pekkinen M., Andersson S., Makitie O. Genetic Variation in GC and CYP2R1 Affects 25-Hydroxyvitamin D Concentration and Skeletal Parameters: A Genome-Wide Association Study in 24-Month-Old Finnish Children. PLoS Genet. 2019;15:e1008530. doi: 10.1371/journal.pgen.1008530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manousaki D., Mitchell R., Dudding T., Haworth S., Harroud A., Forgetta V., Shah R.L., Luan J., Langenberg C., Timpson N.J., et al. Genome-Wide Association Study for Vitamin D Levels Reveals 69 Independent Loci. Am. J. Hum. Genet. 2020;106:327–337. doi: 10.1016/j.ajhg.2020.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revez J.A., Lin T., Qiao Z., Xue A., Holtz Y., Zhu Z., Zeng J., Wang H., Sidorenko J., Kemper K.E., et al. Genome-Wide Association Study Identifies 143 Loci Associated with 25 Hydroxyvitamin D Concentration. Nat. Commun. 2020;11:1647. doi: 10.1038/s41467-020-15421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traglia M., Windham G.C., Pearl M., Poon V., Eyles D., Jones K.L., Lyall K., Kharrazi M., Croen L.A., Weiss L.A. Genetic Contributions to Maternal and Neonatal Vitamin D Levels. Genetics. 2020;214:1091–1102. doi: 10.1534/genetics.119.302792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng J.-S., Luan J., Sofianopoulou E., Sharp S.J., Day F.R., Imamura F., Gundersen T.E., Lotta L.A., Sluijs I., Stewart I.D., et al. The Association between Circulating 25-Hydroxyvitamin D Metabolites and Type 2 Diabetes in European Populations: A Meta-Analysis and Mendelian Randomisation Analysis. PLoS Med. 2020;17:e1003394. doi: 10.1371/journal.pmed.1003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y.A., Yoon J.W., Lee Y., Choi H.J., Yun J.W., Bae E., Kwon S.-H., Ahn S.E., Do A.-R., Jin H., et al. Unveiling Genetic Variants Underlying Vitamin D Deficiency in Multiple Korean Cohorts by a Genome-Wide Association Study. Endocrinol. Metab. 2021;36:1189–1200. doi: 10.3803/EnM.2021.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong J.-S., Dixon-Suen S.C., Han X., An J., Fitzgerald R., Buas M., Gammon M.D., Corley D.A., Shaheen N.J., Hardie L.J., et al. A Comprehensive Re-Assessment of the Association between Vitamin D and Cancer Susceptibility Using Mendelian Randomization. Nat. Commun. 2021;12:246. doi: 10.1038/s41467-020-20368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer N.D., Lu L., Register T.C., Lenchik L., Carr J.J., Hicks P.J., Smith S.C., Xu J., Dimitrov L., Keaton J., et al. Genome-Wide Association Study of Vitamin D Concentrations and Bone Mineral Density in the African American-Diabetes Heart Study. PLoS ONE. 2021;16:e0251423. doi: 10.1371/journal.pone.0251423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallinen R.J., Dethlefsen O., Ruotsalainen S., Mills R.D., Miettinen T.A., Jääskeläinen T.E., Lundqvist A., Kyllönen E., Kröger H., Karppinen J.I., et al. Genetic Risk Score for Serum 25-Hydroxyvitamin D Concentration Helps to Guide Personalized Vitamin D Supplementation in Healthy Finnish Adults. J. Nutr. 2021;151:281–292. doi: 10.1093/jn/nxaa391. [DOI] [PubMed] [Google Scholar]
- 27.Sampathkumar A., Tan K.M., Chen L., Chong M.F.F., Yap F., Godfrey K.M., Chong Y.S., Gluckman P.D., Ramasamy A., Karnani N. Genetic Link Determining the Maternal-Fetal Circulation of Vitamin D. Front. Genet. 2021;12:721488. doi: 10.3389/fgene.2021.721488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng H., Ge J., Xu W., Ma H., Chen L., Xia M., Pan B., Lin H., Wang S., Gao X. Type 2 Diabetes Is Causally Associated With Reduced Serum Osteocalcin: A Genomewide Association and Mendelian Randomization Study. J. Bone Miner. Res. 2021;36:1694–1707. doi: 10.1002/jbmr.4330. [DOI] [PubMed] [Google Scholar]
- 29.Backman J.D., Li A.H., Marcketta A., Sun D., Mbatchou J., Kessler M.D., Benner C., Liu D., Locke A.E., Balasubramanian S., et al. Exome Sequencing and Analysis of 454,787 UK Biobank Participants. Nature. 2021;599:628–634. doi: 10.1038/s41586-021-04103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barton A.R., Sherman M.A., Mukamel R.E., Loh P.-R. Whole-Exome Imputation within UK Biobank Powers Rare Coding Variant Association and Fine-Mapping Analyses. Nat. Genet. 2021;53:1260–1269. doi: 10.1038/s41588-021-00892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin E.J., Dupuis J., Larson M.G., Lunetta K.L., Booth S.L., Govindaraju D.R., Kathiresan S., Keaney J.F., Keyes M.J., Lin J.-P., et al. Genome-Wide Association with Select Biomarker Traits in the Framingham Heart Study. BMC Med. Genet. 2007;8((Suppl. 1)):S11. doi: 10.1186/1471-2350-8-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locke A.E., Steinberg K.M., Chiang C.W.K., Service S.K., Havulinna A.S., Stell L., Pirinen M., Abel H.J., Chiang C.C., Fulton R.S., et al. Exome Sequencing of Finnish Isolates Enhances Rare-Variant Association Power. Nature. 2019;572:323–328. doi: 10.1038/s41586-019-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mbatchou J., Barnard L., Backman J., Marcketta A., Kosmicki J.A., Ziyatdinov A., Benner C., O’Dushlaine C., Barber M., Boutkov B., et al. Computationally Efficient Whole-Genome Regression for Quantitative and Binary Traits. Nat. Genet. 2021;53:1097–1103. doi: 10.1038/s41588-021-00870-7. [DOI] [PubMed] [Google Scholar]
- 34.Sinnott-Armstrong N., Tanigawa Y., Amar D., Mars N., Benner C., Aguirre M., Venkataraman G.R., Wainberg M., Ollila H.M., Kiiskinen T., et al. Genetics of 35 Blood and Urine Biomarkers in the UK Biobank. Nat. Genet. 2021;53:185–194. doi: 10.1038/s41588-020-00757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Q., Graff M., Rowland B., Wen J., Huang L., Miller-Fleming T.W., Haessler J., Preuss M.H., Chai J.-F., Lee M.P., et al. Analyses of Biomarker Traits in Diverse UK Biobank Participants Identify Associations Missed by European-Centric Analysis Strategies. J. Hum. Genet. 2022;67:87–93. doi: 10.1038/s10038-021-00968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson T.G., O’Nunain K., Relton C.L., Smith G.D. Harnessing Whole Genome Polygenic Risk Scores to Stratify Individuals Based on Cardiometabolic Risk Factors and Biomarkers at Age 10 in the Lifecourse. Arterioscler. Thromb. Vasc. Biol. 2022;42:ATVBAHA121316650. doi: 10.1161/ATVBAHA.121.316650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aiba I., Yamasaki T., Shinki T., Izumi S., Yamamoto K., Yamada S., Terato H., Ide H., Ohyama Y. Characterization of Rat and Human CYP2J Enzymes as Vitamin D 25-Hydroxylases. Steroids. 2006;71:849–856. doi: 10.1016/j.steroids.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Zhou A., Selvanayagam J.B., Hyppönen E. Non-Linear Mendelian Randomization Analyses Support a Role for Vitamin D Deficiency in Cardiovascular Disease Risk. Eur. Heart J. 2022;43:1731–1739. doi: 10.1093/eurheartj/ehab809. [DOI] [PubMed] [Google Scholar]
- 40.Collins C.S., Gould S.J. Identification of a Common PEX1 Mutation in Zellweger Syndrome. Hum. Mutat. 1999;14:45–53. doi: 10.1002/(SICI)1098-1004(1999)14:1<45::AID-HUMU6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 41.Rush E.T., Goodwin J.L., Braverman N.E., Rizzo W.B. Low Bone Mineral Density Is a Common Feature of Zellweger Spectrum Disorders. Mol. Genet. Metab. 2016;117:33–37. doi: 10.1016/j.ymgme.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Guerrin M., Ishigami A., Méchin M.-C., Nachat R., Valmary S., Sebbag M., Simon M., Senshu T., Serre G. CDNA Cloning, Gene Organization and Expression Analysis of Human Peptidylarginine Deiminase Type I. Biochem. J. 2003;370:167–174. doi: 10.1042/bj20020870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Méchin M.C., Enji M., Nachat R., Chavanas S., Charveron M., Ishida-Yamamoto A., Serre G., Takahara H., Simon M. The Peptidylarginine Deiminases Expressed in Human Epidermis Differ in Their Substrate Specificities and Subcellular Locations. Cell. Mol. Life Sci. CMLS. 2005;62:1984–1995. doi: 10.1007/s00018-005-5196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobs F.M.J., van der Heide L.P., Wijchers P.J.E.C., Burbach J.P.H., Hoekman M.F.M., Smidt M.P. FoxO6, a Novel Member of the FoxO Class of Transcription Factors with Distinct Shuttling Dynamics *. J. Biol. Chem. 2003;278:35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- 45.Zemva J., Schilbach K., Stöhr O., Moll L., Franko A., Krone W., Wiesner R.J., Schubert M. Central FoxO3a and FoxO6 Expression Is Down-Regulated in Obesity Induced Diabetes but Not in Aging. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2012;120:340–350. doi: 10.1055/s-0031-1297970. [DOI] [PubMed] [Google Scholar]
- 46.Vincent J.B., Skaug J., Scherer S.W. The Human Homologue of Flamingo, EGFL2, Encodes a Brain-Expressed Large Cadherin-like Protein with Epidermal Growth Factor-like Domains, and Maps to Chromosome 1p13.3-P21.1. DNA Res. 2000;7:233–235. doi: 10.1093/dnares/7.3.233. [DOI] [PubMed] [Google Scholar]
- 47.Ichthyosis Vulgaris Disease: Malacards—Research Articles, Drugs, Genes, Clinical Trials. [(accessed on 27 May 2022)]. Available online: https://www.malacards.org/card/ichthyosis_vulgaris.
- 48.Peeling Skin Syndrome 6 Disease: Malacards—Research Articles, Drugs, Genes, Clinical Trials. [(accessed on 27 May 2022)]. Available online: https://www.malacards.org/card/peeling_skin_syndrome_6.
- 49.Kukita T., Wada N., Kukita A., Kakimoto T., Sandra F., Toh K., Nagata K., Iijima T., Horiuchi M., Matsusaki H., et al. RANKL-Induced DC-STAMP Is Essential for Osteoclastogenesis. J. Exp. Med. 2004;200:941–946. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van der Valk R.J.P., Kreiner-Møller E., Kooijman M.N., Guxens M., Stergiakouli E., Sääf A., Bradfield J.P., Geller F., Hayes M.G., Cousminer D.L., et al. A Novel Common Variant in DCST2 Is Associated with Length in Early Life and Height in Adulthood. Hum. Mol. Genet. 2015;24:1155–1168. doi: 10.1093/hmg/ddu510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borges C.R., Jarvis J.W., Oran P.E., Nelson R.W. Population Studies of Vitamin D Binding Protein Microheterogeneity by Mass Spectrometry Lead to Characterization of Its Genotype-Dependent O-Glycosylation Patterns. J. Proteome Res. 2008;7:4143–4153. doi: 10.1021/pr8002936. [DOI] [PubMed] [Google Scholar]
- 52.Hezroni H., Koppstein D., Schwartz M.G., Avrutin A., Bartel D.P., Ulitsky I. Principles of Long Noncoding RNA Evolution Derived from Direct Comparison of Transcriptomes in 17 Species. Cell Rep. 2015;11:1110–1122. doi: 10.1016/j.celrep.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghatnatti V., Vastrad B., Patil S., Vastrad C., Kotturshetti I. Identification of Potential and Novel Target Genes in Pituitary Prolactinoma by Bioinformatics Analysis. AIMS Neurosci. 2021;8:254–283. doi: 10.3934/Neuroscience.2021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.PubChem CPS1—Carbamoyl-Phosphate Synthase 1 (Human) [(accessed on 26 May 2022)]; Available online: https://pubchem.ncbi.nlm.nih.gov/gene/CPS1/human.
- 55.Meech R., Hu D.G., McKinnon R.A., Mubarokah S.N., Haines A.Z., Nair P.C., Rowland A., Mackenzie P.I. The UDP-Glycosyltransferase (UGT) Superfamily: New Members, New Functions, and Novel Paradigms. Physiol. Rev. 2019;99:1153–1222. doi: 10.1152/physrev.00058.2017. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z., Wong T., Hashizume T., Dickmann L.Z., Scian M., Koszewski N.J., Goff J.P., Horst R.L., Chaudhry A.S., Schuetz E.G., et al. Human UGT1A4 and UGT1A3 Conjugate 25-Hydroxyvitamin D3: Metabolite Structure, Kinetics, Inducibility, and Interindividual Variability. Endocrinology. 2014;155:2052–2063. doi: 10.1210/en.2013-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biederer T. Bioinformatic Characterization of the SynCAM Family of Immunoglobulin-like Domain-Containing Adhesion Molecules. Genomics. 2006;87:139–150. doi: 10.1016/j.ygeno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 58.Yan X., Wang Z., Schmidt V., Gauert A., Willnow T.E., Heinig M., Poy M.N. Cadm2 Regulates Body Weight and Energy Homeostasis in Mice. Mol. Metab. 2018;8:180–188. doi: 10.1016/j.molmet.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chun R.F. New Perspectives on the Vitamin D Binding Protein. Cell Biochem. Funct. 2012;30:445–456. doi: 10.1002/cbf.2835. [DOI] [PubMed] [Google Scholar]
- 60.Liang Y., Niederstrasser H., Edwards M., Jackson C.E., Cooper J.A. Distinct Roles for CARMIL Isoforms in Cell Migration. Mol. Biol. Cell. 2009;20:5290–5305. doi: 10.1091/mbc.e08-10-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Canu N., Possenti R., Ricco A.S., Rocchi M., Levi A. Cloning, Structural Organization Analysis, and Chromosomal Assignment of the Human Gene for the Neurosecretory Protein VGF. Genomics. 1997;45:443–446. doi: 10.1006/geno.1997.4945. [DOI] [PubMed] [Google Scholar]
- 62.Benchoula K., Parhar I.S., Hwa W.E. The Molecular Mechanism of Vgf in Appetite, Lipids, and Insulin Regulation. Pharmacol. Res. 2021;172:105855. doi: 10.1016/j.phrs.2021.105855. [DOI] [PubMed] [Google Scholar]
- 63.Hahm S., Fekete C., Mizuno T.M., Windsor J., Yan H., Boozer C.N., Lee C., Elmquist J.K., Lechan R.M., Mobbs C.V., et al. VGF Is Required for Obesity Induced by Diet, Gold Thioglucose Treatment, and Agouti and Is Differentially Regulated in Pro-Opiomelanocortin- and Neuropeptide Y-Containing Arcuate Neurons in Response to Fasting. J. Neurosci. 2002;22:6929–6938. doi: 10.1523/JNEUROSCI.22-16-06929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li R., Zhang L., Qin Z., Wei Y., Deng Z., Zhu C., Tang J., Ma L. High LINC00536 Expression Promotes Tumor Progression and Poor Prognosis in Bladder Cancer. Exp. Cell Res. 2019;378:32–40. doi: 10.1016/j.yexcr.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 65.Fossati M., Assendorp N., Gemin O., Colasse S., Dingli F., Arras G., Loew D., Charrier C. Trans-Synaptic Signaling through the Glutamate Receptor Delta-1 Mediates Inhibitory Synapse Formation in Cortical Pyramidal Neurons. Neuron. 2019;104:1081–1094.e7. doi: 10.1016/j.neuron.2019.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng J.B., Levine M.A., Bell N.H., Mangelsdorf D.J., Russell D.W. Genetic Evidence That the Human CYP2R1 Enzyme Is a Key Vitamin D 25-Hydroxylase. Proc. Natl. Acad. Sci. USA. 2004;101:7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J., Zhou Y., Liu S., Song X., Yang X., Fan Y., Chen W., Akdemir Z.C., Yan Z., Zuo Y., et al. The Coexistence of Copy Number Variations (CNVs) and Single Nucleotide Polymorphisms (SNPs) at a Locus Can Result in Distorted Calculations of the Significance in Associating SNPs to Disease. Hum. Genet. 2018;137:553–567. doi: 10.1007/s00439-018-1910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng S.-Y., Bettany-Saltikov J., Cheung I.Y.K., Chan K.K.Y. The Role of Vitamin D in the Pathogenesis of Adolescent Idiopathic Scoliosis. Asian Spine J. 2018;12:1127–1145. doi: 10.31616/asj.2018.12.6.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donato R., Cannon B.R., Sorci G., Riuzzi F., Hsu K., Weber D.J., Geczy C.L. Functions of S100 Proteins. Curr. Mol. Med. 2013;13:24–57. doi: 10.2174/156652413804486214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cases S., Stone S.J., Zhou P., Yen E., Tow B., Lardizabal K.D., Voelker T., Farese R.V. Cloning of DGAT2, a Second Mammalian Diacylglycerol Acyltransferase, and Related Family Members. J. Biol. Chem. 2001;276:38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 71.Smith S.J., Cases S., Jensen D.R., Chen H.C., Sande E., Tow B., Sanan D.A., Raber J., Eckel R.H., Farese R.V. Obesity Resistance and Multiple Mechanisms of Triglyceride Synthesis in Mice Lacking Dgat. Nat. Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 72.Eckhart L., Schmidt M., Mildner M., Mlitz V., Abtin A., Ballaun C., Fischer H., Mrass P., Tschachler E. Histidase Expression in Human Epidermal Keratinocytes: Regulation by Differentiation Status and All-Trans Retinoic Acid. J. Dermatol. Sci. 2008;50:209–215. doi: 10.1016/j.jdermsci.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 73.Suchi M., Sano H., Mizuno H., Wada Y. Molecular Cloning and Structural Characterization of the Human Histidase Gene (HAL) Genomics. 1995;29:98–104. doi: 10.1006/geno.1995.1219. [DOI] [PubMed] [Google Scholar]
- 74.Welsh M.M., Karagas M.R., Applebaum K.M., Spencer S.K., Perry A.E., Nelson H.H. A Role for Ultraviolet Radiation Immunosuppression in Non-Melanoma Skin Cancer as Evidenced by Gene-Environment Interactions. Carcinogenesis. 2008;29:1950–1954. doi: 10.1093/carcin/bgn160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Landeck L., Jakasa I., Dapic I., Lutter R., Thyssen J.P., Skov L., Braun A., Schön M.P., John S.M., Kezic S., et al. The Effect of Epidermal Levels of Urocanic Acid on 25-Hydroxyvitamin D Synthesis and Inflammatory Mediators upon Narrowband UVB Irradiation. Photodermatol. Photoimmunol. Photomed. 2016;32:214–223. doi: 10.1111/phpp.12249. [DOI] [PubMed] [Google Scholar]
- 76.Simões-costa M.S., Azambuja A.P., Xavier-Neto J. The search for non-chordate retinoic acid signaling: Lessons from chordates. J. Exp. Zoolog. B Mol. Dev. Evol. 2008;310B:54–72. doi: 10.1002/jez.b.21139. [DOI] [PubMed] [Google Scholar]
- 77.Van Lith M., Hartigan N., Hatch J., Benham A.M. PDILT, a Divergent Testis-Specific Protein Disulfide Isomerase with a Non-Classical SXXC Motif That Engages in Disulfide-Dependent Interactions in the Endoplasmic Reticulum. J. Biol. Chem. 2005;280:1376–1383. doi: 10.1074/jbc.M408651200. [DOI] [PubMed] [Google Scholar]
- 78.Kurogi K., Sakakibara Y., Suiko M., Liu M.-C. Sulfation of Vitamin D3 -Related Compounds-Identification and Characterization of the Responsible Human Cytosolic Sulfotransferases. FEBS Lett. 2017;591:2417–2425. doi: 10.1002/1873-3468.12767. [DOI] [PubMed] [Google Scholar]
- 79.Wong T., Wang Z., Chapron B.D., Suzuki M., Claw K.G., Gao C., Foti R.S., Prasad B., Chapron A., Calamia J., et al. Polymorphic Human Sulfotransferase 2A1 Mediates the Formation of 25-Hydroxyvitamin D3-3-O-Sulfate, a Major Circulating Vitamin D Metabolite in Humans. Drug Metab. Dispos. 2018;46:367–379. doi: 10.1124/dmd.117.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prassas I., Eissa A., Poda G., Diamandis E.P. Unleashing the Therapeutic Potential of Human Kallikrein-Related Serine Proteases. Nat. Rev. Drug Discov. 2015;14:183–202. doi: 10.1038/nrd4534. [DOI] [PubMed] [Google Scholar]
- 81.Jones G., Prosser D.E., Kaufmann M. 25-Hydroxyvitamin D-24-Hydroxylase (CYP24A1): Its Important Role in the Degradation of Vitamin D. Arch. Biochem. Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Shi M., Grabner A., Wolf M. Importance of Extra-Renal CYP24A1 Expression for Maintaining Mineral Homeostasis. J. Endocr. Soc. 2021;5:A234. doi: 10.1210/jendso/bvab048.476. [DOI] [Google Scholar]
- 83.Murase R., Taketomi Y., Miki Y., Nishito Y., Saito M., Fukami K., Yamamoto K., Murakami M. Group III Phospholipase A2 Promotes Colitis and Colorectal Cancer. Sci. Rep. 2017;7:12261. doi: 10.1038/s41598-017-12434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karohl C., Su S., Kumari M., Tangpricha V., Veledar E., Vaccarino V., Raggi P. Heritability and Seasonal Variability of Vitamin D Concentrations in Male Twins. Am. J. Clin. Nutr. 2010;92:1393–1398. doi: 10.3945/ajcn.2010.30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Engelman C.D., Fingerlin T.E., Langefeld C.D., Hicks P.J., Rich S.S., Wagenknecht L.E., Bowden D.W., Norris J.M. Genetic and Environmental Determinants of 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D Levels in Hispanic and African Americans. J. Clin. Endocrinol. Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sutherland J.P., Zhou A., Leach M.J., Hyppönen E. Differences and Determinants of Vitamin D Deficiency among UK Biobank Participants: A Cross-Ethnic and Socioeconomic Study. Clin. Nutr. 2021;40:3436–3447. doi: 10.1016/j.clnu.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 87.Enlund-Cerullo M., Koljonen L., Holmlund-Suila E., Hauta-alus H., Rosendahl J., Valkama S., Helve O., Hytinantti T., Viljakainen H., Andersson S., et al. Genetic Variation of the Vitamin D Binding Protein Affects Vitamin D Status and Response to Supplementation in Infants. J. Clin. Endocrinol. Metab. 2019;104:5483–5498. doi: 10.1210/jc.2019-00630. [DOI] [PubMed] [Google Scholar]
- 88.Nissen J., Vogel U., Ravn-Haren G., Andersen E.W., Madsen K.H., Nexø B.A., Andersen R., Mejborn H., Bjerrum P.J., Rasmussen L.B., et al. Common Variants in CYP2R1 and GC Genes Are Both Determinants of Serum 25-Hydroxyvitamin D Concentrations after UVB Irradiation and after Consumption of Vitamin D₃-Fortified Bread and Milk during Winter in Denmark. Am. J. Clin. Nutr. 2015;101:218–227. doi: 10.3945/ajcn.114.092148. [DOI] [PubMed] [Google Scholar]
- 89.Nissen J., Vogel U., Ravn-Haren G., Andersen E.W., Nexø B.A., Andersen R., Mejborn H., Madsen K.H., Rasmussen L.B. Real-Life Use of Vitamin D3-Fortified Bread and Milk during a Winter Season: The Effects of CYP2R1 and GC Genes on 25-Hydroxyvitamin D Concentrations in Danish Families, the VitmaD Study. Genes Nutr. 2014;9:413. doi: 10.1007/s12263-014-0413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frost P. The Problem of Vitamin D Scarcity: Cultural and Genetic Solutions by Indigenous Arctic and Tropical Peoples. Nutrients. 2022;14:4071. doi: 10.3390/nu14194071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davies N.M., Holmes M.V., Smith G.D. Reading Mendelian Randomisation Studies: A Guide, Glossary, and Checklist for Clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burgess S., Davey Smith G., Davies N., Dudbridge F., Gill D., Glymour M., Hartwig F., Holmes M., Minelli C., Relton C., et al. Guidelines for Performing Mendelian Randomization Investigations [Version 2; Peer Review: 2 Approved] Wellcome Open Res. 2020;4:186. doi: 10.12688/wellcomeopenres.15555.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mokry L.E., Ross S., Ahmad O.S., Forgetta V., Smith G.D., Leong A., Greenwood C.M.T., Thanassoulis G., Richards J.B. Vitamin D and Risk of Multiple Sclerosis: A Mendelian Randomization Study. PLOS Med. 2015;12:e1001866. doi: 10.1371/journal.pmed.1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu L., Bennett D.A., Millwood I.Y., Parish S., McCarthy M.I., Mahajan A., Lin X., Bragg F., Guo Y., Holmes M.V., et al. Association of Vitamin D with Risk of Type 2 Diabetes: A Mendelian Randomisation Study in European and Chinese Adults. PLOS Med. 2018;15:e1002566. doi: 10.1371/journal.pmed.1002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vimaleswaran K.S., Cavadino A., Berry D.J., Jorde R., Dieffenbach A.K., Lu C., Alves A.C., Heerspink H.J.L., Tikkanen E., Eriksson J., et al. Association of Vitamin D Status with Arterial Blood Pressure and Hypertension Risk: A Mendelian Randomisation Study. Lancet Diabetes Endocrinol. 2014;2:719–729. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morris M.C., Tangney C.C. A Potential Design Flaw of Randomized Trials of Vitamin Supplements. JAMA. 2011;305:1348–1349. doi: 10.1001/jama.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scragg R. Limitations of Vitamin D Supplementation Trials: Why Observational Studies Will Continue to Help Determine the Role of Vitamin D in Health. J. Steroid Biochem. Mol. Biol. 2018;177:6–9. doi: 10.1016/j.jsbmb.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 98.Sutherland J.P., Zhou A., Hyppönen E. Vitamin D Deficiency Increases Mortality Risk in the UK Biobank: A Non-Linear Mendelian Randomization Study. Ann. Intern. Med. 2022 doi: 10.7326/M21-3324. in press . [DOI] [PubMed] [Google Scholar]
- 99.Sofianopoulou E., Kaptoge S.K., Afzal S., Jiang T., Gill D., Gundersen T.E., Bolton T.R., Allara E., Arnold M.G., Mason A.M., et al. Estimating Dose-Response Relationships for Vitamin D with Coronary Heart Disease, Stroke, and All-Cause Mortality: Observational and Mendelian Randomisation Analyses. Lancet Diabetes Endocrinol. 2021;9:837–846. doi: 10.1016/S2213-8587(21)00263-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Navale S.S., Mulugeta A., Zhou A., Llewellyn D.J., Hyppönen E. Vitamin D and Brain Health: An Observational and Mendelian Randomization Study. Am. J. Clin. Nutr. 2022;116:nqac107. doi: 10.1093/ajcn/nqac107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mokry L.E., Ross S., Forgetta V., Morris J.A., Manousaki D., Richards J.B. Genetically Decreased Vitamin D and Risk of Alzheimer Disease. Neurology. 2016;87:2567–2574. doi: 10.1212/WNL.0000000000003430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang L., Qiao Y., Zhang H., Zhang Y., Hua J., Jin S., Liu G. Circulating Vitamin D Levels and Alzheimer’s Disease: A Mendelian Randomization Study in the IGAP and UK Biobank. J. Alzheimers Dis. JAD. 2020;73:609–618. doi: 10.3233/JAD-190713. [DOI] [PubMed] [Google Scholar]
- 103.Larsson S.C., Traylor M., Malik R., Dichgans M., Burgess S., Markus H.S. Modifiable Pathways in Alzheimer’s Disease: Mendelian Randomisation Analysis. BMJ. 2017;359:j5375. doi: 10.1136/bmj.j5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bjelakovic G., Gluud L.L., Nikolova D., Whitfield K., Wetterslev J., Simonetti R.G., Bjelakovic M., Gluud C. Vitamin D Supplementation for Prevention of Mortality in Adults. Cochrane Database Syst. Rev. 2014:CD007470. doi: 10.1002/14651858.CD007470.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data for Table 2 is available from the UK Biobank upon application.