Abstract
Currently, there are no therapies to prevent severe dengue disease. Essential oils (EOs) can serve as primary sources for research and the discovery of phytomedicines for alternative therapy. Fourteen EOs samples were obtained by distillation from six plants used in Colombian folk medicine. GC/MS analysis identified 125 terpenes. Cytopathic effect (CPE) reduction assays revealed differences in antiviral activity. EOs of Lippia alba, citral chemotype and carvone-rich fraction; Lippia origanoides, phellandrene chemotype; and Turnera diffusa, exhibited strong antiviral activity (IC50: 29 to 82 µg/mL; SI: 5.5 to 14.3). EOs of Piper aduncum, Ocimum basilicum, and L. origanoides, carvacrol, and thymol chemotypes, exhibited weak antiviral activity (32 to 53% DENV-CPE reduction at 100 µg/mL; SI > 5.0). Cluster and one-way ANOVA analyses suggest that the strong antiviral activity of EOs could be attributed to increased amounts of non-phenolic oxygenated monoterpenes and sesquiterpene hydrocarbons. Docking analyses (AutoDock Vina) predicted binding affinity between the DENV-2 E protein and terpenes: twenty sesquiterpene hydrocarbons (−8.73 to −6.91 kcal/mol), eight oxygenated monoterpenes (−7.52 to −6.98 kcal/mol), and seven monoterpene hydrocarbons (−7.60 to −6.99 kcal/mol). This study reports for the first time differences in the antiviral activity of EOs against DENV, corresponding to their composition of monoterpenes and sesquiterpenes.
Keywords: dengue, dengue virus, essential oils, terpenes
1. Introduction
Dengue virus (DENV) is transmitted to humans by infected Aedes mosquitoes. The virus is prevalent in more than 100 countries worldwide [1]. DENV infections manifest in a broad spectrum of presentations, including asymptomatic infection, mild flu-like syndrome, and severe disease [2]. Severe dengue is a life-threatening worsening of dengue symptoms, which remains one of the leading causes of hospitalization in developing and underdeveloped countries, where the surveillance network for disease control is not robust [3]. There are no effective drugs to prevent the development of severe dengue, although intensive research on synthetic antivirals has been ongoing for decades [4,5]. Dengue is a complex disease, which has made the discovery of effective therapies difficult. Studies support the view that herbal medication could be useful for treating DENV infections, which could reduce the risk of severe dengue if started soon after exposure to the virus [6,7,8].
Essential oils (EOs) distilled from aromatic plants have traditionally been used to prepare medicinal herbs to treat human diseases [9,10]. Numerous EOs exhibit in vitro antiviral activity against pathogenic human-enveloped viruses such as herpesvirus, coronavirus, influenza virus, and human immunodeficiency virus [11,12,13]. EOs are also recognized for their anti-inflammatory and stimulatory effects on the human immune system [14,15]. Monoterpenes and sesquiterpenes are the main constituents of EOs and have been considered their active components against pathogenic viruses due to their antiviral and anti-inflammatory properties [9,10]. EOs have been proposed as alternative medicines for the treatment of various antiviral diseases [12,13]. Recently, EOs with established pharmacokinetic and pharmacodynamic properties were proposed as an alternative treatment to prevent clinical complications associated with COVID-19 [16,17].
Dengue is a serious health problem in Colombia. In the last decade, epidemics have occurred with high mortality rates, compared to the average values reported in Latin American countries [3]. Despite the richness of Colombian flora, characterized by more than 30,000 species of higher plants [18], only a small number of species have been studied to discover their potential as primary sources of herbal medicines. Research focusing on the antiviral potential of EOs may contribute to the discovery of alternative therapies to prevent severe dengue.
Plants used in folk medicine in Colombia and Latin American countries were selected for the present study. Lippia alba (Mill.) N.E.Br. ex Britt and Wils (Verbenaceae) is used as a remedy for treating stomach disorders, influenza, and headaches [19]. Lippia origanoides Kunth (Verbenaceae) is employed to prepare gastrointestinal and respiratory remedies [20]. Turnera diffusa Willd ex Schult (Passifloraceae), commonly known as damiana, is used as a tonic and sexual stimulant, and to treat influenza, gastrointestinal and skin disorders [21]. Piper aduncum L. (Piperaceae) is utilized in traditional medicine for its antimicrobial, anti-inflammatory, anthelminthic, and analgesic properties [22]. Varronia curassavica Jacq. (Boraginaceae) is used to treat inflammation, ulcers, arthritis, and pain; its EO is employed to treat myofascial pain and tendonitis [23]. Ocimum basilicum L. (Lamiaceae) has extensive applications in the culinary, cosmetics, nutraceutical, and toiletry industries; its EO is utilized to alleviate mental fatigue, colds, rhinitis, and to treat snakebites [24].
Variations of the biological activities of EOs according to specie, chemotype, and phenological stages of the plant are documented [9,10,25]. Therefore, studies on the variation of the antiviral activity with respect to the chemical composition of EOs could provide information on what determines their effects on virus infectivity. In this study, fourteen EOs obtained from the above-mentioned medicinal plants grown in Colombia were analyzed to determine the variation of antiviral activities against two DENV serotypes and the influence of terpene composition. In addition, molecular docking analyses were performed to predict the interactions of EO chemical constituents with DENV structural proteins.
2. Results
2.1. EO Chemical Composition
Fourteen EOs from six medicinal plants grown in Colombia were studied (Table 1). A total of 125 compounds were identified by GC/MS, most of which are terpenes. Relative amounts measured by GC/FID and the linear retention indices of compounds in order of their elution on the DB-5MS column are listed in Supplementary Table S1. The main constituents of all fourteen EOs are presented in Table 2.
Table 1.
Essential oils studied in this work.
| Plant Material | Voucher Number | EO Identifier | EO Characteristics * |
|---|---|---|---|
| Lippia origanoides Kunth | 22035 | LoP | Phellandrene chemotype, neat EO |
| 22034 | LoC | Carvacrol chemotype, neat EO | |
| 22039 | LoTC | Thymol-carvacrol chemotype, neat EO | |
| 22036 | LoT | Thymol chemotype, neat EO | |
| LoTf | Thymol chemotype, thymol-rich fraction | ||
| Lippia alba (Mill.) N.E.Br. ex Britton & P. Wilson | 22002 | LaCi | Citral chemotype, neat EO |
| LaCif | Citral chemotype, light fraction | ||
| 22031 | LaCaf1 | Carvone chemotype, limonene-rich fraction | |
| LaCaf2 | Carvone chemotype, carvone-rich fraction | ||
| Turnera diffusa Willdenow | 22032 | TdS1 | 2019, neat EO |
| 22037 | TdS2 | 2016, neat EO | |
| Piper aduncum L. | 22033 | PaS1 | Linalool chemotype, neat EO |
| Ocimum basilicum L. | 22227 | ObS1 | Piperitone chemotype, neat EO |
| Varronia curassavica Jacq. | 20892 | VcS1 | Neat EO |
* Based on the chemical profile analysis and the extraction method (see Materials and Methods).
Table 2.
The main chemical constituents of essential oils or their fractions studied in this work.
| Compound | LRIs (DB-5MS Column) | LaCi | LaCif | LaCaf1 | LaCaf2 | LoP | LoC | LoT | LoTf | LoTC | TdS1 | TdS2 | PaS1 | ObS1 | VcS1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | Lit. | |||||||||||||||
| α-Pinene * | 935 | 932 a | 0.3 | 0.2 | 0.2 | - | 1.7 | 0.4 | - | - | 0.4 | - | - | 4.6 | 0.5 | 9.4 |
| α-Phellandrene | 1005 | 1002 a | 0.1 | - | - | - | 7.1 | - | - | - | 0.5 | - | - | 4.4 | - | - |
| p-Cymene * | 1027 | 1024 a | - | - | - | - | 12.6 | 14.4 | 2.3 | 2.0 | 19.1 | 3.0 | 3.6 | 3.0 | - | - |
| Limonene * | 1034 | 1029 a | 2.4 | 5.9 | 82.2 | 0.9 | 2.1 | 0.3 | - | - | 0.9 | - | - | 6.0 | - | 0.8 |
| 1,8-Cineol * | 1036 | 1031 a | - | - | - | - | 13.0 | 1.3 | - | - | - | - | - | 3.6 | 8.1 | 0.8 |
| γ-Terpinene | 1061 | 1059 a | - | - | - | - | 2.4 | 5.3 | 0.9 | 6.9 | 9.2 | 0.6 | 0.7 | 0.8 | - | - |
| Linalool * | 1099 | 1096 a | 1.1 | 2.5 | 0.4 | 0.5 | 0.7 | 1.0 | - | - | 0.3 | - | - | 0.4 | 42.7 | - |
| Estragole * | 1203 | 1196 a | - | - | - | - | - | - | - | - | - | - | - | - | 18.6 | - |
| Neral * | 1246 | 1252 b | 11.9 | 18.1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Carvone * | 1259 | 1258 c | - | - | 12.2 | 78.2 | - | - | - | - | - | - | - | - | - | - |
| Geraniol * | 1260 | 1240 b | 19 | 8.1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Piperitone | 1265 | 1264 c | - | - | - | 4.8 | - | - | - | - | - | - | - | 14.8 | - | - |
| Geranial * | 1272 | 1270 b | 24.5 | 24.8 | - | - | - | - | - | - | - | - | - | - | - | - |
| Thymol * | 1290 | 1290 a | - | - | - | - | 14.0 | 8.0 | 75.3 | 82.9 | 49.4 | - | 0.2 | - | - | - |
| Carvacrol * | 1300 | 1298 a | - | - | - | - | 0.9 | 35 | 4.9 | 1.2 | 2.7 | - | 0.4 | - | - | - |
| Piperitenone | 1347 | 1343 a | - | - | 0.3 | 0.3 | - | - | - | - | - | - | - | 14.8 | - | - |
| α-Copaene | 1385 | 1376 a | - | - | - | 0.3 | 0.6 | 0.7 | - | - | - | - | - | 2.9 | - | 7.0 |
| trans-β-Caryophyllene * | 1433 | 1427 c | 9.1 | 13.3 | - | 0.1 | 15.1 | 4.4 | 5.4 | 7.0 | 1.6 | 4.0 | 4.9 | 7.4 | 0.9 | 19.2 |
| α-Humulene * | 1467 | 1468 c | 2.8 | 2.8 | - | - | 8.1 | 1.1 | 3.2 | - | 0.9 | - | 0.4 | 1.5 | 2.5 | 2.7 |
| Aristolochene | 1483 | 1488 a | - | - | - | - | - | - | - | - | - | 17.9 | 20.9 | - | - | - |
| Germacrene D | 1492 | 1481 a | 4.3 | 1.5 | 0.1 | - | 0.9 | - | - | - | - | - | - | 1.7 | 4.9 | 12.3 |
| β-Selinene | 1502 | 1490 a | - | - | - | - | 0.5 | 0.3 | - | - | - | 5.2 | 5.8 | - | - | - |
| Valencene | 1503 | 1496 a | - | - | - | - | - | - | - | - | - | 7.4 | 6.5 | 1.2 | - | - |
| trans-β-Guaiene | 1517 | 1502 a | 2.2 | - | - | - | - | - | - | - | - | - | - | - | - | 11.8 |
| Dehydrofukinone | 1827 | 1820 c | - | - | - | - | - | - | - | - | - | 25.4 | 19.3 | - | - | - |
LRIs, linear retention indices calculated using n-alkanes C8–C25 mixture on the DB-5MS (non-polar) column. L. alba: neat EO (LaCif) and light fraction (LaCif) of citral chemotype; and limonene-rich fraction (LaCaf1) and carvone-rich fraction (LaCaf2) of carvone chemotype. L. origanoides: neat EO of phellandrene chemotype EO (LoP), carvacrol (LoC), thymol-carvacrol (LoTC), and thymol (LoT) chemotypes; and thymol-rich fraction (LoTf) of the thymol chemotype. Neat EOs of T. diffusa collected in 2019 (TdS1) and in 2016 (TdS2). Neat EOs of O. basilicum (ObS1), P. aduncum (PaS1) and V. curassavica (VcS1). Exp. Experimental. Lit. Literature a Adams, 2007 [26]; b Babushok et al., 2011 [27]; c NIST 2017 [28]. * Standard compounds were used for confirmatory identification.
L. origanoides EOs had more monoterpenes (59.8 to 94.3%) than sesquiterpenes (2.5 to 35.3%). In the LoP (phellandrene chemotype) sample, trans-β-caryophyllene (15.1%), thymol (14%), 1,8-cineol (13%) and p-cymene (12.6%) were the main constituents followed by α-phellandrene (7.1%). In thymol chemotype EOs, thymol was the main constituent: 82.9, 75.3, and 49.4% in LoTf, LoT, and LoTC, respectively. In the LoC (carvacrol chemotype) sample, carvacrol (35%) and p-cymene (14.4%) were the main constituents.
L. alba EOs had a higher content of monoterpenes (66.2 to 99%) than sesquiterpenes (0.6 to 23.5%). In citral chemotype EOs, geranial and geraniol were the main constituents: LaCi, 24.5 and 19.0%; LaCif, 24.8 and 8.1%, respectively. In carvone chemotype EOs, limonene (82.2%) and carvone (78.2%) were the main constituents.
Both T. diffusa EOs (TdS1 and TdS2) can be classified as sesquiterpene-rich EOs (86.2 to 87.2%), aristolochene (17.9 and 20.9%) and dehydrofukinone (25.4 and 19.3%) were their main constituents.
P. aduncum EO (PaS1) was characterized by similar amounts of sesquiterpenes (46.9%) and monoterpenes (44.2%), piperitone (14.8%), and trans-β-caryophyllene (7.4%) were the main constituents.
O. basilicum EO (ObS1) had a higher content of monoterpenes (72.3%) than sesquiterpenes (27.7%), linalool (42.7%) and estragol (18.6%) were the main constituents.
V. curassavica EO (VcS1) was characterized by high (76.2%) content of sesquiterpenes, trans-β-caryophyllene (19.2%), germacrene D (12.3%), and trans-β-guaiene (11.8%) were the principal constituents.
2.2. Antiviral Activity
The crystal violet assay was used to evaluate the cytotoxicity of the EOs against Vero cells (Supplementary Figure S1). No sample of EO reduced cell viability by more than 50%. Maximum non-cytotoxic concentration (MNTC) values were estimated to be in the range of 429 and 512 µg/mL.
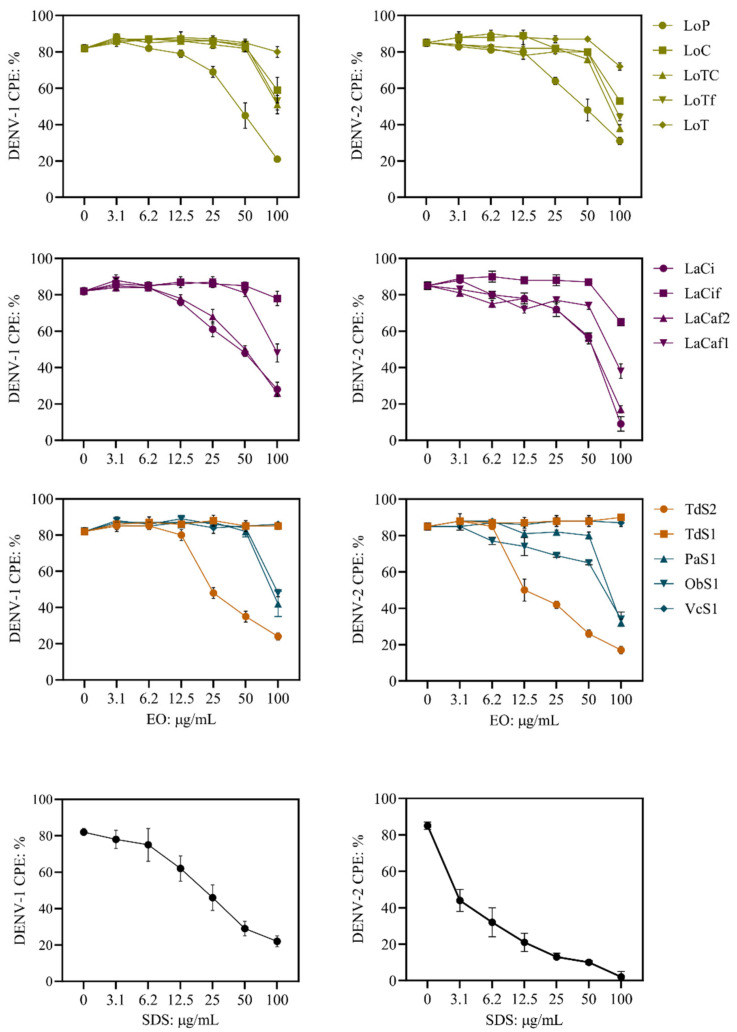
DENV-induced cytopathic effect (DENV CPE) is a surrogate measure of virus replication in vitro [29]. The lower the CPE, the higher the antiviral activity of the test sample. EOs were tested at six-point non-cytotoxic concentrations to evaluate antiviral activity against two serotypes of DENV (DENV-1 and DENV-2) during adsorption into the cell (Figure 1, Table 3). Four EO samples (LaCi and LaCaf2 of L. alba; LoP of L. origanoides; and TdS2 of T. diffusa 2016) reduced the CPE of both DENV serotypes in a dose-dependent manner. The IC50 values range from 29 to 82 µg/mL, and the selectivity indices were 5.5 to 14.3; therefore, these four EOs were classified as having a strong antiviral activity. Six EO samples (LaCaf1 of L. alba; LoC, LoTC, and LoTf of L. origanoides; ObS1 of O. basilicum; and PaS1 of P. aduncum) reduced by 32–53% (one-way ANOVA, p: < 0.0001) the CPE of both DENV serotypes at the highest concentration of 100 µg/mL, but not at the other oil concentrations. Therefore, IC50 values could not be estimated, and these six EOs were classified as having weak antiviral activity. The remaining four EOs (TdS1, LaCif, LoT, and VcS1) were classified as inactive. DENV-CPE (%) of both virus serotypes was not different from the untreated control at all six concentrations of the EOs.
Figure 1.
Results of cytopathic effect (CPE)-based assays to evaluate the antiviral activities of the essential oils against dengue viruses (DENV-1 and DENV-2). Quantification of crystal violet staining was performed to measure virus-induced CPE. % DENV-CPE = [(OD 570 of infected and treated cells/OD 570 of non-infected non-treated]. EO samples (Table 1): L. origanoides (LoP, LoC, LoTC, LoTf, and LoT); L. alba (LaCi, LaCif, LaCaf2, LaCaf1); T. diffusa (TdS1 and TdS2); O. basilicum (ObS1); P. aduncum (PaS1) and V. curassavica (VcS1). Sodium dodecyl sulfate (SDS) is an antiviral compound. Data are expressed as the mean ± SD from six independent measurements.
Table 3.
The activity of the essential oils tested against dengue virus serotypes in the cytopathic effect (CPE) assay.
| Plant Material | Essential Oil Identifier | DENV-1 | DENV-2 |
|---|---|---|---|
| Lippia origanoides | LoP | Strong: IC50 (SI): 77 ± 1.1 (6.6) |
Strong: IC50 (SI): 75 ± 1.0 (6.8) |
| LoC | Weak | Weak | |
| LoTC | Weak | Weak | |
| LoTf | Weak | Weak | |
| LoT | Inactive | Inactive | |
| Lippia alba | LaCi | Strong: IC50 (SI): 78 ± 1.1 (5.5) |
Strong: IC50 (SI): 67 ± 1.2 (6.4) |
| LaCaf2 | Strong: IC50 (SI): 82 ± 1.1 (5.8) |
Strong: IC50 (SI): 72 ± 1.1 (6.6) |
|
| LaCaf1 | Weak | Weak | |
| LaCif | Inactive | Inactive | |
| Turnera diffusa | TdS2 | Strong: IC50 (SI): 54 ± 1.1 (7.7) |
Strong: IC50 (SI): 29 ± 1.1 (14.3) |
| TdS1 | Inactive | Inactive | |
| Piper aduncum | PaS1 | Weak | Weak |
| Ocimum basilucum | ObS1 | Weak | Weak |
| Verronia curassavica | VcS1 | Inactive | Inactive |
Essential oils are described in Table 1. The IC50 (µg/mL) values are the means (±SD) of six measurements from three independent assays. Selectivity index (SI): MNTC in the cytotoxic assay/IC50. Strong activity, reduction of DENV CPE in EO-treated cells in a dose-dependent manner. Weak activity, reduction of DENV CPE in EO-treated cells at 100 µg/mL (DENV CPE: 32 to 53%, respect to 100% untreated control; one-way ANOVA: F: 48–1295, p < 0.001; Dunnett’s post hoc test, p < 0.001), but not at other concentrations. Inactive, there was no reduction of DENV CPE in EO-treated cells at the six concentrations tested.
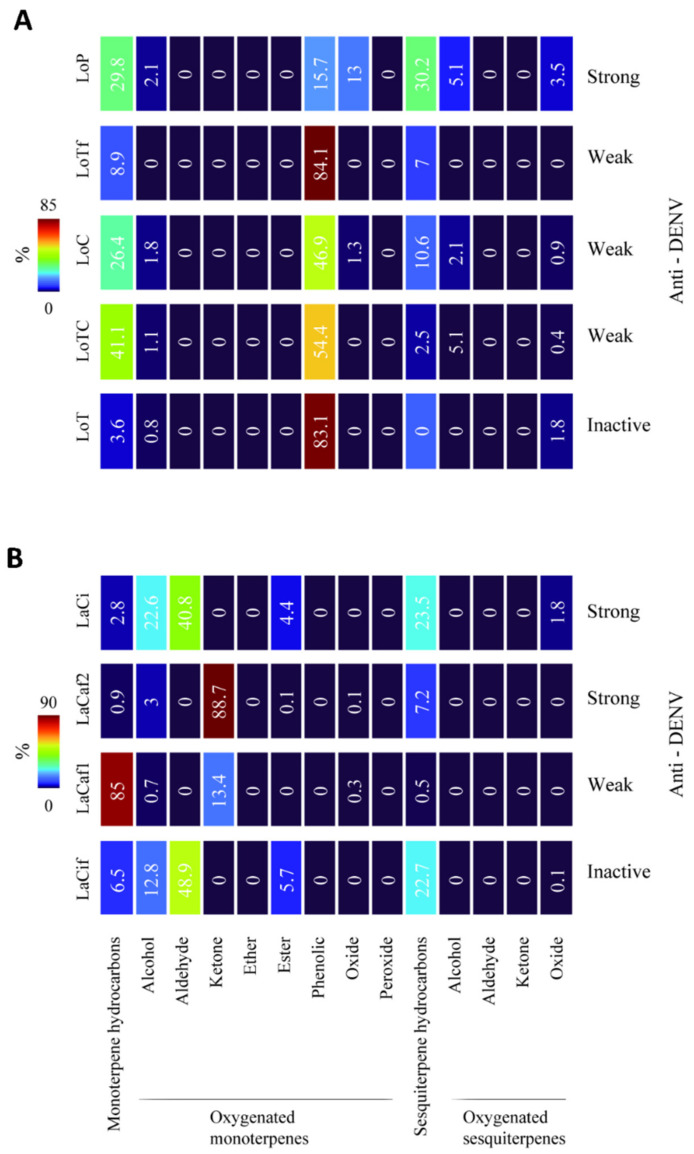
Comparisons of antiviral activities against DENV-2 and terpene content of the EOs are described below: L. origanoides EOs (Figure 2A). LoP (phellandrene chemotype), with strong antiviral activity, had a higher content of sesquiterpene hydrocarbons (30.2%) and oxygenated monoterpenes (alcohols and oxides: 15.1%) compared to the EOs with weak antiviral activity (LoC, carvacrol chemotype; LoTC, thymol-carvacrol chemotype; and LoTf, thymol chemotype, fraction: 2.5 to 10.6% sesquiterpenes and 0 to 3.1% monoterpenes). Conversely, LoP had lower content (15.7%) of phenolic monoterpenes than LoTf (84.1%), LoC (46.9%), and LoTC (54.4%). The LoT EO (thymol chemotype), inactive against both virus serotypes, had the lowest amounts of monoterpene hydrocarbons among the five EO samples (3.6 vs 8.9%, in LoTf to 41.1% in LoTC).
Figure 2.
Antiviral activities of Lippia essential oils against dengue virus and terpene content. Heat maps representing the chemical composition in monoterpene and sesquiterpene types. Antiviral activity (Anti-DENV) was expressed as strong, weak, and inactive (Table 3). (A). EOs of L. origanoides chemotypes: neat EOs phellandrene EO (LoP); carvacrol (LoC); thymol-carvacrol (LoTC) and thymol (LoT); and thymol-rich fraction (LoTf). (B). EOs of L. alba: neat EO (LaCif) and light fraction (LaCif) of the citral chemotype; and limonene-rich fraction (LaCaf1) and carvone-rich fraction (LaCaf2) of the carvone chemotype.
L. alba EOs (Figure 2B). LaCi (citral chemotype) and LaCaf2 (carvone-rich fraction), with strong antiviral activity, had higher amounts of oxygenated monoterpenes (alcohols, aldehydes, and ketones: 63.4% and 91.7%, respectively) compared to LaCaf1 (limonenerich fraction, 14.1%) with weak antiviral activity. On the other hand, the LaCif sample EO (citral, light fraction), which was inactive against both virus serotypes, had lower amounts of monoterpene alcohols (12.8%) and sesquiterpene oxides (0.1%) compared to LaCi (22.6% monoterpenes and 1.8% sesquiterpenes).
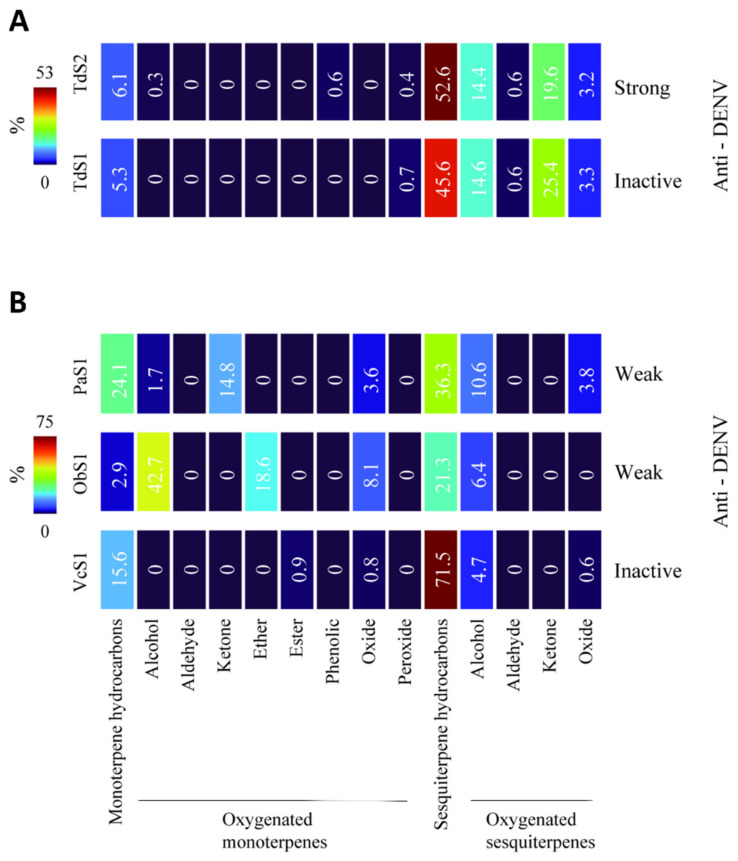
T. diffusa EOs (Figure 3A). TdS2, with strong antiviral activity, but not TdS1, without antiviral activity, had monoterpene alcohols (0.3%) and phenolic monoterpenes (0.6%). Also, TdS2 had a higher content of sesquiterpene hydrocarbons (52.6%) than TdS1 (45.6%)
Figure 3.
Antiviral activities of essential oils against dengue virus and terpene content. Heat maps representing the chemical composition in monoterpenes and sesquiterpenes types. Antiviral activity (Anti-DENV) was expressed as strong, weak, and inactive (Table 3). (A). Neat EOs of T. diffusa collected in 2019 (TdS1) and 2016 (TdS2). (B). Neat EOs of O. basilicum (ObS1) P. aduncum (PaS1) and V. curassavica (VcS1).
P. aduncum (PaS1), O. basilicum (ObS1), and V. curassavica (VcS1) EOs (Figure 3B): PaS1 and ObS1, which exhibited weak antiviral activity, had higher amounts (20.1 and 69.4%, respectively of oxygenated monoterpenes (alcohols, ethers, and oxides) compared to the V. curassavica EO (0.8%), which was inactive against both virus serotypes.
2.3. Chemical Cluster of EOs and Antiviral Activity
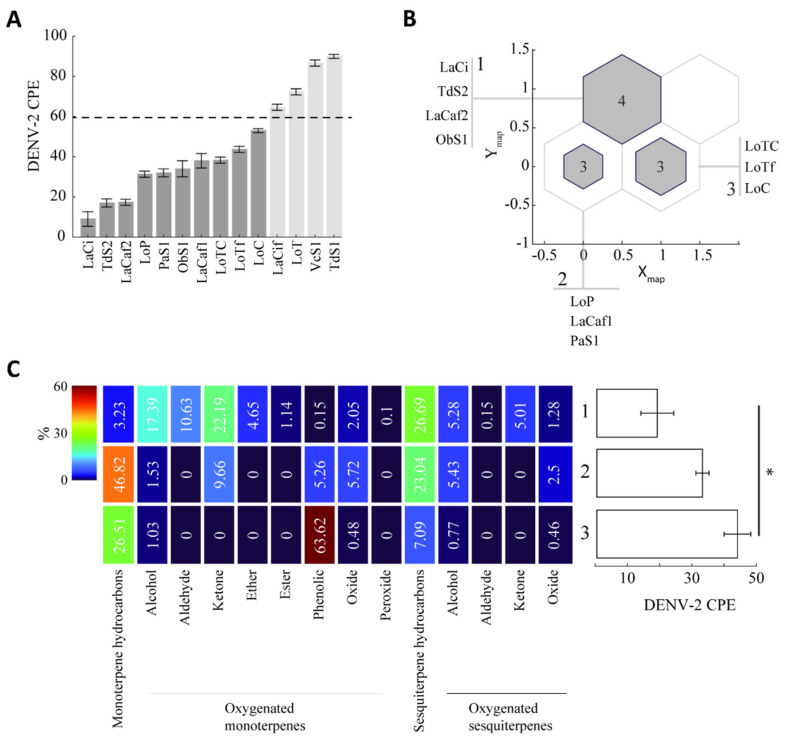
The relationship between antiviral activity and chemical composition was explored to test the hypothesis that, for those EOs showing strong and weak antiviral activity (CPE ≤ 60%, n = 10, Figure 4A), similar terpene content results in similar antiviral activity. Kohonen’s self-organized maps algorithm, using the amounts of terpenes exclusively as input (Figure 2 and Figure 3), grouped EOs in three different clusters (Figure 4B,C).
Figure 4.
Clustering of essential oils based on monoterpene and sesquiterpene contents and their relation to antiviral activity. (A). Cytopathic effect (CPE: %) of DENV-2 in Vero cells, EOs identifiers in Table 1. The dashed line indicates the CPE threshold for further clustering analysis, only EOs with CPE ≤ 60 (dark gray bars) were included. (B). Clustering configuration of selected EOs in A using a 2 × 2 self-organized map. Three non-empty clusters were obtained. The number inside each cluster indicates the number of EOs it contains. (C). Left, heat map representing the chemical composition of clusters 1 (upper row), 2 (middle row), and 3 (lower row). Right, mean CPE of each cluster indicating statistical differences between clusters 1 and 3 (* One-way ANOVA: F2,9 = 6.6092, p = 0.023, post hoc Tukey-Kramer test p = 0.026).
Cluster 1 grouped three EOs (LaCi, LaCaf2, and TdS2) with strong antiviral activity and one EO (ObS1) with weak antiviral activity, which showed a chemical profile characterized by the presence of all eight types of oxygenated monoterpenes, and the highest amounts of sesquiterpenoids, both hydrocarbon and oxygenated sesquiterpenes.
Cluster 2 grouped one EO (LoP) with strong antiviral activity and two EOs (LaCaf1 and PaS1) with weak activity, which showed higher amounts of monoterpene hydrocarbons and lower amounts of oxygenated monoterpenes and sesquiterpenes, compared to cluster 1.
Cluster 3 grouped the three EOs of L. origanoides (LoTC, LoTf and LoC) with weak antiviral activity, which showed higher amounts of phenolic monoterpenes, lower amounts of sesquiterpene hydrocarbons, and had no five oxygenated monoterpenes (aldehydes, ketones, ethers, esters, and peroxides), compared to cluster 1.
A one-way ANOVA of the DENV-2 CPE values of EOs in each cluster revealed significant differences between clusters 1 and 3 (Figure 4C, One-way ANOVA: F2,9 = 6.6092, p = 0.023, post hoc Tukey-Kramer test p = 0.026) but not clusters 1 and 2, suggesting a relation between higher content of non-phenolic oxygenated monoterpenes and sesquiterpenes, and higher antiviral activity.
2.4. Molecular Interactions between EO Compounds and DENV-2 Proteins
The DENV particle contains an RNA genome and capsid surrounded by a lipid envelope, which contains the envelope (E) and pre-membrane (prM/M) proteins [30]. Copies of the capside protein (C) form the viral capsid. Docking analysis was performed to predict the binding affinities between 68 sesquiterpenes and 53 monoterpenes, identified in the fourteen EOs, and the E, C, and prM/M proteins of DENV-2. A binding energy below the upper threshold of −6.8 kcal/mol was considered a cutoff value for predicting the binding affinity between ligand and target [31]. Supplementary Table S2 presents the AutoDock Vina binding energies values of EOs terpenes with DENV-2 proteins
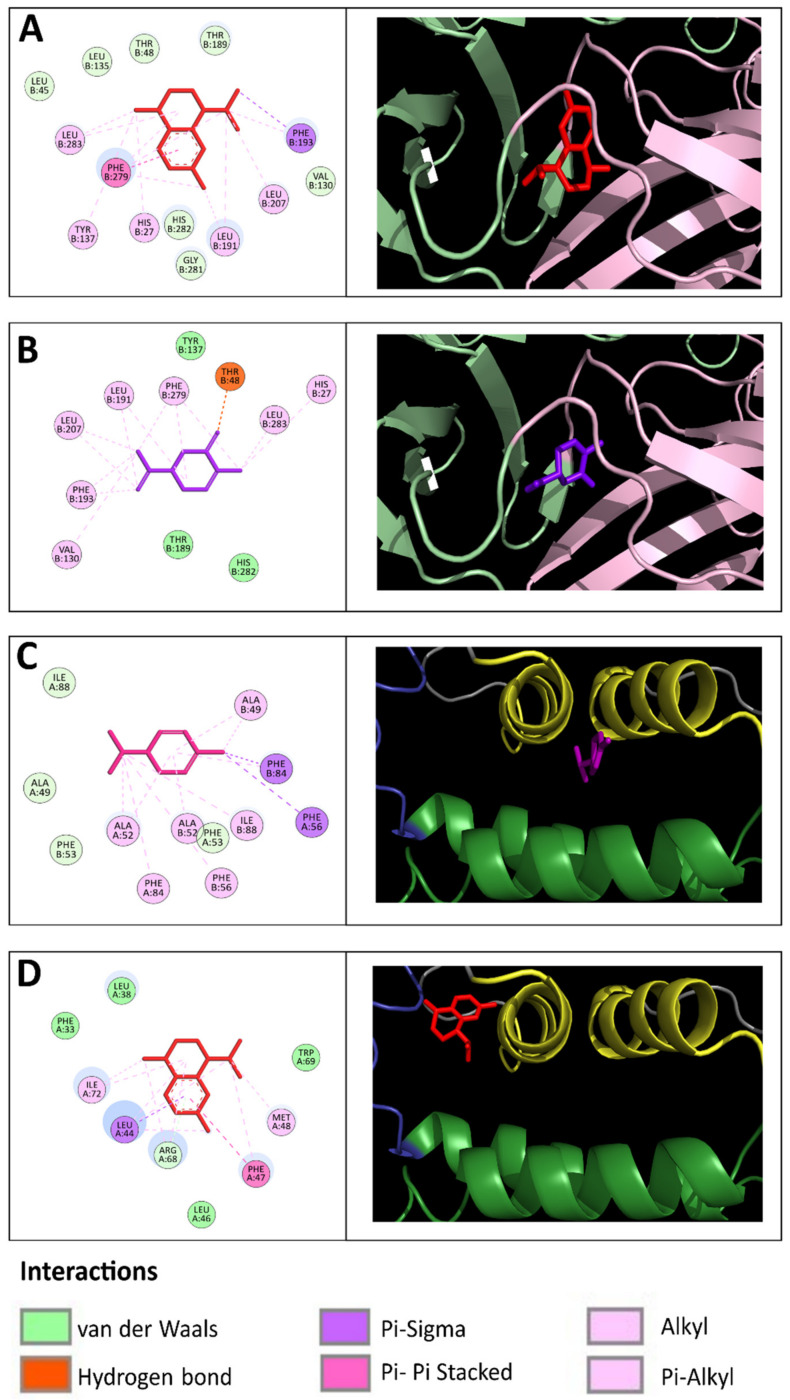
The DENV-2 E protein is organized into three structural domains. The beta-octylglucoside detergent binding site (the "βOG pocket") is in the hinge region formed by DI and DII domains. It plays an essential role in viral entry by mediating fusion between virus and host cell membranes [30,32]. The docking analyses predicted the binding affinities between 38 terpenes and the DENV-2 E protein, which have formed hydrophobic bonds with amino acid residues (especially Thr48, Tyr137, Leu191, Phe193, and Phe279) of the βOG pocket.
Twenty sesquiterpene hydrocarbons docked to DENV-2 E, of which thirteen were bicyclic (−8.73 to −6.91 kcal/mol), five were tricyclic (−8.13 to −7.62 kcal/mol), and two were monocyclic compounds (−7.83 to −6.91 kcal/mol). Two bicyclic sesquiterpene alcohols docked to DENV E (−7.07 to −6.91 kcal/mol), vetiselinenol had formed a hydrogen bond with Thr280 (4.6 Å). Fifteen monoterpenes were predicted to dock into the E-βOG pocket, all of them were monocyclic compounds, of which eight were oxygenated (−7.52 to −6.98 kcal/mol) and seven were hydrocarbons (−7.60 to −6.99 kcal/mol). Three monoterpenes had formed hydrogen bonds: carvone with Thr48 (4.3 Å); carvacrol with His282 (4.6 Å); trans-dihydrocarvone with His27 (4.8 Å) and Thr48 (4.18 Å). Figure 5A,B show the predicted binding site of representative terpenes to DENV-2 E protein.
Figure 5.
Lowest-energy docked poses of sesquiterpenes and monoterpenes with DENV-2 proteins. Docked cis-calamenene (A) and carvone (B) in complex with E protein showing bonds with amino acids at the hinge region. (C). Docked α-phellandrene in complex with C protein showing bonds with amino acids at the α2-α2′ dimer interface hinge region. (D). Docked cis-calamenene in complex with C protein showing bonds with amino acids at the loop that connect α1 and α3 helices.
Based on our docking analysis, the potential monoterpenes and sesquiterpenes candidates that may inhibit DENV-2 E are listed in Table 4. The top five sesquiterpenes were cis-calamenene, δ-cadinene, α-cadinene, α-guaiene, and γ-cadinene, and the top five monoterpenes were α-phellandrene, carvacrol, carvone, γ-terpinene, and limonene.
Table 4.
Essential oil sesquiterpenes and monoterpenes with the lowest binding free energy for DENV-2 E protein.
| No | Compound | Kcal/mol | Structural Formula |
Amino Acid Residues. H-bond in Bold Font |
|---|---|---|---|---|
| 1 | cis-Calamenene | −8.73 |
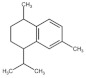
|
Thr189, Leu19, Phe193, Leu167, Phe279. |
| 2 | δ-Cadinene | −8.41 |
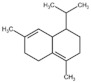
|
Leu45, Thr48, Leu135, Tyr137, Thr189, Leu191, Phe193, Phe279, Leu283. |
| 3 | α-Cadinene | −8.28 |
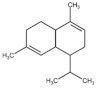
|
Leu45, Thr48, Leu135, Tyr137, Leu191, Phe193, Phe279, Leu283. |
| 4 | α-Guaiene | −8.26 |
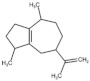
|
Glu26, Leu45, Thr48, Tyr137, Leu191, Phe193, Phe279, Leu283. |
| 5 | γ-Cadinene | −8.19 |
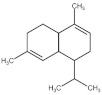
|
Leu45, Thr48, Leu135, Tyr137, Leu191, Phe193, Ohe279, Leu283. |
| 6 | Viridiflorene | −8.13 |
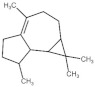
|
Thr48, Leu135, Tyr137, Thr189, Leu191, Phe193, Leu207, Phe279, Leu283. |
| 7 | α-Selinene | −7.98 |
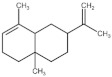
|
Thr48, Leu135, Thr189, Leu191, Phe193, Phe193, Phe279, Leu283. |
| 8 | δ-Amorphene | −7.96 |
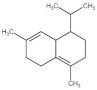
|
Leu45. Thr48, Leu135, Tyr137, Leu191, Phe193, Phe279, Leu283. |
| 9 | β-Bourbonene | −7.95 |
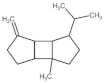
|
Thr48, Val130, Leu135, Tyr137, Leu191, Phe193, Leu207, Phe279, Leu283. |
| 10 | α-Gurjunene | −7.83 |
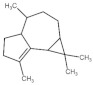
|
Thr48, Val130, Leu135, Tyr137, Thr189, Leu191, Phe193, Leu207, Phe279. |
| 11 | α-Phellandrene | −7.60 |
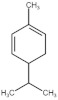
|
Thr48, Leu135, Tyr137, Thr189, Phe193, Phe279, Leu283 |
| 12 | Carvacrol | −7.31 |
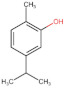
|
Thr189, Leu191, Leu207, Phe279, Leu283, His282 |
| 13 | Carvone | −7.29 |
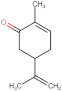
|
Thr48, Thr189, Leu191, Phe193, Leu207, Phe279. |
| 14 | γ-Terpinene | −7.28 |
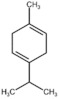
|
Thr48, Val130, Leu191, Leu207, Phe279, Leu283. |
| 15 | p-Cymene | −7.27 |
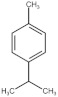
|
Thr48, Val130, Leu191, Leu207, Phe279, Leu283. |
| 16 | Limonene | −7.24 |
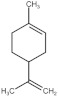
|
Thr48, Leu135, Phe193, Phe279, Leu283. |
| 17 | trans-Dihydrocarvone | −7.23 |
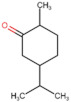
|
His27, Thr48, Leu191, Phe193, Leu207, Phe279. |
| 18 | Thymol-methyl-ether | −7.11 |
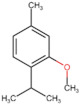
|
Leu191, Phe193, Leu207, Phe279. |
| 19 | α-Terpinene | −7.10 |
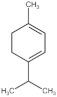
|
Thr48, Val130, Leu191, Phe279, Leu283. |
| 20 | Terpinolene | −7.03 |
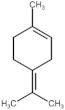
|
Thr48, Leu135, Thr189, Phe193, Leu207, Leu277, Phe279. |
The DENV C protein is organized into two pairs of antiparallel helical interfaces namely, α2–α2′ and α4–α4′, and amino acid residues of the α1 and α3 helices form a concave hydrophobic cleft [30]. The docking analyses predicted binding affinity between α-phellandrene (−7.60 kcal/moL) and cis-calamenene (−6.91 kcal/mol) with DENV-2 C. α-Phellandrene had formed hydrophobic interactions with amino acid residues (especially Ala49, Ala52, Phe53, Phe56, Phe84) of the α2-α2′ interface. Cis-Calamenene had formed hydrophobic interactions with amino acid residues (especially Leu44, Phe47, and Met48) of the loop that connects α1 and α3 helices (Figure 5C,D).
The DENV prM/M protein is believed to be a chaperon for the E protein to assure its conformational changes to produce infectious viral particles [30]. The docking analysis did not predict terpenes to dock the prM/M protein of DENV-2 (docking score: −5.55 to −4.12 kcal/mol).
3. Discussion
Among the medicinal plants growing in Colombia, we have selected six species used in both, folk medicine, and the food and pharmaceutical industries [19,20,21,22,23,24]. We have previously studied the chemical compositions of EOs distilled from L. alba and L. origanoides [33,34], and the chemical profile of the EO samples analyzed in this study resembled, with minor differences. Generally, L. alba and L. origanoides EOs are classified as monoterpene-rich EOs. The two EO samples of T. diffusa in this study showed differences in their chemical constituents from each other and from another sample from Colombia that we analyzed in a previous study [35]. Chemical variability among T. diffusa EOs due to geographic location, vegetative state of the plant, and storage time of the harvested is documented [21]. The chemical composition of the EOs of P. aduncum, O. basilicum, and V. curassavica from Colombia is not yet documented. Three piperitone-rich chemotypes of P. aduncum have been proposed [36,37], and the EO sample analyzed in this study could be classified as piperitone-rich oil. The EO of O. basilicum in this study could belong to both the linalool-rich and European chemotypes [38], characterized by high amounts of linalool. The EO of V. curassavica in this study is similar in chemical composition to EO samples from Brazil [39].
The results of this study showed variability in antiviral activity against DENV among EOs from the same and different plant species. The EOs of L. alba (citral and carvone chemotypes), L. origanoides (phellandrene chemotype), and T. diffusa showed strong antiviral activity against DENV-1 and DENV-2. These EOs reduced the CPE of both virus serotypes at IC50 < 100 µg/mL and SI > 5.0, which are parameters for EOs with antiviral efficacy in vitro [12,13,40]. In a previous study, L. alba EO also exhibited an antiviral effect against all four DENV serotypes in a plaque reduction assay [41]. We are unaware of reports on the antiviral activity of L. origanoides EO against DENV. We have previously documented the antiviral activity of this EO against the yellow fever virus [42], a pathogenic virus similar in structure to DENV. The EO of T. diffusa (2016) showed the highest antiviral activity (SI of 14.3), to our knowledge, there are no published studies demonstrating the antiviral activity of this EO against human viruses. O. basilicum EO showed weak anti-DENV activity; in vitro antiviral efficacy of this EO against pathogenic enveloped viruses has been documented [43,44,45]. P. aduncum EO showed weak anti-DENV activity; we have not found studies on the antiviral efficacy of this EO on enveloped viruses.
It has been well demonstrated that the chemistry of EO determines the bioactivity of EOs [9,10]. In this study, the combination of data from the antiviral CPE reduction assay and GC/MS analysis suggests that terpene content can influence the antiviral activity of the EOs against DENV. The anti-DENV activity of monoterpene-rich EOs increased in oils with high concentrations of monoterpenes alcohols, ethers and ketones, and sesquiterpene hydrocarbons. Conversely, the anti-DENV activity decreased in EOs with higher phenolic monoterpene concentrations and lower amounts of sesquiterpenes and monoterpene alcohols. The antiviral activity of sesquiterpene-rich EOs increased with the concentrations of oxygenated monoterpenes. As for T. diffusa EO samples, TdS2 showed strong anti-DENV activity, whereas TdS1 was found to be inactive. TdS2 but not TdS1 contains four oxygenated monoterpenes (terpinen-4-ol, α-terpineol, carvacrol, and thymol), one oxygenated sesquiterpene (zierone), and two sesquiterpene hydrocarbons (δ-cadinene and α-humulene), of which six compounds were predicted to bind to the E and C proteins of DENV. The different chemical compositions could partly explain the higher antiviral activity of TdS2 EO. V. curassavica EO was inactive, whereas O. basilicum EO showed weak antiviral activity; the former had a much lower content (1.7%) of oxygenated monoterpenes than the latter (69.4%).
The type of monoterpenes and sesquiterpenes has been recognized as a factor influencing the antiviral activity of EOs against pathogenic enveloped viruses [12,13]. The cluster analysis of this study suggests that the oxygenated monoterpenes, especially alcohols, ketones, ethers, and sesquiterpene hydrocarbons, might be associated with the antiviral activity of the EOs against DENV. The DENV envelope layer mainly comprises lipid bilayers, and it has been well demonstrated that alcohol compounds can favorably interact with enveloped viruses and block their infectivity [46]. The sesquiterpene hydrocarbon fraction, rather than the oxygenated fraction, accounted for the antiviral action against the Herpes Simplex-2 virus, an enveloped human pathogenic virus [47]. Furthermore, a mixture containing monoterpene alcohols and sesquiterpene hydrocarbons derived from Melaleuca alternifolia, showed promising in vivo antiviral efficacy against West Nile virus, an enveloped virus similar in structure to DENV [48].
The mechanisms of action of EOs against viruses have not yet been fully elucidated. Data from addition time experiments suggest that EOs mainly inhibit virus adsorption on the cell by acting on cell-free viruses directly [12,13,14]. The antiviral assay in this study evaluated the ability of the EOs to interfere with virus adsorption to the cell. The reduction of DENV CPE suggests that EOs may have exerted their antiviral action by destroying the viral envelope or its masking and, consequently, blocking the interaction of viral particles with the cell membrane. We have found that EOs of Lippia spp. showed better antiviral action before than after viral adsorption [41,42]. EOs also have intracellular modes of action by blocking the viral particle formation and releasing them from the host cell [12,13,14]. EO of L. alba is rich in trans-β-caryophyllene and this isolated sesquiterpene reduced the Zika virus replication, in an enveloped virus similar in structure to DENV, by treatment after virus adsorption to the cell [49]. We could hypothesize that inactive or weakly active EOs in this study, might exhibit strong anti-DENV activity in a cell-based assay that examines the inhibitory effect after virus adsorption to cells.
The in silico analysis of this study allows us to make observations about the contribution of terpene types to the antiviral action of the EOs against DENV. The analysis revealed a tendency of sesquiterpene hydrocarbons and oxygenated monoterpenes to bind to the E protein. The results support the hypothesis that nonpolar sesquiterpenes, rather than oxygenated sesquiterpenes, tend to interact more with viral proteins [13,50]. Sesquiterpenes and monoterpenes present in the EOs studied bound to the βOG pocket of the E protein, which is located at the region for the major conformational change during viral and cellular membrane fusion [29,51]. The βOG pocket has been established as a target for developing antivirals [51]. The efficacy of monoterpenes and sesquiterpenes to suppress DENV replication has not been systematically analyzed in cell-based assays. In a previous study [52], we demonstrated the intracellular antiviral action of β-caryophyllene, citral, p-cymene, and α-phellandrene to a greater extent than carvone, limonene, and nerol. Antiviral activities of numerous oxygenated monoterpenes and sesquiterpene hydrocarbons against many human pathogenic enveloped viruses have been well documented [11,12,13,16].
4. Materials and Methods
4.1. Plant Material and EO Distillation
All six plants used in this study were grown in the experimental plots at the Agroindustrial Pilot Complex of the National Center for Agroindustrialization of Aromatic and Medicinal Tropical Vegetal (CENIVAM). The taxonomic identification of these plants was performed at the Colombian National Herbarium, where their vouchers were placed. The plants were dried in the dark and leaves and stems were crushed and homogenized. The EOs were obtained by hydrodistillation (2 h) of plant material (80 kg) on a Clevenger apparatus as described elsewhere [30,32]. Fractional distillation of EOs under reduced pressure was carried out in a B/R Instruments (Easton, Easton, Maryland, USA) 800 High-Efficiency Micro Distillation device. The following auxiliary equipment was used: Edwards 8 vacuum pump (Edwards Vacuum, WS, Burgess Hill, UK), Alpha RA8 cooling bath (Lauda, Delran, NJ, USA), AREC-F magnetic stirring heating plate (VELP Científica, MB, Usmate Velate, Italy), and a magnetic stirring heating plate AREC-F (VELP Scientific, MB, Usmate Velate, Italy). EO (10–15 g) was deposited into the reboiler balloon of the micro-distiller, which was connected to the fractionation column. EO fractions were collected by opening the reflux valve each time the top temperature remained constant for 30 s. Reduced-pressure at 12 Torr (1.6 kPa) and 7 Torr (0.9 kPa) were used for L. alba citral and carvone chemotypes EO, respectively. As for L. alba carvone chemotype EO, the limonene-rich fraction (LaCaf1) was collected at 65–69 °C, and the carvone-rich fraction (LaCaf2) at 89–93 °C. For L. alba citral chemotype EO, the light fraction (LaCif) was collected at 115 °C. For the L. origanoides EO, the thymol-rich heavy fraction (LoTf) was collected at 10 Torr (1.3 kPa) and 65–69 °C. All EOs and their fractions were kept at 4 °C. For analysis of antiviral activity, stock solutions were prepared in dimethyl sulfoxide (DMSO, 0.5% final concentration) and stored at −20 °C until used.
4.2. Chromatographic Analysis
Essential oil analysis was performed by gas chromatography using mass spectrometric (GC/MS) and flame ionization detection (GC/FID) systems, as described elsewhere [33]. Before the GC analysis, each essential oil or its fraction was dried with sodium sulphate and weighed (50 mg), and then dissolved in dichloromethane (1 mL), with n-tetradecane (0.5 µL) as an internal standard. The injection volume was 2 µL in split mode (30:1) for GC/MS and GC/FID systems. For both systems, the injector temperature was maintained at 250 °C. A 6890 Plus Gas Chromatograph (Agilent Technologies, Palo Alto, CA, USA.) equipped with a mass selective detector MSD 5975 (Electron ionization, EI, 70 eV, AT, Palo Alto, CA, USA.), a 7863 automatic injector and an MSChemStation G1701DA data system (AT, Palo Alto, CA, USA.), were used. A fused-silica capillary column DB-5MS (J&W Scientific, Folsom, CA, USA.) of 60 m × 0.25 mm I.D., coated with 5%-phenyl poly (methyl siloxane) (0.25 μm-film thickness, df) was used. Chromatographic conditions were as follows: GC oven temperature started from 45 °C (5 min) to 150 °C (2 min) at 4 °C/min, then to 250 °C (5 min) at 5 °C/min, and finally, to 275 °C (15 min) at 10 °C/min. The ionization chamber, the quadrupole, and the transfer line temperatures were set at 250 °C, 150 °C, and 285 °C, respectively. Helium (99.99%, AP gas, Messer, Bogota, Colombia) was used as carrier gas, with the initial inlet pressure of 113.5 kPa (27 cm/s linear velocity). Its volumetric flow rate was kept constant (1 mL/min). n-Alkane (C8–C25) and (C8–C40) mixtures (Sigma-Aldrich, San Luis, MO, USA) were used to obtain linear retention indices (LRI). Mass spectra and reconstructed ion chromatograms were obtained by automatic scanning of quadrupole radiofrequency in the m/z 30–300 mass range at 3.58 scan/s. Chromatographic peaks were checked for homogeneity with the aid of the mass spectra for the characteristic fragment ions. Identification of EO compounds was accomplished by comparison of their LRIs, measured on both non-polar (DB-5MS, 60 m × 0.25 mm × 0.25 μm) and polar (DB-WAX, 60 m × 0.25 mm × 0.25 μm) columns (J & W Scientific, Folsom, CA, USA), with those of the available standard compounds and by comparison of their mass spectral fragmentation patterns with those described in the scientific literature and of databases (Wiley-2008, NIST-2017, QUADLIB-2007). For the quantitative analysis (GC relative amounts, %), assuming that all compounds had the same response factor (Rf = 1), the EO samples, prepared as described above, were injected into the GC 6890 Plus Gas Chromatograph (AT, Palo Alto, CA, USA), coupled to the FID and the non-polar 5%-Ph-PDMS capillary column (J&W Scientific, Folsom, CA, USA) of the same dimensions (L, I.D., df) as used for the GC/MS analysis. All chromatographic parameters for the GC/FID analysis (column, split ratio, injection volume, and temperatures) were the same as for the GC/MS experiments. The internal standard was used for result reproducibility checking (retention times and GC areas).
4.3. Virus and Cells
Dengue virus type 1 (DENV-1) strain US/Hawaii/1944 and Dengue virus type-2 (DENV-2) New Guinea C strain (NGC) were used. Viruses were propagated in C6/36 Aedes albopictus cells (Pedro Kourí Institute for Tropical Medicine, La Habana, Cuba) and harvested on day 7 post-infection. Virus stock was titrated in BHK-21 cells (ATCC® CCL-10™) using the plaque method and stored at −80 °C until needed [38,39]. Vero cells (ATCC® CCL-81™) were cultured in Eagle’s Minimum Essential Medium (EMEM) containing 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) at 37 °C in the presence of 5% CO2.
4.4. Cytotoxicity Assay
Cytotoxicity of EOs was evaluated in the crystal violet assay. Vero cells were seeded 96-well plates and allowed to adhere for 24 h at 37 °C and 5% CO2. Next, cells were treated with EO at seven-point concentrations (7.8 to 500 µg/mL) for 72 h at 37 °C. Not-treated cells and cells treated with dimethyl-sulfoxide were run in parallel as a negative and positive control, respectively. After washing with PBS, 100 µL of 0.05% crystal violet solution in 20% ethanol was added and cells were allowed to stain for 20 min at room temperature. After six washing with distilled water, the plates were aspirated and allowed to air-dry at room temperature, and 200 µL of methanol was added to each well for 20 min with shaking. The optical density at 570 nm at each well was measured on a microplate reader to quantify crystal violet staining. Each EO was analyzed in three independent assays in triplicate.
4.5. Cytopathic Effect (CPE)-Based Antiviral Assay
A standard protocol was followed [29]. Vero cells were seeded 96-well plates and allowed to adhere for 24 h at 37 °C and 5% CO2. After washing with PBS, DENV (MOI of 1.0) in a culture medium containing EO at non-cytotoxic concentrations (3.12, 6.25, 12.5, 25, 50, and 100 µg/mL) was adsorbed on cells 1 h at 37 °C; 5% CO2. Next, the cells were washed with PBS, fresh media without EO was added, and the virus was allowed to replicate for 5 days at 37 °C under a 5% CO2 atmosphere. The next steps followed the cytotoxicity assay mentioned above for quantification of crystal violet staining to measure virus-induced CPE indirectly. Untreated DENV-infected cells and SDS-treated DENV-infected cells served as the negative and positive control, respectively. CPE of DENV was estimated using the following formula: DENV CPE (%) = [(OD570 of DENV-infected EO-treated cells/OD570 of non-infected non-treated cells) × 100]. Reduction of CPE was estimated as: DENV CPE reduction (%) = [(OD570 of DENV-infected EO-treated cells − OD570 of negative control)/(OD570 of positive control − OD570 of negative control) × 100]. The 50% inhibition concentration (IC50) was calculated by regression analysis (GraphPad Software, San Diego, CA, USA). Each EO was analyzed in three independent assays in triplicate.
4.6. In Silico Analysis
Target proteins. The three-dimensional (3D) crystallographic structure of DENV-2 proteins was downloaded from the protein data bank (PDB ID 2FOM): E (ID: 1OAN), prM/M (ID: 3C5X), and C (ID: 1R6R). Three-dimensional protein structures were prepared using the PyMOL 2.3.0 software package (Schrodinger, Portland, OR, USA) by removing all the present water molecules and substructures. Energy minimization (1000 kJ/mol) was performed with the OPLS force field-AA/L implemented in the GROMACS 5.0 package (GROMACS development team, Groningen, NL) by using the steepest descent algorithm with a minimization step of 0.01 nm. Three-dimensional structures were prepared for molecular docking by adding hydrogen atoms, Kollman united atom type charges, and solvation parameters using AutoDock Tools [53]. Optimized structures were saved in PDB format and converted to PDBQT files with MGLTools (Center for computational structural biology, La Jolla, CA, USA).
Ligands preparation. All 125 EO compounds identified by GC/MS analysis were selected for docking analysis. Epigallocatechin gallate, with in silico and in vitro anti-DENV activities reported previously in the literature [54], was included as the positive control. Structures of ligands were retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov/ accessed on 30 August 2022) database. All ligands were prepared by adding hydrogen atoms and merging them with non-polar hydrogen atoms. Gasteiger partial charges were added, rotatable bonds were defined, and the energies were minimized using AutoDock Tools. Optimized structures were saved in MOL2 format and converted to PDBQT files with MGLTools.
Docking analysis. Molecular docking was performed using the Autodock Vina 1.5.6 software [55]. Default parameters were used, and the search exhaustiveness parameter was set to 100. For each ligand, 27 docked conformations were generated using global docking simulations, i.e., the grid box was defined to cover all protein structures to search for the best binding site in the protein. Three simulations were performed for each ligand-protein pair by using seeds 6, 12, and 18. The binding free energy was approximated by the average of docking scores for each protein. Discovery Studio Visualizer v21.1.0.20298 (Dassault Systèmes, San Diego, CA, USA) was used to view ligand-protein interaction. The docking protocol was validated using a set of molecules (n = 8) reported in the literature as DENV-2 inhibitors interacting with the E protein (i.e., 2,4-disubstituted pyrimidine 2k, 2,4-disubstituted pyrimidine 3a, 2,4-disubstituted pyrimidine 5c, castanospermine, celgosivir, NITD448, zosteric acid, and cinnamic acid). In addition, 3D structures were downloaded from the NCBI PubChem Database and were processed as mentioned.
4.7. Correlation and Statistical Analysis
To determine the relationship between chemical composition and antiviral activity, each EO was characterized under two independent parameters as follows; (i) chemical composition, the multivariate parameter with an absolute number (%) of different terpene types (Table 2); (ii) antiviral activity, DENV CPE as a unidimensional variable ranging from 0 to 100, low CPE values indicated strong antiviral activity. EOs with CPE ≤ 60 entered the analysis (n = 10). The samples were assigned into clusters according to their terpene compositions using an unsupervised self-organized Kohonen Map [56]. Each EO was represented by a vector with fifteen values represented by Equation (1) where C stands for its chemical composition formed by compounds cn. The algorithm was based on the distance d between vectors Ci and a set of artificial neurons Nj conforming to the network and was represented by vectors of the same size as the samples (Equation (2)). Using an iterative approach, the Nj values were updated to minimize the distance d between neurons and samples (Equation (3)). As a result, EOs with similar terpene types will fall close to the same representative neuron, defining a given cluster. In contrast, EOs with dissimilar compositions will fall close to different neurons, indicating their membership in different clusters.
| (1) |
| (2) |
| (3) |
A one-way ANOVA and a Tukey-Kramer post hoc test of CPE values were used to compare the antiviral potential of each resulting cluster, adopting a significance level of 0.0. Clustering and statistical analysis were performed using Matlab® R2021b (Mathworks Inc., Natick, MA, USA).
5. Conclusions
This study reports for the first time differences in the antiviral activity of the EOs, against DENV, corresponding to their composition of monoterpenes and sesquiterpenes. Fourteen EO samples of six plant species grown in Colombia were studied, which were different in the content of terpenes. As for monoterpene-rich EOs, samples that had monoterpene alcohols (LaCi, citral chemotype) or ketones (LaCaf2, carvone-rich fraction) had the greatest antiviral activity among L. alba EOs; and the sample rich in both sesquiterpene hydrocarbons and oxygenated monoterpene (LoP, phellandrene chemotype) had the highest antiviral activity among L. origanoides EOs. As for sesquiterpene-rich EOs, samples that had monoterpene alcohols showed antiviral activity (EOs of T, diffusa 2016, O. basilicum and P. aduncum), whereas samples that had no monoterpene alcohols and very low amounts of other oxygenated monoterpene were inactive (T. diffusa 2019 and V. curassavica EOs). Sesquiterpene hydrocarbons and oxygenated monoterpenes present in the EOs with antiviral activity showed binding affinities with the E protein of DENV-2, which suggests that these terpene types could act as inhibitors of virus adsorption and entry into the cell. The results of this study provide information on the antiviral activity of EOs and their terpenes on DENV replication in vitro and could be used in the research and development of herbal medicines for the prophylactic treatment of severe dengue. Sesquiterpene hydrocarbons and oxygenated monoterpenes may serve as a starting material for the development of antiviral phytomedicines. Well-controlled activity-guided fractionation, mode-of-action elucidation studies, and pre-clinical trials are encouraged to establish the use of EO-based herbal medicines for preventing severe dengue.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27206844/s1; Table S1: Chemical composition of essential oils studied in this work. Table S2: Docking energies (kcal/mol) of essential oil terpenes with dengue virus serotype 2 proteins. Figure S1: Representative results of the cytotoxicity assay.
Author Contributions
Conceptualization, R.E.O.; methodology, L.S.-T.; E.Q.-R.; P.R.-V.; software, P.R.-V.; S.C.-O.; data curation, S.C.-O.; E.E.S.; writing—original draft preparation, R.E.O.; L.S.-T.; writing—review and editing, E.E.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Ministry of Environment and Sustainable Development of Colombia supported the Universidad Industrial de Santander through the permit to genetic resources and derivatives access for bioprospecting (Contract No 270). The project RC-FP44842-212-2018 was approved by the Scientific Research Ethical Committee (Record No. 15-2017, File No. 4110) from Universidad Industrial de Santander. The experiments and the chemical management were done according to national law (Resolution No 008430-1993) from the Ministry of Health of Colombia and the Institutional Manual of Integrated Management and Processes (PGOR-PGGA.05).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not Available.
Funding Statement
The authors thank funding from the Ministry of Science, Technology and Innovation, the Ministry of Education, the Ministry of Industry, Commerce and Tourism, and ICETEX, Programme Ecosistema Científico-Colombia Científica, from the Francisco José de Caldas Fund; Grant RC-FP44842-212-2018.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zeng Z., Zhan J., Chen L., Chen H., Cheng S. Global, regional, and national dengue burden from 1990 to 2017: A systematic analysis based on the global burden of disease study 2017. EClinicalMedicine. 2021;32:100712. doi: 10.1016/j.eclinm.2020.100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalayanarooj S. Clinical manifestations and management of dengue/DHF/DSS. Trop. Med. Health. 2011;39((Suppl. S4)):83–87. doi: 10.2149/tmh.2011-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutierrez-Barbosa H., Medina-Moreno S., Zapata J., Chua J. Dengue infections in Colombia: Epidemiological trends of a hyperendemic country. Trop. Med. Infect. Dis. 2020;5:156. doi: 10.3390/tropicalmed5040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim S.P. Dengue drug discovery: Progress, challenges and outlook. Antivir. Res. 2019;163:156–178. doi: 10.1016/j.antiviral.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Obi J.O., Gutiérrez-Barbosa H., Chua J.V., Deredge D.J. Current trends and limitations in dengue antiviral research. Trop. Med. Infect. Dis. 2021;6:180. doi: 10.3390/tropicalmed6040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim S.Y.M., Chieng J.Y., Pan Y. Recent insights on anti-dengue virus (DENV) medicinal plants: Review on in vitro, in vivo and in silico discoveries. All Life. 2021;14:1–33. doi: 10.1080/26895293.2020.1856192. [DOI] [Google Scholar]
- 7.Dhiman M., Sharma L., Dadhich A., Dhawan P., Sharma M.M. Traditional knowledge to contemporary medication in the treatment of infectious disease dengue: A review. Front. Pharmacol. 2022;13:750494. doi: 10.3389/fphar.2022.750494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavda V.P., Kumar A., Banerjee R., Das N. Ayurvedic and other herbal remedies for dengue: An update. Clin. Complement. Med. Pharmacol. 2022;2:100024. doi: 10.1016/j.ccmp.2022.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manion C.R., Widder R.M. Essentials of essential oils. Am. J. Health-Syst. Pharm. 2017;74:e153–e162. doi: 10.2146/ajhp151043. [DOI] [PubMed] [Google Scholar]
- 10.Sharifi-Rad J., Sureda A., Tenore G., Daglia M., Sharifi-Rad M., Valussi M., Tundis R., Sharifi-Rad M., Loizzo M., Ademiluyi A., et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules. 2017;22:70. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wani A.R., Yadav K., Khursheed A., Rather M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021;152:104620. doi: 10.1016/j.micpath.2020.104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reichling J. Antiviral and virucidal properties of essential oils and isolated compounds—A scientific approach. Planta Med. 2021;88:587–603. doi: 10.1055/a-1382-2898. [DOI] [PubMed] [Google Scholar]
- 13.Ma L., Yao L. Antiviral effects of plant-derived essential oils and their components: An updated review. Molecules. 2020;25:2627. doi: 10.3390/molecules25112627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anastasiou C., Buchbauer G., Musterman M., Placeholder P. Essential oils as immunomodulators: Some examples. Open Chem. 2017;15:352–370. doi: 10.1515/chem-2017-0037. [DOI] [Google Scholar]
- 15.Sandner G., Heckmann M., Weghuber J. Immunomodulatory activities of selected essential oils. Biomolecules. 2020;10:1139. doi: 10.3390/biom10081139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valussi M., Antonelli M., Donelli D., Firenzuoli F. Appropriate use of essential oils and their components in the management of upper respiratory tract symptoms in patients with COVID-19. J. Herb. Med. 2021;28:100451. doi: 10.1016/j.hermed.2021.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ćavar Zeljković S., Schadich E., Džubák P., Hajdúch M., Tarkowski P. Antiviral activity of selected Lamiaceae essential oils and their monoterpenes against SARS-Cov-2. Front. Pharmacol. 2022;13:893634. doi: 10.3389/fphar.2022.893634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernal R., Gradstein S.R., Celis M., editors. Catálogo de Plantas y Líquenes de Colombia. Instituto de Ciencias Naturales, Universidad Nacional de Colombia; Bogotá, Colombia: 2019. [Google Scholar]
- 19.Mamun-Or-Rashid A.N.M., Sen M.K., Jamal M.A.H., Nasrin S. A Comprehensive Ethnopharmacological Review on Lippia alba M. Int. J. Biomed. Mater. Res. 2013;1:14–20. [Google Scholar]
- 20.Oliveira D.R., Leitão G.G., Fernandes P.D., Leitão S.G. Ethnopharmacological studies of Lippia origanoides. Rev. Bras. Farmacogn. 2014;24:206–214. doi: 10.1016/j.bjp.2014.03.001. [DOI] [Google Scholar]
- 21.Szewczyk K., Zidorn C. Ethnobotany, phytochemistry, and bioactivity of the genus Turnera (Passifloraceae) with a focus on damiana—Turnera diffusa. J. Ethnopharmacol. 2014;152:424–443. doi: 10.1016/j.jep.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Durant-Archibold A.A., Santana A.I., Gupta M.P. Ethnomedical uses and pharmacological activities of most prevalent species of genus Piper in Panama: A review. J. Ethnopharmacol. 2018;217:63–82. doi: 10.1016/j.jep.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Oza M.J., Kulkarni Y.A. Traditional uses, phytochemistry and pharmacology of the medicinal species of the genus Cordia (Boraginaceae) J. Pharm. Pharmacol. 2017;69:755–789. doi: 10.1111/jphp.12715. [DOI] [PubMed] [Google Scholar]
- 24.Sestili P., Ismail T., Calcabrini C., Guescini M., Catanzaro E., Turrini E., Layla A., Akhtar S., Fimognari C. The potential effects of Ocimum basilicum on health: A review of pharmacological and toxicological studies. Expert Opin. Drug Metab. Toxicol. 2018;14:679–692. doi: 10.1080/17425255.2018.1484450. [DOI] [PubMed] [Google Scholar]
- 25.Yuan Y., Huang M., Pang Y.X., Yu F.L., Chen C., Liu L.W., Chen Z.X., Zhang Y.B., Chen X.L., Hu X. Variations in essential oil yield, composition, and antioxidant activity of different plant organs from Blumea balsamifera (L.) DC. at different growth times. Molecules. 2016;21:1024. doi: 10.3390/molecules21081024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 27.Babushok V.I., Linstrom P.J., Zenkevich I.G. Retention Indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data. 2011;40:1–47. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
- 28.NIST Database. National Institute of Standards and Technology; Gaithersburg, MD, USA,: 2017. Version 2.3. [Google Scholar]
- 29.Smee D.F., Hurst B.L., Evans W.J., Clyde N., Wright S., Peterson C., Jung K.H., Day C.W. Evaluation of cell viability dyes in antiviral assays with RNA viruses that exhibit different cytopathogenic properties. J. Virol. Methods. 2017;246:51–57. doi: 10.1016/j.jviromet.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasar S., Rashid N., Iftikhar S. Dengue proteins with their role in pathogenesis, and strategies for developing an effective anti-dengue treatment: A review. J. Med. Virol. 2020;92:941–955. doi: 10.1002/jmv.25646. [DOI] [PubMed] [Google Scholar]
- 31.Shityakov S., Förster C. In silico predictive model to determine vector-mediated transport properties for the blood-brain barrier choline transporter. Adv. Appl. Bioinforma. Chem. 2014;7:23–36. doi: 10.2147/AABC.S63749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modis Y., Ogata S., Clements D., Harrison S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 33.Stashenko E.E., Martínez J.R., Cala M.P., Durán D.C., Caballero D. Chromatographic and mass spectrometric characterization of essential oils and extracts from Lippia (Verbenaceae) aromatic plants. J. Sep. Sci. 2012;36:192–202. doi: 10.1002/jssc.201200877. [DOI] [PubMed] [Google Scholar]
- 34.Vicuña G.C., Stashenko E.E., Fuentes J.L. Chemical composition of the Lippia origanoides essential oils and their antigenotoxicity against bleomycin-induced DNA damage. Fitoterapia. 2010;81:343–349. doi: 10.1016/j.fitote.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Bueno J., Escobar P., Martínez J.R., Leal S.M., Stashenko E.E. Composition of three essential oils, and their mammalian cell toxicity and antimycobacterial activity against drug resistant-tuberculosis and nontuberculous mycobacteria strains. Nat. Prod. Commun. 2011;6:1743–1748. doi: 10.1177/1934578X1100601143. [DOI] [PubMed] [Google Scholar]
- 36.Monzote L., Scull R., Cos P., Setzer W. Essential oil from Piper aduncum: Chemical analysis, antimicrobial assessment, and literature review. Medicines. 2017;4:49. doi: 10.3390/medicines4030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salehi B., Zakaria Z.A., Gyawali R., Ibrahim S.A., Rajkovic J., Shinwari Z.K., Khan T., Sharifi-Rad J., Ozleyen A., Turkdonmez E., et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules. 2019;24:1364. doi: 10.3390/molecules24071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurav T.P., Dholakia B.B., Giri A.P. A glance at the chemodiversity of Ocimum species: Trends, implications, and strategies for the quality and yield improvement of essential oil. Phytochem. Rev. 2021;21:879–913. doi: 10.1007/s11101-021-09767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marques A.P.S., Bonfim F.P.G., Dantas W.F.C., Puppi R.J., Marques M.O.M. Chemical composition of essential oil from Varronia curassavica Jacq. accessions in different seasons of the year. Ind. Crops Prod. 2019;140:111656. doi: 10.1016/j.indcrop.2019.111656. [DOI] [Google Scholar]
- 40.Grienke U., Mair C.E., Kirchmair J., Schmidtke M., Rollinger J.M. Discovery of bioactive natural products for the treatment of acute respiratory infections—An integrated approach. Planta Med. 2018;84:684–695. doi: 10.1055/a-0590-5153. [DOI] [PubMed] [Google Scholar]
- 41.Ocazionez R.E., Meneses R., Torres F.Á., Stashenko E. Virucidal activity of Colombian Lippia essential oils on dengue virus replication in vitro. Mem. Inst. Oswaldo Cruz. 2010;105:304–309. doi: 10.1590/S0074-02762010000300010. [DOI] [PubMed] [Google Scholar]
- 42.Meneses R., Ocazionez R.E., Martínez J.R., Stashenko E.E. Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann. Clin. Microbiol. Antimicrob. 2009;8:8. doi: 10.1186/1476-0711-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tshilanda D.D., Ngoyi E.M., Kabengele C.N., Matondo A., Bongo G.N., Inkoto C.L., Mbadiko C.M., Gbolo B.Z., Lengbiye E.M., Kilembe J.T., et al. Ocimum species as potential bioresources against COVID-19: A review of their phytochemistry and antiviral activity. Int. J. Pathog. Res. 2020;5:42–54. doi: 10.9734/ijpr/2020/v5i430143. [DOI] [Google Scholar]
- 44.Kubiça T.F., Alves S.H., Weiblen R., Lovato L.T. In vitro inhibition of the bovine viral diarrhoea virus by the essential oil of Ocimum basilicum (basil) and monoterpenes. Braz. J. Microbiol. 2014;45:209–214. doi: 10.1590/S1517-83822014005000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh P., Chakraborty P., He D.H., Mergia A. Extract prepared from the leaves of Ocimum basilicum inhibits the entry of Zika Virus. Acta Virol. 2019;63:316–321. doi: 10.4149/av_2019_307. [DOI] [PubMed] [Google Scholar]
- 46.Lin Q., Lim J.Y., Xue K., Yew P.Y., Owh C., Chee P.L., Loh X.J. Sanitizing agents for virus inactivation and disinfection. View. 2020;1:e16. doi: 10.1002/viw2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cagno V., Sgorbini B., Sanna C., Cagliero C., Ballero M., Civra A., Donalisio M., Bicchi C., Lembo D., Rubiolo P. In vitro anti-herpes simplex virus-2 activity of Salvia desoleana Atzei & V. Picci essential oil. PLoS ONE. 2017;12:e0172322. doi: 10.1371/journal.pone.0172322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pliego Zamora A., Edmonds J.H., Reynolds M.J., Khromykh A.A., Ralph S.J. The in vitro and in vivo antiviral properties of combined monoterpene alcohols against West Nile virus infection. Virology. 2016;495:18–32. doi: 10.1016/j.virol.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 49.Nogueira Sobrinho A.C., de Morais S.M., Marinho M.M., de Souza N.V., Lima D.M. Antiviral activity on the Zika virus and larvicidal activity on the Aedes spp. of Lippia alba essential oil and β-caryophyllene. Ind. Crops Prod. 2021;162:11381. doi: 10.1016/j.indcrop.2021.113281. [DOI] [Google Scholar]
- 50.Yarovaya O.I., Salakhutdinov N.F. Mono- and sesquiterpenes as a starting platform for the development of antiviral drugs. Russ. Chem. Rev. 2021;90:488–510. doi: 10.1070/RCR4969. [DOI] [Google Scholar]
- 51.Naresh P., Selvaraj A., Shyam Sundar P., Murugesan S., Sathianarayananan S., Namboori P.K.K., Jubie S. Targeting a conserved pocket (n-octyl-β-D–glucoside) on the dengue virus envelope protein by small bioactive molecule inhibitors. J. Biomol. Struct. Dyn. 2022;40:4866–4878. doi: 10.1080/07391102.2020.1862707. [DOI] [PubMed] [Google Scholar]
- 52.Flechas M.C., Ocazionez R.E., Stashenko E.E. Evaluation of in vitro antiviral activity of essential oil compounds against Dengue Virus. Pharmacogn. J. 2018;10:55–59. doi: 10.5530/pj.2018.1.11. [DOI] [Google Scholar]
- 53.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaihatsu K., Yamabe M., Ebara Y. Antiviral mechanism of action of epigallocatechin-3-O-gallate and its fatty acid esters. Molecules. 2018;23:2475. doi: 10.3390/molecules23102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trott O., Olson A. Autodock Vina: Improving the speed and accuracy of docking. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohonen T., Somervuo P. How to make large self-organizing maps for nonvectorial data. Neural Netw. 2002;15:945–952. doi: 10.1016/S0893-6080(02)00069-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.