Abstract
Cryptosporidium parvum is a protozoan parasite that causes diarrheal illness in a wide range of mammalian hosts, including humans. Characteristic serum immunoglobulin G (IgG) antibody responses to antigens in the 27- and 17-kDa size ranges have been shown to develop after infection, and several enzyme-linked immunosorbent assay (ELISA) and Western blot assay formats have been used to measure these IgG levels in human serum. Using a collection of serial samples from laboratory-confirmed cryptosporidiosis patients, we compared the results obtained by using two new ELISAs with those obtained with two different Western blot assays. When assayed with the large-format Western blot, 97% of the 67 patients had a demonstrable antibody response on at least one occasion. The Cp23 ELISA correctly identified 93% of the samples that had a 27-kDa response by Western blot and 100% of the negative samples. The Triton antigen ELISA detected 77% of the samples that had a 17-kDa response by Western blot and 88% of the negative samples. The sensitivity of the Triton antigen assay was higher for samples collected between 16 and 92 days after the onset of symptoms (96%). The minigel-format Western blot did not compare favorably with the large-format blot for the detection of antibodies to the 27-kDa antigen (71% sensitivity). A half-life of about 12 weeks was estimated for antibodies to both the 27- and 17-kDa antigens. We believe the Cp23 and Triton antigen ELISAs will be useful in epidemiologic studies of the prevalence of Cryptosporidium infection in the population.
Cryptosporidium parvum, a protozoan parasite that invades the intestinal epithelium of a wide range of mammalian hosts, causes a self-limiting but sometimes severe diarrheal illness in immunocompetent humans (2). However, in those with compromised immune systems, the disease can be debilitating, chronic, and life threatening (4, 23). Because of its ubiquitous distribution in the environment, its small size, and its resistance to standard chlorination techniques, C. parvum contamination of drinking water may pose a significant public health risk (1, 11, 12). Numerous outbreaks have been traced to contaminated water, and waterborne outbreaks have even occurred in communities served by state-of-the-art water treatment facilities (3, 8, 9, 15). To conduct epidemiologic studies to assess the risks of C. parvum infection that may be associated with drinking water and other potential sources of exposure, new serologic assays that are rapid, sensitive, and specific are needed.
Characteristic serum immunoglobulin G (IgG) antibody responses have been shown to develop in humans after C. parvum infection. As shown by Western blot analysis of serum samples collected from outbreak patients and human volunteers, the antibody response is consistently directed toward two low-molecular-weight antigen families: one in the 27-kDa size range and a second in the 17-kDa size range (16–19, 25, 26). We recently reported the development of two enzyme-linked immunosorbent assays (ELISAs) for the detection of antibodies to the 17- and 27-kDa antigens (25). The ELISA for antibodies to the 27-kDa antigen uses a recombinant form of the antigen based on the gene sequence reported by Perryman et al. (22), while the assay for antibodies to the 17-kDa antigen uses a Triton X-114 detergent extract of oocysts that is enriched for the native 17-kDa antigen. These assays were shown to be both sensitive and specific for the detection of antigen-specific antibodies compared with the “gold standard” Western blot assay (25). Unfortunately, in our preliminary analysis of the new ELISAs, relatively few serial samples from confirmed cryptosporidiosis patients were available for study.
In the late spring and summer of 1996, four major outbreaks of cryptosporidiosis occurred in the province of British Columbia in western Canada: 29 laboratory-confirmed cases were identified in the city of Cranbrook (population 18,131) in the East Kootenay region of southeastern British Columbia; 157 laboratory-confirmed cases were identified in the city of Kelowna (population 89,442) in the Central Okanagan region of central British Columbia; 86 laboratory-confirmed cases were identified in Kamloops (population 100,850); and 138 laboratory-confirmed cases were identified in Penticton (population 39,754), also in the Central Okanagan region (21). In three of these communities (Cranbrook, Kelowna, and Penticton), the outbreaks were associated with consumption of water from the municipal supply. From these outbreaks, 67 volunteers with laboratory-confirmed cryptosporidiosis were recruited and were asked to provide multiple serum specimens over the course of a 1.5-year study. In this work, we examine the IgG antibody responses to the 17- and 27-kDa C. parvum antigens that are found in these serial specimens, and we compare the results obtained with the new ELISAs to those obtained with two different Western blot assay formats.
MATERIALS AND METHODS
Serum specimens.
Serum specimens were collected from 67 adults with laboratory-confirmed cryptosporidiosis who were infected during waterborne outbreaks in British Columbia in the summer of 1996. All patients also met a case definition for cryptosporidiosis based on the occurrence of three or more loose or watery bowel movements within a 24-h period. Informed consent was obtained from volunteers by procedures reviewed and approved by the Clinical Screening Committee for Research Involving Human Subjects at the University of British Columbia. Single specimens were obtained from 26 patients, and sequential specimens (207 sera) were collected from 41 patients at approximately 3-month intervals. The elapsed time between the date of symptom onset and the date of serum collection was recorded for each of the samples collected from the 41 repeat donors and for two of the samples collected from those who donated only once. Five of the individuals who provided multiple serum samples donated their first sample within 15 days of symptom onset. All sera were aliquoted and stored at −20°C until tested. This study was carried out retrospectively on serum specimens that were coded without personal identifiers.
Crude antigen preparation and Western blots.
A preparation of crude antigen was made from oocysts of the Iowa strain of C. parvum by sonication and freeze-thawing as previously described (20). By using the discontinuous buffer system of Laemmli (13), proteins from this crude preparation were resolved under nonreducing conditions on either a large-format 10 to 22.5% gradient polyacrylamide gel (dimensions, 160 by 200 by 0.75 mm; 78 μg of antigen in a 130-mm-long preparative well) (25) or on a sodium dodecyl sulfate (SDS)-polyacrylamide (15%) minigel (dimensions, 73 by 102 by 0.75 mm; 4 μg of antigen in a 70-mm-long preparative well) (5, 7). The proteins were then electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon P; Millipore Corp., Bedford, Mass.).
The large-format Western blot assay was conducted in the Atlanta laboratory by the method of Priest et al. (25), while the minigel-format blot assay was conducted in the British Columbia laboratory by the method of Frost et al. (5, 7). Briefly, the membrane from the large-format gel was cut into approximately 60 2-mm-wide strips that were then incubated overnight at 4°C with 2 ml of a 1:100 dilution of serum in phosphate-buffered saline (PBS) (0.85% NaCl and 10 mM Na2PO4 at pH 7.2) containing 0.3% Tween 20. One strip on each tray was incubated with a known positive control serum, and one strip was incubated in buffer only. Bound IgG antibodies were visualized with a biotin-labeled mouse monoclonal anti-human IgG antibody (1:1,000 dilution in 0.3% Tween 20–PBS for 1 h at room temperature) (clone HP6017; Zymed Laboratories, South San Francisco, Calif.) and streptavidin-labeled alkaline phosphatase (1:1,000 dilution in 0.3% Tween 20–PBS for 1 h at room temperature) (Life Technologies, Rockville, Md.) as previously described (25). Nitro blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate were used as chromogens.
The membrane from the minigel was placed into a 20-channel Bio-Rad Mini Protean II Multiscreen apparatus (Bio-Rad, Hercules, Calif.), and the membrane contained in the channels was incubated overnight at 4°C with 400 μl of a 1:50 dilution of serum in Tween 20-PBS or with a reagent blank. Two of the channels on each minigel blot were incubated with a known positive control serum, and one channel was incubated with a negative control serum. Bound IgG antibodies were visualized with a biotin-labeled mouse monoclonal anti-human IgG antibody (clone HP6017) (1:500 dilution in 0.3% Tween 20–PBS for 30 min at room temperature) and streptavidin-labeled alkaline phosphatase (1:1,000 dilution in 0.3% Tween 20–PBS for 30 min at room temperature). Membranes were developed with the chromogen described above.
Both the large gel and minigel blots were read independently by members from the British Columbia and Atlanta laboratory groups, and a laboratory consensus was reached for each format for the presence or absence of antibodies to the 17- and 27-kDa antigens. Blots were only considered positive if the positions, patterns, and relative intensities of the bands in the 27- and 17-kDa regions corresponded with those found in the positive control. In cases in which a consensus could not be reached among the readers in a laboratory group, the assay was repeated. If a consensus still could not be reached, the blot result for that sample was considered negative for purposes of analysis. An overall consensus for the presence or absence of antibodies in each sample was determined from the British Columbia and Atlanta laboratory interpretations of the large-gel-format and minigel-format blots. For a serum sample to be considered positive for antibody to one of the antigens, at least two of the four laboratory consensus blot results for that antigen had to be positive.
ELISAs.
Serologic assays for IgG antibodies against the 27- and 17-kDa C. parvum antigens used either a recombinant form of the 27-kDa antigen (Cp23) or a partially purified native antigen fraction isolated from oocysts by Triton X-114 detergent extraction (Triton antigen), respectively (25). The Triton antigen fraction has been shown to be greatly enriched for the 17-kDa antigen. Both assays were performed in Atlanta and British Columbia as previously described (25). Briefly, antigens were diluted in 0.1 M NaHCO3 buffer at pH 9.6 to concentrations of 0.2 μg/ml (Cp23) and 0.28 μg/ml (Triton antigen) and were used to coat 96-well plates overnight at 4°C (50 μl/well; Immunlon 2; Dynatech Industries, McLean, Va.). Plates were washed with 0.3% Tween 20–PBS for 1 h at 4°C and then washed four times with 0.05% Tween 20–PBS. Sera from the British Columbia outbreak patients were diluted 1:50 in 0.05% Tween 20–PBS, loaded in duplicate (50 μl/well), and incubated for 2 h at room temperature. Serial samples collected from individual patients were assayed on the same plate. Three positive control sera, two negative control sera, and two buffer blanks were run in duplicate on each plate. A twofold serial dilution (1:50 to 1:12,800) of a strong positive control was also included on each plate to be able to generate a standard curve. The bound antibodies were quantitated with a biotinylated mouse monoclonal antibody against human IgG (1:1,000 in 0.05% Tween 20–PBS) (clone HP6017) and alkaline phosphatase-labeled streptavidin (1:500 in 0.05% Tween 20–PBS) with p-nitrophenylphosphate substrate (Sigma Chemical Co., St. Louis, Mo.) as previously described (25). A405s were measured with a Molecular Devices (Sunnyvale, Calif.) UVmax kinetic microplate reader.
Antibody levels of the unknown samples (from the mean of duplicate wells) were assigned a unit value based upon the nine-point positive control standard curve with a four-parameter curve fit and were expressed per microliter of serum. The 1:50 dilution of the positive control serum was arbitrarily assigned a value of 6,400 U. Samples that were found to have values above 6,400 arbitrary units (AU) were further diluted and reassayed until they fell within the range of the curve values. If the standard deviation was greater than or equal to 15% of the mean value for the duplicate wells, the assay for that sample was repeated (unless both values were considered negative). Cutoff values were determined by using a relative operating characteristic curve to maximize the sensitivity and specificity of the ELISAs compared with those of the Western blot consensus. A positive ELISA response for antibodies to the 27-kDa antigen was defined as one above 86 AU with the recombinant Cp23 protein, and a positive ELISA response for antibodies to the 17-kDa antigen was defined as one above 35 AU with the Triton-extracted antigen. When applied to an unrelated set of 220 serum samples, these cutoff values compared favorably with the Western blot results when the same standard curve was used (sensitivity and specificity of >87% for the Triton antigen assay and >89% for the Cp23 antigen assay) (J. W. Priest, B. W. Furness, and P. J. Lammie, unpublished observations).
All samples were assayed on three separate occasions with freshly made serum dilutions: twice in the Atlanta laboratory (2 months apart) and once in the British Columbia laboratory. During one of the assay runs in the Atlanta laboratory, the duplicate serum dilutions were placed in random locations on the ELISA plate to eliminate the possibility of bias as a result of the use of adjacent wells. Samples were considered positive for antibodies to the 17- and 27-kDa antigens if at least two out of three of the respective ELISA values were above the threshold.
Statistical analysis.
The sensitivities and specificities of the ELISAs were calculated from a relative operating characteristic curve by using the consensus Western blot result as the gold standard. Spearman's rank order correlation coefficients for the comparison of assay results were calculated with SigmaStat for Windows, version 2.03 (SPSS, Inc.).
Antibody half-lives were calculated by probit analysis of the cumulative percent decrease in antibody levels when the number of nonzero responses was sufficient to produce 95% fiduciary limits. When samples were limited to two nonzero decreasing antibody responses, the half-life was calculated by interpolating the number of days after onset at which response decreased by 50%. Antibody responses among the different ELISAs during specified intervals after exposure were compared by the Kruskal-Wallis test. Unless otherwise stated, all statistical procedures were performed with SAS version 8 statistical software (SAS Institute, Inc., Cary, N.C.).
RESULTS
Western blot analysis.
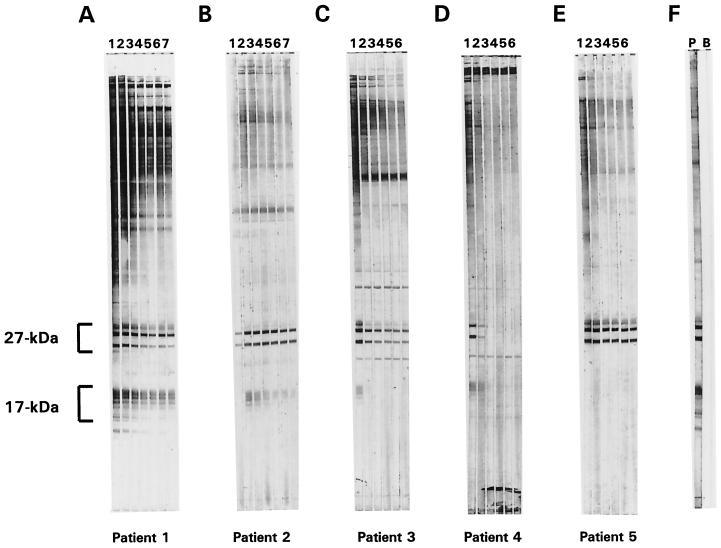
Sera from 67 laboratory-confirmed cryptosporidiosis patients were assayed for antibodies to the 27- and 17-kDa sporozoite surface antigens with the large-format Western blot, and a consensus interpretation of the blot results was reached by the Atlanta laboratory group. Of the 26 patients who donated only a single serum specimen, 24 (92%) were positive for antibodies against both antigens by large-format Western blot (data not shown). One of the two patients who lacked antibodies had a serum collection date less than 15 days after symptom onset and therefore may not have had sufficient time to develop an antibody response. The symptom onset date for the other negative patient was not known. Of the 41 patients who donated more than one serum specimen, all were positive for antibodies to one or both antigens at some point during the first 183 days of the study. As represented by the large-format Western blots shown in Fig. 1, the 41 patients who donated more than one serum specimen could be grouped into five general categories. Figure 1A (patient 1) is representative of the 21 patients (51% of those who donated more than one specimen) who were positive for antibodies to both the 27- and 17-kDa antigens at each time point. A gradual decline over time in the total antibody response was apparent from the intensities of the bands on the blots. Of the five patients (12%) who donated their first serum sample ≤15 days after symptom onset, all were initially positive for antibodies to the 27-kDa antigen, but negative for antibodies to the 17-kDa antigen, and all had a detectable peak antibody response to the 17-kDa antigen by the second time point (3 to 4 months later) (represented by patient 2 in Fig. 1B). Ten patients (24%) represented by patient 3 in Fig. 1C were initially positive for antibodies to both antigens, but later became negative for one of the antibodies. Three patients (7%) who had an antibody response to at least one of the antigens later became completely negative (represented by patient 4 in Fig. 1D). Finally, Fig. 1E (patient 5) is representative of two patients (5%) who were positive for antibodies to the 27-kDa antigen at every time point, but who did not have a detectable response to the 17-kDa antigen in any of the specimens tested (average number of tested specimens per patient, 6). No obvious changes were noted in the intensities of the blot responses to the 27-kDa antigen for these two patients. In summary, changes in the blot responses that were consistent with recent exposure to C. parvum were observed for 39 of the 41 patients (95%) who donated multiple serum samples.
FIG. 1.
Large-gel-format Western blots of sera collected at intervals from five cryptosporidiosis patients. Crude C. parvum antigens (600 ng of protein/mm of gel width) were resolved on an SDS-polyacrylamide (10 to 22.5%) gel under nonreducing conditions and then transferred to a PVDF membrane. Sera that had been collected from five laboratory-confirmed cryptosporidiosis patients (patients 1 to 5 represented in panels A to E, respectively) at intervals of approximately 3 months were diluted 1:100 with 0.3% Tween 20–PBS and incubated (2 ml per well) with 2-mm-wide strips of membrane overnight at 4°C. Seven serum samples were donated by patients 1 and 2 (Western blots shown in temporal order in lanes 1 to 7 of panels A and B, respectively), and six serum samples were donated by patients 3, 4, and 5 (Western blots shown in temporal order in lanes 1 to 6 of panels C, D, and E, respectively). The first sample in each panel (Western blots shown in lane 1) was collected within the first 3 months (A), 15 days (B), 3 months (C), and 1 month (D and E) after symptom onset. The blots were developed with a biotinylated mouse anti-human IgG monoclonal antibody and alkaline phosphatase-labeled streptavidin as described in Materials and Methods. The positions of the 27- and 17-kDa antigen families are indicated. Control strips that were incubated with a positive serum (P) and a buffer blank (B) are shown in panel F.
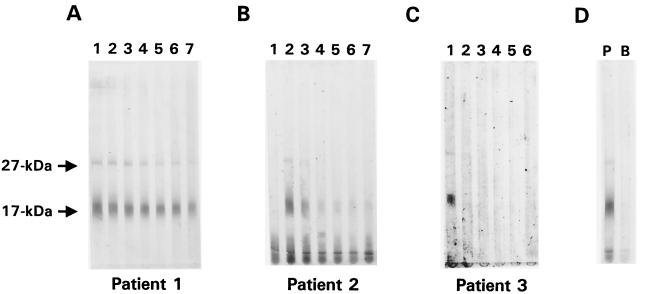
When the sera from the outbreak patients were assayed with the minigel Western blot format, we observed that some of samples previously determined to be positive for antibodies to the 27-kDa antigen when the large-format Western blot was used had responses that appeared to be negative or were very weak and difficult to interpret. The minigel blot results for patients 1 through 3, who were shown in Fig. 1 to have strong antibody responses to the 27-kDa antigen, are presented in Fig. 2. While the 27-kDa antigen responses in samples from patient 1 were clearly positive by minigel blot (Fig. 2A), samples 1 and 7 from patient 2 (Fig. 2B) and samples 2 to 6 from patient 3 (Fig. 2C) were interpreted as negative by both laboratories. This apparent lack of sensitivity was not limited to these two patients: 22 patients had at least one sample in which this discrepancy was observed. Overall, of the 209 samples that were interpreted by both laboratories as positive for antibodies to the 27-kDa antigen by using the large gel blot, 61 were considered negative by both laboratories with the minigel blot. This suggests a sensitivity of the minigel-format blot of only 71% for the detection of antibodies to the 27-kDa antigen compared with that of the large-gel-blot format. In contrast, the minigel blot results for antibodies to the 17-kDa antigen for the three patients shown in Fig. 2 and for most of the other patients were in good agreement with large-gel-blot results. Of the 196 samples that were considered positive for antibodies to the 17-kDa antigen by both laboratories using one blot format, only 11 (6%) were considered negative by both laboratories with the other blot format.
FIG. 2.
Minigel Western blot results for sera collected at intervals from cryptosporidiosis patients 1, 2, and 3. Crude C. parvum antigens (57 ng of protein/mm of gel width) were resolved under nonreducing conditions on an SDS-polyacrylamide (15%) minigel and transferred to a PVDF membrane. Sera from patients 1 (A), 2 (B), and 3 (C) were diluted 1:50 with 0.3% Tween 20–PBS and incubated (400 μl per channel) with separate regions of the membrane overnight at 4°C. Blots were developed as described in Materials and Methods. The positions of the 27- and 17-kDa antigens are indicated. Panel C was scanned and digitally enhanced to improve the resolution of the 27-kDa band in lane 1. Control strips that were incubated with a positive serum (P) and a buffer blank (B) are shown in panel D.
As described in Materials and Methods, an overall blot consensus was reached based upon the two laboratories' interpretations of the responses on the large and minigel format blots. In general, there was good agreement between the two laboratories in the interpretation of the blot results. Eighty-seven percent of the large-format Western blots were assigned the same response for the 17-kDa antigen by both laboratories, and 95% of the blots were assigned the same response by both laboratories for the 27-kDa antigen. Similarly, the two laboratories were in agreement for 96% of the 17-kDa antigen responses on the minigel blots and for 90% of the 27-kDa antigen responses. Table 1 summarizes the overall consensus blot results for the antibody status of the serum samples. Overall, 210 of 233 samples (90%) were positive for antibodies to the 27-kDa antigen (24 from donors who provided only one specimen and 186 from donors who provided sequential specimens), and 175 samples (75%) were positive for antibodies to the 17-kDa antigen (24 from single specimens and 151 from sequential specimens). Of the 41 patients who donated more than one serum specimen, 38 (93%) were positive by consensus for antibodies to the 17-kDa antigen, and 40 (98%) were positive by consensus for antibodies to the 27-kDa antigen (Table 1). A total of 37 patients (90%) were simultaneously positive for antibodies to both antigens on at least one occasion, and 11 of these became negative for antibodies to either the 17-kDa antigen (n = 8), the 27-kDa antigen (n = 1), or both antigens (n = 2) during the course of the study. The consensus blot responses were used as the gold standard for comparisons with the ELISA results presented below.
TABLE 1.
Agreement between consensus ELISA and Western blot results for single and sequential specimens
| Specimen type | No. (%) of positive patientsa | No. of blot-positive samplesb | No. (%)c of ELISA samples
|
No. of blot-negative samplesb | No. (%)d of ELISA samples
|
||
|---|---|---|---|---|---|---|---|
| True positive | False negative | True negative | False positive | ||||
| Single (n = 26) | |||||||
| 17-kDa antigen | 24 (92) | 24 | 23e (96) | 1e | 2 | 2e (100) | 0e |
| 27-kDa antigen | 24 (92) | 24 | 24f (100) | 0f | 2 | 2f (100) | 0f |
| Sequential (n = 207; 41 patients) | |||||||
| 17-kDa antigen | 38 (93) | 151 | 111e (74) | 40e | 56 | 49e (87) | 7e |
| 27-kDa antigen | 40 (98) | 186 | 172f (92) | 14f | 21 | 21f (100) | 0f |
Number of patients positive for the given antigen by consensus Western blot result, with the percentage of positive patients in parentheses.
Consensus of large- and minigel-format Western blot responses.
Percentage of Western blot-positive samples correctly identified by consensus ELISA result.
Percentage of Western blot-negative samples correctly identified by consensus ELISA result.
Triton antigen ELISA detection of 17-kDa antigen.
Cp23 ELISA detection of 27-kDa antigen.
ELISA results.
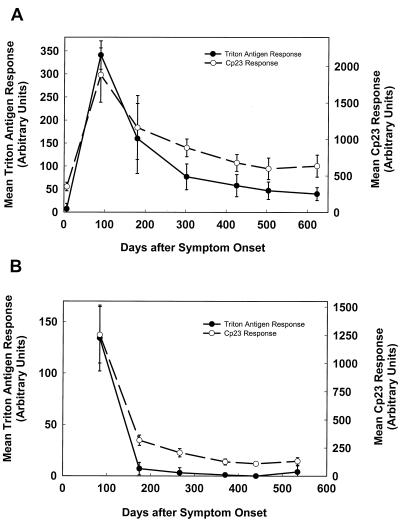
To assess the inter- and intralaboratory variability in the assays, the levels of antibodies to the 27- and 17-kDa antigens were analyzed independently by ELISA once in the British Columbia laboratory and twice in the Atlanta laboratory. In one of the sets of assays conducted in the Atlanta laboratory, the samples were placed in random wells on the ELISA plate so as eliminate the possibility of positional bias. The mean ELISA responses for the patients whose blot responses were shown previously in Fig. 1B and C are shown in Fig. 3A and B, respectively. As expected from the positive 27-kDa antigen blot responses in Fig. 1B, the mean ELISA responses for the Cp23 assay (Fig. 3A, dashed line) were consistently above the 86-AU cutoff value, and except in the first sample (which was negative for antibodies to the 17-kDa antigen by blot), the mean responses for the Triton antigen assay (solid line) were above the 35-AU cutoff value. Similarly, the mean ELISA responses for patient 3 (blot responses shown in Fig. 1C) were consistently positive for the Cp23 assay (Fig. 3B, dashed line), and the response was positive for the initial sample by Triton antigen assay (solid line), in good agreement with the blot results. The results of the three sets of assays for all of the patient samples were also in good agreement with each other: the intralaboratory correlation coefficients for the Cp23 and Triton antigen ELISAs calculated for all 233 samples were 0.978 and 0.913, respectively, and the interlaboratory correlation coefficients were >0.969 and >0.924, respectively. All three assays gave the same result (positive or negative) for 94% of the Cp23 assays and 88% of the Triton antigen assays.
FIG. 3.
Mean Triton antigen and Cp23 ELISA responses for sera collected at intervals from cryptosporidiosis patients 2 and 3. Sera from cryptosporidiosis patients 2 (A) and 3 (B) were assayed independently in Atlanta (twice) and in British Columbia (once) with the Triton antigen and Cp23 ELISAs described in Materials and Methods. The optical densities were converted into an AU value based upon a 9-point standard curve that was included on each ELISA plate. The means of the ELISA values for each time point after symptom onset are plotted for both the Triton antigen ELISAs (solid line) and the Cp23 ELISAs (dashed line). Each bar indicates one standard deviation.
As summarized in Table 1, the agreement between the consensus Cp23 ELISA results and the consensus Western blot results was excellent for both the single and sequential specimens. Compared with the blot, the Cp23 ELISA was 100% sensitive and specific for single specimens and was 92% sensitive and 100% specific for sequential specimens. Similarly, the Triton antigen ELISA response for the single specimens correlated well with the 17-kDa antigen Western blot response (sensitivity of 96% and specificity of 100%). However, the Triton antigen ELISA response for the sequential specimens correctly identified only 74% of the Western blot-positive samples and 87% of the negative samples—somewhat below the 90% sensitivity and 94% specificity we described for this assay in an earlier work (25). The difference may be related to the use of sequential specimens in the current study. The sensitivity of the Triton antigen ELISA was higher for samples collected between 16 and 92 days after symptom onset (96%) than for samples collected in any subsequent 3-month interval (range, 81 to 59%) (data not shown). In addition, many of the false-negative specimens (14 of 40; 35%) were donated by the 10 patients who, by overall blot consensus, converted from positive to negative by Western blot for antibodies to the 17-kDa antigen, and they were followed in sequence by a true blot-negative sample. Thus the low sensitivity may be attributed to a response that rapidly approached the limit of detection for the assay. The low specificity (87%) may be attributed to the fact that six of the seven false-positive serum specimens were donated by a single patient.
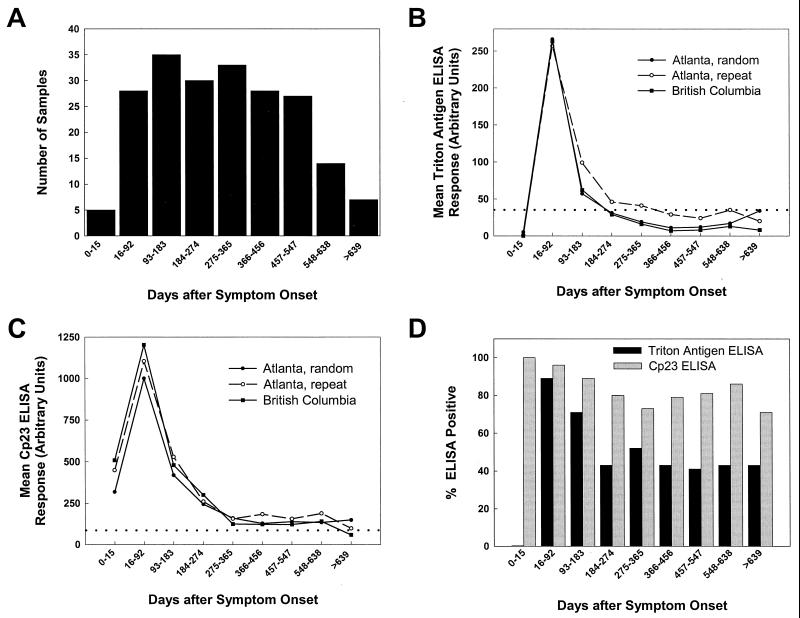
As can be seen in the graphs in Fig. 3, the antibody responses to the Cp23 and Triton antigens observed in patients 2 and 3 tended to rise and decay in parallel, and much of the response was lost within the first year after symptom onset. The same downward trends that were observed in the individual patient responses were also apparent when the responses from the 41 patients who donated multiple samples were plotted versus the time after symptom onset. Figure 4A shows the number of samples collected in each 3-month interval after symptom onset. The five samples that were collected within 15 days of symptom onset were grouped separately because they were collected before these patients had developed an antibody response to the 17-kDa antigen. As shown in Fig. 4B and C, when the geometric means for the Triton antigen and Cp23 ELISA responses were calculated for each time interval after symptom onset, a steady decline after the 16- to 92-day interval was shown. Figure 4B and C also give a visual indication of the low levels of intra- and interlaboratory variation that were observed among the three independent sets of assays. Although the repeat set of assays performed in Atlanta yielded a higher geometric mean value for six of nine time intervals for the Triton antigen assay (open circles, Fig. 4B), a statistical comparison by time interval of the two sets of responses from Atlanta and the set from British Columbia did not reveal a significant difference. The P values for the Triton antigen ELISA response comparisons were greater than 0.09 for each of the sample collection intervals (Kruskal-Wallis test with chi-square approximation), and the P values for the Cp23 ELISA response comparisons were greater than 0.53 for each of the sample collection intervals.
FIG. 4.
ELISA analysis of sera from cryptosporidiosis patients who donated sera on more than one occasion. Panel A shows the total number of serum samples collected during each time interval after symptom onset from 41 confirmed cryptosporidiosis patients who donated more than one specimen. Triton antigen (B) and Cp23 (C) ELISAs were performed with these serum samples on two occasions in Atlanta to assess the intralaboratory variation (Atlanta, random and Atlanta, repeat) and on one occasion in British Columbia to assess the interlaboratory variation (British Columbia). For the assays represented by the “Atlanta, random” results, the duplicate serum samples were placed in random locations on the ELISA plate to eliminate the possibility of positional bias. The geometric means (log scale) of the ELISA responses are plotted for each interval of sample collection, and the threshold for positivity is indicated in each graph by a dotted line. Panel D shows the fraction of the samples from each time interval positive for antibodies in the Triton antigen (black bars) and Cp23 (grey bars) ELISAs. A serum sample was considered ELISA positive if at least two of the responses from the three independent assays were above 35 AU for the Triton antigen assay and above 86 AU for the Cp23 assay.
The prevalence of the samples that were positive by Triton antigen ELISA and Cp23 ELISA versus the time interval of sample collection is shown in Fig. 4D. The percentage of the patients who were positive by the Triton antigen ELISA demonstrated a downward trend consistent with the conversion of some patients from positive to negative for antibodies to the 17-kDa antigen by Western blot, while the percentage of patients who were positive by the Cp23 ELISA was relatively constant, as expected from the Western blot results for the 27-kDa antigen described earlier.
Antibody half-life determination.
A sufficient number of nonzero Cp23 ELISA responses were available from 25 patients to allow the calculation of the antibody half-lives by probit analysis. A mean half-life of 87 days (median, 72 days; range, 50 to 181 days) was obtained for Cp23 reactivity. From the responses of 15 patients, a mean half-life of 84 days (median, 69 days; range, 47 to 215 days) was estimated for the Triton antigen response. The half-lives for the two antibody responses were not significantly different (P = 0.514). For those patients' responses that could not be analyzed by the probit procedure, a half-life estimate was calculated assuming a linear rate of decay. These estimates (mean of 83 days for the Cp23 assay with 10 patients; mean of 68 days for the Triton antigen assay with 17 patients) did not differ significantly from those described above.
DISCUSSION
Several different assay formats have been used to assess the levels of human serum IgG antibodies to C. parvum antigens: an ELISA that uses a crude antigen preparation (29), a large-gel-format Western blot that requires the separation of parasite antigens on a gradient SDS-polyacrylamide gel (6, 17–19, 25), a minigel-format Western blot (5, 7, 10), and ELISAs that use purified recombinant or native antigens (25). A number of laboratories have noted that the results of the large-gel-format Western blot and those of the crude antigen ELISA were not well correlated (6,18, 19, 25, 28; F. J. Frost and G. F. Craun, Letter, Infect. Immun. 66:4008–4009, 1998). In general, the large-gel-format Western blot appeared to be more sensitive than the crude antigen ELISA for the detection of IgG antibodies: more than half of the crude antigen ELISA-negative individuals in the study of Frost et al. (5; Frost and Craun, Letter) were found to be antibody positive by the large-gel-format Western blot. Unfortunately, because of its complexity and expense, the large-gel-format Western blot is not suitable for use in large-scale epidemiologic studies of the prevalence of C. parvum antibodies in the general population. To accomplish this type of work in a more reagent- and cost-efficient manner, several groups have turned to the minigel-format Western blot (5, 7,10). The assay as described by Frost uses 10-fold-less antigen per millimeter of PVDF membrane than the large-format blot assay originally developed in our laboratory (17, 18, 25). While the minigel assay compared favorably in our hands with the large-gel-format blot for the detection of antibodies to the 17-kDa antigen, it failed to detect 29% of the samples that were positive for antibodies to the 27-kDa antigen. Recent studies have suggested that roughly one-third to one-half of those individuals who are positive for antibodies by the large-gel Western blot assay only have an antibody response to the 27-kDa antigen (6, 25). Thus the minigel-format blot (at least in the format currently used) may significantly underestimate the proportion of the population with prior Cryptosporidium parvum exposure. No data yet exist on whether the minigel assay would perform better if more antigen was used.
Using a large collection of serial samples from laboratory-confirmed cryptosporidiosis patients, we have demonstrated that the recombinant Cp23 and Triton antigen ELISAs can be used to monitor changes in the antibody responses to two specific C. parvum surface antigens. The high sensitivities and specificities of the Cp23 and Triton antigen ELISAs in the analysis of the single specimens (>96%) and those of the Cp23 ELISA in the analysis of the sequential specimens (>93%) are similar to what we have previously reported (25), but the Triton antigen results are somewhat lower for the sequential specimens than previously reported (74 and 87% versus 90 and 94%, respectively). The sensitivity of the Triton antigen assay was higher for samples collected between 16 and 92 days of symptom onset (96%) than in subsequent samples, and many of the false-negative samples were donated by patients who eventually converted to negative for antibodies to the 17-kDa antigen by Western blot. We think the lower sensitivity is simply an artifact caused by the repeat sampling of patients who have borderline-positive Western blot responses in samples collected many months after the clearance of the infection. Similarly, the lower specificity of the assay can be attributed to one patient who donated six of seven false-positive specimens. Interestingly, this patient had a very high level of antibodies to the 27-kDa antigen, and a small amount of 27-kDa antigen is normally found in the Triton extract used in the assay (the amount of 27-kDa antigen is 10- to 20-fold lower than that of the 17-kDa antigen) (25). When these sera were checked by Western blotting with Triton-extracted antigen, only the 27-kDa antigen was apparent (data not shown). No other bands were visible. Thus, it is possible that the Triton antigen ELISA is not absolutely specific for antibodies to the 17-kDa antigen when high concentrations of antibodies to the 27-kDa antigen are present. In practice, however, very few patients have been found with a high 27-kDa-antigen response and no 17-kDa-antigen response.
We noted that the antibody response to both antigens increased and decreased in parallel in a time frame consistent with C. parvum infection near the time of symptom onset. The peaks of the responses most likely occurred between 16 and 92 days of symptom onset, but, because of the 3-month interval between sample collections, they could not be further pinpointed. In a recent study in which human volunteers were fed C. parvum oocysts, the peak of the response most likely occurred between 11 and 32 days after ingestion of oocysts (19). We conclude that it is highly unlikely that the antibody responses observed in our study could have resulted from cross-reaction with antibodies from a concurrent or previous infection with another organism. This conclusion is further supported by the observation that most of the antibody response to these two antigens is directed against the protein component of the antigen rather than against a carbohydrate component (24, 25; J. W. Priest and P. J. Lammie, unpublished observations). Robbins et al. (27) have suggested that serum antibodies to the carbohydrate epitopes of surface antigens, especially those whose acquisition is age-related, may result from exposure to cross-reacting species found on normal enteric and respiratory flora, while serum antibodies that recognize surface protein epitopes are most often elicited by infection with the specific pathogen. We believe that the Cp23 and Triton antigen ELISAs are specific for C. parvum infection in humans, and despite our efforts and those of other laboratories, no evidence of cross-reaction has been found to implicate other human parasites, including Toxoplasma gondii, Giardia lamblia, and Isospora sp. (14, 29; Priest and Lammie, unpublished).
Our results indicate that antibodies against both the 17- and 27-kDa antigens are cleared at about the same rate, with half-lives of approximately 12 weeks each, and that both antibody responses reach a fairly constant level about 1 year after the infection. This working estimate will be important for future studies of the prevalence of cryptosporidiosis in the population. To our knowledge, only one other group has attempted to establish an estimate of the duration of the human serum antibody response following Cryptosporidium infection. In a recent work, Frost et al. (5) collected paired serum samples from cryptosporidiosis patients from a waterborne outbreak in Jackson County, Oreg., at 6 months and then again at 2.5 years after the end of the outbreak and analyzed these samples by using the large-gel- and minigel-format Western blot assays. They observed that the antibody response to the 27-kDa antigen declined by 46% (as measured from the blots by densitometry) over this 2-year period, while the response to the 17-kDa antigens declined by only 9%. Their conclusions that the response to the 17-kDa antigen may have declined to baseline levels before the beginning of the serum collection and that the antibody response to the 27-kDa antigen may remain high for an extended period of time appear to be supported by our results. Indeed, had sera been collected from the patients in our study 6 months after symptom onset (184 to 274 days) and again 1 year later (548 to 638 days) (Fig. 4), a pattern similar to that reported by Frost et al. would likely have been observed, since the largest fluctuations in the antibody responses appear to occur within the first 6 months after symptom onset.
Our work supports the suggestion that the postinfection level of the antibody response is higher (relative to the threshold of antibody detection) for the 27-kDa antigen than for the 17-kDa antigen: eight patients with a persistent 27-kDa antigen response became negative by overall blot consensus for antibodies to the 17-kDa antigen, while only one patient with a persistent 17-kDa antigen response became negative for antibodies to the 27-kDa antigen over the course of the study. The geometric means of the responses to the 27-kDa antigen in the various time intervals of our study were consistently above the cutoff threshold, but this was not the case for the responses to the 17-kDa antigen (Fig. 4). We also observed that the five patients who provided a serum sample <15 days after symptom onset either had a very early 27-kDa antigen response or had a preexisting 27-kDa antigen response from a previous infection, since no response to the 17-kDa antigen was apparent until the following time point. In our studies of sera collected from individuals with no known previous exposure to Cryptosporidium, we have, in fact, observed that a significant proportion of the population has an antibody response only to the 27-kDa antigen (25; Priest and Lammie, unpublished). We do not yet understand why the response to the 27-kDa antigen should persist, but it may in some way be related to repeated exposure to the parasite.
In conclusion, we have demonstrated that the Triton antigen and Cp23 ELISAs are useful for monitoring the changes in antibody responses associated with infection with C. parvum and that this technology can be transferred to other laboratories. Given that antibodies to the 27-kDa antigen may persist above the limit of detection, we believe that the Cp23 ELISA may be better suited to the assessment of historic exposure to Cryptosporidium, while the Triton antigen ELISA may be a better choice for the detection of recent infections. For both assays, a high antibody response is likely to be indicative of a recent infection.
ACKNOWLEDGMENTS
We thank Delynn Moss for help with the preparation of the antigen used in this work and James Kwon for assistance in the interpretation of blot strips.
The University of British Columbia gratefully acknowledges that the American Water Works Association Research Foundation is the joint owner of some of the technical information upon which this is work is based. The University of British Columbia thanks the foundation for its financial, technical, and administrative assistance in funding and managing the project through which this work was discovered.
REFERENCES
- 1.Campbell I, Tzipori A S, Hutchinson G, Angus K W. Effect of disinfectants on survival of Cryptosporidium oocysts. Vet Rec. 1982;111:414–415. doi: 10.1136/vr.111.18.414. [DOI] [PubMed] [Google Scholar]
- 2.DuPont H L, Chappel C L, Sterling C R, Okhuysen P C, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 3.Dworkin M S, Goldman D P, Wells T G, Kobayashi J M, Herwaldt B L. Cryptosporidiosis in Washington State: an outbreak associated with well water. J Infect Dis. 1996;174:1372–1376. doi: 10.1093/infdis/174.6.1372. [DOI] [PubMed] [Google Scholar]
- 4.Flanigan T, Whalen C, Turneer J, Soave R, Toerner J, Havlir D, Kotler D. Cryptosporidium infection and CD4 counts. Ann Intern Med. 1992;116:840–842. doi: 10.7326/0003-4819-116-10-840. [DOI] [PubMed] [Google Scholar]
- 5.Frost F J, Calderon R L, Muller T B, Curry M, Rodman J S, Moss D M, De La Cruz A A. A two-year follow-up survey of antibody to Cryptosporidium in Jackson County, Oregon, following an outbreak of waterborne disease. Epidemiol Infect. 1998;121:213–217. doi: 10.1017/s095026889800898x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frost F J, De La Cruz A A, Moss D M, Curry M, Calderon R L. Comparisons of ELISA and Western blot assays for detection of Cryptosporidium antibody. Epidemiol Infect. 1998;121:205–211. doi: 10.1017/s0950268898008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost F, Muller T. Two city Cryptosporidium study. Denver, Colo: American Water Works Research Foundation and American Water Works Association; 1999. [Google Scholar]
- 8.Goldstein S T, Juranek D D, Ravenholt O, Hightower A W, Martin D G, Mesnik J L, Griffiths S D, Bryant A J, Reich R R, Herwaldt B L. Cryptosporidiosis: an outbreak associated with drinking water despite state-of-the-art water treatment. Ann Intern Med. 1996;125:459–468. doi: 10.7326/0003-4819-124-5-199603010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hayes E B, Matte T D, O'Brien T R, McKinley T W, Logsdon G S, Rose J B, Ungar B L P, Word D W, Pinsky P F, Cummings M L, Wilson M A, Long E G, Hurwitz E S, Juranek D D. Large community outbreak of cryptosporidiosis due to contamination of a filtered public water supply. N Engl J Med. 1989;320:1372–1376. doi: 10.1056/NEJM198905253202103. [DOI] [PubMed] [Google Scholar]
- 10.Isaac-Renton J L, Blaterwick J, Bowie W R, Fyfe M, Khan M, Li A, King A, McLean M, Medd L, Morehead W, Ong C S, Robertson W. Epidemic and endemic seroprevalence of antibodies to Cryptosporidium and Giardia in residents of three communities with different drinking water supplies. Am J Trop Med Hyg. 1999;60:578–583. doi: 10.4269/ajtmh.1999.60.578. [DOI] [PubMed] [Google Scholar]
- 11.Juranek D D. Cryptosporidiosis: sources of infection and guidelines for prevention. Clin Infect Dis. 1995;21(Suppl.):S57–S61. doi: 10.1093/clinids/21.supplement_1.s57. [DOI] [PubMed] [Google Scholar]
- 12.Korich D G, Mead J R, Madore M S, Sinclair M A, Sterling C R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl Environ Microbiol. 1990;56:1423–1428. doi: 10.1128/aem.56.5.1423-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lappin M R, Ungar B, Brown-Hahn B, Cooper C M, Spilker M, Thrall M, Hill S L, Cheney J, Taton-Allen G. Enzyme-linked immunosorbent assay for the detection of Cryptosporidium parvum IgG in the serum of cats. J Parasitol. 1997;83:957–960. [PubMed] [Google Scholar]
- 15.MacKenzie W R, Hoxie N J, Proctor M E, Gradus M S, Blair K A, Peterson D E, Kazmierczak J J, Addiss D A, Fox K R, Rose J B, Davis J P. A massive outbreak of cryptosporidiosis infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- 16.Mead J R, Arrowood M J, Sterling C R. Antigens of Cryptosporidium sporozoites recognized by immune sera of infected animals and humans. J Parasitol. 1988;74:135–143. [PubMed] [Google Scholar]
- 17.Moss D M, Bennett S N, Arrowood M J, Hurd M R, Lammie P J, Wahlquist S P, Addiss D G. Kinetic and isotypic analysis of specific immunoglobulins from crew members with cryptosporidiosis on a U.S. Coast Guard cutter. J Eukaryot Microbiol. 1994;41:52S–55S. [PubMed] [Google Scholar]
- 18.Moss D M, Bennett S I, Arrowood M J, Wahlquist S P, Lammie P J. Enzyme-linked immunoelectrotransfer blot analysis of a cryptosporidiosis outbreak on a United States Coast Guard cutter. Am J Trop Med Hyg. 1998;58:110–118. doi: 10.4269/ajtmh.1998.58.110. [DOI] [PubMed] [Google Scholar]
- 19.Moss D M, Chappell C L, Okhuysen P C, DuPont H L, Arrowood M J, Hightower A W, Lammie P J. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J Infect Dis. 1998;178:827–833. doi: 10.1086/515377. [DOI] [PubMed] [Google Scholar]
- 20.Moss D M, Lammie P J. Proliferative responsiveness of lymphocytes from Cryptosporidium parvum-exposed mice to two separate antigen fractions from oocysts. Am J Trop Med Hyg. 1993;49:393–401. doi: 10.4269/ajtmh.1993.49.393. [DOI] [PubMed] [Google Scholar]
- 21.Ong C S, Eisler D L, Goh S H, Tomblin J, Awad-el-Kariem F M, Beard C B, Xiao L, Sulaiman I, Lal A, Fyfe M, King A, Bowie W R, Isaac-Renton J L. Molecular epidemiology of cryptosporidiosis outbreaks and transmission in British Columbia, Canada. Am J Trop Med Hyg. 1999;61:63–69. doi: 10.4269/ajtmh.1999.61.63. [DOI] [PubMed] [Google Scholar]
- 22.Perryman L E, Jasmer D P, Riggs M W, Bohnet S G, McGuire T C, Arrowood M J. A cloned gene of Cryptosporidium parvum encodes neutralization-sensitive epitopes. Mol Biochem Parasitol. 1996;80:137–147. doi: 10.1016/0166-6851(96)02681-3. [DOI] [PubMed] [Google Scholar]
- 23.Pozio E, Rezza G, Boschini A, Pezzotti P, Tamburrini A, Rossi P, Di Fine M, Smacchia C, Schiesari A, Gattei E, Zucconi R, Ballarini P. Clinical cryptosporidiosis and human immunodeficiency virus (HIV)-induced immunosuppression: findings from a longitudinal study of HIV-positive and HIV-negative former injection drug users. J Infect Dis. 1997;176:969–975. doi: 10.1086/516498. [DOI] [PubMed] [Google Scholar]
- 24.Priest J W, Kwon J P, Arrowood M J, Lammie P J. Cloning of the immunodominant 17-kDa antigen from Cryptosporidium parvum. Mol Biochem Parasitol. 2000;106:261–271. doi: 10.1016/s0166-6851(99)00223-6. [DOI] [PubMed] [Google Scholar]
- 25.Priest J W, Kwon J P, Moss D M, Roberts J M, Arrowood M J, Dworkin M S, Juranek D D, Lammie P J. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J Clin Microbiol. 1999;37:1385–1392. doi: 10.1128/jcm.37.5.1385-1392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reperant J-M, Naciri M, Iochmann S, Tilley M, Bout D T. Major antigens of Cryptosporidium parvum recognized by serum antibodies from different infected animal species and man. Vet Parasitol. 1994;55:1–13. doi: 10.1016/0304-4017(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 27.Robbins J B, Schneerson R, Szu S C. Perspective. Hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 28.Ungar B L P, Nash T E. Quantification of specific antibody response to Cryptosporidium antigens by laser densitometry. Infect Immun. 1986;53:124–128. doi: 10.1128/iai.53.1.124-128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ungar B L P, Soave R, Fayer R, Nash T E. Enzyme immunoassay detection of immunoglobulin M and G antibodies to Cryptosporidium in immunocompetent and immunocompromised persons. J Infect Dis. 1986;153:570–578. doi: 10.1093/infdis/153.3.570. [DOI] [PubMed] [Google Scholar]