Abstract
In the last three decades, several flaviviruses of concern that belong to different antigenic groups have expanded geographically. This has resulted in the presence of often more than one virus from a single antigenic group in some areas, while in Europe, Africa and Australia, additionally, multiple viruses belonging to the Japanese encephalitis (JE) serogroup co-circulate. Morphological heterogeneity of flaviviruses dictates antibody recognition and affects virus neutralization, which influences infection control. The latter is further impacted by sequential infections involving diverse flaviviruses co-circulating within a region and their cross-reactivity. The ensuing complex molecular virus–host interplay leads to either cross-protection or disease enhancement; however, the molecular determinants and mechanisms driving these outcomes are unclear. In this review, we provide an overview of the epidemiology of four JE serocomplex viruses, parameters affecting flaviviral heterogeneity and antibody recognition, host immune responses and the current knowledge of the cross-reactivity involving JE serocomplex flaviviruses that leads to differential clinical outcomes, which may inform future preventative and therapeutic interventions.
Keywords: Japanese encephalitis serogroup, Japanese encephalitis virus, Murray valley encephalitis virus, West Nile virus, Usutu virus, envelope protein, cross-reactivity
1. Introduction
Members of the genus flavivirus within the family Flaviviridae cause a substantial global burden of disease and mortality each year and pose a constant threat for future outbreaks. Yet, treatment for human flavivirus infections is lacking [1]. Depending on the type of vector involved in virus transmission, these arthropod-borne animal viruses, or arboviruses, are broadly divided into three groups: the mosquito-borne flaviviruses, the tick-borne flaviviruses and a third group with yet unidentified vectors. Historically, members of the family Flaviviridae were classified based on serological assays such as neutralization tests, hemagglutination inhibition assays, complement fixation and immunodiffusion [2,3]. Eight antigenic groups or serocomplexes in the genus flavivirus within the family Flaviviridae, classified on the basis of serological assays, have been described [4]. The tick-borne encephalitis antigenic group comprises members such as Omsk haemorrhagic fever virus, Russian spring–summer encephalitis virus (RSSE), Louping Ill virus, Kyasanur forest disease virus, Langat virus and Powassan virus. The Japanese encephalitis (JE) serocomplex includes Murray valley encephalitis virus (MVEV), Japanese encephalitis virus (JEV), West Nile virus (WNV), Kunjin virus (KUNV), Usutu virus (USUV), Kokobera virus, Alfuy virus and St. Louis encephalitis virus (SLEV). The four dengue serotypes form the Dengue serocomplex and the Spondwenii serocomplex includes Zika virus (ZIKV) and Spondwenii virus (SPOV). Yellow fever virus (YFV) forms a distinct serogroup. Genome sequencing and subsequent genomic and phylogenetic quantitative and bootstrapping analyses using pair-wise nucleotide sequence identity and clustering reveal the genetic relatedness of newly identified member strains and inform parameters influencing virus evolution, transmission and discovery [5,6]. A second classification system developed by Kuno et al. [5], defining fourteen clades (I–XIV), relies on the nucleotide and amino acid sequence identity of certain genes in the viruses. Similar to the serologic classification, the mosquito-borne viruses form a distinct cluster. Of these, the members of the Japanese encephalitis serocomplex discussed in this review, MVEV, JEV, WNV, KUNV and USUV, fall under clade XIV; DENV serotypes form clade IX; ZIKV and SPOV belong to clade X; the Nataya serocomplex members Bagaza virus, Tembusu and Israel meningo-encephalitis virus are part of clade XI; and YFV (Asibi) is part of clade VII [5,7]. These serological and phylogenetic relationships among flaviviruses provide a framework for understanding the host-immune interplay of viruses within and between antigenic complexes and flavivirus biology. For instance, antigenic classification correlates with the vector species involved in transmission; for the JE serogroup, this is largely the Culex spp., although other vector species are known to transmit these viruses as discussed below (Table 1) [4].
Table 1.
Listed are some vectors and animal hosts of the four viruses from Japanese encephalitis serocomplex and the clinical symptoms in human infections.
| Virus | Mosquito Vectors |
Natural Reservoir/Amplifying Hosts | Animal Hosts/Animals Infected | Human Clinical Symptoms | References |
|---|---|---|---|---|---|
| JEV |
Culex spp. including
Cx. tritaeniorhynchus Cx. vishnui Cx. gelidus Cx. annulirostris Cx. annulus Cx. fuscocephala Cx. sitiens Cx. quinquefasciatus Cx. bitaeniorhynchus; Aedes, Anopheles, Mansonia and Armigeres spp. including Aedes albopictus |
Ardeidae family such as egrets, herons; ducklings, chicken, American crow, house finch, house sparrow, ducklings | Feral and domestic pigs, horses, boars, racoons, dogs, bats, cattle, goats | Febrile illness without any other clinical manifestation; acute encephalitis including headache, vomiting, seizures; flaccid paralysis, facial paralysis, hepatomegaly, splenomegaly, Thrombocytopenia, Guillain Barré syndrome, neurological sequelae |
[8,9] |
| MVEV |
Cx. annulirostris,
Cx. australicus Aedes normanensis Ochlerotatus tremulus |
Ciconiiformes such as herons, egrets | Kangaroos, rabbits, mice, dogs, pigs | Febrile illness, encephalitis, neurological sequelae, flaccid paralysis |
[10] |
| WNV |
Culex spp. including
Cx. tarsalis Cx. quinquefasciatus Cx. stigmatosoma Cx. thriambus Cx. pipiens Cx. nigripalpus |
Corvidae family such as American Crows and blue jays, common grackles, house sparrows, American robins, house finches |
Rabbits, lemurs, hamsters, squirrels, chipmunks | Fever, myalgia, encephalitis, meningo-encephalitis, meningitis, flaccid paralysis | [11,12] |
| USUV |
Culex spp. Including Cx. pipiens Cx. neavei Cx. modestus Cx. perfuscus Cx. quinquefasciatus Aedes, Anopheles Mansonia and Ochlerotatus spp. Aedes albopictus Aedes japonicus |
Passeriformes (such as Eurasian blackbirds, house sparrows and Magpies), Strigiformes (such as Great grey owl), Coraciiformes |
Horses, bats, dogs, cattle, wild boar, deer, rodents, shrew | Fever, skin rash, meningo-encephalitis, facial paralysis Asymptomatic infections/presence in donated blood from healthy adults |
[13,14] |
Arboviruses are zoonotic agents that transmit disease from vertebrate hosts (wild animals) to human beings via arthropod vectors such as mosquitoes and are maintained in the environment in zoonotic transmission cycles [15,16]. In the enzootic or sylvatic cycle, the female mosquito vector feeds on the infected vertebrate host that acts as the reservoir; when the virus replicates and infects the mosquito, the latter transmits the amplified virus to the next vertebrate via its salivary glands during a subsequent blood meal. Vertebrate reservoirs are typically birds, small wild animals or non-human primates; these are the natural hosts of the virus, and frequently transmission does not result in disease. In the epizootic or rural cycle, domestic animals are infected by either a primary or accessory vector which leads to outbreaks in the animal population. When humans are in close proximity, the virus can get transmitted to humans via the vector as well (urban or epidemic cycle). In the case of sufficient viremia, there is enough virus amplified to infect a new mosquito vector; otherwise, humans are dead-end hosts and do not perpetuate the virus transmission in the population. Non-vector modes of flavivirus transmission are less common and these have been described for DENV, JEV, WNV and ZIKV in humans and animals. Examples include transmission via organ transplantation, blood transfusion, oro-nasal secretions and horizontal transmission, and an uncommon, atypical case of WNV transmission via lactation [17,18,19,20,21]. Isolation from non-mosquito vectors such as ticks with transmission has been reported for WNV [22], as well as viral transmission in the absence of mosquito vectors in farmed crocodiles [23]. Sexual transmission of most flaviviruses in humans is uncommon. However, sexual and vertical transmission are established routes of ZIKV infection in humans [24]. Due to the overlapping ecological and geographical distribution of vectors and amplifying hosts, transmission of more than one virus by a single vector, simultaneously or consecutively, is feasible. Therefore, virus particles can adapt and survive the temperature and cellular milieu of multiple host and vector species and thrive in a broad diversity of micro-environments and tissues. Furthermore, multiple sequential infections with the same JE serocomplex virus or with a second flavivirus of the same or different serogroup can influence the host immune response and disease outcomes.
2. Distribution and Disease
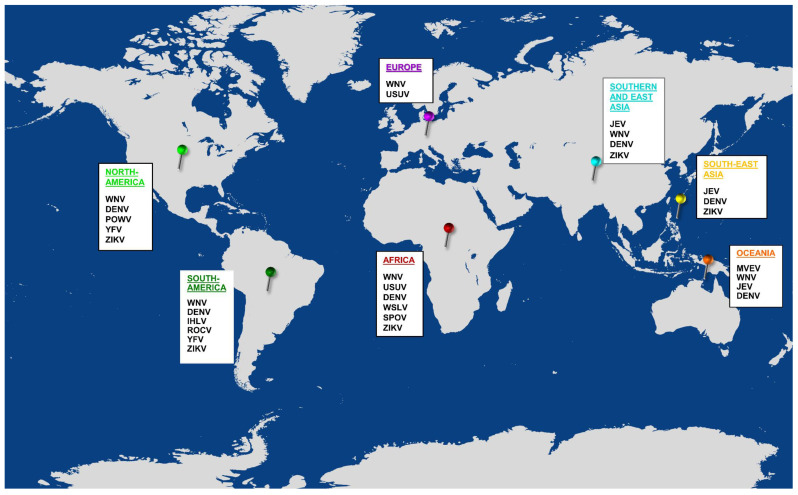
JE serogroup viruses circulate in both temperate and tropical zones, with an expanse corresponding to a population of over three billion people (Figure 1). This wide distribution can be attributed to evolution, high genetic diversity, emergence and re-emergence of strains and natural spread of the virus due to vector proliferation and avian migration [25,26,27]. Distribution of specific strains is directly linked with climate change [25,28]. Based on the geographical distribution and phylogenetic analyses of whole genome, the NS5 or the envelope (E) gene, genotypes 1–4 (G1–4), genotypes I–V (GI–V) and eight lineages each have been described for MVEV, JEV, WNV and USUV, respectively (Table 2).
Figure 1.
Global distribution of flaviviruses. Multiple flaviviruses co-circulate in most continents; of these, at least one virus belongs to the JE serocomplex. Co-circulation of more than one JE serocomplex virus occurs in Africa, Europe, Asia and Australia. The figure was generated using an online tool, URL: https://mapchart.net. (DENV: Dengue virus; IHLV: Ilheus virus; JEV: Japanese encephalitis virus; MVEV: Murray valley encephalitis virus; POWV: Powassan virus; ROCV: Rocio virus; SPOV: Spondwenii virus; USUV: Usutu virus; WSLV: Wesselsbron virus; WNV: West nile virus; YFV: Yellow fever virus; ZIKV: Zika virus).
Table 2.
Listed are the lineages of JEV, MVEV, WNV and USUV with a few representative strains, their geographical distribution and disease incidences.
| Virus Lineage | Alternative Name/Included Strains | Accession Numbers | Geographical Distribution | Disease Prevalence | References |
|---|---|---|---|---|---|
| JEV | |||||
| Genotype I | KV1899 | AY316157 | Asia including Korea, China, Japan, Cambodia, Vietnam, Thailand | Epidemics in temperate regions, predominant in humans |
[8,9,29] |
| Ishikawa | AB051292 | ||||
| HEN0701 | FJ495189 | ||||
| Genotype II | FU | AF217620 | Asia, Australia, Korea | Endemic disease in tropical regions |
[8,9,29] |
| Genotype III | p3 SA14-14-2 Vellore P20778 |
U47032 AF315119 AF080251 |
Asia including India, China, Japan, Korea (Temperate zones) |
Epidemics in temperate regions, endemic activity, predominant in humans | [8,9,29] |
| Genotype IV | JKT6468 | AY184212 | Indonesia and Australia | Endemic disease in tropical regions |
[8,9,29] |
| Genotype V | Muar strain XZ0934 |
HM596272 JF915894 |
Asia including Malaysia, China and Korea |
Re-emerged strain, Human encephalitis |
[8,9,29,30] |
| MVEV | |||||
| Genotype 1 | MVE-1-51 K49077 08-154300 |
AF161266 EF015056 JN119766 |
Australia | Epidemics, sporadic cases |
[31] |
| Genotype 2 | OR156 K6454 |
EF015074 EF015070 |
Australia | Human encephalitis |
[32] |
| Genotype 3 | NG156 | EF015076 | Papua New Guinea |
Human encephalitis |
[10,33,34] |
| Genotype 4 | MK6684 | EF015075 | Papua New Guinea |
- | [31] |
| WNV | |||||
| Lineage I | HNY1999 VLG4 LEIV-Vlg99-27889 Eg101 Kunjin MRM61C |
AF202541 AF317203 AY277252 AF260968 D00246 |
Europe, Russia, North Africa, Israel, United States, Middle-east, Australia and India | Outbreaks in humans | [12,35,36,37] |
| Lineage II | Sarafend Ug37 |
AY688948 NC_001563 |
Sub-saharan Africa including South Africa, Madagascar; Europe including Greece, Russia |
Zoonotic outbreaks in South Africa, avian and human outbreaks in Europe |
[12,35,37,38] |
| Lineage III | Rabensburg virus (RABV/97-103) | AY765264 | Europe | - | [35,36,39] |
| Lineage IV | LEIV-Krnd88-190 | AY277251 | Russia | - | [36] |
| Lineage V | 804994 G16146 |
DQ256376 GQ851605 |
India | Outbreaks in humans | [12] |
| Lineage VI | HU2925/06 | GU047875 | Spain | - | [40] |
| Lineage VII | Koutango virus ArD96655 |
KY703855 | Africa, Malaysia, Senegal | Sporadic outbreaks in Africa | [37] |
| Lineage VIII | ArD94343 | KY703856 | Senegal | Sporadic outbreaks in Africa | [37] |
| Lineage XI | WNV-Uu-LN-AT-2013 | KJ831223 | Austria | - | [37,40] |
| USUV | |||||
| Africa 1 | Central African Republic 1969 (CAR 1969) | KC754958 | Africa | - | [13,41,42] |
| Africa 2 | South Africa1959 (SAAR 1776) Spain 2006 |
AY453412 KF573410 |
Africa Europe |
- | [13,41,42] |
| Africa 3 | Central African Republic (CAR 1981) Senegal 2007 Netherlands 2016 |
KC754955 KC754957 KY128482 |
Africa Europe |
Human illness | [41,43] |
| Europe 1 | Austria 2001 (Vienna 2001) Hungary 2005 |
AY453411 EF206350 |
Europe | Human meningo-encephalitis | [13] |
| Europe 2 | Italy 2009 Austria 2016 Hungary 2016 |
HM569263 MF063042 MF063043 |
Europe | Human meningo-encephalitis, meningitis, asymptomatic blood donors | [42,44,45] |
| Europe 3 | Germany 2011 Belgium 2016 France 2016 |
KJ438769 KX977447 KY128481 |
Europe | Asymptomatic blood donors | [13,42] |
| Europe 4 | Italy 2009 Italy 2010 |
HM138711 JF834562 |
Europe | Asymptomatic blood donors | [13,42] |
| Europe 5 | Germany 2016 France 2016 |
KY113091 LT854220 |
Europe | Atypical human illness | [13,14] |
The geographic distribution of JEV includes countries in Asia and Southeast Asia. JEV causes an estimated annual burden of 68,000 cases every year; the global disease burden is unknown but is estimated to be approximately 20,000 fatalities. JEV encephalitis has a mortality rate of up to 30% and survivors can have permanent neurological or psychological sequelae. GIII used to be the dominant genotype in temperate zones, and it was associated with human outbreaks in the past. GI represents the dominant genotype of JEV in the world [25]. MVEV is spread across the Northern Territory, Western Australia and the south-eastern region in Australia. It infects humans and animals from time to time, causes meningitis and encephalitis in rare cases and varying degrees of brain dysfunction. GI is the dominant strain across all areas of distribution, whereas some others like GIB (a sub-strain of GI) and GII are more restricted [31]. WNV is one of the most widespread viruses in this antigenic complex and has caused outbreaks in many countries. By 2002, WNV lineage I strain, NY99, had spread across the USA with the emergence of new, more virulent strains [35]. WNV lineage II, with a dissemination in Europe around 2008–2009, is currently the dominant strain in the region and responsible for multiple epidemics in humans and animals. Both WNV lineages cause a neuro-invasive disease in 1% of the infected human population [35]. USUV is currently restricted to Europe and is mostly asymptomatic in humans [46]. Two cases of human illness have been reported from Africa, whereas European strains are the cause of severe illness in humans only in rare cases.
The WNV and JEV distribution overlaps with that of other heterotypic flaviviruses such as ZIKV and DENV in parts of Asia and South America, whereas WNV and JEV overlap in Asia. In Europe, WNV and USUV share a large geographic distribution, as well as many vectors and amplifying hosts. The overlap of geographic distribution has implications for disease diagnosis and control and host immune response (discussed below). A multitude of factors add to the ecological interplay, such as the genetic diversity of the virus, co-circulation of multiple strains and viruses and distinct pathology displayed by different lineages.
3. Virus Morphology and Host Interplay
Flavivirus pathogenicity, extensively studied using molecular and structural biology together with animal models in ZIKV as well as in the hemorrhagic DENV, is intricately linked with virus assembly, maturation, host cell interactions, the immune response and membrane fusion [47,48,49,50,51]. Virus morphology affects disposition of structural proteins and surface residues and hence impacts interactions with host receptors and immune cells [52,53]. Previous reports are recommended for an in-depth review of the current understanding of the flavivirus life cycle, domain organization and the structural biology of the envelope protein (E), the premembrane protein (prM), the capsid protein (C), and structures of complexes of these proteins with receptors and antibodies [52,54,55]. Fundamental structural understanding stemming from investigations in DENV, WNV, ZIKV, TBEV and JEV structures suggests that many molecular interactions and mechanisms may share some similarity within flaviviruses [56].
3.1. Distinct Structures Correlate with Specific Stages of the Viral Life Cycle
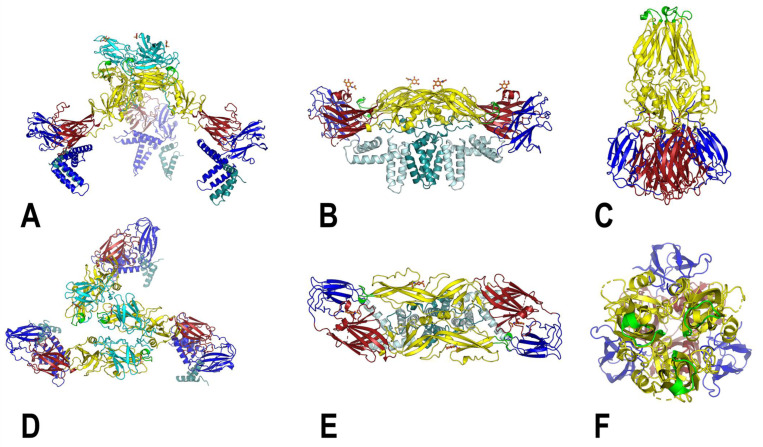
Within infected cells, flavivirus interaction with host proteins in the endoplasmic reticulum (ER) alters its morphology at specific sites where replication of the RNA genome and assembly of new virions takes place [57,58,59]. Immature flavivirus particles are roughly 60 nm in diameter, assemble at neutral pH and bud into the ER lumen [60]. An immature flavivirus is composed of the structural proteins, E, prM and C, and the viral RNA genome; the transmembrane regions of E and prM are embedded in a lipid envelope that surrounds the nucleocapsid [60,61]. Sixty heterotrimers of E and prM form ‘spikes’ that project out from the virus surface; the trimer is positioned at the quasi-threefold of the asymmetric subunit, and the fusion loop (FL) at the distal end of the E:prM trimer is protected by prM (Figure 2) [60,61,62,63,64]. However, FL in the immature virion can be targeted by antibodies and must be accessible [65,66]. Immature virus is non-infectious by virtue of the presence of prM, which shields the fusion loop (FL) of E and prevents membrane fusion [67,68,69]. Low resolution, asymmetric cryo-EM reconstruction suggests that immature virions bud with an eccentrically positioned nucleocapsid core relative to the outer icosahedral glycoprotein shell [70]. The electron density present at the base of the spike formed by transmembrane domains of three E/prM monomers and positioned between the inner leaflet and the nucleocapsid core was observed in immature ZIKV structures; this corresponds to a single capsid protein which interacts with the glycoprotein transmembrane domain [71,72,73].
Figure 2.
Models of the flavivirus structural proteins corresponding to various stages in the virus life cycle. (A–C): Immature DENV1 trimer, mature USUV dimer and the fusogenic DENV hairpin conformation, respectively. Corresponding top views are depicted in (D–F), respectively. For the immature E trimer and the E dimer, the E-stem region is also shown. The models are color coded as follows: DI (red), DII (yellow), DIII (blue), fusion loop (green), glycosylation sites (orange) and prM (cyan); for (B,E), the E-stem is in light blue and M protein is in teal.
As the immature virus traverses the acidic compartments of the trans-Golgi network, conformational changes in E and concomitant processing of prM facilitate flavivirus maturation. Acid-induced conformational rearrangement leads to the formation of an icosahedral shell of 90 E:prM antiparallel heterodimers that lie ‘flat’ on the virus surface, resulting in a smooth outer appearance; prM is subsequently cleaved into ‘pr’ and ‘M’ proteins by the host furin enzyme and ‘pr’ is released when exposed to the neutral pH of the extracellular milieu [62,68,74,75,76]. The mature flavivirus is 50 nm in diameter and displays the ‘herringbone’ array of E rafts, a hallmark of the mature virion (Figure 2) [77]. Mature flavivirus is infectious, multiple molecular determinants within E confer specific tropism and subsequent virus entry in the host cells is mediated via attachment factors [78,79] which represent a primary target for host antibodies. Multiple high-resolution structures showed the absence of capsid density near the E/M transmembrane domain and between the lipid layer and nucleocapsid core, which suggests a rearrangement of C in the mature virus [62,71,74,75]. More significant structural changes occur in the M-TMD than in the E-TMD between the immature and mature forms of flavivirus [80].
Infectious, assembled flavivirus particles are an assortment of mature and mosaic particles that interact with multiple, often redundant, host cell surface receptors/attachment factors to gain entry into a cell [81]. However, purified JE serocomplex viruses expressed in mammalian cells are relatively less heterogeneous than those observed for DENV, which may possibly be a type-specific difference. Known flavivirus receptors/attachment factors, including those for JE serocomplex viruses, fall under the broad classification into families of proteins such as C-type lectins or CLRs (e.g., dendritic cell-specific ICAM-3 grabbing non-integrin or DC-SIGN, a homologue of DC-SIGN named DC-SIGNR, C-type lectin domain family 5 member A or CLEC5A), integrins (e.g., αVβ3, αVβ5, αVβ1), phosphatidyl serine receptors of the TIM/TAM families (T cell immunoglobulin mucin domain; Tyro3, Axl and Mer), heat-shock proteins (e.g., heat shock proteins HSP70 and HSP90, heat shock cognate HSC70) as well as tight junction proteins (Claudin-1), heparin sulfate proteoglycans (HSPGs) and glycosaminoglycans (GAGs), laminin receptors and natural-killer-cell-activating receptor NKp44 [82,83,84,85,86,87]. Interactions with receptors/attachment factors affect cellular and tissue tropism and hence disease manifestation. However, successful cellular infection is a multiple-step process (attachment, recognition, binding, viral entry and virus internalization) that requires both receptors/attachment factors and additional host factors that facilitate viral replication in a permissive cell [87]. For the JE serocomplex, some receptors/attachment factors have been identified using in vitro experiments, but their role in viral entry and the molecular mechanisms of highly specific receptor binding are unclear [50,82]. For instance, JEV is neuroinvasive and known to breach the BBB; it infects pericytes, glial cells and developing neuronal cells, and utilizes CLRs, TIMs, HSPG and GAG, integrins and heat-shock proteins as receptors/attachment factors, yet the JEV receptor responsible for viral entry in the central nervous system remains elusive [50,82,88]. Virus–receptor interactions greatly depend on particle morphology and can trigger cellular changes that facilitate virus internalization and clathrin-mediated endocytosis.
Extensive reviews have described flavivirus characteristics and structural heterogeneity that pertain to viral entry (discussed in Section 3.2) [50,81,87]. Molecular determinants on E confer binding to various receptors/attachment factors, but these are not always well defined. Specific amino acid residues in JEV, MVEV and WNV can drive viral entry and these reside on different domains of the E protein [89]. The surface properties of the E protein, in particular the presence of Lys-rich residues in the DI or DIII domains, confer positive charge, which drives the interaction with the negatively charged GAGs in JEV, WNV and MVEV [90,91], and similarly localized Lys residues in some USUV strains may confer preferential GAG binding [75,92]. In the mature, infectious form, these receptor-binding determinants would need to be exposed for interactions, as is indeed the case for some interactions, while, conceivably, some E elements that participate in interactions may become accessible only during intermediate stages, possibly post-attachment. Known findings on CLR and integrin interactions attest to these differences, for instance, glycosylation at the Asn-154 site on the glycan loop is a marker of virulence in JEV, WNV and MVEV and mediates differential binding to CLRs [93]. On the other hand, while integrins have been implicated in WNV and JEV entry, the role of the RGD motif in mediating WNV entry has been inconclusive from in vitro studies and high-resolution details showed the RGD motif in USUV to be unavailable for interactions [74,75,94,95]. DENV and USUV are the only two flaviviruses that contain a second glycosylation site, at Asn-67 and Asn-118, respectively. All serotypes and strains within the two respective antigenic complexes are known to possess the second glycosylation site; however, other JE serocomplex members including WNV, JEV and MVEV lack this site, which prompts a question of evolutionary conservation and suggests some entry/fitness advantage of the DII glycan site for DENV and USUV. Structural knowledge of E protein with receptors/attachment factors revealing atomic-resolution details of the virus–host interface in complexes is lacking [96]. An integrated structural approach involving X-ray crystallography and cryo-electron microscopy (cryoEM) may be essential to fully decode the structural basis of flavivirus recognition and binding of cognate attachment factors or receptors as well as define the molecular determinants and key interactions involved in viral entry.
Once endocytosed in a newly infected cell, an irreversible, acid-induced conformational change occurs in the mature virus that leads to the realignment of inter-domain interactions and dissociation of the E:M heterodimers; the repositioning of DII, DIII and the E-stem; and subsequently the formation of sixty E:E:E homotrimers [64,97]. Formation of the acid-induced trimeric fusion-competent form is essential for fusion and a requisite prelude to the release of viral RNA into host cytoplasm [98]. E homotrimers expose three FLs at the distal end for insertion into the endosomal membrane (Figure 2) [99]. However, since the process is dynamic and transient, the intermediate states are often captured by complexing with antibody fragments. Complex formation of WNV with monoclonal antibody (mAb) E16, which blocks WNV membrane fusion by inhibiting E-trimer formation, followed by a drop in pH, arrests the virus in a pre-fusion state [100]. The WNV pre-fusion state reveals an expansion of the E shell, with a gap of 60 Å between the E and lipid layers. DENV post-fusion intermediates further detail the “open” and “closed” states that are on the continuum leading to fusion: in the “open state”, the FLs are inserted into the endosomal membrane but the DI-DII hinge angle is the same as that in the E:M heterodimer of mature virus, whereas in the “closed” state, additionally, E-stem helices and DIII are repositioned to facilitate E-stem “zippering” [99,101,102].
3.2. The Sources of Heterogeneity Are Multifactorial
Flavivirus particles produced in an infected cell are a heterogeneous mixture of mature, partially mature and immature virus particles [103,104]. This primarily results from inefficient furin processing of the prM, which has been observed primarily in DENV and JEV [105]. The mature and immature particles contain M and prM, respectively, whereas, in partially immature particles, both M and prM are present in the same particle and prM and E associate as a heterodimer that lies close to the viral surface [106]. The latter may represent an intermediate along the continuum of prM processing; indeed, structural studies confirmed that these particles contain features characteristic of both the mature and immature virions [106,107]. Mutational analysis of DENV prM residues at and around the furin cleavage site and chimeras containing prM segments from JEV, YFV and TBEV showed enhanced cleavage for the JEV chimera and delayed egress of the virions but no change in infectivity [108]. Cleavage efficiency and hence the degree of heterogeneity varies among flaviviruses and may also vary between viruses/strains within the JEV serogroup; for instance, purified SAAR-1776 USUV displayed mostly mature particles with a low number of immature particles and fewer partially immature particles. However, the visual appearance of particles, without further analysis with virometry or mass spectrometry, is insufficient to conclude the nature of heterogeneity [75,105,108]. The cell type used for virus production is important as mammalian cells result in less heterogeneity; however, it remains to be established whether the range of variation in heterogeneity might correlate with the pathogenicity of strains for specific hosts, within and between serogroups [103,109].
Fundamental knowledge of the structural biology of flaviviruses comes from investigations of DENV serotypes and their interactions with host components. However, in the broader context of the structural architecture of flaviviruses that is pertinent for pathogenesis, common features are shared while finer details may differ between type-specific viruses or strains. The morphology of the mature DENV virions spans a broad range: the particles include those with a ‘smooth’ (diameter of ~500 Å) appearance or a rough, ‘bumpy’ appearance (somewhat variable sizes with reported diameters between 360–550 Å), depending on the host infected or types of cells used for virus production, and a non-spherical structure referred to as the club-shaped particles [110,111,112]. Dengue virions change to the ‘bumpy’ morphology above 33 °C, and, therefore, at the physiological body temperature of the human host (37 °C), this form is expected to dominate, whereas in the mosquito vector (28 °C), Aedes spp. mosquitoes for DENV, the smooth form would predominate. This phenomenon of temperature-dependent particle expansion is referred to as viral ‘breathing’. Structurally, the ‘bumpy’ particle shows expansion of the protein shell and some structural rearrangements of the E protein domains compared to the smooth morphology. The protein shell containing E lies at a greater radius from the center of the particle whereas the radial distance of the lipid layer remains unchanged, protrusions of E domains I and III (DI and DIII) are observed between the five- and three-fold icosahedral axes, weakening of E dimer interactions at the icosahedral two-fold shifts the raft arrangement, and a hole is present at the icosahedral three-fold vertices that is surrounded by the DI and DIII domains [111]. Structural studies reveal that the temperature-dependent expansion observed in DENV is serotype-specific and is shown only by DENV2.
Evidence for viral breathing in JE serocomplex viruses comes from functional studies on WNV (described below) using antibodies E60, E16 and E53, and Thr198 of WNV E (and Phe193 in DENV1) [113,114]. In the JE serogroup, cryo-EM structures of mature viruses have been determined for USUV, WNV and JEV (resolutions of 2.4, 3.1 and 4.3 Å, respectively) and for chimeras of Binjari virus with WNV and with MVEV (resolution of 2.9 and 3.7 Å, respectively), where WNV or MVEV structural proteins, respectively, form the icosahedral glycoprotein shell [74,75,115]. USUV structures represent the highest-resolution maps of a mature flavivirus solved to date using cryo-EM, and these are also the highest-resolution structures from the JE serogroup [75]. The maps reveal densities for three lipid sites; none of the other recent structures of mature flaviviruses reveal the presence of all three sites and this could be due, in part, to the higher number of particles used for the reconstruction and the biological component pertaining to the disposition of M helices in the membrane, which is most similar for ZIKV and USUV [75,116]. One of the two sites near the E-stem (referred to as ‘S2’) is also present in the flavivirus cryo-EM structures of ZIKV, SPOV and the chimera of Binjari virus and DENV; therefore, the two sites may be functionally distinct and S2 may represent an essential lipid interaction site across flaviviruses [62,74,75,116,117].
A non-spherical morphology for DENV, called the club-shaped particle, was recently described [118]. Morphologies with a head and a tail (HAT particles), albeit a slack tail, similar to club-shaped particles have been observed in some purified flavivirus samples where the number of HAT particles increased with time and were observed with a concomitant decrease in the number of spherical virions. However, these changes are differentially observed for flaviviruses (unpublished data). A structurally distinguishable subpopulation of USUV was also recently described where the differences were restricted to side-chain conformations of residues and the presence of an FL disulfide bond in one of the three monomers of the asymmetric subunit; the simultaneous existence of virions displaying different conformations in a sample and elucidation of their cryo-EM structures has been reported for other viruses [75,119]. This subpopulation represented about 33% of the sample size in USUV, comparable to the class III particles of the ‘bumpy’ DENV2 extracted for cryo-EM reconstruction, emphasizing that the presence of flavivirus subpopulations within a sample may have functional implications for host interactions [75,111].
3.3. Particle Architecture Affects Host Interactions
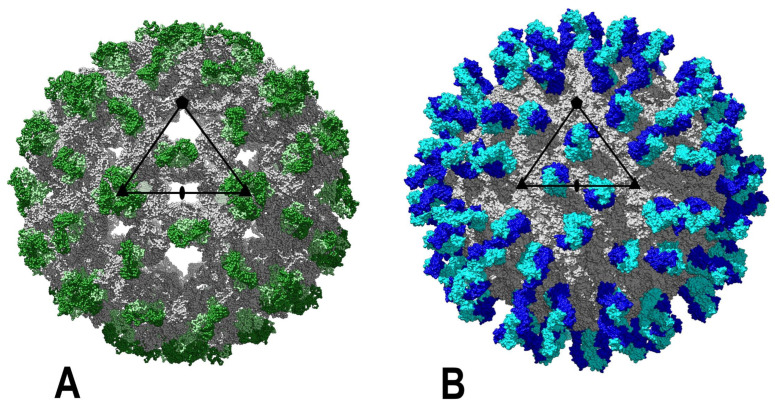
Mature and immature forms of flaviviruses, by virtue of the presence of prM, and the fusion-competent forms of the virus possess distinct particle architecture and hence display differences in the surface-exposed regions of the structural proteins; this has direct implications for antibody recognition and binding. Antibodies can target both structural forms of flaviviruses and antibodies that target all three domains as well as the DI–DII and DII–DIII hinge regions of E have been identified and structurally characterized for DENV [55]. The neutralization potency of the antibodies varies. Some antibodies target the same region in multiple flaviviruses, including DENV and ZIKV, such as the E dimer epitope (EDE)-recognizing monoclonal antibodies that crosslink the two E proteins in the homodimer [55,65]. EDE antibodies can be further classified into EDE-1 and EDE-2 antibodies, where only the latter require the Asn154 glycosylation on the adjacent E of the dimer for recognition. Furthermore, EDE antibodies can bind both mature and partially mature virion, exemplifying the interplay of antibodies and virion architecture. Binding of antibodies such as CR4354 and 14C10, specific for WNV and DENV1, respectively, results from recognition of quaternary epitopes on the E dimer of the mature virion; however, the occupancies of the antibodies on the flaviviruses vary, further specifying the residues recognized by different antibodies on mature virions (Figure 3) [55,120]. The maturation state, virion stability and “viral breathing” affect antibody recognition and hence virus neutralization in WNV and DENV [65]. For instance, DENV E-specific antibody 1A1D-2 binds to the mature virus after incubation at 37 °C as the epitope is not accessible at 4 °C [121].
Figure 3.
Models of some JE serocomplex flavivirus particles with bound Fabs. (A). Immature WNV particle with bound E53, which is cross-reactive and preferentially binds immature flavivirus particle but not the mature particles. The Fab chains are depicted in green and light green. (B). Mature WNV with bound CR4354, which binds two neighboring E molecules and neutralizes by blocking the conformational rearrangement essential for membrane fusion. Fab chains are shown in blue and light blue.
Epitopes recognized by antibodies in the immature virus may become inaccessible when E monomers form dimers in the mature virus. A change in neutralization sensitivity and hence potency was observed in response to WNV maturation as a result of epitopes becoming less accessible in the mature virion [122,123,124]. Conversely, studies in MVEV revealed that prM in the immature particle conceals epitopes accessible in the mature particle and the prM-associated immature particles are more acid-resistant [125]. Another “cryptic” or inaccessible epitope on the immature virions is the fusion loop epitope (FLE) that is recognized by the WNV E53 antibody [126]. These antibodies bind immature virions, as well as the partially mature particles or particles undergoing viral “breathing”, all scenarios where FLE is exposed [65]. FLE–recognizing antibodies in DENV were shown to be strain- and DENV serotype-dependent, suggesting the need for evaluation of individual strains [127]. E53 preferentially recognizes partially mature WNV virions and fails to neutralize mature virus [126].
FL-recognizing antibodies tend to be weakly neutralizing and cross-reactive with other flaviviruses due to the strong conservation of the FL; however, Vogt et al. showed that weakly neutralizing WNV FL antibody E28 conferred protection in mice in vivo via effector activation and phagocytic activity even though E28 showed poor neutralization in vitro [128]. Vogt et al. further speculated that the protective effects of cross-reactive FL antibodies could be protective for secondary WNV infections in geographical areas where more than one flavivirus is circulating; this may be relevant for the observed protection recently reported for WNV lineage II in Europe in patients with pre-existing USUV immunity (discussed later). Immuno-dominance of FLE antibodies is also reported in other flaviviruses in studies using polyclonal sera from infected and vaccinated individuals [129]. While morphology affects infectivity and antigenicity [65,126], antibody binding can affect viral function. The flavivirus humoral response generates antibodies against E and prM; a peculiar feature of some prM antibodies is their ability to render immature particles infectious as these facilitate binding and internalization into cells containing Fcγ receptors [69,130].
4. JE Serocomplex Flavivirus Immune Response
4.1. Innate Immunity
Clinical manifestations of JE serocomplex virus infections in humans span a wide range, from asymptomatic prevalence or mild febrile illness to neuro-invasive disease and encephalitis. Severe neurological disease is more likely to afflict the elderly and immune-compromised individuals. Studies aimed at understanding the molecular basis of pathogenesis and the host immune response in these diverse scenarios, using in vitro and in vivo murine models such as mice with single or double knock-outs of effector molecules, their receptors or other components, helped identify cellular components and biomarkers critical for viral restriction and the spread of infection in peripheral tissues as well as identify factors that may contribute to neuro-invasive disease, immunopathology or exacerbation of outcomes toward severe disease [105,131,132]. In addition to the three structural proteins (E, C and prM), the flavivirus polyprotein encodes seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) [53]. Protection emanating from the innate defense against these viral components generates a non-specific, broad-range response that is essential for viral restriction and clearance and limiting progression of the disease. Pattern recognition receptors (PRRs) on mammalian antigen-presenting cells, Langerhans, human primary keratinocytes and dermal dendritic cells in the skin detect pathogen-associated molecular patterns (PAMPs) such as double-strand viral RNA. Binding of viral components triggers a downstream signaling cascade, beginning with the activation of one or more of the three kinds of receptors: retinoic-acid inducible gene-I (RIG-I)-like receptors (RLRs); melanoma-differentiation-associated-gene 5 (MDA5) in the cytoplasm; and nucleotide oligomerization domain (Nod)-like receptors (NLRs) and Toll-like receptors (TLR; e.g., TLR3, TLR7 and TLR8) in endosomes [132,133,134,135]. Activation of downstream adapter molecules with kinase activity (e.g., NEMO, IKKα, IKKβ; TBK, IKKε) activates transcription factors (e.g., IRF3 and IRF7) and NF-κB in a cell-type-specific manner [132,133]. IRF1, IRF3, IRF5 and IRF7 all restrict WNV replication; however, IRF5 plays a non-redundant, immunomodulatory role in shaping the early immune response events via production of pro-inflammatory cytokines in the lymphoid tissues, but not type I interferons, and also affects the trafficking and activation of immune cells entering the draining lymph node [136]. IRF5 further adversely impacts early antibody response in mice. In vivo experiments revealed that IPS-1, a key adaptor in the RIG-I signaling pathway, and a transcription factor Batf3 regulate inflammation via interactions with T cells [137,138]. Once translocated to the nucleus, each transcription factor induces the expression of specific genes, and, subsequently, the production of inflammatory cytokines (type I (IFN-α, IFN-β), type II (IFN-γ) and type-III (IFN-λ) interferons), and a multitude of interferon-stimulating genes (ISGs), which have antiviral effects as they restrict viral replication and dissemination in the host [105,133,139,140,141,142]. Eliciting production of interferons with a different virus was also shown to reduce WNV titers in vitro [143]. Secreted cytokines further interact with interferon receptors in virus-infected cells (autocrine or paracrine) and activate the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway. The interactions of effectors of this pathway with interferon regulatory factors (IRFs) and binding to interferon-stimulated response elements triggers the expression of ISGs that eventually exert an antiviral state directly through effectors [144].
Known viral features that play a role in conferring neuro-invasiveness in WNV, as in many other flaviviruses, including those of JE serogroup, reside on the structural envelope protein, and N-linked glycosylation specifically is in the E protein [145]. In contrast, in USUV, N-linked glycosylation is present at Asn154 and Asn118 across strains, yet human USUV infections display a wide range of manifestation from asymptomatic presence with antibodies against the virus to meningo-encephalitis, suggesting that, in USUV, other features in the structural proteins or factors may facilitate neuroinvasion. The host milieu and components therein are also known to affect WNV neuro-invasiveness: the blood–brain barrier (BBB) is present at the blood-to-brain interface and is a physiologically and functionally distinct region made up of a vascular basal lamina; brain capillary endothelia cells (BCECs) characterized by the presence of tight junctions, adherens junctions and low vesicular traffic; and the neurovascular unit (NVU) consisting of pericytes, perivascular fibroblasts, glial cells and astrocytes [146]. Breaching the BBB may precede neuroinvasion and occurs via the compromised permeability of BCECs or the NVU, and in WNV infection, the underlying mechanisms of CNS entry and infection may involve cytokines like IL-6 and TNFα, semaphorin 7A, metalloproteinases, a ‘Trojan horse’ route utilizing infected immune cells, direct axonal retrograde transport, infection via the olfactory bulb and direct infection of cells of the NVU or neuron-to-neuron infection [134,147,148,149,150]. The neuroinvasion property differs across WNV strains and may involve variable mechanisms among WNV strains and between JE serocomplex viruses and is yet to be elucidated [151,152].
The innate immune response to WNV is essential for limiting viral dissemination to the CNS. In WNV-infected mice and patients with West Nile virus fever (WNF) and West Nile virus neuro-invasive disease (WNND), upregulation and elevated concentrations of a multitude of cytokines and chemokines were observed in sera and cerebrospinal fluid (CSF), with higher levels of pro-inflammatory cytokines in patients with WNND compared to those with WNF, and also during JEV infection [153,154,155]. These included markers of inflammation (such as IL1α, IL4, IFNα and TNFα), type 2 cytokines (such as IL4 and IL13) and IL10, which may be associated with the exacerbated immune response in patients with WNND. Neurological damage in WNND results from both neuro-inflammation and direct viral infection of the brain cells such as astrocytes, microglia and neurons. Studies in mouse models revealed an essential role for interferon γ (IFN-γ) in restricting viral replication, viral infection of peripheral tissues and the early onset of CNS infection. While the innate immune response is best described for WNV and investigated in JEV, these pathways are yet to be understood in-depth for USUV; however, animal models for in vivo and in vitro studies of USUV pathogenesis have been described [156]. Emerging research provides comparable insights into USUV and WNV pathogenesis and the immune response within a system: the use of distinct cellular tropism that involves specific receptors (langerin and DC-SIGN) and differential infection, replication and activation and the susceptibility of the innate immune response in different cell types such as Langerhans cells or human neural stem cells (hNSC) [157,158,159]. For instance, both WNV and USUV (strain Vienna_2001) induce a robust innate response in hNSC with high levels of type I and III IFNs and caspase-3, but WNV may be more efficient in evading the host immune response; the latter effect was also observed in dendritic cells [157,158]. JE serocomplex viruses, like other flaviviruses, have adapted multiple mechanisms to evade some innate immune response; therefore, these are not primary targets for therapeutic development [133,139].
4.2. Adaptive Immunity
The essential roles of CD4+ and CD8+ cells in modulation, control and protection in JE flavivirus infections have been described for JEV and WNV [105,142,160]. These involve viral clearance by CD4+ cells via its multitude effector functions including cytokine production, CD8+ response enhancement and maintenance of antibody response, and CD8+ cell-mediated lysis of infected cells by secretion of effectors such as Fas receptor, perforin or granzymes; however, the determinants of T cell immunity are better explored for WNV than for JEV or other JE serocomplex flaviviruses. For instance, in a mouse model of JEV encephalitis, the role of T helper cells was essential to maintain humoral immunity and counteract infection and lethality, and a robust CD8+ activation was marked by an increase in CD69 and CD25. However, the CD8+ response didn’t uniquely contribute to animal survival while the viral burden in the CNS of mice lacking this response was higher [161]. On the other hand, CD8+ is essential for clearance of WNV and in the CNS and recovery in mouse models [162], and variation in susceptibility was observed in mice deficient in Fas- or perforin-dependent cytolytic pathways between WNV lineage I and II, JEV and MVEV [161], which highlights the importance of elucidating a T cell response for specific flaviviruses of the same antigenic complex. Recent studies highlighted the important role of CD8+ cells in eliciting antibody-mediated protection in response to JEV vaccination in mice and in protecting JEV-infected mice via granule-mediated cytotoxic effects with a contribution of γ/δ TCR expressing T cells [163,164]. The T cell immune response in MVEV and USUV infections is less thoroughly defined. Some insights into the host T cell response to USUV infection comes from retrospective analyses of asymptomatic blood donors in WNV- or USUV-infected individuals, and a USUV-specific T cell response could be distinguished with high accuracy [165].
A significant protective defense is mounted by the host humoral immunity that generates neutralizing antibodies targeted against non-structural and structural proteins, including NS1, NS3, NS5, prM and the structural protein E on the virus surface. A number of epitopes of flavivirus E proteins have been mapped and some of the most potent neutralizing antibodies in flaviviruses like DENV and ZIKV are known to be directed against DIII and its lateral ridge comprising strand A, BC-, DE- and FG-loops [65]. Both B and T cell epitopes have been determined for JE viruses but fewer have been for JEV and MVEV than WNV, and a much lower number of epitopes has been identified for JE serocomplex viruses compared to DENV2 [166,167]. On the E protein, B cell epitopes in WNV map to the DIII lateral ridge, DI lateral ridge, DI linker region, DII hinge interface, DII dimer interface, DII central interface, DII lateral ridge and DII [113,168,169]. Unlike DENV, WNV neutralization is not dominated by DIII-lateral-ridge-directed antibodies, as studied in horses and humans and for the WNV strains associated with recent outbreaks [168,170,171], and includes a large repertoire directed at the fusion loop (discussed below). While epitopes are identified using many methods not limited to yeast display, structural biology techniques of cryogenic electron microscopy, nuclear magnetic resonance and X-ray crystallography, binding and neutralization assays, identification and investigations of escape mutants, the methods inform different aspects of antigen–antibody interactions and structural biology techniques can provide unique insights into quaternary epitopes. Secondly, current data show that, especially for B cell epitopes, sequence conservation of an epitope may not be sufficient to predict binding and/or neutralization, emphasizing a need for independent resolution of the underlying mechanisms of neutralization for different viruses [170]. For instance, E16 is a potent WNV antibody. Based on yeast display, critical residues and regions for antibody interaction were initially identified [172], the interactions were subsequently confirmed using structural studies [173]. However, substitution of a few residues within these regions renders E16 ineffective for USUV SAAR-1776 neutralization and shows an altered pattern for USUV CAR-1969 [75,174]. Polyclonal antibodies generated against WNV in humans have been reported to show narrow-specificity targeting regions in DI and DII rather than DIII, with the majority displaying broad cross-reactivity [168,170]; whether this is a feature of all members the JE serogroup including MVEV and USUV is unclear.
Flavivirus neutralization is affected by many factors, not all of which are well defined for a virus/strain. For instance, neutralization by mouse monoclonal antibodies (MAbs) against DENV4 was shown to be strain- and genotype-dependent; this neutralization varied at different temperatures (37 °C or 40 °C), whereas after incubation at 37 °C, cross-reactive antibodies against FL of DENV1–3 weakly neutralized multiple strains of DENV4 [127]. Known factors that affect binding and neutralization include accessibility of the epitope, affinity of the antibody, maturation and conformational state of the virus and the specific stoichiometry of the antibody to achieve neutralization [175,176]. Sub-neutralizing antibodies may lead to neutralization at higher concentrations [177]. The cryo-EM structure of WNV virus in complex with the therapeutic antibody E16 showed that the antibody targets sixteen residues on loops of DIII and forms a network of hydrogen bond interactions [173,178]; E16 was shown to neutralize by blocking acid-induced membrane fusion. In a separate study, two potent antibodies against JEV, 2F2 and 2H4, were also shown to bind quaternary epitopes; the binding interface spans three adjacent E monomers in the asymmetric subunit, with four such interfaces locking the E monomers of a raft [179]. On the E protein, the interfacing residues map to DIII and DI-DII hinge regions, which tend to differ between flaviviruses but are conserved among the genotypes of JEV. The two antibodies blocked receptor attachment and JEV entry as well as endosomal membrane fusion.
4.3. Flavivirus Cross-Reactivity
The impact of flavivirus cross-reactivity is pertinent for the development of therapeutics because of the presence of multiple flaviviruses circulating in any given region and hence the possibility of simultaneous or sequential (homotypic and especially secondary heterotypic) human infections. The changing geographic distribution of flavivirus strains/genotypes is in part due to changing global climate, as well as new introductions [180]. Complexity in the adaptive immune control of sequential, secondary homo- or heterotypic flavivirus infections results from: (1) Amino acid sequence conservation within the antigen affecting antigenic diversity. The highly cross-reactive FL of flavivirus E protein is also one of the most conserved regions across flaviviruses and mutations in this region result in lower cross-reactivity in in vitro studies [4,181,182,183]; (2) the original antigenic sin, which means that the secondary exposure to a variant, non-identical antigen is not recognized as such by the B cells (or the cytotoxic T lymphocytes) and the immune system relies on its ‘memory’ of the original, primary antigen to mount a response to the variant, which is inadequate and ineffective. This altered memory recall results in production of sub-neutralizing antibodies that fail to control the secondary infection or additionally leads to a worse clinical outcome [184]; (3) antibody-dependent enhancement (ADE), resulting from the original antigenic sin. When less effective, non-neutralizing antibodies are generated in response to an often heterotypic secondary flavivirus infection. These antibodies can be internalized and sequestered into monocytes, macrophages and mast cells containing fragment γ receptors (Fcγ) and complement receptors. This amplifies viral replication and leads to the worsening of disease. ADE is a hallmark of DENV and various flavivirus infections, including those involving vaccination-induced immunity in human infections and mouse models [185,186]. Antibody-facilitated infection enhancement via routes not involving cellular receptors has been shown in experiments and can also occur when antibody-bound infected cells are lysed by cytotoxic natural killer cells, referred to as antibody-dependent cellular cytotoxicity [185,187,188,189,190,191].
Cross-reactive immune responses to flavivirus infections are, therefore, a double-edged sword both for flavivirus diagnostics as well as therapeutics. While epitopes on non-structural proteins elicit adaptive immune responses, the neutralizing humoral response against the structural E protein constitutes a dominant avenue of protection against flavivirus infections; these antibodies are primarily directed toward epitopes localized on DIII and the lateral ridge and tend to be type-specific [169]. In vitro assays showed greater cross-reactivity and neutralizing titers when E protein was used as a marker rather than NS1, although titers of the neutralizing antibodies may not directly correlate with protection in natural infections [192]. In mouse experiments with WNV, DIII-epitope-generated antibodies were shown to comprise a fraction of the total initial antibody response and were overshadowed by cross-reactive, sub-neutralizing antibodies [193]. The latter can confer protection at higher concentrations or via the antibody effector functions involving complement fixation and antibody-mediated cytotoxicity, emphasizing the importance of B cell memory recall; therefore, lack of or partial neutralization in vivo may not be an ideal indicator of protection for outcomes for natural secondary infections [177,194,195]. Cross-reactive B cell epitopes are often characterized by distinct structural attributes such as cryptic presence on E and localization on the DII domain, near the FL, and these epitopes include distinct, highly conserved amino acid residues; peptide fragments in DII; and distinct as well as overlapping regions in the E protein, not limited to amino acid residues of the fusion loop [171,192,196,197]. Cross-reactive T cell epitopes associated with CD8 and CD4 T cells have been localized to the non-structural proteins as well as the E protein and induce either cross-protection or immunopathology in homotypic as well as heterotypic infections involving JE serocomplex viruses [4,198]. Because these epitopes elicit heterotypic cross-protection, there’s a need to define the molecular and immunological determinants of cross-reactive T cell-based immunity in animal models that can be used to inform the development of a pan-flavivirus or pan-JE-serocomplex-virus vaccine [199,200,201].
Pre-existing immunity to a flavivirus can result from asymptomatic viral exposure, natural viral infection or immunization. Four licensed vaccines for human use exist for JEV infection (Table 3) and vaccines for human use are unlikely to be developed for other JE serocomplex viruses that cause infrequent outbreaks. These licensed vaccines and those under development were utilized to explore cross-reactive immune responses in animal experiments. JEV vaccination using JE-ADVAX or a DNA vaccine derived from the expression of prM/E proteins induced protection against a lethal JEV challenge in animal experiments, resulting primarily from humoral and cellular immunity while the CD8 T cell immunity was dispensable for survival [161,202,203]. Similar experiments in mice using recombinant vaccinia virus that carried the genes for the E and NS1 proteins of MVEV showed complete protection with a subsequent challenge with MVEV by eliciting generation of neutralizing antibodies, rather than a CD8+ immune response when E protein was used; passive transfer of MVEV-infected human sera also conferred protection with a subsequent MVEV challenge [204]. Cross-protection due to neutralizing antibodies resulting from CD4 T cell expansion following infection after JEV immunization, but not with YF immunization, has also been reported against ZIKV and DENV [205,206,207]. Li et al. reported cross-protection against all four serotypes of DENV following JEV immunization, using more than one JEV vaccine [208]. Chimeric vaccines using components of the JEV live vaccine strain SA-14-14-2 have been reported to protect against YF and TBEV infections, with dual protection against YFV and JEV in case of the former [209]. Determinants of cross-protection via CD4 T cell immunity map to two helices in the capsid protein and regions of E [210,211]. Koblischke et al. [210] reported peptide regions that form the immune-dominant WNV E epitopes, and one such region (E149) is unique and absent in DENV and ZIKV and lies on a structurally divergent region in WNV. Multiple research findings from mouse experiments and human studies revealed the essential role of cellular immunity and neutralizing antibodies in cross-protection between primary JEV or WNV exposure and secondary DENV and ZIKV infections [212]. The sequence of infection is known to influence disease outcomes; additionally, immune-dominant mechanisms of protection for each dyad of viral infections can be distinct and essential to elucidate if these outcomes are to inform therapeutics. For instance, in the sequential JEV-ZIKV infection, unlike the JEV-JEV infection, JEV vaccination-induced CD8 T cell immunity was found to be essential for conferring cross-protection in mouse models compared to passive transfer of serum [213,214]. Alternatively, cross-protective outcomes in JEV-DENV1 infection in mouse models required the cooperative effects of humoral and T cell responses, whereas in human infections, secondary DENV following anti-JEV immunity resulted in manifestation of increased viral symptoms [206,212,215].
Table 3.
Cross-reactivity among the four JE serocomplex viruses, JEV, MVEV, WNV and USUV, and the outcomes for disease are depicted. Viruses listed across represent preexisting immunity, primary challenge or vaccination and viruses down the column represent secondary infection. Flaviviruses circulating in distinct areas with no currently reported co-existence are denoted by ‘Distinct geography’.
| Virus (Infection or Vaccination) | Outcome | JEV | MVEV | WNV | USUV |
|---|---|---|---|---|---|
| JEV | Protection Pathology |
[203] [195] - |
[216] [186] [217] [218] [186] |
[203] [217] [219] [220] |
Distinct geography |
| MVEV | Protection Pathology |
[221] [217] [195] [186] |
[216] [204] - |
TBD/Unknown TBD/Unknown |
Distinct geography |
| WNV | Protection Pathology/no protection |
[222] [223] [195] [224] [217] [219] [225] [226] |
TBD/Unknown - |
[227] [228] - |
[229] [230] [231] - |
| USUV | Protection Pathology |
Distinct geography TBD/Unknown |
Distinct geography TBD/Unknown |
[227] [232] TBD/Unknown |
[233] [234] - |
Multiple studies explored the outcomes of sequential homotypic infections with JE serocomplex viruses (Table 3 and Table 4). Vaccination of different mouse models with JEV (JE-ADVAX, live-chimeric JEV vaccine) or sera from adult mice that were infected sub-lethally with JEV revealed the generation of cross-protective humoral and cellular immunity and the protection of homotypic secondary MVEV and WNV infections [195,216,219,221,222]. In some studies, protection was observed in the absence of detectable neutralizing antibodies and dispensable CD8 T cell immunity, emphasizing the role of memory B cells in conferring long-term protection against the secondary MVEV or WNV infections virus [195]. Animal studies in macaques revealed that, while immunization with JEV vaccine completely protected against a secondary WNV infection, immunizing with the latter protected partially against disease severity [217]. These studies emphasize the potential of using JEV vaccines against a range of JE serocomplex viruses. In the human population in Europe, USUV antibodies have been found in patients with severe WNV neuro-invasive disease [235]. Blazquez et al. [229] reported differential susceptibilities of adult and suckling mice to USUV infection, neutralization of secondary USUV following WNV infection and protection against neuro-invasive WNV disease in mice pre-infected with USUV. WNV recombinant subviral particles were able to induce cross-reactive humoral response in USUV, albeit at low levels, and a USUV-based recombinant DNA vaccine could also elicit neutralizing antibodies in adult mice deficient in interferon alpha/beta receptor [227,233].
Table 4.
Vaccines for JE serocomplex viruses that are licensed for use in humans.
| Virus | Strain | Vaccine | Platform | References |
|---|---|---|---|---|
| JEV | Genotype III | JE-VAX | Inactivated, derived from mouse brain | [236,237,238] |
| JEV | Genotype III | SA-14-14-2 | Live attenuated strain SA-14-14-2, derived from cell culture | [239] |
| JEV | Genotype III | IXIARO, JEBIK V | Inactivated, derived from cell culture | [240,241,242] |
| JEV | Genotype III | IMOJEV | Attenuated, chimeric, derived from cell culture | [241] |
Understanding the factors that drive disease enhancement in sequential JE serocomplex viruses is essential to design therapeutic interventions for multiple viruses and because human outbreaks of some JE serocomplex viruses are too infrequent to support development of virus-specific vaccines. Experimental studies [220] caution the possibility of ADE when the effect of sub-optimal concentrations of neutralizing antibodies against JEV were used to explore the effect on a secondary infection with MVEV. Similarly, immunization with MVEV, using killed virus or vector-delivered structural-protein-based subunit vaccine, followed by challenge with JEV in BALB/c mice showed enhanced disease and protection, respectively [186,220]. A low dose of inactivated JEV vaccine or sera from mice that were sub-lethally infected with MVEV caused ADE when subsequently challenged with MVE. However, in a different study, utilizing inactivated JEV vaccine in animals elicited partial protection against WNV infection, emphasizing the importance of consideration of dosage of the priming virus [216,221]. Sub-lethal immunization with KUNV instead of WNV and passive transfer of sera of mice infected with MVEV resulted in enhanced secondary MVEV disease [216]. However, disease enhancement in the human population in response to a JE vaccine eliciting humoral response with or without augmenting T cell immunity is seemingly unlikely [194,220,243] and the robust immune responses generated to cross-protect JE viruses suggest a strong consideration for harnessing cross-protective immunity for vaccine development.
5. Summary
The end goal of flavivirus research centers on developing vaccines and effective therapeutics. With the climate crisis and changing distribution of vectors and epidemiology of pathogens, this need is urgent. To this end, convergence of research on drivers of epidemiological changes, flavivirus structural biology, studies in animal models and natural exposure in humans, and immunology can advance our understanding. Connecting the dots between immunological correlates of cross-protection with molecular mechanisms and interactions is challenging, and is limited, in part, by the resolution and availability of structures of complexes of virus and host components. Recent high-resolution structures of JE serocomplex flaviviruses, advances in methods in cryo-electron microscopy and deep learning approaches may enable greater understanding to design effective strategies in the near future.
Author Contributions
Conceptualization and ideation, B.K. and R.J.K.; Literature search and data analysis, B.K.; Original draft preparation and editing, B.K.; review, B.K. and R.J.K. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by NIH National Institute of Allergy and Infectious Diseases awards AI073755 and AI095366 to R.J.K.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Daep C.A., Muñoz-Jordán J.L., Eugenin E.A. Flaviviruses, an expanding threat in public health: Focus on dengue, West Nile, and Japanese encephalitis virus. J. NeuroVirol. 2014;20:539–560. doi: 10.1007/s13365-014-0285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke D.H., Casals J. Techniques for Hemagglutination and Hemagglutination-Inhibition with Arthropod-Borne Viruses. Am. J. Trop. Med. Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 3.De Madrid A.T., Porterfield J.S. The Flaviviruses (Group B Arboviruses): A Cross-neutralization Study. J. Gen. Virol. 1974;23:91–96. doi: 10.1099/0022-1317-23-1-91. [DOI] [PubMed] [Google Scholar]
- 4.Rathore A.P.S., St John A.L. Cross-Reactive Immunity among Flaviviruses. Front. Immunol. 2020;11:334. doi: 10.3389/fimmu.2020.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuno G., Chang G.-J.J., Tsuchiya K.R., Karabatsos N., Cropp C.B. Phylogeny of the Genus Flavivirus. J. Virol. 1998;72:73–83. doi: 10.1128/JVI.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakonyi T., Gould E.A., Kolodziejek J., Weissenböck H., Nowotny N. Complete genome analysis and molecular characterization of Usutu virus that emerged in Austria in 2001: Comparison with the South African strain SAAR-1776 and other flaviviruses. Virology. 2004;328:301–310. doi: 10.1016/j.virol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Benzarti E., Linden A., Desmecht D., Garigliany M. Mosquito-borne epornitic flaviviruses: An update and review. J. Gen. Virol. 2019;100:119–132. doi: 10.1099/jgv.0.001203. [DOI] [PubMed] [Google Scholar]
- 8.Turtle L., Solomon T. Japanese encephalitis—the prospects for new treatments. Nat. Rev. Neurol. 2018;14:298–313. doi: 10.1038/nrneurol.2018.30. [DOI] [PubMed] [Google Scholar]
- 9.Le Flohic G., Porphyre V., Barbazan P., Gonzalez J.-P. Review of Climate, Landscape, and Viral Genetics as Drivers of the Japanese Encephalitis Virus Ecology. PLOS Negl. Trop. Dis. 2013;7:e2208. doi: 10.1371/journal.pntd.0002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackenzie J.S., Lindsay M.D., Coelen R.J., Broom A.K., Hall R.A., Smith D.W. Arboviruses causing human disease in the Australasian zoogeographic region. Arch. Virol. 1994;136:447–467. doi: 10.1007/BF01321074. [DOI] [PubMed] [Google Scholar]
- 11.Colpitts T., Rodenhuis-Zybert I., Moesker B., Wang P., Fikrig E., Smit J.M. prM-antibody renders immature West Nile virus infectious in vivo. J. Gen. Virol. 2011;92:2281–2285. doi: 10.1099/vir.0.031427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chancey C., Grinev A., Volkova E., Rios M. The Global Ecology and Epidemiology of West Nile Virus. BioMed Res. Int. 2015;2015:376230. doi: 10.1155/2015/376230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clé M., Beck C., Salinas S., Lecollinet S., Gutierrez S., Van de Perre P., Baldet T., Foulongne V., Simonin Y. Usutu virus: A new threat? Epidemiol. Infect. 2019;147:e232. doi: 10.1017/S0950268819001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonin Y., Sillam O., Carles M.J., Gutierrez S., Gil P., Constant O., Martin M.F., Grard G., Van de Perre P., Salinas S., et al. Human Usutu Virus Infection with Atypical Neurologic Presentation, Montpellier, France, 2016. Emerg. Infect. Dis. 2018;24:875–878. doi: 10.3201/eid2405.171122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Go Y.Y., Balasuriya U.B.R., Lee C.-K. Zoonotic encephalitides caused by arboviruses: Transmission and epidemiology of alphaviruses and flaviviruses. Clin. Exp. Vaccine Res. 2014;3:58–77. doi: 10.7774/cevr.2014.3.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver S.C. Infectious Diseases from Nature: Mechanisms of Viral Emergence and Persistence. Springer; Berlin/Heidelberg, Germany: 2005. Host range, amplification and arboviral disease emergence; pp. 33–44. [DOI] [PubMed] [Google Scholar]
- 17.Chen L.H., Wilson M.E. Update on non-vector transmission of dengue: Relevant studies with Zika and other flaviviruses. Trop. Dis. Travel Med. Vaccines. 2016;2:1–6. doi: 10.1186/s40794-016-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricklin M.E., García-Nicolás O., Brechbühl D., Python S., Zumkehr B., Nougairede A., Charrel R.N., Posthaus H., Oevermann A., Summerfield A. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat. Commun. 2016;7:10832. doi: 10.1038/ncomms10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belgrave R.L. Chapter 35—West Nile Virus. In: Sprayberry K.A., Robinson N.E., editors. Robinson’s Current Therapy in Equine Medicine. 7th ed. W.B. Saunders; St. Louis, MO, USA: 2015. pp. 152–154. [Google Scholar]
- 20.Banet-Noach C., Simanov L., Malkinson M. Direct (non-vector) transmission of West Nile virus in geese. Avian Pathol. 2003;32:489–494. doi: 10.1080/0307945031000154080. [DOI] [PubMed] [Google Scholar]
- 21.Motta I.J., Spencer B.R., Cordeiro da Silva S.G., Arruda M.B., Dobbin J.A., Gonzaga Y.B., Arcuri I.P., Tavares R.C., Atta E.H., Fernandes R.F., et al. Evidence for Transmission of Zika Virus by Platelet Transfusion. N. Engl. J. Med. 2016;375:1101–1103. doi: 10.1056/NEJMc1607262. [DOI] [PubMed] [Google Scholar]
- 22.Lwande O.W., Venter M., Lutomiah J., Michuki G., Rumberia C., Gakuya F., Obanda V., Tigoi C., Odhiambo C., Nindo F., et al. Whole genome phylogenetic investigation of a West Nile virus strain isolated from a tick sampled from livestock in north eastern Kenya. Parasites Vectors. 2014;7:542. doi: 10.1186/s13071-014-0542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habarugira G., Moran J., Colmant A.M., Davis S.S., O’Brien C.A., Hall-Mendelin S., McMahon J., Hewitson G., Nair N., Barcelon J., et al. Mosquito-Independent Transmission of West Nile virus in Farmed Saltwater Crocodiles (Crocodylus porosus) Viruses. 2020;12:198. doi: 10.3390/v12020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blitvich B.J., Magalhaes T., Laredo-Tiscareño S.V., Foy B.D. Sexual Transmission of Arboviruses: A Systematic Review. Viruses. 2020;12:933. doi: 10.3390/v12090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuh A.J., Ward M.J., Brown A.J.L., Barrett A.D.T. Dynamics of the Emergence and Establishment of a Newly Dominant Genotype of Japanese Encephalitis Virus throughout Asia. J. Virol. 2014;88:4522–4532. doi: 10.1128/JVI.02686-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao X., Liu H., Li X., Fu S., Cao L., Shao N., Zhang W., Wang Q., Lu Z., Lei W., et al. Changing Geographic Distribution of Japanese Encephalitis Virus Genotypes, 1935–2017. Vector-Borne Zoonotic Dis. 2019;19:35–44. doi: 10.1089/vbz.2018.2291. [DOI] [PubMed] [Google Scholar]
- 27.Gill C.M., Beckham J.D., Piquet A.L., Tyler K.L., Pastula D.M. Five Emerging Neuroinvasive Arboviral Diseases: Cache Valley, Eastern Equine Encephalitis, Jamestown Canyon, Powassan, and Usutu. Semin. Neurol. 2019;39:419–427. doi: 10.1055/s-0039-1687839. [DOI] [PubMed] [Google Scholar]
- 28.Bellone R., Failloux A.-B. The Role of Temperature in Shaping Mosquito-Borne Viruses Transmission. Front. Microbiol. 2020;11:584846. doi: 10.3389/fmicb.2020.584846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang G.-D., Huanyu W. Epidemiology of Japanese encephalitis: Past, present, and future prospects. Ther. Clin. Risk Manag. 2015;11:435–448. doi: 10.2147/TCRM.S51168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao X., Liu H., Li M., Fu S., Liang G. Insights into the evolutionary history of Japanese encephalitis virus (JEV) based on whole-genome sequences comprising the five genotypes. Virol. J. 2015;12:43. doi: 10.1186/s12985-015-0270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams D.T., Diviney S.M., Niazi A.-U., Durr P.A., Chua B.H., Herring B., Pyke A., Doggett S.L., Johansen C.A., Mackenzie J.S. The Molecular Epidemiology and Evolution of Murray Valley Encephalitis Virus: Recent Emergence of Distinct Sub-lineages of the Dominant Genotype 1. PLOS Negl. Trop. Dis. 2015;9:e0004240. doi: 10.1371/journal.pntd.0004240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell J.S., Caly L., Kostecki R., McGuinness S.L., Carter G., Bulach D., Seemann T., Stinear T.P., Baird R., Catton M., et al. The First Isolation and Whole Genome Sequencing of Murray Valley Encephalitis Virus from Cerebrospinal Fluid of a Patient with Encephalitis. Viruses. 2018;10:319. doi: 10.3390/v10060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.French E.L., Anderson S.G., Price A.V., Rhodes F.A. Murray Valley encephalitis in New Guinea. I. Isolation of Murray Valley encephalitis virus from the brain of a fatal case of encephalitis occurring in a Papuan native. Am. J. Trop. Med. Hyg. 1957;6:827–828. doi: 10.4269/ajtmh.1957.6.827. [DOI] [PubMed] [Google Scholar]
- 34.Williams D.T., Diviney S.M., Corscadden K.J., Chua B.H., Mackenzie J.S. Complete Genome Sequences of the Prototype Isolates of Genotypes 2, 3, and 4 of Murray Valley Encephalitis Virus. Genome Announc. 2014;2:e00581-14. doi: 10.1128/genomeA.00581-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulbert S. West Nile Virus: The Complex Biology of an Emerging Pathogen. Intervirology. 2011;54:171–184. doi: 10.1159/000328320. [DOI] [PubMed] [Google Scholar]
- 36.Bakonyi T., Ivanics É., Erdélyi K., Ursu K., Ferenczi E., Weissenböck H., Nowotny N. Lineage 1 and 2 Strains of Encephalitic West Nile Virus, Central Europe. Emerg. Infect. Dis. 2006;12:618–623. doi: 10.3201/eid1204.051379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fall G., Di Paola N., Faye M., Dia M., de Melo Freire C.C., Loucoubar C., de Andrade Zanotto P.M., Faye O., Sall A.A. Biological and phylogenetic characteristics of West African lineages of West Nile virus. PLOS Negl. Trop. Dis. 2017;11:e0006078. doi: 10.1371/journal.pntd.0006078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakonyi T., Ferenczi E., Erdélyi K., Kutasi O., Csörgő T., Seidel B., Weissenböck H., Brugger K., Bán E., Nowotny N. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet. Microbiol. 2013;165:61–70. doi: 10.1016/j.vetmic.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Bakonyi T., Hubálek Z., Rudolf I., Nowotny N. Novel Flavivirus or New Lineage of West Nile Virus, Central Europe. Emerg. Infect. Dis. 2005;11:225–231. doi: 10.3201/eid1102.041028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pachler K., Lebl K., Berer D., Rudolf I., Hubalek Z., Nowotny N. Putative New West Nile Virus Lineage in Uranotaenia unguiculata Mosquitoes, Austria, 2013. Emerg. Infect. Dis. 2014;20:2119–2122. doi: 10.3201/eid2012.140921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolay B., Diallo M., Boye C.S.B., Sall A.A. Usutu Virus in Africa. Vector-Borne Zoonotic Dis. 2011;11:1417–1423. doi: 10.1089/vbz.2011.0631. [DOI] [PubMed] [Google Scholar]
- 42.Vilibic-Cavlek T., Petrovic T., Savic V., Barbic L., Tabain I., Stevanovic V., Klobucar A., Mrzljak A., Ilic M., Bogdanic M., et al. Epidemiology of Usutu Virus: The European Scenario. Pathogens. 2020;9:699. doi: 10.3390/pathogens9090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hönig V., Palus M., Kaspar T., Zemanova M., Majerova K., Hofmannova L., Papezik P., Sikutova S., Rettich F., Hubalek Z., et al. Multiple Lineages of Usutu Virus (Flaviviridae, Flavivirus) in Blackbirds (Turdus merula) and Mosquitoes (Culex pipiens, Cx. modestus) in the Czech Republic (2016–2019) Microorganisms. 2019;7:568. doi: 10.3390/microorganisms7110568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pecorari M., Longo G., Gennari W., Grottola A., Sabbatini A.M., Tagliazucchi S., Savini G., Monaco F., Simone M.L., Lelli R., et al. First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Eurosurveillance. 2009;14:19446. doi: 10.2807/ese.14.50.19446-en. [DOI] [PubMed] [Google Scholar]
- 45.Nagy A., Mezei E., Nagy O., Bakonyi T., Csonka N., Kaposi M., Koroknai A., Szomor K., Rigó Z., Molnár Z., et al. Extraordinary increase in West Nile virus cases and first confirmed human Usutu virus infection in Hungary, 2018. Eurosurveillance. 2019;24:28. doi: 10.2807/1560-7917.ES.2019.24.28.1900038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pacenti M., Sinigaglia A., Martello T., De Rui M.E., Franchin E., Pagni S., Peta E., Riccetti S., Milani A., Montarsi F., et al. Clinical and virological findings in patients with Usutu virus infection, northern Italy, 2018. Eurosurveillance. 2019;24:1900180. doi: 10.2807/1560-7917.ES.2019.24.47.1900180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Apte-Sengupta S., Sirohi D., Kuhn R.J. Coupling of replication and assembly in flaviviruses. Curr. Opin. Virol. 2014;9:134–142. doi: 10.1016/j.coviro.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierson T.C., Kielian M. Flaviviruses: Braking the entering. Curr. Opin. Virol. 2013;3:3–12. doi: 10.1016/j.coviro.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez-Garcia M.D., Mazzon M., Jacobs M., Amara A. Pathogenesis of Flavivirus Infections: Using and Abusing the Host Cell. Cell Host Microbe. 2009;5:318–328. doi: 10.1016/j.chom.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Nain M., Abdin M.Z., Kalia M., Vrati S. Japanese encephalitis virus invasion of cell: Allies and alleys. Rev. Med Virol. 2016;26:129–141. doi: 10.1002/rmv.1868. [DOI] [PubMed] [Google Scholar]
- 51.Barnard T.R., Abram Q.H., Lin Q.F., Wang A.B., Sagan S.M. Molecular Determinants of Flavivirus Virion Assembly. Trends Biochem. Sci. 2021;46:378–390. doi: 10.1016/j.tibs.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Hasan S.S., Sevvana M., Kuhn R.J., Rossmann M.G. Structural biology of Zika virus and other flaviviruses. Nat. Struct. Mol. Biol. 2018;25:13–20. doi: 10.1038/s41594-017-0010-8. [DOI] [PubMed] [Google Scholar]
- 53.Dey D., Poudyal S., Rehman A., Hasan S.S. Structural and biochemical insights into flavivirus proteins. Virus Res. 2021;296:198343. doi: 10.1016/j.virusres.2021.198343. [DOI] [PubMed] [Google Scholar]
- 54.Mukhopadhyay S., Kuhn R.J., Rossmann M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 55.Sevvana M., Kuhn R.J. Mapping the diverse structural landscape of the flavivirus antibody repertoire. Curr. Opin. Virol. 2020;45:51–64. doi: 10.1016/j.coviro.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pulkkinen L.I.A., Barrass S.V., Domanska A., Överby A.K., Anastasina M., Butcher S.J. Molecular Organisation of Tick-Borne Encephalitis Virus. Viruses. 2022;14:792. doi: 10.3390/v14040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morita E., Suzuki Y. Membrane-Associated Flavivirus Replication Complex—Its Organization and Regulation. Viruses. 2021;13:1060. doi: 10.3390/v13061060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajah M.M., Monel B., Schwartz O. The entanglement between flaviviruses and ER-shaping proteins. PLOS Pathog. 2020;16:e1008389. doi: 10.1371/journal.ppat.1008389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gillespie L.K., Hoenen A., Morgan G., Mackenzie J.M. The Endoplasmic Reticulum Provides the Membrane Platform for Biogenesis of the Flavivirus Replication Complex. J. Virol. 2010;84:10438–10447. doi: 10.1128/JVI.00986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y., Kaufmann B., Chipman P.R., Kuhn R.J., Rossmann M.G. Structure of Immature West Nile Virus. J. Virol. 2007;81:6141–6145. doi: 10.1128/JVI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y., Corver J., Chipman P.R., Zhang W., Pletnev S.V., Sedlak D., Baker T.S., Strauss J.H., Kuhn R.J., Rossmann M.G. Structures of immature flavivirus particles. EMBO J. 2003;22:2604–2613. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Renner M., Dejnirattisai W., Carrique L., Martin I.S., Karia D., Ilca S.L., Ho S.F., Kotecha A., Keown J.R., Mongkolsapaya J., et al. Flavivirus maturation leads to the formation of an occupied lipid pocket in the surface glycoproteins. Nat. Commun. 2021;12:1238. doi: 10.1038/s41467-021-21505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu I.-M., Zhang W., Holdaway H.A., Li L., Kostyuchenko V.A., Chipman P.R., Kuhn R.J., Rossmann M.G., Chen J. Structure of the Immature Dengue Virus at Low pH Primes Proteolytic Maturation. Science. 2008;319:1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y., Zhang W., Ogata S., Clements D., Strauss J.H., Baker T.S., Kuhn R.J., Rossmann M.G. Conformational Changes of the Flavivirus E Glycoprotein. Structure. 2004;12:1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rey F.A., Stiasny K., Vaney M., Dellarole M., Heinz F.X. The bright and the dark side of human antibody responses to flaviviruses: Lessons for vaccine design. EMBO Rep. 2018;19:206–224. doi: 10.15252/embr.201745302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rey F.A., Lok S.-M. Common Features of Enveloped Viruses and Implications for Immunogen Design for Next-Generation Vaccines. Cell. 2018;172:1319–1334. doi: 10.1016/j.cell.2018.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heinz F.X., Stiasny K., Püschner-Auer G., Holzmann H., Allison S.L., Mandl C.W., Kunz C. Structural Changes and Functional Control of the Tick-Borne Encephalitis Virus Glycoprotein E by the Heterodimeric Association with Protein prM. Virology. 1994;198:109–117. doi: 10.1006/viro.1994.1013. [DOI] [PubMed] [Google Scholar]
- 68.Li L., Lok S.-M., Yu I.-M., Zhang Y., Kuhn R.J., Chen J., Rossmann M.G. The Flavivirus Precursor Membrane-Envelope Protein Complex: Structure and Maturation. Science. 2008;319:1830–1834. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]
- 69.Rodenhuis-Zybert I., Van Der Ende-Metselaar H., Wilschut J., Smit J.M. Functional importance of dengue virus maturation: Infectious properties of immature virions. J. Gen. Virol. 2008;89:3047–3051. doi: 10.1099/vir.0.2008/002535-0. [DOI] [PubMed] [Google Scholar]
- 70.Therkelsen M.D., Klose T., Vago F., Jiang W., Rossmann M.G., Kuhn R.J. Flaviviruses have imperfect icosahedral symmetry. Proc. Natl. Acad. Sci. 2018;115:11608–11612. doi: 10.1073/pnas.1809304115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan T.Y., Fibriansah G., Kostyuchenko V.A., Ng T.-S., Lim X.-X., Zhang S., Wang J., Shi J., Morais M.C., Corti D., et al. Capsid protein structure in Zika virus reveals the flavivirus assembly process. Nat. Commun. 2020;11:895. doi: 10.1038/s41467-020-14647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang X., Zhang Y., Jia R., Wang M., Yin Z., Cheng A. Structure and function of capsid protein in flavivirus infection and its applications in the development of vaccines and therapeutics. Vet. Res. 2021;52:98. doi: 10.1186/s13567-021-00966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blazevic J., Rouha H., Bradt V., Heinz F.X., Stiasny K. Membrane Anchors of the Structural Flavivirus Proteins and Their Role in Virus Assembly. J. Virol. 2016;90:6365–6378. doi: 10.1128/JVI.00447-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardy J.M., Newton N.D., Modhiran N., Scott C.A.P., Venugopal H., Vet L.J., Young P.R., Hall R.A., Hobson-Peters J., Coulibaly F., et al. A unified route for flavivirus structures uncovers essential pocket factors conserved across pathogenic viruses. Nat. Commun. 2021;12:3266. doi: 10.1038/s41467-021-22773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khare B., Klose T., Fang Q., Rossmann M.G., Kuhn R.J. Structure of Usutu virus SAAR-1776 displays fusion loop asymmetry. Proc. Natl. Acad. Sci. USA. 2021;118:e2107408118. doi: 10.1073/pnas.2107408118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng A., Yuan F., Kleinfelter L.M., Kielian M. A toggle switch controls the low pH-triggered rearrangement and maturation of the dengue virus envelope proteins. Nat. Commun. 2014;5:1–9. doi: 10.1038/ncomms4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuhn R.J., Zhang W., Rossmann M.G., Pletnev S.V., Corver J., Lenches E., Jones C.T., Mukhopadhyay S., Chipman P.R., Strauss E.G., et al. Structure of Dengue Virus: Implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/S0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaufmann B., Rossmann M.G. Molecular mechanisms involved in the early steps of flavivirus cell entry. Microbes Infect. 2011;13:1–9. doi: 10.1016/j.micinf.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sirohi D., Kuhn R.J. Zika Virus Structure, Maturation, and Receptors. J. Infect. Dis. 2017;216:S935–S944. doi: 10.1093/infdis/jix515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kostyuchenko V.A., Zhang Q., Tan J.L., Ng T.-S., Lok S.-M. Immature and Mature Dengue Serotype 1 Virus Structures Provide Insight into the Maturation Process. J. Virol. 2013;87:7700–7707. doi: 10.1128/JVI.00197-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rey F.A., Stiasny K., Heinz F.X. Flavivirus structural heterogeneity: Implications for cell entry. Curr. Opin. Virol. 2017;24:132–139. doi: 10.1016/j.coviro.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laureti M., Narayanan D., Rodriguez-Andres J., Fazakerley J.K., Kedzierski L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018;9:2180. doi: 10.3389/fimmu.2018.02180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tassaneetrithep B., Burgess T.H., Granelli-Piperno A., Trumpfheller C., Finke J., Sun W., Eller M.A., Pattanapanyasat K., Sarasombath S., Birx D.L., et al. DC-SIGN (CD209) Mediates Dengue Virus Infection of Human Dendritic Cells. J. Exp. Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang S., Zhang Q., Tiwari S.K., Lichinchi G., Yau E.H., Hui H., Li W., Furnari F., Rana T.M. Integrin αvβ5 Internalizes Zika Virus during Neural Stem Cells Infection and Provides a Promising Target for Antiviral Therapy. Cell Rep. 2020;30:969.e4–983.e4. doi: 10.1016/j.celrep.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu Z., Mesci P., Bernatchez J.A., Gimple R.C., Wang X., Schafer S.T., Wettersten H.I., Beck S., Clark A.E., Wu Q., et al. Zika Virus Targets Glioblastoma Stem Cells through a SOX2-Integrin αvβ5 Axis. Cell Stem Cell. 2020;26:187.e10–204.e10. doi: 10.1016/j.stem.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perera-Lecoin M., Meertens L., Carnec X., Amara A. Flavivirus Entry Receptors: An Update. Viruses. 2013;6:69–88. doi: 10.3390/v6010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yun S.-I., Lee Y.-M. Early Events in Japanese Encephalitis Virus Infection: Viral Entry. Pathogens. 2018;7:68. doi: 10.3390/pathogens7030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nain M., Mukherjee S., Karmakar S.P., Paton A.W., Paton J.C., Abdin M.Z., Basu A., Kalia M., Vrati S. GRP78 Is an Important Host Factor for Japanese Encephalitis Virus Entry and Replication in Mammalian Cells. J. Virol. 2017;91:e02274-16. doi: 10.1128/JVI.02274-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu T., Wu Z., Wu S., Chen S., Cheng A. The key amino acids of E protein involved in early flavivirus infection: Viral entry. Virol. J. 2021;18:136. doi: 10.1186/s12985-021-01611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prow N., May F., Westlake D.J., Hurrelbrink R.J., Biron R.M., Leung J.Y., McMINN P.C., Clark D.C., MacKenzie J.S., Lobigs M., et al. Determinants of attenuation in the envelope protein of the flavivirus Alfuy. J. Gen. Virol. 2011;92:2286–2296. doi: 10.1099/vir.0.034793-0. [DOI] [PubMed] [Google Scholar]
- 91.Lee E., Hall R.A., Lobigs M. Common E Protein Determinants for Attenuation of Glycosaminoglycan-Binding Variants of Japanese Encephalitis and West Nile Viruses. J. Virol. 2004;78:8271–8280. doi: 10.1128/JVI.78.15.8271-8280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Francese R., Civra A., Donalisio M., Volpi N., Capitani F., Sottemano S., Tonetto P., Coscia A., Maiocco G., Moro G.E., et al. Anti-Zika virus and anti-Usutu virus activity of human milk and its components. PLOS Negl. Trop. Dis. 2020;14:e0008713. doi: 10.1371/journal.pntd.0008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carbaugh D.L., LaZear H.M. Flavivirus Envelope Protein Glycosylation: Impacts on Viral Infection and Pathogenesis. J. Virol. 2020;94:94. doi: 10.1128/JVI.00104-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chu J.J.-H., Ng M.-L. Interaction of West Nile Virus with αvβ3 Integrin Mediates Virus Entry into Cells. J. Biol. Chem. 2004;279:54533–54541. doi: 10.1074/jbc.M410208200. [DOI] [PubMed] [Google Scholar]
- 95.dos Reis V.P., Keller M., Schmidt K., Ulrich R.G., Groschup M.H. αVβ3 Integrin Expression Is Essential for Replication of Mosquito and Tick-Borne Flaviviruses in Murine Fibroblast Cells. Viruses. 2021;14:18. doi: 10.3390/v14010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pokidysheva E., Zhang Y., Battisti A.J., Bator-Kelly C.M., Chipman P.R., Xiao C., Gregorio G.G., Hendrickson W.A., Kuhn R.J., Rossmann M.G. Cryo-EM Reconstruction of Dengue Virus in Complex with the Carbohydrate Recognition Domain of DC-SIGN. Cell. 2006;124:485–493. doi: 10.1016/j.cell.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 97.Stiasny K., Kössl C., Heinz F.X. Differences in the Postfusion Conformations of Full-Length and Truncated Class II Fusion Protein E of Tick-Borne Encephalitis Virus. J. Virol. 2005;79:6511–6515. doi: 10.1128/JVI.79.10.6511-6515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bressanelli S., Stiasny K., Allison S.L., Stura E., Duquerroy S., Lescar J., Heinz F.X., Rey F. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004;23:728–738. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang X., Sheng J., Austin S.K., Hoornweg T.E., Smit J.M., Kuhn R.J., Diamond M.S., Rossmann M.G. Structure of Acidic pH Dengue Virus Showing the Fusogenic Glycoprotein Trimers. J. Virol. 2015;89:743–750. doi: 10.1128/JVI.02411-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaufmann B., Chipman P.R., Holdaway H.A., Johnson S., Fremont D.H., Kuhn R.J., Diamond M.S., Rossmann M.G. Capturing a Flavivirus Pre-Fusion Intermediate. PLOS Pathog. 2009;5:e1000672. doi: 10.1371/journal.ppat.1000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Modis Y., Ogata S., Clements D., Harrison S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 102.Fritz R., Blazevic J., Taucher C., Pangerl K., Heinz F.X., Stiasny K. The Unique Transmembrane Hairpin of Flavivirus Fusion Protein E Is Essential for Membrane Fusion. J. Virol. 2011;85:4377–4385. doi: 10.1128/JVI.02458-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pierson T.C., Diamond M.S. Degrees of maturity: The complex structure and biology of flaviviruses. Curr. Opin. Virol. 2012;2:168–175. doi: 10.1016/j.coviro.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wengler G. Cell-associated West Nile flavivirus is covered with E+pre-M protein heterodimers which are destroyed and reorganized by proteolytic cleavage during virus release. J. Virol. 1989;63:2521–2526. doi: 10.1128/jvi.63.6.2521-2526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valiakos G., Athanasiou V.L., Touloudi A., Papatsiros V., Spyrou V., Petrovska L., Billinis L.P.A.C. Viral Replication. Intech Open; Rijeka, Croatia: 2013. West Nile Virus: Basic Principles, Replication Mechanism, Immune Response and Important Genetic Determinants of Virulence; pp. 38–46. [DOI] [Google Scholar]
- 106.Junjhon J., Edwards T.J., Utaipat U., Bowman V.D., Holdaway H.A., Zhang W., Keelapang P., Puttikhunt C., Perera R., Chipman P.R., et al. Influence of pr-M Cleavage on the Heterogeneity of Extracellular Dengue Virus Particles. J. Virol. 2010;84:8353–8358. doi: 10.1128/JVI.00696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plevka P., Battisti A.J., Junjhon J., Winkler D., Holdaway H.A., Keelapang P., Sittisombut N., Kuhn R.J., Steven A.C., Rossmann M.G. Maturation of flaviviruses starts from one or more icosahedrally independent nucleation centres. EMBO Rep. 2011;12:602–606. doi: 10.1038/embor.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Keelapang P., Sriburi R., Supasa S., Panyadee N., Songjaeng A., Jairungsri A., Puttikhunt C., Kasinrerk W., Malasit P., Sittisombut N. Alterations of pr-M Cleavage and Virus Export in pr-M Junction Chimeric Dengue Viruses. J. Virol. 2004;78:2367–2381. doi: 10.1128/JVI.78.5.2367-2381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zicari S., Arakelyan A., Fitzgerald W., Zaitseva E., Chernomordik L.V., Margolis L., Grivel J.-C. Evaluation of the maturation of individual Dengue virions with flow virometry. Virology. 2015;488:20–27. doi: 10.1016/j.virol.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fibriansah G., Lim X.-N., Lok S.-M. Morphological Diversity and Dynamics of Dengue Virus Affecting Antigenicity. Viruses. 2021;13:1446. doi: 10.3390/v13081446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X., Sheng J., Plevka P., Kuhn R.J., Diamond M.S., Rossmann M.G. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc. Natl. Acad. Sci. USA. 2013;110:6795–6799. doi: 10.1073/pnas.1304300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rey F.A. Two hosts, two structures. Nature. 2013;497:443–444. doi: 10.1038/497443a. [DOI] [PubMed] [Google Scholar]
- 113.Austin S.K., Dowd K.A. B Cell Response and Mechanisms of Antibody Protection to West Nile Virus. Viruses. 2014;6:1015–1036. doi: 10.3390/v6031015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goo L., VanBlargan L.A., Dowd K.A., Diamond M.S., Pierson T.C. A single mutation in the envelope protein modulates flavivirus antigenicity, stability, and pathogenesis. PLOS Pathog. 2017;13:e1006178. doi: 10.1371/journal.ppat.1006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang X., Li S.-H., Zhu L., Nian Q.-G., Yuan S., Gao Q., Hu Z., Ye Q., Li X.-F., Xie D.-Y., et al. Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability. Nat. Commun. 2017;8:1–9. doi: 10.1038/s41467-017-00024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sevvana M., Long F., Miller A.S., Klose T., Buda G., Sun L., Kuhn R.J., Rossmann M.G. Refinement and Analysis of the Mature Zika Virus Cryo-EM Structure at 3.1 Å Resolution. Structure. 2018;26:1169.e3–1177.e3. doi: 10.1016/j.str.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.DiNunno N.M., Goetschius D.J., Narayanan A., Majowicz S.A., Moustafa I., Bator C.M., Hafenstein S.L., Jose J. Identification of a pocket factor that is critical to Zika virus assembly. Nat. Commun. 2020;11:4953. doi: 10.1038/s41467-020-18747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morrone S.R., Chew V.S.Y., Lim X.-N., Ng T.-S., Kostyuchenko V.A., Zhang S., Wirawan M., Chew P.-L., Lee J., Tan J.L., et al. High flavivirus structural plasticity demonstrated by a non-spherical morphological variant. Nat. Commun. 2020;11:3112. doi: 10.1038/s41467-020-16925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shah P.N.M., Filman D.J., Karunatilaka K.S., Hesketh E.L., Groppelli E., Strauss M., Hogle J.M. Cryo-EM structures reveal two distinct conformational states in a picornavirus cell entry intermediate. PLOS Pathog. 2020;16:e1008920. doi: 10.1371/journal.ppat.1008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lok S.-M. The Interplay of Dengue Virus Morphological Diversity and Human Antibodies. Trends Microbiol. 2016;24:284–293. doi: 10.1016/j.tim.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 121.Lok S.-M., Kostyuchenko V., Nybakken G.E., Holdaway H.A., Battisti A.J., Sukupolvi-Petty S., Sedlak D., Fremont D.H., Chipman P.R., Roehrig J., et al. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat. Struct. Mol. Biol. 2008;15:312–317. doi: 10.1038/nsmb.1382. [DOI] [PubMed] [Google Scholar]
- 122.Nelson S., Jost C.A., Xu Q., Ess J., Martin J.E., Oliphant T., Whitehead S.S., Durbin A.P., Graham B.S., Diamond M.S., et al. Maturation of West Nile Virus Modulates Sensitivity to Antibody-Mediated Neutralization. PLOS Pathog. 2008;4:e1000060. doi: 10.1371/journal.ppat.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.VanBlargan L.A., Milutinovic P.S., Goo L., DeMaso C.R., Durbin A.P., Whitehead S.S., Pierson T.C., Dowd K.A. Dengue Virus Serotype 1 Conformational Dynamics Confers Virus Strain-Dependent Patterns of Neutralization by Polyclonal Sera. J. Virol. 2021;95:e0095621. doi: 10.1128/JVI.00956-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dowd K.A., Mukherjee S., Kuhn R.J., Pierson T.C. Combined Effects of the Structural Heterogeneity and Dynamics of Flaviviruses on Antibody Recognition. J. Virol. 2014;88:11726–11737. doi: 10.1128/JVI.01140-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guirakhoo F., Bolin R.A., Roehrig J.T. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology. 1992;191:921–931. doi: 10.1016/0042-6822(92)90267-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cherrier M.V., Kaufmann B., Nybakken G.E., Lok S.-M., Warren J., Chen B.R., Nelson C.A., Kostyuchenko V., Holdaway H.A., Chipman P.R., et al. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J. 2009;28:3269–3276. doi: 10.1038/emboj.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sukupolvi-Petty S., Brien J.D., Austin S.K., Shrestha B., Swayne S., Kahle K., Doranz B.J., Johnson S., Pierson T.C., Fremont D.H., et al. Functional Analysis of Antibodies against Dengue Virus Type 4 Reveals Strain-Dependent Epitope Exposure That Impacts Neutralization and Protection. J. Virol. 2013;87:8826–8842. doi: 10.1128/JVI.01314-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vogt M.R., Dowd K.A., Engle M., Tesh R.B., Johnson S., Pierson T.C., Diamond M.S. Poorly Neutralizing Cross-Reactive Antibodies against the Fusion Loop of West Nile Virus Envelope Protein Protect In Vivo via Fcγ Receptor and Complement-Dependent Effector Mechanisms. J. Virol. 2011;85:11567–11580. doi: 10.1128/JVI.05859-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jarmer J., Zlatkovic J., Tsouchnikas G., Vratskikh O., Strauß J., Aberle J.H., Chmelik V., Kundi M., Stiasny K., Heinz F.X. Variation of the Specificity of the Human Antibody Responses after Tick-Borne Encephalitis Virus Infection and Vaccination. J. Virol. 2014;88:13845–13857. doi: 10.1128/JVI.02086-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mukherjee S., Lin T.-Y., Dowd K.A., Manhart C.J., Pierson T.C. The Infectivity of prM-Containing Partially Mature West Nile Virus Does Not Require the Activity of Cellular Furin-Like Proteases. J. Virol. 2011;85:12067–12072. doi: 10.1128/JVI.05559-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Slon-Campos J.L., Mongkolsapaya J., Screaton G.R. The immune response against flaviviruses. Nat. Immunol. 2018;19:1189–1198. doi: 10.1038/s41590-018-0210-3. [DOI] [PubMed] [Google Scholar]
- 132.van Leur S.W., Heunis T., Munnur D., Sanyal S. Pathogenesis and virulence of flavivirus infections. Virulence. 2021;12:2814–2838. doi: 10.1080/21505594.2021.1996059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Quicke K.M., Suthar M.S. The Innate Immune Playbook for Restricting West Nile Virus Infection. Viruses. 2013;5:2643–2658. doi: 10.3390/v5112643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suthar M.S., Diamond M.S., Gale M., Jr. West Nile virus infection and immunity. Nat. Rev. Microbiol. 2013;11:115–128. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- 135.Diamond M.S., Gale M. Cell-intrinsic innate immune control of West Nile virus infection. Trends Immunol. 2012;33:522–530. doi: 10.1016/j.it.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thackray L.B., Shrestha B., Richner J.M., Miner J.J., Pinto A., Lazear H., Gale M., Diamond M.S. Interferon Regulatory Factor 5-Dependent Immune Responses in the Draining Lymph Node Protect against West Nile Virus Infection. J. Virol. 2014;88:11007–11021. doi: 10.1128/JVI.01545-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suthar M.S., Brassil M.M., Blahnik G., McMillan A., Ramos H.J., Proll S.C., Belisle S.E., Katze M.G., Gale M. A Systems Biology Approach Reveals that Tissue Tropism to West Nile Virus Is Regulated by Antiviral Genes and Innate Immune Cellular Processes. PLOS Pathog. 2013;9:e1003168. doi: 10.1371/journal.ppat.1003168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hildner K., Edelson B.T., Purtha W.E., Diamond M., Matsushita H., Kohyama M., Calderon B., Schraml B.U., Unanue E.R., Diamond M.S., et al. Batf3 Deficiency Reveals a Critical Role for CD8α + Dendritic Cells in Cytotoxic T Cell Immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Klein R.S., Diamond M.S. Immunological headgear: Antiviral immune responses protect against neuroinvasive West Nile virus. Trends Mol. Med. 2008;14:286–294. doi: 10.1016/j.molmed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 140.Saiz J.-C., Martín-Acebes M.A., Blázquez A.B., Escribano-Romero E., Poderoso T., de Oya N.J. Pathogenicity and virulence of West Nile virus revisited eight decades after its first isolation. Virulence. 2021;12:1145–1173. doi: 10.1080/21505594.2021.1908740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fadnis P.R., Ravi V., Desai A., Turtle L., Solomon T. Innate Immune Mechanisms in Japanese Encephalitis Virus Infection: Effect on Transcription of Pattern Recognition Receptors in Mouse Neuronal Cells and Brain Tissue. Viral Immunol. 2013;26:366–377. doi: 10.1089/vim.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Samuel M.A., Diamond M.S. Pathogenesis of West Nile Virus Infection: A Balance between Virulence, Innate and Adaptive Immunity, and Viral Evasion. J. Virol. 2006;80:9349–9360. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ahlers L.R.H., Goodman A.G. The Immune Responses of the Animal Hosts of West Nile Virus: A Comparison of Insects, Birds, and Mammals. Front. Cell. Infect. Microbiol. 2018;8:96. doi: 10.3389/fcimb.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schoggins J.W., Rice C.M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 2011;1:519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Beasley D.W.C., Whiteman M.C., Zhang S., Huang C.Y.-H., Schneider B.S., Smith D.R., Gromowski G.D., Higgs S., Kinney R.M., Barrett A.D.T. Envelope Protein Glycosylation Status Influences Mouse Neuroinvasion Phenotype of Genetic Lineage 1 West Nile Virus Strains. J. Virol. 2005;79:8339–8347. doi: 10.1128/JVI.79.13.8339-8347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Benz F., Liebner S. Structure and Function of the Blood–Brain Barrier (BBB) Handb. Exp. Pharmacol. 2022;273:3–31. doi: 10.1007/164_2020_404. [DOI] [PubMed] [Google Scholar]
- 147.Cho H., Diamond M.S. Immune Responses to West Nile Virus Infection in the Central Nervous System. Viruses. 2012;4:3812–3830. doi: 10.3390/v4123812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liou M.-L., Hsu C.-Y. Japanese encephalitis virus is transported across the cerebral blood vessels by endocytosis in mouse brain. Cell Tissue Res. 1998;293:389–394. doi: 10.1007/s004410051130. [DOI] [PubMed] [Google Scholar]
- 149.Chang C.-Y., Li J.-R., Chen W.-Y., Ou Y.-C., Lai C.-Y., Hu Y.-H., Wu C.-C., Chang C.-J., Chen C.-J. Disruption of in vitro endothelial barrier integrity by Japanese encephalitis virus-Infected astrocytes. Glia. 2015;63:1915–1932. doi: 10.1002/glia.22857. [DOI] [PubMed] [Google Scholar]
- 150.Liu T.-H., Liang L.-C., Wang C.-C., Liu H.-C., Chen W.-J. The blood-brain barrier in the cerebrum is the initial site for the Japanese encephalitis virus entering the central nervous system. J. NeuroVirol. 2008;14:514–521. doi: 10.1080/13550280802339643. [DOI] [PubMed] [Google Scholar]
- 151.Beasley D.W., Li L., Suderman M.T., Barrett A.D. Mouse Neuroinvasive Phenotype of West Nile Virus Strains Varies Depending upon Virus Genotype. Virology. 2002;296:17–23. doi: 10.1006/viro.2002.1372. [DOI] [PubMed] [Google Scholar]
- 152.Andrews D.M., Matthews V.B., Sammels L.M., Carrello A.C., McMinn P.C. The Severity of Murray Valley Encephalitis in Mice Is Linked to Neutrophil Infiltration and Inducible Nitric Oxide Synthase Activity in the Central Nervous System. J. Virol. 1999;73:8781–8790. doi: 10.1128/JVI.73.10.8781-8790.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Constant O., Barthelemy J., Nagy A., Salinas S., Simonin Y. West Nile Virus Neuroinfection in Humans: Peripheral Biomarkers of Neuroinflammation and Neuronal Damage. Viruses. 2022;14:756. doi: 10.3390/v14040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Winter P.M., Dung N.M., Loan H.T., Kneen R., Wills B., Thu L.T., House D., White N.J., Farrar J., Hart C.A., et al. Proinflammatory Cytokines and Chemokines in Humans with Japanese Encephalitis. J. Infect. Dis. 2004;190:1618–1626. doi: 10.1086/423328. [DOI] [PubMed] [Google Scholar]
- 155.Ravi V., Parida S., Desai A., Chandrmuki A., Gourie-Devi M., Grau E. Correlation of tumor necrosis factor levels in the serum and cerebrospinal fluid with clinical outcome in Japanese encephalitis patients. J. Med. Virol. 1997;51:132–136. doi: 10.1002/(SICI)1096-9071(199702)51:2<132::AID-JMV8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 156.Benzarti E., Garigliany M. In Vitro and In Vivo Models to Study the Zoonotic Mosquito-Borne Usutu Virus. Viruses. 2020;12:1116. doi: 10.3390/v12101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Riccetti S., Sinigaglia A., Desole G., Nowotny N., Trevisan M., Barzon L. Modelling West Nile Virus and Usutu Virus Pathogenicity in Human Neural Stem Cells. Viruses. 2020;12:882. doi: 10.3390/v12080882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cacciotti G., Caputo B., Selvaggi C., la Sala A., Vitiello L., Diallo D., Ceianu C., Antonelli G., Nowotny N., Scagnolari C. Variation in interferon sensitivity and induction between Usutu and West Nile (lineages 1 and 2) viruses. Virology. 2015;485:189–198. doi: 10.1016/j.virol.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 159.Martin M.-F., Maarifi G., Abiven H., Seffals M., Mouchet N., Beck C., Bodet C., Lévèque N., Arhel N.J., Blanchet F.P., et al. Usutu Virus escapes langerin-induced restriction to productively infect human Langerhans cells, unlike West Nile virus. Emerg. Microbes Infect. 2022;11:761–774. doi: 10.1080/22221751.2022.2045875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aberle J.H., Koblischke M., Stiasny K. CD4 T cell responses to flaviviruses. J. Clin. Virol. 2018;108:126–131. doi: 10.1016/j.jcv.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Larena M., Regner M., Lee E., Lobigs M. Pivotal Role of Antibody and Subsidiary Contribution of CD8 + T Cells to Recovery from Infection in a Murine Model of Japanese Encephalitis. J. Virol. 2011;85:5446–5455. doi: 10.1128/JVI.02611-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lobigs M., Müllbacher A., Regner M. CD4 + and CD8 + T-Cell Immune Responses in West Nile Virus Infection. In: Diamond M.S., editor. West Nile Encephalitis Virus Infection: Viral Pathogenesis and the Host Immune Response. Springer New York; New York, NY, USA: 2009. pp. 287–307. [Google Scholar]
- 163.Kalia A., Agrawal M., Gupta N. CD8 + T cells are crucial for humoral immunity establishment by SA14-14-2 live attenuated Japanese encephalitis vaccine in mice. Eur. J. Immunol. 2020;51:368–379. doi: 10.1002/eji.202048745. [DOI] [PubMed] [Google Scholar]
- 164.Jain N., Oswal N., Chawla A.S., Agrawal T., Biswas M., Vrati S., Rath S., George A., Bal V., Medigeshi G.R. CD8 T cells protect adult naive mice from JEV-induced morbidity via lytic function. PLOS Negl. Trop. Dis. 2017;11:e0005329. doi: 10.1371/journal.pntd.0005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Percivalle E., Cassaniti I., Sarasini A., Rovida F., Adzasehoun K.M.G., Colombini I., Isernia P., Cuppari I., Baldanti F. West Nile or Usutu Virus? A Three-Year Follow-Up of Humoral and Cellular Response in a Group of Asymptomatic Blood Donors. Viruses. 2020;12:157. doi: 10.3390/v12020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vaughan K., Greenbaum J., Blythe M., Peters B., Sette A. Meta-analysis of All Immune Epitope Data in the Flavivirus Genus: Inventory of Current Immune Epitope Data Status in the Context of Virus Immunity and Immunopathology. Viral Immunol. 2010;23:259–284. doi: 10.1089/vim.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.De Filette M., Chabierski S., Andries O., Ulbert S., Sanders N.N. T Cell Epitope Mapping of the E-Protein of West Nile Virus in BALB/c Mice. PLoS ONE. 2014;9:e115343. doi: 10.1371/journal.pone.0115343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Diamond M.S., Pierson T.C., Fremont D.H. The structural immunology of antibody protection against West Nile virus. Immunol. Rev. 2008;225:212–225. doi: 10.1111/j.1600-065X.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oliphant T., Diamond M.S. The molecular basis of antibody-mediated neutralization of West Nile virus. Expert Opin. Biol. Ther. 2007;7:885–892. doi: 10.1517/14712598.7.6.885. [DOI] [PubMed] [Google Scholar]
- 170.Chabierski S., Makert G.R., Kerzhner A., Barzon L., Fiebig P., Liebert U.G., Papa A., Richner J., Niedrig M., Diamond M.S., et al. Antibody Responses in Humans Infected with Newly Emerging Strains of West Nile Virus in Europe. PLoS ONE. 2013;8:e66507. doi: 10.1371/journal.pone.0066507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Crill W.D., Trainor N.B., Chang G.-J.J. A detailed mutagenesis study of flavivirus cross-reactive epitopes using West Nile virus-like particles. J. Gen. Virol. 2007;88:1169–1174. doi: 10.1099/vir.0.82640-0. [DOI] [PubMed] [Google Scholar]
- 172.Oliphant T., Engle M., Nybakken G.E., Doane C., Johnson S., Huang L., Gorlatov S., Mehlhop E., Marri A., Chung K.M., et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nybakken G.E., Oliphant T., Johnson S., Burke S., Diamond M.S., Fremont D.H. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nat. 2005;437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nikolay B., Fall G., Boye C.S.B., Sall A.A., Skern T. Validation of a structural comparison of the antigenic characteristics of Usutu virus and West Nile virus envelope proteins. Virus Res. 2014;189:87–91. doi: 10.1016/j.virusres.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 175.Pierson T.C., Fremont D.H., Kuhn R.J., Diamond M.S. Structural Insights into the Mechanisms of Antibody-Mediated Neutralization of Flavivirus Infection: Implications for Vaccine Development. Cell Host Microbe. 2008;4:229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pierson T.C., Diamond M.S. Progress in Molecular Biology and Translational Science. Elsevier Inc.; Amsterdam, The Netherlands: 2015. A Game of Numbers: The stoichiometry of antibody-mediated neutralization of flavivirus infection; pp. 141–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pierson T.C., Xu Q., Nelson S., Oliphant T., Nybakken G.E., Fremont D.H., Diamond M.S. The Stoichiometry of Antibody-Mediated Neutralization and Enhancement of West Nile Virus Infection. Cell Host Microbe. 2007;1:135–145. doi: 10.1016/j.chom.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thompson B.S., Moesker B., Smit J.M., Wilschut J., Diamond M.S., Fremont D.H. A Therapeutic Antibody against West Nile Virus Neutralizes Infection by Blocking Fusion within Endosomes. PLOS Pathog. 2009;5:e1000453. doi: 10.1371/journal.ppat.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qiu X., Lei Y., Yang P., Gao Q., Wang N., Cao L., Yuan S., Huang X., Deng Y., Ma W., et al. Structural basis for neutralization of Japanese encephalitis virus by two potent therapeutic antibodies. Nat. Microbiol. 2018;3:287–294. doi: 10.1038/s41564-017-0099-x. [DOI] [PubMed] [Google Scholar]
- 180.Xu L., Ma Z., Li Y., Pang Z., Xiao S. Antibody dependent enhancement: Unavoidable problems in vaccine development. Adv. Immunol. 2021;151:99–133. doi: 10.1016/bs.ai.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.VanBlargan L.A., Goo L., Pierson T.C. Deconstructing the Antiviral Neutralizing-Antibody Response: Implications for Vaccine Development and Immunity. Microbiol. Mol. Biol. Rev. 2016;80:989–1010. doi: 10.1128/MMBR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Berneck B.S., Rockstroh A., Fertey J., Grunwald T., Ulbert S. A Recombinant Zika Virus Envelope Protein with Mutations in the Conserved Fusion Loop Leads to Reduced Antibody Cross-Reactivity upon Vaccination. Vaccines. 2020;8:603. doi: 10.3390/vaccines8040603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Maciejewski S., Pierson T.C. Cross-Reactive Flavivirus Antibody: Friend and Foe? Cell Host Microbe. 2018;24:622–624. doi: 10.1016/j.chom.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 184.Vatti A., Monsalve D.M., Pacheco Y., Chang C., Anaya J.-M., Gershwin M.E. Original antigenic sin: A comprehensive review. J. Autoimmun. 2017;83:12–21. doi: 10.1016/j.jaut.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 185.St. John A.L., Rathore A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 2019;19:218–230. doi: 10.1038/s41577-019-0123-x. [DOI] [PubMed] [Google Scholar]
- 186.Lobigs M. Cross-protective and infection-enhancing immunity in mice vaccinated against flaviviruses belonging to the Japanese encephalitis virus serocomplex. Vaccine. 2003;21:1572–1579. doi: 10.1016/S0264-410X(02)00743-0. [DOI] [PubMed] [Google Scholar]
- 187.Salazar V., Jagger B.W., Mongkolsapaya J., Burgomaster K.E., Dejnirattisai W., Winkler E.S., Fernandez E., Nelson C.A., Fremont D.H., Pierson T.C., et al. Dengue and Zika Virus Cross-Reactive Human Monoclonal Antibodies Protect against Spondweni Virus Infection and Pathogenesis in Mice. Cell Rep. 2019;26:e1584. doi: 10.1016/j.celrep.2019.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wen J., Tang W.W., Sheets N., Ellison J., Sette A., Kim K., Shresta S. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat. Microbiol. 2017;2:17036. doi: 10.1038/nmicrobiol.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Regla-Nava J.A., Elong Ngono A., Viramontes K.M., Huynh A.-T., Wang Y.-T., Nguyen A.-V.T., Salgado R., Mamidi A., Kim K., Diamond M.S., et al. Cross-reactive Dengue virus-specific CD8+ T cells protect against Zika virus during pregnancy. Nat. Commun. 2018;9:3042. doi: 10.1038/s41467-018-05458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Haslwanter D., Blaas D., Heinz F.X., Stiasny K. A novel mechanism of antibody-mediated enhancement of flavivirus infection. PLOS Pathog. 2017;13:e1006643. doi: 10.1371/journal.ppat.1006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Amarilla A.A., Fumagalli M.J., Figueiredo M.L., Lima-Junior D.S., Santos-Junior N.N., Alfonso H.L., Lippi V., Trabuco A.C., Lauretti F., Muller V.D., et al. Ilheus and Saint Louis encephalitis viruses elicit cross-protection against a lethal Rocio virus challenge in mice. PLoS ONE. 2018;13:e0199071. doi: 10.1371/journal.pone.0199071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Endale A., Medhin G., Darfiro K., Kebede N., Legesse M. Magnitude of Antibody Cross-Reactivity in Medically Important Mosquito-Borne Flaviviruses: A Systematic Review. Infect. Drug Resist. 2021;14:4291–4299. doi: 10.2147/IDR.S336351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Oliphant T., Nybakken G.E., Austin S.K., Xu Q., Bramson J., Loeb M., Throsby M., Fremont D.H., Pierson T.C., Diamond M.S. Induction of Epitope-Specific Neutralizing Antibodies against West Nile Virus. J. Virol. 2007;81:11828–11839. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lobigs M., Diamond M.S. Feasibility of cross-protective vaccination against flaviviruses of the Japanese encephalitis serocomplex. Expert Rev. Vaccines. 2012;11:177–187. doi: 10.1586/erv.11.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Larena M., Prow N.A., Hall R.A., Petrovsky N., Lobigs M. JE-ADVAX Vaccine Protection against Japanese Encephalitis Virus Mediated by Memory B Cells in the Absence of CD8 + T Cells and Pre-Exposure Neutralizing Antibody. J. Virol. 2013;87:4395–4402. doi: 10.1128/JVI.03144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Crill W.D., Chang G.-J.J. Localization and Characterization of Flavivirus Envelope Glycoprotein Cross-Reactive Epitopes. J. Virol. 2004;78:13975–13986. doi: 10.1128/JVI.78.24.13975-13986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stiasny K., Kiermayr S., Holzmann H., Heinz F.X. Cryptic Properties of a Cluster of Dominant Flavivirus Cross-Reactive Antigenic Sites. J. Virol. 2006;80:9557–9568. doi: 10.1128/JVI.00080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dos Santos Franco L., Gushi L.T., Luiz W.B., Amorim J.H. Seeking Flavivirus Cross-Protective Immunity. Front. Immunol. 2019;10:2260. doi: 10.3389/fimmu.2019.02260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Subramaniam K., Lant S., Goodwin L., Grifoni A., Weiskopf D., Turtle L. Two Is Better Than One: Evidence for T-Cell Cross-Protection Between Dengue and Zika and Implications on Vaccine Design. Front. Immunol. 2020;11:517. doi: 10.3389/fimmu.2020.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ngono A.E., Shresta S., Ngono A.E., Shresta S. Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights into Vaccine Development. Front. Immunol. 2019;10:1316. doi: 10.3389/fimmu.2019.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hassert M., Brien J.D., Pinto A.K. CD8 T cell cross-reactivity during heterologous flavivirus infection results in cross-reactive immunodomination and enhanced cytolytic capacity at the expense of virus-specific responses. J. Immunol. 2020;204:95–99. [Google Scholar]
- 202.Zheng X., Yu X., Wang Y., Cui M., Wang R., Yin C. Immune responses and protective effects against Japanese encephalitis induced by a DNA vaccine encoding the prM/E proteins of the attenuated SA14-14-2 strain. Infect. Genet. Evol. 2020;85:104443. doi: 10.1016/j.meegid.2020.104443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bosco-Lauth A., Mason G., Bowen R. Pathogenesis of Japanese Encephalitis Virus Infection in a Golden Hamster Model and Evaluation of Flavivirus Cross-Protective Immunity. Am. J. Trop. Med. Hyg. 2011;84:727–732. doi: 10.4269/ajtmh.2011.11-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hall R.A., Brand T.N.H., Lobigs M., Sangster M., Howard M.J., MacKenzie J.S. Protective immune responses to the E and NS1 proteins of Murray Valley encephalitis virus in hybrids of flavivirus-resistant mice. J. Gen. Virol. 1996;77:1287–1294. doi: 10.1099/0022-1317-77-6-1287. [DOI] [PubMed] [Google Scholar]
- 205.Lima N.S., Moon D., Darko S., De La Barrera R.A., Lin L., Koren M.A., Jarman R.G., Eckels K.H., Thomas S.J., Michael N.L., et al. Pre-existing Immunity to Japanese Encephalitis Virus Alters CD4 T Cell Responses to Zika Virus Inactivated Vaccine. Front. Immunol. 2021;12:640190. doi: 10.3389/fimmu.2021.640190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Saron W.A.A., Rathore A.P.S., Ting L., Ooi E.E., Low J., Abraham S.N., John A.L.S. Flavivirus serocomplex cross-reactive immunity is protective by activating heterologous memory CD4 T cells. Sci. Adv. 2018;4:eaar4297. doi: 10.1126/sciadv.aar4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang R., Zhen Z., Turtle L., Hou B., Li Y., Wu N., Gao N., Fan D., Chen H., An J. T cell immunity rather than antibody mediates cross-protection against Zika virus infection conferred by a live attenuated Japanese encephalitis SA14-14-2 vaccine. Appl. Microbiol. Biotechnol. 2020;104:6779–6789. doi: 10.1007/s00253-020-10710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li J., Gao N., Fan D., Chen H., Sheng Z., Fu S., Liang G., Ziyang S. Cross-protection induced by Japanese encephalitis vaccines against different genotypes of Dengue viruses in mice. Sci. Rep. 2016;6:19953. doi: 10.1038/srep19953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mishra N., Boudewijns R., Schmid M.A., Marques R.E., Sharma S., Neyts J., Dallmeier K. A Chimeric Japanese Encephalitis Vaccine Protects against Lethal Yellow Fever Virus Infection without Inducing Neutralizing Antibodies. mBio. 2020;11:2. doi: 10.1128/mBio.02494-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Koblischke M., Spitzer F., Florian D.M., Aberle S.W., Malafa S., Fae I., Cassaniti I., Jungbauer C., Knapp B., Laferl H., et al. CD4 T Cell Determinants in West Nile Virus Disease and Asymptomatic Infection. Front. Immunol. 2020;11:16. doi: 10.3389/fimmu.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Landry S.J., Moss D.L., Cui D., Ferrie R.P., Fullerton M.L., Wells E.A., Yang L., Zhou N., Dougherty T., Mettu R.R. Structural Basis for CD4+ T Cell Epitope Dominance in Arbo-Flavivirus Envelope Proteins: A Meta-Analysis. Viral Immunol. 2017;30:479–489. doi: 10.1089/vim.2017.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hou B., Chen H., Gao N., An J. Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases. Viruses. 2022;14:1213. doi: 10.3390/v14061213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Watanabe S., Tan N.W.W., Chan K.W.K., Vasudevan S. Dengue Virus and Zika Virus Serological Cross-reactivity and Their Impact on Pathogenesis in Mice. J. Infect. Dis. 2019;219:223–233. doi: 10.1093/infdis/jiy482. [DOI] [PubMed] [Google Scholar]
- 214.Zhang W., Xu Y., Zhao F., Tarbe M., Zhou S., Wang W., Zhang S., Zhang W., Xu Q., Shi L., et al. The pre-existing cellular immunity to Japanese encephalitis virus heterotypically protects mice from Zika virus infection. Sci. Bull. 2020;65:402–409. doi: 10.1016/j.scib.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 215.Anderson K.B., Gibbons R.V., Thomas S.J., Rothman A.L., Nisalak A., Berkelman R.L., Libraty D.H., Endy T.P. Preexisting Japanese Encephalitis Virus Neutralizing Antibodies and Increased Symptomatic Dengue Illness in a School-Based Cohort in Thailand. PLOS Negl. Trop. Dis. 2011;5:e1311. doi: 10.1371/journal.pntd.0001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Broom A.K., Wallace M.J., Mackenzie J.S., Smith D.W., Hall R.A. Immunisation with gamma globulin to Murray Valley encephalitis virus and with an inactivated Japanese encephalitis virus vaccine as prophylaxis against Australian encephalitis: Evaluation in a mouse model. J. Med. Virol. 2000;61:259–265. doi: 10.1002/(SICI)1096-9071(200006)61:2<259::AID-JMV13>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 217.Goverdhan M.K., Kulkarni A.B., Gupta A.K., Tupe C.D., Rodrigues J.J. Two-way cross-protection between West Nile and Japanese encephalitis viruses in bonnet macaques. Acta Virol. 1992;36:277–283. [PubMed] [Google Scholar]
- 218.Williams D.T., Lunt R.A., Wang L.F., Daniels P.W., Newberry K.M., MacKenzie J.S. Experimental infections of pigs with Japanese encephalitis virus and closely related Australian flaviviruses. Am. J. Trop. Med. Hyg. 2001;65:379–387. doi: 10.4269/ajtmh.2001.65.379. [DOI] [PubMed] [Google Scholar]
- 219.Kanesa-Thasan N., Ludwig G.V., Saluzzo J.E., Putnak J.R., Mangiafico J.A. Short report: Absence of protective neutralizng antibodies to West Nile virus in subjects following vaccination with Japanese encephalitis or dengue vaccines. Am. J. Trop. Med. Hyg. 2002;66:115–116. doi: 10.4269/ajtmh.2002.66.115. [DOI] [PubMed] [Google Scholar]
- 220.Wallace M.J., Smith D.W., Broom A.K., MacKenzie J.S., Hall R.A., Shellam G.R., McMINN P.C. Antibody-dependent enhancement of Murray Valley encephalitis virus virulence in mice. J. Gen. Virol. 2003;84:1723–1728. doi: 10.1099/vir.0.18980-0. [DOI] [PubMed] [Google Scholar]
- 221.Lobigs M., Larena M., Alsharifi M., Lee E., Pavy M. Live Chimeric and Inactivated Japanese Encephalitis Virus Vaccines Differ in Their Cross-Protective Values against Murray Valley Encephalitis Virus. J. Virol. 2009;83:2436–2445. doi: 10.1128/JVI.02273-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Petrovsky N., Larena M., Siddharthan V., Prow N.A., Hall R.A., Lobigs M., Morrey J. An Inactivated Cell Culture Japanese Encephalitis Vaccine (JE-ADVAX) Formulated with Delta Inulin Adjuvant Provides Robust Heterologous Protection against West Nile Encephalitis via Cross-Protective Memory B Cells and Neutralizing Antibody. J. Virol. 2013;87:10324–10333. doi: 10.1128/JVI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yamshchikov G., Borisevich V., Kwok C.W., Nistler R., Kohlmeier J., Seregin A., Chaporgina E., Benedict S., Yamshchikov V. The suitability of yellow fever and Japanese encephalitis vaccines for immunization against West Nile virus. Vaccine. 2005;23:4785–4792. doi: 10.1016/j.vaccine.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 224.Tesh R.B., Da Rosa A.P.T., Guzman H., Araujo T.P., Xiao S.-Y. Immunization with Heterologous Flaviviruses Protective Against Fatal West Nile Encephalitis. Emerg. Infect. Dis. 2002;8:245–251. doi: 10.3201/eid0803.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Takasaki T., Yabe S., Nerome R., Ito M., Yamada K.-I., Kurane I. Partial protective effect of inactivated Japanese encephalitis vaccine on lethal West Nile virus infection in mice. Vaccine. 2003;21:4514–4518. doi: 10.1016/S0264-410X(03)00507-3. [DOI] [PubMed] [Google Scholar]
- 226.Tang F., Zhang F., Zhang J.-S., Liu W., Ly H., Cao W.-C., Wu X.-M., Yang H., Zhao Q.-M. Failure of Japanese encephalitis vaccine and infection in inducing neutralizing antibodies against West Nile virus, People’s Republic of China. Am. J. Trop. Med. Hyg. 2008;78:999–1001. doi: 10.4269/ajtmh.2008.78.999. [DOI] [PubMed] [Google Scholar]
- 227.Merino-Ramos T., Blázquez A.-B., Escribano-Romero E., Cañas-Arranz R., Sobrino F., Saiz J.-C., Martín-Acebes M.A. Protection of a Single Dose West Nile Virus Recombinant Subviral Particle Vaccine against Lineage 1 or 2 Strains and Analysis of the Cross-Reactivity with Usutu Virus. PLoS ONE. 2014;9:e108056. doi: 10.1371/journal.pone.0108056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hill A.B., Müllbacher A., Parrish C., Coia G., Westaway E.G., Blanden R.V. Broad cross-reactivity with marked fine specificity in the cytotoxic T cell response to flaviviruses. J. Gen. Virol. 1992;73:1115–1123. doi: 10.1099/0022-1317-73-5-1115. [DOI] [PubMed] [Google Scholar]
- 229.Blazquez A.-B., Escribano-Romero E., Martin-Acebes M.A., Petrovic T., Saiz J.-C. Limited susceptibility of mice to Usutu virus (USUV) infection and induction of flavivirus cross-protective immunity. Virology. 2015;482:67–71. doi: 10.1016/j.virol.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 230.Schoenenwald A.K.J., Pletzer M., Skern T. Structural and antigenic investigation of Usutu virus envelope protein domain III. Virology. 2020;551:46–57. doi: 10.1016/j.virol.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 231.Sinigaglia A., Pacenti M., Martello T., Pagni S., Franchin E., Barzon L. West Nile virus infection in individuals with pre-existing Usutu virus immunity, northern Italy, 2018. Eurosurveillance. 2019;24:1900261. doi: 10.2807/1560-7917.ES.2019.24.21.1900261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Salgado R., Hawks S.A., Frere F., Vázquez A., Huang C.Y.-H., Duggal N.K. West Nile Virus Vaccination Protects against Usutu Virus Disease in Mice. Viruses. 2021;13:2352. doi: 10.3390/v13122352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Martín-Acebes M.A., Blázquez A.-B., Cañas-Arranz R., Vázquez-Calvo Á., Merino-Ramos T., Escribano-Romero E., Sobrino F., Saiz J.-C. A recombinant DNA vaccine protects mice deficient in the alpha/beta interferon receptor against lethal challenge with Usutu virus. Vaccine. 2016;34:2066–2073. doi: 10.1016/j.vaccine.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 234.Böszörményi K., Hirsch J., Kayere G.K., Fagrouch Z., Heijmans N., Garcia R.R., Dwarka S., van Dijke A., Aaldijk B., Limpens R., et al. A Bacterially-Expressed Recombinant Envelope Protein from Usutu Virus Induces Neutralizing Antibodies in Rabbits. Vaccines. 2021;9:157. doi: 10.3390/vaccines9020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Vilibic-Cavlek T., Kaic B., Barbic L., Pem-Novosel I., Slavic-Vrzic V., Lesnikar V., Kurecic-Filipovic S., Babic-Erceg A., Listes E., Stevanovic V., et al. First evidence of simultaneous occurrence of West Nile virus and Usutu virus neuroinvasive disease in humans in Croatia during the 2013 outbreak. Infection. 2014;42:689–695. doi: 10.1007/s15010-014-0625-1. [DOI] [PubMed] [Google Scholar]
- 236.Furuya-Kanamori L., Xu C., Doi S.A., Clark J., Wangdi K., Mills D.J., Lau C.L. Comparison of immunogenicity and safety of licensed Japanese encephalitis vaccines: A systematic review and network meta-analysis. Vaccine. 2021;39:4429–4436. doi: 10.1016/j.vaccine.2021.06.023. [DOI] [PubMed] [Google Scholar]
- 237.Hegde N.R., Gore M.M., Hegde N.R., Gore M.M. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vaccines Immunother. 2017;13:1–18. doi: 10.1080/21645515.2017.1285472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Halstead S.B., Thomas S.J. New Japanese encephalitis vaccines: Alternatives to production in mouse brain. Expert Rev. Vaccines. 2011;10:355–364. doi: 10.1586/erv.11.7. [DOI] [PubMed] [Google Scholar]
- 239.Muangchana C., Henprasertthae N., Nurach K., Theppang K., Yoocharoen P., Varinsathien P., Techathawat S., Sanohsieng S., Anantapreecha S. Effectiveness of mouse brain-derived inactivated Japanese encephalitis vaccine in Thai National Immunization Program: A case–control study. Vaccine. 2012;30:361–367. doi: 10.1016/j.vaccine.2011.10.083. [DOI] [PubMed] [Google Scholar]
- 240.Erra E.O., Kantele A. The Vero cell-derived, inactivated, SA14-14-2 strain-based vaccine (Ixiaro) for prevention of Japanese encephalitis. Expert Rev. Vaccines. 2015;14:1167–1179. doi: 10.1586/14760584.2015.1061939. [DOI] [PubMed] [Google Scholar]
- 241.Feroldi E., Boaz M., Yoksan S., Chokephaibulkit K., Thisyakorn U., Pancharoen C., Monfredo C., Bouckenooghe A. Persistence of Wild-Type Japanese Encephalitis Virus Strains Cross-Neutralization Five Years following JE-CV Immunization. J. Infect. Dis. 2016;215:221–227. doi: 10.1093/infdis/jiw533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Erra E.O., Askling H.H., Yoksan S., Rombo L., Riutta J., Vene S., Lindquist L., Vapalahti O., Kantele A. Cross-Protective Capacity of Japanese Encephalitis (JE) Vaccines Against Circulating Heterologous JE Virus Genotypes. Clin. Infect. Dis. 2012;56:267–270. doi: 10.1093/cid/cis883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ulbert S. West Nile virus vaccines—current situation and future directions. Hum. Vaccines Immunother. 2019;15:2337–2342. doi: 10.1080/21645515.2019.1621149. [DOI] [PMC free article] [PubMed] [Google Scholar]