Abstract
The current studies were focused on the phytochemical profiling of two local wild Artemisia species, Artemisia scoparia and Artemisia absinthium leaves’ essential oils, extracted via the hydro distillation method along with evaluation of their antioxidant as well as antimicrobial effects. The constituents of EOs were identified using a combined gas chromatography-mass spectrometric (GC-MS) technique. A total of 25 compounds in A. scoparia essential oil (EOAS) were identified, and 14 compounds with percentage abundance of >1% were tabulated, the major being tocopherol derivatives (47.55%). A total of nine compounds in Artemisia absinthium essential oil (EOAA) were enlisted (% age > 1%), the majority being oleic acid derivatives (41.45%). Strong antioxidant effects were pronounced by the EOAS in DPPH (IC50 = 285 ± 0.82 µg/mL) and in ABTS (IC50 = 295 ± 0.32 µg/mL) free radical scavenging assays. Both the EOs remained potent in inhibiting the growth of bacterial species; Escherichia coli (55–70%) and Shigella flexneri (60–75%) however remained moderately effective against Bacillus subtilis as well as Staphylococcus aureus. Both EOAS and EOAA strongly inhibited the growth of the tested fungal species, especially Aspergillus species (up to 70%). The oils showed anti-cholinesterase potential by inhibiting both Acetylcholinesterase (AChE; IC50 = 30 ± 0.04 µg/mL (EOAS), 32 ± 0.05 µg/mL (EOAA) and Butyrylcholinesterase (BChE; IC50 = 34 ± 0.07 µg/mL (EOAS), 36 ± 0.03 µg/mL (EOAA). In conclusion, the essential oils of A. scoparia and A. absinthium are promising antioxidant, antimicrobial and anticholinergic agents with a different phytochemical composition herein reported for the first time.
Keywords: Artemisia scoparia, Artemisia absinthium, essential oils (EOs), EOAS, EOAA, phytochemical profiling, GC-MS, antioxidant effects, DPPH, ABTS, antibacterial, antifungal activities
1. Introduction
Essential oils (EOs) are the volatile constituents mainly found in the flowers and fresh leaves of plants, often stored in the epidermal tissues [1]. EOs have been used as flavoring factors in the food industries, perfumes, beverages, cosmetics, pharmaceuticals and nutraceuticals, etc. [2]. Chemically, EOs are mixtures of low molecular weight organic compounds such as mono and sesquiterpenoids, phenylpropenes, isothiocyanates, etc. [3] and possess a broad spectrum of potent biological effects including antifungal, antibacterial, anti-diabetic, anticancer, antiviral and, most recently, have been considered effective against COVID-19 respiratory infections [4,5,6]. Approximately 3000 different EOs have been obtained from more than 2000 plant species with production of 40,000–60,000 tons per year and revenue generation of about 7.5 billion US$ in 2018 with a 9% increase until 2026 [7].
Artemisia is a diversified genus of flowering plants, comprising approximately 500 species related to the daisy family of Asteraceae [8]. Artemisia contains various herbs, plants and shrubs, which are famous due to its antimalarial constituent, artemisinin [9] and rich in essential oils [10]. Two of the species including Artemisia scoparia (Local name = Tarkha) and Artemisia absinthium (Local name = Zoon) have been found in abundance in the Dir districts, KP, Pakistan [11,12]. The different types of Artemisia are locally used to treat inflammation, jaundice, malaria, tumors and various microbial infections and their essential oils possess strong antispasmodic, anti-hepatotoxic and anti-jaundice activities [13]. Some of the major constituents of Artemisia essential oils are caryophyllene oxide, borneol, camphor, thujane, thujanol, myrtenol, linalyl acetate, limonene and pinene etc. [13]. It has been observed that various factors such as genetic mutation, plant nutrition, geographic location, seasonal variation, surrounding climate, growth, and maturity affect the constitution of EOs of Artemisia. Moreover, the production of EOs also depends upon the nature of plant materials and methods of extraction. The current study is proposed to investigate the essential oils composition, antioxidant, antimicrobial and cholinesterase potential of two local Artemisia species, A. scoparia and A. absinthium essential oils.
2. Results and Discussion
2.1. GC-MS Analysis of the Artemisia scoparia Essential Oil (EOAS)
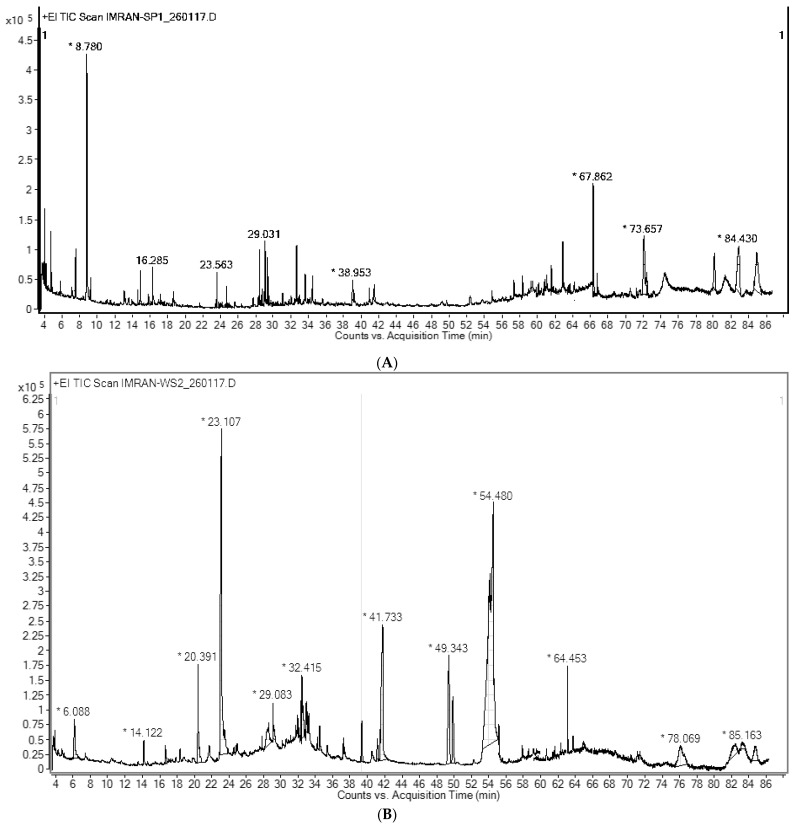
A total of 31 peaks were recorded for EOAS by gas chromatography (GC) in which 25 compounds were analyzed and 14 were quantified (% > 1%) (Figure 1A). The resolutions were obtained by comparison with the system GC-MS library, the mainlib and riplib databases, as well as estimated through a non-polar retention index (n-alkane scale), available literature as well as NIST online library. These findings are summarized in Table 1. The most abundant compound found was 7-Hydroxy-bicyclo[3.3.1] non-2-en-9-one (17.05%), while Tocopherol-β-D-mannoside was detected in 16.21% quantity. The other major constituents obtained were D-α-Tocopherol (13.65%), (+)-α-Tocopherol acetate (12.54%), and 18,19-Secoyohimban-19-oic acid was in an 8.24% ratio. α-Tocopherol acetate was found to exist in 5.15%, while the other constituents were present in very minor amounts. Overall, the essential oil displayed a strange composition as its major components were derivatives of tocopherol, a strong antioxidant. These tocopherol derivatives have never been detected in such quantities earlier nor have been reported, to the best of our knowledge. Similarly, amine derivatives such as 18,19-Secoyohimban-19-oic acid has been found for the first time in the essential oil of this species. The majority of terpenoids found were sesquiterpenenes and their oxygenated derivatives, while the monoterpenoids and their oxygenated derivatives were found in relatively small quantities. The presence of chromene derivatives suggested the possibility of flavone type constituents. According to previous studies, the essential oils of A. scoparia contained 1,8-cineole, p-cyamene, limonene, β-myrecene, camphor, β-pinene, terpenine, α, β-thujone etc. [14].
Figure 1.
(A) Total Ion Chromatogram (TIC) of EOAS; (B) Total Ion Chromatogram (TIC) of EOAA. * = identification matched >50% with database.
Table 1.
Quantification assessment of major phytochemicals in EOAS.
| S. No. | Area Sum% |
Compound Name | RT a | NIST # | DB ID * = Mainlib ** = Replib |
|---|---|---|---|---|---|
| 1 | 2.66 | 2-Ethenyl-bicyclo[2.1.1]hexan-2-ol | 8.924 | 221,372 | 50,226 * |
| 2 | 17.05 | 7-Hydroxy-bicyclo[3.3.1]non-2-en-9-one | 8.930 | 193,435 | 41,812 * |
| 3 | 1.65 | Decane | 14.867 | 114,147 | 21,679 * |
| 4 | 1.37 | 2,6,11,15-tetramethyl-hexadecane, (Crocetane) | 23.559 | 114,255 | 22,806 * |
| 5 | 1.22 | 1-Isopropyl-4,7-dimethyl-1,3,4,5,6,8a-hexahydro-4a(2H)-naphthalenol (cubenol) | 27.628 | 140,968 | 80,121 * |
| 6 | 2.11 | 4,5,9,10-dehydro-isolongifolene | 28.380 | 151,550 | 105,281 * |
| 7 | 2.99 | 2,4-bis(1,1-dimethylethyl)-phenol | 29.031 | 228,966 | 140,190 * |
| 8 | 1.73 | β-Himachalenoxide | 30.996 | 140,216 | 74,268 * |
| 9 | 1.6 | Estra-1,3,5(10)-trien-17β-ol | 41.42 | 254,855 | 7219 * |
| 10 | 8.24 | 18,19-Secoyohimban-19-oic acid | 67.862 | 48,433 | 21,782 * |
| 11 | 13.65 | d-α-Tocopherol | 73.657 | 151,382 | 122,577 * |
| 12 | 5.15 | dl-α-Tocopherol | 81.652 | 230,590 | 123,023 |
| 13 | 12.54 | (+)-α-Tocopherol acetate | 84.43 | 154,596 | 28,227 ** |
| 14 | 16.21 | Tocopherol-β-D-mannoside | 86.48 | 156,682 | 123,458 * |
| Total area = 91.83% |
a = RI calculated. Comparison with literature from # NIST, mainlib and riplib databases. Estimated non-polar retention index (n-alkane scale).
2.2. GC-MS Analysis of the Artemisia absinthium Essential Oil (EOAA)
The nine compounds having a percentage abundance of >1% were identified and quantified by gas chromatography-mass spectral (GC-MS) analysis (Figure 1B). These findings are summarized in Table 2. The most abundant compound found was Oleic acid (41.45%), while 2-Furancarboxaldehyde, 5-(hydroxymethyl) was detected in 14.24% quantity. The other major constituents identified included 9,12-Octadecadienoic acid (Z,Z)-, methyl ester (9.01%), Estra-1,3,5(10)-trien-17β-ol (8.75%), 9,9-Dimethoxybicyclo[3.3.1]nona-2,4-dione (7.74%) and N-(1-methoxycarbonyl-1-methylethyl)-4-methyl-2-aza-1,3-dioxane was in a 5.5% ratio, while the remaining were detected in very minute quantities. These types of constituents have been reported for the first time from this species, especially the amine derivative N-(1-methoxycarbonyl-1-methylethyl)-4-methyl-2-aza-1,3-dioxane.
Table 2.
GC and GC-MS analysis of A. absinthium essential oil (AAEO).
| S. No. | Area Sum% | Compound Name | RT a | NIST # | DB ID * = Mainlib ** = Replib |
|---|---|---|---|---|---|
| 1 | 7.74 | 9,9-Dimethoxybicyclo[3.3.1]nona-2,4-dione | 14.122 | 106,223 | 7209 * |
| 2 | 2.85 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | 20.391 | 281,424 | 9854 * |
| 3 | 5.55 | N-(1-Methoxycarbonyl-1-methylethyl)-4-methyl-2-aza-1,3-dioxane | 20.471 | 146,417 | 105,697 * |
| 4 | 14.24 | 5-(hydroxymethyl)-2-Furancarboxaldehyde | 23.227 | 231,276 | 60,271 * |
| 5 | 2.2 | Guanosine | 28.555 | 212,407 | 2164 ** |
| 6 | 2.69 | 3,4-diethyl-1,1′-Biphenyl | 32.375 | 62,426 | 150,478 * |
| 7 | 8.75 | Estra-1,3,5(10)-trien-17β-ol | 41.791 | 254,855 | 7219 * |
| 8 | 9.01 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 49.253 | 333,205 | 28,886 * |
| 9 | 41.45 | Oleic Acid | 54.227 | 228,066 | 4483 ** |
| Total area = 94.48% |
a = RI calculated. Comparison with literature from # NIST, mainlib and riplib databases. Estimated non-polar retention index (n-alkane scale).
2.3. Antioxidant Activity
The antioxidant potential of both EOAS and EOAA were measured using both DPPH and ABTS scavenging assays in a dose dependent manner. The EOAS of A. Scoparia was extremely active in inhibiting DPPH, 85 ± 0.91% and ABTS, 83 ± 0.43% at the dose of 500 µg/mL (Table 3). The IC50 values were obtained as 285 ± 0.82 µg/mL against DPPH and 295 ± 0.32 µg/mL against ABTS, respectively. However, at the same concentration, the EOAA of A. absinthium showed low antioxidant effect against DPPH (60 ± 0.31%) and ABTS (57 ± 0.47%), respectively. The potent antioxidant effect of the A. scoparia EO in both the assays could be attributed to the presence of huge quantities of tocopherol derivatives as well as sesquiterpene derivatives. The A. Scoparia essential oils previously showed strong antioxidant effects in scavenging both hydroxyl ions as well as hydrogen peroxide at doses of 25–200 µg/mL [15]. Similarly, the huge quantities of oleic acid derivatives could be responsible for the antioxidant effects of A. absinthium EO since higher IC50 values have been reported earlier in its antioxidant activities with different phytochemical profiles (DPPH= 29.72 mg/mL and ABTS = 38.83 mg/mL) [16]. In a recent study, the EO obtained from A. aragonesis was an effective antioxidant against DPPH (IC50 340 μg/mL); however, our results are in contradiction with the reported concentrations where the results in this study demonstrated IC50 values of 285 ± 0.82 µg/mL (EOAS against DPPH) and IC50 values of 295 ± 0.32 µg/mL (EOAS, against ABTS), respectively [17]. Similarly, the EO obtained from A. Judaica showed strong antioxidant activities by enhancing the catalytic functions of superoxide dismutase and catalase enzymes [18].
Table 3.
DPPH and ABTS free radical scavenging effects of EOAS and EOAA.
| Concentration | % Inhibition (500 µg/mL) |
IC50 Value |
|---|---|---|
| DPPH | ||
| EOAS | 85 ± 0.91 | 285 ± 0.82 µg/mL |
| EOAA | 60 ± 0.31 | 416 ± 0.45 µg/mL |
| Standard drug a | 100 | 55 ± 0.56 µg/mL |
| ABTS | ||
| EOAS | 83 ± 0.43 | 295 ± 0.32 µg/mL |
| EOAA | 57 ± 0.47 | 433 ± 0.65 µg/mL |
| Standard drug b | 100 | 1.0 ± 0.63 µg/mL |
a = Ascorbic acid in DPPH assay; b = Trolox for ABTS assay.
Reactive oxygen species (ROS) can cause serious oxidative harmful effects on macromolecules, especially the biomolecules. These are the chemically reactive ions/radicals produced as byproducts from the primary metabolism and include singlet oxygen, hydrogen peroxide, hydroxyl radicals or superoxide anion radicals. An excess of such ROS in bodies leads to the damage of the enzymatic processes as well as alters the function of various proteins, lipids, and even DNA which result in cerebral dementia type diseases such as Parkinson’s disease (PD), Alzheimer’s disease (AD), diabetes mellitus (DM) as well as cancer development [19,20,21,22]. The balancing of “oxidative stress” induced to the body by ROS could be achieved by using antioxidants as medications or supplements. The EO of various Artemisia species have previously been explored for their antioxidant effects and often correlated to the presence of oxygenated mono or sesquiterpenoids [23]. Another study revealed that the presence of eucalyptol, linalool as well as β-myrecene in A. absinthium essential oils could impart potent antioxidant and herbicidal effects [24]. It has also been observed that an increase in the antioxidant effect usually follows a dose dependent manner, especially in case of essential oils [25]. Our results reveal the presence of tocopherol derivatives in EOAS. Tocopherol and its acetate derivatives have been used as reference drugs for measuring the antioxidant effects in various studies [26], and thus EOAS proved to be best candidate for use as an antioxidant in further studies. Oleic acid and its derivatives have previously been used for the measurement of antioxidant effects in various studies, [27] hence, EOAA could be a possible new antioxidant agent.
2.4. Antibacterial Activities
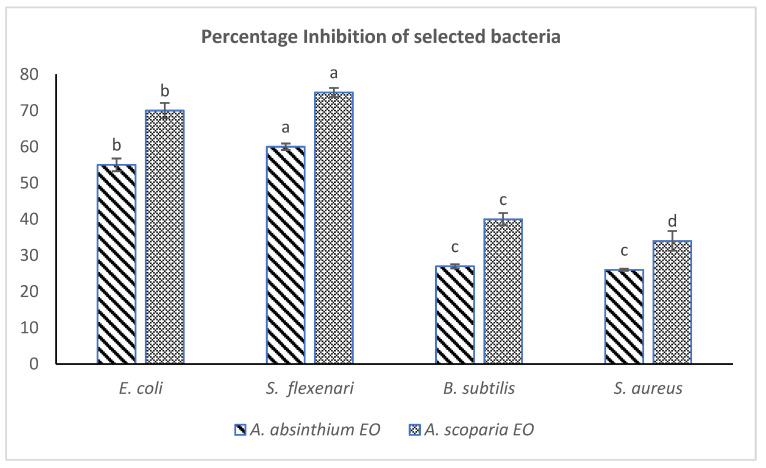
Both of the plants EOs remained effective in imparting strong antibacterial effects against the tested bacteria, especially the Gram-negative EOAS inhibited E. coli (70%) and S. flexneri 75%, while EOAA inhibited E. coli 55% and S. flexneri 60% respectively at the same doses (500 µg/mL). The percentage inhibition calculated from the measurements of zones of inhibitions have been provided in Figure 2. The EOs of A. scoparia have earlier been reported to possess strong antibacterial effects against 15 oral bacteria while various species of Salmonella and Bacillus were found suspectable to the oils of A. absinthium. [28,29]. It has been observed that EO of many Artemisia species exert strong antibacterial effects. The EO of A. abrotanum and A. afra has been effective against Staphylococcus aureus and other pathogenic organisms [30,31]. The oils of A. persica and A. asiatica showed strong antibacterial effects, especially against E. coli [32,33,34]. Studies on A. absinthium EOs suggested strong antibacterial activities (MIC ranged from <800 µ/mL) against some species [16]. Moreover, the presence of phenolic/alcoholic groups in the essential oil components (thymol, carvacrol) have been shown to possess strong antibacterial effects especially on Bacillus species as well as Campylobacter species such as C. jejuni and C. coli [35,36]. Most recently, the EO obtained from A. aragonesis through hydro distillation was effective in inhibiting various pathogenic bacteria such as E. coli, B. subtills and S. aureus with MIC < 7 µg/mL, much strongly than EOAS/EOAA. This may be due to the presence of different phytochemicals (Camphor, 24%) in its EOs [17].
Figure 2.
Comparative antibacterial effect (% inhibition) of EOAS and EOAA (dose of 500 µg/mL DMSO). One same letter shows no significant difference.
These may be used as alternative antibiotics in animals. The formulations of small quantities of such essential oils have been proven to inhibit the production of Streptococcus species, E. coli, and have been successfully been formulated into skin care creams against dermatitis [37]. Moreover, the EO of many other species of Dir-Kohistan have also been investigated for their phytochemical profiles as well as biological activities [38].
Recently, plant essential oils are getting attention as promising antibacterial agents due to low toxicity, smaller dose and resistance of pathogenic bacteria to the existing antibiotics. The evidence of pharmacological activities, especially against antimicrobial action, has been well established for essential oil components (especially the major ones) that possess hydrophilic functionalities in their lipophilic skeletons [39]. Moreover, various essential oils have been considered good antimicrobial agents towards Gram-positive rather than Gram-negative bacteria. Gram-negative bacteria constitute cell envelopes of the outer lipopolysaccharide layer over their thin cell walls composed of the peptidoglycan layer. This structural feature acts as a barrier towards the penetration of hydrophobic compounds and limits their diffusion into bacterium cytoplasms [40]. In addition, the Lipid A component of their complex lipopolysaccharide layer produces toxicity towards the human immune defense system [41]. Our findings suggest that both EOAS and EOAA can inhibit the growth of Gram-negative bacteria, probably with different modes of actions. One of the most probable pathways includes either the ease of entrance of the smaller constituents of EOs through the outer membranes of Gram-negative bacteria or the incorporation of these constituents with bacterial enzyme systems [42].
It has been observed that the EO of Artemisia from their leaves differed in their phytochemicals in comparison with literature. e.g., EO of A. firgida, A. cana, A. tridentata. The oils overall showed a weak pharmacological effect (anti-inflammatory) in activating the human neutrophils; however, the relatively higher effect was showed by A. tridentata due to the presence of farnesene (smaller amounts) [43]. A recent study of various Artemisia species suggested the presence of sufficient amounts of oxygenated monoterpenoids that imparted potential insecticidal activities (LD50 = 17–52 μg/mL), a possible therapeutic application for environmentally friendly pesticides [44].
2.5. Antifungal Activity
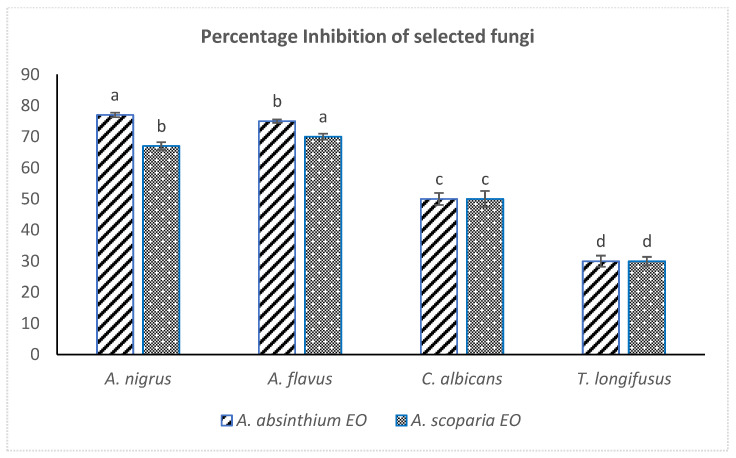
Both the EOAS and EOAA showed strong antifungal effects against the Aspergillus species (70% inhibition of Aspergillus niger and 67% Aspergillus flavus), while they remained ineffective against Candida albicans and Trichophyton longifusus. The linear growth and control linear growth were effectively measured for calculating the percentage inhibition of EOs against the tested fungal strains (Figure 3). The results are consistent with the reported antifungal effects of EOs of A. judaica, A. absintium and A. biennis against Aspergillus niger, however, no reports have been documented on the antifungal activity of A. scoparia EO against A. flavus so far [45]. Strong antifungal effects have been documented in the literature for Artemisia essential oils along with their cytotoxic, antihepatotoxic and antimalarial activities [46,47]. The EO obtained from A. Judaica was potent against the Candida and Aspergillus species [17].
Figure 3.
Comparative antifungal effects (% inhibition) of EOAS and EOAA (dose of 500 µg/mL DMSO). One same letter shows no significant difference.
Fungi are mostly tough targets for the essential oils to inhibit at micro and macro levels. The pathogenic fungi including Aspergillus and Candida species are notorious and harmful for large populations, causing serious infections, especially in those with immune deficiencies, hence the need for greater attention [48]. Various human pathogenic fungi including Candida albicans show susceptibility to the essential oils of Apiaceae plants, cinnamon, lemongrass, clove oil etc. [49,50]. The oil is rich in sesquiterpenoids such as bisabolol, inhibits the growth of Aspergillus niger, A. flavus and Candida species, and thus confirms our findings [8].
2.6. Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) Inhibition Activities
The results against AChE and BChE suggested that both the EOs successfully inhibited these enzymes in vitro by showing IC50 values of 30 ± 0.04 µg/mL (EOAS), 32 ± 0.05 µg/mL (EOAA) against AChE and 34 ± 0.07 µg/mL (EOAS), 36 ± 0.03 µg/mL (EOAA) against BChE respectively (Table 4). More promising results have been noted in BChE inhibition where the IC50 values of EOAS (34 ± 0.07 µg/mL) and EOAA (36 ± 0.03 µg/mL) were close to the IC50 of allanzanthane (IC50 24.32 ± 0.04).
Table 4.
Cholinesterase inhibition data of EOAS and EOAA.
| Sample | AChE ± SEM a (IC50 in µg/mL) | BChE ± SEM (IC50 in µg/mL) |
|---|---|---|
| EOAS | 30 ± 0.04 | 34 ± 0.07 |
| EOAA | 32 ± 0.05 | 36 ± 0.03 |
| Allanzanthane b | 6.38 ± 0.04 | 24.32 ± 0.04 |
| Galantamine b | 8.22 ± 0.03 | 17.03 ± 0.05 |
a = Standard Error Mean of five assays; b = positive controls; data shown are values from triplicate experiments.
These promising results are in contrast to the previous results obtained for the Artemisia species, where the IC50 ranged from 100 to 200 µg/mL [51]. This potent effect could be attributed to the different chemical profiling of our EOs, especially the presence of tocopherol derivatives in EOAS as well as the occurrence of oleic acid in the EOAA. Tocopherol is a reversible inhibitor of AChE and has kinetically been estimated to slowly bind to human enzymes with 2 min of residence time on target. Moreover, the molecular docking studies suggest that tocopherol non-specifically interacts with the alkylene chain of enzymes without producing any conformational changes [15]. A. scoparia has also demonstrated a well-established anti-Alzheimer’s disease (AD) effect by lowering β-amyloid precursor protein levels through cleaving related enzymes as well as suppressing the expression of phosphorylated glycogen synthase kinases in vivo, thus making this plant a good candidate against AD and related dementias [52].
EOAA was enriched (41%) in oleic acid contents, which is a remarkable selective inhibitor of BChE in earlier reports. It has been observed that oleic acid binds to different allostatic sites of enzyme by interaction to OH and NH3 functional groups at cystine 319 and tyrosine 320 and show some non-competitive reversible inhibition [53]. The literature suggests that various plant extracts containing smaller amounts of oleic acid have shown promising anti-cholinesterase effects [54,55]. However, the major effect has been observed against BChE in many cases. Our study suggests that EOAA strongly inhibits both the enzymes which render a potential use of Artemisia based formulations against AD and related diseases.
3. Materials and Methods
3.1. Identification of Plant Material and Collection
The plants A. scoparia and A. absinthium were identified by plant taxonomist, Prof. Dr. Ali Hazrat, University of Malakand, Lower Dir, KPK, Pakistan. Both the plants are wild and not being cultivated. The voucher specimens (accession number A-039; A-040) were deposited in the herbarium of Natural Product Laboratory, Department of Chemistry, SBBU, Sheringal. The collection of aerial parts (leaves and shoots) of A. scoparia was made from Dog Dara (altitude 5367 feet), Sheringal, Dir (Upper), Pakistan (35.33° N latitude; 71.97° E longitude) while that of A. absinthium were collected from Munda, Dir lower (35.56° N latitude; 72.20° E longitude; 8100 feet altitude).
3.2. Isolation of Essential Oils (EOs)
The oils were extracted from the fresh leaves of both Artemisia species through hydro-distillation methods using a Clevenger apparatus [56]. Fresh mature leaves (250 g) of each plant were separated, sliced and combined with distilled water in a round bottom flask (1 L) fixed with a glass column (60 cm length) and connected with a condenser. Each combination was boiled and the oils from the hydro-distillate were decanted from the snout of the condenser. The procedure was repeated four times for each species with fresh leaves. A. scoparia yielded pale yellow colored oil (0.2% w/w), while A. absinthium yielded a light greenish color oil (0.35% w/w). The collected oils were dried over anhydrous magnesium sulphate and kept in the dark at 4 °C for further analysis.
3.3. Gas Chromatography and Mass Spectrometry (GC/MS) Analysis
The phytochemical profile of essential oil samples was determined through GC/MS analysis. An Agilent USB=393752 GC (Agilent Technologies, Palo Alto, CA, USA) connected to an Agilent HP 5973 Mass Spectrometer (mass selective detector) in electron impact (EI, 70 eV) mode was used to obtain GC/MS data. The GC was equipped with a HHP-5MS 5% phenylmethylsiloxane column (capillary column, 30 m × 0.25 mm × 0.25 μm film thickness, Restek, PaloAlto, CA, USA) having an FID detector. Helium gas was used as carrier gas (flow rate 1 mL/min) while a dynamic oven temperature range was used. The initial temperature was retained at 70 °C for 1 min followed by a 6 °C/min increase up to 180 °C. The temperature was again retained for 5 min at 180 °C and then increased at a rate of 5 °C up to 280 °C and retained for 20 min. The samples were injected in a splitless mood (1/1000 in pentane, v/v). The scan range was 50 m/z to 800 m/z. The individual compounds were identified through comparative analysis with their retention times and spectral pattern on the same conditions from our database, NIST libraries as well as Kovat retention indexes (relative calculation with n-alkane series on same columns).
3.4. 1,1-diphenyl-2-picryl-hydrazyl (DPPH) Free Radical Scavenging Assay
DPPH was purchased from Sigma Aldrich (Burlington, MA, USA), while all the other solvents used were of analytical grade. The assays of EOAS and EOAA were carried according to the method described in the literature with slight modifications [57]. Briefly, the EOs stock solution was prepared by the addition of 500 μg EO (each) with 1 mL methanol while 0.075 mM DPPH solution was prepared in methanol. To initiate the reaction, 0.1 mL of EOs solution was added into 3.9 mL of DPPH solution, homogenized at 2500 rpm for 2 min to remove any cloudy substance, and the absorbance was measured at 517 nm (t = 0) on a BMS spectrophotometer (VIS-1100, New York, USA). The mixtures were then incubated in the dark for 30 min at room temperature and then their absorbance was again measured at 517 nm. The control sample was prepared by mixing 0.1 mL methanol in a 3.9 mL DPPH solution. IC50 values for the mixture samples were obtained by serial dilution and individual measurements. The following formula was applied in calculating the percent absorbance.
| (1) |
3.5. 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) Method
An ABTS assay was performed for further measurements of the antioxidant potential of the essential oils [58]. According to this method, a 10 mL ABTS substrate was mixed with 25 μL of 3% hydrogen peroxide to obtain the standard curve (A). In the next step, 10 μL of trolox (reference standard) was added in wells with the addition of 20 μL of myoglobin working solution to obtain a trolox reference curve (B). In the next step, 10 μL test sample + 20 μL of myoglobin working solution were added to the wells from which the test samples (C) were collected. Finally, the reactions were started with the addition of 150 μL ABTS substrate solution to each well (having A, B, C solutions), incubated for 5 min, and, after that, 100 μL of stop solution was added. The endpoint was monitored at 405 nm using a plate reader. The concentration of EO used for this assay was 500 µg/mL in DMSO. The following formula was used for measurement of ABTS effect.
| (2) |
3.6. Antibacterial Assay
The agar disc diffusion procedure was used for the measurement of antibacterial activity of the essential oils against the selected strains e.g., Escherichia coli (ATCC 15224), Bacillus subtilis (ATCC 6663), Staphylococcus aureus (ATCC 29213) and Shigella flexneri (ATCC 14028). The stock solutions for the sample were prepared in 500 µg/mL concentrations in DMSO, while 100 and 200 μL of each dilution were added to the concerned wells (control contained = 100 and 200 μL DMSO). The reference drug used was clarithromycin. The diameters for the zone of inhibition were measured in mm, converted into a percentage while the MIC of the antibiotic was measured using standard methods [59].
3.7. Fungicidal Assay
The standard method, “agar dilution”, was performed to measure the antifungal activity of the essential oils [60]. The strain used in this assay included Aspergillus flavus (ATTC 32611), Aspergillus niger, clinically isolated, obtained from the microbiology lab, Agha Khan University Hospital, Karachi, Pakistan), Candida albicans (ATTC 2091) and Trichophyton longifusus (ATTC 22397). The 500 µg/mL concentration of the sample was dissolved in DMSO (sterile). The linear growth of the fungal strains was monitored visually after seven days (incubation in humid conditions at 37 °C). Media nourishment growth was detected by measuring linear growth (mm), and growth inhibition was calculated with reference to the negative control (also presented in %), (miconazole as reference drug).
3.8. Cholinesterase Inhibitory Assay and IC50 Values Determination
A modified spectroscopic method for the assessment of cholinergic potential of essential oils was adopted [61]. The enzymes used in this assay were acetylcholinesterase (AChE, EC 3.1.1.7) and butyrylcholinesterase (BChE, EC. 3.1.1.8), the substrates used were, acetylcholine iodide and butyrylcholine chloride, while the standard used was Allanzanthane and Galantamine. 5-5′-dithiobis[2-nitrobenzoic acid] (DTNB) acted as the colorimetric reagent. All the chemicals used were of analytical grade. Accordingly, 40 µL of AChE/BChE and 40 µL of test solution were mixed in 880 µL of sodium phosphate buffer (pH = 8) while 0.2 mM DTNB was also added to each sample solution. The reaction was started by the addition of 40 µL of acetylcholine iodide or butyrylcholine chloride to each sample followed by incubation for 15 min and then monitoring the yellow color of solutions at 412 nm due to the formation of 5-thio-2-nitrobenzene as the product of hydrolysis of acetylthiocholine and butyryl thiocholine. All of the experiments were performed in replicas of five assays. The IC50 values were determined from various concentrations using the serial dilution method. A double beam BMS spectrophotometer (USA) was used for λmax measurements.
3.9. Statistical Analysis
The data were evaluated statistically by a Student’s t-test. p values less than 0.05 and 0.01 were taken as significant. Values are expressed as mean ± S.E.M.
4. Conclusions
The current studies have been carried out to identify the constituents of essential oils (EOs) obtained from the leaves of Artemisia scoparia and A. absinthium as well as their antioxidant and antimicrobial effects. The oils were obtained by the hydro-distillation method. A total of 14 compounds were detected in A. scoparia with a major portion composed of tocopherol derivatives, while oleic acid derivatives were the dominant constituents in A. absinthium. Strong antioxidant effects were pronounced by the EOs of A. scoparia; however, A. absinthium showed lower antioxidant effects. Both of the EOs potentially inhibited the growth of the tested Gram-negative bacteria, however, remained low potent against Gram-positive. Both the oils were active in inhibiting the Aspergillus species. The oils showed pronounced cholinergic potential in inhibiting the tested enzymes, acetylcholinesterase and butyrylcholinesterase in vitro. Our findings reveal that the essential oils of A. scoparia and A. absinthium are promising antioxidant, antimicrobial and cholinergic agents, even if devoid of aroma components.
Acknowledgments
The authors like to thank Taif University, Taif, Saudi Arabia for their support through Taif University Researchers Supporting Project number TURSP-2020/80.
Author Contributions
Conceptualization, F.A.K. and N.M.K.; methodology, N., R.A. and I.U.; software, S.A.; validation, F.A.K.; formal analysis, F.A.K. and S.A.; investigation, R.A. and I.U.; resources, M.A. (Mazen Almehmadi); data curation, M.A. (Mazen Almehmadi) and A.A.A.; writing—original draft preparation, F.A.K., N. and I.U.; writing—review and editing, A.A., A.A.A. and M.A. (Mamdouh Allahyani); visualization, N.M.K.; supervision, F.A.K. and N.M.K.; project administration, F.A.K.; funding acquisition, M.A. (Mazen Almehmadi), M.A. (Mamdouh Allahyani) and S.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aljaafari M.N., Alali A.O., Baqais L., Alqubaisy M., Alali M., Molouki A., Ong-Abdullah J., Abushelaibi A., Lai K.S., Lim S.H.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules. 2021;26:628. doi: 10.3390/molecules26030628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Göksen G., Fabra M.J., Pérez-Cataluña A., Ekiz H.I., Sanchez G., López-Rubio A. Biodegradable active food packaging structures based on hybrid cross-linked electrospun polyvinyl alcohol fibers containing essential oils and their application in the preservation of chicken breast fillets. Food Packag. Shelf Life. 2021;27:100613. doi: 10.1016/j.fpsl.2020.100613. [DOI] [Google Scholar]
- 3.Dawood M.A.O., El Basuini M.F., Zaineldin A.I., Yilmaz S., Hasan M.T., Ahmadifar E., El Asely A.M., Abdel-Latif H.M.R., Alagawany M., Abu-Elala N.M., et al. Antiparasitic and Antibacterial Functionality of Essential Oils: An Alternative Approach for Sustainable Aquaculture. Pathogens. 2021;10:185. doi: 10.3390/pathogens10020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasheminya S.M., Dehghannya J. Chemical composition, antioxidant, antibacterial, and antifungal properties of essential oil from wild Heracleum rawianum. Biocatal. Agric. Biotechnol. 2021;31:101913. doi: 10.1016/j.bcab.2021.101913. [DOI] [Google Scholar]
- 5.Ansari M.H., Mahapatra D.K. Natural Products Pharmacology and Phytochemicals for Health Care. Apple Academic Press; Palm Bay, FL, USA: 2021. A short overview on Anti-diabetic natural products: Reviewing the herbotherapeutic potentials; pp. 1–22. [DOI] [Google Scholar]
- 6.Arena M.E., Alberto M.R., Cartagena E. Potential use of Citrus essential oils against acute respiratory syndrome caused by coronavirus. J. Essent. Oil Res. 2021;33:330–341. doi: 10.1080/10412905.2021.1912839. [DOI] [Google Scholar]
- 7.Sharmeen J.B., Mahomoodally F.M., Zengin G., Maggi F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules. 2021;26:666. doi: 10.3390/molecules26030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisht D., Kumar D., Kumar D., Dua K., Chellappan D.K. Phytochemistry and pharmacological activity of the genus Artemisia. Arch. Pharmacal Res. 2021;44:439–474. doi: 10.1007/s12272-021-01328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni L., Wu H., Du C., Li X., Li Y., Xu C., Wang P., Li S., Zhang J., Chen X. Effects of allelochemical artemisinin in Artemisia annua on Microcystis aeruginosa: Growth, death mode and microcystin-LR changes. Environ. Sci. Pollut. Res. 2021;28:45253–45265. doi: 10.1007/s11356-021-13793-x. [DOI] [PubMed] [Google Scholar]
- 10.Romeilah R.M., El-Beltagi H.S., Shalaby E.A., Younes K.M., El Moll H., Rajendrasozhan S., Mohamed H.I. Antioxidant and cytotoxic activities of Artemisia monosperma L. and Tamarix aphylla L. essential oils. Not. Bot. Horti Agrobot. Cluj-Napoca. 2021;49:12233. doi: 10.15835/nbha49112233. [DOI] [Google Scholar]
- 11.Jan G., Ajab Khan M., Gul F., Ahmad M., Jan M., Zafar M. Ethnobotanical study of common weeds of Dir Kohistan Valley, Khyber Pakhtoonkhwa, Pakistan. Pak. J. Weed Sci. Res. 2010;16:81–88. [Google Scholar]
- 12.Hazrat A., Nisar M.O., Shah J., Ahmad S. Ethnobotanical study of some elite plants belonging to Dir, Kohistan valley, Khyber Pukhtunkhwa, Pakistan. Pak. J. Bot. 2011;43:787–795. [Google Scholar]
- 13.Van Hung P., Lan Phi N.T., Vy Vy T.T. Effect of debranching and storage condition on crystallinity and functional properties of cassava and potato starches. Starch–Stärke. 2012;64:964–971. doi: 10.1002/star.201200039. [DOI] [Google Scholar]
- 14.Esposito E.R., Bystrek M.V., Klein J.S. An elective course in aromatherapy science. Am. J. Pharm. Educ. 2014;78:79. doi: 10.5688/ajpe78479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J., Wang L., He C., Zhao J., Si L., Huang H. Artemisia scoparia: Traditional uses, active constituents and pharmacological effects. J. Ethnopharmacol. 2021;273:113960. doi: 10.1016/j.jep.2021.113960. [DOI] [PubMed] [Google Scholar]
- 16.Mihajilov-Krstev T., Jovanović B., Jović J., Ilić B., Miladinović D., Matejić J., Rajković J., Äorević L., Cvetković V., Zlatković B. Antimicrobial, Antioxidative, and Insect Repellent Effects of Artemisia absinthium Essential Oil. Planta Med. 2014;80:1698–1705. doi: 10.1055/S-0034-1383182. [DOI] [PubMed] [Google Scholar]
- 17.Chebbac K., Ghneim H.K., El Moussaoui A., Bourhia M., El Barnossi A., Benziane Ouaritini Z., Guemmouh R. Antioxidant and antimicrobial activities of chemically-characterized essential oil from Artemisia aragonensis Lam. against drug-resistant microbes. Molecules. 2022;27:1136. doi: 10.3390/molecules27031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammed H.A., Qureshi K.A., Ali H.M., Al-Omar M.S., Khan O., Mohammed S.A. Bio-Evaluation of the Wound Healing Activity of Artemisia judaica L. as Part of the Plant’s Use in Traditional Medicine; Phytochemical, Antioxidant, Anti-Inflammatory, and Antibiofilm Properties of the Plant’s Essential Oils. Antioxidants. 2022;11:332. doi: 10.3390/antiox11020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duarte-Jurado A.P., Gopar-Cuevas Y., Saucedo-Cardenas O., Loera-Arias M.D.J., Montes-De-oca-luna R., Garcia-Garcia A., Rodriguez-Rocha H. Antioxidant Therapeutics in Parkinson’s Disease: Current Challenges and Opportunities. Antioxidants. 2021;10:453. doi: 10.3390/antiox10030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasconcelos L.H.C., Ferreira S.R.D., Silva M.D.C.C., Ferreira P.B., Souza I.L.L.D., Cavalcante F.D.A., Silva B.A.D. Uncovering the Role of Oxidative Imbalance in the Development and Progression of Bronchial Asthma. Oxid. Med. Cell. Longev. 2021;2021:6692110. doi: 10.1155/2021/6692110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reily-Bell M., Bahn A., Katare R. Reactive Oxygen Species-Mediated Diabetic Heart Disease: Mechanisms and Therapies. Antiox. Redox Signal. 2022;36:608–630. doi: 10.1089/ars.2021.0098. [DOI] [PubMed] [Google Scholar]
- 22.Kang R., Tang D. Cancer, Oxidative Stress and Dietary Antioxidants. 2nd ed. Acadamic Press; Elsevier; Cambridge, MA, USA: 2021. Chapter 14: Ferroptosis, free radicals, and cancer; pp. 149–158. [DOI] [Google Scholar]
- 23.Singh H.P., Kaur S., Mittal S., Batish D.R., Kohli R.K. In vitro screening of essential oil from young and mature leaves of Artemisia scoparia compared to its major constituents for free radical scavenging activity. Food Chem.Toxicol. 2010;48:1040–1044. doi: 10.1016/j.fct.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Jiang C., Zhou S., Liu L., Toshmatov Z., Huang L., Shi K., Shao H. Evaluation of the phytotoxic effect of the essential oil from Artemisia absinthium. Ecotoxicol. Environ. Saf. 2021;226:112856. doi: 10.1016/j.ecoenv.2021.112856. [DOI] [PubMed] [Google Scholar]
- 25.Chaqroune A., Sakar E.H., Mahjoubi F., Chaouch M., Chaqroune A., Taleb M. Effects of Extraction Technique and Solvent on Phytochemicals, Antioxidant, and Antimicrobial Activities of Cultivated and Wild Rosemary (Rosmarinus officinalis L.) from Taounate Region (Northern Morocco) Biointerface Res. App. Chem. 2022;12:8441–8452. doi: 10.33263/BRIAC126.84418452. [DOI] [Google Scholar]
- 26.Kulisic T., Radonic A., Katalinic V., Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633–640. doi: 10.1016/j.foodchem.2003.07.024. [DOI] [Google Scholar]
- 27.Ayaz M., Junaid M., Ullah F., Sadiq A., Subhan F., Khan M.A., Ahmad W., Ali G., Imran M., Ahmad S. Molecularly characterized solvent extracts and saponins from Polygonum hydropiper L. show high anti-angiogenic, anti-tumor, brine shrimp, and fibroblast NIH/3T3 cell line cytotoxicity. Front. Pharmacol. 2016;7:74. doi: 10.3389/fphar.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cha J.D., Jeong M.R., Jeong S.I., Moon S.E., Kim J.Y., Kil B.S., Song Y.H. Chemical composition and antimicrobial activity of the essential oils of Artemisia scoparia and A. capillaris. Planta Med. 2005;71:186–190. doi: 10.1055/s-2005-837790. [DOI] [PubMed] [Google Scholar]
- 29.Dulger B., Ceylan M., Alitsaous M., Uğurlu E. Artemisia absinthium L. (Pelin)’un antimikrobiayal aktivitesi. Turk. J. Biol. 1999;23:377–384. [Google Scholar]
- 30.Liu C.Z., Murch S.J., El-Demerdash M., Saxena P.K. Artemisia judaica L. Micropropagation and antioxidant activity. J. Biotechnol. 2004;110:63–71. doi: 10.1016/j.jbiotec.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Misire C. Ph.D. Thesis. University of Eldoret; Eldoret, Kenya: 2021. Bioactivity of Crude Essential Oils and Blends of Artemisia afra, Ocimum Kilimandscharicum AND Tagetes Minuta against Anopheles Gambiae SS. [DOI] [Google Scholar]
- 32.Muyima N.Y.O., Zulu G., Bhengu T., Popplewell D. The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour Fragr. J. 2002;17:258–266. doi: 10.1002/ffj.1093. [DOI] [Google Scholar]
- 33.Khezri S., Khezerlou A., Dehghan P. Antibacterial activity of Artemisia persica Boiss essential oil against Escherichia coli O157: H7 and Listeria monocytogenes in probiotic Doogh. J. Food Process. Preserv. 2021;45:e15446. doi: 10.1111/jfpp.15446. [DOI] [Google Scholar]
- 34.Kalemba D., Kusewicz D., Świa̧der K. Antimicrobial properties of the essential oil of Artemisia asiatica Nakai. Phyther. Res. 2002;16:288–291. doi: 10.1002/ptr.856. [DOI] [PubMed] [Google Scholar]
- 35.Mutlu-Ingok A., Catalkaya G., Capanoglu E., Karbancioglu-Guler F. Antioxidant and antimicrobial activities of fennel, ginger, oregano and thyme essential oils. Food Front. 2021;2:508–518. doi: 10.1002/fft2.77. [DOI] [Google Scholar]
- 36.Wannissorn B., Jarikasem S., Siriwangchai T., Thubthimthed S. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia. 2005;76:233–236. doi: 10.1016/j.fitote.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Reichling J., Fitzi J., Hellmann K., Wegener T., Bucher S., Saller R. Topical tea tree oil effective in canine localised pruritic dermatitis--a multi-centre randomised double-blind controlled clinical trial in the veterinary practice. Dtsch. Tierarztl. Wochenschr. 2004;111:408–414. [PubMed] [Google Scholar]
- 38.Jan A.K., Hazrat A., Ahmad S., Jan T., Jan G. In vitro antifungal, antibacterial, phytotoxic, brine shrimp, insecticidal activities and composition of essential oil of Tagetes minuta from Dir-kohistan, Pakistan. Pak. J. Bot. 2019;51:201–204. doi: 10.30848/PJB2019-1(19). [DOI] [Google Scholar]
- 39.Griffin S.G., Wyllie S.G., Markham J.L., Leach D.N. The role of structure and molecular properties of terpenoids in determining their antimicrobial activity. Flavour Fragr. J. 1999;14:322–332. doi: 10.1002/(SICI)1099-1026(199909/10)14:5<322::AID-FFJ837>3.0.CO;2-4. [DOI] [Google Scholar]
- 40.Zeroual A., Sakar E.H., Eloutassi N., Mahjoubi F., Chaouch M., Chaqroune A. Wild chamomile [Cladanthus mixtus (L.) chevall.] collected from central-northern Morocco: Phytochemical profiling, antioxidant, and antimicrobial activities. Bioint. Res. App. Chem. 2021;11:11440–11457. [Google Scholar]
- 41.Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis: A brief review. Virulence. 2014;5:213–218. doi: 10.4161/viru.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Schepetkin I.A., Özek G., Özek T., Kirpotina L.N., Khlebnikov A.I., Klein R.A., Quinn M.T. Neutrophil Immunomodulatory Activity of Farnesene, a Component of Artemisia dracunculus Essential Oils. Pharmaceuticals. 2022;15:642. doi: 10.3390/ph15050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J.W., Li B.Y., Lu X.X., Zheng Y., Wang D., Zhang Z., Du S.S. Chemical Diversity and Anti-Insect Activity Evaluation of Essential Oils Extracted from Five Artemisia Species. Plants. 2022;11:1627. doi: 10.3390/plants11131627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stojanović G.S., Ickovski J.D., Đorđević A.S., Petrović G.M., Stepić K.D., Palić I.R., Stamenković J.G. The First Report on Chemical Composition and Antimicrobial Activity of Artemisia scoparia Waldst. et Kit. Extracts. Nat. Prod. Commun. 2020;15:1934578X20915034. doi: 10.1177/1934578X20915034. [DOI] [Google Scholar]
- 46.Bora K.S., Sharma A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011;49:101–109. doi: 10.3109/13880209.2010.497815. [DOI] [PubMed] [Google Scholar]
- 47.Badea M.L., Delian E., Dobrescu A., Bădulescu L., Alexandru Mihai C. Investigation the quantity and quality of essential oil of Artemisia vulgaris L. Sci. Pap.-Ser. B Hortic. 2020;64:628–632. [Google Scholar]
- 48.Yahia S., Alsayed A.A., Mokhtar G. Fungal profile of otomycosis in a sample of Egyptian patients in Zagazig university hospitals: A prospective study. Microbes Infect. Dis. 2021;2:143–151. doi: 10.21608/mid.2020.48586.1082. [DOI] [Google Scholar]
- 49.Sahal G., Woerdenbag H.J., Hinrichs W.L.J., Visser A., Tepper P.G., Quax W.J., van der Mei H.C., Bilkay I.S. Antifungal and biofilm inhibitory effect of Cymbopogon citratus (lemongrass) essential oil on biofilm forming by Candida tropicalis isolates; an in vitro study. J. Ethnopharmacol. 2020;246:112188. doi: 10.1016/j.jep.2019.112188. [DOI] [PubMed] [Google Scholar]
- 50.Shahrivari S., Alizadeh S., Ghassemi-Golezani K., Aryakia E. A comprehensive study on essential oil compositions, antioxidant, anticholinesterase and antityrosinase activities of three Iranian Artemisia species. Sci. Rep. 2022;12:1–12. doi: 10.1038/s41598-022-11375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Promyo K., Cho J.Y., Park K.H., Jaiswal L., Park S.Y., Ham K.S. Artemisia scoparia attenuates amyloid β accumulation and tau hyperphosphorylation in spontaneously hypertensive rats. Food Sci. Biotechnol. 2017;26:775–782. doi: 10.1007/s10068-017-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Youn K., Yun E.Y., Lee J., Kim J.Y., Hwang J.S., Jeong W.S., Jun M. Oleic acid and linoleic acid from Tenebrio molitor larvae inhibit BACE1 activity in vitro: Molecular docking studies. J. Med. Food. 2014;17:284–289. doi: 10.1089/jmf.2013.2968. [DOI] [PubMed] [Google Scholar]
- 53.Çayan F., Tel G., Duru M.E., Öztürk M., Türkoğlu A., Harmandar M. Application of GC, GC-MSD, ICP-MS and spectrophotometric methods for the determination of chemical composition and in vitro bioactivities of Chroogomphus rutilus: The edible mushroom species. Food Anal. Methods. 2014;7:449–458. doi: 10.1007/s12161-013-9644-2. [DOI] [Google Scholar]
- 54.da Silva G.G., Pimenta L.P.S., Melo J.O.F., Mendonça H.D.O.P., Augusti R., Takahashi J.A. Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents. Molecules. 2022;27:1892. doi: 10.3390/molecules27061892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orhan I.E., Senol F.S., Ozturk N., Celik S.A., Pulur A., Kan Y. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L.(dill) samples cultivated under organic and conventional agricultural conditions. Food Chem. Toxicol. 2013;59:96–103. doi: 10.1016/j.fct.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 56.Jennan S., Fouad R., Nordine A., Farah A., Bennani B., Moja S., Greche H., Mahjoubi F. Chemical Composition and Antibacterial Screening of Aerial Parts of Essential Oils of Three Satureja species (Satureja briquetti, Satureja atlantica and Satureja alpina) Growing Wild in the Middle Atlas Mountains of Morocco. J. Essent. Oil-Bear. Plants. 2018;21:741–748. doi: 10.1080/0972060X.2018.1486230. [DOI] [Google Scholar]
- 57.Sharma O.P., Bhat T.K. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202–1205. doi: 10.1016/j.foodchem.2008.08.008. [DOI] [Google Scholar]
- 58.Huang B., Ban X., He J., Tong J., Tian J., Wang Y. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010;120:873–878. doi: 10.1016/j.foodchem.2009.11.020. [DOI] [Google Scholar]
- 59.Rahman S., Imra M., Muhammad N., Hassan N., Khan S. Antibacetial screening of leaves and stem of Carica papaya. J. Med. Plants Res. 2011;5:5167–5171. doi: 10.5897/JMPR.9000119. [DOI] [Google Scholar]
- 60.Szekely A., Johnson E.M., Warnock D.W. Comparison of E-Test and broth microdilution methods for antifungal drug susceptibility testing of molds. J. Clin. Microbiol. 1999;37:1480–1483. doi: 10.1128/JCM.37.5.1480-1483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.