Abstract
Expression of the chemokine receptors CXCR4 and CCR5 was monitored using EDTA-anticoagulated whole blood held for different time periods prior to fluorescent-antibody staining. When left overnight CXCR4 expression on leukocytes was substantially increased, whereas CCR5 expression was reduced. The results were similar when heparin and acid-citrate-dextrose were used as anticoagulants.
The G protein-coupled chemokine receptors, CXCR4 and CCR5, are the major human immunodeficiency virus (HIV) coreceptors that, in addition to CD4, are utilized for viral entry (5–7). These receptors play an important role in maintaining an effective immune response through chemotaxis in response to their specific ligands; CXCR4-expressing cells migrate in response to SDF-1α (1), while CCR5-expressing cells are recruited by MIP-1α, MIP-1β, and RANTES (4). Therefore, alterations in receptor expression in vivo could lead to dysregulation of cellular trafficking and alterations in cell permissiveness for HIV type 1 (HIV-1).
Over the past few years, a number of studies have evaluated the expression of CXCR4 and CCR5 using whole blood. It has been demonstrated that CXCR4 expression is decreased in CD4+ and CD8+ T cells and that CCR5 expression is elevated in CD4+ T cells of HIV-1-infected persons compared with uninfected controls (9, 12). Antiretroviral therapy significantly increases the cellular expression of CXCR4 and decreases that of CCR5 compared with pretherapy levels (9). CCR5 density on CD4+ T cells has further been shown to be a determining factor of virus load in HIV-1-infected individuals (13). A recent study, but using isolated peripheral blood mononuclear cells, has shown that thalidomide can reduce the upregulation of CXCR4 and CCR5 induced by bacterial and mycobacterial antigens (11). Flow cytometric analysis of these chemokine receptors is therefore a valuable tool, particularly in HIV-1 disease, and is likely to be utilized in the future for predictive purposes and for monitoring the success of various therapies.
As part of a study to investigate the effect that infection with HIV-1 has on CXCR4 and CCR5 receptor expression, we had stained EDTA-anticoagulated blood samples from a cohort of HIV-1-infected patients (n = 9); these samples had been delayed due to transportation difficulties. To control for possible effects due to standing time, we compared these results with those from a cohort (comparable with respect to age, sex, race, CD4 cell count, and viral load) where samples were stained within 6 h (n = 11). Using two- and three-color staining, different subsets of mononuclear cells expressing either CXCR4 or CCR5 (CXCR4-phycoerythrin [PE] and CCR5-PE; Pharmingen, San Diego, Calif.) were identified with markers that distinguish T cells (CD3-peridinin chlorophyll protein [PerCP]), CD4+ and CD8+ T cells (CD4-PerCP, CD8-PerCP, CD3-fluorescein isothiocyanate [FITC]), B cells (CD19-FITC; Becton Dickinson, San Jose, Calif.), monocytes (CD14-FITC; Coulter, Hileah, Fla.), and CD16+ CD56+ CD3− natural killer (NK) cells (CD16-FITC [Becton Dickinson] and CD56-FITC [Serotec]). B cells and NK cells were analyzed after gating for lymphocytes based on CD45 staining and forward scatter (FSC) and side scatter (SSC) properties. CD4+ and CD8+ T cells were identified by initial gating on total CD3+ cells and SSC. Monocytes and granulocytes were identified using their FSC and SSC properties and the presence or absence of CD14 staining, respectively. In order to control for nonspecific staining, quadrants were set using isotype-matched controls immunoglobulin G1 (IgG1)-PerCP and IgG2a-PE (Becton Dickinson), IgG1-FITC (Dako), and IgG2a-FITC (Serotec). Ten thousand events were acquired per sample. Calibrite beads (Becton Dickinson) were run on a weekly basis to ensure the stability of the flow cytometer. When we compared CXCR4 staining of whole blood within 6 h (t = 0) with staining after overnight (ON) incubation there were significant increases in CXCR4 expression on all cell subsets, as reflected in both the percentage of fluorescing cells (except for on polymorphonuclear neutrophils) and in the mean fluorescence intensity (MFI). In contrast, there was a trend toward a decrease in CCR5 expression in the samples that were stained after ON incubation compared with those stained within 6 h (data not shown).
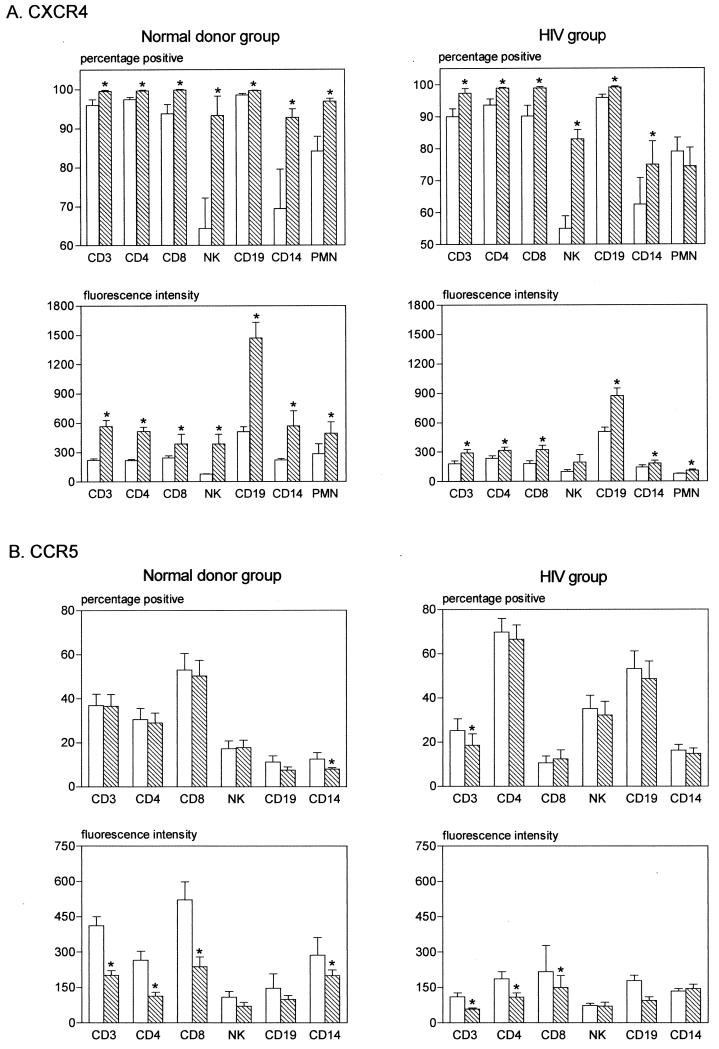
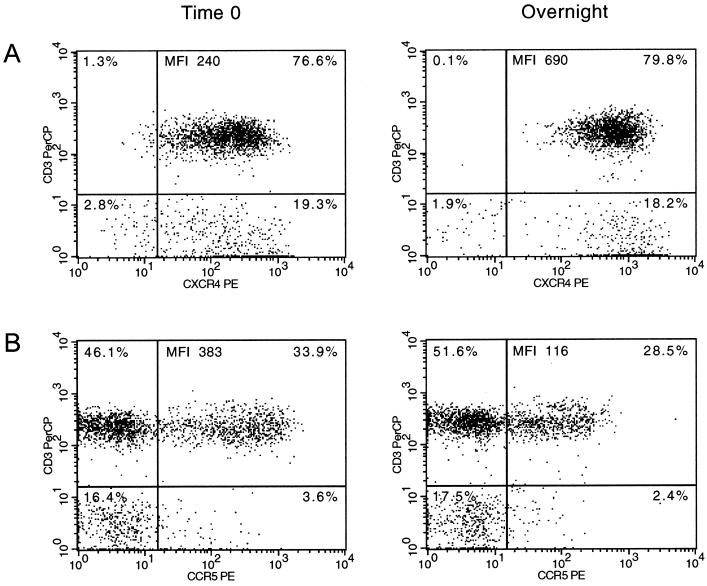
In order to confirm these observations, longitudinal analysis was performed on whole blood samples obtained from six healthy individuals (normal donor group) and from seven HIV-1-infected individuals (HIV group). These samples were stained within 6 h (t = 0) after blood was drawn and again after being left at room temperature ON. As shown in Fig. 1A, delaying the sample staining resulted in a significant upregulation of CXCR4 expression on all cell types, as shown in both proportions of cells expressing CXCR4 as well as in the MFI. Exceptions were the proportions of CXCR4-expressing polymorphonuclear neutrophils and MFI of CXCR4 on NK cells in the HIV group. Conversely, CCR5 expression tended to be reduced within all cell subsets evaluated except for CD14+ monocytes (MFI) in the HIV group (Fig. 1B). However, significance was only attained for intensity of CCR5 fluorescence on CD3+, CD4+, and CD8+ lymphocytes in both groups; proportions of CCR5-expressing CD14+ monocytes in the normal donor group; and proportions of CCR5-expressing CD3+ lymphocytes in the HIV group. Figure 2 shows representative dot plots of data (CXCR4 and CCR5 expression on CD3+ cells) from a normal donor.
FIG. 1.
Longitudinal analysis of CXCR4 (A) and CCR5 (B) receptor expression on cellular subsets from healthy individuals (normal donor group) and HIV-1-infected individuals (HIV group) stained within 6 h (t = 0) (open bars) or left ON (hatched bars). The results are expressed as the percentage of positive cells as well as the MFI (fluorescence intensity). The values represent the mean ± the standard error of the mean. The Wilcoxon test for related samples was used to determine whether the ON samples were significantly different from the samples at t = 0. ∗, P < 0.05.
FIG. 2.
Representative dot plots of normal donor CD3+ lymphocytes expressing CXCR4 (A) and CCR5 (B) from time zero (left) and ON samples (right). The percentage of cells in each quadrant, as well as the MFI, of the CD3+CXCR4+ and CD3+CCR5+ are indicated.
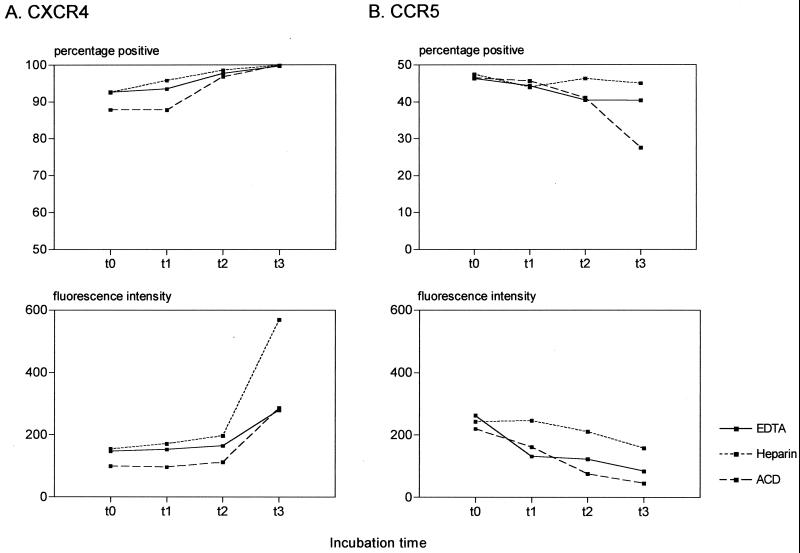
These findings raised the question as to whether the use of anticoagulants other than EDTA would give similar results. We used CD3+ cells as the representative subset for the comparisons since these cells showed significant alterations in both CXCR4 and CCR5 expression in the cross-sectional and the longitudinal analyses. As shown in Fig. 3A, CXCR4 upregulation occurred over the times indicated with all three anticoagulants evaluated, with the most dramatic increase in fluorescence intensity occurring after overnight incubation. In contrast to the upregulation of CXCR4, CCR5 expression was again downregulated with respect to the proportions of CD3+ cells expressing CCR5 as well as in the MFI (Fig. 3B).
FIG. 3.
Longitudinal analysis of CXCR4 (A) and CCR5 (B) expression on CD3+ cells. Whole blood samples anticoagulated with EDTA, heparin, and acid-citrate-dextrose were stained immediately on being drawn (t = 0), at 3 h (t = 1), at 6 h (t = 2), and after standing ON (t = 3). The data are representative of experiments using two healthy blood donors.
The extent of the modulation of these receptors with time was surprising. The rapid upregulation of CXCR4 expression may be due to the fact that various leukocytes have been shown to contain large intracellular stores of this receptor (8). CXCR4 is localized in endosomal compartments from where it can recycle to the cell surface (14), and CXCR4 has been shown to be increased on the surface of lymphocytes after only a few hours of culture (2). Studies on the effect of activation of peripheral blood leukocytes on CXCR4 expression have been contradictory, with phytohemagglutinin being shown by some groups to decrease CXCR4 expression (2, 8, 14), while others have reported a rapid upregulation of expression (3).
In summary, our results have clearly demonstrated that CXCR4 and CCR5 expression was reciprocally altered with sample standing time. This has been confirmed in another study (10). What was also apparent was that CXCR4 was more easily modulated, with significant alterations being found for most cell subsets, while CCR5 expression was most significantly altered in CD3+, CD4+, and CD8+ cells. Furthermore, CXCR4 and CCR5 were modulated similarly in both the normal-donor and HIV cohorts. Moreover, these alterations in receptor expression occurred with all three anticoagulants examined. Since it is often impossible to stain patient samples immediately due to logistical constraints, we recommend using 6 h as a cutoff time in which samples should be processed. In conclusion, the data presented here demonstrate that, in order to avoid compromising the accuracy of results due to ex vivo effects, careful consideration of time of venesection should be taken, particularly in the case of longitudinal samples from the same patient that are to be compared. It should be further emphasized that awareness in this regard would preclude inconsistencies in findings of receptor expression that are likely to occur within and between different laboratories.
REFERENCES
- 1.Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermejo M, Martin-Serrano J, Oberlin E, Pedraza M A, Serrano A, Santiago B, Caruz A, Loetscher P, Baggiolini M, Arenzana-Seisdedos F, Alcami J. Activation of blood T lymphocytes down-regulates CXCR4 expression and interferes with propagation of X4 HIV strains. Eur J Immunol. 1998;28:3192–3204. doi: 10.1002/(SICI)1521-4141(199810)28:10<3192::AID-IMMU3192>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 3.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 5.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 6.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 7.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 8.Förster R, Kremmer E, Schubel A, Breitfeld D, Kleinschmidt A, Nerl C, Bernhardt G, Lipp M. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160:1522–1531. [PubMed] [Google Scholar]
- 9.Giovannetti A, Ensoli F, Mazzetta F, De Cristofaro M, Pierdominici M, Muratori D S, Fiorelli V, Aiuti F. CCR5 and CXCR4 chemokine receptor expression and beta-chemokine production during early T cell repopulation induced by highly active anti-retroviral therapy. Clin Exp Immunol. 1999;118:87–94. doi: 10.1046/j.1365-2249.1999.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hultin L E, Lua M, Hultin P, Hausner M A, Giorgi J V. Sample preparation induced variation in chemokines receptor measurement. Cytometry. 1999;38:333. [Google Scholar]
- 11.Juffermans N P, Verbon A, Olszyna D P, van Deventer S J, Speelman P, van Der Poll T. Thalidomide suppresses up-regulation of human immunodeficiency virus coreceptors CXCR4 and CCR5 on CD4+ T cells in humans. J Infect Dis. 2000;181:1813–1816. doi: 10.1086/315478. [DOI] [PubMed] [Google Scholar]
- 12.Ostrowski M A, Justement S J, Catanzaro A, Hallahan C A, Ehler L A, Mizell S B, Kumar P N, Mican J A, Chun T W, Fauci A S. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J Immunol. 1998;161:3195–3201. [PubMed] [Google Scholar]
- 13.Reynes J, Portales P, Segondy M, Baillat V, André P, Réant B, Avinens O, Couderc G, Benkirane M, Clot J, Eliaou J F, Corbeau P. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J Infect Dis. 2000;181:927–932. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 14.Signoret N, Oldridge J, Pelchen-Matthews A, Klasse P J, Tran T, Brass L F, Rosenkilde M M, Schwartz T W, Holmes W, Dallas W, Luther M A, Wells T N, Hoxie J A, Marsh M. Phorbol esters and SDF-1 induce rapid endocytosis and downmodulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]