Abstract
Infectious bursal disease (IBD) is an acute, highly contagious, immunosuppressive disease of chickens caused by the virus (IBDV), which critically threatens the development of the global chicken industry and causes huge economic losses. As a large country in the poultry industry, the epidemic history of IBDV in China for more than 40 years has been briefly discussed and summarized for the first time in this report. The first classic strain of IBDV appeared in China in the late 1970s. In the late 1980s and early 1990s, the very virulent IBDV (vvIBDV) rapidly swept across the entirety of China, threatening the healthy development of the poultry industry for more than 30 years. Variants of IBDV, after long-term latent circulation with the accumulation of mutations since the early 1990s, suddenly reappeared as novel variant strains (nVarIBDV) in China in the mid-2010s. Currently, there is a coexistence of various IBDV genotypes; the newly emerging nVarIBDV of A2dB1 and persistently circulating vvIBDV of A3B3 are the two predominant epidemic strains endangering the poultry industry. Continuous epidemiological testing and the development of new prevention and control agents are important and require more attention. This report is of great significance to scientific cognition and the comprehensive prevention and control of the IBDV epidemic.
Keywords: infectious bursal disease virus, vvIBDV, nVarIBDV, epidemic, China
1. Introduction
Infectious bursal disease (IBD) is an acute, highly contagious disease that affects chickens worldwide. It is caused by the infectious bursal disease virus (IBDV), which causes tissue damage and immunosuppression in infected flocks, making them susceptible to secondary or concurrent disease. The classic strain of IBDV infection first occurred in Gumboro, USA, in 1957 [1]. Approximately 30 years later, an antigenic variant strain of IBDV, evading the protection provided by the classic strain vaccine, was reported in Delaware, USA, in 1985 and is now predominantly epidemic in North America and Australia. Following this, the very virulent IBDV (vvIBDV) suddenly occurred in Belgium, Europe, in 1989 [2]. The vvIBDV is highly transmissible, and rapidly spread across Europe, Asia, Africa, and South America [3]. In China, IBD was first detected in Beijing and Guangdong in 1979 and rapidly spread to the main poultry areas of this country [4,5,6]. In recent years, the sudden prevalence of a novel variant of IBDV (nVarIBDV) in eastern Asia, including China, causing subclinical symptoms in chickens, has brought new threats and challenges to the poultry industry [7,8,9,10,11,12,13].
IBDV is a member of the genus Avianbirnavirus, a family of Birnaviridae, and is an icosahedral non-enveloped virus [14]. IBDV is a polyploid RNA virus with a genome consisting of two segments: A (3.2 kb) and B (2.7 kb). Segment A contains two partially overlapping open reading frames (ORFs): the smaller ORF in the front encodes the non-structural protein VP5, and the larger ORF in the back encodes the polyprotein, which is subsequently cleaved into a precursor protein pVP2, a viral serine protease VP4, and a capsid scaffolding protein VP3. Furthermore, pVP2 is processed into the mature capsid protein VP2 [15]. A hypervariable region (HVR, aa 206–350) of VP2 is responsible for the virulence, antigenic variation, and cell tropism of IBDV, playing a key role in its genetic evolution [16,17,18,19]. Segment B has only one ORF encoding the protein VP1, which has RNA-dependent RNA polymerase activity and is an essential protein for viral transcription and replication. Segment B and its VP1 also play an important role in the genetic evolution of IBDV and have a significant impact on its virulence [20,21,22,23].
As an RNA virus, the antigenic drift and escape caused by mutations pose great challenges to the prevention and control of IBDV. China has a large chicken industry, and there have been many clinical case reports of IBD in China. However, a systematic review of the overall epidemic characteristics of IBDV has not yet been reported. In this report, the genetic evolution history over the past 40 years and the current epidemic situation of IBDV in China have been systematically analyzed and summarized for the first time; this is of great significance to scientific cognition and the comprehensive prevention and control of IBDV epidemics.
2. The Coexistence of Various Strains of IBDV in China
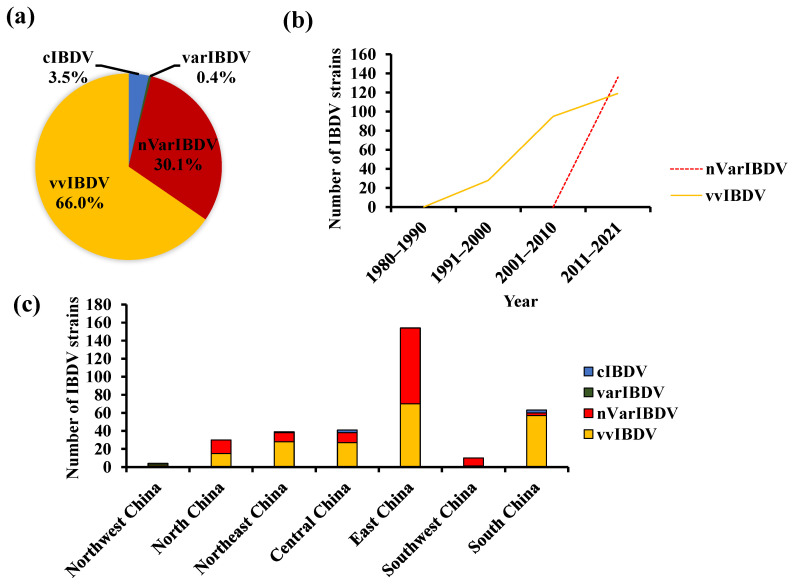
Currently, circulating IBDVs include the classic (cIBDV), variant (varIBDV), very virulent (vvIBDV), and recently emerging novel variant strains (nVarIBDV). To understand the prevalence of IBDV in China, we collected IBDV nucleotide sequences from GenBank (https://www.ncbi.nlm.nih.gov/ (accessed on 31 December 2021)). As of 31 December 2021, there were 804 IBDV nucleotide sequences from China, including 520 VP2, 255 VP1, and 29 other genes. For genetic evolution analysis, excluding attenuated vaccine strains, 451 VP2 nucleotide sequences covering a 435 bp fragment of HVR (bp 746–1180, aa 206–350), and 255 VP1 nucleotide sequence, and covering a 430 bp fragment of B-marker (bp 441–867, aa 110–252) were selected. Traditionally, the VP2 HVR has often been used for genetic evolution analysis. Based on the VP2 sequence analysis of 451 Chinese strains of IBDV, we found the highest number of vvIBDV (60.0%, 297/451), followed by nVarIBDV (30.1%, 136/451), with relatively few cIBDV (3.5%, 16/451) and varIBDV (0.4%, 2/451) (Figure 1a).
Figure 1.
Spatiotemporal analysis of infectious bursal disease virus (IBDV) strains with various phenotypes in China. (a) The proportion of IBDV strains with various phenotypes. (b) Temporal distribution. (c) Geographical distribution.
To clarify the current status of IBDV strains in China, we analyzed the spatiotemporal distribution of IBDV strains within various phenotypes in China (Figure 1). The epidemic of IBD can be traced back to the late 1970s [4,5], and the earliest report of IBDV in the classic strain CJ801 in China, with available gene sequences in GenBank, was published in 1980 [4,24]. Sequence analysis based on the VP2 gene from GenBank (Figure 1) showed that varIBDV and vvIBDV appeared in China in the early 1990s, and that the VP2 sequences of vvIBDV submitted to GenBank increased rapidly in the following 30 years. Since 2017, atypical IBD caused by nVarIBDV has become widespread in China, and nVarIBDV sequence numbers have shown a rapid growth trend, even exceeding the number of vvIBDV sequences (Figure 1b). Currently, the newly emerging nVarIBDV, and persistently circulating vvIBDV are the two predominant epidemic strains endangering the healthy development of the poultry industry, which is consistent with a recent clinical epidemiological survey from 2019 to 2020 [25]. The geographical distribution of IBDV in China was also analyzed. The isolation rates for IBDV in different regions were as follows: northwest (1%, 4/368), north (9%, 34/368), northeast (11%, 39/368), central (11%, 42/368), east (45%, 165/368), southwest (3%, 10/368), and South China (20%, 74/368). The IBDV number in East China (Anhui, Fujian, Jiangsu, Shandong, Shanghai, and Zhejiang provinces) was the highest (Figure 1c) because it is the most developed poultry industry in this region.
3. Genotype Classification of IBDV
Scientific classification is critical to correctly understanding the epidemic characteristics of viruses. Virus classification is not a one-step task but a process of gradual improvement. Traditionally, according to viral pathogenicity and antigenicity, serotype I strain of IBDVs have been divided into four phenotypes: classic [1], variant [26], very virulent [2], and attenuated IBDV. With the dazzling molecular characteristics of emerging strains owing to cumulative mutations, the traditional descriptive classification method can no longer meet the classification and definition of novel IBDV strains [27,28,29]. To solve this dilemma, Michel and Jackwood proposed a genogroup classification scheme in which serotype I strain of IBDVs were classified into seven genogroups according to VP2 [30]. However, as a segmented virus, an IBDV classification scheme based only on a VP2 coded by segment A is non-comprehensive.
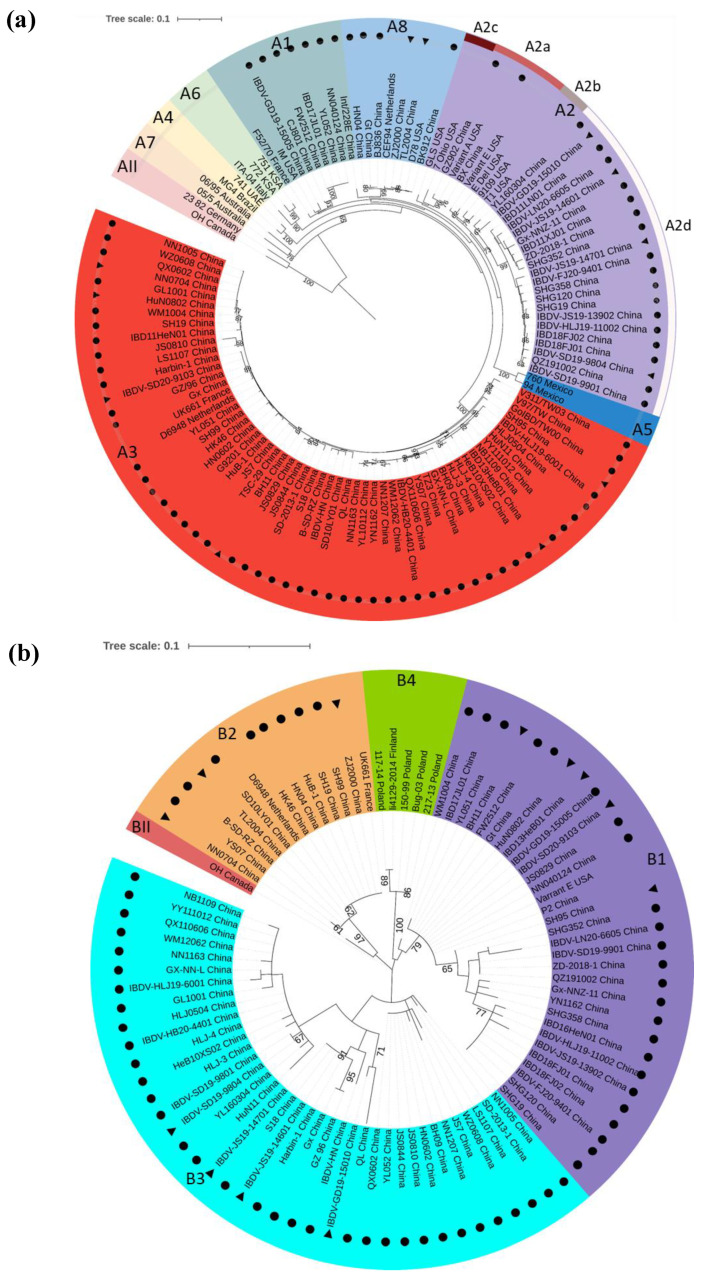
Recently, a similar improved scheme for IBDV genotype classifications based on both segments was proposed [31,32], which is highly useful for the molecular epidemiology of IBDV. According to this scheme [31], based on the nucleotide sequences of the VP2 HVR and VP1 B-markers, 86 representative strains isolated in China and 29 (VP2) or 13 (VP1) reference strains were used for phylogenetic analysis. The phylogenetic tree displayed that IBDVs were classified into nine genogroups of A (Figure 2a) and five genogroups of B (Figure 2b); the genogroup A2 was further divided into four lineages. Collectively, IBDVs circulating in China were classified as A1B1, A2dB1, A3B2/A3B3, and A8B1 genotypes corresponding to the classic, variant, very virulent, and attenuated IBDVs (Table 1), which showed the diversity of the epidemic strains in China.
Figure 2.
Phylogenetic analysis of the nucleotide sequences of the representative strains of infectious bursal disease virus (IBDV) in China. (a) Nine genogroups based on the hypervariable region (HVR) of VP2 gene in segment A. (b) Five genogroups based on the B-marker of the VP1 gene in segment B. The trees are generated by the maximum-likelihood method using MEGA7 software and are visualized using iTOL. The trees are drawn to scale with branch lengths measured in the number of substitutions per site. Only branches supported by a bootstrap value above 60% are displayed. A total of 86 representative strains isolated in China and 29 (VP2) or 13 (VP1) reference strains are involved in this analysis. All Chinese strains are highlighted with a solid circle (●), of which 13 stains with segment-reassortant characteristics are marked with a solid triangle (▲).
Table 1.
The genotype classification corresponding to the traditional phenotype of IBDV.
| Phenotype | Genotype | Reference Strain | GenBank No. (A, B) |
|---|---|---|---|
| Classic strains | A1B1 | YL052 China | DQ656521, KC968889 |
| FW2512 China | DQ656499, KC986359 | ||
| NN040124 China | DQ656502, KC968872 | ||
| IBD17JL01 China | MN604241, MN604242 | ||
| IBDV-GD19-15005 China | MW682890, MW863620 | ||
| Variant strains | A2aB1 | Variant E USA | AF133904, AF133905 |
| A2bB1 | 9109 USA | AY462027, AY459321 | |
| A2cB1 | GLS USA | AY368653, AY368654 | |
| Novel variant strains | A2dB1 | SHG19 China | MH879045, MH879092 |
| SHG120 China | MH879063, MH879110 | ||
| ZD-2018-1 China | MN485882, MN485883 | ||
| Gx-NNZ-11 China | JX134483, JX134484 | ||
| QZ191002 China | MZ066613, MZ066615 | ||
| vvIBDV | A3B2 | NN0704 China | FJ615511, KC968858 |
| YS07 China | FJ695138, FJ695139 | ||
| B-SD-RZ China | GQ166972, GQ166971 | ||
| SD10LY01 China | KF569803, KF569804 | ||
| HuB-1 China | KF569805, KF569804 | ||
| A3B3 | Gx China | AY444873, AY705393 | |
| S18 China | MK472711, MK472712 | ||
| QL China | JX682709, JX682710 | ||
| GL1001 China | KC968831, HQ452814 | ||
| HLJ0504 China | GQ166972, GQ451331 | ||
| Attenuated strains | A8B1 | Gt China | DQ403248, DQ403249 |
| BH15 China | DQ656498, KC968825 | ||
| JD1 China | AF321055, AY103464 | ||
| HuN0804 China | FJ615498, KC968842 | ||
| QX110603 China | KC918849, KC968876 |
4. The Persistently Circulating vvIBDV
Under the trend of a global vvIBDV pandemic in the late 1980s, the vvIBDV in China was first reported in the form of research articles in 1991 [6] and the first vvIBDV of the G9201 strain, with the gene sequence available in GenBank, was isolated in Guangdong province in 1992 [24]. Since then, with the rapid development of the chicken industry, vvIBDV has rapidly swept across almost the entire country of China (Figure 1). Owing to the high mortality and severe immunosuppression associated with this disease, vvIBDV has become one of the most important threats to the healthy development of the poultry industry in the past 30 years in China [24,25,27,33,34,35,36,37,38]. IBD is an important disease that must be immunized and prevented in all chicken farms in China.
Unlike other countries, it is highly interesting that there are two genotypes of vvIBDV, A3B2 and A3B3, as shown in Table 2. The genotype A3B2 of vvIBDV (typical vvIBDV) has been prevalent worldwide, including in China, since its first outbreak in Belgium in the late 1980s. Genotype A3B3 of vvIBDV (HLJ0504-like vvIBDV) is the most prevalent strain in China [34,39,40,41,42,43]. A high prevalence (86.0%) of A3B3-vvIBDV was reported in Southern China between 2000 and 2012 [34]. In a recent study of IBDV molecular epidemiology in China from 2019 to 2020, almost all detected vvIBDVs belonged to the genotype A3B3 [25]. Among the 86 vvIBDV strains with both VP2 and VP1 sequences analyzed in this study, genotypes A3B2 and A3B3 accounted for 34.9% (30/86) and 65.1% (56/86), respectively. Recently, it was reported that A3B3-vvIBDV has also been circulating in other countries, including Pakistan [44], India [45], Bangladesh [32,46], Thailand, Vietnam [30], Korea [9], and Venezuela [47]. Genotypes A3B2 and A3B3 of vvIBDV have the same segment A but different segment B of the genome. It has been speculated that segment B of the A3B3 strain originated from an unidentified ancestral virus circulating in avian or wild birds [40,48,49]. Although the homology of its segment B is between that of A3B2-vvIBDV and the attenuated strain, A3B3-vvIBDV has a very high mortality rate of over 60% in chickens as A3B2-vvIBDV. In recent years, vvIBDV infections have gradually been controlled due to the use of vaccines, as well as improvements in feeding and biosecurity management; however, the number of isolated vvIBDVs remain high, as illustrated in Figure 1B Therefore, the prevention and control of vvIBDV should be considered.
Table 2.
Segment reassortment strains of IBDV.
| Genotype | Feature 1 | Reference Strain | GenBank No. (A, B) |
|---|---|---|---|
| A3B1 | vv-A/att-B | SH95 China | AY134874, AY134875 |
| 77.5% (31/40) | TSC-2(9) China | DQ656519, KC968881 | |
| BH11 China | DQ656497, KC968823 | ||
| NB1109 China | KC918838, KC968855 | ||
| JS0829 China | FJ615508, KC968853 | ||
| JS0822 China | FJ615507, KC968852 | ||
| JS0821 China | FJ615506, KC968851 | ||
| JS0819 China | FJ615505, KC968850 | ||
| JS0811 China | FJ615504, KC968849 | ||
| JS0809 China | FJ615502, KC968847 | ||
| GL0902 China | HQ452817, KC968830 | ||
| GL0901 China | HQ452816, KC968829 | ||
| NN0603 China | FJ615509, KC968856 | ||
| JS0806 China | FJ615501, KC968856 | ||
| HuN0802 China | FJ615496, KC968840 | ||
| HuN0801 China | FJ615495, KC968839 | ||
| WM1004 China | JQ260883, KC968883 | ||
| YN1162 China | JQ260876, KC968894 | ||
| YN1161 China | JQ260875, KC968894 | ||
| NN1166 China | JQ260874, KC968866 | ||
| NN1165 China | JQ260873, KC968865 | ||
| NN1164 China | JQ260872, KC968864 | ||
| YL051 China | DQ656506, KC968888 | ||
| IBDV-HN China | KT884486, KY948019 | ||
| IBDV-SD20-9103 China | MZ766382, MZ766407 | ||
| IBDV-SD20-9104 China | MZ766383, MZ766408 | ||
| IBDV-SD20-9102 China | MZ766381, MZ766406 | ||
| IBD13HeB01 China | KP676467, KP676468 | ||
| NN0704 China | FJ615511, KC968858 | ||
| NN1007 China | JQ260882, KC968860 | ||
| NN1005 China | JQ260881, KC968859 | ||
| A2dB3 | nVar-A/uniq-B | IBDV-JS19-14701 China | MW700332, MW700333 |
| 12.5% (5/40) | IBDV-JS19-14601 China | MW682905, MW863641 | |
| IBDV-GD1915010 China | MW682891, MW863621 | ||
| IBDV-SD19-9801 China | MW682907, MW863642 | ||
| IBDV-SD19-9804 China | MW682908, MW863643 | ||
| A8B2 | att-A/vv-B | ZJ2000 China | AF321056, DQ166818 |
| 10% (4/40) | TL2004 China | DQ088175, DQ118374 | |
| HN04 China | KC109816, KC109815 | ||
| YL160304 China | MZ066614, MZ066616 |
1 vv—very virulent IBDV; nVar—novel variant IBDV; att—attenuated strain; uniq—unique strain as HLJ0504-like IBDV.
5. The Newly Emerging nVarIBDV
In recent years, farmers in China have consistently addressed the challenge of atypical IBD. This pathogen was identified as nVarIBDV for the first time in our laboratory [7]. The nVarIBDV belongs to genotype A2dB1, which is different from the early North American varIBDV (genotypes A2aB1, A2dB1, and A2dB1) [7,42]. Although varIBDV appeared in China in the early 1990s [24,50], most of their genetic information was not available. Sequence analysis showed that only two varIBDV strains, GZ902 and BX, had a high homology with the early North American varIBDV of genogroup A2a (Figure 2a). It has been reported that the variant IBDV spread from North America to China in the late 1980s and the early 1990s but did not cause a large-scale epidemic. After long-term latent circulation with the accumulation of amino acid mutations, it reappeared suddenly as nVarIBDV of the genotype A2dB1 in China in the mid-2010s [51].
Since 2017, nVarIBDV has been widely prevalent and has caused critical damage to major poultry breeding regions in China [7,10,52] and has also been reported in Japan [8], Korea [9,13], and Malaysia [11,12]. Atypical IBD caused by nVarIBDV pose a new threat to the poultry industry. It is well known that vvIBDV can cause the acute death of infected chickens with high mortalities within 3–5 days. Comparatively, nVarIBDV does not cause obvious appearance symptoms and death; however, the central immune organ bursa is severely destroyed causing severe immunosuppression in infected chickens and production performance is reduced. It was reported that nVarIBDV could suppress immune responses to vaccines against both highly pathogenic avian influenza [7] and Newcastle disease [53], the two most severe infectious diseases threatening poultry farming. In one study, the weight of nVarIBDV-infected broilers was dramatically reduced by approximately 16% compared to that of the control at 42 days of age, indicating huge economic losses [54]. Moreover, coinfection with nVarIBDV and other pathogens may further aggravate damage [55].
It was confirmed that nVarIBDV could partly circumvent the immune protection of existing vvIBDV vaccines, which is the key reason for the spread of nVarIBDV in immunized flocks [52,54]. Furthermore, residues 318 and 323 of the viral capsid protein are deeply involved in the antigenicity difference between the newly emerging nVarIBDV and persistently circulating vvIBDV [56]. Additional mechanisms of genetic variation and immune escape need to be further explored.
6. The Segment Reassortment and Gene Recombination among Circulating Strains
Segment reassortment among circulating strains is an important evolutionary characteristic that cannot be ignored for IBDV with a double-segmented genome. As shown in Figure 2 and Table 2, two types of segment-reassortment IBDVs between the vvIBDV and attenuated strains were observed among the Chinese strains. The genotype A3B1 of reassortment IBDV combines segment A from vvIBDV and segment B from the attenuated strain [57,58], and has also been reported in many countries, including Korea [59], India [60], Poland [61], Nigeria [62], Zambia [63], and Venezuela [47]. Another type of segment-reassortment IBDV (genotype A8B2) contains attenuated strains A and vvIBDV-B [64,65].
Most recently, segment reassortments of the two predominant epidemic strains, nVarIBDV and vvIBDV have also been observed. This reassortment strain (genotype A2dB3) has a nVarIBDV-A but an HLJ0504-like vvIBDV-B, which shows a similar pathogenicity to specific-pathogen-free (SPF) chickens as nVarIBDV [42]. However, another A2dB3 reassortment strain of YL160304 containing nVarIBDV-A and HLJ0504-like vvIBDV-B, isolated in southern China, could enhance its pathogenicity with 10% mortality compared to that of nVarIBDV [43].
In addition to segment reassortment, gene recombination may play an important role in the evolution of IBDV. Recently, a homologous recombination between nVarIBDV and an intermediate vaccine strain was identified, in which the 3′ regions of segment A in nVarIBDV were replaced by the corresponding region of an intermediate vaccine strain. Compared with the pathogenicity of nVarIBDV, this recombinant strain showed an increased viral pathogenicity in chick embryos [66]. Segment recombination and gene reassortment among the circulating strains increased the epidemiological complexity, potential harm and raised the difficulty of preventing and controlling IBDV.
7. Immune Prevention and Control of IBDV
Vaccines are an effective method for preventing and controlling vvIBDV infections. In China, the vvIBDV vaccine is a necessary routine vaccine, especially for large-scale intensive chicken farms. However, the vvIBDV vaccines currently available in China also require improvement. First, there are obvious biological safety risks due to the widespread use of intermediate and hot vaccine strains [67]. Second, most of the vaccine strains in use are non-domestic strains with possible incomplete antigenicity matches.
Additionally, the sudden prevalence of nVarIBDV poses a great challenge for the comprehensive control of IBD. To some extent, nVarIBDV can escape the immune protection of existing vvIBDV vaccines because the vvIBDV-antiserum cannot neutralize nVarIBDV well; therefore, there is an urgent need to develop a novel vaccine that completely matches the antigenicity of nVarIBDV [52,54,56]. It was reported that a tailored vaccine based on reverse genetics could provide 100% protection against newly emerging nVarIBDV and the persistently circulating vvIBDV [68]. Recently, viral-like particle (VLP) candidate vaccines have also been developed [69,70], one of which could provide complete immune protection against the two predominant epidemic strains of the homologous nVarIBDV and the heterologous vvIBDV [69].
For modern intensive poultry-farming, to some extent, immunity is productivity. In addition to vaccines, there are several other factors that are very important for improving the immunity of poultry flocks. First, biosafety: strict biosafety measures will greatly reduce the prevalence of epidemic diseases and secondary infections. The second is feeding management. Good nutrition ratios and appropriate temperatures, moderation, and ventilation can improve the resistance of chickens to disease and reduce conditional disease. Third, animal welfare: reducing resistance, replacing resistance, and reasonably adjusting breeding densities are a green breeding concept that must be adhered to.
In conclusion, according to the literature and sequence analysis, the epidemic history of IBDV in China for more than 40 years has been briefly reviewed and summarized for the first time. IBDV of the classic strain appeared in China in the late 1970s. In the late 1980s and early 1990s, vvIBDV rapidly swept through China, threatening the healthy development of the poultry industry for more than 30 years. The variant IBDV, after long-term latent circulation with the accumulation of mutations, reappeared suddenly as nVarIBDV in China in the mid-2010s. Currently, there is a coexistence of various IBDV genotypes; the newly emerging nVarIBDV of A2dB1 and the persistently circulating vvIBDV of A3B3 are the two predominant epidemic strains endangering the poultry industry. Continuous epidemiological testing and the development of new prevention and control agents are important and require more attention.
Author Contributions
Conceptualization, X.Q. and W.Z.; writing—original draft preparation, W.Z.; writing—review and editing, X.Q.; supervision, project administration, and funding acquisition, X.Q., Y.G. and X.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be requested by writing to the author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Heilongjiang Provincial Natural Science Foundation of China (grant number ZD2020C006), the National Natural Science Foundation of China (grant number 32072852), the Key Research and Development Program of Heilongjiang Province (grant number GA21B004), China Agriculture Research System (CARS-41-G15).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cosgrove A.S. An Apparently New Disease of Chickens: Avian Nephrosis. Avian Dis. 1962;6:385. doi: 10.2307/1587909. [DOI] [Google Scholar]
- 2.Chettle N., Stuart J.C., Wyeth P.J. Outbreak of virulent infectious bursal disease in East Anglia. Vet. Rec. 1989;125:271–272. doi: 10.1136/vr.125.10.271. [DOI] [PubMed] [Google Scholar]
- 3.Di J., Rossini L., Eterradossi N., Toquin M.D., Gardin Y. European-like pathogenic infectious bursal disease viruses in Brazil. Vet. Rec. 1999;145:203–204. [PubMed] [Google Scholar]
- 4.Zhou J., Liu F., Chen L., Wang S. Isolation of pathogen of infectious bursal disease in Beijing area. Chin. J. Vet. Med. 1982;8:25–26. [Google Scholar]
- 5.Bi Y. A study on infectious bursal disease of the chicken in Guangdong area. J. South China Agric. Univ. 1985;1:46–61. [Google Scholar]
- 6.Li D., Wu Z. Isolation and preliminary identification of a supervirulent strain of infectious bursal disease virus. Chin. J. Prev. Vet. Med. 1991;6:3–6. [Google Scholar]
- 7.Fan L., Wu T., Hussain A., Gao Y., Zeng X., Wang Y., Gao L., Li K., Wang Y., Liu C., et al. Novel variant strains of infectious bursal disease virus isolated in China. Vet. Microbiol. 2019;230:212–220. doi: 10.1016/j.vetmic.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Myint O., Suwanruengsri M., Araki K., Izzati U.Z., Pornthummawat A., Nueangphuet P., Fuke N., Hirai T., Jackwood D.J., Yamaguchi R. Bursa atrophy at 28 days old caused by variant infectious bursal disease virus has a negative economic impact on broiler farms in Japan. Avian Pathol. 2021;50:6–17. doi: 10.1080/03079457.2020.1822989. [DOI] [PubMed] [Google Scholar]
- 9.Thai T.N., Jang I., Kim H.A., Kim H.S., Kwon Y.K., Kim H.R. Characterization of antigenic variant infectious bursal disease virus strains identified in South Korea. Avian Pathol. 2021;50:174–181. doi: 10.1080/03079457.2020.1869698. [DOI] [PubMed] [Google Scholar]
- 10.Xu A., Pei Y., Zhang K., Xue J., Ruan S., Zhang G. Phylogenetic analyses and pathogenicity of a variant infectious bursal disease virus strain isolated in China. Virus Res. 2020;276:197833. doi: 10.1016/j.virusres.2019.197833. [DOI] [PubMed] [Google Scholar]
- 11.Aliyu H.B., Hair-Bejo M., Omar A.R., Ideris A. Genetic Diversity of Recent Infectious Bursal Disease Viruses Isolated From Vaccinated Poultry Flocks in Malaysia. Front. Vet. Sci. 2021;8:643976. doi: 10.3389/fvets.2021.643976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aliyu H.B., Hamisu T.M., Hair Bejo M., Omar A.R., Ideris A. Comparative Pathogenicity of Malaysian Variant and Very Virulent Infectious Bursal Disease Viruses in Chickens. Avian Pathol. 2022;51:76–86. doi: 10.1080/03079457.2021.2006604. [DOI] [PubMed] [Google Scholar]
- 13.Thai T.N., Yoo D.S., Jang I., Kwon Y.K., Kim H.R. Dynamics of the Emerging Genogroup of Infectious Bursal Disease Virus Infection in Broiler Farms in South Korea: A Nationwide Study. Viruses. 2022;14:1604. doi: 10.3390/v14081604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown M.D., Skinner M.A. Coding Sequences of Both Genome Segments of a European “very Virulent” Infectious Bursal Disease Virus. Virus Res. 1996;40:1–15. doi: 10.1016/0168-1702(95)01253-2. [DOI] [PubMed] [Google Scholar]
- 15.Petit S., Lejal N., Huet J., Delmas B. Active Residues and Viral Substrate Cleavage Sites of the Protease of the Birnavirus Infectious Pancreatic Necrosis Virus. J. Virol. 2000;74:2057–2066. doi: 10.1128/JVI.74.5.2057-2066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackwood D.J. Advances in Vaccine Research against Economically Important Viral Diseases of Food Animals: Infectious Bursal Disease Virus. Vet. Microbiol. 2017;206:121–125. doi: 10.1016/j.vetmic.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Letzel T., Coulibaly F., Rey F.A., Delmas B., Jagt E., van Loon A.A.M.W., Mundt E. Molecular and Structural Bases for the Antigenicity of VP2 of Infectious Bursal Disease Virus. J. Virol. 2007;81:12827–12835. doi: 10.1128/JVI.01501-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi X., Gao H., Gao Y., Qin L., Wang Y., Gao L., Wang X. Naturally Occurring Mutations at Residues 253 and 284 in VP2 Contribute to the Cell Tropism and Virulence of Very Virulent Infectious Bursal Disease Virus. Antivir. Res. 2009;84:225–233. doi: 10.1016/j.antiviral.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Qi X., Zhang L., Chen Y., Gao L., Wu G., Qin L., Wang Y., Ren X., Gao Y., Gao H., et al. Mutations of Residues 249 and 256 in VP2 Are Involved in the Replication and Virulence of Infectious Bursal Disease Virus. PLoS ONE. 2013;8:e70982. doi: 10.1371/journal.pone.0070982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escaffre O., Le Nouën C., Amelot M., Ambroggio X., Ogden K.M., Guionie O., Toquin D., Müller H., Islam M.R., Eterradossi N. Both Genome Segments Contribute to the Pathogenicity of Very Virulent Infectious Bursal Disease Virus. J. Virol. 2013;87:2767–2780. doi: 10.1128/JVI.02360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao L., Li K., Qi X., Gao H., Gao Y., Qin L., Wang Y., Shen N., Kong X., Wang X. Triplet Amino Acids Located at Positions 145/146/147 of the RNA Polymerase of Very Virulent Infectious Bursal Disease Virus Contribute to Viral Virulence. J. Gen. Virol. 2014;95:888–897. doi: 10.1099/vir.0.060194-0. [DOI] [PubMed] [Google Scholar]
- 22.Yang H., Wang Y., Ye C. Rapid Generation of Attenuated Infectious Bursal Disease Virus from Dual-Promoter Plasmids by Reduction of Viral Ribonucleoprotein Activity. J. Virol. 2020;94:e01569-19. doi: 10.1128/JVI.01569-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pikuła A., Śmietanka K., Perez L.J. Emergence and expansion of novel pathogenic reassortant strains of infectious bursal disease virus causing acute outbreaks of the disease in Europe. Transbound. Emerg. Dis. 2020;67:1739–1744. doi: 10.1111/tbed.13510. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y.C., Yeung W.S., Law M., Bi Y.Z., Leung F.C., Lim B.L. Molecular Characterization of Seven Chinese Isolates of Infectious Bursal Disease Virus: Classical, Very Virulent, and Variant Strains. Avian Dis. 1998;42:340–351. doi: 10.2307/1592484. [DOI] [PubMed] [Google Scholar]
- 25.Jiang N., Wang Y., Zhang W., Niu X., Huang M., Gao Y., Liu A., Gao L., Li K., Pan Q., et al. Genotyping and Molecular Characterization of Infectious Bursal Disease Virus Identified in Important Poultry-Raising Areas of China During 2019 and 2020. Front. Vet. Sci. 2021;8:759861. doi: 10.3389/fvets.2021.759861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackwood D.H., Saif Y.M. Antigenic Diversity of Infectious Bursal Disease Viruses. Avian Dis. 1987;31:766–770. doi: 10.2307/1591028. [DOI] [PubMed] [Google Scholar]
- 27.Jackwood D.J. Molecular Epidemiologic Evidence of Homologous Recombination in Infectious Bursal Disease Viruses. Avian Dis. 2012;56:574–577. doi: 10.1637/10053-010912-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 28.Hernández M., Tomás G., Marandino A., Iraola G., Maya L., Mattion N., Hernández D., Villegas P., Banda A., Panzera Y., et al. Genetic Characterization of South American Infectious Bursal Disease Virus Reveals the Existence of a Distinct Worldwide-Spread Genetic Lineage. Avian Pathol. J. WVPA. 2015;44:212–221. doi: 10.1080/03079457.2015.1025696. [DOI] [PubMed] [Google Scholar]
- 29.Lupini C., Giovanardi D., Pesente P., Bonci M., Felice V., Rossi G., Morandini E., Cecchinato M., Catelli E. A Molecular Epidemiology Study Based on VP2 Gene Sequences Reveals That a New Genotype of Infectious Bursal Disease Virus Is Dominantly Prevalent in Italy. Avian Pathol. J. WVPA. 2016;45:458–464. doi: 10.1080/03079457.2016.1165792. [DOI] [PubMed] [Google Scholar]
- 30.Michel L.O., Jackwood D.J. Classification of Infectious Bursal Disease Virus into Genogroups. Arch. Virol. 2017;162:3661–3670. doi: 10.1007/s00705-017-3500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Fan L., Jiang N., Gao L., Li K., Gao Y., Liu C., Cui H., Pan Q., Zhang Y.-P., et al. An improved scheme for infectious bursal disease virus genotype classification based on both genome-segments A and B. J. Integr. Agr. 2021;20:1372–1381. doi: 10.1016/S2095-3119(20)63424-4. [DOI] [Google Scholar]
- 32.Islam M.R., Nooruzzaman M., Rahman T., Mumu T.T., Rahman M.M., Chowdhury E.H., Eterradossi N., Müller H. A Unified Genotypic Classification of Infectious Bursal Disease Virus Based on Both Genome Segments. Avian Pathol. J. WVPA. 2021;50:190–206. doi: 10.1080/03079457.2021.1873245. [DOI] [PubMed] [Google Scholar]
- 33.Chen H., Zhou Q., Zhang M. Sequence analysis of the VP2 hyperirable region of nine infectious bursal disease viruses from Mainland China. Avian Dis. 1998;42:762–776. doi: 10.2307/1592713. [DOI] [PubMed] [Google Scholar]
- 34.He X., Xiong Z., Yang L., Guan D., Yang X., Wei P. Molecular Epidemiology Studies on Partial Sequences of Both Genome Segments Reveal That Reassortant Infectious Bursal Disease Viruses Were Dominantly Prevalent in Southern China during 2000–2012. Arch. Virol. 2014;159:3279–3292. doi: 10.1007/s00705-014-2195-z. [DOI] [PubMed] [Google Scholar]
- 35.Liu D., Zhang X., Yan Z., Chen F., Ji J., Qin J., Li H., Lu J., Xue Y., Liu J., et al. Molecular Characterization and Phylogenetic Analysis of Infectious Bursal Disease Viruses Isolated from Chicken in South China in 2011. Trop. Anim. Health Prod. 2013;45:1107–1112. doi: 10.1007/s11250-012-0333-8. [DOI] [PubMed] [Google Scholar]
- 36.Meng D., Yu W., Wu G., Ren J., Cao S., Zhao J., Meng F., Liu Y., Ma Z. Isolation and identification of infectious bursal disease virus in chickens in Shanxi Province. China Prog. Vet. Med. 2016;37:70–74. [Google Scholar]
- 37.Qi X., Qin L., Gao Y., Gao H., Li Y., Gao L., Lu Z., Wang N., Chen Y., Zhang L., et al. Genetic Analysis of the VP2 hypervariable region of thirty-six infectious bursal disease virus Isolates in China. Agric. Sci. Technol. 2015;16:1565–1569. [Google Scholar]
- 38.Wang X., Zeng X., Gao H., Fu C., Wei P. Changes in VP2 Gene during the Attenuation of Very Virulent Infectious Bursal Disease Virus Strain Gx Isolated in China. Avian Dis. 2004;48:77–83. doi: 10.1637/7061. [DOI] [PubMed] [Google Scholar]
- 39.Gao H., Wang X., Gao Y., Fu C. Direct evidence of reassortment and mutant spectrum analysis of a very virulent infectious bursal disease virus. Avian Dis. 2007;51:893–899. doi: 10.1637/7626-042706R1.1. [DOI] [PubMed] [Google Scholar]
- 40.Qi X., Gao L., Qin L., Deng X., Wu G., Zhang L., Yu F., Ren X., Gao Y., Gao H., et al. Genomic sequencing and characterization of a very virulent strain of infectious bursal disease virus isolated in China. Agric. Sci. Technol. 2011;12:1950–1953. [Google Scholar]
- 41.Xia R.X., Wang H.Y., Huang G.M., Zhang M.F. Sequence and phylogenetic analysis of a chinese very virulent infectious bursal disease virus. Arch. Virol. 2008;153:1725–1729. doi: 10.1007/s00705-008-0140-8. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., Jiang N., Fan L., Niu X., Zhang W., Huang M., Gao L., Li K., Gao Y., Liu C., et al. Identification and Pathogenicity Evaluation of a Novel Reassortant Infectious Bursal Disease Virus (Genotype A2dB3) Viruses. 2021;13:1682. doi: 10.3390/v13091682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W., Huang Y., Zhang Y., Qiao Y., Deng Q., Chen R., Chen J., Huang T., Wei T., Mo M., et al. The Emerging Naturally Reassortant Strain of IBDV (Genotype A2dB3) Having Segment A from Chinese Novel Variant Strain and Segment B from HLJ 0504-like Very Virulent Strain Showed Enhanced Pathogenicity to Three-Yellow Chickens. Transbound. Emerg. Dis. 2022;69:e566–e579. doi: 10.1111/tbed.14336. [DOI] [PubMed] [Google Scholar]
- 44.Hussain A., Wu T., Li H., Fan L., Li K., Gao L., Wang Y., Gao Y., Liu C., Cui H., et al. Pathogenic Characterization and Full Length Genome Sequence of a Reassortant Infectious Bursal Disease Virus Newly Isolated in Pakistan. Virol. Sin. 2019;34:102–105. doi: 10.1007/s12250-019-00082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel A.K., Pandey V.C., Pal J.K. Evidence of Genetic Drift and Reassortment in Infectious Bursal Disease Virus and Emergence of Outbreaks in Poultry Farms in India. Virusdisease. 2016;27:161–169. doi: 10.1007/s13337-016-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nooruzzaman M., Hossain I., Rahman M.M., Uddin A.J., Mustari A., Parvin R., Chowdhury E.H., Islam M.R. Comparative Pathogenicity of Infectious Bursal Disease Viruses of Three Different Genotypes. Microb. Pathog. 2022;169:105641. doi: 10.1016/j.micpath.2022.105641. [DOI] [PubMed] [Google Scholar]
- 47.Nouën C.L., Rivallan G., Toquin D., Darlu P., Morin Y., Beven V., de Boisseson C., Cazaban C., Comte S., Gardin Y., et al. Very Virulent Infectious Bursal Disease Virus: Reduced Pathogenicity in a Rare Natural Segment-B-Reassorted Isolate. J. Gen. Virol. 2006;87:209–216. doi: 10.1099/vir.0.81184-0. [DOI] [PubMed] [Google Scholar]
- 48.Fagbohun O., Owoade A., Oluwayelu D., Olayemi F. Serological survey of infectious bursal diseases virus antibodies in cattle egrets, pigeons and Nigerian laughing doves. Afr. J. Biomed. Res. 2000;3:191–192. [Google Scholar]
- 49.Jackwood D.J., Gough R.E., Sommer S.E. Nucleotide and Amino Acid Sequence Analysis of a Birnavirus Isolated from Penguins. Vet. Rec. 2005;156:550–552. doi: 10.1136/vr.156.17.550. [DOI] [PubMed] [Google Scholar]
- 50.Li S., Huang S., Lin Z., Lin Y., Bi Y., Huang J., Guan S., Dai C., Huang B., Zeng J. Isolation of serotype I virus subtype strains of infectious bursal disease in chickens. Chin. J. Prev. Vet. Med. 1991;5:7–11. [Google Scholar]
- 51.Wang W., He X., Zhang Y., Qiao Y., Shi J., Chen R., Chen J., Xiang Y., Wang Z., Chen G., et al. Analysis of the Global Origin, Evolution and Transmission Dynamics of the Emerging Novel Variant IBDV (A2dB1b): The Accumulation of Critical Aa-Residue Mutations and Commercial Trade Contributes to the Emergence and Transmission of Novel Variants. Transbound. Emerg. Dis. 2022;69:e2832–e2851. doi: 10.1111/tbed.14634. [DOI] [PubMed] [Google Scholar]
- 52.Hou B., Wang C., Luo Z., Shao G. Commercial Vaccines Used in China Do Not Protect against a Novel Infectious Bursal Disease Virus Variant Isolated in Fujian. Vet. Rec. 2022:e1840. doi: 10.1002/vetr.1840. [DOI] [PubMed] [Google Scholar]
- 53.Fan L., Wang Y., Jiang N., Chen M., Gao L., Li K., Gao Y., Cui H., Pan Q., Liu C., et al. Novel Variant Infectious Bursal Disease Virus Suppresses Newcastle Disease Vaccination in Broiler and Layer Chickens. Poult. Sci. 2020;99:6542–6548. doi: 10.1016/j.psj.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan L., Wu T., Wang Y., Hussain A., Jiang N., Gao L., Li K., Gao Y., Liu C., Cui H., et al. Novel Variants of Infectious Bursal Disease Virus Can Severely Damage the Bursa of Fabricius of Immunized Chickens. Vet. Microbiol. 2020;240:108507. doi: 10.1016/j.vetmic.2019.108507. [DOI] [PubMed] [Google Scholar]
- 55.Xu A., Sun L., Tu K., Teng Q., Xue J., Zhang G. Experimental Co-Infection of Variant Infectious Bursal Disease Virus and Fowl Adenovirus Serotype 4 Increases Mortality and Reduces Immune Response in Chickens. Vet. Res. 2021;52:61. doi: 10.1186/s13567-021-00932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan L., Wang Y., Jiang N., Gao Y., Niu X., Zhang W., Huang M., Bao K., Liu A., Wang S., et al. Residues 318 and 323 in Capsid Protein Are Involved in Immune Circumvention of the Atypical Epizootic Infection of Infectious Bursal Disease Virus. Front. Microbiol. 2022;13:909252. doi: 10.3389/fmicb.2022.909252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu Z., Zhang L., Wang N., Chen Y., Gao L., Wang Y., Gao H., Gao Y., Li K., Qi X., et al. Naturally Occurring Reassortant Infectious Bursal Disease Virus in Northern China. Virus Res. 2015;203:92–95. doi: 10.1016/j.virusres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Sun J., Lu P., Yan Y., Hua X., Jiang J., Zhao Y. Sequence and Analysis of Genomic Segment A and B of Very Virulent Infectious Bursal Disease Virus Isolated from China. J. Vet. Med. Ser. B. 2003;50:148–154. doi: 10.1046/j.1439-0450.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- 59.Lee H., Jang I., Shin S., Lee H., Choi K. Genome Sequence of a Novel Reassortant and Very Virulent Strain of Infectious Bursal Disease Virus. Genome Announc. 2017;5:e00730-17. doi: 10.1128/genomeA.00730-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raja P., Senthilkumar T.M.A., Parthiban M., Thangavelu A., Gowri A.M., Palanisammi A., Kumanan K. Complete Genome Sequence Analysis of a Naturally Reassorted Infectious Bursal Disease Virus from India. Genome Announc. 2016;4:e00709-16. doi: 10.1128/genomeA.00709-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pikuła A., Lisowska A., Jasik A., Śmietanka K. Identification and Assessment of Virulence of a Natural Reassortant of Infectious Bursal Disease Virus. Vet. Res. 2018;49:89. doi: 10.1186/s13567-018-0586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nwagbo I.O., Shittu I., Nwosuh C.I., Ezeifeka G.O., Odibo F.J.C., Michel L.O., Jackwood D.J. Molecular Characterization of Field Infectious Bursal Disease Virus Isolates from Nigeria. Vet. World. 2016;9:1420–1428. doi: 10.14202/vetworld.2016.1420-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kasanga C.J., Yamaguchi T., Munang’andu H.M., Ohya K., Fukushi H. Genomic Sequence of an Infectious Bursal Disease Virus Isolate from Zambia: Classical Attenuated Segment B Reassortment in Nature with Existing Very Virulent Segment A. Arch. Virol. 2013;158:685–689. doi: 10.1007/s00705-012-1531-4. [DOI] [PubMed] [Google Scholar]
- 64.Cui P., Ma S., Zhang Y., Li X., Gao X., Cui B., Chen H. Genomic Sequence Analysis of a New Reassortant Infectious Bursal Disease Virus from Commercial Broiler Flocks in Central China. Arch. Virol. 2013;158:1973–1978. doi: 10.1007/s00705-013-1682-y. [DOI] [PubMed] [Google Scholar]
- 65.Wei Y., Yu X., Zheng J., Chu W., Xu H., Yu X., Yu L. Reassortant Infectious Bursal Disease Virus Isolated in China. Virus Res. 2008;131:279–282. doi: 10.1016/j.virusres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 66.Wu T., Wang Y., Li H., Fan L., Jiang N., Gao L., Li K., Gao Y., Liu C., Cui H., et al. Naturally Occurring Homologous Recombination between Novel Variant Infectious Bursal Disease Virus and Intermediate Vaccine Strain. Vet. Microbiol. 2020;245:108700. doi: 10.1016/j.vetmic.2020.108700. [DOI] [PubMed] [Google Scholar]
- 67.Ingrao F., Rauw F., Lambrecht B., van den Berg T. Infectious Bursal Disease: A Complex Host–Pathogen Interaction. Dev. Comp. Immunol. 2013;41:429–438. doi: 10.1016/j.dci.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 68.Fan L., Wang Y., Jiang N., Gao L., Li K., Gao Y., Cui H., Pan Q., Liu C., Zhang Y., et al. A Reassortment Vaccine Candidate of the Novel Variant Infectious Bursal Disease Virus. Vet. Microbiol. 2020;251:108905. doi: 10.1016/j.vetmic.2020.108905. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y., Jiang N., Fan L., Gao L., Li K., Gao Y., Niu X., Zhang W., Cui H., Liu A., et al. Development of a Viral-Like Particle Candidate Vaccine Against Novel Variant Infectious Bursal Disease Virus. Vaccines. 2021;9:142. doi: 10.3390/vaccines9020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li G., Kuang H., Guo H., Cai L., Chu D., Wang X., Hu J., Rong J. Development of a Recombinant VP2 Vaccine for the Prevention of Novel Variant Strains of Infectious Bursal Disease Virus. Avian Pathol. J. WVPA. 2020;49:557–571. doi: 10.1080/03079457.2020.1791314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be requested by writing to the author.