Abstract
The recent advancements of poly(methyl methacrylate) (PMMA) as a transparent flexible polymer material have been utilized in numerous areas of engineering and materials science. PMMA-based copolymers demonstrate outstanding mechanical and optical properties owing to high transparency, lightweight nature, high impact resistance, and stress relaxation across glass transition temperature. These copolymers have unique characteristics of retaining optical and microstructural integrities during successive bending or elongations which make them an attractive choice for materials of stretchable electronics. In particular, there has been an escalated rise in the use of methyl methacrylate (MMA)-based transparent and stretchable copolymer films during the recent decades. Therefore, we have highlighted these recent developments into a comprehensive review in order to aid the future progress in these diverse fields. Herein, we have highlighted the scope of MMA as an important building block for the synthesis of highly transparent and flexible materials. The synthetic pathways of these copolymer materials and the resulting mechanical properties have been discussed. Moreover, the immense scope of these copolymer films has been highlighted by virtue of their applications in various industries.
1. Introduction
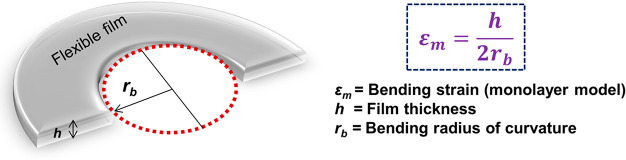
There has been an unprecedented demand for developing highly flexible materials in modern-day electronics, healthcare, energy storage systems, and other such industries. Flexible materials have the advantage of reversibly bending or stretching and can undergo multiple mechanical deformations. However, practical usage of such materials requires that they simultaneously have high yield strength and a low elastic modulus.1 Recent efforts by researchers have been focused on the design and manufacture of novel materials which have a desired balance between flexibility and strength performance.2 Flexibility of a material can be best described as the ability of a material to bend along a radius of curvature without undergoing any permanent deformation. Hence, flexibility can be quantified in terms of the minimum bending radius of curvature (1/rb), where a smaller rb value signifies a more flexible material. In the case of a single layer of a flexible film, the bending strain of the film can be easily calculated from the bending radius and the film thickness (as shown in Figure 1).3
Figure 1.
Schematic representation and equation to determine the bending strain for a monolayer flexible film.
The current market survey reveals the commonly used flexible substrates to be metallic foils, ultrathin glass, and organic polymers.4 Over the decades, flexible polymeric substrates are gradually advancing on a path to replace the traditional rigid substrates such as glass and silicon (Si) substrates. Most notably, organic polymers such as, polyethylene naphthalate (PEN), polyimides (PI), polyethylene terephthalate (PET), polydimethylsiloxane (PDMS), cellulose composites, etc. have been developed as a flexible base for a number of applications.5,6 However, these pliable substrates almost always require a coating of inorganic materials to imbibe the desired property in the material.7 One of the earlier reliable thermoelectric materials, silicon-germanium (Si-Ge) alloy has been commonly used in semiconducting devices.8 However, severe limitations associated with manufacturing expenses and high activating temperatures are often tackled by coating the silica alloy in the form of thin films on flexible substrates.9 The selection of the flexible substrate is influenced by the requirement of the application, i.e., conformability, lightweight, malleability, ease of storage, flexibility, etc.10
Another property that is analogous with flexibility is transparency of the materials. Flexible materials with a high percentage of light transmittance are essentially used in electronic displays, building windows, wearable devices, aerospace vehicles, food packaging, etc.11,12 The optical clarity for such applications should be such that the light transmittance is above 85% over the visible spectrum (400–800 nm) and the percentage of haze should be <0.7%.13 Few polymers which meet the demands of both flexibility and transparency are PET, PEN, polycarbonates (PCs), and poly(methyl methacrylate) (PMMA). In particular, PMMA is a transparent homopolymer of methyl methacrylate (MMA) which has gradually created a niche in different display and packaging applications. The main focus of this review article is to discuss the MMA-based polymers that have high transparency and flexibility. We have tried to provide a comprehensive understanding of the recent advances in the synthesis and applications of these flexible and transparent copolymers.
2. Polymerization of Methyl Methacrylate: Properties and Scope
Methyl methacrylate (MMA), also known as methacrylic acid methyl ester is the key monomer that is involved in the synthesis of a wide array of acrylics and plastics.14 MMA is a popular monomer choice in polymer chemistry owing to its contribution toward durability, thermal stability, optical clarity, impact strength, and scratch/abrasion resistance in the polymers.15 It is an acrid smelling liquid that is prone to undergo self-polymerization, generating considerable amounts of heat. Hence, the commercially available MMA contains a small amount of inhibitor (∼0.1%) to allow for its safe storage.16 Since the first reported synthesis of methacrylic esters in the 1930s, MMA has been extensively employed as cost-effective starting materials for glazing, sheet applications, exterior paints, and paper coatings.
2.1. Homopolymers of Methacrylate Derivatives
Methacrylic acid and its derived alkyl methacrylates are high production volume chemicals, i.e., they are widely used in the production of different consumer items or industrial materials.17 In addition, many alkyl methacrylate polymers act as additives to enhance the rheological characteristics of lubricants.18 Methacrylate homopolymers such as, poly(2-hydroxyethyl methacrylate),19,20 poly(2-phenylethyl methacrylate),21 2-(acetoacetoxy)ethyl methacrylate,22 and poly(benzyl methacrylate),23 are widely used in the development of hydrogels, medical implants, and adhesives, respectively. However, the most widely used and well-known alkyl methacrylate polymer is PMMA. The first reported synthesis of PMMA is generally credited to Hill and Crawford during the early 1930s.24 However, it was Rohm who was responsible for popularizing the homopolymer of MMA as two commercially available thermoplastic polymers (Plexiglas and Acryloid).25,26 In the next decade, with the advent of World War II, PMMA became the preferred material for the construction of aircraft windows and bombardier canopies. PMMA is also very useful in surgical and other biomedical applications as acrylic-based bone cement and biocompatible implants.27
2.2. Physical and Chemical Properties of PMMA
Nowadays, PMMA is known to the general populace by its commercial trade names, i.e., Plexiglas, Akrylon, Diakon, Perspex, etc. The popularity of PMMA among researchers and industrialists alike is often attributed to its attractive physical, chemical, and optical properties. PMMA is a clear and colorless polymer that is amorphous in nature. PMMA is also known to withstand temperatures within the range of −70 to 100 °C, which makes it suitable for use in different climates and environments.28 Usually, polymeric materials used in displays, optical devices, solar cells, and packaging are subjected to extreme temperatures and weather conditions. In this regard, PMMA has been proven to be highly suitable due to its high resistance to UV-induced and humidity damage. Yildirim et al. have studied the effects of outdoor weathering conditions in Turkey on different thermoplastic polymers and have determined that PMMA is more resistive toward natural weather damage than acrylonitrile butadiene styrene (ABS) and acrylonitrile styrene acrylate (ASA).29 The stereochemistry of the adjacent chiral carbons of a polymer backbone also influences the physical characteristics of a polymeric material. This can be explained by taking a look at the polymer tacticities of PMMA. The different spatial arrangement of the ester groups of the MMA moieties is used to distinguish PMMA as syndiotactic, isotactic, or atactic (Figure 2).30 The tacticity of PMMA is also responsible for determining the amorphous nature and glass transition temperatures (Tg); syndiotactic PMMA has the highest Tg (130 °C), followed by atactic (120 °C) and isotactic (55 °C).31 The higher stability of the syndiotactic and atactic polymers are inherently responsible for increasing the Tg.32 The spatial configuration of the MMA moieties also plays a role in influencing the chemical reactivity and solubility of PMMA.
Figure 2.
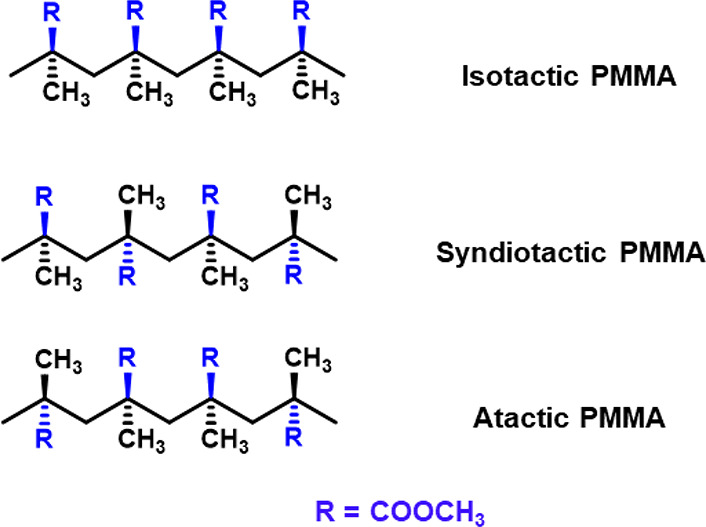
Representation of the three tacticities of PMMA.
Besides tacticity, the solubility of PMMA is also governed by the polarity of the solute and solvents. PMMA is generally soluble in polar organic solvents such as, ethyl acetate, cyclohexanone, chloroform, tetrahydrofuran (THF), trichloroethylene, N,N-dimethylformamide (DMF), etc.33,34 Apart from these common solvents, recent research has also shown the solubility of PMMA in certain hydrophobic ionic liquids (e.g., 1-butyl-3-methylimidazolium hexafluorophosphate (C4mimPF6) and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (C4mimNTf2)), resulting in different ionic gels.35 Furthermore, PMMA is also highly stable in strong acids and bases under ambient temperature and pressure conditions.
2.3. Electrical Properties of PMMA
Exploring polymer materials with appropriate dielectric behavior can show immense promise in electronics and energy storage applications. Tailoring polymers with high dielectric constant, low dielectric loss, and high breakdown strength is essential for designing an efficient gate dielectric material.36 The bulk properties of the polymer can modify the surface interactions arising in a dielectric material. Hence, continuous efforts have been directed toward controlling the polymer structures to tune the molecular weights, surface packing, composition of the polymer, etc. PMMA possess a high resistivity value (∼1015 Ω cm), appropriate dielectric constant (at low frequencies), and impressive film processability. However, the dielectric constant of PMMA is found to be lacking at high frequencies (∼3 at 1 MHz), which limit the more practical applications of pure PMMA as a dielectric material.37 In addition, the thermoplastic PMMA acts as an insulator which is characteristic of such polymers. However, the dielectric properties and electrical conductivity of PMMA have been modified by introducing different ceramic fillers into the polymer framework.38
2.4. Optical and Mechanical Properties of PMMA
The high optical clarity and impressive mechanical properties of PMMA are responsible for their increasing utilization in the development of transparent and flexible materials. The key parameters required for these applications are high total light transmittance, compatible refractive index, good Young’s modulus, high tensile strength, and a small elongation at break. The values of these parameters for pure PMMA are described in Table 1.
Table 1. Some of the Optical and Mechanical Properties of Commercial PMMA39.
| Sl. No. | Property | PMMA |
|---|---|---|
| 1 | Color | Colorless |
| 2 | Refractive index | 1.49 |
| 3 | Total light transmittance | 92% |
| 4 | Glass transition temperature | 120–130 °C |
| 5 | Young’s modulus | 3.10 GPa |
| 6 | Elongation at break | 2–10% |
| 7 | Tensile strength | 40–110 MPa |
2.5. Retention of Properties of PMMA Post Deformation/Modification
The interesting physicochemical properties of PMMA have influenced its applications in various industries. However, the real-life impact of these materials can be effectively judged by evaluating the retention of these properties after undergoing external stress or deformation. PMMA polymers possess both elastic and viscous properties during stress-induced deformation. The viscoelastic properties of PMMA are linked with the Tg of the polymer. The addition of an ionizable compound in the polymer matrix of PMMA can increase the Tg and modify its viscoelastic properties.40 Different creep recovery experiments have been employed by researchers in the past to investigate the effects of thermal and mechanical deformation on the physical properties of PMMA and its composites. Interestingly, PMMA polymers have been known to retain their morphological properties when subjected to different forms of mechanical stress. It is important to note that the structural integrity of PMMA starts to soften as the temperature approaches its Tg value. The tensile extent of PMMA can also be increased on increasing the temperature. However, heating the homopolymer beyond its Tg can lead to degradation of the structural integrity and render the polymer incompetent for practical use.41 McLoughlin and Tobolsky have extensively studied the viscoelastic behavior of different forms of PMMA over a range of different temperatures.42 The stress relaxation curves of PMMA reveal a strong temperature dependence of the relaxation rate near the Tg. This implies the increase in elastic nature of the polymer around its glass transition temperature.
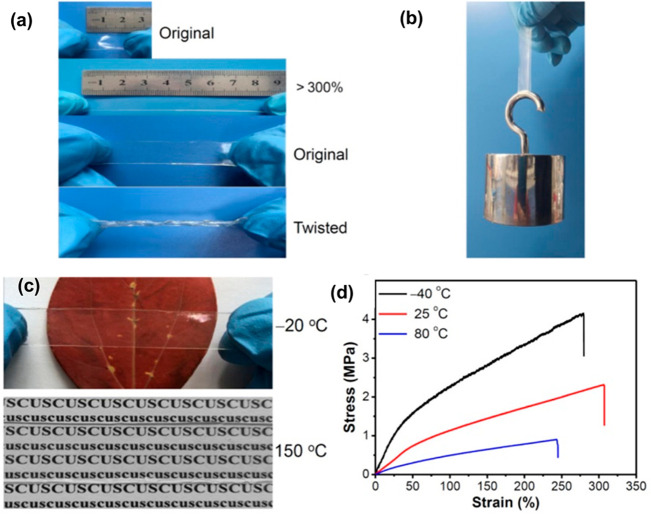
PMMA can also be utilized to imbibe improved mechanical properties and optical transparency in biopolymer composites.43,44 In this regard, efforts have been extended to study the retention of the optical properties of PMMA-based architectures after application of external stress. Lan et al. have developed PMMA containing ionogels that have displayed high transparency of >93%.45 The optical clarity of these ionogels was found to remain intact even after undergoing different levels of mechanical deformations and thermal conditions (Figure 3). PMMA-based stretchable materials have also been reported to show dynamic self-healing properties subject to the proper tuning of the interactions between the different comonomers/components of the material.46 The copolymerization of MMA opens up multiple avenues for combining two or more desirable properties in a single polymeric architecture.
Figure 3.
(a) Digital photographs of the PMMA-based ionogel in its original form and under different mechanical deformations. (b) Photograph of the PMMA-based ionogel stretched with a load of 1 kg. (c) Photographs depicting the optical clarity of the PMMA-based ionogel at two different temperatures (i.e., −20 and 150 °C). (d) Stress–strain curves of the ionogel at different temperatures. Reproduced with permission from ref (45). Copyright 2020, American Chemical Society.
3. Flexible and Transparent PMMA-Based Copolymers
The current status of PMMA research is concerned with improving the viscoelasticity and electrical properties of synthetic PMMA. This has opened up expanding avenues into the synthesis of PMMA copolymers, hybrids, composites, and blends. In the consecutive sections, we will provide a thorough discussion on the synthesis and characterization of PMMA copolymers and copolymer hybrids.
3.1. PMMA-Based Graft Copolymers
Graft copolymers consist of randomly branched monomer side chains between linear polymer backbones.47 Graft copolymerization of MMA has been employed by researchers in the past to tailor the interfacial properties between the layers of different materials.48,49 One of the commonly used synthetic methods for producing graft copolymers is via the “macromonomer” technique. The surface modification of common synthetic polymers can be done in a facile manner by this technique. The surface of poly(vinyl chloride) (PVC) plastic had been earlier modified by blending with PDMS.50 However, the complete PDMS coverage over the PVC surface was somewhat limited due to the phase separation between the two polymers. To counter this problem, Gorelova et al. have grafted PDMS on to PMMA to improve the compatibility between the modifier and the substrate (PVC).51 PMMA-based graft copolymers possess increased mechanical strength than their polymer blend counterparts. Tomić et al. have investigated the adhesive and mechanical properties of a poly(ethylene-co-vinyl acetate) (EVA) grafted PMMA copolymer.52 Pure PMMA coatings on optical fibers have a tendency to form cracks on bending. Hence, PMMA was grafted on the adhesive EVA to develop polymer coatings with better mechanical strength. The grafted PMMA polymer displayed a higher degree of miscibility, minimal phase separation, greater tensile strength, and improved flexibility. PMMA has also been grafted on biopolymers (cellulose, chitosan, etc.) to be utilized for a number of medical or biological applications.53 Chitosan-g-PMMA copolymers were synthesized and thermopressed into flexible membranes by Prashanth and Tharanathan.54 The authors have studied the influence of polyacrylonitrile (PAN) and PMMA grafts on the structural framework and thermal properties of chitosan. It was observed that chitosan-g-PMMA had better grafting efficiency and better flexibility than chitosan-g-PAN. Besides improving the flexibility, it is also possible to retain the high transparency of PMMA in grafted copolymers. Shih et al. have developed PMMA nanocomposites and improved the impact resistance of the materials by grafting with cellulose nanofibers.55 The tensile test of the materials revealed that the grafted polymer possessed better tensile strength and low elongation at break than the PMMA homopolymer. In addition, the total light transmittance (TLT) of the PMMA composites was only slightly lower than pristine PMMA. Later, Liu and co-workers have also worked on modified PMMA grafted cellulose nanocrystal powders to develop a transparent film for UV shielding and oil/water separation membrane applications.56 The transparent film showed a low transmittance (below 25%) in the ultraviolet region and a high transmittance (83%) in the visible range.
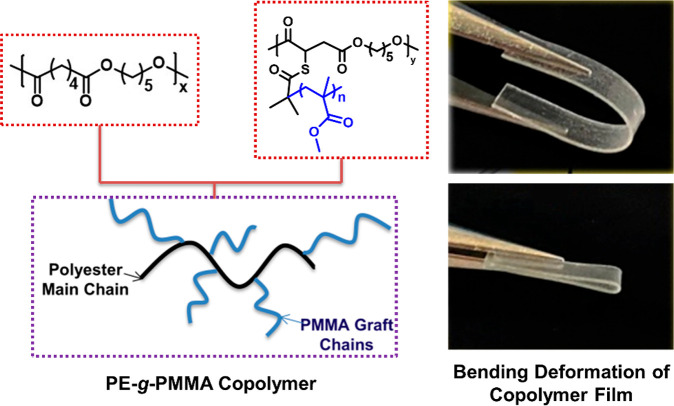
Recently, Mesbah et al. have tuned the ionic properties of a cellulose acetate grafted poly(AMPS-co-MMA) flexible proton conducting membrane.57 The transparent and flexible graft copolymer membranes displayed a high proton conductivity value (6.4 × 10–3 S cm–1) which opens up many avenues for the application of PMMA-based flexible copolymers. One of the recent examples of flexible PMMA-based graft copolymers was reported by Hayashi et al., where the ductility of a glassy polymer like PMMA was enhanced with the help of graft architectures (Figure 4).58 The grafted PMMA fragments constituted a major portion of the copolymer and were responsible for significantly reducing the shear stress of the material. The flexible polyester grafted PMMA (PE-g-PMMA) showed a Young’s modulus (EY) of 1.2 GPa which is similar to pure PMMA homopolymer. In addition, the grafted copolymer had a low fracture strain (20–30%) when compared to other glassy polymers. This signified the enhanced ductile nature of PMMA grafted copolymers and emphasizes on their scope of application in different flexible devices.
Figure 4.
PMMA grafted on a flexible polyester main chain to develop a transparent and highly flexible polymer film. Reproduced with permission from ref (58). Copyright 2021, Elsevier.
3.2. PMMA-Based Block Copolymers
The monomer chains in a block copolymer are chemically distinct, and the monomer blocks are linearly aligned.59 Block copolymers offer a wide array of morphologies and physicochemical properties on the basis of the choice of the block architecture. Depending on the number of distinguishable building blocks and their spatial configuration, block copolymers can be differentiated as diblock, triblock, multiblock, and starblock copolymers.60 In the past decades, PMMA-based block copolymers have been synthesized via different polymerization techniques. The MMA unit in the block copolymers is chosen to imbue the polymer with impressive optical properties. The unique morphologies of PMMA block copolymers also undergo interesting self-assemblies to form various nanostructure formations.61,62 This phenomenon occurs due to the interconnected framework and the thermodynamic incompatibilities of the monomer blocks. The self-assembly of polystyrene-block-poly(methyl methacrylate) (PS-b-PMMA) was used to modify the surface of graphene nanomesh by Park et al.63 They applied directed self-assembly of PS-b-PMMA thin films as a lithography template for creating two different nanostructured graphene arrays. The high transparency of the modified graphene nanomesh can be further employed to develop a semiconducting transparent film for wearable devices.
Graphene (GO)-reinforced PMMA block copolymers have also been reported to display enhanced flexibility without compromising on the optical clarity of the thin film. Song et al. have synthesized GO and pyrene modified PMMA-b-PDMS copolymers.64 The subsequent effects of these modifications on the physicochemical properties of PMMA were investigated in detail. Increasing the GO content in the polymer films beyond 0.5 wt % was detrimental to the transparency of the film and resulted in an opaque polymer film. The optimized GO content was maintained at 0.05 wt % to increase the light transmittance and decrease the percentage of haze. The incorporation of graphene in the block copolymer was found to increase the tensile strength of the PMMA-based copolymer film. PMMA block copolymers have also been synthesized with PCs, since PCs show a similar level of transparency (89%) and impact resistance as PMMA. Jang and co-workers have synthesized a series of PMMA-co-PC copolymers with different ratios of the monomers via a radical polymerization technique.65 The optimized copolymer (PMMA-PC-40) produced a highly flexible film when solution casted onto a mold. Hosseini et al. have also employed the free radical polymerization technique to synthesize four poly(methyl methacrylate-co-methacrylic acid) (PMMA-co-MAA) copolymers with different compositions.66 The PMMA-co-MAA coatings show immense potential for being applied in biodiagnostic devices owing to the high transparency, ease of surface functionalization, and tunability of the surface properties. In addition, the copolymer coatings possessed uniform thickness and enhanced hydrophilicity. Another important comonomer of PMMA-based flexible films is polylactic acid (PLA), which is characterized by a clear and transparent appearance. Choochottiros and Chin have employed a PMMA-based copolymer, i.e., poly(butadiene-co-lactide-co-methyl methacrylate-co-butyl methacrylate) (BLMB), as an impact modifier for PLA.67 The content of polybutadiene was optimized to obtain a balance between the transparency, impact strength, and flexibility. The optimized BLMB copolymer could improve the impact strength to 25% more than pure PLA and retained the optical clarity of the monomer components.
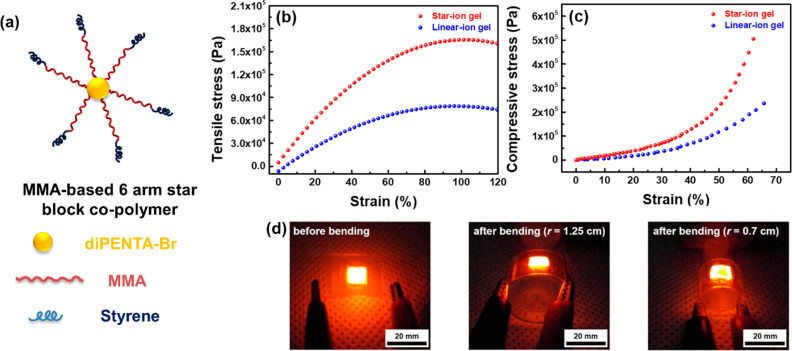
Triblock or starblock copolymers consisting of one or more ionic liquid (IL) units have been employed to synthesize flexible ionogels and polyionic. Hwang et al. have synthesized a group of ionogels based on a PMMA-co-PS star shaped copolymer and an ionic liquid (Figure 5).68 The prepared ionogel was utilized as a solid-state electrolyte that displays impressive mechanical properties. The PMMA-based electrolyte had high conductivity (1.54 mS cm–1) and good elastic modulus (2.5 × 104 Pa). The combination of PMMA copolymers and different ionic liquids involved in the preparation of flexible films will be discussed in detail in the subsequent section.
Figure 5.
(a) Schematic illustration of poly(MMA-co-St) star block copolymer with a bromine functionalized dipentaerythritol (diPENTA-Br) core. (b) Tensile stress–strain and (c) compressive stress–strain curves for the star-block and linear block copolymer-based ionogels. (d) Digital images of the fabricated flexible device before and after bending. Reproduced with permission from ref (68). Copyright 2019, American Chemical Society.
3.3. PMMA-Based Polyionic Liquids
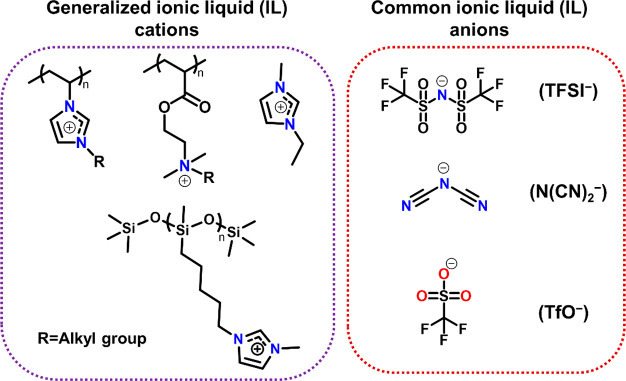
Solid-state ionic liquids or ionogels are comprised of a room-temperature ionic liquid suspended in a solid substrate (polymer or silica-based gels).69 Polymerized ionic liquids (PILs) are a novel class of polymeric materials that combine the ionic nature and high conductivity of ionic liquids with the macromolecular properties of polymers.70 PILs are prepared through radical/living polymerization of ionic monomers or encapsulation of ionic liquids in a polymer framework. The feasibility of PIL-based flexible gels or films is judged on the basis of Tg. The Tg of PILs determines whether their nature will be rubbery or brittle and glassy at a given temperature. The ionic conductivity of PILs can also be correlated to the Tg because the rate of mobility of polymer segments depends on the Tg.71 The choice of the ionic monomer in PILs is vital for engineering the desired flexibility or ductility in the material (Figure 6).
Figure 6.
Common examples of ionic liquid motifs utilized in the synthesis of PMMA-based polyionic liquids.
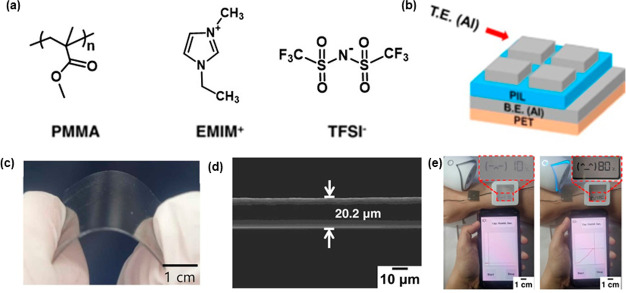
PMMA-based copolymers provide an interesting opportunity for integrating different morphologies in PILs.72 On the other hand, the influence of the ionic monomer composition on the conductivity and structure of the PIL was well-explained by Gwee et al.73 Herein, a PMMA and styrene copolymer (denoted as SbMMA) was combined with an ionic liquid, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMIm-TFSI), and the mixture was solution casted to form a transparent and flexible film (SbMMA/EMIm-TFSI). The PMMA film was expectedly transparent but brittle. On the other hand, with increasing EMIm-TFSI concentration, the SbMMA/IL film becomes more flexible as a result of the decrease in the Tg. The highest ionic conductivity was observed for 50 wt % EMIm-TFSI content at 2 × 10–5 S cm–1. The dependence of the viscoelastic properties of a PMMA/ionic liquid mixture on the Tg was well-explored by Mok and co-workers.74 The authors have conducted extensive calorimetric and rheology experiments at different PMMA concentrations and temperatures. The transition of PMMA/IL mixtures from brittle to flexible to terminal was clearly observed over a range of composition ratios and temperatures. In addition to high flexibility, incorporating ionic liquids in PMMA polymers imbue the material with impressive conductive properties. Grishina et al. have prepared heterogeneous polymer films from a PMMA homopolymer, methyl methacrylate-vinylpyrrolidone copolymer, and 1-butyl-3-methylimidazolium hexafluorophosphate (BMIPF6) ionic liquid.75 The heterogeneous films were prepared by a solution casting technique using chloroform or acetone. The polymer films were semitransparent and flexible when the BMIPF6 content was maintained below 50 wt %. Additionally, the PMMA/BMIPF6 film shows high specific conductivity (0.6 × 10–5 S cm–1) at room temperature. The real-life application of such polymer/ionic liquid flexible films has been explored by Park and co-workers.76 The authors have prepared a 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [EMIM][TFSI]/PMMA-based wearable humidity sensor, as shown in Figure 7. The flexible polymer-based dielectric film was bar printed on PET substrates to assemble the final sensing device. The [EMIM][TFSI]-embedded polymer films also displayed an increase in the capacitive properties over a range of relative humidity. The sensing ability and capacitive properties of the film were even observed at a 30% bending strain, which suggests the formation of a highly flexible film.
Figure 7.
(a) Components of the PMMA/ionic liquid copolymer. (b) Schematic design of PIL-based device. (c) Photograph of transparent and flexible copolymer sheet. (d) Thickness of copolymer film. (e) Wearable flexible humidity sensor. Reproduced with permission from ref (76). Copyright 2022, Elsevier.
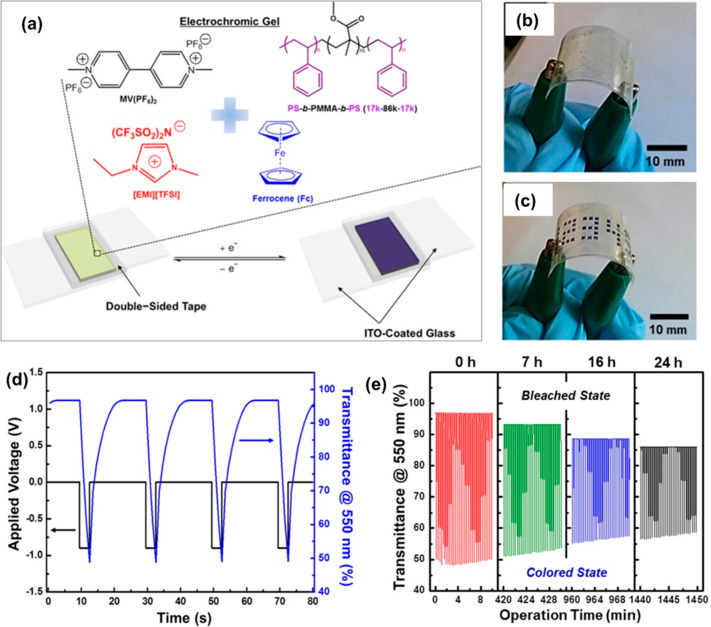
Copolymer networks of PMMA can absorb different ionic liquids to produce ionogels with good mechanical integrity, low volatility, and high conductivity.77 These polymer-based ionogels are particularly sought-after in stretchable or wearable electronics owing to their intrinsic transparency and stretchability. Moreover, the flexible ionogels are capable of sustaining their conductive nature under high mechanical deformation.78 Gayet et al. have synthesized SiO2 functionalized copolymers of MMA and methacryloxypropyltrimethoxysilane, which were combined with an imidazolium ionic liquid to develop hybrid ionogels.79 The authors posit that the presence of the ionic liquid can promote greater specific interactions between the ion-pair and the inorganic oxide interface. The PMMA-based ionogel showed good flexibility as observed from the low Young’s modulus. PMMA-based ionogels have also been combined with redox-active species to introduce a new dimensionality to the material. Moon et al. have synthesized a PMMA and polystyrene (PS) block copolymer (PS-b-PMMA-b-PS) and enhanced its functionality with 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMI][TFSI]) ionic liquid and redox active methyl viologen (Figure 8).80 The rubber-like nature of the synthesized ionogel was capitalized to assemble a transparent and flexible electrochromic device by a patterning technique. The square wave profile and transmittance curves of the bleached and colored states of the device suggest a good efficiency and transmittance contrast.
Figure 8.
(a) Schematic illustration of the electrochromic device assembled with the PS-b-PMMA-b-PS based ionogel. Photographs of the ionogel incorporated device after bending in the (b) bleached state and (c) colored state. (d) Symmetric square wave profile and corresponding transmittance at a wavelength of 550 nm against time. (e) Change in the transmittance of electrochromic device as a function of the operation time. Reproduced with permission from ref (80). Copyright 2015, American Chemical Society.
Recently, Tao et al. have developed a luminescent ionogel by confining a 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([Bmim][NTf2]) ionic liquid within a PMMA and organosilica polymer network.81 The polymer/silica interface imbibed the ionogel with adjustable mechanical properties, good transparency, and high flexibility. On the other hand, the ionic liquid immobilized in the hybrid polymer gel was responsible for the high conductivity value of 0.04 S cm–1 at 50 °C.
3.4. PMMA/Inorganic Metal Oxide Hybrids
The coexistence of an organic polymeric phase and an inorganic phase in the micro or nanoscale provides a promising avenue to design advanced materials with well-controlled properties. The interactions between the components of a hybrid material can be either class I type (weak interactions like hydrogen bonding or van der Waals interaction) or class II type (strong ionic or covalent interactions).82 In particular, incorporating inorganic particles in a PMMA matrix couples the desirable characteristics of both the polymer (transparency and thermal stability) and inorganic phases (mechanical strength).83 Hybrid materials of PMMA and inorganic oxides (like SiO2, ZnO, TiO2, etc.) have attracted considerable attention in diverse fields.84 Inorganic oxides have been known to imbue organic polymers with better dielectric and mechanical properties. PMMA/inorganic hybrids are conventionally prepared through the sol–gel process. Koziej and co-workers have prepared sol–gel-mediated transparent PMMA-TiO2 nanoparticle hybrid films.85 The TiO2 nanoparticles exhibit good compatibility with the PMMA matrix, and hence the inherent optical clarity of PMMA was retained even hybrid films containing 9.3 wt % TiO2. However, the hybrid films showed some increase in UV absorbance (∼1%) after they were illuminated for 8 h under an UV lamp due to the photocatalytic nature of anatase TiO2. The UV absorbing phenomenon displayed by the films can be further utilized in UV shielding applications. Morales-Acosta et al. have also employed a sol–gel technique to synthesize PMMA-SiO2 hybrid films, and studied the different optical and mechanical properties of the material.86 Here, different concentrations of 3-(trimethoxysilyl)propyl methacrylate (TMSPM) were used as a coupling agent between the PMMA polymer and the SiO2 particles. The optical transmittance of PMMA was maintained over the entire range of TMSPM concentrations (>90%). It was also observed that the coupling agent had a role in controlling the dielectric properties of the hybrid films.
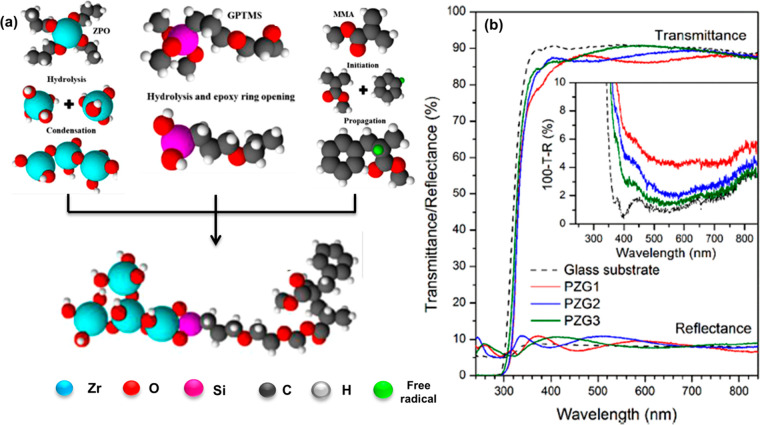
Recently, Garibay-Martínez et al. carried out similar investigations into the PMMA-GPTMS-ZrO2 hybrid films (Figure 9).87 The authors used 3-glycidyloxypropyl(trimethoxy)silane (GPTMS) as the coupling agent between PMMA and ZrO2. The dual presence of silyl end groups and epoxy rings of GPTMS causes an enhancement in the mechanical and adhesion properties of the polymer/inorganic hybrid. The UV–vis spectra of the hybrid films depict high optical transmittance (85–90%), low optical loss due to absorbance (<5%), and optical reflectance that is comparable to glass (10%). The hybrid films were mechanically strong, which is ascertained from the elastic modulus (123 GPa) of the material. Another recent example of PMMA/inorganic nanoparticle hybrid film was reported by Kong et al.88 The authors prepared transparent and flexible PMMA-based hybrid films with high contents of ZnO nanoparticles. The investigation of the mechanical properties of the hybrid films revealed a nearly 150% increase in the tensile strength when compared with a pure PMMA film. At 20 vol % ZnO content, the hybrid film possessed a high tensile strength of 24.3 MPa. The elastic ZnO nanoparticles behave as stress releasing points in the PMMA matrix, resulting in higher flexibility of the material. The optical experiments also suggest that the hybrid films show high light transmittance in the visible region (above 500 nm wavelength) and high UV absorbance in the UV region.
Figure 9.
(a) Mechanistic pathway for the formation of PMMA-GPTMS-ZrO2 polymer hybrids. (b) Total light transmittance and reflectance spectra of the synthesized hybrid films and commercial glass (inset displaying the 100–T–R (%) spectra). Reproduced with permission from ref (87). Copyright 2021, Elsevier.
3.5. PMMA/PDMS Hybrids
PDMS is another common contemporary of PMMA which is used in the development of flexible materials. MMA has been copolymerized with a PDMS macromonomer to prepare a series of polymer films with variable monomer ratios by Smith et al.89 The low Tg, good transparency, and high stability against oxygen degradation of PDMS have been used to enhance the overall surface properties of the copolymer films. However, the optical properties of the copolymer films like total transmittance and reflectance were not investigated in detail. The excellent optical properties of PMMA/PDMS hybrid materials have been demonstrated by Sugimoto et al.90 The highest %transmittance was observed for the copolymer of MMA and modified PDMS macromonomer with methacrylate side chain (SigUMA3), P(MMA-co-SigUMA3). However, the mechanical properties were limited for the composite films with increasing content of the siloxane monomer due to aggregating nature of the silyl groups. PDMS can also function as a transparent and flexible substrate for assembling a variety of practical device applications. Yuan et al. have developed PMMA and metal–organic framework (MOF)-based composite films.91 The synthesized composite films were later embedded on the PDMS substrates to assemble a novel MOF-PMMA-PDMS hybrid film. The formation of the final composite films is responsible for transforming the brittle nature of the MOF film into a more viscoelastic state.
4. Applications of PMMA-Based Materials
Considering the many benefits of PMMA-based copolymers/hybrid materials, they have been involved in different industry applications. Generally, the use of amorphous form of PMMA is prevalent in the manufacture of window glass, packaging materials, etc. Gradually, PMMA-based advanced materials are being developed by researchers to widen their scope and feasibility of application. In this section, we have discussed some particular applications of PMMA-based copolymers/hybrid materials.
4.1. Aviation Industry
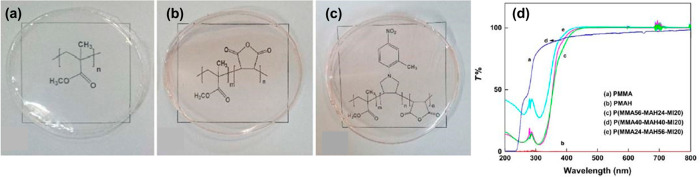
The canopies of aircraft cockpits are designed to ensure high visibility and resistance against damage from foreign objects. The materials used for manufacture of aircraft canopies also need to be lightweight and cost-effective. Therefore, amorphous PMMA is one of the leading candidates for designing modern day defense and passenger aircrafts.92 However, there are some limitations of aero-grade PMMA regarding the brittleness, slow processing, and low ductility of the material.93 In this regard, the properties of PMMA are modified by forming copolymers or hybrids with different organic/inorganic moieties. Atabaki et al. have copolymerized PMMA with maleic anhydride (MAH) and a maleimide derivative (MI) to enhance the thermal stability and transparency of PMMA (Figure 10).94 MAH acts as a plasticizer of PMMA owing to the noncovalent-type interactions of MAH with the carbonyl sites of PMMA. On the other hand, N-2-methyl-4-nitrophenyl maleimide increases the thermal stability of vinyl monomers during copolymerization. The crystalline nature of MAH was responsible for achieving ∼100% transparency in the copolymers. The synthesized copolymers were blended with aero-grade amorphous PMMA to improve the flexibility of PMMA by generating free volume spaces in the polymer matrix.
Figure 10.
Digital photographs of transparent films prepared from (a) pure PMMA, (b) poly(MMA-co-MAH) copolymer, and (c) P(MMA-co-MAH-co-MI) terpolymer. (d) Percentage of light transmittance in the synthesized copolymers and terpolymers. Reproduced with permission from ref (94). Copyright 2018, Wiley Periodicals, LLC.
Another issue faced in the manufacture of display windows of aircrafts is the dispersion of static charge buildup at high altitudes.95 The usual methods employed for enhancing the antistatic property of PMMA lead to compromising the transparency of the material. The surface of pure plastic PMMA has a tendency to accumulate static charges. Chen et al. have developed an antistatic PMMA copolymer with high tensile strength and transparency.96 A terpolymer of PMMA with methacrylic acid (MAA) and 3-sulfopropyl methacrylate potassium salt was synthesized after selecting the monomers through theoretical simulations. The surface resistance and tensile strength of the terpolymer were higher than pure PMMA. At the same time, the optical transparency and haze of the terpolymers were comparable to PMMA.
4.2. Solid-State Electronic Devices
The ever-increasing demand for portable electronics has fueled the search for flexible polymer materials with good conductivity and ion transport properties. Moreover, the applied materials should be resistant to damage during mechanical deformation of the device. Most thermoplastic polymers (like PMMA) are electrically insulating in nature. The ionic conductivity of PMMA polymers can be improved by (i) copolymerizing with a charged monomer, (ii) mixing with an ionic liquid, or (iii) doping with conductive fillers.97,98 The main challenge associated with these techniques is balancing high conductivity with good optical clarity and high tensile strength.
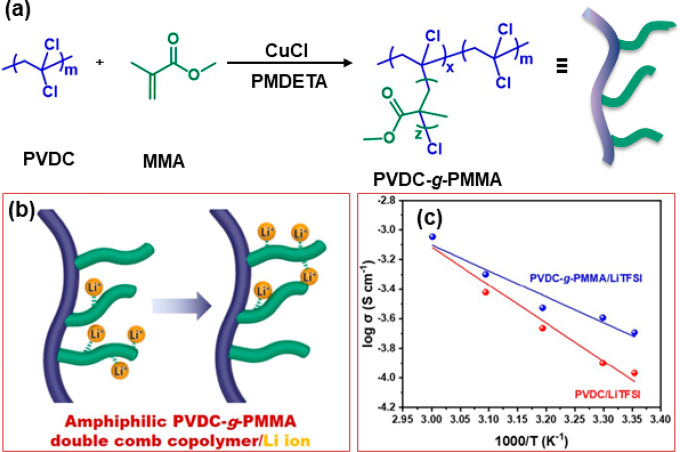
Moon et al. have prepared amphiphilic double comb graft copolymers of poly(vinylidene chloride) (PVDC) and PMMA for solid-state electrolyte application (Figure 11).99 Here, the good mechanical strength of PVDC was complemented with the transparent and amorphous properties of PMMA. The graft copolymers were used in the preparation of flexible solid-state electrolyte membranes via complexation with bis(trifluoromethylsulfonyl)amine lithium salt and lithium perchlorate. The high mobility of lithium salts has further enhanced the flexibility of the polymer membrane. Moreover, the free-standing membranes showed high flexibility and ion conductivity even after undergoing mechanical deformation. PMMA copolymer films have shown immense application in energy conversion and energy storage devices (batteries, supercapacitors, etc.). Ghosh and Kofinas have synthesized a conductive electrolyte matrix consisting of a poly(ethylene oxide) and PMMA block copolymer.100 The second block of the synthesized copolymer was composed of random segments of an ionic PMMA derivative. The lithium counteranion in the polymer segments is immobile and acts as a secondary source of lithium in the battery. The conductivity measurements of the assembled flexible battery revealed improved lithium transport in the PEO homopolymer.
Figure 11.
(a) Synthetic scheme of amphiphilic PVDC-g-PMMA comb copolymer. (b) Illustration of Li ion mobility in the PVDC-g-PMMA solid-state membrane. (c) Variation of conductivity against temperature for the synthesized copolymer membranes. Reproduced with permission from ref (99). Copyright 2021, Elsevier.
PMMA-based viscoelastic copolymers have also shown great promise in other solid-state devices such as organic field effect transistors (OFETs) and light-emitting diodes.101 Polymer dielectrics are used in flexible OFETs as the gate dielectric material and are required to possess high dielectric constant, low film thickness, and small leakage current. A PMMA grafted poly(vinylidene fluoride) (PVDF) copolymer was developed by Shin et al. as high-performance dielectric materials for OFETs.102 The flexible transistors with the graft copolymer dielectric had better electrical characteristics and improved mechanical properties than bare PVDF or PMMA materials. In addition, the PMMA grafted copolymer dielectrics possessed higher operational stability than the traditionally used polymer dielectrics.
4.3. Food Packaging Application
In today’s modern world, thermoplastic polymers constitute a major portion of the materials used in the packaging industry. In particular, transparency and flexibility are two of the major requirements for food packaging materials. In addition, certain characteristics, such as antifogging property, high degree of adhesion, and antibacterial property, are desirable when engineering advanced food packaging materials. Pure PMMA is not sufficient enough for industrial-grade food packaging.
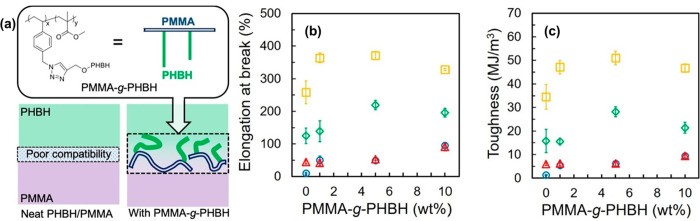
Developing PMMA blends with similar polymers have been previously explored to enhance the mechanical properties of PMMA. However, the ductility and adhesive properties of the polymer blends are compromised due to the incompatibility of different components. To counter this limitation, PMMA has been copolymerized to generate better compatibility between the different monomers. Tamiya et al. have synthesized a graft copolymer comprising of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) and PMMA to increase the interfacial adhesion in PHBH/PMMA blend (Figure 12).103 The graft copolymer acts as a compatibilizer by restricting the phase separation between PHBH and PMMA. The graft copolymer also successfully increases the Young’s modulus and mechanical toughness of the polymer films.
Figure 12.
(a) Structure of PMMA-g-PHBH and illustration depicting the interfacial adhesion between the components of PHBH/PMMA blend and graft copolymer. (b) Plot of elongation at break. (c) Plot of material toughness of PMMA-g-PHBH copolymer at different monomer weight ratios. Reproduced with permission from ref (103). Copyright 2020, Elsevier.
4.4. Nanolithography
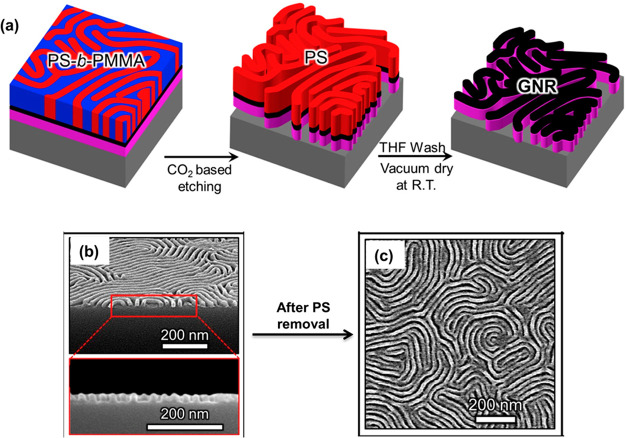
Block copolymers hold a special interest in nanolithography techniques owing to their tendency to self-assemble, control over domain size and density, and morphological uniformity.104 Block copolymer lithography provides an emerging alternative to the traditional photolithography techniques for the fabrication of microprocessors and integrated circuits.105 The current industry standard for block copolymer lithography is the PS and PMMA block copolymer (PS-b-PMMA). PS-b-PMMA copolymers possess perpendicularly oriented domains when they are annealed over a neutral substrate at a temperature of ∼180 °C.106 Katsumata et al. have utilized the perpendicular orientation of PS-b-PMMA domains for the nanopatterning of graphene nano ribbons (Figure 13).107 The PS-b-PMMA copolymers coated on graphene substrates were annealed to form perpendicular arrays and were utilized as an etch mask to transfer the pattern to the underlying graphene. However, the relatively low value of the block–block interaction parameter (χ) of PS-b-PMMA limits the scope of variation in domain sizes.108 This limitation hinders the large-scale production of higher generation microdevices using PS-b-PMMA.
Figure 13.
(a) Schematic representation of the graphene nano ribbon array fabrication by PMMA-based block copolymer lithography. Scanning electron microscope images of (b) perpendicular polystyrene (PS) patterns after PMMA etching and (c) graphene nano ribbon arrays after the removal of PS. Reproduced with permission from ref (107). Copyright 2017, Elsevier.
Another alternative lithography technique for microfabrication is the hot embossing lithography technique. Here, polymers are softened by heating them barely above the glass transition temperatures and stamping the desired nanopattern on the softened polymer. Different copolymers of PMMA, acrylic acid, and 2,2,2-trifluoroethyl methacrylate were utilized by Palacios-Cuesta and group as versatile platforms to create nanopatterned microstructures via hot embossing lithography.109 The use of these copolymers enabled the simultaneous modification of surface topology and chemical composition of the patterned surface. Nanolithography and hot embossing techniques using PMMA copolymers offer a cost-effective solution for the rapid prototyping of microfabricated devices.
4.5. Biomedical Technologies
The research on polymer materials for use in various biomedical applications has been on the forefront due to the many attractive properties of polymers. Modification of conventional polymers like PMMA has been carried out to tailor the desired properties according to the concerned application. The optically transparent PMMA has shown high potential in drug delivery systems,110,111 synthetic bone cement,112 cornea implants,113 optical lens,114 dental implants,115 etc. due to its good biological compatibility, low toxicity, and adhesive property. The biocompatibility of PMMA has been further improved by polymerizing it with biosourced polymers such as chitosan. Hemalatha et al. have investigated the scope of chitosan grafted PMMA in corneal tissue engineering.116 The synthesized copolymer films showed a maximum optical clarity of 88% and facilitated good cell attachment. The grafted copolymer films possessed a tensile strength of 41 MPa and 37% elongation at break. In another example, Zhao and co-workers have explored the antifogging properties of poly(methyl methacrylate-co-(2-(dimethylamino)-ethyl methacrylate)) (PMMA-co-DMAEMA).117 The transparent copolymer coatings were further cross-linked with the polymerized ethylene glycol dimethacrylate network. The partially quaternized copolymer films showed good antifogging and antimicrobial properties. Therefore, the PMMA-based copolymer films/coatings are extensively utilized in biocompatible and biomedical technologies.
5. Conclusion and Future Perspectives
In summary, we have systematically highlighted the recent developments of MMA-based advanced copolymer materials and reviewed their optical and viscoelastic properties. Modern day applications demand the smart engineering of multifaceted materials which are imbibed with different attractive characteristics like optical clarity, flexibility, mechanical strength, thermal stability, etc. (Figure 14). From the previous literature reports, it can be surmised that polymers of MMA display a considerable degree of these properties and, hence, have been utilized for various industry applications. However, pure PMMA is brittle in nature and, therefore, fails to be useful in the thin film form. As a result, researchers have focused their endeavors toward modifying the limitations of PMMA via copolymerizing it with various compatible monomers. The type of copolymer synthesized was also observed to play a vital role in tailoring the physicochemical characteristics of the materials. An array of evidence suggests the need to balance the high transparency and amorphous nature of PMMA with the mechanical strength of the employed comonomers. Different literature reports have successfully attempted to enhance the transparency, flexibility, tensile strength, and conductivity of PMMA copolymers. In this review, we have discussed the advantages offered by a wide variety of PMMA-based copolymers and polymer hybrids. A select few commercial applications of transparent and flexible PMMA-based copolymer films have been described. These reports can emphasize the importance of more in-depth investigation in the synthesis and properties of PMMA-based copolymer materials.
Figure 14.
Illustration summarizing the various properties and applications of MMA-based copolymer films.
On the basis of the current status of PMMA research, significant advancement of well-engineered PMMA-based flexible materials can be anticipated in the future. The majority of the current research in this field is focused on the enhancement of either the flexibility or conductivity of the copolymer materials. The materials for electronics have been unfolded from brittle to flexible and in recent years advanced to stretchable for wearable plastic electronics. In some cases, this improvement of flexibility or conductivity comes at the cost of optical clarity of PMMA. The development of novel strategies for the synthesis and characterization of PMMA copolymers that exhibit multifaceted optical, mechanical, and electrical properties is the need of the hour. The innovative strategies to design and control the polymeric microstructures at molecular level enable the retention of mechanical and optoelectronic properties during successive stretching. In addition, there is a significant mismatch between the results obtained in a laboratory setup and the actual application in industry. Therefore, we believe that there is substantial space and scope for future research in the evolution of the next-generation of PMMA-based flexible films. Several new advancements of transparent and stretchable active MMA base copolymer matrices have been reviewed, and the importance of polymer chemistry to achieve an array of remarkable properties has been highlighted in this review. However, several technological and polymer processing challenges still remain to be addressed for stretchable transparent matrices. Although significant progress has been made to control the morphology and orientation of polymeric chains, the multiscale ordering of the copolymers and mechanical reversibility against the cyclic strain largely limit the use of PMMA for stretchable optics applications. The multicomponent material systems have shown some promise, and the introduction of nanofiller opens up a wide range of avenues to regulate the chain dynamics and to tune physical properties to maintain the mobility during stretching of polymers. We also believe that this review provides a thorough summation of the current progress in the field and will inspire future advancement to address the prevalent challenges associated with this area.
Acknowledgments
P.D. acknowledges Defence Materials and Stores Research and Development Establishment (DMSRDE), Kanpur, Government of India (sanction no. and date: TR/0569/CARS/128) for the financial support. A.B. thanks the Council of Scientific and Industrial Research (CSIR), Government of India, for his senior research fellowship (SRF).
The authors declare no competing financial interest.
References
- Peng J.; Snyder G. J. A figure of merit for flexibility. Science 2019, 366, 690–691. 10.1126/science.aaz5704. [DOI] [PubMed] [Google Scholar]
- Ritchie R. O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. 10.1038/nmat3115. [DOI] [PubMed] [Google Scholar]
- Mao L.; Meng Q.; Ahmad A.; Wei Z. Mechanical analyses and structural design requirements for flexible energy storage devices. Adv. Energy Mater. 2017, 7, 1700535. 10.1002/aenm.201700535. [DOI] [Google Scholar]
- Ramanujam J.; Bishop D. M.; Todorov T. K.; Gunawan O.; Rath J.; Nekovei R.; Artegiani E.; Romeo A. Flexible CIGS, CdTe and a-Si: H based thin film solar cells: A review. Prog. Mater. Sci. 2020, 110, 100619. 10.1016/j.pmatsci.2019.100619. [DOI] [Google Scholar]
- Malik A.; Kandasubramanian B. Flexible polymeric substrates for electronic applications. Polym. Rev. 2018, 58, 630–667. 10.1080/15583724.2018.1473424. [DOI] [Google Scholar]
- Lu Q.- H.; Zheng F.. Polyimides for electronic applications. In Advanced Polyimide Materials; Elsevier: Amsterdam, 2018; pp 195–255. [Google Scholar]
- Kranz L.; Gretener C.; Perrenoud J.; Schmitt R.; Pianezzi F.; Mattina F. L.; Blösch P.; Cheah E.; Chirilă A.; Fella C. M.; Hagendorfer H.; Jäger T.; Nishiwaki S.; Uhl A. R.; Buecheler S.; Tiwari A. N. Doping of polycrystalline CdTe for high-efficiency solar cells on flexible metal foil. Nat. Commun. 2013, 4, 2306. 10.1038/ncomms3306. [DOI] [PubMed] [Google Scholar]
- Bhandari C. M.; Rowe D. M. Silicon–germanium alloys as high-temperature thermoelectric materials. Contemp. Phys. 1980, 21, 219–242. 10.1080/00107518008210957. [DOI] [Google Scholar]
- Kusano K.; Yamamoto A.; Nakata M.; Suemasu T.; Toko K. Thermoelectric inorganic SiGe film synthesized on flexible plastic substrate. ACS Appl. Energy Mater. 2018, 1 (10), 5280–5285. 10.1021/acsaem.8b00899. [DOI] [Google Scholar]
- MacDonald W. A.; Looney M. K.; MacKerron D.; Eveson R.; Adam R.; Hashimoto K.; Rakos K. Latest advances in substrates for flexible electronics. J. Soc. Inf. Dispersion 2007, 15, 1075–1083. 10.1889/1.2825093. [DOI] [Google Scholar]
- Chen T.; Peng H.; Durstock M.; Dai L. High-performance transparent and stretchable all-solid supercapacitors based on highly aligned carbon nanotube sheets. Sci. Rep. 2014, 4, 3612. 10.1038/srep03612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; Ma G.; Qin F.; Hu L.; Luo B.; Liu T.; Yin X.; Su Z.; Zeng Z.; Jiang Y.; Wang G.; Li Z. High-conductivity, flexible and transparent PEDOT:PSS electrodes for high performance semi-transparent supercapacitors. Polymers 2020, 12, 450. 10.3390/polym12020450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald W. A. Engineered films for display technologies. J. Mater. Chem. 2004, 14, 4–10. 10.1039/b310846p. [DOI] [Google Scholar]
- Brydson J. A.Acrylic Plastics. In Plastic Materials, 7th ed., Butterworth Heinemann: Oxford, 1999; pp 398–424. [Google Scholar]
- Mete S.; Goswami K. G.; De P. Composition-dependent crystallization behavior of copolyperoxides from methyl methacrylate and 4-vinylbenzyl stearate. J. Polym. Sci. 2020, 58, 766–778. 10.1002/pol.20200029. [DOI] [Google Scholar]
- De P.; Sathyanarayana D. N.; Sadasivamurthy P.; Sridhar S. Reactivity ratios for the oxidative copolymerizations of indene with methyl methacrylate and methacrylonitrile. Eur. Polym. J. 2002, 38, 847–855. 10.1016/S0014-3057(01)00271-3. [DOI] [Google Scholar]
- Albertini R. J. The lower alkyl methacrylates: Genotoxic profile of non-carcinogenic compounds. Regul. Toxicol. Pharmacol. 2017, 84, 77–93. 10.1016/j.yrtph.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Ghosh P.; Das T.; Nandi D. Synthesis characterization and viscosity studies of homopolymer of methyl methacrylate and copolymer of methyl methacrylate and styrene. J. Solution Chem. 2011, 40, 67–78. 10.1007/s10953-010-9632-8. [DOI] [Google Scholar]
- Wichterle O.; Lím D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. 10.1038/185117a0. [DOI] [Google Scholar]
- Choudhury N.; Das S.; Samadder S.; De P. Phenylalanine-tethered pH-responsive poly (2-Hydroxyethyl Methacrylate). Chem.—Asian J. 2021, 16, 1016–1024. 10.1002/asia.202100136. [DOI] [PubMed] [Google Scholar]
- Ratner B. D.Biomedical applications of synthetic polymers. In Comprehensive Polymer Science and Supplements; Pergamon: Oxford, 1989; pp 201–247. [Google Scholar]
- Pal S.; Das A.; Maiti S.; De P. Synthesis and characterization of a biodegradable polymer prepared via radical copolymerization of 2-(acetoacetoxy) ethyl methacrylate and molecular oxygen. Polym. Chem. 2012, 3, 182–189. 10.1039/C1PY00419K. [DOI] [Google Scholar]
- Fang C.; Zhou F.; Zhu X. The application research of benzyl methacrylate (BzMA) in acrylate latex pressure sensitive adhesives. Int. J. Adhes. Adhes. 2021, 107, 102861. 10.1016/j.ijadhadh.2021.102861. [DOI] [Google Scholar]
- Kennedy C.ICI: The company that changed our lives; SAGE Publications Limited: Thousand Oaks, CA, 1993. [Google Scholar]
- Neher H. T. Acrylic resins. Ind. Eng. Chem. 1936, 28, 267–271. 10.1021/ie50315a002. [DOI] [Google Scholar]
- Chisholm M. S. Artificial glass-the versatility of poly(methyl methacrylate) from its early exploitation to the new millennium. J. Chem. Educ. 2000, 77, 841–845. 10.1021/ed077p841. [DOI] [Google Scholar]
- Dunne N.; Clements J.; Wang J.-S.. Acrylic cements for bone fixation in joint replacement. In Joint Replacement Technology, 2nd ed.; Woodhead Publishing: Sawston, UK, 2014; pp 212–256. [Google Scholar]
- Ali U.; Karim K. J.; Buang N. A. A review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. 10.1080/15583724.2015.1031377. [DOI] [Google Scholar]
- Yildirim F. F.; Hicyilmaz A. S.; Yildirim K. The effects of the weathering methods on the properties of the ABS, ASA and PMMA polymers. Polym. Test. 2022, 107, 107484. 10.1016/j.polymertesting.2022.107484. [DOI] [Google Scholar]
- Shah Z. M.; Khanday F. A.; Malik G. F. A.; Jhat Z. A.. Fabrication of polymer nanocomposite-based fractional-order capacitor: a guide. In Fractional-Order Design; Academic Press: Cambridge, MA, 2022; pp 437–483. [Google Scholar]
- Chat K.; Tu W.; Beena Unni A.; Adrjanowicz K. Influence of tacticity on the glass-transition dynamics of poly(methyl methacrylate) (PMMA) under elevated pressure and geometrical nanoconfinement. Macromolecules 2021, 54, 8526–8537. 10.1021/acs.macromol.1c01341. [DOI] [Google Scholar]
- Forte M. A.; Silva R. M.; Tavares C. J.; Silva R. F. Is poly(methyl methacrylate) (PMMA) a suitable substrate for ALD?: A Review. Polymers 2021, 13, 1346. 10.3390/polym13081346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evchuk I. Y.; Musii R. I.; Makitra R. G.; Pristanskii R. E. Solubility of polymethyl methacrylate in organic solvents. Russ. J. Appl. Chem. 2005, 78, 1576–1580. 10.1007/s11167-005-0564-9. [DOI] [Google Scholar]
- Roy S. G.; Bauri K.; Pal S.; Goswami A.; Madras G.; De P. Synthesis, characterization and thermal degradation of dual temperature- and pH-sensitive RAFT-made copolymers of N,N-(dimethylamino)ethyl methacrylate and methyl methacrylate. Polym. Int. 2013, 62, 463–473. 10.1002/pi.4335. [DOI] [Google Scholar]
- Ueno K.; Fukai T.; Nagatsuka T.; Yasuda T.; Watanabe M. Solubility of poly(methyl methacrylate) in ionic liquids in relation to solvent parameters. Langmuir 2014, 30, 3228–3235. 10.1021/la404797g. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Huang X.; Li T.; Li L.; Guo X.; Jiang P. Polymer-based gate dielectrics for organic field-effect transistors. Chem. Mater. 2019, 31, 2212–2240. 10.1021/acs.chemmater.8b03904. [DOI] [Google Scholar]
- Na M.; Rhee S.-W. Electronic characterization of Al/PMMA [poly (methyl methacrylate)]/p-Si and Al/CEP (cyanoethyl pullulan)/p-Si structures. Organ. Electron. 2006, 7, 205–212. 10.1016/j.orgel.2006.02.003. [DOI] [Google Scholar]
- da Silva Leite Coelho P. H.; Marchesin M. S.; Morales A. R.; Bartoli J. R. Electrical percolation, morphological and dispersion properties of MWCNT/PMMA nanocomposites. Mater. Res. 2014, 17, 127–32. 10.1590/S1516-14392014005000059. [DOI] [Google Scholar]
- Peters E. N.Plastics: Thermoplastics, thermosets, and elastomers. In Handbook of Materials Selection; Wiley: Hoboken, NJ, 2002; pp 335–355. [Google Scholar]
- Miyagawa A.; Ayerdurai V.; Nobukawa S.; Yamaguchi M. Viscoelastic properties of poly (methyl methacrylate) with high glass transition temperature by lithium salt addition. J. Polym. Sci. B Polym. Phys. 2016, 54, 2388–2394. 10.1002/polb.24227. [DOI] [Google Scholar]
- Jiang J.; Li C.; Zhu S.; Chen Z.; Fu M.; He D.; Wang Y. Optical properties of PMMA inverse opal structures with anisotropic geometries by stretching. Mater. Res. Express 2020, 7, 045801. 10.1088/2053-1591/ab88fe. [DOI] [Google Scholar]
- McLoughlin J. R.; Tobolsky A. V. The viscoelastic behavior of polymethyl methacrylate. J. Colloid Sci. 1952, 7, 555–568. 10.1016/0095-8522(52)90039-1. [DOI] [Google Scholar]
- Li Y.; Fu Q.; Yu S.; Yan M.; Berglund L. Optically transparent wood from a nanoporous cellulosic template: Combining functional and structural performance. Biomacromolecules 2016, 17, 1358–1364. 10.1021/acs.biomac.6b00145. [DOI] [PubMed] [Google Scholar]
- Kai D.; Jiang S.; Low Z. W.; Loh X. J. Engineering highly stretchable lignin-based electrospun nanofibers for potential biomedical applications. J. Mater. Chem. B 2015, 3, 6194–6204. 10.1039/C5TB00765H. [DOI] [PubMed] [Google Scholar]
- Lan J.; Li Y.; Yan B.; Yin C.; Ran R.; Shi L.-Y. Transparent stretchable dual-network ionogel with temperature tolerance for high-performance flexible strain sensors. ACS Appl. Mater. Interfaces 2020, 12, 37597–37606. 10.1021/acsami.0c10495. [DOI] [PubMed] [Google Scholar]
- Song P. N.; Hong J.-L. Highly-stretchable, self-healable random copolymers for loading large amounts of multiwall carbon nanotubes (MWCNTs) for the preparation of stretchable and healable electric sensors. J. Mater. Chem. C 2019, 7, 13161–13175. 10.1039/C9TC03735G. [DOI] [Google Scholar]
- Rudin A.; Choi P. Introductory concepts and definitions, In The Elements of Polymer Science & Engineering, 3rd ed.; Academic Press: Cambridge, MA, 2013; pp 1–62. [Google Scholar]
- Mutlutürk E.; Caykara T. Grafting parameters and surface free energy components of photosensitive poly(methacrylated spiropyran) brushes. J. Macromol. Sci. A 2022, 59, 307–314. 10.1080/10601325.2022.2033124. [DOI] [Google Scholar]
- Men J.; Dong C.; Han Y.; Yang Y.; Wang J.; Lv Z.; Wang L.; Wang Y. Preparation of grafted adsorbent CPVA-g-PMAA and its adsorption performance for amlodipine. J. Macromol. Sci. A 2022, 59, 354–365. 10.1080/10601325.2022.2041030. [DOI] [Google Scholar]
- Gorelova M. M.; Pertsin A. J.; Levin V. Y.; Makarova L. I.; Filimonova L. V. Surface behavior of blends of siloxane and siloxane containing copolymers in poly (vinyl chloride). J. Appl. Polym. Sci. 1992, 45, 2075–2078. 10.1002/app.1992.070451202. [DOI] [Google Scholar]
- Gorelova M. M.; Pertsin A. J.; Muzafarov A. M.; Gritsenok O. T.; Vasilenko N. G. Surface modification of poly (vinyl chloride) with poly (methyl methacrylate)/poly (dimethyl siloxane) graft copolymers. J. Appl. Polym. Sci. 1995, 55, 1131–1135. 10.1002/app.1995.070550716. [DOI] [Google Scholar]
- Tomić N. Z.; Veljović Đ.; Trifković K.; Međo B.; Rakin M.; Radojević V.; Jančić-Heinemann R. Numerical and experimental approach to testing the adhesive properties of modified polymer blend based on EVA/PMMA as coatings for optical fibers. Int. J. Adhes. Adhes. 2017, 73, 80–91. 10.1016/j.ijadhadh.2016.11.010. [DOI] [Google Scholar]
- Thakur V. K.; Thakur M. K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustainable Chem. Eng. 2014, 2, 2637–2652. 10.1021/sc500634p. [DOI] [Google Scholar]
- Prashanth K. V. H.; Tharanathan R. N. Studies on graft copolymerization of chitosan with synthetic monomers. Carbohydr. Polym. 2003, 54, 343–351. 10.1016/S0144-8617(03)00191-7. [DOI] [Google Scholar]
- Shih Y. F.; Chou M. Y.; Lian H. Y.; Hsu L. R.; Chen-Wei S. M. Highly transparent and impact-resistant PMMA nanocomposites reinforced by cellulose nanofibers of pineapple leaves modified by eco-friendly methods. Express Polym. Lett. 2018, 12, 844–854. 10.3144/expresspolymlett.2018.72. [DOI] [Google Scholar]
- Liu Y.; Zhou L.; Wang L.; Pan X.; Wang K.; Shu J.; Liu L.; Zhang H.; Lin L.; Shi X.; Schlarb A. K.; Zhang J. Air-dried porous powder of polymethyl methacrylate modified cellulose nanocrystal nanocomposite and its diverse applications. Compos. Sci. Technol. 2020, 188, 107985. 10.1016/j.compscitech.2019.107985. [DOI] [Google Scholar]
- Mesbah F.; El Gayar D.; Farag H.; Tamer M. T.; Omer A. M.; Mohy-Eldin M. S.; Khalifa R. E. Development of highly ionic conductive cellulose acetate-g-poly (2-acrylamido-2-methylpropane sulfonic acid-co-methyl methacrylate) graft copolymer membranes. J. Saudi Chem. Soc. 2021, 25, 101318. 10.1016/j.jscs.2021.101318. [DOI] [Google Scholar]
- Hayashi M.; Shibata K.; Nobukawa S. Advantage of graft architecture with a flexible main chain for implantation of ductile nature into brittle amorphous acrylic glass. Polymer 2021, 236, 124316. 10.1016/j.polymer.2021.124316. [DOI] [Google Scholar]
- Quirk R. P.; Kinning D. J.; Fetters L. J.. Block copolymers. In Comprehensive Polymer Science and Supplements; Pergamon: Oxford, 1989; pp 1–26. [Google Scholar]
- Khimani M.; Patel H.; Patel V.; Parekh P.; Vekariya R. L. Self-assembly of stimuli-responsive block copolymers in aqueous solutions: an overview. Polym. Bull. 2020, 77, 5783–5810. 10.1007/s00289-019-03046-w. [DOI] [Google Scholar]
- Bates F. S.; Fredrickson G. H. Block copolymer thermodynamics: Theory and experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. 10.1146/annurev.pc.41.100190.002521. [DOI] [PubMed] [Google Scholar]
- Blanco M.; López M.; Kortaberria G.; Mondragon I. Nanostructured thermosets from self-assembled amphiphilic block copolymer/epoxy resin mixtures: effect of copolymer content on nanostructures. Polym. Int. 2010, 59, 523–528. 10.1002/pi.2731. [DOI] [Google Scholar]
- Park S.; Yun J. M.; Maiti U. N.; Moon H.-S.; Jin H. M.; Kim S. O. Device-oriented graphene nanopatterning by mussel-inspired directed block copolymer self-assembly. Nanotechnology 2014, 25, 014008. 10.1088/0957-4484/25/1/014008. [DOI] [PubMed] [Google Scholar]
- Song S.; Wan C.; Zhang Y. Non-covalent functionalization of graphene oxide by pyrene-block copolymers for enhancing physical properties of poly (methyl methacrylate). RSC Adv. 2015, 5, 79947–79955. 10.1039/C5RA14967C. [DOI] [Google Scholar]
- Jang H.; Ahmed F.; Joo H.; Ryu T.; Yang H.; Yoon S.; Kim W.; Jeon H.-S. Polycarbonate-co-PMMA block copolymers via atom transfer radical polymerization reaction. J. Nanoscience Nanotechnol. 2017, 17, 7381–7386. 10.1166/jnn.2017.14810. [DOI] [Google Scholar]
- Hosseini S.; Ibrahim F.; Djordjevic I.; Koole L. H. Polymethyl methacrylate-co-methacrylic acid coatings with controllable concentration of surface carboxyl groups: A novel approach in fabrication of polymeric platforms for potential bio-diagnostic devices. Appl. Surf. Sci. 2014, 300, 43–50. 10.1016/j.apsusc.2014.01.203. [DOI] [Google Scholar]
- Choochottiros C.; Chin I.-J. Potential transparent PLA impact modifiers based on PMMA copolymers. Eur. Polym. J. 2013, 49, 957–966. 10.1016/j.eurpolymj.2012.12.008. [DOI] [Google Scholar]
- Hwang H.; Park S. Y.; Kim J. K.; Kim Y. M.; Moon H. C. Star-shaped block copolymers: Effective polymer gelators of high-performance gel electrolytes for electrochemical devices. ACS Appl. Mater. Interfaces 2019, 11, 4399–4407. 10.1021/acsami.8b20004. [DOI] [PubMed] [Google Scholar]
- Taghavikish M.; Subianto S.; Gu Y.; Sun X.; Zhao X. S.; Choudhury N. R. A poly(ionic liquid) gel electrolyte for efficient all solid electrochemical double-layer capacitor. Sci. Rep. 2018, 8, 10918. 10.1038/s41598-018-29028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J.; Antonietti M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. 10.1016/j.polymer.2011.01.043. [DOI] [Google Scholar]
- Angell C. A. Fast ion motion in glassy and amorphous materials. Solid State Ion. 1983, 9–10, 3–16. 10.1016/0167-2738(83)90206-0. [DOI] [Google Scholar]
- Zhang Z.; Krajniak J.; Ganesan V. A multiscale simulation study of influence of morphology on ion transport in block copolymeric ionic liquids. Macromolecules 2021, 54, 4997–5010. 10.1021/acs.macromol.1c00025. [DOI] [Google Scholar]
- Gwee L.; Choi J.-H.; Winey K. I.; Elabd Y. A. Block copolymer/ionic liquid films: The effect of ionic liquid composition on morphology and ion conduction. Polymer 2010, 51, 5516–5524. 10.1016/j.polymer.2010.09.026. [DOI] [Google Scholar]
- Mok M. M.; Liu X.; Bai Z.; Lei Y.; Lodge T. P. Effect of concentration on the glass transition and viscoelastic properties of poly(methyl methacrylate)/ionic liquid solutions. Macromolecules 2011, 44, 1016–1025. 10.1021/ma102503j. [DOI] [Google Scholar]
- Grishina E. P.; Ramenskaya L. M.; Mudrov A. N. Conductivity and dielectric properties of heterogeneous films based on homo- and copolymers of methyl (methacrylate) and vinyl pyrrolidone doped with ionic liquid. Eur. Polym. J. 2014, 59, 247–253. 10.1016/j.eurpolymj.2014.07.041. [DOI] [Google Scholar]
- Park S.-J.; Jeon J.-Y.; Ha T.-J. Wearable humidity sensors based on bar-printed poly(ionic liquid) for real-time humidity monitoring systems. Sens. Actuators B Chem. 2022, 354, 131248. 10.1016/j.snb.2021.131248. [DOI] [Google Scholar]
- Li M.; Li J.; Na H.; Vlassak J. J. Mechanical behavior of poly (methyl methacrylate)-based ionogels. Soft Matter 2014, 10, 7993–8000. 10.1039/C4SM01466A. [DOI] [PubMed] [Google Scholar]
- Keplinger C.; Sun J.-Y.; Foo C. C.; Rothemund P.; Whitesides G. M.; Suo Z. Stretchable, transparent, ionic conductors. Science 2013, 341, 984–987. 10.1126/science.1240228. [DOI] [PubMed] [Google Scholar]
- Gayet F.; Viau L.; Leroux F.; Monge S.; Robin J.-J.; Vioux A. Polymer nanocomposite ionogels, high-performance electrolyte membranes. J. Mater. Chem. 2010, 20, 9456–9462. 10.1039/c000033g. [DOI] [Google Scholar]
- Moon H. C.; Lodge T. P.; Frisbie C. D. Solution processable, electrochromic ion gels for sub-1 V, flexible displays on plastic. Chem. Mater. 2015, 27, 1420–1425. 10.1021/acs.chemmater.5b00026. [DOI] [Google Scholar]
- Tao L.; Liu Y.; Wu D.; Wei Q.-H.; Taubert A.; Xie Z. Luminescent ionogels with excellent transparency, high mechanical strength, and high conductivity. Nanomater. 2020, 10, 2521. 10.3390/nano10122521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickelbick G.Introduction to hybrid materials. In Hybrid materials: synthesis, characterization and applications; John Wiley & Sons: Hoboken, NJ, 2006, 1, pp 2–46. [Google Scholar]
- Ansaloni L.; Deng L.. Advances in polymer-inorganic hybrids as membrane materials. In Recent Developments in Polymer Macro, Micro and Nano Blends; Woodhead Publishing: Sawston, UK, 2017; pp 163–206. [Google Scholar]
- Shin K.; Kim H.-N.; Cho J.-C.; Moon S. J.; Kim J. W.; Suh K.-D. Fabrication of monodisperse polymer/silica hybrid microparticles for improving light diffusion properties. Macromol. Res. 2012, 20, 385–390. 10.1007/s13233-012-0045-y. [DOI] [Google Scholar]
- Koziej D.; Fischer F.; Kränzlin N.; Caseri W. R.; Niederberger M. Nonaqueous TiO2 nanoparticle synthesis: A versatile basis for the fabrication of self-supporting, transparent, and UV-absorbing composite films. ACS Appl. Mater. Interfaces. 2009, 1, 1097–104. 10.1021/am9000584. [DOI] [PubMed] [Google Scholar]
- Morales-Acosta M. D.; Alvarado-Beltrán C. G.; Quevedo-López M. A.; Gnade B. E.; Mendoza-Galván A.; Ramírez-Bon R. Adjustable structural, optical and dielectric characteristics in sol–gel PMMA–SiO2 hybrid films. J. Non-Cryst. Solids 2013, 362, 124–135. 10.1016/j.jnoncrysol.2012.11.025. [DOI] [Google Scholar]
- Garibay-Martínez F.; Syamala Rao M. G.; Cortázar-Martínez O.; Hurtado-Macías A.; Quevedo-López M. A.; Ramírez-Bon R. Optical, mechanical and dielectric properties of sol-gel PMMA-GPTMS-ZrO2 hybrid thin films with variable GPTMS content. J. Non-Cryst. Solids 2021, 563, 120803. 10.1016/j.jnoncrysol.2021.120803. [DOI] [Google Scholar]
- Kong L.; Rau A.; Yang N.; Lu K. Flexible ZnO nanoparticle-poly(methyl methacrylate) hybrid films and their ultraviolet shielding behaviors. JOM 2021, 73, 432–440. 10.1007/s11837-020-04454-4. [DOI] [Google Scholar]
- Smith S. D.; DeSimone J. M.; Huang H.; York G.; Dwight D. W.; Wilkes G. L.; McGrath J. E. Synthesis and characterization of poly(methyl methacrylate)-g-poly(dimethylsiloxane) copolymers. 1. Bulk and surface characterization. Macromolecules 1992, 25, 2575–2581. 10.1021/ma00036a002. [DOI] [Google Scholar]
- Sugimoto H.; Nishino G.; Koyama H.; Daimatsu K.; Inomata K.; Nakanishi E. Preparation and morphology of transparent poly(methyl methacrylate)–poly(dimethylsiloxane) hybrid materials using multifunctional silicone macromonomer. J. Appl. Polym. Sci. 2012, 124, 1316–1322. 10.1002/app.33903. [DOI] [Google Scholar]
- Yuan K.; Song T.; Yang C.; Guo J.; Sun Q.; Zou Y.; Jiao F.; Li L.; Zhang X.; Dong H.; Li L.; Hu W. Polymer assisted space-confined strategy for the foot-scale synthesis of flexible metal–organic framework-based composite films. J. Am. Chem. Soc. 2021, 143, 17526–17534. 10.1021/jacs.1c07033. [DOI] [PubMed] [Google Scholar]
- Raghuwanshi R. K.; Verma V. K. Mechanical and thermal characterization of aero grade polymethyl methacrylate polymer used in aircraft canopy. Int. J. Eng. Adv. Technol. 2014, 3, 216–219. [Google Scholar]
- Mao Z.; Jiang T.; Zhang X.; Jiang G.; Zhang J. Co-continuous phase structure formed in melt processing inducing shear bands to prevent crack propagation: Significant improvement in impact toughness of PMMA. Polym. Test. 2020, 85, 106425. 10.1016/j.polymertesting.2020.106425. [DOI] [Google Scholar]
- Atabaki F.; Shokrolahi A.; Pahnavar Z. Methyl methacrylate based copolymers and terpolymers: Preparation, identification, and plasticizing capability for a poly(methyl methacrylate) used in aviation. J. Appl. Polym. Sci. 2018, 135, 46603. 10.1002/app.46603. [DOI] [Google Scholar]
- Yadav R.; Tirumali M.; Wang X.; Naebe M.; Kandasubramanian B. Polymer composite for antistatic application in aerospace. Def. Technol. 2020, 16, 107–118. 10.1016/j.dt.2019.04.008. [DOI] [Google Scholar]
- Chen S.; Xu C.; Ma M.; Shi Y.; He H.; Yuan K.; Xu R.; Wang X. Application of solubility parameters in the preparation of PMMA with permanent antistatic, high toughness, and excellent optical properties. Polym. Adv. Technol. 2021, 32, 3750–3758. 10.1002/pat.5394. [DOI] [Google Scholar]
- Farooq U.; Upadhyaya L.; Shakeel A.; Martinez G.; Semsarilar M. pH-responsive nano-structured membranes prepared from oppositely charged block copolymer nanoparticles and iron oxide nanoparticles. J. Membr. Sci. 2020, 611, 118181. 10.1016/j.memsci.2020.118181. [DOI] [Google Scholar]
- Santos A. M.; Elaïssari A.; Martinho J. M. G.; Pichot C. Synthesis of cationic poly(methyl methacrylate)-poly(N-isopropyl acrylamide) core-shell latexes via two-stage emulsion copolymerization. Polymer 2005, 46, 1181–1188. 10.1016/j.polymer.2004.11.069. [DOI] [Google Scholar]
- Moon J.; Cho S.; Song E.; Park K. W.; Chae Y.; Park J. T. Designing double comb copolymer as highly lithium ionic conductive solid-state electrolyte membranes. React. Funct. Polym. 2021, 169, 105093. 10.1016/j.reactfunctpolym.2021.105093. [DOI] [Google Scholar]
- Ghosh A.; Kofinas P. PEO based block copolymer as solid-state lithium battery electrolyte. ECS Trans. 2008, 11, 131. 10.1149/1.2938916. [DOI] [Google Scholar]
- Cho K. G.; Lee J. I.; Lee S.; Hong K.; Kang M. S.; Lee K. H. Light-emitting devices based on electrochemiluminescence gels. Adv. Funct. Mater. 2020, 30, 1907936. 10.1002/adfm.201907936. [DOI] [Google Scholar]
- Shin E.-Y.; Cho H. J.; Jung S.; Yang C.; Noh Y.-Y. A high-k fluorinated P(VDF-TrFE)-g-PMMA gate dielectric for high-performance flexible field-effect transistors. Adv. Funct. Mater. 2018, 28, 1704780. 10.1002/adfm.201704780. [DOI] [Google Scholar]
- Tamiya T.; Cui X.; Hsu Y.-I.; Kanno T.; Asoh T.-A.; Uyama H. Enhancement of interfacial adhesion in immiscible polymer blend by using a graft copolymer synthesized from propargyl-terminated poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Eur. Polym. J. 2020, 130, 109662. 10.1016/j.eurpolymj.2020.109662. [DOI] [Google Scholar]
- Stoykovich M. P.; Kang H.; Daoulas K. C.; Liu G.; Liu C.-C.; de Pablo J. J.; Müller M.; Nealey P. F. Directed self-assembly of block copolymers for nanolithography: fabrication of isolated features and essential integrated circuit geometries. ACS Nano 2007, 1, 168–175. 10.1021/nn700164p. [DOI] [PubMed] [Google Scholar]
- Bates C. M.; Maher M. J.; Janes D. W.; Ellison C. J.; Willson C. G. Block copolymer lithography. Macromolecules 2014, 47, 2–12. 10.1021/ma401762n. [DOI] [PubMed] [Google Scholar]
- Peters R. D.; Yang X. M.; Kim T. K.; Sohn B. H.; Nealey P. F. Using self-assembled monolayers exposed to X-rays to control the wetting behavior of thin films of diblock copolymers. Langmuir 2000, 16, 4625–4631. 10.1021/la991500c. [DOI] [Google Scholar]
- Katsumata R.; Yogeesh M. N.; Wong H.; Zhou S. X.; Sirard S. M.; Huang T.; Piner R. D.; Wu Z.; Li W.; Lee A. L.; Carlson M. C.; Maher M. J.; Akinwande D.; Ellison C. J. Large area fabrication of graphene nanoribbons by wetting transparency-assisted block copolymer lithography. Polymer 2017, 110, 131–138. 10.1016/j.polymer.2016.12.034. [DOI] [Google Scholar]
- Wan L.; Ruiz R.; Gao H.; Patel K. C.; Albrecht T. R.; Yin J.; Kim J.; Cao Y.; Lin G. The limits of lamellae-forming PS-b-PMMA block copolymers for lithography. ACS Nano 2015, 9, 7506–7514. 10.1021/acsnano.5b02613. [DOI] [PubMed] [Google Scholar]
- Palacios-Cuesta M.; Vasiev I.; Gadegaard N.; Rodríguez-Hernández J.; García O. Direct micrometer patterning and functionalization of polymer blend surfaces by using hot embossing. Eur. Polym. J. 2014, 59, 333–340. 10.1016/j.eurpolymj.2014.07.020. [DOI] [Google Scholar]
- Bettencourt A.; Almeida A. J. Poly(methyl methacrylate) particulate carriers in drug delivery. J. Microencapsul. 2012, 29, 353–367. 10.3109/02652048.2011.651500. [DOI] [PubMed] [Google Scholar]
- Kumbhakar K.; Dey A.; Mondal A.; De P.; Biswas R. Interactions and dynamics in aqueous solutions of pH-responsive polymers: A combined fluorescence and dielectric relaxation study. J. Phys. Chem. B 2021, 125, 6023–6035. 10.1021/acs.jpcb.1c03435. [DOI] [PubMed] [Google Scholar]
- Santos J. G. F. Jr; Peixoto L. S.; Nele M.; Melo P. A.; Pinto J. C. Theoretical and experimental investigation of the production of PMMA-based bone cement. Macromol. Symp. 2006, 243, 1–12. 10.1002/masy.200651101. [DOI] [Google Scholar]
- Riau A. K.; Venkatraman S. S.; Dohlman C. H.; Mehta J. S. Surface modifications of the PMMA optic of a keratoprosthesis to improve biointegration. Cornea 2017, 36, S15–S25. 10.1097/ICO.0000000000001352. [DOI] [PubMed] [Google Scholar]
- Wei Y.; Chen Y.; Liu P.; Gao Q.; Sun Y.; Huang C. Surface modification of hydrophobic PMMA intraocular lens by the immobilization of hydroxyethyl methacrylate for improving application in ophthalmology. Plasma Chem. Plasma Process. 2011, 31, 811–825. 10.1007/s11090-011-9323-2. [DOI] [Google Scholar]
- Frazer R. Q.; Byron R. T.; Osborne P. B.; West K. P. PMMA: An essential material in medicine and dentistry. J. Long Term Eff. Med. Implants 2005, 15, 629–639. 10.1615/JLongTermEffMedImplants.v15.i6.60. [DOI] [PubMed] [Google Scholar]
- Hemalatha T.; Yadav S.; Krithiga G.; Sastry T. P. Chitosan as a matrix for grafting methyl methacrylate: Synthesis, characterization and evaluation of grafts for biomedical applications. Polym. Bull. 2016, 73, 3105–3117. 10.1007/s00289-016-1644-0. [DOI] [Google Scholar]
- Zhao J.; Ma L.; Millians W.; Wu T.; Ming W. Dual-functional antifogging/antimicrobial polymer coating. ACS Appl. Mater. Interfaces 2016, 8, 8737–8742. 10.1021/acsami.6b00748. [DOI] [PubMed] [Google Scholar]