Abstract
We previously reported differential humoral responses to glucosyltransferases (GTFs), with significantly higher saliva and serum antibody levels to GtfD than to GtfB or GtfC. To test the hypothesis that cellular immune responses to these molecules also may differ, peripheral blood mononuclear cell (PBMC) and T-cell proliferative responses in young adults and children with distinct genetic backgrounds were determined using purified recombinant GtfC and GtfD. PBMCs from all of the volunteers responded to GtfC and -D, but responses were directed predominantly towards GtfD and were major histocompatibility class II antigen dependent. A predominant T-cell response to GtfD, over GtfC, was detectable at various antigen concentrations ranging from 1 to 20 μg/ml and correlated with the differential serum immunoglobulin G (IgG) and salivary IgA antibody responses to the GTFs. Therefore, in naturally sensitized humans, Streptococcus mutans GTFs stimulate differential humoral and cellular immune responses, with the secreted form of GtfD eliciting a stronger response than the cell wall-associated form of GtfC.
Human dental caries, an infectious disease of bacterial origin, has been found to be preventable by mucosal immunization (for reviews, see references 10 and 20). Mutans streptococci are the primary etiological agents, and within this group, Streptococcus mutans and Streptococcus sobrinus are the two most prevalent isolates from the human oral cavity (17). Secretory immunoglobulin A (S-IgA)-mediated protection against dental caries has been focused on interference with S. mutans adherence and inhibition of virulence factors involved in colonization. S. mutans cell surface protein antigen I/II (AgI/II) and glucosyltransferases (GTFs) (EC 2.4.1.5) are ideal candidates for a dental caries vaccine because of their essential role in bacterial adherence and vulnerability to blocking by S-IgA in saliva (9, 25). An ideal approach to a dental caries vaccine is to develop subunit vaccines that induce S-IgA against protective epitopes in AgI/II or GTFs. Protective epitopes are those molecular domains associated with adhesion and colonization and which are accessible to antibody. A subunit vaccine also precludes the induction of antibodies to irrelevant or unwanted epitopes. Toward this goal, protective B-cell and dominant T-cell epitopes of AgI/II have been mapped in selected human populations (16, 19). However, analogous studies for GTFs are limited.
GTFs are enzymes responsible for the synthesis from sucrose of water-soluble and insoluble glucose polymers (glucans). Glucans, along with the GTFs, enhance the colonization of tooth surfaces and formation of biofilm by the cariogenic microflora (12). S. mutans expresses three GTFs (1, 13, 14) with distinct functions and localizations. GtfB and GtfC are cell wall associated (7) and synthesize primarily insoluble glucan, whereas GtfD is secreted and synthesizes water-soluble glucan. GTFs from either S. mutans or S. sobrinus were able to elicit protective immunity against experimental dental caries caused by implanted mutans streptococcal species (29). In human trials, oral and/or local administration of GTFs could induce salivary IgA antibody responses, which correlated with interference with reaccumulation of indigenous mutans streptococci (24, 27). In these studies, anti-GTF antibody-mediated protection was related to functional inhibition of the enzymatic activities of GTFs through antibody binding. Concomitantly, three peptides of around 20 amino acid residues, CAT, GLU, and Gtf-P1, derived from N- and C-terminal regions corresponding to either the putative catalytic or glucan-binding domains of GTFs, were demonstrated to induce protective immunity against experimental dental caries in a rat model (23, 26, 28). The immunogenicity of GLU was poor in people from the United States, since fewer than 15% of parotid saliva samples which were tested had naturally induced IgA antibody activity against GLU (26). Interestingly, naturally induced salivary IgA antibodies in Chinese individuals readily recognized Gtf-P1. In addition, anti-Gtf-P1 IgA levels correlated with disease activity, suggesting a protective role (3). However, the immunogenicity of these peptides in humans is still not clear.
Several interesting observations were made for the naturally occurring serum and salivary antibody responses to GTFs in human populations (3). First, young adults were found to exhibit differential salivary or serum antibodies against GTFs, with significantly higher antibody levels to GtfD than to GtfB or -C. Second, positive correlations were found between the history of dental caries and the levels of the salivary anti-GtfB and -C and anti-Gtf-P1, but not anti-GtfD, antibodies. These findings suggested that S. mutans, in order to evade immune surveillance, may direct host antibody responses to irrelevant antigenic determinants. The finding that S. mutans, along with other, noncariogenic streptococci, colonizes the oral cavity despite a readily detectable systemic and mucosal antibody response supports this hypothesis (6). As an initial step to test the hypothesis that the various GTFs of S. mutans may direct differential responses at the cellular level as well, we analyzed the T-cell proliferative responses to different GTFs from naturally sensitized humans of different age groups.
The volunteers who participated in the present study were 30 healthy students, 20 to 22 years of age, from National Taiwan University and 6 children, 6 to 8 years of age, from the Pediatric Department of National Taiwan University Hospital. Umbilical blood was collected routinely from the Gynecology Department of National Taiwan University Hospital. The statement on the informed consent form for use of human sera and umbilical blood samples followed the regulation of the university hospital committee. Unstimulated whole saliva was collected from students by direct expectoration into sterile 15-ml containers. The saliva samples were clarified by centrifugation at 6,500 × g for 30 min; sediments were discarded, and aliquots of clarified saliva were stored frozen at −70°C until use for enzyme-linked immunosorbent assay or Western blot analysis, as described previously (3). Total IgA concentrations in the saliva samples were measured by nephrometry (Behring, Marburg, Germany). Genetic typing of HLA-DRB1 of the volunteers was conducted by PCR and sequence-specific oligonucleotide probe (SSOP) hybridization (21). In brief, HLA-generic and HLA-DRB1-specific PCRs were carried out to amplify the second exons of generic DRB and HLA-DRB1 genes. The PCR product was denatured with NaOH-EDTA and dotted onto nylon membranes (Pall, Glen Cove, N.Y.). Panels of 5′-biotinylated SSOPs were used to characterize the polymorphic regions in the exon. The membrane was hybridized with the 5′-biotinylated SSOPs in the presence of streptavidin-peroxidase and subsequently subjected to stringent washing. The hybridization signal was viewed by chemiluminence generated by the ECL gene detection system (Amersham). Using DRB-generic and DRB1-specific PCR together with 25 SSOPs, more than 90% of HLA-DRB1 alleles present in the Taiwanese population could be determined (15). Subspecificities of HLA-DR2 (DRw15 and DRw16), -DR7, and -DR9 types were not characterized further.
Recombinant GtfC and GtfD expressed in Escherichia coli were purified by chromatography on an Ni2+ affinity resin. The gtfC coding sequence in pNH3 (13) was digested with PshAI and inserted into the NheI-HindIII sites of plasmid pRSETA (Invitrogen, Carlsbad, Calif.). The resulting plasmid, pRSETAgtfC, expressing gtfC with a deletion of its signal sequence (amino acids 1 to 43) and an N-terminal 6-His tag, was introduced into E. coli BL21(DE3) (Novagen Inc., Madison, Wis.), which contains the T7 polymerase gene on the chromosome under the control of the lacUV5 promoter. Plasmid pYND72 (22), expressing gtfD under the control of the lac promoter, was altered to introduce a 7-His tag. Cloning of the His tag into PYND72 was carried out by insertion of a DNA fragment encoding nine amino acid residues into the SalI site in gtfD. The inserted DNA sequence was constructed by annealing two oligonucleotides, 5′-TCGAGGCATCATCATCATCATCATCAT-3′ and 5′-TCGAATGATGATGATGATGATGATGCC-3′, which are complementary and encode seven His residues. The resulting plasmid, which expresses gtfD with a seven-His tag immediately C terminal to the putative signal sequence (amino acids 1 to 29), was termed pYND72-His. E. coli harboring pRSETAgtfC or pYND72-His was grown to an A550 of 0.4 to 0.5, and the T7 or lac promoter was induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 2.0 mM. The cultures were grown for an additional 4 h and then harvested. The pellets were resuspended in binding buffer (5 mM imidazole, 0.5 M NaCl, 20 mM Tris-HCl [pH 7.9]), and a cell lysate was prepared by disrupting the cells with sonication. The cell debris was removed by centrifugation at 15,000 × g for 30 min. Further steps in the purification of His-GtfC and -GtfD were performed according to the pET instruction manual provided by the manufacturers. Homogeneity of the purified proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by silver staining or activity staining using periodic acid-Schiff reagent (31). The bands were analyzed with an Electrophoresis Documentation and Analysis System 120 (Scientific Imaging Systems, Eastman Kodak Co., Rochester, N.Y.). Protein concentrations were determined using a modification of the method of Lowry et al. (18), with bicinchoninic acid as the colorimetric detection reagent (BCA Protein Assay Reagent; Pierce). GTF activity was determined by the [14C]glucose-sucrose (New England Nuclear Corp., Boston, Mass.) incorporation assay as described previously (5). After purification, the purity of the GtfD was found to be 98.5%. However, for GtfC, a degradation product of lower molecular weight was consistently found immediately after elution. Only minor bands from the E. coli host were observed in the final purified GtfC and GtfD, following prolonged exposure of sodium dodecyl sulfate-polyacrylamide gels to silver staining reagent. The authenticity of GtfC and -D was confirmed by Western blot analysis using PJS-2 and PJS-3 antibodies (2), and their biological activities were detectable by activity gel staining.
S. mutans MT-8148 was grown in brain heart infusion broth (Difco Laboratories, Detroit, Mich.). The extracellular protein antigens (EXP-A) and cell wall-associated protein antigens (CWP-A) were prepared as described previously (4). Phytohemagglutinin (PHA) and staphylococcal enterotoxin B (SEB) were purchased from Sigma Chemical Co. (St. Louis, Mo.). Glutaraldehyde-inactivated tetanus toxoid (TT) was provided by Ming-Yi Liau of the Department of Health, Center for Disease Control, Vaccine Center, Taiwan, Republic of China. Monoclonal antibody (MAb) MCA477 for HLA-DR DP DQ blocking was purchased from Serotec Ltd. (Oxford, United Kingdom). Mononuclear cells or enriched T cells were analyzed using a FACScan (Becton Dickinson, San Jose, Calif.), and data analysis was performed using the LYSYS II software program. All antigens, including purified GtfC and -D and reagents used for proliferation assays, exhibited undetectable endotoxin levels (<30 pg/ml) as determined by the Limulus amebocyte lysate assay (Sigma).
Mononuclear cells were isolated from peripheral blood or umbilical blood specimens from healthy children or adult volunteers by Ficoll-Hypaque centrifugation. Irradiated human peripheral blood mononuclear cells (PBMC) were used as accessory cells. Suspensions (2 × 105 cells per 50 μl) of PBMC in RPMI 1640 medium (Gibco BRL Laboratories, Grand Island, N.Y), supplemented with 10% fetal calf serum (Gibco BRL), (complete RPMI medium) were irradiated at 4,500 rads with an X-ray irradiator (Hitachi Medical Co., Tokyo, Japan) to inhibit proliferation and used as accessory cells in T-cell proliferation assays. For enrichment of T cells, human PBMC or mononuclear suspensions in complete RPMI 1640 medium were passed through a nylon wool column to deplete B cells and macrophages. Alternatively, T cells were enriched directly from whole blood by antibody-mediated separation with RosetteSep (StemCell Technologies Inc., Vancouver, Canada). The enriched T-cell fractions were collected and used in the proliferation assays.
PBMC or mononuclear cells were washed and resuspended in AIM-V (Gibco BRL) supplemented with 2 mM l-glutamine, penicillin (100 μg/ml), streptomycin sulfate (100 μg/ml), and 2% serum replacement TCH (Celox, St. Paul, Mich.). PBMC (2 × 105 cells per well) were cultured in 96-well round-bottomed plates (Costar, Cambridge, Mass.) in a total volume of 200 μl. Purified T cells (1 × 105 cells per well) were cultured in the presence of irradiated autologous PBMC (2 × 105 cells per well) in RPMI 1640 supplemented with 2% fetal calf serum, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, penicillin (100 μg/ml), streptomycin sulfate (100 μg/ml), and 2% TCH (Celox). Three replicates of each culture were incubated with various concentrations (1 to 40 μg/ml) of recombinant GtfC and -D or crude extracts of CWP and EXP or were unsupplemented controls. Incubation was at 37°C in a humidified atmosphere with 5% CO2 for 4 days. Each culture received 0.2 μCi (7.4 kBq) of [3H]thymidine (Amersham International, Little Chalfont, United Kingdom) 18 h before harvesting. Cultures were harvested onto 96-unifilter GF/C plates using a FilterMate cell harvester (Packard, Meriden, Conn.) and dried at 50°C for 30 min, and 30 μl of Microscint (Packard) was added per well. [3H]thymidine incorporation was measured with a Packard microplate scintillation counter. Proliferation was expressed as the stimulation index (SI), which was calculated as the mean counts per minute of antigen-stimulated cultures divided by the counts per minute of antigen-free cultures. PHA (10 μg/ml), SEB (0.01 to 20 ng/ml), and TT (5 to 20 μg/ml) were used with every culture as positive controls. The major histocompatibility complex (MHC) dependency of PBMC proliferative responses to GtfC and -D were determined by culturing lymphocytes with antigens, as described above, in the presence of MAb MCA477, an anti-MHC class II MAb. Cultures were incubated with [3H]thymidine and harvested, and [3H]thymidine uptake was determined as described above.
Saliva and plasma samples from 23 volunteers were initially analyzed by Western blotting using purified GtfC and -D. The majority of the samples exhibited significantly (two- to threefold) higher levels of salivary IgA and serum IgG antibodies to S. mutans GtfD than to GtfC. The differential antibody responses to the S. mutans GTFs were more pronounced in the saliva IgA. These results confirmed our earlier findings that salivary and serum antibody responses to the S. mutans GTFs are different, although the proteins share extensive homology in their primary amino acid sequences.
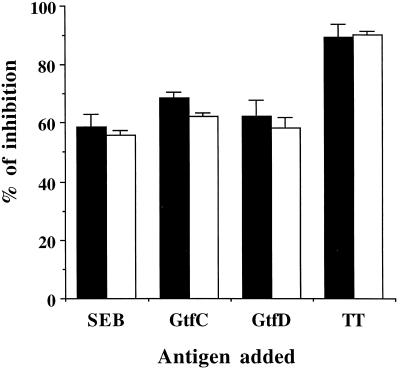
To test the hypothesis that individual cellular immune responses to GTFs may occur, PBMC from 22 adults and 6 children were examined following stimulation in vitro with purified GtfC and -D. PBMC from either adults or children responded well to the GtfC and -D and TT, with an SI significantly higher than that following stimulation by CWP-A or EXP-A (Table 1). In parallel to the antibody responses, all individuals exhibited significantly higher responses to GtfD than to GtfC at doses of 5 to 20 μg/ml, with peak responses generally at 20 μg/ml (data not shown). Although considerable variation in SI was observed with the different antigens, on the whole, GtfD stimulated PBMC proliferation about two-and-a-half-fold higher than GtfC (P < 0.01) in children and adults. Although the recombinant GtfC and -D preparations included traces of components derived from E. coli, which may themselves induce proliferative responses as indicated by the stimulation of cord blood mononuclear cells (Table 1), no differential stimulatory effects were observed for GtfC and GtfD. Moreover, GtfC and -D stimulated proliferation of these cells to an extent far below that of the mitogen PHA or superantigen SEB, in terms of antigen concentration or SI. These results suggested that recombinant GtfC and -D preparations stimulated proliferation of populations of cells other than T cells nonspecifically. Antigen-dependent stimulation of T cells by GtfC, GtfD, and TT was confirmed by inhibition experiments using MAb blocking of MHC class II molecules (Fig. 1). The percentages of inhibition for GtfC, GtfD, and TT were 57.9, 62.8, and 86.6, respectively. In addition, the dominant cellular response to GtfD, compared to GtfC, could be observed in individuals with distinct HLA-DR antigens. DRB1 genotypes in the tested individuals are randomly distributed.
TABLE 1.
Mononuclear cell response to bacterial antigensa
| Antigen (concn, μg/ml) | Proliferative response (mean SI ± SD)
|
||
|---|---|---|---|
| Mononuclear cells from cord blood (n = 6) | PBMC from:
|
||
| Children (n = 6) | Adults (n = 22) | ||
| GtfC (20) | 13.07 ± 7.38 | 8.08 ± 2.26 | 8.61 ± 10.73 |
| GtfD (20) | 11.78 ± 7.13 | 18.64 ± 6.18b | 18.55 ± 15.78b |
| CWP-A (20) | 7.12 ± 5.48 | 4.12 ± 3.4 | 3.73 ± 3.01 |
| EXP-A (20) | 2.45 ± 1.03c | 2.71 ± 2.49c | 2.68 ± 1.71c |
| TT (10) | 0.98 ± 0.72 | 6.64 ± 3.82 | 8.79 ± 5.88 |
| SEB (0.01) | 35.5 ± 5.20 | NDd | 35.5 ± 8.92 |
Positive responses of different groups to the recombinant GtfC and -D and other antigens were those with SIs of greater than the mean plus two standard deviations of the response to the control protein TT in the cord blood group. With medium only, the response was 678 ± 85 cpm. PHA (10 μg/ml) stimulated PBMC efficiently, with a mean SI ranging from 170 to 180 in the three different groups. Control eluents from E. coli harboring a nonrecombinant plasmid exhibited nondetectable stimulation in all tested groups.
P < 0.01 compared to the value for GtfC by student's t test.
no response.
ND, not determined.
FIG. 1.
Inhibition of proliferation by anti-MHC class II antibody. Mononuclear cells from cord blood (□) or adult PBMC (■) were preincubated with MAb MCA477, and assays of proliferation to different antigens were carried out. Inhibition was expressed as the SI for treated cells compared to that for untreated cells, which was normalized to 100%. Each bar represents the mean and standard deviation from three assays.
To further confirm the differential cellular responses stimulated by GtfD versus GtfC, proliferation experiments were carried out using enriched T cells from adults. As a control, T cells were enriched from cord blood samples. Analysis of the homogeneity of the enriched populations using cell sorting indicated that total T cells, enriched by nylon column or antibody depletion methods, are 83 and 93% pure, respectively. T cells enriched from cord blood exhibited negligible responses to either GtfD or GtfC (Table 2). These results confirmed our previous observation that the stimulation of cord blood mononuclear cells by GtfC and -D (Table 1) was due to proliferation of cells other than T cells, and, accordingly, such proliferative responses could not be blocked completely by anti-MHC class II antibodies. On the other hand, analogous to the responses of PBMC, enriched T cells responded better to GtfD than to GtfC. The intensity of T-cell proliferative responses to GtfD was comparable to that with TT but was far less than that with SEB (Table 2). The fold increase in T-cell proliferation induced by GtfD versus GtfC exhibited a positive correlation with the IgA or IgG levels quantitated on the Western blots (r = 0.85). These results confirmed our hypothesis that cellular responses to GTF molecules may differ in a manner similar to that of levels of saliva and serum antibody GtfD or -C.
TABLE 2.
T-cell response to bacterial antigensa
| Antigen (concn, μg/ml) | T-cell response (mean SI ± SD)
|
|
|---|---|---|
| Cord blood (n = 4) | Adult (n = 8) | |
| GtfC (10) | NDb | 3.63 ± 2.3 |
| GtfC (20) | 1.7 ± 0.38 | 5.25 ± 3.3 |
| GtfD (1) | ND | 3.51 ± 1.5 |
| GtfD (10) | 1.8 ± 0.4 | 12.51 ± 7.6c |
| CWP-A (20) | 1.24 ± 0.09 | 1.20 ± 1.35d |
| TT (10) | 1.02 ± 0.12 | 11.02 ± 0.23 |
| SEB (0.01) | 36.8 ± 4.12 | 43.5 ± 6.8 |
Positive responses in T cells from the adult group to different antigens were those with. SIs of greater than mean plus two standard deviations of the response to the same protein in the cord blood group. With irradiated PBMC only, the response was 108 ± 25 cpm.
ND, not determined
P < 0.01 compared to the value for GtfC at 10 μg/ml by Student's t test.
No response.
The proliferative responses of either PBMC or enriched T-cell populations were primary responses without prior antigen feeding or stimulation during culture. In addition, all tested individuals, regardless of age group, responded well to GtfD, with an SI comparable to that of the potent immunogen TT. These results confirmed that, in naturally sensitized humans, differential cellular immune responses to S. mutans GTFs exist in an antigen-specific manner at the T-cell level, with a stronger response to the secreted form of GtfD than to the cell wall-associated form of GtfC. Recombinant, His-tagged GtfC and -D both were biologically active. Thirty-four of the 42 amino acids deleted from the N terminus of GtfC are predicted to comprise a signal sequence, and it is unlikely, therefore, that differences in cellular immune responses to GtfC and -D resulted from truncation of the N terminus of GtfC. Genetic and biochemical analyses revealed that GtfB and GtfC share an overall 79% identity in amino acid residues and about 58% homology with GtfD. Considering the linear nature of T-cell epitopes, we are currently investigated the possibility of identifying major T-cell epitopes, which are recognized by most of the population, by constructing truncated GtfD fragments.
Although cell wall polysaccharides from S. mutans showed mitogenic activity on B cells (11), protein antigens of cell wall-associated or secreted forms does not contain mitogenic or superantigenic integredients, as indicated by the proliferative responses of either PBMC or T cells to CWP-A and EXP-A. By analogy, GtfB and -C do not possess superantigenic effects, as confirmed by the lack of response in the T cells from cord blood samples (Table 2). Therefore, the nonspecific stimulation by either GtfC or GtfD observed on the PBMC was probably due to minor contaminating fractions from the E. coli host, although the final preparation revealed that lipopolysaccharide integredients were undetectable. This nonspecific stimulation was class II MHC independent and might result from cell populations other than T cells, as suggested by the anti-MHC antibody blocking experiments and the nonresponsiveness of the T cells from cord blood samples (Table 2). Therefore, the primary responses measured with the enriched T-cell samples were antigen specific.
An interesting question is why GTFs should elicit differential antibody and cellular immune responses. It has been demonstrated previously that GtfB and -C are more important, structurally and functionally, than is GtfD in establishing bacterial adherence to smooth surfaces (14). Additional studies from two independent groups also reported that GtfC is the molecule essential for adherence and colonization (8, 30). We have shown previously that levels of salivary antibody to GtfC were significantly higher in caries-free than in caries-active young adults. This suggests that GtfC may serve as a protective antigen, although the responses in saliva and serum induced by this protein are lower than those induced by GtfD. Results from the present study confirmed that GtfC and -D stimulate different T-cell responses. Taking the results together, one possible speculation might be that S. mutans directs antibody and cellular immune responses away from molecules essential for adherence and colonization to evade immune surveillance. In other words, extracellular secretion of GtfD may provide a decoy antigen to neutralize circulating IgA or IgG, preventing reaction with cell wall-associated GtfC and evading antibody clearance. However, it should be pointed out that despite the higher response to GtfD, there is a robust response to GtfC as well.
Acknowledgments
We thank H. K. Kuramitsu for providing plasmids pNH3 and pYND72, Wen-Main Wu for technical assistance, and Ping-Ning Hsu for helpful discussion. We thank Ming-Yi Liau for providing TT. We thank Tim J. Harrison, Royal Free and University College Medical School, for his kind review and help in the preparation of the manuscript.
This work was supported in part by the National Science Council (grant NSC-892314-B002-184) and National Health Research Institute (grant NHRI-GT-EX89B814C).
REFERENCES
- 1.Aoki H, Shiroza T, Hayakawa M, Sato S, Kuramitsu H K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986;53:587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chia J S, Lin S W, Hsu T Y, Chen J Y, Kwan H W, Yang C S. Analysis of a DNA polymorphic region in the gtfB and gtfC genes of Streptococcus mutans strains. Infect Immun. 1993;61:1563–1566. doi: 10.1128/iai.61.4.1563-1566.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chia J S, Lin S W, Yang C S, Chen J Y. Antigenicity of a synthetic peptide from glucosyltransferase of Streptococcus mutans in human. Infect Immun. 1997;65:1126–1130. doi: 10.1128/iai.65.3.1126-1130.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chia J S, Lin R H, Lin S W, Chen J Y, Yang C S. Inhibition of glucosyltransferase activities of Streptococcus mutans by a monoclonal antibody to a subsequence peptide. Infect Immun. 1993;61:4689–4695. doi: 10.1128/iai.61.11.4689-4695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chia J S, Yang C S, Chen J Y. Functional analysis of a conserved region in glucosyltransferases from Streptococcus mutans. Infect Immun. 1998;65:1126–1130. doi: 10.1128/iai.66.10.4797-4803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole M F, Bryan S, Evans M K, Pearce C L, Sheridan M J, Sura P A, Wientzen R L, Bowden G H. Humoral immunity to commensal oral bacteria in human infants: salivary secretory immunoglobulin A antibodies reactive with Streptococcus mitis biovar 1, Streptococcus oralis, Streptococcus mutans, and Enterococcus faecalis during the first two years of life. Infect Immun. 1999;67:1878–86. doi: 10.1128/iai.67.4.1878-1886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara T, Kawabata S, Hamada S. Molecular characterization and expression of the cell-associated glycosyltransferase gene from Streptococcus mutans. Biochem Biophys Res Commun. 1992;187:1432–1438. doi: 10.1016/0006-291x(92)90462-t. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara T, Tamesada M, Bian Z, Kawabata S, Kimura S, Hamada S. Deletion and reintroduction of glycosyltransferase gene of Streptococcus mutans and role of their gene products in sucrose dependent cellular adherence. Microb Pathog. 1992;20:225–233. doi: 10.1006/mpat.1996.0021. [DOI] [PubMed] [Google Scholar]
- 9.Hajishengallis G, Nikolova E, Russell M W. Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyapatite by human secretory immunoglobulin A (IgA) antibodies to the cell surface protein antigen I/II: reversal by IgA protease cleavage. Infect Immun. 1992;60:5057–5064. doi: 10.1128/iai.60.12.5057-5064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajishengallis G, Michalek S M. Current status of a mucosal vaccine against dental caries. Oral Microbiol Immunol. 1999;14:1–20. doi: 10.1034/j.1399-302x.1999.140101.x. [DOI] [PubMed] [Google Scholar]
- 11.Hamada S, McGhee J R, Kiyono H, Torii M, Michalek S M. Lymphoid cell responses to bacterial cell wall components: mitogenic responses of murine B cells to Streptococcus mutans carbohydrate antigens. J Immunol. 1981;126:2279–2283. [PubMed] [Google Scholar]
- 12.Hamada S, Slade H D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanada N, Kuramitsu H K. Isolation and characterization of the Streptococcus mutans gtfC gene, coding for synthesis of both soluble and insoluble glucans. Infect Immun. 1988;56:1999–2005. doi: 10.1128/iai.56.8.1999-2005.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanada N, Kuramitsu H K. Isolation and characterization of the Streptococcus mutans gtfD gene, coding for primer-dependent soluble glucan synthesis. Infect Immun. 1989;57:2079–2085. doi: 10.1128/iai.57.7.2079-2085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu C Y, Allen M, Chuang L M, Lin B J, Gyllensten U. Association of insulin-dependent diabetes mellitus in Taiwan with HLA DQB1 and DRB1 alleles. Hum Immunol. 1993;38:105–114. doi: 10.1016/0198-8859(93)90526-7. [DOI] [PubMed] [Google Scholar]
- 16.Kelly C, Todryk S, Kendal H L, Munro G H, Lehner T. T-cell adhesion and B-cell epitopes of the cell surface Streptococcus mutans protein antigen I/II. Infect Immun. 1995;63:3649–3658. doi: 10.1128/iai.63.9.3649-3658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Matsushita K, Nisizawa T, Nagaoka S, Kawagoe M, Koga T. Identification of antigenic epitopes in a surface protein antigen of Streptococcus mutans in humans. Infect Immun. 1994;62:4034–4042. doi: 10.1128/iai.62.9.4034-4042.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell M W, Hajishengallis G, Childers N K, Michalek S M. Secretory immunity in defense against cariogenic mutans streptococci. Caries Res. 1999;33:4–15. doi: 10.1159/000016490. [DOI] [PubMed] [Google Scholar]
- 21.Scharf J S, Griffith R L, Erlich H A. Rapid typing of DNA sequence polymorphism at the HLA-DRB1 locus using the polymerase chain reaction and non-radio-active oligonucleotide probes. Hum Immunol. 1991;30:190–201. doi: 10.1016/0198-8859(91)90034-7. [DOI] [PubMed] [Google Scholar]
- 22.Shimamura A, Nakano Y J, Mukasa H, Kuramitsu H K. Identification of amino acid residues in Streptococcus mutans glucosyltransferase influencing the structure of the glucan product. J Bacteriol. 1994;176:4845–4850. doi: 10.1128/jb.176.16.4845-4850.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith D J, Shoushtari B, Heschel R L, King W F, Taubman M A. Immunogenicity and protective immunity induced by synthetic peptides associated with a catalytic subdomain of mutans group streptococcal glucosyltransferase. Infect Immun. 1997;65:4424–4430. doi: 10.1128/iai.65.11.4424-4430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith D J, Taubman M A. Oral immunization of humans with Streptococcus sobrinus glucosyltransferase. Infect Immun. 1987;55:2562–2569. doi: 10.1128/iai.55.11.2562-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith D J, Taubman M A, Ebersole J L. Salivary IgA antibody to glucosyltransferase in man. Clin Exp Immunol. 1985;61:416–424. [PMC free article] [PubMed] [Google Scholar]
- 26.Smith D J, Taubman M A, Holmberg C F, Eastcott J, King W F, Ali-Salaam P. Antigenicity and immunogenicity of a synthetic peptide derived from a glucan-binding domain of mutans streptococcal glucosyltransferase. Infect Immun. 1993;61:2899–2905. doi: 10.1128/iai.61.7.2899-2905.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith D J, Taubman M A, King W F. Effect of local deposition of antigen on salivary immune responses and reaccumulation of mutans streptococci. J Clin Immunol. 1990;10:273–281. doi: 10.1007/BF00916703. [DOI] [PubMed] [Google Scholar]
- 28.Smith D J, Taubman M A, King W F, Eida S, Powell J R, Eastcott J. Immunological characteristics of a synthetic peptide associated with a catalytic domain of mutans streptococcal glucosyltransferase. Infect Immun. 1994;62:5470–5476. doi: 10.1128/iai.62.12.5470-5476.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taubman M A, Smith D J. Effects of local immunization with glucosyltransferase from Streptococcus mutans on experimental dental caries. J Immunol. 1977;118:710–720. [PubMed] [Google Scholar]
- 30.Tsumori H, Kuramitsu H. The role of the Streptococcus mutans glucosyltransferase in the sucrose-dependent attachment to smooth surfaces: essential role of the GtfC enzyme. Oral Microbiol Immunol. 1997;12:274–280. doi: 10.1111/j.1399-302x.1997.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 31.Zacharius R M, Zell T E, Morrison J H, Woodlock J J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969;30:148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]