Abstract
Background
This paper reports results from the 5th International Conference “Controversies in Vitamin D” that was held in Stresa, Italy, 15–18 September 2021. The conference is part of this series that started in 2017 and has been conducted annually since. The objective of these conferences is to identify timely and controversial topics related to Vitamin D. Dissemination of the results of the conference through publications in peer-reviewed journals is an important means by which the most up to date information can be shared with physicians, investigators, and other health care professionals. Vitamin D and aging, the subject of this paper was featured at the conference.
Methods
Participants were selected to review available literature on assigned topics related to vitamin D and aging and to present their findings with illustrative material, the intent of which was to stimulate discussion and to arrive at a consensus. The presentations were directed towards the following areas: impact of aging on vitamin D production and levels; skeletal effects of vitamin D deficiency in the older population; falls and vitamin D in the aging; potential extra skeletal effects of vitamin D; and strategies to prevent vitamin D deficiency. A final topic was related to how vitamin D might influence the efficacy of vaccines for Covid-19.
Results
Hypovitaminosis D can lead to several skeletal and extra-skeletal outcomes. Older adults are at risk for vitamin D deficiency as both production and metabolism of vitamin D change with aging due to factors, such as reduced sun exposure and reduced production capacity of the skin. Skeletal consequences of these age-related changes can include reduced bone mineral density, osteomalacia and fractures. Potential extra-skeletal effects can include added risks for falls, reduced muscle strength, diabetes, cancer, and cardiovascular disease. Strategies to avoid these vitamin D deficiency-related negative outcomes include sun exposure, food fortification, and supplementation. While aging does not diminish sufficient reserve capacity for cutaneous vitamin D production, concerns about skin cancers and practical matters for the institutionalized elderly limit this option. Supplementation with vitamin D is the best option either pharmacologically or through food fortification. Regardless of treatment strategies, interventions to restore sufficient vitamin D status will show positive results only in those who are truly deficient. Thus, treatment goals should focus on avoiding 25(OH)D serum levels <30 nmol/l, with a goal to reach levels >50 nmol/l.
Conclusions
The results of this conference has led to consensus on several issues. Vitamin D supplementation should be combined with calcium to reduce fractures in the older population. The goal for adequate Vitamin D status should be to reach a serum level of 25(OH)D >50 nmol/l. It appears that daily low-dose vitamin D regimens reduce the risk of falling, especially in the elderly, compared with infrequent, large bolus doses that may increase it. The role of Vitamin D supplementation on muscle strength remains to be clarified. On the other hand, supplementation decreases the risk of progression to T2D from prediabetes among those who are Vitamin Ddeficient. Of three possible strategies to establish vitamin D sufficiency – sunshine exposure, food fortification, and supplementation – the latter seems to be the most effective and practical in the aging population.
Keywords: Vitamin D, Aging, Older people, Vitamin D deficiency, Vitamin D supplementation
Introduction
The 5th International Conference “Controversies in Vitamin D” was held in Stresa, Italy, 15–18 September 2021 as part of this series that started in 2017 [1–7]. The objective of this conference, which featured international experts and leaders, was to review and discuss controversial topics regarding vitamin D. Four sessions addressed different major aspects of vitamin D: aging, gastrointestinal system, guidelines, and COVID-19. Before the event, participants reviewed the available literature on their assigned topic and presented their findings at the time of the conference. After each presentation, open sessions enabled full discussion to reach a consensus. A separate document was prepared for each component of the conference.
This paper summarizes the deliberations of the experts on aging and Vitamin D. Regarding the literature review, there were no limitations in the types of articles that were included. Randomized clinical trials were given preference; when these were not available, observational, experimental, or opinion studies were also considered. Finally, although it is rather difficult to define the terms “older population” or “older subjects” as age cut-offs vary within the studies and can range from >65 years to >75 years, in general many studies define it as >65 years.
Impact of age on vitamin D production and levels
Both the production and metabolism of vitamin D change with aging. Causes include decreased sun exposure and reduced capacity of the skin to produce vitamin D. In the skin, 7-dehydrocholesterol is converted to previtamin D3 by the sun’s ultraviolet (UV-B) light with a wavelength of 290–315 nm. As demonstrated in skin samples, the concentration of 7-dehydrocholesterol decreases by more than 50% from ages 20 to 80 years. Moreover, isolated, aged skin produces approximately 40% less vitamin D than younger skin [8, 9]. A more recent study by Chalcraft and colleagues calculated an age-related reduction in vitamin D production of 13% per decade, demonstrating production at 70 years to be half of that at 20 years (Fig. 1) [10].
Fig. 1.
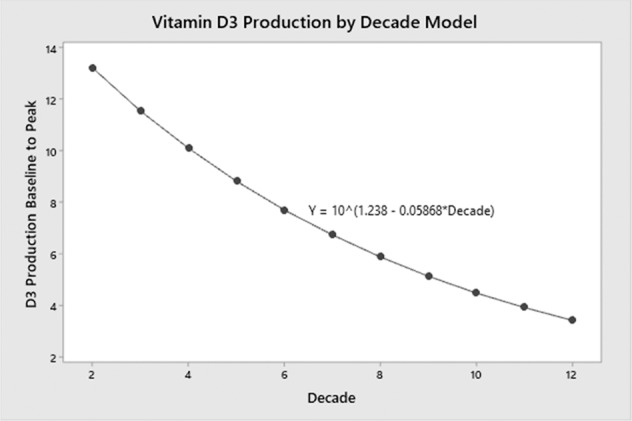
Vitamin D3 production age continuum modeling. The simple linear regression model with decade as the independent variable and log D3 production demonstrated the 13% decrease in D3 production per decade of life. D3 production at age 70 years is approximately half that produced at age 20 years. The graph also demonstrates that D3 production occurs even in the later decades of life. Source: Chalcraft et al. 2020 [10]. Reproduced from MDPI under an open access Creative Common CC BY license
These issues are even more apparent in the housebound elderly and nursing home residents [11]. Additional factors accounting for the risk of vitamin D deficiency in the older population relate to relative vitamin D resistance to stimulating calcium absorption in the gastrointestinal tract and age-related renal function reduction. The aging kidney is also less able to produce 1,25-dihydroxyvitamin D from 25-hydroxyvitamin D (25[OH]D) [12].
Despite issues related to aging, a single 15-minute sun exposure (>40% body area) results in considerable vitamin D production in the skin, not only in younger volunteers but also in older ones [10]. The authors concluded that age accounts only for 20% of the variation in D3 production. Thus, while aging may reduce cutaneous synthesis, sunlight exposure is still a significant source of vitamin D3 [10]. Other studies in nursing home residents support this conclusion. It has been demonstrated that irradiation with artificial UV light with half the minimal erythematous dose three-times per week on a surface of 1000 cm2 of the back increased mean serum 25(OH)D from 25 nmol/l to 60 nmol/l within 3 months, similar to daily oral vitamin D3 400 IU supplementation [13]. This observation suggests that under those conditions, 1000 cm2 exposure of skin every day may result in an increase of serum 25(OH)D comparable to daily supplementation of about 800 IU of vitamin D, such as daily recommendations for those >70 years. As the skin surface amounts to more than 15,000 cm2, the theoretical amount of vitamin D that could be produced in the skin is potentially high. For example, in another study from the same center, eight psychogeriatric vitamin D-deficient patients underwent half-body UV irradiation once a week with half of the minimal erythematous dose (2 min) for 8 weeks. The median serum 25(OH)D increased from 26.5 nmol/l (range 12–58) at baseline to 43.5 nmol/l (range 36–71) [14]. Thus, UV exposure effectively triggers cutaneous vitamin D synthesis even in older patients.
The potential of vitamin D production in the skin depends, of course, on a series of well-known factors that can facilitate or mitigate this process. Season, time of the day, latitude, altitude, cloudiness, air pollution [15], skin type, clothing, sunscreen [16], and lifestyle can all influence the ability of the sun’s UV-B energy to stimulate skin synthesis of vitamin D.
Beyond the skin, other factors influence vitamin D levels in the aging population. Smoking, for example, may decrease serum 25(OH)D concentrations [17], although the mechanism is unknown. A higher percentage of total body fat also results in lower circulating 25(OH)D levels. Fat content persists as a variable even after adjustment for age, season, and smoking in men and women. The two mechanisms appear to be reduced production and higher distribution volume of vitamin D [18]. As the aging population is experiencing a worldwide increase in BMI, especially in rural areas [19], obesity looms large as an increasingly important factor to account for reduced levels of 25(OH)D. Along with the increase in BMI, trends from the Longitudinal Aging Study Amsterdam (LASA) also show a secular decrease in physical activity in men and women, which may be another factor [20]. Indeed, physical activity is positively related to serum 25(OH)D levels [21, 22]. With regard to gender, it is well known that vitamin D deficiency is more prevalent in men than in women in the general population. This is true also in older populations [23–25], although the difference between older men and women seems to become of smaller magnitude [23].
In summary, despite the reduction in vitamin D production with aging, cutaneous reserve capacity for production should be ample. It is, therefore, possible that most older people can produce sufficient vitamin D from the sun. Nonetheless, meeting vitamin D requirements for housebound and institutionalized people almost always requires vitamin D supplementation. Factors that might restrict the availability of vitamin D should be avoided, particularly in older individuals.
Skeletal effects of vitamin D deficiency in the older population
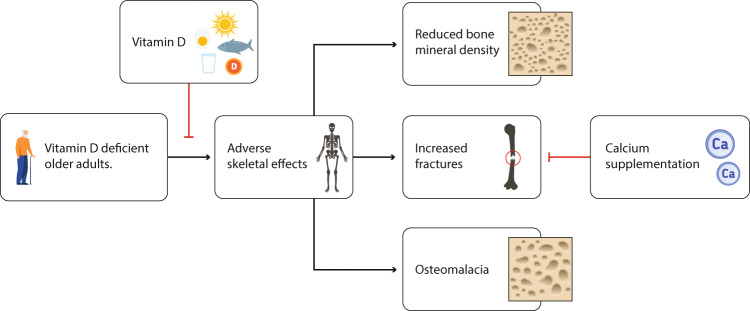
While it is undisputed that severe vitamin D deficiency has adverse skeletal effects (Fig. 2) [4], including osteomalacia, high bone turnover and bone loss, and an increased risk of hip fractures in the elderly, skeletal effects of milder degrees of vitamin D deficiency have recently come under question. Milder degrees of vitamin D deficiency contribute to the development of osteoporosis, but supplementation with vitamin D alone does not appear to reduce fractures. In this regard, it is worth noting that vitamin D is a threshold nutrient. Skeletal benefits in the elderly will not likely be seen in those who are mildly vitamin D deficient and certainly not in those whose serum 25(OH)D is above the threshold value. On the other hand, in severely vitamin D-deficient individuals, the beneficial effects of vitamin D to reduce fracture risk are more likely to be appreciated. The best example of this expectation comes from the pivotal trial of Chapuy et al. [26]. Daily supplementation with vitamin D (800 IU) and calcium (1200 mg) of older, ambulatory French nursing home and apartment-dwelling women resulted in a reduction of hip and other non-vertebral fractures by 43% and 32%, respectively, over 18 months [26]. In this population, the mean baseline serum 25(OH)D concentration was only 36 nmol/L. However, tests were conducted with an old competitive protein binding assay with 80% higher values than high-performance liquid chromatography. Cross-calibration with high-performance liquid chromatography confirmed this and yielded even lower concentrations, mean value around 20 nmol/L, clearly indicating that most participants had moderate or severe vitamin D deficiency [27].
Fig. 2.
Skeletal effects of vitamin D deficiency in the elderly and role of vitamin D in preventing them
Another contributory factor that places severely vitamin D-deficient individuals at risk is vitamin D deficiency-related increases in parathyroid hormone (PTH) levels. This secondary hyperparathyroidism could contribute, at least in part, to the pathophysiology of bone loss and fracture risk among severely vitamin D-deficient individuals.
Two older studies have provided insight into a possible threshold value for 25(OH)D below which PTH levels are likely to rise. In the MORE trial [28], serum PTH was higher in two groups of women with different degrees of vitamin D deficiency (serum 25(OH)D < 25 nmol/L and 25(OH)D 25–50 nmol/L; 4.8 ± 2.2 and 4.1 ± 1.8 pmol/L, respectively) compared with women whose serum 25(OH)D was >50 nmol/L (3.5 ± 1.5 pmol/L). Both groups showed a significant decrease in serum PTH after vitamin D treatment, while serum PTH did not decrease after vitamin D treatment in the group with serum 25(OH)D > 50 nmol/L. A study based on data collected within the LASA [29] found a 25(OH)D threshold value ranging from 40 to 60 nmol/l. In another study in osteoporotic postmenopausal women [30], the threshold for a secondary increase in PTH also appeared to be at a serum 25(OH)D concentration around 50 nmol/L.
Some controversy continues to exist about the precise 25(OH)D level below which PTH levels increase. If such a threshold could be clearly identified, one might define the extent of vitamin D deficiency by the extent of the secondary hyperparathyroidism induced by vitamin D deficiency. However, this threshold may also depend on other factors, such as calcium intake and physical activity.
Bone mineral density
Attempts to define a threshold have been recently published. A sub-study of the New Zealand Vitamin D Assessment (ViDA) study of older community-dwelling men and women showed that monthly dosing of 100,000 IU vitamin D for 2 years did not prevent bone loss from the femoral neck and hip [31]. However, mean baseline serum 25(OH)D levels were 55 nmol/L, indicating a non-deficient population. A pre-planned exploratory analysis showed clinically meaningful reductions in bone loss at the spine, femoral neck and total hip, which were statistically significant at the spine and femoral neck, in participants with a baseline serum 25(OH)D < 30 nmol/L. By contrast, smaller reductions in bone loss at the total hip alone were seen in those with baseline serum 25(OH)D > 30 nmol/L. PTH levels were not measured in this study. In the entire cohort of the ViDA study, with a mean baseline serum 25(OH)D concentration of 66 nmol/L and an adequate calcium intake, vitamin D3 supplementation over 3.3 years did not reduce the incidence of fractures [32].
In the second study, the Aberdeen study [33], recruited 305 postmenopausal women in late winter and randomized them to receive vitamin D 400 IU/day or 1000 IU/day, or placebo over 1 year. A post-hoc analysis showed significant beneficial effects of vitamin D 1000 IU/day on both spine and hip BMD in those with baseline 25(OH)D ≤ 30 nmol/L, but no significant effects in those with baseline 25(OH)D above this level. These studies support the notion that adverse skeletal effects are most likely in older individuals with serum 25(OH)D levels <30 nmol/L, in whom supplementation with vitamin D would have the most definitive skeletal benefits.
Vitamin D and calcium
Dietary sources of vitamin D are scarce. Most frail older individuals following a western diet have a low dietary intake (i.e., around 150 IU) of vitamin D each day [34–36]. As noted above, they are limited in their sun exposure and capacity for cutaneous vitamin D synthesis [8–10]. They also may consume insufficient amounts of calcium-containing foods [37]. Older adults, thus, are likely to be at risk for both vitamin D deficiency and insufficient dietary calcium intake. Therefore, most older individuals should benefit from vitamin D and calcium supplementation. Meta-analyses of randomized controlled trials (RCTs) have shown that vitamin D, when combined with calcium and a compliance rate of >80%, decreases the incidence of hip fractures and other non-vertebral fractures by 16% and 14%, respectively. The effect on fractures is greater in 70- to >80-year-olds compared with 60–70-year-olds and in institutionalized rather than in the community-dwelling older population [38, 39]. A more recent meta-analysis by Yao and colleagues resulted in a similar conclusion, with vitamin D showing a 6% reduction in risk of any fracture and a 16% reduction in risk of hip fracture, but only if taken with concomitant calcium supplementation [40].
Based upon our current knowledge, there is general agreement that serum 25(OH)D < 30 nmol/L in the older population should be avoided, as skeletal effects of vitamin D deficiency, such as a decrease in BMD, secondary hyperparathyroidism, and mineralization defects (osteomalacia) appear to be most evident, and are most likely to occur, below this threshold. Treatment goals should focus on avoiding 25(OH)D serum levels <30 nmol/l, with a goal to reach levels >50 nmol/ to ensure that the adverse effects of vitamin D deficiency are avoided. To reduce fractures in the elderly, vitamin D and calcium sufficiency are necessary (Fig. 2).
Falls
Many interventional studies have addressed the relationship between vitamin D and falls, but the results are variable and inconsistent. The large D-Health trial in Australia reported that supplementation with 60,000 IU of D3 per month, when compared with placebo, had no significant effect on the risk of falling over a mean treatment period of 4.3 years in 15,416 men and women aged 60–84 years (OR 1.02; 95% CI: 0.95–1.10) [41]. It is worth noting that the intra-study mean serum 25(OH)D level in a subset of participants in the placebo group was 77.5 nmol/L, reflecting a vitamin D-sufficient state. The mean serum 25(OH)D was 114.8 nmol/L in a subset of treated participants. Among participants with a BMI < 25 kg/m2, there was a concern that vitamin D increased the risk of falling (OR 1.25; 95% CI: 1.09–1.43). This finding is consistent with an earlier observation that treatment with 60,000 IU of vitamin D3 monthly increased the risk of falling compared with treatment with 24,000 IU per month [42].
Many meta-analyses have combined trials testing: (1) different doses; (2) some testing calcium + vitamin D and others vitamin D only (vs placebo); (3) different dose schedules (including daily and bolus dosing); (4) different intervention periods ranging from a few months to several years; and (5) study populations that differ in initial vitamin D status and differ in age and degree of mobility. These variables make it difficult to determine the relationship between vitamin D status and fall risk and which population segments may benefit from supplementation. Adding to the uncertainty is the recent meta-analysis by Bislev et al., finding no significant effect of vitamin D supplementation on a series of muscle performance measures, but the study did not include falls [43].
Thus, despite conflicting findings from meta-analyses, it appears that modest doses of vitamin D (700–1000 IU per day) may reduce the risk of falling in deficient older adults [44]. In contrast, infrequent larger bolus doses may increase fall risk [41, 42, 45]. A randomized prospective, placebo-controlled multi-dose vitamin D trial in postmenopausal women with baseline 25(OH)D levels <50 nmol/L found that treatment with low doses of vitamin D decreased the risk of falling whereas doses of 4000 and 4800 IU daily increased the risk of falling [46]. Since many regions of the world have widespread vitamin D deficiency, a large proportion of the world’s population would likely benefit from daily low- to medium-dose supplementation with vitamin D. High doses, given either daily or intermittently, should be avoided.
Extra-skeletal effects
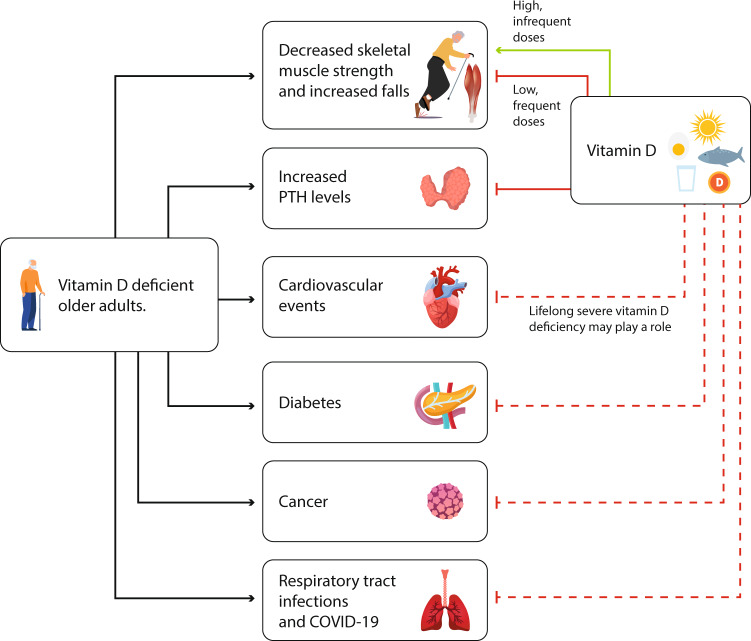
Although vitamin D is metabolized into about 50 metabolites, it is the active form, 1,25(OH)2D, that serves as the ligand for the vitamin D receptor (VDR). The VDR is expressed in most tissues and its activation results in the altered expression of thousands of genes [4]. The ubiquity of the VDR is the basis for the hypothesis that vitamin D has many extra-skeletal effects, a topic that was reviewed in several recent publications generated from previous meetings of our group (Fig. 3) [1, 3–7].
Fig. 3.
Extra skeletal effects of vitamin D deficiency in the elderly and possible role of vitamin D in preventing them. Continuous lines indicate a known effect. Dashed lines indicate a possible effect
Skeletal muscle
The relationship between skeletal muscle and vitamin D has been investigated experimentally utilizing global knock-out or muscle-specific VDR null mice. These mice develop a muscle phenotype, suggesting that the total absence of vitamin D negatively impacts skeletal muscle [47]. Previous meta-analyses suggested that vitamin D supplementation slightly improved muscle strength [48]. However, several intervention studies have been withdrawn [43]. A novel meta-analysis excluding such data and including more recent studies revealed that vitamin D supplementation did not have beneficial effects on various aspects of muscle strength and had a negative impact in some cases [43].
Muscle strength is important in the older population since it can influence falls (see above). While the relationship between falls and vitamin D deficiency has been explored above, adjunctive factors, like muscle strength, might be important. Classically, we consider vitamin D deficiency as affecting two distinct musculoskeletal pathways, one involving effects on neuromuscular tissue leading to falls and fractures and the other through decreased calcium absorption, leading to increased levels of PTH, increased bone resorption, and bone loss also leading to increased fracture risk. A related possibility is that the increased circulating PTH levels, seen in vitamin D and calcium deficiency, have a direct untoward dysfunctional effect on muscle. Preclinical and clinical evidence for a direct effect of PTH on muscle exists. Intact, bovine PTH and the synthetic 1–34 fragment of PTH increased muscle degradation and release of newly synthesized alanine and glutamine from skeletal muscle in rats [49]. Clinically, neuromuscular signs and symptoms and muscle weakness have been described in patients with advanced primary hyperparathyroidism, with muscle weakness reversing soon after successful parathyroid surgery [50, 51]. Two groups of older women with similar clinical characteristics and low 25(OH)D levels but different PTH levels (above or within range) performed differently in several muscle strength and function tests. The group with higher PTH levels had lower knee flexion strength, lower maximal muscle force production and reduced postural stability. In contrast, several other muscle strength and balance measures did not differ in the two groups [52]. One small observational study examined the role of PTH in falls in 83 nursing home residents with a mean age of 84 years, a mean 25(OH)D level of 27 nmol/L and a median PTH level of 5.2 pmol/L (reference range 1–6.5 pmol/L), during which 33 participants fell at least once [53]. Those who fell had lower 25(OH)D levels and higher PTH levels, whereas the 1,25(OH)2D levels in the two groups did not differ significantly. Logistic regression analysis indicated that the PTH level was an independent determinant of falling [53]. Sambrook et al. performed a large observational study in 637 generally vitamin D-deficient older adults, mean age 86 years, who resided in intermediate- and full-care institutions in Australia [54]. Serum 25(OH)D levels in the fallers and non-fallers were 28.8 and 33.2 nmol/L, respectively, and PTH levels 64.8 were 57.0 pg/ml, respectively. Logistic regression revealed that PTH was an independent predictor of falls.
In conclusion, a low daily dose of vitamin D will likely reduce fall risk in deficient older adults. Further examination of the role of PTH as an independent predictor of falls is warranted.
Cardiovascular events
Preclinical data link poor vitamin D status to cardiovascular risks. Experimentally, lack of VDR causes high-renin hypertension, cardiac muscle hypertrophy and fibrosis. Thrombosis is enhanced [55]. Two large RCTs (VIDA and VITAL) clearly demonstrated that vitamin D supplementation did not decrease cardiovascular events [56, 57]. A sub-study of the VIDA trial showed some minor beneficial effects on central blood pressure [58]. Mendelian randomization (MR) studies did not find a link between genetically low serum 25(OH)D concentrations and CV events, but the combined polymorphism in these studies did not permit any predictive value greater than 5% of the variation in serum 25(OH)D [59–62]. The overall results of a recent large MR study confirmed this conclusion, but by combining MR and serum 25(OH)D levels, this study showed that genetically low serum 25(OH)D in the group with severe vitamin D deficiency at the time of the study (<10 ng/ml) increased CV events and mortality [63].
Overall, it is unlikely that vitamin D status is a major contributor to the burden of CV diseases, but lifelong severe vitamin D deficiency may play a role.
Cancer
Many genes regulated by the vitamin D endocrine system are involved in regulating cell cycle control and cell differentiation. Animal and preclinical data strongly suggest that the total absence of vitamin D action predispose to cancer when combined with other carcinogenic events and a preventive effect of vitamin D supplementation early during carcinogenesis. Poor vitamin D status is associated with many cancers, especially colon, breast and prostate. Two major, large RCTs (VITAL and VIDA) did not find an effect of long-term vitamin D supplementation on cancer incidence. However, cancer-related mortality is significantly lower in patients receiving daily supplements with 2000 IU of vitamin D, as suggested by a meta-analysis of the VITAL and four other similar studies. In the D-Health study, by contrast, there was an increased risk of death from cancer in those randomized to higher dose (60,000 IU) monthly vitamin D supplementation [64]. These findings are rather surprising as the preclinical data suggested a preventive effect early during carcinogenesis.
Diabetes
Many preclinical and observational studies suggest a link between low vitamin D status and type 2 diabetes (T2D). However, in the large Vitamin D and Type 2 Diabetes Study (D2d) RCT [65], vitamin D supplementation only showed a non-significant trend to slow the progression of prediabetes into T2D (0.88 CI of 0.75–1.04; p = 0.12). On the other hand, in a D2d post-hoc analysis, a significant effect was observed in subjects with either a baseline BMI below 30, severe vitamin D deficiency at baseline, perfect compliance during the study, or achieving serum 25(OH)D above 100 nmol/L throughout the study [66]. Moreover, two other trials [67–69], specifically designed to prevent diabetes, showed that vitamin D supplementation, compared with placebo, reduced the risk of developing diabetes by 10–13% in persons with prediabetes not selected for vitamin D deficiency [70]. This is in line with two recent meta-analyses concluding that vitamin D supplementation decreased the risk of progress to T2D by about 10%, especially when using doses above 1000 IU/day and in non-obese subjects [71, 72]. Participant-level meta-analysis of these trials may better estimate risk reduction and identify patient populations likely to benefit the most from vitamin D supplementation to prevent diabetes.
Cognitive impairment
Data on cognitive impairment and vitamin D status are often conflicting and further studies are needed to clarify this relationship. It seems that older people with a sufficient vitamin D status have a lower prevalence of cognitive impairment [73]. Significant associations, however, were also found between vitamin D and Mini-Mental State Examination independently of the presence or absence of the cognitive impairment. These were confirmed also adjusting by age, sex, frailty, and diagnoses of cognitive impairment. Furthermore, patients affected by dementia show lower levels of vitamin D compared to those with only a mild cognitive impairment [74]. It should be noted that vitamin D exerts a variety of favorable effects on neural and endothelial dysfunctions, which could potentially explain a protective role against neurodegenerative processes [75, 76]. Placebo controlled trials seem to confirm these findings. Vitamin D supplementation in older subjects with dementia or mild cognitive impairment was shown to improve cognitive functions measured with full scale intelligence quotient, and information, digit span, vocabulary, block design, and picture arrangement scores compared to placebo, even after adjustment for confounding factors [77–80]. Moreover, supplementation was able to reduce amyloid β-related biomarkers in patients with Alzheimer disease [78]. Finally, vitamin D seems to have differential effects on domain-specific cognitive measures and that a higher dose may negatively affect reaction time. Participants taking 2000 IU/day performed better in learning and memory tests compared to other regimens, yet dosages of 4000 IU/day resulted in slower reaction time compared to the 600 IU/d group [79]. Larger and longer trials taking into account various dosage regimens and the different cognitive domains will help clarify the role of vitamin D in tackling cognitive impairments.
To conclude, a very recent review summarized the results of RCTs and MR studies that took place between 2017 and 2020. The study concluded that supplementation of vitamin D-replete individuals does not provide demonstrable health benefits for global health or major diseases or medical events such as cancer, cardiovascular events, T2D, falls or fractures. However, it appears that supplementation with vitamin D might have some extra-skeletal benefits—namely reduced progression to T2D, decreased numbers of upper respiratory tract infections, increased lung function and decreased cancer or overall mortality—especially in severe deficient populations [81].
Strategies to prevent vitamin D deficiency
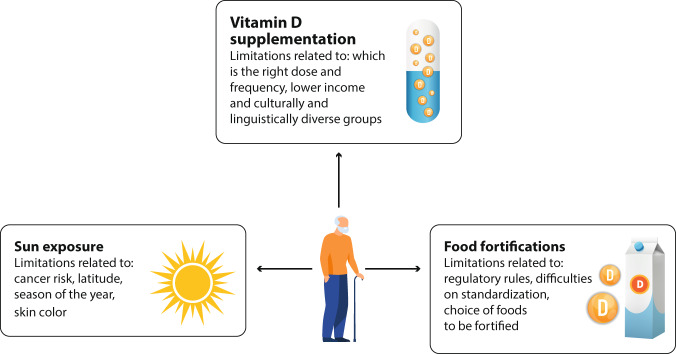
A timely topic among countries at this time is how public health policies can be implemented to prevent vitamin D deficiency in the elderly. Beyond an adequate diet, key strategies are sun exposure, food fortification and supplementation (Fig. 4).
Fig. 4.
Strategies to avoid vitamin D deficiency in the elderly and related limitations
Sunshine exposure
Improving vitamin D status with higher UV exposure is controversial. The World Health Organization assumes that the health threats caused by UV exposure might outweigh the health risks induced by vitamin D deficiency. Non-melanoma skin cancer accounts for one-third of all cancers worldwide and is present mainly in the older population, with UV exposure being their main cause [82]. Although this strategy is debated, it should be recognized clearly that there is a worldwide seasonal variation of serum 25(OH)D levels, increasing after summer. Sunny countries have better vitamin D status, with a lower prevalence of rickets and osteomalacia. Therefore, the amount of local UV radiation is relevant to providing a greater supply of vitamin D from nature. Older skin is well capable of producing vitamin D, as already discussed [82].
Consistent with this, in a study performed in Sao Paulo, Brazil (latitude 23oS), 110 adults older than 55 years (mean ± SD: 67.6 ± 5.4 years), and regularly registered in an outdoor physical activity program, had an average serum 25(OH)D levels of 78.9 ± 31 nmol/l during winter, which improved to 91.6 ± 32 nmol/l in summer, without supplementation [83]. Severe vitamin D deficiency (serum 25(OH)D < 25 nmol/L) was seen in less than 4% of subjects, and only during winter, but no summer improvement was seen in women, in those older than 70 years old and black individuals. On the other hand, at the same time and city, 177 Brazilians living in nursing homes had much lower serum levels of 25(OH)D even after summer (42.1 ± 26 nmol/l), with 50% of them with levels <25 nmol/l [83].
In a study performed in Sweden [84], at a higher latitude, only a slight but significant serum 25(OH)D increase (+11 nmol/l) was observed in the group assigned to stay outdoors 5 days/week for 20–30 min for 2 summer months. By contrast, investigators of a cluster RCT carried out in Australia with older persons living in a nursing home concluded that vitamin D supplementation appears to be a much more practical approach because of the low adherence to the sunlight exposure protocols (only 17% of all participants attended more than 50% of the UV sessions) [85]. Serum 25(OH)D slightly increased proportionally to the number of sessions, and a fall reduction was observed among those who attended more than 50% of the sessions [85].
Age and sunlight exposure are important factors that influence serum 25(OH)D levels. The outdoor activity could be considered a reasonable measure to prevent severe vitamin D deficiency and improve other health and well-being outcomes. However, the population must be educated about the risks of excessive sun exposure to avoid sunburn, about the use of sunscreen, and how to identify cancerous skin lesions early. Despite these considerations, a change in lifestyle as already known from other chronic conditions, such as obesity, is difficult to be persistently achieved and rarely finds adequate space in medical consultations.
Food and food fortification
The natural sources for vitamin D in food are scarce (around 150 IU/day in western diets) [86, 87]. Therefore, food fortification with vitamin D can be a valid strategy to be applied to large populations to avoid severe deficiency, especially for countries with limited sun exposure.
In the last 3 years, two important statements have been published, one from the 2nd Rank Prize Funds Forum on vitamin D [36] and one from the European Calcified Tissue Society [88]. These highlighted the public health aim to decrease the risk of rickets and osteomalacia and avoid serum 25(OH)D levels below 25 nmol/L. These two documents concluded that fortification of foods is an urgent, to-be-implemented strategy, especially for groups at risk for severe vitamin D deficiency, such as children and adolescents, ethnic minority groups, and the institutionalized elderly. There are two very good examples supporting this strategy. In Finland, milk products were fortified with vitamin D from 2003 onwards. In 2000–2011, this public health policy increased serum 25(OH)D from 49.7 to 66.3 nmol/l in people aged 65–74 years, and similarly from 43.0 to 65.1 nmol/l in people of 75 years of age or older [89, 90]. This improvement was uniform across seasons, educational status, smoking status and BMI [90]. In Canada, where milk fortification with vitamin D is mandatory, there is a lower prevalence of serum levels below 25 nmol/l, compared with the UK, US and Germany [89].
Biofortification of animal feed with vitamin D could improve its content in eggs or meat. Additionally, the increase in the vegan population will require an increase in the use of plant-based vitamin D.
However, the implementation of a food fortification program is challenging. The lack of standardization about the recommended intake, which varies between countries in Europe (from 200 to 800 IU/day according to age), the regulatory rules for food fortification, which is permitted in some countries (e.g., UK, Austria, Finland and Sweden) but not in others (e.g., Norway and Denmark), the implications for the industrial production and quality control (e.g., differences between content stated in the label and actual content), are examples of the many difficulties to be overcome [36, 88].
Vitamin D supplementation
Considering these options, vitamin D supplementation seems to be the easiest way to achieve vitamin D sufficiency in an efficient manner, especially for older and institutionalized populations [7]. Most of the various regimens that have been tested demonstrate a dose-dependent increase in serum 25(OH)D levels, but there is great individual variability. As some studies using intermittent high-dose vitamin D supplementation have described an increased risk of falls and fractures, daily or weekly doses are preferred. However, the ViDA study that administered 100,000 IU of vitamin D monthly did not report an increase in fractures or falls [91].
To further shed light on vitamin D supplementation, Cashman and colleagues calculated the daily dose to reach the target serum 25(OH)D level in the blood, based on a meta-regression analysis of individual participants from seven winter-based RCTs, including 882 patients aged from 4 to 90 years [92]. They concluded that the daily dose to avoid severe deficiency (i.e., reaching 25 nmol/l in 97.5% of the individuals) is 400 IU. In comparison, 1000 IU daily is needed to reach the safer level of 50 nmol/l; however, 1000 IU daily is a higher dose than earlier recommended by the Institute of Medicine (IOM) and other regulatory agencies.
While vitamin D supplementation is the best option for the institutionalized (as they are likely to get it freely), in the general population, especially in the lower-income and culturally and linguistically diverse groups, including migrants and refugees, supplementation may be challenging, even with free distribution. In contrast, food fortification will increase vitamin D status over the population as a whole, preventing severe deficiency and avoiding rickets and osteomalacia in large populations, especially for high latitude countries or in groups at high risk. Ideally, a combination of fortification and supplementation is required to tackle vitamin D deficiency and raise serum 25(OH)D levels to 50 nmol/L throughout the entire population.
COVID-19 vaccination
Finally, no published evidence points to an improved immune response with vitamin D supplementation to COVID-19 vaccination. However, the label for the Pfizer vaccine does mention a possible positive role of concomitant immunomodulation [93]. Indeed, low vitamin D levels have been associated with increased susceptibility to SARS-CoV-2 infection and increased COVID-19 clinical impact (Fig. 3) [94, 95]. Moreover, vitamin D supplementation was shown to boost antigen-specific immunity in older adults with suboptimal vitamin D status [96], and to decrease the severity of COVID-19, as demonstrated by a reduced need for intensive care and decreased mortality risks [97]. To this point, vitamin D might be helpful, specifically in areas, such as southern Europe, where hypovitaminosis D is prevalent. If vitamin D is to be utilized to improve the host response to COVID-19 vaccination, this may present a unique opportunity to address the widespread prevalence of hypovitaminosis D. Repleting populations that are being immunized against COVID-19 with vitamin D may address the double pandemic of COVID-19 and vitamin D deficiency [98].
Conclusion
The Aging session of the Conference reviewed some of the most debated matters that center on vitamin D and the older population. In this manuscript, we report the findings emphasized during the meetings.
Vitamin D production decreases with age, but the aging skin can produce sufficient vitamin D when exposed to UV light. However, this remains a challenge, especially in the institutionalized. Vitamin D supplementation should be combined with calcium to reduce fractures in the older population. The goal for adequate vitamin D status should be to reach a serum level of 25(OH)D > 50 nmol/l. It appears that daily low-dose vitamin D regimens reduce the risk of falling, especially in the elderly, compared with infrequent, large bolus doses that may increase it. The role of vitamin D supplementation on muscle strength remains to be clarified. On the other hand, supplementation decreases the risk of progression to T2D from prediabetes among those who are vitamin D deficient. Finally, of the three possible strategies to gain vitamin D sufficiency—sunshine exposure, food fortification, and supplementation—the latter seems to be the most effective and practical. Better-designed clinical trials in the future especially targeted to those who are truly vitamin D deficient, may help us unravel the many controversies revolving around vitamin D and the aged population.
Acknowledgements
We would like to acknowledge the support of Fabio Perversi (Polistudium, Milan, Italy) in drafting the first version of the manuscript and Aashni Shah (Polistudium, Milan, Italy) for editorial assistance. We wish to acknowledge all participants in the Conference: Adrian Martineau, Neil Binkley, Anna Maria Formenti, Ghada El-Hajj Fuleihan, Angelo Fassio, Hector F. De Luca, Annemieke C. Heijboer, Daniel D. Bikle, Salvatore Minisola, Silvia Trasciatti, Nicola Napoli, Giulia Martina Cavestro, Giovanni Latella, David Feldman, Salvatore Minisola, Anastassios G. Pittas, René Rizzoli, and Fabio Massimo Ulivieri.
Author contributions
Study conception and design: J.P.B., A.G.; collection and interpretation of data from literature: J.P.B., P.R.E., R.B., B.D.H., M.L.-C., P.L., C.M.; manuscript drafting and editing: J.P.B., A.G., P.R.E., R.B., B.D.H., M.L.-C., P.L., C.M.; approval to submit: J.P.B., A.G., P.R.E., R.B., B.D.H., M.L.-C., P.L., C.M.
Funding
This work was supported, in part, by International Vitamin D Expert Association (IDEA). The conference and editorial assistance were supported by an unrestricted educational grant by Abiogen Pharma, Pisa, Italy. The sponsors had no role in the selection of discussion topics, speakers, or authors, preparation, or review of this paper.
Compliance with ethical standards
Conflict of interest
A.G. is consultant for Abiogen and Takeda and received research grant to Institution from Takeda, R.B. received lecture fees from Abiogen (Italy), FAES Farma (Spain) and Ceres (Belgium) outside of the submitted work. The other authors have no conflicts of interest to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giustina A, Adler RA, Binkley N, Bouillon R, Ebeling PR, Lazaretti-Castro M, Marcocci C, Rizzoli R, Sempos CT, Bilezikian JP. Controversies in Vitamin D: summary statement from an international conference. J. Clin. Endocrinol. Metab. 2019;104:234–240. doi: 10.1210/jc.2018-01414. [DOI] [PubMed] [Google Scholar]
- 2.Sempos CT, Heijboer AC, Bikle DD, Bollerslev J, Bouillon R, Brannon PM, DeLuca HF, Jones G, Munns CF, Bilezikian JP, Giustina A, Binkley N. Vitamin D assays and the definition of hypovitaminosis D: Results from the first international conference on controversies in vitamin D. Br. J. Clin. Pharmacol. 2018;84:2194–2207. doi: 10.1111/bcp.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebeling PR, Adler RA, Jones G, Liberman UA, Mazziotti G, Minisola S, Munns CF, Napoli N, Pittas AG, Giustina A, Bilezikian JP, Rizzoli R. Management of endocrine disease: Therapeutics of vitamin D. Eur. J. Endocrinol. 2018;179:R239–R259. doi: 10.1530/EJE-18-0151. [DOI] [PubMed] [Google Scholar]
- 4.Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, Lips P, Munns CF, Lazaretti-Castro M, Giustina A, Bilezikian J. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019;40:1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giustina A, Adler RA, Binkley N, Bollerslev J, Bouillon R, Dawson-Hughes B, Ebeling PR, Feldman D, Formenti AM, Lazaretti-Castro M, Marcocci C, Rizzoli R, Sempos CT, Bilezikian JP. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020;21:89–116. doi: 10.1007/s11154-019-09532-w.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giustina A, Bouillon R, Binkley N, Sempos C, Adler RA, Bollerslev J, Dawson-Hughes B, Ebeling PR, Feldman D, Heijboer A, Jones G, Kovacs CS, Lazaretti-Castro M, Lips P, Marcocci C, Minisola S, Napoli N, Rizzoli R, Scragg R, White JH, Formenti AM, Bilezikian JP. Controversies in Vitamin D: a statement from the third international conference. JBMR. 2020;4:e10417. doi: 10.1002/jbm4.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilezikian JP, Formenti AM, Adler RA, Binkley N, Bouillon R, Lazaretti-Castro M, Marcocci C, Napoli N, Rizzoli R, Giustina A. Vitamin D: dosing, levels, form, and route of administration: does one approach fit all. Rev. Endocr. Metab. Disord. 2021;22:1201–1218. doi: 10.1007/s11154-021-09693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J. Bone Miner. Res. 2007;22:V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 10.Chalcraft JR, Cardinal LM, Wechsler PJ, Hollis BW, Gerow KG, Alexander BM, Keith JF, Larson-Meyer DE. Vitamin D synthesis following a single bout of sun exposure in older and younger men and women. Nutrients. 2020;12:2237. doi: 10.3390/nu12082237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 12.de Jongh RT, van Schoor NM, Lips P. Changes in vitamin D endocrinology during aging in adults. Mol. Cell. Endocrinol. 2017;453:144–150. doi: 10.1016/j.mce.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Chel VG, Ooms ME, Popp-Snijders C, Pavel S, Schothorst AA, Meulemans CC, Lips P. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J. Bone Miner. Res. 1998;13:1238–1242. doi: 10.1359/jbmr.1998.13.8.1238. [DOI] [PubMed] [Google Scholar]
- 14.Chel VG, Ooms ME, Pavel S, de Gruijl F, Brand A, Lips P. Prevention and treatment of vitamin D deficiency in Dutch psychogeriatric nursing home residents by weekly half-body UVB exposure after showering: a pilot study. Age Ageing. 2011;40:211–124. doi: 10.1093/ageing/afq159. [DOI] [PubMed] [Google Scholar]
- 15.Mousavi SE, Amini H, Heydarpour P, Amini Chermahini F, Godderis L. Air pollution, environmental chemicals, and smoking may trigger vitamin D deficiency: Evidence and potential mechanisms. Environ. Int. 2019;122:67–90. doi: 10.1016/j.envint.2018.11.052. [DOI] [PubMed] [Google Scholar]
- 16.Passeron T, Bouillon R, Callender V, Cestari T, Diepgen TL, Green AC, van der Pols JC, Bernard BA, Ly F, Bernerd F, Marrot L, Nielsen M, Verschoore M, Jablonski NG, Young AR. Sunscreen photoprotection and vitamin D status. Br. J. Dermatol. 2019;181:916–931. doi: 10.1111/bjd.17992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cutillas-Marco E, Fuertes-Prosper A, Grant WB, Morales-Suarez-Varela M. Vitamin D deficiency in South Europe: effect of smoking and aging. Photodermatol. Photoimmunol. Photomed. 2012;28:159–161. doi: 10.1111/j.1600-0781.2012.00649.x. [DOI] [PubMed] [Google Scholar]
- 18.Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J. Clin. Endocrinol. Metab. 2005;90:4119–4123. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 19.NCD Risk Factor Collaboration (NCD-RisC) Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569:260–264. doi: 10.1038/s41586-019-1171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinders I, van Schoor NM, Deeg DJH, Huisman M, Visser M. Trends in lifestyle among three cohorts of adults aged 55-64 years in 1992/1993, 2002/2003 and 2012/2013. Eur. J. Public Health. 2018;28:564–570. doi: 10.1093/eurpub/ckx173. [DOI] [PubMed] [Google Scholar]
- 21.van Dam RM, Snijder MB, Dekker JM, Stehouwer CD, Bouter LM, Heine RJ, Lips P. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study. Am. J. Clin. Nutr. 2007;85:755–761. doi: 10.1093/ajcn/85.3.755. [DOI] [PubMed] [Google Scholar]
- 22.van den Heuvel EG, van Schoor N, de Jongh RT, Visser M, Lips P. Cross-sectional study on different characteristics of physical activity as determinants of vitamin D status; inadequate in half of the population. Eur. J. Clin. Nutr. 2013;67:360–365. doi: 10.1038/ejcn.2013.22. [DOI] [PubMed] [Google Scholar]
- 23.Vallejo MS, Blümel JE, Arteaga E, Aedo S, Tapia V, Araos A, Sciaraffia C, Castelo-Branco C. Gender differences in the prevalence of vitamin D deficiency in a southern Latin American country: a pilot study. Climacteric. 2020;23:410–416. doi: 10.1080/13697137.2020.1752171. [DOI] [PubMed] [Google Scholar]
- 24.Sanghera DK, Sapkota BR, Aston CE, Blackett PR. Vitamin D status, gender differences, and cardiometabolic health disparities. Ann. Nutr. Metab. 2017;70:79–87. doi: 10.1159/000458765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Z. Wu, C.A. Camargo Jr., I.R. Reid, A. Beros, J.D. Sluyter, D. Waayer, C.M.M. Lawes, L. Toop, K.T. Khaw, R. Scragg, What factors modify the effect of monthly bolus dose vitamin D supplementation on 25-hydroxyvitamin D concentrations. ? J. Steroid. Biochem. Mol. Biol. 201, 105687 (2020). 10.1016/j.jsbmb.2020.105687 [DOI] [PubMed]
- 26.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 27.Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos. Int. 1999;9:394–397. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]
- 28.Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J. Clin. Endocrinol. Metab. 2001;86:1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 29.Sohl E, de Jongh RT, Heymans MW, van Schoor NM, Lips P. Thresholds for serum 25(OH)D concentrations with respect to different outcomes. J. Clin. Endocrinol. Metab. 2015;100:2480–2488. doi: 10.1210/jc.2015-1353. [DOI] [PubMed] [Google Scholar]
- 30.Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J. Bone Miner. Res. 2009;24:693–701. doi: 10.1359/jbmr.081209. [DOI] [PubMed] [Google Scholar]
- 31.Reid IR, Horne AM, Mihov B, Gamble GD, Al-Abuwsi F, Singh M, Taylor L, Fenwick S, Camargo CA, Stewart AW, Scragg R. Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. J. Intern. Med. 2017;282:452–460. doi: 10.1111/joim.12651. [DOI] [PubMed] [Google Scholar]
- 32.Khaw KT, Stewart AW, Waayer D, Lawes CMM, Toop L, Camargo CA, Jr., Scragg R. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol. 2017;5:438–447. doi: 10.1016/S2213-8587(17)30103-1. [DOI] [PubMed] [Google Scholar]
- 33.Macdonald HM, Reid IR, Gamble GD, Fraser WD, Tang JC, Wood AD. 25-Hydroxyvitamin D threshold for the effects of vitamin D supplements on bone density: secondary analysis of a randomized controlled trial. J. Bone Miner. Res. 2018;33:1464–1469. doi: 10.1002/jbmr.3442. [DOI] [PubMed] [Google Scholar]
- 34.de França NA, Camargo MB, Lazaretti-Castro M, Peters BS, Martini LA. Dietary patterns and bone mineral density in Brazilian postmenopausal women with osteoporosis: a cross-sectional study. Eur. J. Clin. Nutr. 2016;70:85–90. doi: 10.1038/ejcn.2015.27. [DOI] [PubMed] [Google Scholar]
- 35.Lips P, van Ginkel FC, Jongen MJ, Rubertus F, van der Vijgh WJ, Netelenbos JC. Determinants of vitamin D status in patients with hip fracture and in elderly control subjects. Am. J. Clin. Nutr. 1987;46:1005–1010. doi: 10.1093/ajcn/46.6.1005. [DOI] [PubMed] [Google Scholar]
- 36.Buttriss JL, Lanham-New SA, Steenson S, Levy L, Swan GE, Darling AL, Cashman KD, Allen RE, Durrant LR, Smith CP, Magee P, Hill TR, Uday S, Kiely M, Delamare G, Hoyland AE, Larsen L, Street LN, Mathers JC, Prentice A. Implementation strategies for improving vitamin D status and increasing vitamin D intake in the UK: current controversies and future perspectives: proceedings of the 2nd Rank Prize Funds Forum on vitamin D. Br. J. Nutr. 2022;127:1567–1587. doi: 10.1017/S0007114521002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balk EM, Adam GP, Langberg VN, Earley A, Clark P, Ebeling PR, Mithal A, Rizzoli R, Zerbini CAF, Pierroz DD, Dawson-Hughes B. International Osteoporosis Foundation Calcium Steering Committee.: Global dietary calcium intake among adults: a systematic review. Osteoporos. Int. 2017;28:3315–3324. doi: 10.1007/s00198-017-4230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016;27:367–376. doi: 10.1007/s00198-015-3386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avenell A, Mcak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev. 2014;4:CD000227. doi: 10.1002/14651858.CD000227.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao P, Bennett D, Mafham M, Lin X, Chen Z, Armitage J, Clarke R. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw. Open. 2019;2:e1917789. doi: 10.1001/jamanetworkopen.2019.17789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waterhouse M, Sanguineti E, Baxter C, Duarte Romero B, McLeod DSA, English DR, Armstrong BK, Ebeling PR, Hartel G, Kimlin MG, O’Connell RL, Pham H, van der Pols JC, Venn AJ, Webb PM, Whiteman DC, Neale RE. Vitamin D supplementation and risk of falling: outcomes from the randomized, placebo-controlled D-Health trial. J. Cachexia Sarcopenia Muscle. 2021;12:1428–1439. doi: 10.1002/jcsm.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern. Med. 2016;176:175–183. doi: 10.1001/jamainternmed.2015.7148. [DOI] [PubMed] [Google Scholar]
- 43.Bislev LS, Grove-Laugesen D, Rejnmark L. Vitamin D and muscle health: a systematic review and meta-analysis of randomized placebo-controlled trials. J. Bone Miner. Res. 2021;36:1651–1660. doi: 10.1002/jbmr.4412. [DOI] [PubMed] [Google Scholar]
- 44.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 46.Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: a randomized clinical trial. J. Steroid Biochem. Mol. Biol. 2017;173:317–322. doi: 10.1016/j.jsbmb.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144:5138–5144. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 48.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster JY, Bruyère O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014;99:4336–4345. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 49.Garber AJ. Effects of parathyroid hormone on skeletal muscle protein and amino acid metabolism in the rat. J. Clin. Invest. 1983;71:1806–1821. doi: 10.1172/jci110936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patten BM, Bilezikian JP, Mallette LE, Prince A, Engel WK, Aurbach GD. Neuromuscular disease in primary hyperparathyroidism. Ann. Intern. Med. 1974;80:182–193. doi: 10.7326/0003-4819-80-2-182. [DOI] [PubMed] [Google Scholar]
- 51.Joborn C, Joborn H, Rastad J, Akerström G, Ljunghall S. Maximal isokinetic muscle strength in patients with primary hyperparathyroidism before and after parathyroid surgery. Br. J. Surg. 1988;75:77–80. doi: 10.1002/bjs.1800750128. [DOI] [PubMed] [Google Scholar]
- 52.Bislev LS, Langagergaard Rødbro L, Sikjær T, Rejnmark L. Effects of elevated parathyroid hormone levels on muscle health, postural stability and quality of life in vitamin D-insufficient healthy women: a cross-sectional study. Calcif. Tissue Int. 2019;105:642–650. doi: 10.1007/s00223-019-00612-2. [DOI] [PubMed] [Google Scholar]
- 53.Stein MS, Wark JD, Scherer SC, Walton SL, Chick P, Carlantonio MD, Zajac JD, Flicker L. Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. JAGS. 1999;47:1195–1201. doi: 10.1111/j.1532-5415.1999.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 54.Sambrook PN, Chen JS, March LM, Cameron ID, Cumming RG, Lord SR, Schwarz J, Seibel MJ. Serum parathyroid hormone is associated with increased mortality independent of 25-hydroxy vitamin d status, bone mass, and renal function in the frail and very old: a cohort study. J. Clin. Endocrinol. Metab. 2004;89:5477–5481. doi: 10.1210/jc.2004-0307. [DOI] [PubMed] [Google Scholar]
- 55.Pilz S, Kienreich K, Tomaschitz A, Lerchbaum E, Meinitzer A, März W, Zittermann A, Dekker JM. Vitamin D and cardiovascular disease: update and outlook. Scand. J. Clin. Lab. Invest. 2012;Suppl.:83–91. doi: 10.3109/00365513.2012.681972. [DOI] [PubMed] [Google Scholar]
- 56.Scragg R. The Vitamin D assessment (ViDA) study – design and main findings. J. Steroid Biochem. Mol. Biol. 2020;198:105562. doi: 10.1016/j.jsbmb.2019.105562. [DOI] [PubMed] [Google Scholar]
- 57.Manson JE, Bassuk SS, Cook NR, Lee IM, Mora S, Albert CM, Buring JE, VITAL Research Group Vitamin D, Marine n-3 fatty acids, and primary prevention of cardiovascular disease current evidence. Circ. Res. 2020;126:112–128. doi: 10.1161/CIRCRESAHA.119.314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sluyter JD, Camargo CA, Jr., Stewart AW, Waayer D, Lawes CMM, Toop L, Khaw KT, Thom SAM, Hametner B, Wassertheurer S, Parker KH, Hughes AD, Scragg R. Effect of monthly, high-dose, long-term vitamin D supplementation on central blood pressure parameters: A randomized controlled trial substudy. J. Am. Heart Assoc. 2017;6:e006802. doi: 10.1161/JAHA.117.006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manousaki D, Mokry LE, Ross S, Goltzman D, Richards JB. Mendelian randomization studies do not support a role for vitamin D in coronary artery disease. Circ. Cardiovasc. Genet. 2016;9:349–356. doi: 10.1161/CIRCGENETICS.116.001396. [DOI] [PubMed] [Google Scholar]
- 60.Brøndum-Jacobsen P, Benn M, Afzal S, Nordestgaard BG. No evidence that genetically reduced 25-hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study. Int. J. Epidemiol. 2015;44:651–661. doi: 10.1093/ije/dyv078. [DOI] [PubMed] [Google Scholar]
- 61.Vimaleswaran KS, Cavadino A, Berry DJ, LifeLines Cohort Study investigators. Jorde R, Dieffenbach AK, Lu C, Alves AC, Heerspink HJ, Tikkanen E, Eriksson J, Wong A, Mangino M, Jablonski KA, Nolte IM, Houston DK, Ahluwalia TS, van der Most PJ, Pasko D, Zgaga L, Thiering E, Vitart V, Fraser RM, Huffman JE, de Boer RA, Schöttker B, Saum KU, McCarthy MI, Dupuis J, Herzig KH, Sebert S, Pouta A, Laitinen J, Kleber ME, Navis G, Lorentzon M, Jameson K, Arden N, Cooper JA, Acharya J, Hardy R, Raitakari O, Ripatti S, Billings LK, Lahti J, Osmond C, Penninx BW, Rejnmark L, Lohman KK, Paternoster L, Stolk RP, Hernandez DG, Byberg L, Hagström E, Melhus H, Ingelsson E, Mellström D, Ljunggren O, Tzoulaki I, McLachlan S, Theodoratou E, Tiesler CM, Jula A, Navarro P, Wright AF, Polasek O, Caroline Hayward, Wilson JF, Rudan I, Salomaa V, Heinrich J, Campbell H, Price JF, Karlsson M, Lind L, Michaëlsson K, Bandinelli S, Frayling TM, Hartman CA, Sørensen TI, Kritchevsky SB, Langdahl BL, Eriksson JG, Florez JC, Spector TD, Lehtimäki T, Kuh D, Humphries SE, Cooper C, Ohlsson C, März W, de Borst MH, Kumari M, Kivimaki M, Wang TJ, Power C, Brenner H, Grimnes G, van der Harst P, Snieder H, Hingorani AD, Pilz S, Whittaker JC, Järvelin MR, Hyppönen E. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:719–729. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skaaby T, Husemoen LL, Martinussen T, Thyssen JP, Melgaard M, Thuesen BH, Pisinger C, Jørgensen T, Johansen JD, Menné T, Carlsen B, Szecsi PB, Stender S, Fenger RV, Fenger M, Linneberg A. Vitamin D status, filaggrin genotype, and cardiovascular risk factors: a Mendelian randomization approach. PLoS One. 2013;8:e57647. doi: 10.1371/journal.pone.0057647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Emerging Risk Factors Collaboration/EPIC-CVD/Vitamin D Studies Collaboration. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2021;9:837–846. doi: 10.1016/S2213-8587(21)00263-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Neale RE, Baxter C, Romero BD, McLeod DSA, English DR, Armstrong BK, Ebeling PR, Hartel G, Kimlin MG, O’Connell R, van der Pols JC, Venn AJ, Webb PM, Whiteman DC, Waterhouse M. The D-Health Trial: a randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol. 2022;10:120–128. doi: 10.1016/S2213-8587(21)00345-4. [DOI] [PubMed] [Google Scholar]
- 65.Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee R, Desouza C, Dolor R, Foreyt J, Fuss P, Ghazi A, Hsia DS, Johnson KC, Kashyap SR, Kim S, LeBlanc ES, Lewis MR, Liao E, Neff LM, Nelson J, O’Neil P, Park J, Peters A, Phillips LS, Pratley R, Raskin P, Rasouli N, Robbins D, Rosen C, Vickery EM, Staten M. D2d Research Group.: Vitamin D supplementation and prevention of type 2 diabetes. N. Engl. J. Med. 2019;381:520–530. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dawson-Hughes B, Staten MA, Knowler WC, Nelson J, Vickery EM, LeBlanc ES, Neff LM, Park J, Pittas AG, D2d Research Group. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the vitamin D and type 2 diabetes (D2d) study. Diabetes Care. 2020;43:2916–2922. doi: 10.2337/dc20-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, Fuskevåg OM, Figenschau Y, Hutchinson MY. Vitamin D 20,000 IU per week for five years does not prevent progression from prediabetes to diabetes. J. Clin. Endocrinol. Metab. 2016;101:1647–1655. doi: 10.1210/jc.2015-4013. [DOI] [PubMed] [Google Scholar]
- 68.Kawahara T, Suzuki G, Inazu T, Mizuno S, Kasagi F, Okada Y, Tanaka Y. Rationale and design of Diabetes prevention with active Vitamin D (DPVD): a randomised, double-blind, placebo-controlled study. BMJ Open. 2016;6:e011183. doi: 10.1136/bmjopen-2016-011183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawahara. T.: Eldecalcitol, a vitamin D analogue, for diabetes prevention in impaired glucose tolerance (DPVD study). Diabetes Care. 67 (2018).
- 70.A.G. Pittas, R. Jorde, T. Kawahara, B. Dawson-Hughes, Vitamin D supplementation for prevention of type 2 diabetes mellitus: To d or not to D. ? J. Clin. Endocrinol. Metab. 105, 3721–3733 (2020). 10.1210/clinem/dgaa594 [DOI] [PMC free article] [PubMed]
- 71.Barbarawi M, Zayed Y, Barbarawi O, Bala A, Alabdouh A, Gakhal I, Rizk F, Alkasasbeh M, Bachuwa G, Manson JE. Effect of vitamin D supplementation on the incidence of diabetes mellitus. J. Clin. Endocrinol. Metab. 2020;105:dgaa335. doi: 10.1210/clinem/dgaa335. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Tan H, Tang J, Li J, Chong W, Hai Y, Feng Y, Lunsford LD, Xu P, Jia D, Fang F. Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: A systematic review and meta-analysis. Diabetes Care. 2020;43:1650–1658. doi: 10.2337/dc19-1708. [DOI] [PubMed] [Google Scholar]
- 73.Matsuo LH, Confortin SC, Ceolin G, Soar C, Xavier AJ, D’Orsi E, Moreira JD. Association between lower serum vitamin D (25-hydroxy-cholecalciferol) concentrations and cognitive impairment in older adults: data from a populational-based cohort study in a middle-income country. Public Health Nutr. 2022;25:2507–2516. doi: 10.1017/S1368980021004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arosio B, Rossi PD, Ferri E, Cesari M, Vitale G. Characterization of Vitamin D status in older persons with cognitive impairment. Nutrients. 2022;14:1142. doi: 10.3390/nu14061142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bivona G, Lo Sasso B, Gambino CM, Giglio RV, Scazzone C, Agnello L, Ciaccio M. The role of vitamin D as a biomarker in alzheimer’s disease. Brain Sci. 2021;11:334. doi: 10.3390/brainsci11030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perez L, Heim L, Sherzai A, Jaceldo-Siegl K. Nutrition and vascular dementia. J. Nutr. Health Aging. 2012;16:319–324. doi: 10.1007/s12603-012-0042-z. [DOI] [PubMed] [Google Scholar]
- 77.Hu J, Jia J, Zhang Y, Miao R, Huo X, Ma F. Effects of vitamin D3 supplementation on cognition and blood lipids: a 12-month randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry. 2018;89:1341–1347. doi: 10.1136/jnnp-2018-318594. [DOI] [PubMed] [Google Scholar]
- 78.Jia J, Hu J, Huo X, Miao R, Zhang Y, Ma F. Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: a randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry. 2019;90:1347–1352. doi: 10.1136/jnnp-2018-320199. [DOI] [PubMed] [Google Scholar]
- 79.Castle M, Fiedler N, Pop LC, Schneider SJ, Schlussel Y, Sukumar D, Hao L, Shapses SA. Three doses of vitamin d and cognitive outcomes in older women: a double-blind randomized controlled trial. J. Gerontol. A. Biol. Sci. Med. Sci. 2020;75:835–842. doi: 10.1093/gerona/glz041.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang T, Wang H, Xiong Y, Chen C, Duan K, Jia J, Ma F. Vitamin D supplementation improves cognitive function through reducing oxidative stress regulated by telomere length in older adults with mild cognitive impairment: a 12-month randomized controlled trial. J. Alzheimers Dis. 2020;78:1509–1518. doi: 10.3233/JAD-200926. [DOI] [PubMed] [Google Scholar]
- 81.Bouillon R, Manousaki D, Rosen C, Trajanoska K, Rivadeneira F, Richards JB. The health effects of vitamin D supplementation: evidence from human studies. Nat. Rev. Endocrinol. 2022;18:96–110. doi: 10.1038/s41574-021-00593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barysch MJ, Hofbauer GF, Dummer R. Vitamin D, ultraviolet exposure, and skin cancer in the elderly. Gerontology. 2010;56:410–413. doi: 10.1159/000315119. [DOI] [PubMed] [Google Scholar]
- 83.Maeda SS, Kunii IS, Hayashi LF, Lazaretti-Castro M. Increases in summer serum 25-hydroxyvitamin D (25OHD) concentrations in elderly subjects in São Paulo, Brazil vary with age, gender and ethnicity. BMC Endocr. Disord. 2010;10:12. doi: 10.1186/1472-6823-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Samefors M, Tengblad A, Östgren CJ. Sunlight exposure and vitamin D levels in older people- an intervention study in Swedish nursing homes. J. Nutr. Health Aging. 2020;24:1047–1052. doi: 10.1007/s12603-020-1435-z. [DOI] [PubMed] [Google Scholar]
- 85.Sambrook PN, Cameron ID, Chen JS, Cumming RG, Durvasula S, Herrmann M, Kok C, Lord SR, Macara M, March LM, Mason RS, Seibel MJ, Wilson N, Simpson JM. Does increased sunlight exposure work as a strategy to improve vitamin D status in the elderly: a cluster randomised controlled trial. Osteoporos. Int. 2012;23:615–624. doi: 10.1007/s00198-011-1590-5. [DOI] [PubMed] [Google Scholar]
- 86.Pinheiro MM, Schuch NJ, Genaro PS, Ciconelli RM, Ferraz MB, Martini LA. Nutrient intakes related to osteoporotic fractures in men and women–the Brazilian Osteoporosis Study (BRAZOS) Nutr. J. 2009;8:6. doi: 10.1186/1475-2891-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buttriss JL, Lanham-New SA. Is a vitamin D fortification strategy needed? Nutr. Bull. 2020;45:115–122. doi: 10.1111/nbu.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, El-Hajj Fuleihan G, Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019;180:P23–P54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 89.Cashman KD. Vitamin D Deficiency: defining, prevalence, causes, and strategies of addressing. Calcif. Tissue Int. 2020;106:14–29. doi: 10.1007/s00223-019-00559-4. [DOI] [PubMed] [Google Scholar]
- 90.Jääskeläinen T, Itkonen ST, Lundqvist A, Erkkola M, Koskela T, Lakkala K, Dowling KG, Hull GL, Kröger H, Karppinen J, Kyllönen E, Härkänen T, Cashman KD, Männistö S, Lamberg-Allardt C. The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data. Am. J. Clin. Nutr. 2017;105:1512–1520. doi: 10.3945/ajcn.116.151415. [DOI] [PubMed] [Google Scholar]
- 91.Pilz S, Zittermann A, Trummer C, Theiler-Schwetz V, Lerchbaum E, Keppel MH, Grübler MR, März W, Pandis M. Vitamin D testing and treatment: a narrative review of current evidence. Endocr. Connect. 2019;8:R27–R43. doi: 10.1530/EC-18-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cashman KD, Ritz C, Kiely M, Odin Collaborators. Improved dietary guidelines for vitamin D: Application of individual participant data (IPD)-level meta-regression analyses. Nutrients. 2017;9:469. doi: 10.3390/nu9050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.https://www.fda.gov/media/151707/download Accessed 16 Sept 2021
- 94.Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, Madhavan MV, Nair N, Babalyan V, Hutchings N, Napoli N, Accili D, Binkley N, Landry DW, Giustina A. Mechanisms in endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020;183:R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.di Filippo L, Allora A, Doga M, Formenti AM, Locatelli M, Rovere Querini P, Frara S, Giustina A. Vitamin D levels are associated with blood glucose and BMI in COVID-19 patients, predicting disease severity. J. Clin. Endocrinol. Metab. 2022;107:e348–e360. doi: 10.1210/clinem/dgab599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chambers ES, Vukmanovic-Stejic M, Turner CT, Shih BB, Trahair H, Pollara G, Tsaliki E, Rustin M, Freeman TC, Mabbott NA, Noursadeghi M, Martineau AR, Akbar AN. Vitamin D3 replacement enhances antigen-specific immunity in older adults. Immunother. Adv. 2021;1:ltaa008. doi: 10.1093/immadv/ltaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bouillon R, Quesada-Gomez JM. Vitamin D endocrine system and COVID-19. JBMR. 2021;5:e10576. doi: 10.1002/jbm4.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ulivieri FM, Banfi G, Camozzi V, Colao A, Formenti AM, Frara S, Lombardi G, Napoli N, Giustina A. Vitamin D in the Covid-19 era: a review with recommendations from a G.I.O.S.E.G. expert panel. Endocrine. 2021;72:597–603. doi: 10.1007/s12020-021-02749-3. [DOI] [PMC free article] [PubMed] [Google Scholar]