Abstract
We report a case of cerebral venous sinus thrombosis, bilateral adrenal hemorrhage, and thrombocytopenia in a 70-year-old man found dead. He had previously received the ChAdOx1 nCoV-19 vaccine (Vaxzevria®, AstraZeneca) 18 days before, and had since developed unspecific and undiagnosed characteristics of what proved to be a rare case of vaccine-associated thrombocytopenia with thrombosis syndrome (TTS). He was found dead 1 week after the beginning of symptoms (day 25 post-vaccine). Autopsy yielded venous hemorrhagic infarction with the presence of thrombi within dural venous sinuses, and extensive hemorrhagic necrosis of the central part of the adrenal glands. Antibodies against platelet factor 4 (PF4) were strongly positive in postmortem fluids, as measured with an enzyme-linked immunosorbent assay (ELISA). This difficult diagnosis is usually made during the patient’s lifetime. After eliminating differential diagnoses, we concluded on a fatal case of vaccine-induced immune TTS with positive anti-PF4 antibodies in cadaveric blood, 3 weeks after ChAdOx1 nCoV-19 vaccination. Specific search for anti-PF4 antibodies in cadaveric blood appears therefore paramount to assess postmortem cases of TTS associated with anti-COVID vaccines.
Keywords: Antibodies against platelet factor 4 (PF4), Thrombocytopenia syndrome (TTS), Vaccination, Death, AstraZeneca, COVID-19, Forensic medicine
Introduction
The AstraZeneca adenovirus vector Vaxzevria® anti-COVID vaccine (ChAdOx1 nCoV-19) received a European conditional marketing authorization in January 2021 for active immunization against COVID-19 to prevent severe disease and death. The benefits of Vaxzevria® outweigh its risks in adults of all age groups. However, very rare cases of severe thromboembolic events of unusual typology (cerebral and splanchnic venous thrombosis), associated with thrombocytopenia and sometimes leading to death [1–3], have occurred following vaccination with Vaxzevria® [4–6]. This led to an age-restriction use in most European countries, and suspension in some, after both the European Drug Agency (EMA) and the Food and Drugs Administration (FDA) required that this rare risk be mentioned in the SmPC [7]. This rare syndrome is associated with the presence of antibodies directed against platelet factor 4 (PF4), like those observed in heparin-induced thrombocytopenia (HIT), although without any previous heparin treatment. These antibodies lead to the subsequent occurrence of thrombosis and thrombocytopenia. This entity is known as vaccine-induced thrombosis with thrombocytopenia (VITT) or thrombocytopenia with thrombosis syndrome (TTS) [8–11]. What was initially observed with Vaxzevria® was soon followed with reports of cerebral venous sinus thrombosis (CVST) with the Janssen COVID-19 vaccine [12] and also quite recently with the heterologous recombinant Gam-COVID-Vac Sputnik [13].
Currently, the literature on COVID-19-associated deaths is contrasting with that of death-associated events observed after vaccination against COVID-19. Among the studies published on fatal cases concurring with a preceding COVID-19 vaccination, a mere 13 cases were causally related to ChAdOx1 nCoV-19 vaccine administration [1, 4, 14–20].
Not all cases of vaccine-associated TTS or CVST, fortunately, lead to death, and the diagnosis is usually made during hospitalization of the patient. Positive antibodies against PF4 when present confirm the diagnosis [21]. To our knowledge, there is no report, yet, of exclusive postmortem diagnosis of VITT or TTS after SARS-CoV-2 vaccination; few studies evoke this diagnosis in the postmortem period [2, 16]. Nevertheless, cases of sudden or unexplained death, especially without a prior stay in intensive care, may be associated with anti-COVID 19 vaccines.
We report the results of the autopsy of a 70-year-old man who died at home 25 days after receiving a first dose of the adenovirus vector vaccine ChAdOx1 nCoV-19 (Vaxzevria®, AstraZeneca). We could establish a causal link between vaccination and this fatal adverse event. Incidentally, in departments of forensic pathology, we foster the investigation of a possible vaccine causality in cases of sudden or unexplained death.
Methods
A thorough postmortem analysis was performed, including gross microscopic, toxicological, and microbiological testing. SARS-COV-2 nasal swab testing was performed using reverse transcriptase-polymerase chain reaction (RT-PCR). Since public prosecutors and forensic institutes had previously agreed to add the PF4 assay to the panel of assays to be performed following the occurrence of deaths associated with anti-COVID vaccination, antibodies against PF4 were measured in postmortem fluids: 2 mL of citrated blood taken during autopsy was immediately centrifuged, and the supernatant was transferred into a vial, before being analyzed by a manual enzyme-linked immunosorbent assay (ELISA). A resulting optical density less than 0.5 is deemed as negative. We also collected and measured anti-PF4 in the blood from different medico legal corpses for which the cause of death was either traumatic or toxic (160 cases were studied, attributed to drowning (7 cases), gunshot injuries (29 cases), hanging (15 cases), pharmaceutical overdoses (45 cases), drugs-of-abuse overdoses (23 cases), and traffic accidents (41 cases)), for which the delay between death and femoral blood sampling during autopsy was less than 10 days.
Case report
The patient, who suffered from mild chronic systemic hypertension, had received a first dose of adenovirus vector vaccine ChAdOx1 nCoV-19 on day 0. On day 5 post-vaccination he reported feeling ill, with nausea and mild diarrhea. He was admitted to a local hospital on day 19, where he presented with abdominal pain without fever or headache. The physical examination was unremarkable except for bilateral lumbar pain and a resting high blood pressure measured at 185/88 mm Hg. The abdominal pain decreased after daily administration of oral paracetamol (3 g/day). A CT scan showed a hypertrophy of his adrenal glands. The platelet count was 80,000 per cubic millimeter (as compared to 154,000 before vaccination); D-dimers were not tested. The patient was released from the hospital with a diagnosis of non-specific abdominal pain and prescribed oral paracetamol treatment. He had had no previous exposure to heparin. One week later (day 25 post-vaccine), he was found dead at home by his relatives, who had not heard from him for over a week. No signs of burglary or violence were found in the apartment.
Autopsy was performed 3 days after death; the corpse was kept at + 4 °C to avoid postmortem modifications. The external examination of the body revealed a thin subject, with no traumatic skin lesions. The autopsy revealed a diffuse subdural hemorrhage with cerebral edema, no fracture or contusion of the scalp, and macroscopic signs of CVST. Both adrenal glands were significantly enlarged (6 cm by 3 cm), with a necrotic and hemorrhagic aspect (Fig. 1) and no macroscopic thrombosis in the splanchnic, kidney, or spleen vessels. No intra-abdominal or intra-thoracic effusion was observed, nor compression, which may produce blood stasis and facilitate the occurrence of venous thrombosis. No lung infection was noted. RT-PCR of a tracheal swab was negative for SARS-CoV-2 at autopsy. Atheromatous deposits with calcified plaques were present in the aorto-lumbar and iliac region.
Fig. 1.
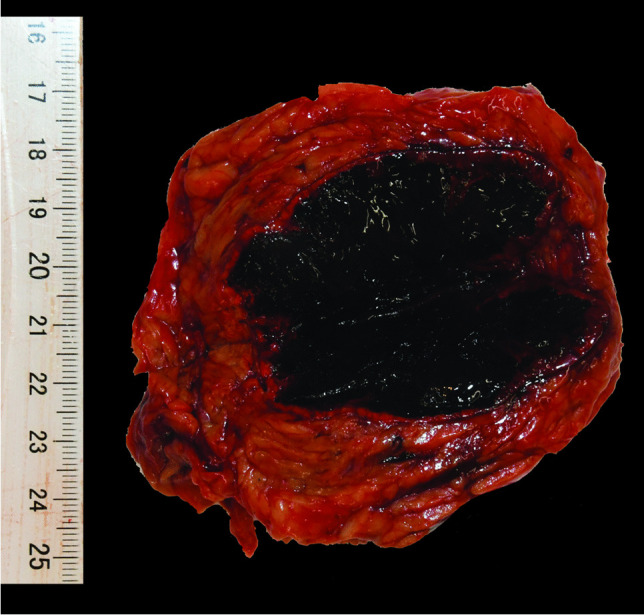
Macroscopic hemorrhagic necrosis of the adrenal gland
Samples taken at autopsy were fixed in buffered formaldehyde diluted 10% for at least 4 weeks. For macroscopic examination multiple samples of each organ were obtained and then paraffin-embedded. Slides of each sample, 5 µm thick and stained with hematoxylin eosin saffron, were prepared for routine optic microscopy.
On macroscopic examination, the brain and adrenal gland samples showed the most lesions. In the brain, a left parieto-temporal subarachnoid hemorrhage was associated with parenchymal hemorrhages. There was an organized heterogeneous brown and adherent thrombus in the sagittal sinus of the dura matter. The vessels of the Willis polygon were uncharacteristic. The adrenal glands were enlarged with bilateral hemorrhagic necrosis of the tissue. The other organs did not present any macroscopic particularity.
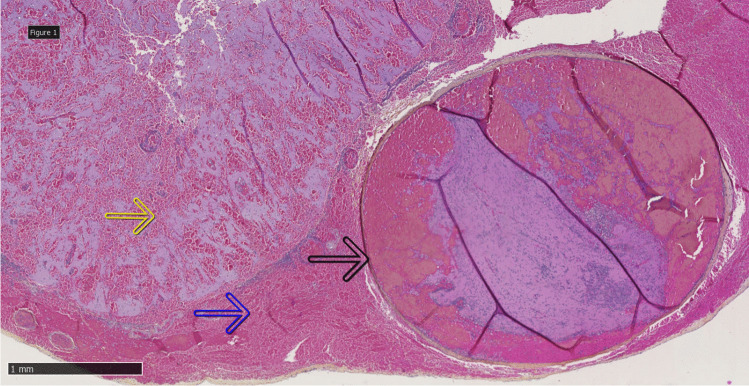
On microscopic examination the organized thrombosis of the longitudinal sinus was confirmed, with multiple organized and adherent thrombi of leptomeningeal vessels localized near the subarachnoid hemorrhage (Fig. 2).
Fig. 2.
HPS (hematoxylin phloxine saffron) staining × 2.5: thrombosis of the leptomeningeal vessel (dark arrow), subarachnoid hemorrhage (blue arrow), and hemorrhagic parenchymal venous infarction (yellow arrow)
In several locations, a neutrophilic infiltration of subarachnoid tissues was present, in hemorrhagic and non-hemorrhagic territories alike.
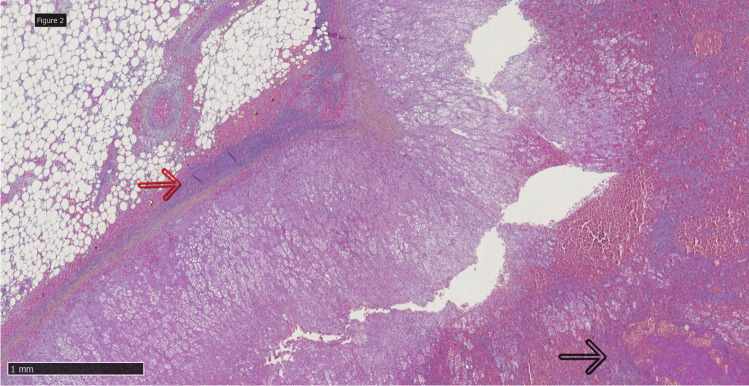
In the left parietal and temporal territory, the cortical parenchyma was the site of venous hemorrhagic infarction. Numerous microthrombi were present in perforative vessels. Gram and Grocott staining proved negative as well as PCR for Neisseria meningitidis, Streptococcus pneumoniae, and the 16S RNA (multibacteria) on deparaffinized tissue sections. PCR of LCR and blood also proved negative for Neisseria meningitidis. The rest of the examination of the brain showed non-specific anoxic lesions. Examination of the adrenal glands showed a similar aspect of both glands (Fig. 3) with extensive hemorrhagic necrosis of their central part, more organized fibrino-inflammatory remodeling of the peri-adrenal tissue, sometimes associated with poorly organized fibrosis. Multiple microthrombi were present in the adrenal small vessels. The remaining histological examination of other organs retrieved thrombi within the pulmonary microcirculation and histological signs of prolonged cardiocirculatory failure such as, apart from cerebral anoxo-ischemia lesions, kidney acute tubular necrosis and severe hepatic sinusoidal distension.
Fig. 3.
HPS (hematoxylin phloxine saffron) staining × 2.5: hemorrhagic necrosis of the central part of the adrenal gland (dark arrow) with inflammation of the peri-adrenal tissue (red arrow)
Toxicological analyses detected concentrations of paracetamol, tramadol, and metoclopramide within the therapeutic range. Antibodies against PF4 proved strongly positive (optical density: 2.17). Blood samples from 160 cases, whose cause of death was either traumatic or toxic, did not contain antibodies against PF4 with a mean optical density of 0.197 ± 0.104 and no result > 0.500.
Our conclusion was that the most likely cause of death was a fatal vaccine-induced immune TTS, associated with the Vaxzevria® vaccine administered 3 weeks before, and confirmed by the presence of PF4 antibodies. Acute adrenal insufficiency due to massive bilateral hemorrhage of the adrenal glands, observed as complications of coagulation disorders, was the final cause of death with histological signs of prolonged cardiocirculatory failure.
Discussion
We report herein the case of a patient who presented with meningeal hemorrhage and microthrombi in multiple organs 3 weeks after receiving a first injection of the anti-COVID ChAdOx1 nCoV-19 Vaxzevria® vaccine. Vaccine-induced TTS is a rare complication occurring with an estimated rate of ~ 1 in 100,000 vaccinated people [22]. This rare effect is caused by antibodies against PF4. To date, this reaction has been reported with the adenovirus vector vaccines Vaxzevria® [4–6], Janssen [12, 23], and more recently the heterologous recombinant Gam-COVID-Vac Sputnik [13].
Despite the absence of heparin exposure, TTS shares common features with heparin-induced thrombocytopenia (HIT) [24–26], including venous or arterial thrombosis at odd sites (like splanchnic) and varying degrees of thrombocytopenia, coexisting with antibodies against PF4 [27, 28]. The mechanism invokes IgG antibodies that recognize PF4 and activate platelets through their Fcγ receptors [29], as they classically do in HIT associated with platelet-activating anti-PF4/heparin antibodies [30]. TTS, which becomes clinically apparent approximately 5 to 20 days after vaccination, led to a “Dear Doctor Letter” by the EMA to alert healthcare professionals of such a rare but serious possible vaccine-associated adverse event. Indeed, most cases, if not fatal, result in a severe course of thrombosis events. The platelet nadir as well as the subsequent thrombosis and co-morbidities have significantly been associated with a risk of death [31].
The current patient consulted for abdominal pain 18 days after vaccination, likely due to adrenal necrosis, as his blood pressure was relatively high and no ionic disorder was reported. Bilateral adrenal necrosis is a difficult diagnosis as the only symptom can be isolated abdominal pain, without any signs of adrenal insufficiency. It can be evoked in severe infections or in the setting of coagulation disorders. In the current case, the diagnosis was not evoked probably because the causality of the adenoviral vaccines in its occurrence was fairly recent. Headache was not mentioned despite the cerebral hemorrhage observed at autopsy, but thrombotic events may shift in time. Pomara et al. [19] described a similar patient with abdominal pain as the first symptom followed by cerebral hemorrhage. Pathological examination found multiple organized and adherent thrombosis in the brain and adrenal glands suggesting an underlying coagulopathy. In our case, specific search for PF4 antibodies helped make the diagnosis of post-vaccinal TTS.
Differential diagnosis must be ruled out to determine a link between vaccination and a fatal adverse event [19].
The assessment of our case included the verification of cardinal facts, i.e., that (i) the vaccine was administered before the occurrence of symptoms, (ii) no adverse event linked to the vaccination procedure was ever declared, (iii) no quality defect of the vaccine concerned was declared, (iv) the patient never tested positive for COVID-19, and (v) the patient was not known to have experienced any previous vaccination-associated disease. Hence, no history of venous thrombosis, severe cardiomyopathy, or infection prevailed. A negative clinical history and the normal blood count of routine tests performed just prior to the event also ruled out a myeloproliferative neoplasm. It is yet unclear whether previous thrombosis increases the risk of TTS with Vaxzevria® but clotting disorders were not documented in many of the previously validated cases of death [2, 15, 17].
An interesting differential diagnosis concerns the Waterhouse–Friderichsen syndrome, which associates inflammatory meningeal changes and bilateral adrenal necrosis. Though, a search for many microorganisms proved negative in both the cerebrospinal fluid and tissue sections. All bacteriological, parasitological, and mycologic analyses proved negative.
In cases of serious or fatal adverse events after vaccination, positive levels of PF4 antibodies are usually obtained antemortem [28]. Postmortem positive antibodies against PF4 are investigated exceptionally since diagnosis is usually made before death [16]. Exclusive postmortem diagnosis is difficult, since laboratory data are lacking in a significant number of cases, postmortem thrombocytopenia cannot be used to investigate these patients, and measurement of antibodies against PF4 are far from systematic during medico legal autopsies. Such analyses are the hallmark of TTS in living patients. We gathered the results of 160 other medico legal results of people whose cause of death was unrelated to anti-COVID vaccination, as public prosecutors and forensic institutes agreed to add the PF4 assay to the panel of previously performed assays (following the occurrence of deaths after anti-COVID vaccination). Postmortem blood samples of these 160 other medico legal results did not detect antibodies against PF4. Greinacher et al. [4] have shown the usefulness of this test with serum from people still alive at the time of investigation, but also on postmortem serum.
We concluded that there was no evidence for other causes and confirmed a fatal TTS complication of the ChAdOx1 nCoV-19 anti-COVID vaccine.
Conclusion
Since early 2021, rare thrombosis with thrombocytopenia syndrome (TTS) has been documented following the administration of adenovirus vector-based anti-COVID 19 vaccines, including the ChAdOx1 nCoV-19 (Oxford-AstraZeneca), the AD26.COV2.S (Johnson & Johnson-Janssen), and recently the heterologous recombinant Gam-COVID-Vac (Sputnik®).
Our report of the autopsy of a patient who received the Vaxevria® vaccine provided the classical features of TTS, as previously described [14, 28], but associated with positive levels of PF4 antibodies found in postmortem blood. Comparable postmortem findings are rarely found in the scientific literature. Autopsy is paramount to establish a causality relationship between vaccine administration and death. It is difficult to make a diagnosis for all subjects found dead without an obvious cause who have not been hospitalized with informative data. It is essential to provide a causal link between vaccination and fatal TTS, for cases with signs suggestive of this diagnosis. The level of PF4 antibodies is of interest to departments of forensic pathology to diagnose or confirm TTS after death, as our control series did not retrieve any false positive.
Declarations
Ethical approval
Not applicable.
Research involving human participants and/or animals
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wiedmann M, Skattør T, Stray-Pedersen A, et al. Vaccine induced immune thrombotic thrombocytopenia causing a severe form of cerebral venous thrombosis with high fatality rate: a case series. Front Neurol. 2021;12:721146. doi: 10.3389/fneur.2021.721146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sessa F, Salerno M, Esposito M, et al. Autopsy findings and causality relationship between death and COVID-19 vaccination: a systematic review. J Clin Med. 2021;10:5876. doi: 10.3390/jcm10245876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindhoff E, Schoenborn L, Piorkowski M, at al, Heterogeneity of vaccine-induced immune thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination and safety of second vaccination with BNT162b2. Thromb Haemost. 2022;122:304–307. doi: 10.1055/a-1701-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fromme M, Zimmermann HW, Bruns T. Splanchnic vein thrombosis with thrombopenia in a young, otherwise healthy patient. Gastroenterology. 2021;0016–5085:03136-X. doi: 10.1053/j.gastro.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines agency (2021) COVID-19 vaccine AstraZeneca: risk of thrombocytopenia and coagulation disorders. Dissemination 24 mars 2021
- 8.Muir KL, Kallam A, Koepsell SA, et al. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384:1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uzun G, Althaus K, Bakchoul T. No correlation between anti-PF4 and anti-SARS-CoV-2 antibodies after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;385:1334–1336. doi: 10.1056/NEJMc2111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alam W. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: a review of the potential mechanisms and proposed management. Sci Prog. 2021;104:368504211025927. doi: 10.1177/00368504211025927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Medicines agency (2021) EMA raises awareness of clinical care recommendations to manage suspected thrombosis with thrombocytopenia syndrome
- 12.Oliver SE, Wallace M, See I, Mbaeyi S, et al. Use of the Janssen (Johnson & Johnson) COVID-19 vaccine: updated interim recommendations from the Advisory Committee on Immunization Practices — United States, December 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):90–95. doi: 10.15585/mmwr.mm7103a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera-Comoglio R, Lane S. Vaccine-induced immune thrombocytopenia and thrombosis after the Sputnik V vaccine. N Engl J Med. 2022;387:1431. doi: 10.1056/NEJMc2210813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Althaus K, Möller P, Uzun G, et al. Antibody-mediated procoagulant platelets in SARS-CoV-2- vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021;106:2170–2179. doi: 10.3324/haematol.2021.279000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauriello A, Scimeca M, Amelio I, et al. Thromboembolism after COVID-19 vaccine in patients with preexisting thrombocytopenia. Cell Death Dis. 2021;12:762. doi: 10.1038/s41419-021-04058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjørnstad-Tuveng TH, Rudjord A, Anker P (2021) Fatal cerebral haemorrhage after COVID-19 vaccine. TidsskrNor Laegeforen 141. 10.4045/tidsskr.21.0312 [DOI] [PubMed]
- 17.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Günther A, Brämer D, Pletz MW, et al. Complicated long term vaccine induced thrombotic immune thrombocytopenia—a case report. Vaccines. 2021;9:1344. doi: 10.3390/vaccines9111344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomara C, Sessa F, Ciaccio M, et al. COVID-19 vaccine and death: causality algorithm according to the WHO eligibility diagnosis. Diagnostics. 2021;11:955. doi: 10.3390/diagnostics11060955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider J, Sottmann L, Greinacher A, et al. Postmortem investigation of fatalities following vaccination with COVID-19 vaccines. Int J Legal Med. 2021;135:2335–2345. doi: 10.1007/s00414-021-02706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiele T, Ulm L, Holtfreter S, et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021;138:299–303. doi: 10.1182/blood.2021012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Violi F, Cammisotto V, Pastori D, et al. Thrombosis in pre- and post-vaccination phase of COVID-19. Eur Heart J Suppl. 2021;23(Suppl E):E184–E188. doi: 10.1093/eurheartj/suab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cari L, Alhosseini MN, Fiore P, Pierno S, et al. Cardiovascular, neurological, and pulmonary events following vaccination with the BNT162b2, ChAdOx1 nCoV-19, and Ad26.COV2.S vaccines: an analysis of European data. J Autoimmun. 2021;125:102742. doi: 10.1016/j.jaut.2021.102742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warkentin TE, Makris M, Jay RM, et al. A spontaneous prothrombotic disorder resembling heparin-induced thrombocytopenia. Am J Med. 2008;121:632–636. doi: 10.1016/j.amjmed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344:1286–1292. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 26.Krauel K, Potschke C, Weber C, et al. Platelet factor 4 binds to bacteria, [corrected] inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood. 2011;117:1370–1378. doi: 10.1182/blood-2010-08-301424. [DOI] [PubMed] [Google Scholar]
- 27.Vayne C, Rollin J, Gruel Y, et al. Immunoassays in vaccine-induced thrombotic thrombocytopenia. N Engl J Med. 2021;385(4):376–378. doi: 10.1056/NEJMc2106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greinacher A, Selleng K, Mayerle J, et al. Anti-platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood. 2021;138:1269–1277. doi: 10.1182/blood.2021012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcucci R, Marietta M. Intern Emerg Med Vaccine-induced thrombotic thrombocytopenia: the elusive link between thrombosis and adenovirus-based SARS-CoV-2 vaccines. Intern Emerg Med. 2021;16(5):1113–1119. doi: 10.1007/s11739-021-02793-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 31.Hafeez MU, Ikram M, Shafiq Z, Sarfraz Z, et al. Vaccine-associated thrombosis with thrombocytopenia syndrome (TTS): a systematic review and post hoc analysis. Clin Appl Thromb Hemost. 2021;27:10760296211048815. doi: 10.1177/10760296211048815. [DOI] [PMC free article] [PubMed] [Google Scholar]