Abstract
Cytokine expression in the brain has been suggested to mediate various sickness behaviors. Here we report that intraperitoneal injection of a Corynebacterium parvum antigen in C57BL/6 mice was followed by prolonged upregulation of cytokines in the cerebral cortex and subcortical structures in a time course that coincided with reduced spontaneous running activity.
The response of the immune system to microbial invasion is often accompanied by a series of sickness behaviors including reduced physical activity, daytime sleepiness, diminished food intake, and a decline in social activity (4, 8). The exact mechanism(s) underlying these sickness behaviors is unknown. However, upregulation of cytokine expression in the central nervous system (CNS) has been postulated to mediate a number of these behavioral responses to infection (3, 5, 10, 12).
The sensation of fatigue or exhaustion is a prominent symptom in a number of infectious and autoimmune diseases and has been a disabling manifestation of certain idiopathic disorders, such as chronic fatigue syndrome (6). We have developed a mouse model to study immunologically mediated fatigue using heat-killed Corynebacterium parvum as the immune stimulant (1, 11). Our previous studies have demonstrated that intraperitoneal (i.p.) inoculation of C. parvum greatly reduced spontaneous running activity for as long as 2 to 3 weeks and that interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) mRNA expression was observed in the brain at 10 days postinoculation (11). We have also shown that peripheral administration of neutralizing antibodies against IL-1β or TNF-α failed to restore the animals' running activity (11), suggesting that cytokines in the CNS may play an important mediating role in immunologically mediated fatigue. In this study, we sought to investigate the kinetics of C. parvum-induced expression of cytokines in selected brain regions that may be involved in immunologically mediated fatigue.
Time course of cytokine mRNA expression in the mouse brain.
Previously, we had shown, by using reverse transcription-PCR (RT-PCR) analysis, that i.p. inoculation of a commercially purchased C. parvum antigen in C57BL/6 mice was associated with expression of TNF-α, IL-1β, and transforming growth factor β (TGF-β) mRNA in the brain at 10 days postinoculation (11). We now used a quantitative RNase protection assay (RPA) to evaluate the expression of mRNA of these cytokines and of IL-1 and TNF receptors in the mouse brain at different time points after a single i.p. injection of C. parvum antigen (2 mg/mouse). Fifty micrograms of extracted total RNA (2) was used in the multiprobe RPA according to the manufacturer's protocol (PharMingen, San Diego, Calif.). Results were quantified for each cytokine (IL-1β, TNF-α, TGF-β), cytokine receptor (IL-1 receptor, TNF receptor), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, used as an internal control) in control (saline injection) and C. parvum-challenged mice. The average ratio of the mRNA of each cytokine or cytokine receptor to GAPDH mRNA in the control mice was used as the baseline.
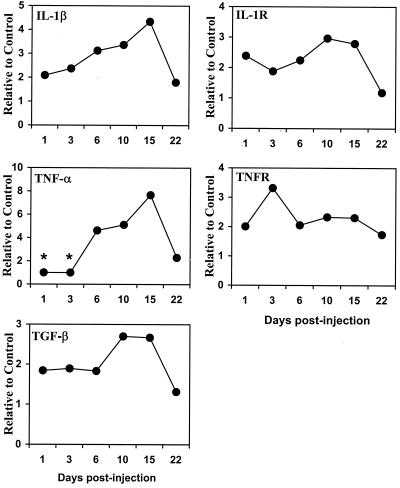
The C. parvum antigen was prepared in our laboratory according to the protocol from Ribi Immuno-Chem Research (Hamilton, Mont.) and was shown at a dose of 2 mg/mouse inoculated i.p. to induce a >50% reduction in daily spontaneous running activity over a 2-week period using an exercise wheel to measure running distance (1) (Fig. 1). The level of IL-1β mRNA expression, relative to that for the control, increased about 2-fold at 24 h after C. parvum antigen injection, peaked at 4.5-fold 15 days after C. parvum inoculation, and then gradually returned to control levels at 22 days postinjection (Fig. 2). TNF-α mRNA expression was first detected at 6 days postinjection, peaked at a level eightfold higher than control values 15 days postinjection, and returned to near the control level by day 22. A similar profile was found for TGF-β mRNA, although at a lesser magnitude. In contrast to IL-1β, TNF-α, and TGF-β, no change in IL-6 mRNA was detected (data not shown). Expression levels of IL-1 receptor mRNA and TNF receptor mRNA also showed two- to threefold increases after C. parvum injection and remained elevated throughout the sampling periods, except that IL-1 receptor mRNA declined to near-control levels at day 22. The time course of expression of IL-1β, TNF-α, TGF-β, and IL-1 receptor mRNA levels appeared to coincide with the reduction of spontaneous running behavior shown in Fig. 1.
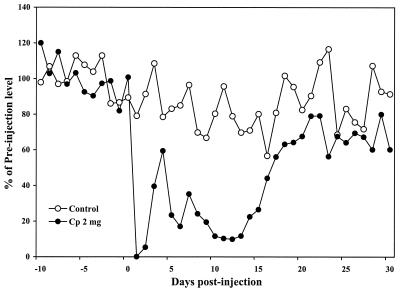
FIG. 1.
C. parvum (Cp) antigen-induced reduction of spontaneous running activity. C. parvum antigen at 2 mg/mouse was injected i.p. (0.2 ml) in mice (day 0) after 15 days of running adaptation. Control mice were injected with saline (0.2 ml). Running activity was recorded for 30 days after injection. The mean of preinjection running distances of individual mice was used as the baseline. Ten mice were used per group.
FIG. 2.
Cytokine mRNA expression in the brains of C. parvum antigen-challenged mice. Total RNA (50 μg) from control (saline injection) or C. parvum antigen-challenged mouse brains at various time points was used in the multitemplate RPA. Expression of cytokines, their receptors, and GAPDH (as an internal control) in individual mouse brain was quantified. The average ratio of cytokine or cytokine receptor to GAPDH in the control mouse was used as a baseline. There were four mice per group per time period. Asterisks, expression below detectable threshold.
Cytokine proteins in specific brain regions.
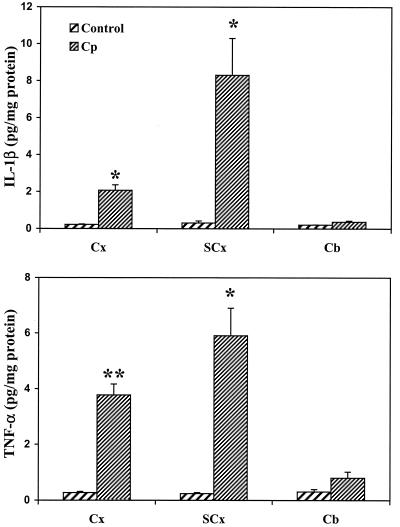
To determine whether upregulation of cytokine mRNA following inoculation of C. parvum antigen was associated with the production of cytokines at the protein level, an enzyme-linked immunosorbent assay (ELISA) method was used to measure cytokines in brain tissue (dissected into cortex, subcortex, and cerebellum) at day 10 post-C. parvum antigen injection. A marked increase in IL-1β and TNF-α protein production was found in the cerebral cortex and the subcortical brain region, while no significant change was seen in the cerebellum (Fig. 3). Consistent with the mRNA findings, no increase in levels of IL-6, which was undetectable, was found in any of the three brain regions (data not shown).
FIG. 3.
Cytokine protein production in the cortex (Cx), subcortex (SCx), and cerebellum (Cb) from C. parvum (Cp) antigen-challenged mice. Protein extracts from three brain regions of control (saline injection) or C. parvum antigen-challenged mice at day 10 postinoculation were assayed for cytokine levels (expressed as picograms per milligram of brain tissue protein) by ELISA. ∗, P < 0.05; ∗∗, P < 0.001. Significance was calculated for differences from the respective control (Student's t test; n = 5 per group).
Taken together, the results of these experiments indicate that the immunologic response to an i.p. challenge with C. parvum antigen is associated with a relatively rapid and sustained upregulation of IL-1β and TNF-α and/or their cognate receptors within cortical and subcortical regions of the brain. These findings contrast with those of studies of lipopolysaccharide as an immune stimulant, which have shown a rapid but transient upregulation of TNF-α and IL-1β in the mouse brain (7, 9). The observation that cytokine upregulation in the mouse brain following C. parvum challenge was associated with a decrease in running activity supports the notion that cytokines within the CNS serve as mediators of behavioral responses that are seen in a variety of infectious and immunologic disorders. Further investigation is needed, however, to determine more specifically which brain structures and cell types are involved in cytokine expression and whether this upregulation of brain cytokines indeed plays a pivotal mediator role in the decreased physical activity observed as a neurobehavioral response to the sensation of fatigue.
Acknowledgments
This study was supported by U.S. Public Health grant AI35110.
REFERENCES
- 1.Chao C C, DeLahunt M, Hu S, Close K, Peterson P K. Immunologically mediated fatigue: a murine model. Clin Immunol Immunopathol. 1992;64:161–165. doi: 10.1016/0090-1229(92)90194-s. [DOI] [PubMed] [Google Scholar]
- 2.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 3.Dantzer R, Auber A, Bluthe R, Gheusi G, Cremona S, Laye S, Konsman J, Parnet P, Kelley K W. Mechanisms of the behavioral effects of cytokines. Adv Exp Med Biol. 1999;461:83–105. doi: 10.1007/978-0-585-37970-8_6. [DOI] [PubMed] [Google Scholar]
- 4.Dantzer R, Bluthe R M, Gheusi G, Cremona S, Laye S, Parnet P, Kelley K W. Molecular basis of sickness behavior. Ann N Y Acad Sci. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 5.Dantzer R, Bluthe R M, Laye S, Bret-Dibat J L, Parnet P, Kelley K W. Cytokines and sickness behavior. Ann N Y Acad Sci. 1998;840:586–590. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda K, Straus S E, Hickie I, Sharpe M C, Dobbin J G, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Gabellec M M, Griffais R, Fillion G, Haour F. Expression of interleukin 1 alpha, interleukin 1 beta and interleukin 1 receptor antagonist mRNA in mouse brain: regulation by bacterial lipopolysaccharide (LPS) treatment. Brain Res Mol Brain Res. 1995;31:122–130. doi: 10.1016/0169-328x(95)00042-q. [DOI] [PubMed] [Google Scholar]
- 8.Hart B L. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 9.Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 10.Maier S F, Watkins L R. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 11.Sheng W S, Hu S, Lamkin A, Peterson P K, Chao C C. Susceptibility to immunologically mediated fatigue in C57BL/6 versus BALB/c mice. Clin Immunol Immunopathol. 1996;81:161–167. doi: 10.1006/clin.1996.0172. [DOI] [PubMed] [Google Scholar]
- 12.Watkins L R, Nguyen K T, Lee J E, Maier S F. Dynamic regulation of pro-inflammatory cytokines. Adv Exp Med Biol. 1999;461:153–179. doi: 10.1007/978-0-585-37970-8_10. [DOI] [PubMed] [Google Scholar]