Abstract
BNT162b2 is one of the effective COVID-19 vaccines. However, some researchers have also reported some neurological adverse events after the vaccination. Here, we present a case of a 52-year-old female who developed aquaporin (AQP) 4-IgG-positive neuromyelitis optica spectrum disorder (NMOSD) 14 days after the first dose of BNT162b2. She experienced the neck pain, weakness of the left arm and leg, numbness of the left hand, and impaired temperature sensation of the right leg. MRI showed T2WI hyperintense lesions in the area postrema and cervical spinal cord ranging from C1 to C6 level and Gd-enhanced lesions from C3 to C5 level; especially left lateral column was predominantly enhanced. Cell-based assays showed anti-AQP4 antibody (AQP4Ab) was positive. We diagnosed AQP4-IgG-positive NMOSD. After high-dose glucocorticoid therapy, she is showing improved symptoms. The present case was characterized by the findings that a Gd-enhanced lesion in the cervical cord localized dominantly at the left lateral column, consistent with the side of the shoulder where the vaccine was injected. Many studies suggested that AQP4-IgG-positive NMOSD development has multistep mechanisms following the blood–brain barrier (BBB) breakdown. We suspected that immune responses following vaccination lead to BBB disruptions. Through the limitedly damaged BBB, the plasma cells producing AQP4Abs might be recruited to CNS, and AQP4Abs might bind to the cervical cord and the area postrema. A population-based study revealed that neurological events following COVID-19 vaccination were less likely to be observed than COVID-19 infectious symptoms. Considering rare adverse events, clinicians need to observe any changes in patient condition.
Keywords: Neuromyelitis optica spectrum disorder, Myelitis, AQP4, COVID-19 vaccine
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen that causes coronavirus disease (COVID-19), has been responsible for hospitalizations and deaths globally. COVID-19 presents with acute respiratory symptoms and also leads to long-term neurological ones. In order to combat the COVID-19 pandemic, several vaccines have been developed and utilized worldwide.
BNT162b2 (Comirnaty©, BioNTech/Pfizer) is one of the effective COVID-19 vaccines. Many randomized trials and real-world studies have revealed that the vaccines are key to reducing COVID-19 infections, transmissions, hospitalizations, and death. However, some researchers have also reported that the vaccination leads to some neurological events. Here, we report a case of a patient who developed aquaporin (AQP) 4-IgG-positive neuromyelitis optica spectrum disorder (NMOSD) 2 weeks after vaccination with the first dose of BNT162b2.
Case presentation
A 52-year-old right-handed female experienced a first attack of NMOSD after vaccination with the first dose of BNT162b2. She had a mild fever the day after vaccination but no other inflammation reactions, including local reactions in her left arm around the injection site for 13 days. Fourteen days after vaccination, she began to feel pain ranging from the neck to the left shoulder, weakness of the left arm, numbness of the left hand, and impaired temperature sensation of the right leg. Seventeen days after vaccination, she complained of weakness of the left leg.
Twenty-one days after vaccination, she was admitted to our department. On admission, she had no visual impairment. Ophthalmological checkups showed no remarkable findings suggesting optic neuritis. The other cranial nerves were intact. Distal-dominant moderate to severe weakness of the left upper extremity, mild weakness of the left lower extremity, and hypesthesia of the abdomen and the right thigh were found. Her left grasp power was 4.5 kg compared with 19.0 kg for her right. Lhermitte sign was positive. Neither nausea nor hiccups were observed.
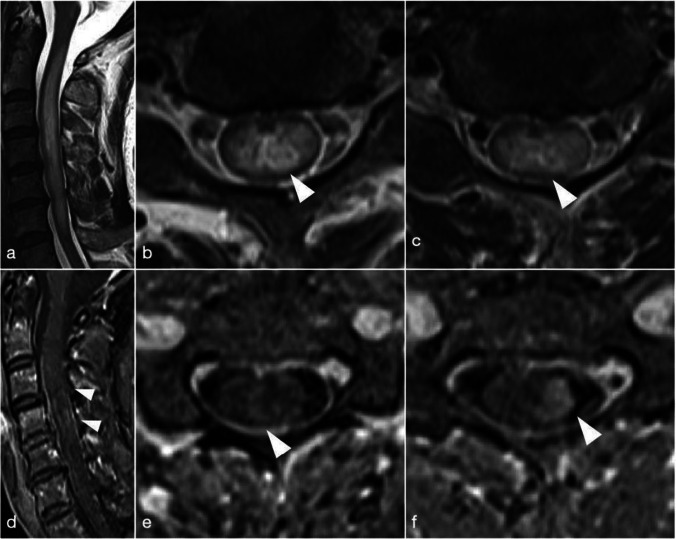
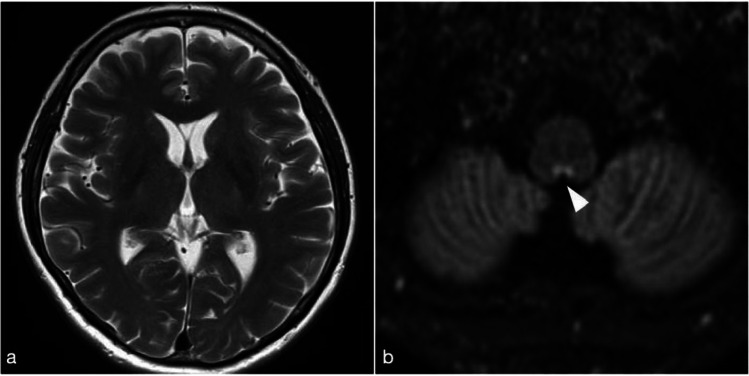
Magnetic resonance imaging (MRI) of the spinal cord showed that T2-weighted (T2WI) and fluid-attenuated inversion recovery (FLAIR) hyperintense lesions reached from the C1 to C6 level. Gadolinium (Gd)-enhancement lesion was located from the C3 to C5 level, and, especially, left lateral fasciculus was enhanced predominantly (Fig. 1). Cerebral MRI showed T2-weighted and double inversion recovery (DIR) hyperintense lesions in the area postrema and the obex of the medulla (Fig. 2). These lesions were not enhanced. No other remarkable signal changes were detected in the cortices or the optic nerves.
Fig. 1.
Spinal MRI 21 days after vaccination. a T2WI showed hyperintense lesions from the C1 to C6 level. b C3 level. c C5 level. d Gd-enhancement image showed lesions from the C3 to C5 level. e C3 level. f C5 level. The left column predominantly enhanced at the C4 and C5 level
Fig. 2.
Brain MRI 21 days after vaccination. a T2WI showed no signal changes in the cortex. b DIR image showed hyperintense lesions in the area postrema and the obex of medulla
Routine blood tests detected no remarkable abnormal values. Cell-based assays showed that anti-AQP4 antibody (AQP4Ab) was positive. Other autoantibodies were absent. On cerebrospinal fluid (CSF) analysis, mild pleocytosis (9 cells/µL), in which mononuclear cells dominated (7 cells/µL), mildly increased protein (49 mg/dL), and elevated myelin basic protein (MBP) (1550 pg/mL) were found. IgG index was normal (0.54), and oligoclonal bands were negative.
We ruled out SARS-CoV-2 infection by a negative polymerase chain reaction (PCR) test and the absence of antibodies against the SARS-CoV-2 N protein. In addition, she had no complaints of fever, cough, or other known COVID-19 symptoms before admission during the pandemic periods.
The patient had a history of Guillain-Barré syndrome (GBS). At the age of 35, she developed weakness of the bilateral lower extremities. The onset was about a month after suffering a cervical sprain because of a car accident. These symptoms progressed for about a month until her admission to our department. On admission, a nerve conduction study (NCS) showed temporal dispersion findings and other demyelinating patterns at multiple nerves. MRI showed no remarkable signal changes in the spinal cord. We diagnosed GBS, and after intravenous immunoglobulin therapy, she fully recovered. She had experienced no similar symptoms until the present admission. The present MRI showed no enlargements of nerve roots or enhancement lesions in the cauda equina, and NCS showed no demyelinating patterns in the bilateral median nerves and no other findings suggesting peripheral neuropathy. The family history was negative for any neurologic disorders and autoimmune diseases.
After other differential diagnoses were excluded, following the 2015 International Consensus Diagnostic Criteria, we diagnosed AQP4-IgG-positive neuromyelitis optica spectrum disorder. We conducted two cycles of high-dose glucocorticoid therapy (each 1000 mg methylprednisolone i.v. for 3 days; the first cycle was initiated at 21 days after vaccination; the second cycle was at 28 days) and oral administration of 40 mg prednisolone for 16 days and a tapering dose for about 2 weeks.
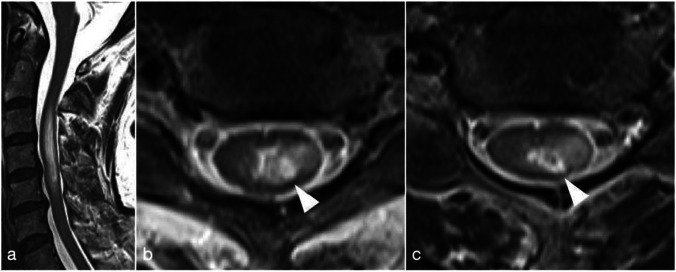
Twenty-eight days after vaccination, T2WI hyperintense lesions shrank to locate from the C3 to C5 level in the cervical spinal cord, and lateralization pattern at the left lateral column remained (Fig. 3). Currently, the patient is taking 25 mg of prednisolone orally and showing improved symptoms.
Fig. 3.
Spinal MRI 28 days after vaccination. a T2WI showed hyperintense lesions from the C3 to C5 level in the cervical spinal cord. b C3 level. c C5 level. The lesions were lateralized to left lateral column from the C3 to C5 level
Discussion
To our knowledge, this is the first case of AQP4-IgG-positive NMOSD development following the initial dose of BNT162b2. The present case was noteworthy in terms of its temporal association with the vaccine, the type of vaccine, and AQP4-IgG status. Previously, there have been some reports of cases of NMOSD after COVID-19 vaccination, such as AQP4-IgG-positive NMOSD development 2 months after administration of inactivated vaccine [1], AQP4-IgG-positive after Gam-COVID-Vac [2], and AQP4-IgG-negative after mRNA-1273 [3]. In the present case, the duration from vaccination to development was 2 weeks. Considering myelitis and other neurological disorders following the vaccination occurred approximately at the tenth day, ranging from 1 to 2 weeks after vaccination in other previous reports, it is reasonable to assume that the present case followed the timecourse of the post-vaccine adverse events. Additionally, the available vaccines against COVID-19 include mRNA vaccines, BNT162b2 and mRNA-1273, and adenovirus vector vaccines, ChAdOx1nCoV and Gam-COVID-Vac. Recently, a large population-based study in the UK compared neurological events between BNT162b2 and ChAdOx1nCoV and showed that these have different risks of adverse events [4]. This study suggested that distinct mechanisms underlie adverse events following these two types of vaccine. Furthermore, AQP4Ab is a major disease-specific biomarker of NMOSD; simultaneously, AQP4Ab plays a direct role in astrocyte damage in NMOSD. The pathophysiology behind AQP4-IgG-positive NMOSD lies in astrocyte lysis, not demyelination, which is thought to underlie another subtype of NMOSD, namely MOG-IgG positive NMOSD. Clinically, AQP4-IgG serological status is incorporated into the International Diagnostic Criteria. In these regards, the present case is significant as the first manifestation of AQP4-IgG-positive NMOSD following BNT162b2.
The patient had a history of GBS in the present case. After intravenous immunoglobulin therapy, she recovered and had no experiences of similar symptoms for about 17 years. In addition, a month before GBS development, she suffered a cervical sprain because of a car accident. We are unsure whether this episode was related to the present NMOSD pathophysiology. At least, we assumed that the patient has a predisposition to humoral immunologic reactions triggered by exogenous factors.
Moreover, the present case was characterized by the finding that a Gd-enhancement lesion in the cervical spinal cord localized dominantly at the left lateral column, consistent with the side of the shoulder where the vaccine was injected. No local inflammation reactions occurred in her left arm. Although this feature may be only a coincidence, it does however need to be discussed in terms of pathophysiology. Many in vivo and in vitro studies showed that AQP4-IgG-positive NMOSD development has multistep mechanisms, including complement activations and astrocyte lysis following the blood–brain barrier (BBB) breakdown [5]. It is hypothesized that interleukin-6 (IL-6) signaling pathways and humoral factors lead the BBB to increased permeability and decreased integrity with glial cells in the acute phase. Through damaged BBB, the plasma cells producing AQP4Abs and other inflammatory mediator cells are recruited to the central nervous system. AQP4Abs binding to AQP4 interact with complements, and astrocyte lysis follows by the classical complement cascade. It is also known that AQP4 is highly expressed in the area postrema, which was the lesion other than the cervical spinal cord in the present case. Taken together, we suspected that immune responses following vaccination lead to BBB disruptions. Through the limitedly damaged BBB, the plasma cells producing AQP4Abs or the other mediators might be recruited to the CNS, and AQP4Abs might bind to the cervical cord and the area postrema. One of the possible mechanisms underlying the present event could be the IL-6 signaling pathways. This concordance remains to be discussed, and further research is needed.
As mentioned above, there have been some reported cases of neurological events following COVID-19 vaccination as well as symptoms caused by infection. It has also been reported that some patients with NMOSD tended to be reluctant to receive vaccines. However, a large population-based study in the UK recently revealed that neurological events following COVID-19 vaccination including BNT162b2 and ChAdOx1nCoV-19 were less likely to be observed than ones caused by COVID-19 infection [4]. For example, there was an increased risk of encephalitis, meningitis, and myelitis 1–28 days after COVID-19 infection (incident rate ratio (IRR): 2.70) but not after vaccination (IRR: 1.14 (BNT162b2); 1.07 (ChAdOx1nCoV-19)). Therefore, weighing these two different risks, we recommend vaccination. To encourage this, healthcare professionals have to provide more information about the risks of COVID-19-related neurological complications compared to those following COVID-19 vaccination. At the same time, considering rare adverse events reported, clinicians need to observe any changes in their condition after vaccination.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The study was performed in accordance with the Helsinki II Declaration and approved by the Institutional Review Board of our hospital.
Consent to participate
All participants (or their legal representatives) gave written informed consent.
Consent for publication
We thank the patient reported here for the consent given to describe and publish the case.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen S, Fan XR, He S, Zhang JW, Li SJ. Watch out for neuromyelitis optica spectrum disorder after inactivated virus vaccination for COVID-19. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2021;42(9):3537–3539. doi: 10.1007/s10072-021-05427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badrawi N, Kumar N, Albastaki U. Post COVID-19 vaccination neuromyelitis optica spectrum disorder: case report & MRI findings. Radiol Case Rep. 2021;16(12):3864–3867. doi: 10.1016/j.radcr.2021.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujikawa P, Shah FA, Braford M, Patel K, Madey J. Neuromyelitis optica in a healthy female after severe acute respiratory syndrome coronavirus 2 mRNA-1273 vaccine. Cureus. 2021;13(9):e17961. doi: 10.7759/cureus.17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patone M, Handunnetthi L, Saatci D, Pan J, Katikireddi SV, Razvi S, Hunt D, Mei XW, Dixon S, Zaccardi F, Khunti K, Watkinson P, Coupland C, Doidge J, Harrison DA, Ravanan R, Sheikh A, Robertson C, Hippisley-Cox J. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021 doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takai Y, Misu T, Suzuki H, Takahashi T, Okada H, Tanaka S, Okita K, Sasou S, Watanabe M, Namatame C, Matsumoto Y, Ono H, Kaneko K, Nishiyama S, Kuroda H, Nakashima I, Lassmann H, Fujihara K, Itoyama Y, Aoki M. Staging of astrocytopathy and complement activation in neuromyelitis optica spectrum disorders. Brain J Neurol. 2021;144(8):2401–2415. doi: 10.1093/brain/awab102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.