Abstract
Excessive release of proinflammatory cytokines from cells stimulated with lipopolysaccharide (LPS) or staphylococcal exotoxin (SE) mediates the pathophysiologic manifestations of septic shock. Tricyclodecan-9-yl (D609), an inhibitor of phosphatidylcholine-specific phospholipase C, suppressed LPS- or SE-induced cytokines and chemokines in human peripheral blood mononuclear cells. These data suggest a potential role for D609 in the treatment of septic shock.
Lipopolysaccharide (LPS) and staphylococcal exotoxins (SE) are among the most common etiological agents causing septic shock (2, 10, 18). Although these bacterial products interact with host cells through different receptors, they trigger excessive systemic release of inflammatory cytokines, resulting in septic shock. LPS from gram-negative bacteria binds to serum LPS-binding protein, which then facilitates its binding to the cell surface protein CD14 on monocytes/macrophages and other cells (21). The subsequent interaction of LPS-CD14 complex with Toll-like receptors on these cells initiates transmembrane signaling and cellular activation to secrete cytokines and chemokines (15).
Staphylococcal toxic shock syndrome toxin 1 (TSST-1), and the structurally related enterotoxins, are bacterial exotoxins that bind directly to major histocompatibility complex class II molecules on antigen-presenting cells (APC) (4, 8, 19) and stimulate T cells expressing specific Vβ elements (5, 9). These toxins are called superantigens because of their ability to polyclonally activate large populations of T cells (10). Interaction of SE with APC and T cells also results in hyperproduction of cytokines and chemokines, which is responsible for the pathogenesis of toxic shock (16). These cytokines include tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and interleukin 1 (IL-1), proinflammatory mediators with potent immunoenhancing effects (12). In addition, levels of circulating TNF-α and IL-1 correlate with the clinical symptoms of sepsis or septic shock (3).
In the last decade, numerous clinical trials conducted with antagonists to key mediators such as TNF-α (1, 7) or IL-1 (6) have not yielded significant therapeutic benefits for septic patients partly because sepsis is not the result of the release of any one cytokine. Thus, inhibitors that suppress multiple proinflammatory cytokines may be more efficacious for the treatment of septic shock. Recent studies show tricyclodecan-9-yl (D609), a specific inhibitor of phosphatidylcholine-specific phospholipase C (PC-PLC), protects mice from lethal shock induced either by TNF-α, LPS, or staphylococcal enterotoxin B (SEB) (14). Surprisingly, D609 did not reduce the SEB-induced IL-1, TNF-α, or IFN-γ levels in these animals (14). The proposed mechanism of action of D609 is via blockade of the cytotoxic action of TNF-α. In another study, D609 improved the survival of mice with LPS-induced shock (20), accompanied by a reduction in the levels of IL-1 and IL-6, but not TNF-α, in serum. In the present study, the modulatory effect of D609 on various cytokines and chemokines induced by both bacterial LPS and superantigens in human peripheral blood mononuclear cells (PBMC) was further examined in vitro.
Purified SEB and TSST-1 were obtained from Toxin Technology (Sarasota, Fla.). The endotoxin content of these preparations was <1 ng of endotoxin/mg of protein, as determined by the Limulus amoebocyte lysate assay (BioWhittaker, Walkersville, Md.). LPS (Escherichia coli O55:B5) was obtained from Difco (Detroit, Mich.). Recombinant human TNF-α (rhTNF-α), antibodies against hTNF-α, peroxidase-conjugated anti-rabbit immunoglobulin G (IgG), and peroxidase-conjugated anti-goat IgG were obtained from Boehringer Mannheim (Indianapolis, Ind.). Recombinant monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1α (MIPγ-1α) and MIP-1β, and antibodies against hIL-1β, hIL-6, hMIP-1α, and MIP-1β were purchased from R&D Systems (Minneapolis, Minn.). rhIL-1β was kindly provided by J. Oppenheim (National Cancer Institute, Frederick, Md.). rhIFN-γ and rhIL-6 were obtained from Collaborative Research (Boston, Mass.). Antibodies against hIFN-γ and MCP-1 were obtained from Pharmingen (San Diego, Calif.). D609 was purchased from Alexis (San Diego, Calif.) and dissolved in phosphate-buffered saline, pH 7.4. All other reagents were from Sigma (St. Louis, Mo.).
Human PBMC were isolated by Ficoll-Hypaque density gradient centrifugation of heparinized blood from normal human donors. PBMC (106 cells/ml) were cultured at 37°C in 24-well plates containing RPMI 1640 medium and 10% heat-inactivated fetal bovine serum. Cells were incubated with either SEB (150 ng/ml), TSST-1 (150 ng/ml), or LPS (100 ng/ml) for 16 h, and the supernatants were harvested and analyzed for IL-1β, TNF-α, IL-6, IFN-γ, MCP-1, MIP-1α, and MIP-1β. Cytokines and chemokines were measured by an enzyme-linked immunosorbent assay (ELISA) with cytokine- or chemokine-specific antibodies according to the manufacturer's instructions (11, 13). Human recombinant cytokines and chemokines (20 to 1,000 pg/ml) were used as standards for calibration on each plate. The detection limit of each assay was 20 pg/ml. The cytokine and chemokine data were expressed as the mean reading ± standard deviation (SD) for duplicate samples. D609, when present, was added simultaneously with the stimulating agent. Cytotoxicity was measured by the release of lactate dehydrogenase (LDH) from the cytosol into the culture supernatant. LDH was quantitated by a colorimetric cytotoxicity kit (Boehringer Mannheim) according to the instructions of the manufacturer. The maximum amount of releasable LDH (100%) was obtained by lysing cells with 1% Triton X-100. Apoptotic cell death was determined by ELISA of cytoplasmic histone-associated-DNA fragments after programmed cell death (Cell Death ELISA; Boehringer Mannheim) according to the manufacturer's instructions. T-cell proliferation assays were performed with PBMC (105 cells/well), plated in triplicate with SEB or TSST-1 (150 ng/ml), with or without D609, for 48 h at 37°C in 96-well microtiter plates. Cells were pulsed with 1 μCi of [3H]thymidine (New England Nuclear, Boston, Mass.)/well during the last 5 h of culture as described previously (11). Cells were harvested onto glass fiber filters, and incorporated [3H]thymidine was measured by liquid scintillation. All data were analyzed for significant differences by Student's t test with Stata (Stata Corp., College Station, Tex.). Differences between the D609-treated group and untreated controls were considered significant if P was <0.02.
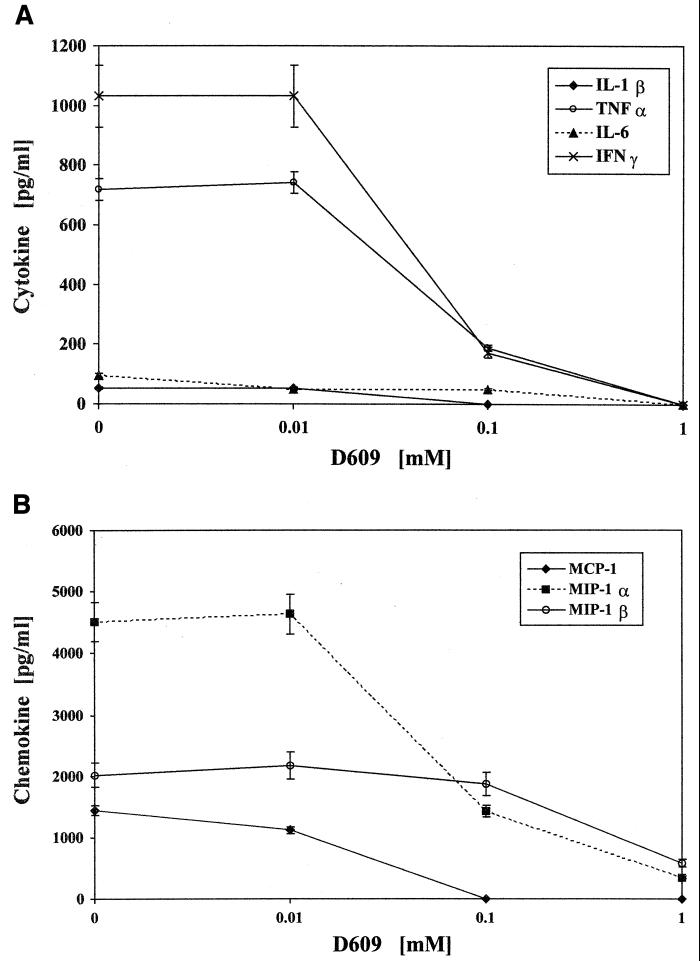
Figure 1 shows that D609 blocks the production of cytokines IL-1β, TNF-α, IL-6, and IFN-γ and of chemokines MCP-1, MIP-1α, and MIP-1β from PBMC incubated with 150 ng of SEB/ml in a dose-dependent manner. Complete inhibition of these cytokines and chemokines was observed at high doses of D609 (0.5 mM). Similar dose-response inhibition by D609 was observed at lower concentrations of SEB (1 and 10 ng/ml) and LPS (10 and 100 ng/ml) (data not shown). Good cell viability (>90%) was evident at low concentrations of D609 (<0.4 mM) as judged by trypan blue exclusion, and subsequent experiments were performed at 0.2 mM D609. Cytotoxicity was also determined by LDH release at various concentrations of D609 at 16 and 48 h. No cytotoxicity was observed from 0.01 to 0.8 mM of D609, as LDH release was not different from that for the medium control up to 0.8 mM D609. At 1 mM D609, 30% of maximum LDH release was indicated. Cellular apotosis as determined by histone-DNA complexes was absent at these concentrations of D609.
FIG. 1.
Dose-response inhibition of IL-1β, TNF-α, IL-6, and IFN-γ production (A) and of MCP-1, MIP-1α, and MIP-1β production (B) by PBMC stimulated with 150 ng of SEB/ml in the presence of various D609 concentrations. Values represent means ± standard errors of the means for duplicate samples from two experiments. Inhibition at all concentrations of D609, with the exception of 0.01 mM, was statistically significant by comparison to control SEB-stimulated cells (P < 0.02).
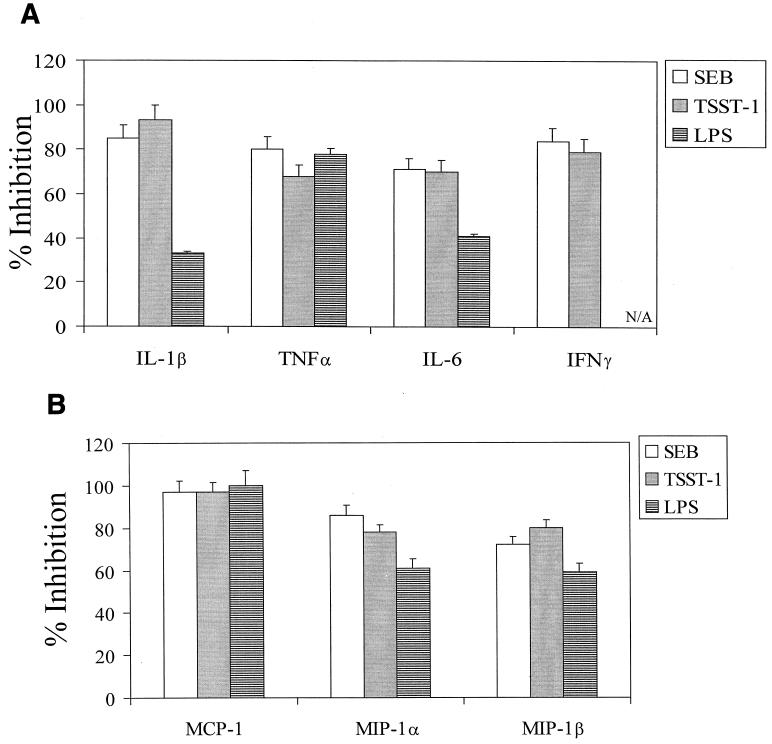
The effect of D609 on cytokine and chemokine production by TSST-1- or LPS-stimulated PBMC was compared with that on SEB-stimulated cells from 12 blood donors (Fig. 2). Production of IL-1β, TNF-α, IL-6, and IFN-γ by TSST-1-stimulated cells was inhibited 93, 68, 70, and 79%, respectively, by 0.2 mM D609. D609 also decreased the levels of MCP-1, MIP-1α, and MIP-1β 97, 78, and 80%, respectively, in TSST-1-stimulated PBMC. Inhibition of cytokines and chemokines in SEB-stimulated cells was similar to that for TSST-stimulated cells. With LPS-activated cells, IL-1β, TNF-α, IL-6, MCP-1, MIP-1α, and MIP-1β production were blocked 33, 78, 41, 99, 61, and 59%, respectively, by D609. The inhibition of SEB-, TSST-, or LPS-induced chemokines by D609 is probably direct, since previous reports indicated that both SEB and LPS can stimulate PBMC to release these chemokines directly (13, 22).
FIG. 2.
Inhibition of production of IL-1β, TNF-α, IL-6, and IFN-γ (A) and of MCP-1, MIP-1α, and MIP-1β (B) by PBMC stimulated with either SEB (150 ng/ml), TSST-1 (150 ng/ml), or LPS (100 ng/ml) in the presence of 0.2 mM D609. Values represent means ± standard errors of the means for PBMC cultures from 12 blood donors for experiments with SEB and TSST, and a subset of 3 donors for LPS. Results are statistically significant (P < 0.02) between SE and SE-plus-D609 samples and between LPS and LPS-plus-D609 samples. N/A, not applicable.
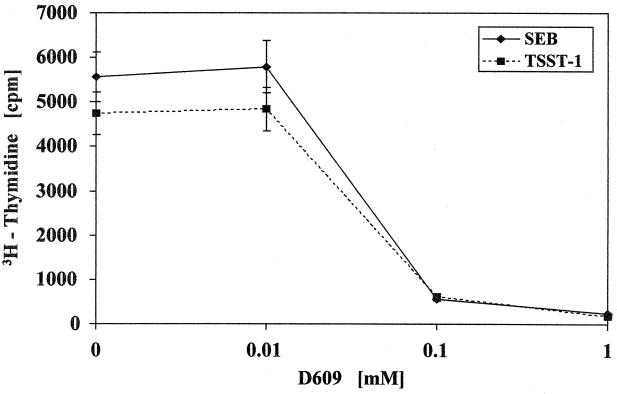
Because superantigens also cause T-cell proliferation, the effect of D609 on SE-induced T-cell proliferation was investigated. Figure 3 shows that D609 dose-dependently inhibited SEB- and TSST-1-stimulated T-cell proliferation, achieving 99% inhibition at 0.1 mM D609.
FIG. 3.
Inhibition of T-cell proliferation in PBMC stimulated with SEB or TSST-1 at 150 ng/ml by varying concentrations of D609. Values are mean counts ± standard errors of the means for triplicate cultures and represent five experiments. Results are statistically significant (P < 0.01) between SE and SE-plus-D609 samples.
This study demonstrated that D609 effectively inhibited LPS-and superantigen-mediated production of cytokines and chemokines by human PBMC in vitro. T-cell proliferation induced by staphylococcal superantigens was also suppressed completely. Downregulation of proinflammatory cytokines and chemokines by D609 in LPS-, SEB-, and TSST-1-stimulated PBMC suggested that D609 may affect the pathophysiology of septic shock. These observations are in agreement with a recent report that D609 inhibits LPS-induced TNF-α and IL-6 production in alveolar macrophages (17). Previous studies indicated that D609 prevents LPS- and SEB-induced septic shock but could not demonstrate a direct inhibition of the same inflammatory cytokines in vivo (14, 20). Nevertheless, the success of D609 in improving survival of murine septic shock in these studies provides further evidence of the utility of this therapeutic compound for septic shock. Discrepancies between the inhibition of TNF-α by D609 in this study and the lack of inhibition by D609 in mice exposed to LPS or SEB could be the result of species differences in sensitivity to D609 and/or the complexity of lethal septic shock in animal models. However, the present findings with human PBMC showed that D609 inhibited multiple proinflammatory cytokines and chemokines induced by bacterial LPS and superantigens, thus suggesting its potential utility for treating human septic shock. It is likely that D609 and other agents that block signal transduction pathways of LPS-or SE-activated cells are more efficacious in countering increased cytokine levels and the ill effects of these mediators.
Acknowledgments
I thank Marilyn Buckley for excellent technical assistance, and Lorraine Farinick and Quentisha Mason for preparation of graphs.
REFERENCES
- 1.Abraham E, Glauser M P, Butler T, et al. P55 tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock: a randomized controlled multicenter clinical trial. JAMA. 1997;277:1531–1538. [PubMed] [Google Scholar]
- 2.Bergdoll M S, Schlievert P M. Toxic shock syndrome toxin. Lancet. 1984;ii:691. [Google Scholar]
- 3.Calandra T, Baumgartner J D, Grau G E, et al. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. J Infect Dis. 1990;161:982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- 4.Chatila T, Geha R S. Signal transduction of microbial superantigens via MHC class II molecules. Immunol Rev. 1993;131:43–59. doi: 10.1111/j.1600-065x.1993.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 5.Choi Y, Kotzin B, Hernon L, Callahan J, Marrack P, Kappler J. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc Natl Acad Sci USA. 1989;86:8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher C J, Jr, Dhainaut J F, Opal S M, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. JAMA. 1994;271:1836–1843. [PubMed] [Google Scholar]
- 7.Fisher C J, Jr, Agosti J M, Opal S M, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 8.Fraser J D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989;339:221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- 9.Gascoigne N R J, Ames K T. Direct binding of secreted TCR β chain to superantigen associated with MHC class II complex protein. Proc Natl Acad Sci USA. 1991;88:613–616. doi: 10.1073/pnas.88.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotzin B L, Leung D Y M, Kappler J, Marrack P A. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 11.Krakauer T. Inhibition of toxic shock syndrome toxin-induced cytokine production and T cell activation by interleukin 10, interleukin 4, and dexamethasone. J Infect Dis. 1994;172:988–992. doi: 10.1093/infdis/172.4.988. [DOI] [PubMed] [Google Scholar]
- 12.Krakauer T, Vilcek J, Oppenheim J J. Proinflammatory cytokines: TNF and IL-1 families, chemokines, TGFβ and others. In: Paul W, editor. Fundamental immunology. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1998. pp. 775–811. [Google Scholar]
- 13.Krakauer T. Induction of CC chemokines in human peripheral blood mononuclear cells by staphylococcal exotoxins and its prevention by pentoxifylline. J Leukoc Biol. 1999;66:158–164. doi: 10.1002/jlb.66.1.158. [DOI] [PubMed] [Google Scholar]
- 14.Machleidt T, Kramer B, Adam D, Neumann B, Schutze S, Weiegmann K, Kronke M. Function of the p55 tumor necrosis factor receptor “death domain” mediated by phosphatidylcholine-specific phospholipase C. J Exp Med. 1996;184:725–733. doi: 10.1084/jem.184.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 16.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer P H, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen SEB: critical role of TNF. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monick M M, Cater A B, Gudmundsson G, Mallampalli R, Powers L S, Hunninghake G W. A phosphatidylcholine-specific phospholipase C regulates activation of p42/44 mitogen-activated protein kinases in lipopolysaccharide-stimulated human alveolar macrophages. J Immunol. 1999;162:3005–3012. [PubMed] [Google Scholar]
- 18.Morrison D C, Ryan J L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–438. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 19.Scholl P, Diez A, Mourad W, Parsonnet J, Geha R S, Chatila T. Toxic shock syndrome toxin-1 binds to major histocompatibility complex class II molecules. Proc Natl Acad Sci USA. 1989;86:4210–4214. doi: 10.1073/pnas.86.11.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschaikowsky K, Schmidt J, Meisner M. Modulation of mouse endotoxin shock by inhibition of phosphatidylcholine-specific phospholipase C. J Pharm Exp Ther. 1998;285:800–804. [PubMed] [Google Scholar]
- 21.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–456. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z M, Liu C, Dziarski R. Chemokines are the main proinflammatory mediators in human monocytes activated by Staphylococcus aureus, peptidoglycan, and endotoxin. J Biol Chem. 2000;275:20260–20267. doi: 10.1074/jbc.M909168199. [DOI] [PubMed] [Google Scholar]