Abstract
Monkeypox (MPX), a zoonotic infection caused by the monkeypox virus (MPXV), has re-emerged worldwide with numerous confirmed cases with person-to-person transmission through close contacts, including in sexual networks. Therefore, this study aimed to determine the epidemiological situation of monkeypox transmission by possible sexual contact. A systematic literature review was conducted using PubMed, Scopus, Web of Science, and Embase databases until 18 August 2022. The key search terms used were “monkeypox”, “sexual contact”, “sexual intercourse” and “sexual transmission”. A total of 1291 articles were retrieved using the search strategy. After eliminating duplicates (n = 738) and examining by title, abstract, and full text, 28 studies reporting case reports of monkeypox with a detailed description of clinical features, sexually transmitted diseases, method of diagnosis, location and course of skin lesions, and treatment were included. A total of 4222 confirmed cases of monkeypox have been reported, of which 3876 monkeypox cases are the result of transmission by sexual contact distributed in twelve countries: 4152 cases were male with a mean age of 36 years. All confirmed cases of monkeypox were diagnosed by reverse transcriptase-polymerase chain reaction (RT-PCR). The most frequent clinical manifestations were fever, lymphadenopathy, headache, malaise, and painful perianal and genital lesions. The most frequent locations of the lesions were perianal, genital, oral, trunk, upper and lower extremities. Patients were in good clinical condition, with treatment based on analgesics and antipyretics to relieve some symptoms of monkeypox. A high proportion of STIs and frequent anogenital symptoms were found, suggesting transmissibility through local inoculation during close skin-to-skin or mucosal contact during sexual activity. The highest risk of monkeypox transmission occurs in men who have sex with men, and MPXV DNA could be recovered in seminal fluid. It is essential to establish health policies for the early detection and management of patients with monkeypox.
Keywords: Monkeypox, sexual contact, orthopoxvirus, sex with men, monkeypox virus
1. Introduction
Monkeypox (MPX) has re-emerged on a global scale with numerous cases confirmed across the globe in 2022 [1]. The rapid spread of cases across different countries has raised serious concern among public health officials worldwide, prompting accelerated investigations aimed at identifying the origins and cause of the rapid spread of cases [2,3]. As of 25 August 2022, 46,724 confirmed cases of monkeypox have been reported in 98 countries worldwide [4]. According to the World Health Organization (WHO), the outbreak continues to primarily affect men who have sex with men, who have reported recent sex with one or more male partners [5]. In addition, the most frequently reported and suspected route of transmission among known contacts has been through possible sexual contact.
MPX is a zoonotic viral disease caused by the monkeypox virus (MPXV) [6,7]. MPXV is a double-stranded DNA virus of the genus Orthopoxvirus of the family Poxviridae [8,9], first identified as a human pathogen in the Democratic Republic of Congo (DRC, formerly Zaire) in 1970 [10,11]. MPXV has two distinct genetic clades: the Central African clade (Congo basin) and the West African clade [11,12]. The mortality rate varies between 1% and 10%, depending on the clade, and children, pregnant women, and immunocompromised individuals are at high risk of a fatal outcome [13].
Individuals with MPX have an incubation period of 7 to 21 days before the onset of clinical manifestations [14], such as fever, headache, muscle aches, back pain, chills, rash, and lymphadenopathy [15,16]. Complications of MPX may include pneumonitis, encephalitis, visibly life-threatening keratitis, and secondary bacterial infections [17,18].
MPX is transmitted to humans by direct contact with an infected person or animal or by contact with virus-contaminated material [15,19,20]. The virus is spread by oral and nasopharyngeal fluid exchange or intradermal injection; it replicates rapidly at the site of inoculation and spreads to adjacent lymph nodes [21]. In addition, most of these patients presented atypical skin lesions with lesions in the genital and perianal region [22]. Therefore, it is important to evaluate sexual transmission and to take into consideration patients with human immunodeficiency virus (HIV) and sexually transmitted infections (STI) [23].
Currently, monkeypox has no definitive vaccine or drug; it is treated by controlling symptoms and preventing or ameliorating complications [24]. However, the United States has recommended a licensed vaccine, JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for vaccination of persons at risk of occupational exposure to Orthopoxviruses [25].
The objective of the present study is to determine the epidemiological situation of monkeypox transmission by possible sexual contact.
2. Materials and Methods
2.1. Protocol and Registration
This protocol follows the recommendations established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [26], and it has been reported in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42022340855).
2.2. Eligibility Criteria
To assess the prevalence of sexual contact transmission of monkeypox, we included peer-reviewed published articles with study designs of case reports, case series, and observational studies (cohort and nonrandomized intervention studies). No language limit was established for the articles and publications were included until 18 August 2022. Systematic review articles, narrative reviews, randomized clinical trials, editorials, letters to the editor, and conference proceedings were excluded.
2.3. Information Sources and Search Strategy
A systematic search was carried out in PubMed, Scopus, Web of Science and Embase. The search terms used were: (“Monkeypox” OR “Monkey Pox”) AND (“sexual contact” OR “sexual intercourse” OR “sexual behavior” OR “transmission” OR “sexual transmission” OR “Sexual Intercourse” OR “Intercourse, Sexual” OR “Coital” OR “Copulation” OR “Sexual relations”) (Table 1). The searches were completed on 18 August 2022, and four different investigators independently evaluated the search results.
Table 1.
Bibliographic search strategy.
| Base | Search Strategy |
|---|---|
| PUBMED | #1 (“Monkeypox” OR “Monkey Pox”) #2 (“sexual contact” OR “sexual intercourse” OR “sexual behavior” OR “transmission” OR “sexual transmission” OR “Sexual Intercourse” OR “Intercourse, Sexual” OR Coital OR Copulation OR “Sexual relations”) #3 = #1 AND #2 |
| SCOPUS | #1 TITLE-ABS-KEY (“Monkeypox” OR “Monkey Pox”) #2 TITLE-ABS-KEY (“sexual contact” OR “sexual intercourse” OR “sexual behavior” OR “transmission” OR “sexual transmission” OR “Sexual Intercourse” OR “Intercourse, Sexual” OR Coital OR Copulation OR “Sexual relations”) #3 = #1 AND #2 |
| WEB OF SCIENCE |
#1 ALL = (“Monkeypox” OR “Monkey Pox”) #2 ALL = (“sexual contact” OR “sexual intercourse” OR “sexual behavior” OR “transmission” OR “sexual transmission” OR “Sexual Intercourse” OR “Intercourse, Sexual” OR Coital OR Copulation OR “Sexual relations”) #3 = #1 AND #2 |
| EMBASE | #1 ‘monkeypox’/exp OR ‘monkeypox’ OR ‘monkeypox virus’/exp OR ‘monkeypox virus’ #2 ‘sexual contact’ OR ‘sexual behavior’ OR transmission OR ‘sexual transmission’ OR ‘sexual intercourse’ OR ‘intercourse, sexual’ OR coital OR copulation OR ‘sexual relations’ #3 = #1 AND #2 |
2.4. Study Selection
Three investigators (D.A.L.F., E.G.V., J.J.B.) created a database based on the electronic searches, managed with the appropriate management software (EndNote), and duplicates were removed. Then, through Rayyan QCRI [27] three researchers (M.T.D.M., M.D.T. and O.C.S.) carried out the screening process, analyzing the titles and abstracts provided by the search independently, choosing those that appeared to meet the inclusion criteria and, if necessary, evaluating the full text. In case of disagreement, the investigators will discuss until a consensus is reached; in case of dispute, a fourth investigator will be invited to the discussion to help resolve it.
The authors (D.K.B.A. and A.J.R.M.) reviewed the full-text reports and analyzed the inclusion criteria to reach a decision.
2.5. Outcomes
The primary outcome was to report the epidemiological situation of monkeypox transmission by possible sexual contact.
2.6. Data Collection Process and Data Items
Four investigators independently extracted data from the selected studies into a Microsoft Excel spreadsheet. The following data were extracted from the selected studies: author data, date of publication, study design, country, sex, age, sexual behavior, sexually transmitted infections (STIs), signs and symptoms, diagnostic test, days from systemic symptoms to lesion onset, location of skin lesions, evolution of lesions, and treatment. A fifth investigator checked the list of articles and data extractions to ensure that there were no duplicate articles or duplicate information and resolved discrepancies about study inclusion.
3. Results
3.1. Study Selection
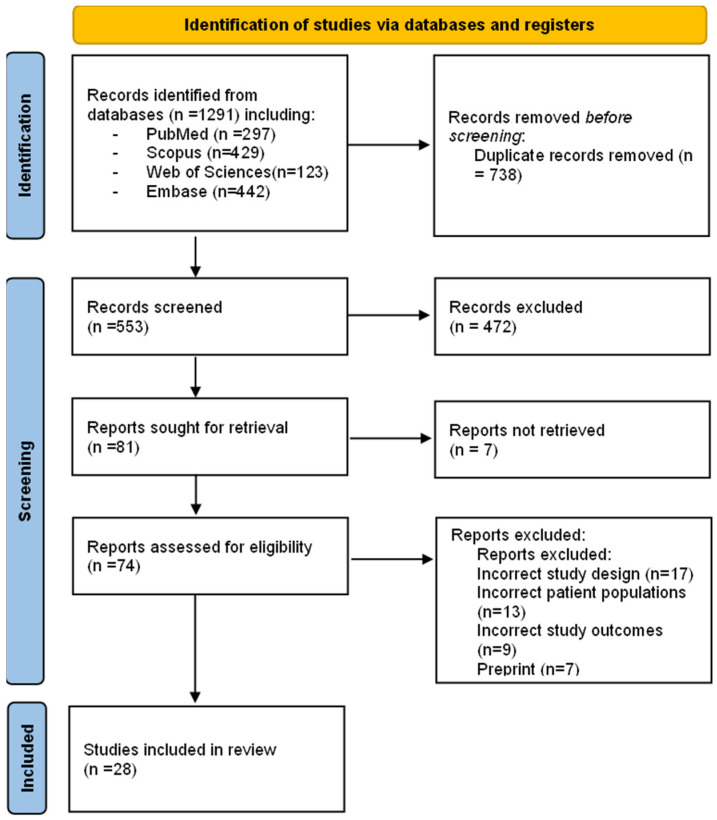
A total of 1291 articles were retrieved using the search strategy. The selection strategy is shown in the prism flow chart (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [26]. After the removal of duplicates (n = 738), 553 articles were screened by the reviewers. After filtering the titles and reading the abstracts, 74 articles were selected for full-text reading, and 28 were considered eligible for inclusion in this systematic review [7,8,22,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] (Figure 1).
Figure 1.
PRISMA flow chart of the studies selection process.
3.2. Study Characteristics
The main characteristics of the articles included in this review are summarized in Table 2. Our review included 28 studies that were published between 1 January and 18 August 2022 [7,8,22,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. The studies (n = 28) reported case reports of sexually transmitted monkeypox with a detailed description of the number of cases, clinical manifestations, history of sexually transmitted diseases, method of diagnosis, location and course of skin lesions, and treatment (Table 2 and Table 3). A total of 4222 confirmed cases of monkeypox were reported [7,8,22,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52], of which 3876 cases of monkeypox were the result of sexual contact transmission, distributed in twelve countries: Germany (n = 357) [38,43,48], Korea (n = 1) [39], Spain (n = 1924) [41,42,44,46,51], Italy (n = 42) [7,8,50], United Kingdom (n = 364) [31,33,37,47,52], Australia (n = 1) [32], Nigeria (n = 16) [45], United States (n = 1140) [22,30], Portugal (n = 28) [29,34], France (n = 1) [35], Romania (n = 1) [36], and Czech Republic (n = 1) [28] (Table 1). Spain was the country with the highest number of cases of sexually transmitted monkeypox, followed by the United States and the United Kingdom.
Table 2.
Characteristics of included studies and description of case reports of monkeypox.
| Authors | Year | Design | Country | Number of Cases (N) | Cases by Sexual Contact (N) | Age (Years) | Sex (M/F) | Sexual Behavior |
Previous STIs | HIV | Recent Sexual Exposure | Diagnostic Method for Monkeypox |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antinori, A. et al. [8] | 2022 | Case reports | Italy | 4 | 1 | Median: 30 | M | MSM | Hepatitis C, syphilis | Positive | Yes | RT-PCR |
| 2 | M | MSM | Syphilis | Negative | Yes | RT-PCR | ||||||
| 3 | M | MSM | Syphilis, hepatitis B | Positive | Yes | RT-PCR | ||||||
| 4 | M | MSM | Hepatitis A | Negative | Yes | RT-PCR | ||||||
| Heskin, J. et al. [31] | 2022 | Case reports | United Kingdom | 2 | 1 | NR | M | MSM | None | Negative | Yes | RT-PCR |
| 2 | NR | M | MSM | None | Positive | Yes | RT-PCR | |||||
| Hammerschlag, Y. et al. [32] | 2022 | Case report | Australia | 1 | 1 | 30 | M | MSM | Syphilis | Positive | Yes | RT-PCR |
| Minhaj, F.S. et al. [22] | 2022 | Case reports | United States | 17 | 16 | Median 40 (28–61) | NR | GBMSM | NR | NR | Yes | RT-PCR |
| Vivancos, R. et al. [33] | 2022 | Case reports | United Kingdom | 86 | 66 | Median: 38 (32–43) | M (79/79) | GBMSM (66/79) |
NR | NR | Yes | RT-PCR |
| Perez Duque, M. et al. [34] | 2022 | Case reports | Portugal | 27 | 27 | Median: 33 (22–51) | M | MSM (18/19), MSW (1/19) | NR | Positive (n = 14) | Yes | RT-PCR |
| Vallée, A. et al. [35] | 2022 | Case report | France | 1 | 1 | NR | M | MSM | HIV | Positive | Yes | RT-PCR |
| Oprea, C. et al. [36] | 2022 | Case report | Romania | 1 | 1 | 26 | M | MSM | HIV | Positive | Yes | RT-PCR |
| Bížová, B. et al. [28] | 2022 | Case report | Czech Republic | 1 | 1 | 34 | M | MSM | Syphilis | Positive | Yes | RT-PCR |
| Patrocinio-Jesus, R. et al. [29] | 2022 | Case report | Portugal | 1 | 1 | 31 | M | MSM | HIV | Positive | Yes | RT-PCR |
| Basgoz, S.N. et al. [30] | 2022 | Case report | United States | 1 | 1 | 31 | M | MSM | Syphilis, herpes simplex | Negative | Yes | RT-PCR |
| Mileto, D. et al. [7] | 2022 | Case report | Italy | 1 | 1 | 33 | M | MSM | HIV | Positive | Yes | RT-PCR |
| Girometti, N. et al. [37] | 2022 | Cohort study | United Kingdom | 54 | 54 | Median: 41 (34–45) | M | MSM | HIV (n = 13) syphilis (n = 14), herpes simplex (n = 24) and gonorrhea (n = 13) |
Positive | Yes | RT-PCR |
| Noe, S. et al. [38] | 2022 | Case report | Germany | 2 | 1 | 26 | M | MSM | HIV | Positive | Yes | RT-PCR |
| 2 | 32 | M | MSM | NR | NR | NR | RT-PCR | |||||
| Jang, Y.R. et al. [39] | 2022 | Case report | Korea | 1 | 1 | 34 | M | MSM | None | NR | NR | RT-PCR |
| Maronese, C.A. et al. [40] | 2022 | Case report | Italy | 1 | 1 | 44 | M | MSM | Hepatitis C, HIV, syphilis | Positive | Yes | RT-PCR |
| Peiró-Mestres, A. et al. [41] | 2022 | Case report | Spain | 12 | 1 | 30 | M | MSM | None | Positive (n = 4) | Yes | RT-PCR |
| 2 | 30 | M | MSM | Syphilis | Yes | RT-PCR | ||||||
| 3 | 40 | M | MSM | None | Yes | RT-PCR | ||||||
| 4 | 40 | M | MSM | None | Yes | RT-PCR | ||||||
| 5 | 40 | M | MSM | None | Yes | RT-PCR | ||||||
| 6 | 30 | M | MSM | None | Yes | RT-PCR | ||||||
| 7 | 40 | M | MSM | None | Yes | RT-PCR | ||||||
| 8 | 50 | M | MSM | Syphilis | Yes | RT-PCR | ||||||
| 9 | 40 | M | MSM | None | Yes | RT-PCR | ||||||
| 10 | 30 | M | MSM | None | Yes | RT-PCR | ||||||
| 11 | 30 | M | MSM | None | Yes | RT-PCR | ||||||
| 12 | 30 | M | MSM | Chlamydia y gonorrhea | Yes | RT-PCR | ||||||
| Iñigo Martínez, J. et al. [42] | 2022 | Case report | Spain | 508 | 427 | Median: 35 (18–67) | M (n = 503) F (n = 5) |
MSM (n = 397) | NR | Positive (n = 225) | Yes | RT-PCR |
| Selb, R. et al. [43] | 2022 | Case report | Germany | 521 | 349 | Median: 38 (32–44) | M | MSM (n = 349) | NR | NR | Yes | RT-PCR |
| Tarín-Vicente, E.J. et al. [44] | 2022 | Cohort study | Spain | 181 | 181 | Median: 37 (31–42) | M (n = 175) F (n = 6) |
MSM (n = 166) MSW (n = 15) |
HIV (n = 72) | Positive | Yes | RT-PCR |
| Ogoina, D. et al. [45] | 2022 | Cross-sectional study | Nigeria | 16 | 16 | Median: 28 (22–43) | M (n = 12)F (n = 4) | MSW (n = 16) | HIV (n = 3) | Positive (n = 3) | Yes | RT-PCR |
| Orviz, E. et al. [46] | 2022 | Observational study | Spain | 48 | 48 | Median: 35 (29–44) | M | MSM (n = 42) | HIV (n = 19) | Positive (n = 19) | Yes | RT-PCR |
| Patel, A. et al. [47] | 2022 | Case report | United Kingdom | 197 | 197 | Median: 38 (32–42) | M | MSM | HIV (n = 70) | Positive (n = 70) | Yes | RT-PCR |
| Pfäfflin, F. et al. [48] | 2022 | Case report | Germany | 1 | 1 | Range (41–50) | M | MSM | None | Positive | Yes | RT-PCR |
| 2 | 2 | Range (21–30) | M | MSM | None | Negative | Yes | RT-PCR | ||||
| 3 | 3 | Range (31–40) | M | MSM | None | Negative | Yes | RT-PCR | ||||
| 4 | 4 | Range (31–40) | M | MSM | Syphilis (blood), gonorrhea (rectal) | Negative | Yes | RT-PCR | ||||
| 5 | 5 | Range (21–30) | M | MSM | Gonorrhea, Ureaplasma, Mycoplasma hominis (all urethral) | Negative | Yes | RT-PCR | ||||
| 6 | 6 | Range (31–40) | M | MSM | Gonorrhea (rectal) | Positive | Yes | RT-PCR | ||||
| Philpott, D. et al. [49] | 2022 | Case report | United States | 1195 | 1123 | Median: 35 (30–41) | M (n = 1178) F (n = 5) |
MSM | HIV (n = 490) | Positive (n = 490) | Yes | RT-PCR |
| Raccagni, A.R. et al. [50] | 2022 | Case report | Italy | 36 | 36 | Median: 41.5 (31.25–35.5) | M | MSM | HIV (n = 15) | Positive (n = 15) | Yes | RT-PCR |
| Rodríguez, B.S. et al. [51] | 2022 | Case report | Spain | 1256 | 1256 | Median: 37 | M (n = 1242) F (n = 14) |
MSM | NR | NR | Yes | RT-PCR |
| Vusirikala, A. et al. [52] | 2022 | Case report | United Kingdom | 45 | 45 | Median: 37 | M | GBMSM | HIV (n = 11) | Positive (n = 11) | Yes | RT-PCR |
MSM: men who have sex with men; MSW: men who have sex with women; GBMSM: gay or bisexual or other men who have sex with men; STI: sexually transmitted infection; HIV: human immunodeficiency virus; RT-PCR: Polymerase chain reaction with reverse transcriptase; M/F: Male/Female; NR: No report.
Table 3.
Characteristics of eligible studies. Clinical manifestations, localization, the evolution of lesions, and treatment of monkeypox cases.
| Authors | Number of Cases (N) | Symptoms and Findings in Physical Examination | Days from Systemic Symptoms to Appearance of Lesion | Localization of Skin Lesions | Evolution of Lesions | Treatment |
|---|---|---|---|---|---|---|
| Antinori, A. et al. [8] | 1 | No | NR | Genital, thorax and calf area. | Asynchronous | Ciprofloxacin, acyclovir, and benzylpenicillin |
| 2 | Fever | 3 | Anal, back, legs and foot sole. | Asynchronous | NR | |
| 3 | Fever | 3 | Anal, head, thorax, legs, arms, hand, and genital area. | Asynchronous | anti-inflammatories and antihistamines | |
| 4 | Myalgia | 2 | Genital and pubic area. | Asynchronous | NR | |
| Heskin, J. et al. [31] | 1 | Lymphadenopathy, fever, headache, and diarrhea. Perioral white patches and painful lesions with perianal blisters. |
1 | Perioral and perianal. | Asynchronous | Intravenous ceftriaxone |
| 2 | Lymphadenopathy, fever, headache, and diarrhea. Perioral papules, papules on the mons pubis and penile shaft that evolved into painful ulcers. |
2 | Genital, pubic and tongue, oral and buccal mucous membranes. | Asynchronous | Intravenous ceftriaxone, antibiotic therapy. | |
| Hammerschlag, Y. et al. [32] | 1 | Fever and general malaise | 3 | Penis, trunk, face, extremities, hand, calf, nasal throat. | Asynchronous | Intramuscular ceftriaxone, oral doxycycline, oral cephalexin, intravenous cephalorin and oral analgesia. |
| Minhaj, F.S. et al. [22] | 17 | Rash (n = 17), Fatigue or malaise (n = 13), Chills (n = 12), Lymphadenopathy (n = 9), Headache (n = 8), Fever (n = 7), Body aches (n = 6), Sore throat or cough (n = 5), Sweat (n = 4). | NR | Arm (n = 9), Trunk (n = 9), Leg (n = 8), Face (n = 7), Hand (n = 6), Perianal (n = 6), Oral (n = 5), Neck (n = 5), Genital (penis or vagina) (n = 4), Feet (n = 4). | Asynchronous | NR |
| Vivancos, R. et al. [33] | 86 | NR | NR | NR | NR | NR |
| Perez Duque, M. et al. [34] | 27 | Exanthema (n = 14), inguinal lymphadenopathy (n = 14), fever (n = 13), genital ulcers (n = 6) | NR | Anus (n = 14) and genitalia (n = 12) | Asynchronous | NR |
| Vallée, A. et al. [35] | 1 | Fever, severe fatigue, chills, myalgia, sore throat, severe anal pain, and lymphadenopathy. | 5 | None | Asynchronous | No specific treatment. |
| Oprea, C. et al. [36] | 1 | High fever (up to 39 degrees Celsius), chills, rectal pain, vesiculopustular rash, dysphagia, severe pain in the anorectal region, marked hyperemia of the pharynx, with pseudomembranous appearance, and palatal petechiae, aphthous ulcers, lymphadenopathy. | 4 | Anogenital, buttocks, neck, trunk, upper and lower limbs, and sole of one foot. | Asynchronous | Symptomatic, fluid, and topical treatment for aphthous ulcers and pharyngeal hyperemia. |
| Bížová, B. et al. [28] | 1 | High fever, chills, lymphadenopathy, rash, painless perianal erosions, and perianal umbilicated papules. | 3 | The perianal and left side of the body | Asynchronous | Antibiotic therapy |
| Patrocinio-Jesus, R. et al. [29] | 1 | Painless genital rash, fever, sore throat, macular rash, lymphadenopathy. | 2 | Genitals and hands | Asynchronous | No specific intervention |
| Basgoz, N. et al. [30] | 1 | Rectal pain, vesiculopustular rash, rectal bleeding, foul-smelling and mucopurulent discharge, fever, chills, lymphadenopathy, and swelling in the groin. | 3 | Perianal, penis, arms, and legs. | Asynchronous | Penicillin G benzathine, ceftriaxone, valacyclovir, doxycycline, and intravenous acyclovir. |
| Mileto, D. et al. [7] | 1 | Asthenia, fever, general malaise, anorexia, papular lesions on both elbows, ulcerated perianal lesion, pharyngodynia, bilateral inguinal lymphadenopathy. | 3 | Perianal, face, both elbows, trunk, buttock, and right foot. | Asynchronous | Dolutegravir, rilpivirine, isolated in a negative pressure room. |
| Girometti, N. et al. [37] | 54 | Fatigue (n = 36), fever (n = 31), myalgia (n = 16), sore throat (n = 11), lymphadenopathy (n = 30) and skin lesions (n = 54). | 3 | Skin (n = 54), genitalia (n = 33), perianal (n = 24), upper and lower extremities (n = 27), facial (n = 11), oropharyngeal (n = 4) and torso (n = 14). | Asynchronous | No specific treatment was recorded and all individuals improved clinically. |
| Noe, S. et al. [38] | 1 | General malaise, fever, arthralgia, myalgia and back pain, headache, dysphagia, and presence of white spots on his tonsils. | 2 | Tonsils, trunk, limbs, and head. | Asynchronous | No specific treatment was recorded. |
| 2 | Fever, fatigue, cough, inguinal lymphadenopathy, and anal pain. | 2 | Trunk | Asynchronous | No specific treatment was recorded. | |
| Jang, Y.R. et al. [39] | 1 | Headache, fever, rash, lymphadenopathy, and chills. | 3 | Penis, oropharynx, nasopharynx, face, abdomen, and trunk. | Asynchronous | No specific treatment was recorded. |
| Maronese, C.A. et al. [40] | 1 | Fever, headache, malaise, and lymphadenopathy. | 5 | Penis, scrotum, and extremities. | Asynchronous | No specific treatment was recorded. |
| Peiró-Mestres, A. et al. [41] | 1 | Myalgia, fatigue | NR | Arm, perianal area and trunk | Asynchronous | No specific treatment was recorded. |
| 2 | Odynophagia, general malaise | Genital area | Asynchronous | No specific treatment was recorded. | ||
| 3 | Myalgia, fever, Proctitis | Anal area | Asynchronous | No specific treatment was recorded. | ||
| 4 | Proctalgia, odynophagia, general malaise | Perianal, chest and trunk | Asynchronous | No specific treatment was recorded. | ||
| 5 | Fever, myalgia, general malaise | Chest and legs | Asynchronous | No specific treatment was recorded. | ||
| 6 | Fever, proctitis | Wrist, pectoral, fingers, hand and perianal area | Asynchronous | No specific treatment was recorded. | ||
| 7 | Headache, general malaise | Ulcerated ventral tongue | Asynchronous | No specific treatment was recorded. | ||
| 8 | General malaise, fever | Trunk and genital area | Asynchronous | No specific treatment was recorded. | ||
| 9 | Myalgia, general malaise | Genital lesions | Asynchronous | No specific treatment was recorded. | ||
| 10 | General malaise, myalgia, proctitis | Perianal area | Asynchronous | No specific treatment was recorded. | ||
| 11 | NR | Genital area | Asynchronous | No specific treatment was recorded. | ||
| 12 | Myalgia, general malaise | Genital and anal area | Asynchronous | No specific treatment was recorded. | ||
| Iñigo Martínez, J. et al. [42] | 508 | Exanthema (n = 498), fever (n = 324), lymphadenopathy (n = 311), asthenia (n = 238), myalgia (n = 185), headache (n = 162), odynophagia (n = 143), and proctitis (n = 81) | NR | Anogenital and/or perineal area (n = 359), legs and/or arms (n = 222), face (n = 177), chest and/or abdomen (n = 159), back (n = 132), palms and/or plants (n = 124). | Asynchronous | No specific treatment was recorded. |
| Selb, R. et al. [43] | 521 | NR | NR | NR | NR | NR |
| Tarín-Vicente, E.J. et al. [44] | 181 | Influenza-like illness (n = 147), Fever (n = 131), Headache (n = 96), Sore throat (n = 66) and lymphadenopathy (n = 153) | NR | Genital (n = 100), Perianal (n = 66), Oral ulcer (n = 45), Perioral (n = 51), Hands and feet (n = 108), Trunk and extremities (n = 104) | Asynchronous | No specific treatment was recorded. |
| Ogoina, D. et al. [45] | 16 | Fever (n = 9), Genital rash (n = 4), facial rash (n = 3) | NR | Genital (n = 13) | Asynchronous | No specific treatment was recorded. |
| Orviz, E. et al. [46] | 48 | Fever (n = 25), Asthenia (n = 32), Myalgia (n = 25), Inguinal lymphadenopathies (n = 30), Other location of lymphadenopathies (n = 9), Headache (n = 25), Proctitis (n = 13), Urethritis (n = 7), Rash (n = 4), Nasal congestion (n = 4), and Cough (n = 8) | NR | Vesicular-umbilicated skin lesions location (n = 45), Genitals (n = 26), Upper extremities (n = 20), Perianal (n = 17), Trunk (n = 16), Facial (n = 12), Periorally (n = 9), Lower extremities (n = 10), and Palms and soles (n = 2) | Asynchronous | No specific treatment was recorded. |
| Patel, A. et al. [47] | 197 | Mucocutaneous manifestations (n = 197), Fever (n = 122), Headache (n = 49), Fatigue/lethargy (n = 46), Myalgia (n = 62), Arthralgia (n = 21), Back pain (n = 21), Rectal pain or pain on defecation (n = 71), and Lymphadenopathy (n = 114) | NR | Face (n = 71), Trunk (n = 70), Arms/legs (n = 74), Hands/feet (n = 56), Genitals (n = 111), Anus or perianal area (n = 82), and Oropharyngeal (n = 27) | Asynchronous | No specific treatment was recorded. |
| Pfäfflin, F. et al. [48] | 1 | Fever, Perianal pain, Anal abscess, and Lymphadenopathy | NR | Limbs | Asynchronous | Ibuprofen |
| 2 | Fever, malaise, anal pain, and anal fissure | NR | Left arm | Asynchronous | Metamizole, tramadol, lidocaine | |
| 3 | Anal pain, Rectal ulcer, and proctitis | NR | Limbs | Asynchronous | Ibuprofen, metamizole, lidocaine | |
| 4 | Fatigue, Anal pain, and Anal ulcer | NR | Arms, trunk, genital | Asynchronous | Metamizole, lidocaine, Penicillin G benzathine, ceftriaxone | |
| 5 | Fever, malaise, myalgia, sweats, Anal pain, Inflammation of sigmoid, rectum and anal canal | NR | Head, neck, trunk, limbs | Asynchronous | Metamizole, lidocaine, Ceftriaxone, azithromycin | |
| 6 | Myalgia, fever, malaise, Anal pain, Anal ulcer, proctitis | NR | Legs | Asynchronous | Metamizole, lidocaine, Ceftriaxone, azithromycin | |
| Philpott, D. et al. [49] | 1195 | Rash (n = 1004), Fever (n = 596), Chills (n = 550), Lymphadenopathy (n = 545), Malaise (n = 531), Myalgia (n = 507), Headache (n = 469), Rectal pain (n = 201), Pus or blood in stools (n = 184), Abdominal pain (n = 96), Rectal bleeding (n = 90), Tenesmus (n = 90), and vomiting or nausea (n = 83) | NR | Genitals (n = 333), Arms (n = 284), Face (n = 276), Legs (n = 265), Perianal (n = 225), Mouth, lips, or oral mucosa (n = 179), Palms of hands (n = 157), Trunk (n = 156), Neck (n = 130), Head (n = 97), and Soles of feet (n = 77) | Asynchronous | No specific treatment was recorded. |
| Raccagni, A.R. et al. [50] | 36 | NR | NR | Genital (n = 13), Rectal (n = 18), cutaneous (n = 20) | Asynchronous | No specific treatment was recorded. |
| Rodríguez, B.S. et al. [51] | 1256 | Report of some cases (n = 530): Fever (n = 302), lymphadenopathy (n = 216), Asthenia (n = 224), Muscle pain (n = 167), Throat pain (n = 136), and Headache (n = 140) | NR | Report of some cases (n = 530): Anogenital (n = 355), other than anogenital or oro/peribuccal (n = 293) | Asynchronous | No specific treatment was recorded. |
| Vusirikala, A. et al. [52] | 45 | NR | NR | NR | Asynchronous | No specific treatment was recorded. |
NR: No report.
3.3. Demographical Characteristics and Diagnostic Method for Monkeypox
Of the total number of cases (n = 4222) registered with monkeypox [7,8,22,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52], 4152 cases were found to be male [7,8,22,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. The average age of reported cases with monkeypox was 36 years. Of the reported cases with monkeypox, 3479 had a sexual behavior of being men who have sex with men [7,8,28,29,30,31,32,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] and 112 cases had a sexual behavior of being gay or bisexual or men who have sex with men [22,33,52]. In addition to the cases reported with monkeypox transmitted by sexual contact: Syphilis (n = 24) [8,28,30,32,37,40,41], Gonorrhea (n = 4) [41,48] and herpes simplex (n = 25) [8,28,30,32,37,48] were the most prevalent sexually transmitted infections and 949 patients tested positive for human immunodeficiency virus [7,8,28,29,31,32,34,35,36,37,38,40,42,44,45,46,47,48,49,50,51,52]. All confirmed cases of monkeypox were diagnosed by Polymerase chain reaction with reverse transcriptase (RT-PCR) [7,8,22,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] (Table 2).
3.4. Clinical Manifestations, Localization of Skin Lesions and Treatment
The most frequent clinical manifestations in patients confirmed with monkeypox were fever (n = 1521) [7,8,22,28,29,30,31,32,34,35,36,38,39,40,41,42,44,45,46,47,48,49,51], lymphadenopathy (n = 1385) [7,22,28,29,30,31,34,35,36,37,38,39,40,42], headache (n = 956) [22,31,32,38,39,40,41,42], skin lesions or rash (n = 1925) [28,29,34,36,37,39,42,44,45,46,47,48,49,51], painful perianal and genital lesions (n = 499) [7,28,29,30,31,34,35,36,44,45,46,47,48,49,51] (Table 3). The average number of days from systemic symptoms to the appearance of lesions was 3 (Table 3). The most frequent lesion locations were perianal (n = 1209) [7,8,22,28,30,31,34,36,38,41,42,44,45,46,47,48,49,50,51], genital (n = 1373) [8,22,29,30,31,32,34,36,37,39,40,41,42,44,45,46,47,48,49,50,51], oral (n = 812) [22,31,32,37,38,39,41,44,46,47,48,49,51], trunk (n = 640) [7,8,22,32,36,44,46,47,48,49,51] and upper and lower extremities (n = 491) [7,8,22,29,30,32,36,37,44,46,47,48,49] (Table 3). The evolution of these lesions was asynchronous. Most of the patients did not report a specific treatment, but simply followed their treatments for the sexually transmitted diseases they were suffering from [7,8,28,29,30,31,32,35,36,37,38,39,40,41,42,44,45,46,47,48,49,50,51,52].
4. Discussion
Currently, MPX represents the most recent emerging zoonotic disease worldwide [53]. For this reason, the main objective of the present systematic review is to determine the epidemiological situation of monkeypox transmission by possible sexual contact. It is important to have knowledge of the clinical characteristics, sexual behavior, localization and evolution of skin lesions, diagnosis, and correct management of these patients.
This study reported 3876 cases of monkeypox through possible sexual contact transmission distributed in twelve countries. It was found that 85% of the reported cases were from Europe, with Spain being the country with the most reports. All patients were diagnosed by RT-PCR. The majority of patients reported an average age of 36 years and were male. The most recent outbreak of Monkeypox (MPXV) in 2022 has brought new light to the importance of this sexual transmission mechanism in the spread of an emerging pathogen [54,55]. All reported patients had sexual risk behaviors, of which men who have sex with men (MSM) was the most prevalent.
According to the WHO, current epidemiological data show a predominance of the involvement of young males, with 98.2% (20,138/20,500) of cases with available data on gender being male with a median age of 36 years (interquartile range: 30–43 years). Among cases with declared sexual orientation, 95.8% (9484/9899) identified as men who have sex with men. Sexual encounters were the most common type of transmission, accounting for 5954 of 7250 (82.1%) of all transmission cases [56]. In the recently released study by Thornhill JP et al., 528 instances of monkeypox were documented, of which 98% were homosexual or bisexual men who had engaged in risky sexual activity, and 41% had human immunodeficiency virus infection [57].
The incubation period has been estimated at 5 to 21 days and the duration of symptoms and signs at 2 to 5 weeks [58]. The disease begins with nonspecific symptoms and signs, the most frequent symptoms reported in the study cases were fever, lymphadenopathy, headache, malaise, and general lesions. All lesions had an asynchronous evolution, with the genital and anal regions being the most frequent locations. This suggests that contact in sexual intercourse could be a risk factor for transmission [8] because it can occur through contact with infected humans, or with human body material containing the virus [59]; therefore, sexual intercourse without the use of a condom could be another risk factor, since there are other viruses found in semen [60]. However, there are still no studies demonstrating the presence of Monkeypox in this body secretion, except for case reports from Italy and Germany [7,8,51].
To determine a rapid and definitive diagnosis of MPX, the exudate from lesions can provide the best sample [61]. This is performed through direct recognition of viral DNA by real-time PCR, allowing rapid discrimination between smallpox and other poxviruses [61,62,63,64,65]. In addition, it is important to understand that MPXV DNA could be recovered in blood, urine, upper respiratory tract, and seminal fluid [8,16,61].
According to the study by Ranjit Sah et al., monkeypox virus is highly prevalent in seminal samples from monkeypox cases, supporting the idea that the disease is sexually transmitted. However, since the virus can reproduce in this environment, this high prevalence rate does not always suggest viral contagiousness [66]. The infectivity of seminal monkeypox virus remains debatable and requires further investigation.
Sixty-nine percent of the cases presented had a previous STI, the most frequent being syphilis and hepatitis. In addition, most of them were HIV positive, which led us to infer that this history could be a risk factor that may contribute to infection [54]. MPX can be confused with some sexually transmitted infections (STIs) that can cause skin rashes, for example, syphilis, human immunodeficiency virus (HIV), chancroid, condyloma acuminate, disseminated gonorrhea, and herpes [67].
Most of the patients had symptomatic treatment, although some did not require any specific treatment. Recently, some drugs were developed in the United States to treat smallpox infection. These antiviral agents are also active against MPXV. The Food and Drug Administration (FDA) approved tecovirimat in 2018, which acts by inhibiting the viral protein p27, thus preventing viral egress from infected cells, and oral brincidofovir in 2021, which blocks viral DNA polymerase [68].
It is important to follow up on the contacts of the reported cases to avoid the spread of this disease, taking into account the number of days from the general symptoms to the appearance of lesions, which ranged from 1 to 5 days in the cases reported. The study did not report any deaths in cases of monkeypox potentially transmitted by sexual contact, although this also depends on the immunological status of the patient and associated complications.
The situation of this new zoonotic disease, which now appears to be emerging as an STI, is of great concern and warrants further study to understand the multiple effects of this virus, which is currently affecting several continents and with possible new routes of transmission, including during the COVID-19 pandemic that has not yet ended [69].
5. Conclusions
The reemerging zoonotic disease (monkeypox) has spread rapidly throughout the world and has shown unusual reports of person-to-person transmission through possible sexual contact. The prevalence of STIs and the frequent occurrence of anogenital symptoms point to local inoculation during intimate skin-to-skin or mucosal contact during sexual activity. Men who have sex with men are most at risk of spreading monkeypox, and MPXV DNA can be found in seminal fluid. The establishment of health policies is crucial for the early identification and treatment of people with monkeypox.
Author Contributions
Conceptualization, D.A.L.-F., E.A.G.-V., D.K.B.-A., M.D.-T., J.J.B., H.M.S.-C., M.T.D.-M., O.C.-S.C. and A.J.R.-M.; methodology, J.J.B., H.M.S.-C., M.T.D.-M., O.C.-S.C. and A.J.R.-M.; software, D.A.L.-F., E.A.G.-V., D.K.B.-A. and J.J.B.; validation, D.A.L.-F., E.A.G.-V., D.K.B.-A. and J.J.B.; formal analysis, D.A.L.-F., E.A.G.-V., D.K.B.-A. and A.J.R.-M.; investigation, D.A.L.-F., E.A.G.-V., J.J.B., H.M.S.-C. and A.J.R.-M.; resources, D.A.L.-F. and A.J.R.-M.; data curation, D.A.L.-F.; writing—original draft preparation, D.A.L.-F., E.A.G.-V., D.K.B.-A., M.D.-T., J.J.B., H.M.S.-C., M.T.D.-M., O.C.-S.C. and A.J.R.-M.; writing—review and editing, D.A.L.-F., J.J.B. and A.J.R.-M.; visualization, D.A.L.-F., E.A.G.-V., D.K.B.-A., M.D.-T., J.J.B., H.M.S.-C., M.T.D.-M., O.C.-S.C. and A.J.R.-M.; supervision, D.A.L.-F.; project administration, A.J.R.-M.; funding acquisition, J.J.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This section provides details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., Steffen R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16:e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhawan M., Bin Emran T., Islam F. The Resurgence of Monkeypox Cases: Reasons, Threat Assessment, And Possible Preventive Measures. Travel Med. Infect. Dis. 2022;49:102367. doi: 10.1016/j.tmaid.2022.102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monkeypox n.d. [(accessed on 29 June 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox.
- 4.Monkeypox Outbreak Global Map|Monkeypox|Poxvirus|CDC n.d. [(accessed on 29 June 2022)]; Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html?fbclid=IwAR1JReLa6ZZivFHO0JDDfBFjZ5xMn-fDtkdHtcmdlcqM6t1EqGfSYR9NMWM.
- 5.Multi-Country Outbreak of Monkeypox, External Situation Report #1–6 July 2022-World|ReliefWeb n.d. [(accessed on 7 July 2022)]. Available online: https://reliefweb.int/report/world/multi-country-outbreak-monkeypox-external-situation-report-1-6-july-2022.
- 6.Gong Q., Wang C., Chuai X., Chiu S. Monkeypox virus: A re-emergent threat to humans. Virol Sin. 2022;37:477–482. doi: 10.1016/j.virs.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mileto D., Riva A., Cutrera M., Moschese D., Mancon A., Meroni L., Giacomelli A., Bestetti G., Rizzardini G., Gismondo M.R., et al. New Challenges in Human Monkeypox Outside Africa: A Review and Case Report from Italy. Travel Med. Infect. Dis. 2022;49:102386. doi: 10.1016/j.tmaid.2022.102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antinori A., Mazzotta V., Vita S., Carletti F., Tacconi D., Lapini L.E., D’Abramo A., Cicalini S., Lapa D., Pittalis S., et al. Epidemiological, Clinical and Virological Characteristics of Four Cases of Monkeypox Support Transmission Through Sexual Contact, Italy, May 2022. Eurosurveillance. 2022;27:2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore M., Zahra F. StatPearls. StatPearls; Tampa, FL, USA: 2022. Monkeypox. [Google Scholar]
- 10.Bonilla-Aldana D.K., Rodrigue-Morales A.J. Is Monkeypox Another Reemerging Viral Zoonosis with Many Animal Hosts Yet to Be Defined? Veter-Q. 2022;42:148–150. doi: 10.1080/01652176.2022.2088881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauldin M.R., McCollum A.M., Nakazawa Y.J., Mandra A., Whitehouse E.R., Davidson W., Zhao H., Gao J., Li Y., Doty J., et al. Exportation of Monkeypox Virus from the African Continent. J. Infect. Dis. 2020;225:1367–1376. doi: 10.1093/infdis/jiaa559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.León-Figueroa D.A., Bonilla-Aldana D.K., Pachar M., Romaní L., Saldaña-Cumpa H.M., Anchay-Zuloeta C., Diaz-Torres M., Franco-Paredes C., Suárez J.A., Ramirez J.D., et al. The Never-Ending Global Emergence of Viral Zoonoses After COVID-19? The Rising Concern of Monkeypox in Europe, North America and beyond. Travel Med. Infect. Dis. 2022;49:102362. doi: 10.1016/j.tmaid.2022.102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diseases T.L.I. Monkeypox: A Neglected Old Foe. Lancet Infect. Dis. 2022;22:913. doi: 10.1016/S1473-3099(22)00377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amao L.K., Olatunji D.I., Igbodo G., Okoli S.C., Amaechi I., Goni M.I., Ehiakhamen O., Aderinola O., Ogunleye A., Ogunbode O., et al. Trend and enhanced surveillance of Monkeypox during COVID-19 pandemic in Nigeria. J. Public Health Afr. 2022;13:2184. doi: 10.4081/jphia.2022.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxena S.K., Ansari S., Maurya V.K., Kumar S., Jain A., Paweska J.T., Tripathi A.K., Abdel-Moneim A.S. Re-Emerging Human Monkeypox: A Major Public-Health Debacle. J. Med. Virol. 2022;1 doi: 10.1002/jmv.27902. [DOI] [PubMed] [Google Scholar]
- 16.Adler H., Gould S., Hine P., Snell L.B., Wong W., Houlihan C.F., Osborne J.C., Rampling T., Beadsworth M.B., Duncan C.J., et al. Clinical Features and Management of Human Monkeypox: A Retrospective Observational Study in the UK. Lancet Infect. Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huhn G.D., Bauer A.M., Yorita K., Graham M.B., Sejvar J., Likos A., Damon I.K., Reynolds M., Kuehnert M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 18.Jezek Z., Grab B., Szczeniowski M., Paluku K.M., Mutombo M. Clinico-Epidemiological Features of Monkeypox Patients with an Animal or Human Source of Infection. Bull. World Health Organ. 1988;66:459–464. [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang Y., White A. Monkeypox Virus Emerges from the Shadow of Its More Infamous Cousin: Family Biology Matters. Emerg. Microbes Infect. 2022;11:1768–1777. doi: 10.1080/22221751.2022.2095309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peter O.J., Kumar S., Kumari N., Oguntolu F.A., Oshinubi K., Musa R. Transmission Dynamics of Monkeypox Virus: A Mathematical Modelling Approach. Model. Earth Syst. Environ. 2021;8:3423–3434. doi: 10.1007/s40808-021-01313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar N., Acharya A., Gendelman H.E., Byrareddy S.N. The 2022 Outbreak and the Pathobiology of the Monkeypox Virus. J. Autoimmun. 2022;131:102855. doi: 10.1016/j.jaut.2022.102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minhaj F.S., Ogale Y.P., Whitehill F., Schultz J., Foote M., Davidson W., Hughes C.M., Wilkins K., Bachmann L., Chatelain R., et al. Monkeypox Outbreak—Nine States, May 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022;71:764–769. doi: 10.15585/mmwr.mm7123e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Morales A.J., Lopardo G. Monkeypox: Another Sexually Transmitted Infection? Pathogens. 2022;11:713. doi: 10.3390/pathogens11070713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farahat R.A., Abdelaal A., Shah J., Ghozy S., Sah R., Bonilla-Aldana D.K., Rodriguez-Morales A.J., McHugh T.D., Leblebicioglu H. Monkeypox Outbreaks During COVID-19 Pandemic: Are We Looking at An Independent Phenomenon or an Overlapping Pandemic? Ann. Clin. Microbiol. Antimicrob. 2022;21:1–3. doi: 10.1186/s12941-022-00518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao A.K., Petersen B.W., Whitehill F., Razeq J.H., Isaacs S.N., Merchlinsky M.J., Campos-Outcalt D., Morgan R.L., Damon I., Sánchez P.J., et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022;71:734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bížová B., Veselý D., Trojánek M., Rob F. Coinfection of Syphilis and Monkeypox in HIV Positive Man in Prague, Czech Republic. Travel Med. Infect. Dis. 2022;49:102368. doi: 10.1016/j.tmaid.2022.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrocinio-Jesus R., Peruzzu F. Monkeypox Genital Lesions. N. Engl. J. Med. 2022;387:66. doi: 10.1056/NEJMicm2206893. [DOI] [PubMed] [Google Scholar]
- 30.Basgoz N., Brown C.M., Smole S.C., Madoff L.C., Biddinger P.D., Baugh J.J., Shenoy E.S. Case 24-2022: A 31-Year-Old Man with Perianal and Penile Ulcers, Rectal Pain, and Rash. N. Engl. J. Med. 2022;387:547–556. doi: 10.1056/NEJMcpc2201244. [DOI] [PubMed] [Google Scholar]
- 31.Heskin J., Belfield A., Milne C., Brown N., Walters Y., Scott C., Bracchi M., Moore L.S., Mughal N., Rampling T., et al. Transmission of Monkeypox Virus Through Sexual Contact—A Novel Route of Infection. J. Infect. 2022;85:334–363. doi: 10.1016/j.jinf.2022.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammerschlag Y., MacLeod G., Papadakis G., Sanchez A.A., Druce J., Taiaroa G., Savic I., Mumford J., Roberts J., Caly L., et al. Monkeypox Infection Presenting as Genital Rash, Australia, May 2022. Eurosurveillance. 2022;27:2200411. doi: 10.2807/1560-7917.ES.2022.27.22.2200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vivancos R., Anderson C., Blomquist P., Balasegaram S., Bell A., Bishop L., Brown C.S., Chow Y., Edeghere O., Florence I., et al. Community Transmission of Monkeypox in the United Kingdom, April to May 2022. Eurosurveillance. 2022;27:2200422. doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duque M.P., Ribeiro S., Martins J.V., Casaca P., Leite P.P., Tavares M., Mansinho K., Duque L.M., Fernandes C., Cordeiro R., et al. Ongoing Monkeypox Virus Outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance. 2022;27:2200424. doi: 10.2807/1560-7917.es.2022.27.22.2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallée A., Farfour E., Zucman D. Monkeypox Virus: A Novel Sexually Transmitted Disease? A Case Report from France. Travel Med. Infect. Dis. 2022;49:102394. doi: 10.1016/j.tmaid.2022.102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oprea C., Ianache I., Piscu S., Tardei G., Nica M., Ceausu E., Popescu C.P., Florescu S.A. First Report of Monkeypox in a Patient Living with HIV From Romania. Travel Med. Infect. Dis. 2022;49:102395. doi: 10.1016/j.tmaid.2022.102395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girometti N., Byrne R., Bracchi M., Heskin J., McOwan A., Tittle V., Gedela K., Scott C., Patel S., Gohil J., et al. Demographic and Clinical Characteristics of Confirmed Human Monkeypox Virus Cases in Individuals Attending a Sexual Health Centre in London, UK: An Observational Analysis. Lancet Infect. Dis. 2022;22:1321–1328. doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noe S., Zange S., Seilmaier M., Antwerpen M.H., Fenzl T., Schneider J., Spinner C.D., Bugert J.J., Wendtner C.-M., Wölfel R. Clinical and Virological Features of First Human Monkeypox Cases in Germany. Infection. 2022:1–6. doi: 10.1007/s15010-022-01874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang Y.R., Lee M., Shin H., Kim J.-W., Choi M.-M., Kim Y.M., Kim J., Na H.K. The First Case of Monkeypox in the Republic of Korea. J. Korean Med. Sci. 2022;37 doi: 10.3346/jkms.2022.37.e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maronese C.A., Beretta A., Avallone G., Boggio F.L., Marletta D.A., Murgia G., Cusini M., Gori A., Carrera C.G., Di Benedetto A., et al. Clinical, Dermoscopic and Histopathological Findings in Localized Human Monkeypox: A Case from Northern Italy. Br. J. Dermatol. 2022;10:1342. doi: 10.1111/bjd.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peiró-Mestres A., Fuertes I., Camprubí-Ferrer D., Marcos M., Vilella A., Navarro M., Rodriguez-Elena L., Riera J., Català A., Martínez M.J., et al. Frequent Detection of Monkeypox Virus DNA In Saliva, Semen, And Other Clinical Samples From 12 Patients, Barcelona, Spain, May to June 2022. Eurosurveillance. 2022;27:2200503. doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martínez J.I., Gil Montalbán E., Bueno S.J., Martínez F.M., Juliá A.N., Díaz J.S., Marín N.G., Deorador E.C., Forte A.N., García M.A., et al. Monkeypox Outbreak Predominantly Affecting Men Who Have Sex with Men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance. 2022;27:2200471. doi: 10.2807/1560-7917.ES.2022.27.27.2200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selb R., Werber D., Falkenhorst G., Steffen G., Lachmann R., Ruscher C., McFarland S., Bartel A., Hemmers L., Koppe U., et al. A Shift from Travel-Associated Cases to Autochthonous Transmission with Berlin as Epicentre of the Monkeypox Outbreak in Germany, May to June 2022. Eurosurveillance. 2022;27:2200499. doi: 10.2807/1560-7917.ES.2022.27.27.2200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarín-Vicente E.J., Alemany A., Agud-Dios M., Ubals M., Suñer C., Antón A., Arando M., Arroyo-Andrés J., Calderón-Lozano L., Casañ C., et al. Clinical Presentation and Virological Assessment of Confirmed Human Monkeypox Virus Cases in Spain: A Prospective Observational Cohort Study. Lancet. 2022;400:661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogoina D., Yinka-Ogunleye A. Sexual history of human monkeypox patients seen at a tertiary hospital in Bayelsa, Nigeria. Int. J. STD AIDS. 2022;2022:095646242211193. doi: 10.1177/09564624221119335. [DOI] [PubMed] [Google Scholar]
- 46.Orviz E., Negredo A., Ayerdi O., Vázquez A., Muñoz-Gomez A., Monzón S., Clavo P., Zaballos A., Vera M., Sánchez P., et al. Monkeypox outbreak in Madrid (Spain): Clinical and virological aspects. J. Infect. 2022 doi: 10.1016/j.jinf.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel A., Bilinska J., Tam J.C.H., Fontoura D.D.S., Mason C.Y., Daunt A., Snell L.B., Murphy J., Potter J., Tuudah C., et al. Clinical Features and Novel Presentations of Human Monkeypox in a Central London Centre During the 2022 Outbreak: Descriptive Case Series. BMJ. 2022;378:e072410. doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfäfflin F., Wendisch D., Scherer R., Jürgens L., Godzick-Njomgang G., Tranter E., Tober-Lau P., Stegemann M.S., Corman V.M., Kurth F., et al. Monkeypox In-Patients with Severe Anal Pain. Infection. 2022:1–5. doi: 10.1007/s15010-022-01896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philpott D., Hughes C.M., Alroy K.A., Kerins J.L., Pavlick J., Asbel L., Crawley A., Newman A.P., Spencer H., Feldpausch A., et al. Epidemiologic and Clinical Characteristics of Monkeypox Cases—United States, May 17 July 22, 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022;71:1018–1022. doi: 10.15585/mmwr.mm7132e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raccagni A.R., Candela C., Mileto D., Canetti D., Bruzzesi E., Rizzo A., Castagna A., Nozza S. Monkeypox infection among men who have sex with men: PCR testing on seminal fluids. J. Infect. 2022 doi: 10.1016/j.jinf.2022.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodríguez B.S., Herrador B.R.G., Franco A.D., Fariñas M.P.S.-S., Valero J.D.A., Llorente A.H.A., de Agreda J.P.A.P., Malonda R.C., Castrillejo D., López M.D.C., et al. Epidemiologic Features and Control Measures during Monkeypox Outbreak, Spain, June 2022-Volume 28, Number 9—September 2022-Emerging Infectious Diseases journal-CDC. Emerg. Infect. Dis. 2022;5:6. doi: 10.3201/eid2809.221051. [DOI] [Google Scholar]
- 52.Vusirikala A., Charles H., Balasegaram S., Macdonald N., Kumar D., Barker-Burnside C., Cumiskey K., Dickinson M., Watson M., Olufon O., et al. Epidemiology of Early Monkeypox Virus Transmission in Sexual Networks of Gay and Bisexual Men, England, 2022-Volume 28, Number 10—October 2022-Emerging Infectious Diseases journal-CDC n.d. Emerg. Infect. Dis. 2022;28 doi: 10.3201/eid2810.220960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bragazzi N.L., Kong J.D., Mahroum N., Tsigalou C., Khamisy-Farah R., Converti M., Wu J. Epidemiological Trends and Clinical Features of the Ongoing Monkeypox Epidemic: A Preliminary Pooled Data Analysis and Literature Review. J. Med. Virol. 2022 doi: 10.1002/jmv.27931. [DOI] [PubMed] [Google Scholar]
- 54.Zambrano P.G., Acosta-España J.D., Moyano F.M., Jara J.B.A. Sexually or intimately transmitted infections: A look at the current outbreak of monkeypox in 2022. Travel Med. Infect. Dis. 2022;49:102383. doi: 10.1016/j.tmaid.2022.102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kupferschmidt K. Why monkeypox is mostly hitting men who have sex with men. Science. 2022;376:1364–1365. doi: 10.1126/science.add5966. [DOI] [PubMed] [Google Scholar]
- 56.Multi-country outbreak of monkeypox-External Situation Report 4, published 24 August 2022-World|ReliefWeb n.d. [(accessed on 25 August 2022)]. Available online: https://reliefweb.int/report/world/multi-country-outbreak-monkeypox-external-situation-report-4-published-24-august-2022.
- 57.Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., Palich R., Nori A., Reeves I., Habibi M.S., et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 58.Petersen E., Kantele A., Koopmans M., Asogun D., Yinka-Ogunleye A., Ihekweazu C., Zumla A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019;33:1027–1043. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brockmeyer N. As Monkeypox Goes Sexual: A Public Health Perspective. J. Eur. Acad. Dermatol. Venereol. 2022;36:1164–1166. doi: 10.1111/jdv.18301. [DOI] [PubMed] [Google Scholar]
- 60.Matusali G., D’Abramo A., Terrosi C., Carletti F., Colavita F., Vairo F., Savellini G.G., Gandolfo C., Anichini G., Lalle E., et al. Infectious Toscana Virus in Seminal Fluid of Young Man Returning from Elba Island, Italy. Emerg. Infect. Dis. 2022;28:865–869. doi: 10.3201/eid2804.211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y., Olson V.A., Laue T., Laker M.T., Damon I.K. Detection of Monkeypox Virus with Real-Time PCR assays. J. Clin. Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shchelkunov S., Gavrilova E., Babkin I. Multiplex PCR Detection and Species Differentiation of Orthopoxviruses Pathogenic to Humans. Mol. Cell. Probes. 2005;19:1–8. doi: 10.1016/j.mcp.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kulesh D.A., Loveless B.M., Norwood D.A., Garrison J., Whitehouse C.A., Hartmann C., Mucker E., Miller D., Wasieloski L.P., Huggins J.W., et al. Monkeypox Virus Detection in Rodents Using Real-Time 3′-Minor Groove Binder Taqman® Assays on the Roche LightCycler. Lab. Investig. 2004;84:1200–1208. doi: 10.1038/labinvest.3700143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olson V.A., Laue T., Laker M.T., Babkin I.V., Drosten C., Shchelkunov S., Niedrig M., Damon I.K., Meyer H. Real-Time PCR System for Detection of Orthopoxviruses and Simultaneous Identification of Smallpox Virus. J. Clin. Microbiol. 2004;42:1940–1946. doi: 10.1128/JCM.42.5.1940-1946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shchelkunov S.N., Shcherbakov D.N., Maksyutov R.A., Gavrilova E.V. Species-Specific Identification of Variola, Monkeypox, Cowpox, And Vaccinia Viruses by Multiplex Real-Time PCR Assay. J. Virol. Methods. 2011;175:163–169. doi: 10.1016/j.jviromet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sah R., Abdelaal A., Reda A., Brakat A., Lashin B., Abdelazeem B., Mohanty A., Rodriguez-Morales A. Monkeypox Viral Detection in Semen Specimens of Confirmed Cases: A Systematic Review and Meta-Analysis. 2022. [(accessed on 28 July 2022)]. Available online: https://www.researchsquare.com/article/rs-1970704/v1. [DOI] [PubMed]
- 67.Koenig K.L., Beÿ C.K., Marty A.M. Monkeypox 2022 Identify-Isolate-Inform: A 3I Tool for Frontline Clinicians for a Zoonosis with Escalating Human Community Transmission. One Health. 2022;15:100410. doi: 10.1016/j.onehlt.2022.100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patauner F., Gallo R., Durante-Mangoni E. Monkeypox Infection: An Update for the Practicing Physician. Eur. J. Intern. Med. 2022 doi: 10.1016/j.ejim.2022.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdelmoez Farahat R., Sah R., El-Sakka A.A., Yasmine Benmelouka A., Kundu M., Labieb F., Sameh Shaheen R., Abdelaal A., Abdelazeem B., Bonilla-Aldana D.K., et al. Human Mon-Keypox Disease (MPX) InfezMed. 2022;13:1–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This section provides details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study.