Abstract
Although a serum thermolabile β-2 macroglycoprotein (TMG) may play a role in host defense as a lectin, little is known of its related physiological functions, mainly due to a lack of appropriate methods for tracing the functions of TMG. We identified a polysaccharide from Aerococcus viridans, PSA, which reacts with TMG, and based on this finding, we developed an enzyme-linked immunosorbent assay to trace the functions of TMG. Using ethanol precipitation and DEAE-Sepharose and Sephacryl S-400 column chromatographies, we isolated PSA from cultured medium of A. viridans, and it exhibited specific binding against TMG in blood samples. In sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the isolated PSA showed ladder bands that implied the existence of repeating units composed of d-glucose, N-acetyl-d-glucosamine, d-mannose, and d-xylose, as confirmed by gas chromatography-mass spectrometry. SDS-PAGE and immunochemical analysis, using rabbit anti-TMG antibody, showed that PSA specifically binds solely to intact serum TMG but not to TMG heated at 56°C for 30 min, a condition under which antigenicity is lost. TMG in serum samples bound to PSA in a dose-dependent manner, and this binding was clearly suppressed by addition of PSA. These observations indicate that PSA is a useful adsorbent to TMG and can be used to develop appropriate methods for tracing the functions of TMG.
A thermolabile β-2 macroglycoprotein (TMG), designated the Hakata antigen, is a novel serum glycoprotein that was found to react to antibody in the sera of some patients with systemic lupus erythematosus (SLE) (3, 13). A similar thermolabile substance was reported by Epstein and Tan (1), but it is not known if the two proteins are one and the same. TMG is present in almost all human sera at levels of 7 to 23 μg/ml (13). The sera from 100% of 10,050 Japanese blood donors, 99.99% of 751,352 Japanese patients, and 99.98% of 41,430 Swedish patients contained TMG (4). TMG is a multimeric molecule with a mass of approximately 520 to 650 kDa, composed of identical units of 35-kDa polypeptide chains, and whose antigenicity is lost when it is heated at 56°C for 1 min (13). In sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis under reducing conditions, TMG was found in the form of a single band of 35 kDa, but under nonreducing conditions, it showed ladder bands from 35 kDa to nearly the top of the gel. cDNA encoding TMG has been identified previously (11). Based on the primary structure, TMG contains a collagenous domain similar to those of C1q, ficolins, and collectins.
Anti-TMG antibody appears during the active hypocomplementemic phase in some SLE patients (1, 3, 4). TMG may be one factor related to the pathogenesis of this disease, and it is also a useful marker of hepatic functions in chronic liver diseases (2, 14). The anti-TMG antibody was found in 4.3% of SLE patients and in 0.3% of patients in the active stages of other autoimmune diseases (4). Although these studies suggested that TMG plays a role in host defense, little is known of its related physiological functions, mainly because there is a lack of rapid and reliable methods for tracing the functions of TMG; hence, immunochemical methods using an anti-TMG antibody have been used here.
In the present study, we identified in stored blood samples a polysaccharide produced by Aerococcus viridans, PSA, with specific binding activity to TMG. Through these analyses, we found PSA to be a useful adsorbent against serum TMG, and we developed an enzyme-linked immunosorbent assay (ELISA) for tracing the functions of TMG.
Initial observation of precipitin line-forming activity.
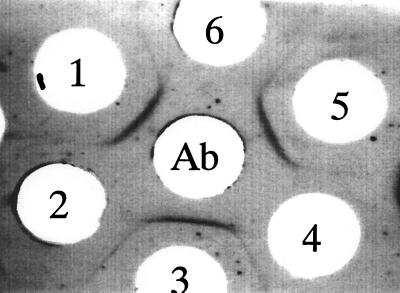
In an agarose gel double-diffusion assay of TMG using a 0.8% agarose gel, we found a unique precipitin line between fresh donor serum and refrigerator-stored serum, in addition to a representative precipitin line produced between fresh donor serum and rabbit anti-TMG antibody (Fig. 1). This unique precipitin line completely fused with the one between fresh serum TMG and the rabbit anti-TMG antibody. Furthermore, the precipitin line that should form between the stored serum and the rabbit anti-TMG antibody was lost. Thus, TMG in the stored serum may have been consumed by bacterial contamination in the serum sample.
FIG. 1.
Immunoprecipitation pattern produced between human sera and rabbit anti-TMG antibody. The center well was filled with the rabbit anti-TMG antibody. Wells 1, 3, and 5 were filled with fresh blood donor serum, and wells 2, 4, and 6 contained serum samples which had been stored in a refrigerator at 4°C for several weeks.
Identification of a gram-positive A. viridans strain.
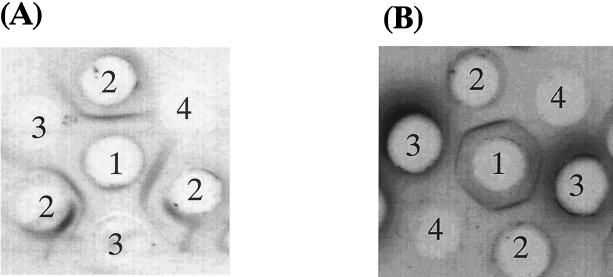
To identify the contaminating bacteria in the stored sample which formed the new precipitin line, we cloned the bacteria from the stored serum sample. The serum sample was added to 1 liter of Nissui brain heart infusion broth (Nissui Seiyaku Co. Ltd., Tokyo, Japan) and was grown at 37°C for 12 h. The precipitin line-forming activity in the supernatant of each clone was confirmed by an agarose gel double-diffusion assay. The activity was observed in the supernatant of the bacterial clone but not in uninoculated brain heart infusion broth (Fig. 2A). Table 1 summarizes the characteristics of the bacterial clone that produced the precipitin line when fresh sera were used. The activity was observed in the supernatant from the clone composed of spherical cells, and which formed tetrads in liquid media. In addition, this clone was gram positive, nonmotile, facultatively anaerobic, and catalase positive and there were green colonies on the blood agar. Based on these observations, this clone was identified as A. viridans.
FIG. 2.
(A) Immunoprecipitation pattern produced by the supernatant of a bacterial clone contaminating a stored blood sample. Well 1, rabbit anti-TMG antibody; wells 2, fresh serum sample from a blood donor, wells 3, supernatant of the broth of A. viridans; well 4, uninoculated brain heart infusion broth. (B) Immunoprecipitation pattern produced between purified substance and fresh serum from a blood donor. Well 1, fresh serum from a blood donor, wells 2, rabbit anti-TMG antibody, wells 3, supernatant of the broth of A. viridans, wells 4, purified substance from supernatant of the broth.
TABLE 1.
Characteristics of contaminant bacteria in stored blood samples
| Test or characteristic | Result |
|---|---|
| Gram staining | + |
| Morphology | Spherical (tetrads or pairs) |
| Catalase reaction | + |
| Pyrolidonyl allylamidase reaction | + |
| Leucine allylamidase reaction | − |
| Growth under anaerobic conditions | + |
| Growth in air or reduced oxygen tension | + |
| Growth in 6.5% NaCl at 10°C | + |
| Growth in 6.5% NaCl at 45°C | − |
| Growth on blood agar | Green colonies |
| Growth on nutrient agar | Flat, white colonies |
| Gas production during growth | − |
| Voges-Proskauer test in culture for 7 days | − |
Purification of bacterial polysaccharide.
To clarify the component responsible for the precipitin line-forming activity of A. viridans, we purified the component from the supernatant of the brain heart infusion broth of A. viridans. After incubation at 37°C for 12 h, the supernatant was adjusted to pH 5.5 with a 3 M sodium acetate solution. Next, we added it to 2.5 liters of cold ethanol and left the preparation to stand at −80°C for 30 min. After centrifugation, the precipitate was dissolved in 20 mM Tris-HCl buffer, pH 8.0, and was dialyzed overnight against Tris-HCl buffer. This dialysate was digested with DNase I and RNase A, extracted with phenol, concentrated with ethanol precipitation, and then dialyzed against a 10 mM phosphate buffer, pH 7.2 (PB). This dialysate was applied to a DEAE-Sepharose column (Fast flow [2.0 by 20 cm]; Amersham Pharmacia Biotech, Uppsala. Sweden), equilibrated with PB, washed with PB containing 0.2 M NaCl, and then eluted with 200 ml of PB containing 0.3 M NaCl. Eluates collected in each 5-ml fraction were checked for the potential to raise the precipitin line in an agarose gel double-diffusion assay. Fractions which did raise the precipitin line against TMG were pooled, concentrated with ethanol precipitation, and dialyzed against 10 mM phosphate-buffered saline, pH 7.2 (PBS). This dialysate was applied to a Sephacryl S-400 column equilibrated with PBS. Fractions raising the precipitin line were again pooled, dialyzed against distilled water, and lyophilized. We confirmed the precipitin line-forming activity of the substance isolated from A. viridans against a fresh serum sample from a blood donor (Fig. 2B). The isolated substance formed a clear precipitin line against human serum, and this line completely fused with the one from the supernatant of the A. viridans broth and the rabbit anti-TMG antibody.
To assess the biochemical characteristics of the component produced by A. viridans, the stored sample was treated with trypsin, DNase I, RNase A, and NaIO4. NaIO4 treatment markedly reduced the activity, but other enzyme digestions did not reduce it. This indicates that the precipitin line formation activity is due to the carbohydrate component and not to a protein or a nucleic acid.
Composition analysis of PSA.
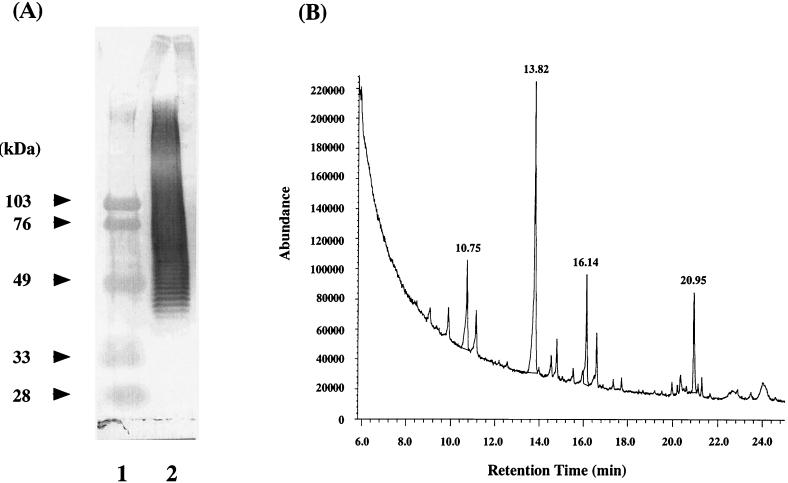
Figure 3A shows the SDS-PAGE pattern of PSA after blotting onto a nitrocellulose membrane. In this experiment, the blotting sheet was first incubated with human serum. The TMG trapped by bound PSA on the sheet was stained using the rabbit anti-TMG antibody and goat antibodies to rabbit IgG conjugated with horseradish peroxidase. PSA revealed ladder bands from approximately 40 kDa to nearly the top of the gel, suggesting that the component in A. viridans is a polymer. The monosaccharide composition of PSA was analyzed after methanolysis, re-N-acetylation, and trimethylsilylation (9). The lyophilized sample (1 mg) was hydrolyzed in 200 μl of 5% methanol-HCl (Wako Junyaku, Osaka, Japan) at 80°C for 2 h, re-N-acetylated, and trimethylsilylated in 200 μl of TCMI-C (GL Science Inc., Tokyo, Japan) at 80°C for 30 min; then the derivative was analyzed using a Hewlett-Packard G1800A GCD system (Avondale, Pa.) equipped with an electron ionization detector and a DB-5 capillary column (Jaw Scientific, Folsom, Calif.). The gas chromatography-mass spectrometry chromatogram of the PSA contained at least four monosaccharide signals, showing the possible presence of d-xylose at a retention time of 10.75 min, d-mannose at 13.82 min, d-glucose at 16.14 min, and N-acetyl-d-glucosamine at 20.95 min (Fig. 3B). These monosaccharide components of PSA were identified by comparing their retention times and mass spectra with those of monosaccharide reference standards. Taken together, PSA was found to consist of heteropolysaccharide-repeating units composed of d-glucose, d-mannose, N-acetyl-d-glucosamine, and d-xylose.
FIG. 3.
(A) Immunoblot analysis of PSA. Lane 1, size markers; lane 2, PSA. (B) Gas chromatography-mass spectrometry chromatogram of derivatized PSA. Peaks: 10.75 min, d-xylose; 13.82 min, d-mannose; 16.14 min, d-glucose; 20.95 min, N-acetyl-d-glucosamine.
Specific TMG binding of PSA.
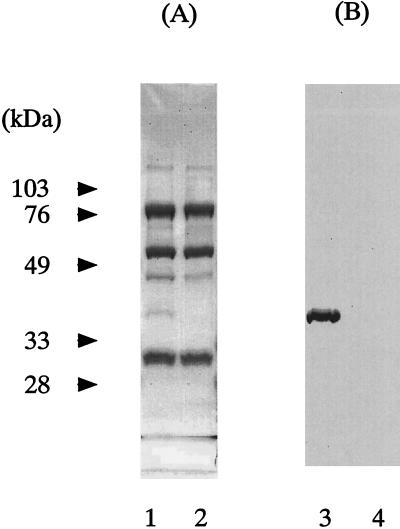
To assess the binding specificity of PSA, Sepharose 6B conjugated with PSA was incubated with serum samples with or without heat treatment at 56°C for 30 min and then analyzed by SDS-PAGE (Fig. 4). Sepharose 6B bead-conjugated PSA was prepared by the incubation of 1 mg of PSA and 0.3 g (dry weight) of epoxy-activated Sepharose 6B (Amersham Pharmacia Biotech) according to the manufacturer's instructions. The beads were incubated with serum for 1 h at 4°C. After being washed extensively with PBS, the beads were mixed with 30 μl of SDS-PAGE sample buffer (250 mM Tris-HCl buffer [pH 6.7], 4% SDS, 0.05% bromophenol blue, and 50% glycerol) in the presence of 10 mM β-mercaptoethanol, incubated for 2 min at 100°C, and applied to SDS-PAGE (10% acrylamide). After electrophoresis, substances on the gel were transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Richmond, Calif.). The sheet was stained with a rabbit anti-TMG antibody and goat antibody to rabbit immunoglobulin G (IgG) conjugated with horseradish peroxidase. Stained by Coomassie brilliant blue, a 35-kDa protein band was observed in intact serum, while this band was not found in the serum sample subjected to heat treatment at 56°C for 30 min, which caused the antigenicity of TMG to be lost (Fig. 4A). Protein bands other than TMG were mainly from nonspecifically bound IgG and IgM, as confirmed by using goat antibodies to human IgG and IgM. The 35-kDa protein was confirmed to be TMG by immunostaining using the rabbit anti-TMG antibody (Fig. 4B). A single band corresponding to the molecular size of the TMG monomer was seen only in the case of the intact serum sample. These observations indicate that PSA specifically binds intact TMG but not heat-denatured TMG.
FIG. 4.
Immunostaining of binding products of Sepharose 6B beads conjugated with PSA. (A) Protein staining with 0.1% Coomassie brilliant blue. (B) TMG stained with rabbit anti-TMG antibody and goat antibody to rabbit IgG conjugated with horseradish peroxidase. Lanes 1 and 3 are intact serum samples, and lanes 2 and 4 are serum samples heated at 56°C for 30 min.
ELISA.
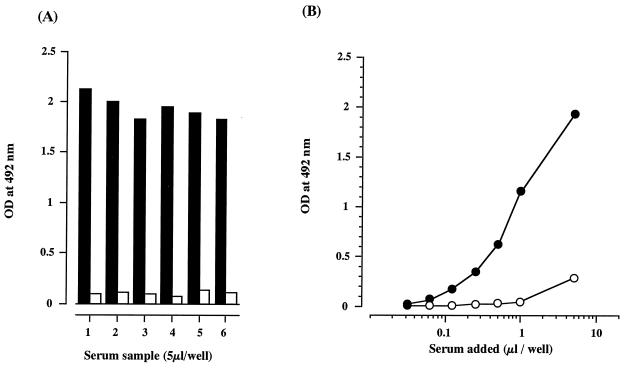
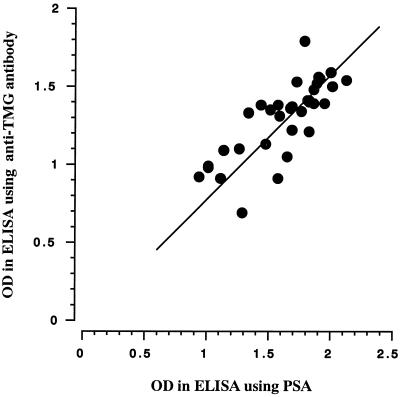
Based on these observations, we set up an ELISA to detect TMG, using PSA as adsorbent reagent. In this ELISA, 2 μg of PSA or 500 ng of the rabbit anti-TMG antibody in 10 mM NaHCO3 buffer, pH 9.55, was added to the wells of a 96-well microtiter plate (Nunc Inc., Naperville, Ill.) and was left overnight at 4°C. Unreacted sites on the solid phase were blocked with 1% bovine serum albumin dissolved in PBS at room temperature for 3 h. After being washed with PBS, serum samples added to the coated wells were incubated at 37°C for 1 h. The plate was washed with PBS, and then it was incubated with the rabbit anti-TMG antibody or the biotinylated rabbit anti-TMG antibody at 37°C for 1 h. The rabbit anti-TMG antibody was biotinylated according to the manufacturer's instructions (Pierce, Rockford, Ill.). The plates were washed three times with PBS and then incubated with goat antibody to rabbit IgG conjugated with horseradish peroxidase (MBL, Nagoya, Japan) or streptavidin conjugated with horseradish peroxidase (BioSource, Camarillo, Calif.) at 37°C for 30 min. The peroxidase activity was measured by the colorimetric method using o-phenylenediamine at 492 nm. Figure 5 shows the ELISA results. TMG binding to PSA was seen in the intact serum sample, whereas binding was not seen in the inactivated serum sample exposed to 56°C for 30 min (Fig. 5A). TMG bound to PSA in a dose-dependent manner, and this binding was clearly suppressed with the addition of 50 μg of PSA per ml (Fig. 5B). Figure 6 shows a comparison of ELISAs using PSA or the rabbit anti-TMG antibody as an adsorbent against TMG. The results obtained by ELISA using PSA were practically equivalent to those obtained by ELISA using the rabbit anti-TMG antibody, which suggests that PSA seems to be specific for TMG recognition by the antibody.
FIG. 5.
Characteristics of TMG binding of PSA in ELISA. All data are the means of triplicate experiments. (A) Optical density (OD) values in ELISA. Solid bar, intact serum; open bar, serum heated at 56°C for 30 min. (B) Binding of TMG in the presence or absence of PSA in the reaction medium. Closed circle, in the absence of PSA; open circle, in the presence of PSA (50 μg/ml).
FIG. 6.
Comparison of the results of ELISAs using PSA and the anti-TMG antibody as adsorbent against TMG. OD, optical density.
To assess the TMG binding potential of PSA, we examined the lectin activity of TMG in the presence of constituent monosaccharides of PSA and other monosaccharides. TMG was purified from sera of healthy blood donors according to Yae et al. (13) but with minor modifications. Ten milliliters of human serum was applied to a Sepharose 4B column (1 ml) conjugated with the rabbit anti-TMG antibody and eluted with 3 M sodium thiocyanate. TMG-active fractions were pooled and then passed through a protein G-Sepharose 4 FF column (Amersham Pharmacia Biotech) equilibrated with PBS to remove human IgG. Next, TMG-active fractions were applied to high-performance liquid chromatography (Waters 626 pump equipped with a 996 photodiode array detector: Millipore, Milford, Mass.), using a TSK GEL G4000SWXL column (7.7 by 300 mm) equilibrated with PBS. TMG binding activity of PSA at a concentration of 40 μg/ml was strongly suppressed by PSA. However, monosaccharides (d-glucose, d-galactose, d-xylose, d-mannose, d-fucose, N-acetyl-d-glucosamine, and N-acetyl-d-galactosamine) did not inhibit the TMG binding of PSA at concentrations up to 5 mM. These results indicate that the conformation of PSA may play a critical role in its association with TMG. The lectin activity of TMG against PSA was Ca2+ independent (data not shown).
The objective of this study was to develop appropriate methods for tracing the functions of TMG, which may play an important role in host defense as serum lectin, based on the specific TMG binding of PSA. Although the molecular mechanism of this TMG binding of PSA is not clear, our results demonstrate that PSA specifically binds to native serum TMG. First, an agarose gel double-diffusion assay showed that the precipitin line between PSA and fresh serum completely fused with the line formed between fresh serum and rabbit anti-TMG antibody, whereas it was lost in serum samples that were heat inactivated at 56°C for 30 min. Second, SDS-PAGE analysis demonstrated that Sepharose 6B beads conjugated with PSA selectively bind to intact TMG in fresh serum but not to heat-denatured TMG. Third, the ELISA using PSA as adsorbent gave almost the same findings as those obtained with the rabbit anti-TMG antibody, and PSA-TMG binding was specifically suppressed by addition of PSA to the reaction mixture. Taken together, these results show that PSA is a good adsorbent against native serum TMG and is useful in ELISA for TMG detection.
Recently, cDNA encoding Hakata antigen (TMG) was identified (11). TMG consists of a collagen-like domain in the middle position and a fibrinogen-like domain in the C terminus, both of which are homologous to two lectin activity-possessing proteins, human ficolin-1 and opsonin p35. To date, human plasma proteins and protein complexes that bind to carbohydrate have been categorized into two groups by Ca2+ dependency in its lectin activity. In the present study, we found that TMG specifically binds to PSA, composed of d-glucose, d-mannose, N-acetyl-d-glucosamine and d-xylose, but unlike opsonin p35 (8) and mannose-binding protein (5), the lectin activity of TMG was Ca2+ independent, similar to that of ficolin (7). Sugimoto et. al. (11) reported that TMG bound to lipopolysaccharide from Salmonella enterica serovar Typhimurium and that this binding was inhibited by a mono/oligosaccharide consisting of N-acetyl-d-glucosamine and N-acetyl-d-galactosamine at 38.9 mM and d-fucose at 2 mM . The TMG binding potential of PSA from A. viridans was not affected by constituent monosaccharides of PSA, N-acetyl-d-galactosamine and d-fucose. Therefore, the TMG binding manner of PSA may differ from that of lipopolysaccharides. To study the physiological function of TMG as a lectin in host defense, it is important to determine the association between PSA and TMG. PSA can be prepared easily and economically with consistent quality and high specificity to native TMG. For the reasons given above, PSA is a much better reagent than antibody for tracing the function of TMG.
Mannose-binding protein binds to specific carbohydrate structures on the surface of microorganisms in a Ca2+-dependent manner and exhibits antibacterial activity through killing mediated by lytic complement components or by promoting phagocytosis (6, 10). Furthermore, the level of mannose-binding protein in plasma is associated with frequent infections in children (12). These observations prompted us to examine the level of TMG in serum of healthy blood donors. We are proceeding with the screening of TMG levels in serum, using ELISA with PSA.
TMG is a novel serum glycoprotein whose levels, in a range of 7 to 23 μg/ml (13), are similar to those of some complement proteins, such as C1q, C2, and C5–9 (20 to 80 μg/ml). Some SLE patients (4.3%) and patients in the active stages of other autoimmune diseases (0.3%) carry the antibody against TMG. Transient TMG deficiency was found in these patients, and a strong correlation of this deficiency with SLE was observed. We found the remarkable specific binding of TMG to a polysaccharide derived from A. viridans. This gram-positive bacterium is common as an airborne organism, but its pathogenicity to humans is not well understood. TMG and A. viridans may possibly be involved in autoantibody production in autoimmune diseases such as SLE.
REFERENCES
- 1.Epstein W V, Tan M. An antibody-like material in systemic lupus erythematosus directed toward a thermolabile serum macroprotein. Arthritis Rheum. 1973;16:43–51. doi: 10.1002/art.1780160107. [DOI] [PubMed] [Google Scholar]
- 2.Fukutomi T, Ando B, Sakamoto S, Sakai H, Nawata H. Thermolabile β-2 macroglycoprotein (Hakata antigen) in liver disease: biochemical and immunohistochemical study. Clin Chim Acta. 1996;255:93–106. doi: 10.1016/0009-8981(96)06393-0. [DOI] [PubMed] [Google Scholar]
- 3.Inaba S, Okochi K. On a new precipitating antibody against normal human serum found in two patients with SLE. Igaku No Ayumi. 1978;107:690–691. . (In Japanese.) [Google Scholar]
- 4.Inaba S, Okochi K, Yae Y, Niklasson F, De-Verdier C H. Serological studies of an SLE-associated antigen-antibody system discovered as a precipitation reaction in agarose gel: the Hakata antigen-antibody system. Fukuoka Acta Med. 1990;81:284–291. [PubMed] [Google Scholar]
- 5.Kawasaki N, Kawasaki T, Yamashina I. Isolation and characterization of a mannan binding protein from human serum. J Biochem. 1983;94:937–947. doi: 10.1093/oxfordjournals.jbchem.a134437. [DOI] [PubMed] [Google Scholar]
- 6.Kuhlman M, Joiner K, Ezekowitz A. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Y, Lee S H, Kon O L, Lu J. Human L-ficolin: plasma levels, sugar specificity, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett. 1998;425:367–370. doi: 10.1016/s0014-5793(98)00267-1. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita M, Endo Y, Taira S, Sato Y, Fujita T, Ichikawa N, Nakata M, Mizouchi T. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–2454. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 9.Merkle R K, Poppe I. Carbohydrate composition analysis of glycoconjugates by gas-liquid chromatography/mass spectrometry. Methods Enzymol. 1994;230:1–15. doi: 10.1016/0076-6879(94)30003-8. [DOI] [PubMed] [Google Scholar]
- 10.Schweinie J E, Ezaekowitz R A B, Tenner A J, Kuhlman M, Joiner K A. Human mannose-binding protein activates the alternative complement pathway and enhances serum bacteriocidal activity on a mannose-rich isolate of Salmonella. J Clin Investig. 1989;84:1821–1829. doi: 10.1172/JCI114367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto R, Yae Y, Akaiwa M, Kitajima S, Shibata Y, Sato H, Hirata J, Okochi K, Izuhara K, Hamasaki N. Cloning and characterization of the Hakata antigen, a member of the Ficolin/Opsonin p35 lectin family. J Biol Chem. 1998;273:20721–20727. doi: 10.1074/jbc.273.33.20721. [DOI] [PubMed] [Google Scholar]
- 12.Super M, Thiel S, Lu J, Levinsky R J, Turner M W. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 1989;ii:1236–1239. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 13.Yae Y, Inaba S, Sato H, Okochi K, Tokunaga F, Iwanaga S. Isolation and characterization of a thermolabile β-2 macroglycoprotein (“thermolabile substance” or “Hakata antigen”) detected by precipitating (auto) antibody in sera of patients with systemic lupus erythematosus. Biochim Biophys Acta. 1991;1078:369–376. doi: 10.1016/0167-4838(91)90158-v. [DOI] [PubMed] [Google Scholar]
- 14.Yoshizawa S, Nagasawa K, Yae Y, Niho Y, Okochi K. A thermolabile β-2 macroglycoprotein (TMG) and the antibody against TMG in patients with systemic lupus erythematosus. Clin Chim Acta. 1997;264:219–225. doi: 10.1016/s0009-8981(97)00078-8. [DOI] [PubMed] [Google Scholar]