Abstract
Objective
This study was initiated to evaluate mammalian target of rapamycin (mTOR) activation in renal tissue of LN patients.
Methods
This retrospective study included 187 LN patients, 20 diabetic nephropathy (DN) patients, 10 minimal change disease (MCD) patients and 10 normal controls (NCs). Seven of 187 LN patients had repeated renal biopsies. mTORC1/2 activation was evaluated by immunohistochemistry and multiplexed immunofluorescence. The association of mTORC1/2 activation with the clinicopathologic indices and prognostic outcomes was analysed among 187 LN patients. Proteomics was performed in renal biopsies of 20 LN patients. Proteomics was employed to comprehensively evaluate the impact of mTOR activation on intrarenal gene expression.
Results
mTORC1/2 was significantly activated in podocytes, mesangial cells, endothelial cells and tubular epithelial cells of LN patients as compared with those with MCD or NC. The glomerular mTORC1 activation was higher in LN patients compared with DN patients. mTORC1, but not mTORC2, activation strongly correlated with serum albumin, complement C3, proteinuria and the following pathological biomarkers of LN: crescent formation, interstitial inflammation and fibrosis. Moreover, mTORC1 activation was identified as a prognostic marker in LN patients. Bioinformatic analyses of proteomics and immunohistochemical data unveiled increased complement activation, antigen presentation and phagocytosis in LN patients with mTORC1 activation.
Conclusion
Renal mTORC1 activation could be a biomarker to reveal disease activity and predict clinical prognosis in LN patients.
Keywords: mTOR, lupus nephritis, proteomics, bioinformatics, rapamycin
Rheumatology key messages.
In LN, renal mTORC1 activation was significantly correlated with the disease activity.
Renal mTORC1 activation could be a prognostic marker in LN patients.
Introduction
SLE is a chronic multisystem autoimmune disorder in which LN is the most common cause of morbidity and mortality over the disease course [1–3]. However, the conventional treatment for LN comprises CS combined with nonspecific immunosuppression, with 50–70% of patients achieving remission and 5–20% experiencing progression to end-stage renal disease within 10 years after diagnosis [1, 4, 5]. Furthering understanding of the molecular pathomechanism underlying LN could allow for the identification of novel and more precise therapeutic targets for LN.
The mammalian target of rapamycin (mTOR) pathway controls organismal growth and homeostasis. It is divided into two distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), based on the complex components [6]. mTORC1, a selective target of rapamycin, is involved in cell proliferation, tissue construction and organism growth, and has emerged as an important regulator of the pathogenesis of SLE/LN [7]. mTORC1 activation is increased in T cells extracted from SLE patients and causes the loss of TCRzeta in lupus T cells [8]. Double-negative T cells have a significantly higher mTORC1 activation level, which results in the expansion of IL-4+ double-negative T cells and Th17 cells, and inhibition of Tregs [9]. Hyperactivated mTORC1 has been found among CD19+ B cells in SLE patients, atypical memory B cells in SLE/LN patients and mesangial cells in the kidneys of class II LN patients [10–12]. Zhang et al. detected mTORC1 activation in the glomeruli of 10 active LN patients and found that p-mTOR (Ser2448) was predominantly colocalized with mesangial cells and glomerular endothelial cells and, to a lesser extent, with podocytes [13]. Animal studies also showed that mTORC1 activation is increased in the podocytes and endothelial cells in the kidneys of NZBW/F1 mice [14]. Interestingly, reduced activity of mTORC2 was found in SLE T cells, particularly CD8+ T cells, and in the livers of 4‐week‐old Murphy Roths Large/lymphoproliferation strain (MRL/lpr) mice [9, 15].
More importantly, a previous clinical trial evaluating rapamycin (a selective mTORC1 inhibitor) showed that 55% of SLE patients who were unresponsive to, or intolerant of, conventional medications demonstrated progressive improvement when treated with rapamycin, which indicated that rapamycin could be a novel and attractive therapeutic option for SLE patients [16, 17]. Rapamycin was also demonstrated to be effective for reducing renal immune complex deposition and improving both disease manifestations and the survival rate of lupus-prone mice [13, 14, 18], and long-term rapamycin therapy in some proliferative LN patients showed acceptable tolerability and therapeutic efficacy [19]. However, the activation pattern of mTORC1 in the kidneys of LN patients and its clinicopathological significance remain to be further elucidated.
Herein, we performed a comprehensive study of mTOR activation in the kidneys of LN patients based on a well-defined Chinese cohort, with the purpose of uncovering the significance of mTOR activation for disease progression.
Methods
Patients and sample collection
Sample size and power were calculated via PASS 11 software. From March 2003 to August 2018, a total of 187 patients with biopsy-proven LN at Peking University First Hospital were selected. The patients were monitored at our outpatient clinic specifically for LN. Renal specimens from 20 patients with diabetic nephropathy (DN) and 10 patients with minimal change disease (MCD) were collected as the disease controls. Renal tissues from normal areas of nephrectomized kidneys from 10 patients with solitary renal cell carcinoma were collected as the normal controls (NCs). These samples were identified as normal via regular light microscopy, immunofluorescence and electron microscopy. All LN patients included in this study met the 1997 ACR revised criteria for SLE [20]. For evaluation of renal outcomes, the composite endpoints were death, end-stage renal disease and ≥30% reduction from baseline estimated glomerular filtration rate (eGFR). Written informed consent was obtained from each patient. The research was in compliance with the declaration of Helsinki, and the design of this work was specifically approved by the ethical committees of Peking University First Hospital (approval number: 2017[1333]).
Renal histopathology
The renal histopathology of LN patients was evaluated according to the International Society of Nephrology/Renal Pathology Society classification system [21]. Pathologic parameters, including activity indices and chronicity indices, were determined by renal pathologists according to a previously reported system involving semiquantitative scoring of specific biopsy features. Activity indices comprise endocapillary hypercellularity, neutrophils/karyorrhexis, fibrinoid necrosis, hyaline deposits, cellular-fibrocellular crescents and interstitial inflammation. Chronicity indices comprise total glomerulosclerosis score, fibrous crescents, tubular atrophy and interstitial fibrosis. The tubulointerstitial lesions were semiquantitatively scored according to the affected area of the tubulointerstitium.
Clinical information
The clinical data of LN patients were extracted from the electronic medical records of Peking University First Hospital, including age, sex, hypertension, fever, malar rash, photosensitivity, oral ulcer, alopecia, arthritis, serositis, neurological disorder, anaemia, acute renal injury, nephrotic syndrome, SLEDAI, leukocytopenia, thrombocytopenia, haematuria, leukocyturia, serum creatinine, eGFR, serum C3, albumin, 24 h proteinuria and anti-dsDNA antibody. Informed consent was obtained from all participants for use of the clinical and pathologic data in future studies.
Immunohistochemistry of renal sections
Formaldehyde-fixed renal slides from LN patients, disease controls and NCs were first dewaxed in xylene ethanol at room temperature and rehydrated through graded ethanol. Antigen retrieval was then performed by heating the slides in citrate buffer (0.01 M, pH 6.0)/EDTA buffer (1×, pH 9.0) for 2 min and 30 s. After cooling to room temperature and washing three times with PBS, the slides were immersed in freshly prepared 3% hydrogen peroxide for 10 min to quench endogenous peroxidase activity. The cells were blocked with 3% BSA in PBS at room temperature for 60 min and then incubated with primary antibodies, including p-RPS6 (Ser235/236) (Cell Signalling Technology, Danvers, MA, USA), p-AKT (Ser473) (Cell Signalling Technology, Danvers, MA, USA), CD4 (Abcam, Boston, MA, USA) and CD8 (Abcam, Boston, MA, USA). The primary antibodies were detected with the appropriate secondary antibodies (ZSGB-Bio, PV9001, Beijing, China). Peroxidase activity was determined by 3–3′-diamino-benzidine-tetrahydrochloride. Cell nuclei were stained with haematoxylin. Finally, the slides were dehydrated in ethanol and xylene and sealed with neutral gum.
The immunohistochemistry staining results were evaluated by Image-Pro Plus (version 6.0; Media Cybernetics, Dallas, TX, USA) as the mean optical density (integrated option density/area). All glomeruli in a section and 10 fields of tubulointerstitial vision per kidney section at ×400 were observed blindly for quantitative assessments of immunohistochemical staining.
Immunofluorescence
The renal sections were incubated with anti-p-RPS6 (Ser235/236) antibodies (Cell Signalling Technology, Danvers, MA, USA), anti-p-AKT (Ser473) antibodies (Cell Signalling Technology, Danvers, MA, USA), anti-synaptopodin (R&D Systems, Minneapolis, MN, USA), anti-CD31 (Santa Cruz Biotechnology, Dallas, Texas, USA), anti-integrin-α8 (Santa Cruz Biotechnology, Dallas, Texas, USA), anti-CD68 (Abcam, Boston, MA, USA), anti-MPO (Abcam, Boston, MA, USA), anti-C1q (Abcam, Boston, MA, USA) and anti-HLA-DRB1 (Abnova, Taipei, China) antibodies. Then, the sections were incubated with goat anti-rabbit IgG H&L 594 (Abcam, Boston, MA, USA), goat anti-mouse IgG H&L FITC (Abcam, Boston, MA, USA) or Lotus Tetragonolobus Lectin (LTL) (Vector Laboratories, California, USA) antibodies, and DAPI (ASGB-BIO, Beijing, China). Fluorescence images were collected using a fluorescence microscope (90i, Nikon, Japan).
GFR estimates
The serum creatinine level was used for the GFR estimates following the Chronic Kidney Disease Epidemiology Collaboration eGFR equation [22].
Proteomics
Renal biopsies from 20 LN patients were collected for quantitative proteomic analysis. The detailed procedures were conducted as described previously [23, 24]. After staining of p-RPS6 (Ser235/236) by IHC, the LN patients were divided into two groups: a high activation group (10 patients) and a low activation group (10 patients). Proteins that met the inclusion criteria for pathway analysis had at least a 1.5-fold change, and P < 0.05 was used to identify differentially expressed proteins. Gene set enrichment analysis (GSEA) (http://software.broadinstitute.org/gsea/index.jsp) was employed to verify the biological processes.
Statistical analysis
SPSS version 25.0 (SPSS, Chicago, IL, USA) and Prism 8 software (GraphPad, San Diego, CA, USA) were used for statistical analysis, as appropriate. Experimental statistics are expressed as the mean (s.d.) or median with range (minimum–maximum). Differences in quantitative parameters among groups were assessed with Student’s t-test or one-way ANOVA for two or more independent samples as appropriate. The correlations between parametric variables were analyzed using Pearson’s test, while the correlations among nonparametric variables were analysed using Spearman’s test. Kaplan–Meier curves were generated to analyse the risk factors for prognosis and compared by using the log-rank test. The Cox proportional hazards regression model was used to quantify the relationship between exposures and the composite outcomes. The results were expressed as hazard ratio (HR) with a 95% CI. P < 0.05 was considered to be statistically significant. The potential confounders included age, sex, eGFR, proteinuria, chronicity indices and hypertension. Harrell’s concordance index (C-index), continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were compared with evaluate the incremental prognostic value of mTORC1 activation for the composite outcome in addition to established risk factors. Differences were considered significant if P was <0.05.
Results
mTOR activation in the kidneys of LN patients
The general data of 187 LN patients from the Peking University First Hospital are listed in supplementary Table S1, available at Rheumatology online.
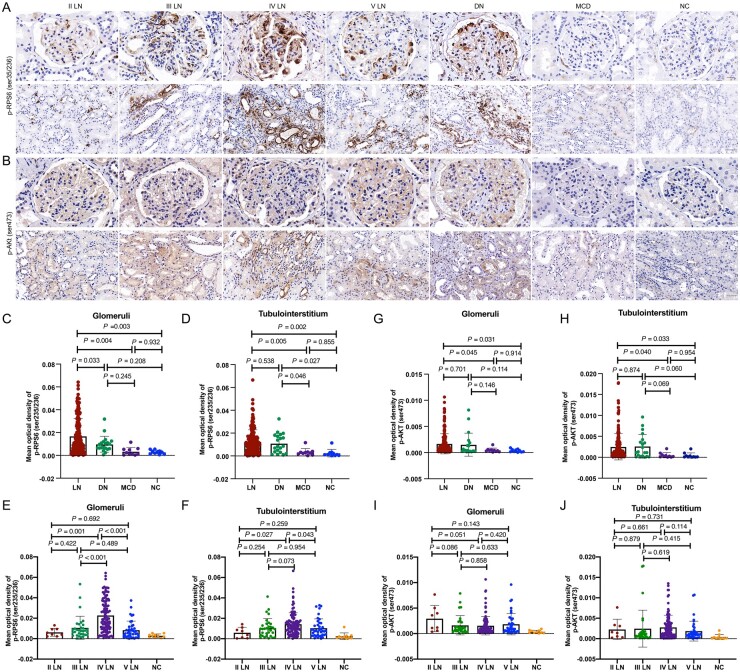
The expression patterns of p-RPS6 (Ser235/236) (the mTORC1 activation marker) and p-AKT (Ser473) (the mTORC2 activation marker) are displayed in Fig. 1A and B. mTORC1 activation was significantly higher in 187 LN patients than in that of 10 MCD patients and 10 NC samples (Fig. 1C and D). Interestingly, we found that the mTORC1 activation was more significant in the glomerular area of the LN group compared with the DN group, while the difference was not significant in the tubulointerstitial area (glomeruli: P = 0.033, tubulointerstitium: P = 0.538). Furthermore, glomerular mTORC1 activation in the class IV LN subgroup was higher than those in the other subgroups [the mean optical density levels of the glomerular mTORC1 activation were 0.0062 (0.0039) in class II LN patients, 0.0106 (0.0113) in class III LN patients, 0.0227 (0.0161) in class IV LN patients and 0.0084 (0.0088) in class V LN patients] (II vs IV, P = 0.001; III vs IV, P < 0.001; V vs IV, P < 0.001, respectively), and mTORC1 activation in the tubulointerstitium of class IV LN patients was higher than that in class II and V LN patients [the mean optical density levels of the tubulointerstitial mTORC1 activation were 0.0059 (0.0053) in class II LN patients, 0.0105 (0.0092) in class III LN patients, 0.0143 (0.0112) in class IV LN patients and 0.0104 (0.0091) in class V LN patients] (II vs IV, P = 0.027; V vs IV, P = 0.043) (Fig. 1E and F).
Fig. 1.
Immunohistochemistry staining for mTOR activation in the glomeruli and tubulointerstitial area of renal specimens
(A, B) Immunohistochemical staining of p-RPS6 (Ser235/236) (A) and p-AKT (Ser473) (B) in the glomeruli (above) and tubulointerstitium (below) of LN patients, DN patients, MCD patients and NCs. (C, D) The mean optical density of p-RPS6 (Ser235/236) (C) and p-AKT (Ser473) (D) in the glomeruli. (E, F) The mean optical density of p-RPS6 (Ser235/236) in the glomeruli (E) and tubulointerstitium (F) of different subclasses. (G, H) The mean optical density of p-AKT (Ser473) in the glomeruli (G) and tubulointerstitium (H). (I, J) The mean optical density of p-AKT (Ser473) in the glomeruli (I) and tubulointerstitium (J) of different subclasses. mTOR: mammalian target of rapamycin; DN: diabetic nephropathy; MCD: minimal change disease; NCs: normal controls.
mTORC2 activation was significantly higher in LN patients than in MCD patients and NC samples (Fig. 1G and H). No significant difference in mTORC2 activation was found between LN and DN groups. Moreover, no significant difference of mTORC2 activation in the glomeruli and tubulointerstitium was found between each pathological subgroup (Fig. 1I and J).
Colocalization of mTOR activation with renal cells in LN patients
A double immunofluorescence assay was conducted to measure the colocalization of mTOR activation and renal cells. As displayed in supplementary Fig. S1 (available at Rheumatology online), mTOR activation was well colocalized with podocytes, mesangial cells, endothelial cells and proximal tubular cells. mTORC1 also colocalized with monocytes in both the glomeruli and tubulointerstitium.
Clinicopathological significance of mTOR activation in the LN cohort
mTORC1 activation exerted significantly positive correlations with a few clinical indices, as listed in supplementary Table S2, available at Rheumatology online. Higher activation of mTORC1 in the glomeruli was associated with higher levels of serum creatinine (r = 0.447, P < 0.001), proteinuria (r = 0.274, P < 0.001) and SLEDAI scores (r = 0.325, P < 0.001), and lower levels of baseline eGFR (r = −0.450, P < 0.001), C3 (r = −0.312, P < 0.001) and albumin (r = −0.201, P = 0.019). mTORC1 activation was also correlated with acute kidney injury (P < 0.001), nephrotic syndrome (P < 0.001), anaemia (P < 0.001), haematuria (P = 0.007), leukocyturia (noninfectious) (P = 0.003) and anti-dsDNA antibodies (P < 0.001). Similar associations were found between tubulointerstitial mTORC1 activation and the above features (supplementary Table S2, available at Rheumatology online). Furthermore, our patients were divided into two groups (high activation group and low activation group) based on the mean optical density of mTORC1 activation. The differences in clinical characteristics between two groups are displayed in supplementary Table S3 (available at Rheumatology online), and the results demonstrated that high renal mTORC1 activation was associated with severe disease activity and renal involvement.
mTORC2 activation in the glomeruli was only positively correlated with higher levels of serum creatinine (r = 0.180, P = 0.014). Higher mTORC2 activation in the tubulointerstitium was associated with higher levels of serum creatinine (r = 0.176, P = 0.016) and SLEDAI scores (r = 0.220, P = 0.003), and lower eGFR values (r = −0.233, P = 0.001) (supplementary Table S4, available at Rheumatology online).
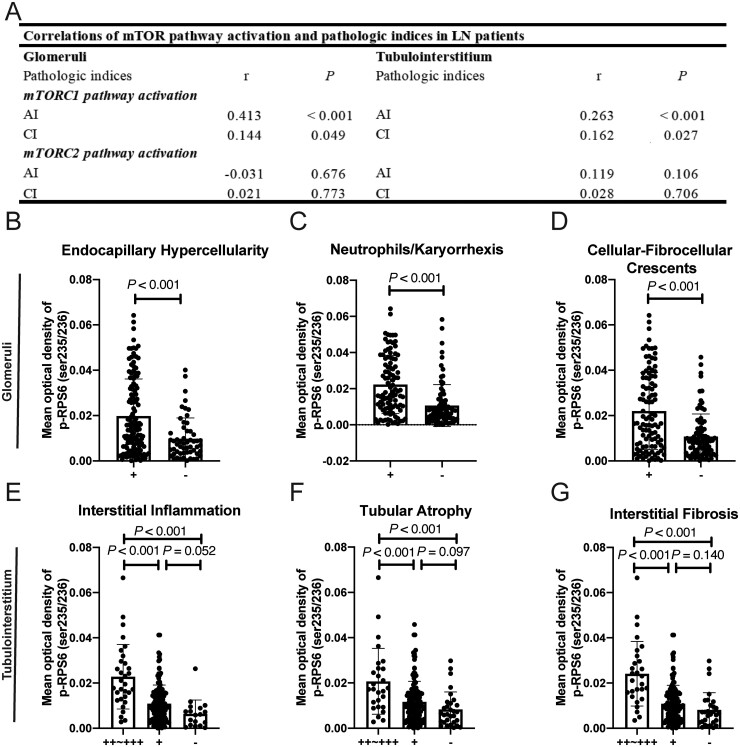
In the pathological evaluations, significant positive correlations were observed between mTORC1 activation in the glomeruli and several activity/chronicity indices as follows: endocapillary hypercellularity, neutrophils/karyorrhexis, cellular-fibrocellular crescents (Fig. 2A–D). In particular, we found that the staining for mTORC1 activation was elevated in the cellular-fibrocellular crescents areas and the level of glomerular mTORC1 activation was positively correlated with the ratio of cellular-fibrocellular crescents (r = 0.463, P < 0.001) (supplementary Fig. S2, available at Rheumatology online).
Fig. 2.
The association of mTOR activation with renal pathologic indices in LN patients
(A) The association between mTORC1 and mTORC2 activation in glomeruli and tubulointerstitium, and the total AI and CI. (B–D) mTORC1 activation in glomeruli with pathologic indices, including endocapillary hypercellularity (B), neutrophils/karyorrhexis (C) and cellular-fibrocellular crescents (D). (E–G) mTORC1 activation in the tubulointerstitium with pathological indices, including interstitial inflammation (E), tubular atrophy (F) and interstitial fibrosis (G). mTOR: mammalian target of rapamycin; AI: activity indices; CI: chronicity indices.
mTORC1 activation in the tubulointerstitium was positively correlated with activity/chronicity indices scores (Fig. 2A). The existence of interstitial fibrosis, interstitial inflammation and tubular atrophy corresponded to higher activation of mTORC1 in the tubulointerstitium (Fig. 2E–G).
These results were verified in seven LN patients who received repeated renal biopsies, including six with disease deterioration and one in remission (supplementary Fig. S3A–D, available at Rheumatology online). The detailed pathologic indices and treatments for these patients are listed in supplementary Table S5, both available at Rheumatology online.
No significant correlation was found between mTORC2 activation and any renal pathologic indices (Fig. 2A).
Association of mTORC1 activation with the prognosis of LN patients
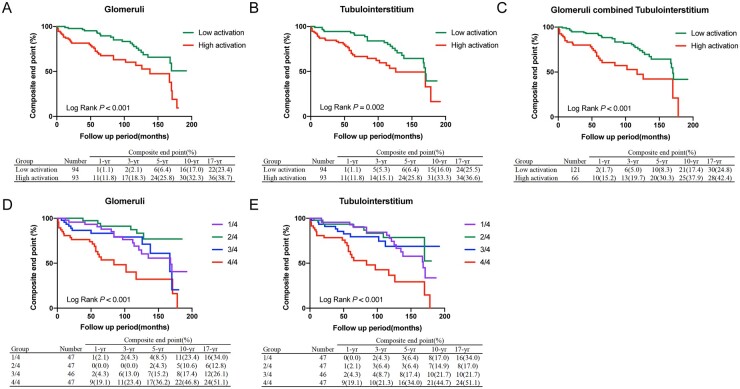
The univariate Cox regression analysis showed that high mTORC1 activation in the glomeruli and tubulointerstitium was a risk factor for prognosis (Table 1). The Kaplan–Meier curve showed that high mTORC1 activation in the glomeruli, tubulointerstitium or both predicted worse prognosis (Fig. 3A–C). Furthermore, the quartile analysis showed that the patients with the highest mTORC1 activation level (4/4) in the glomeruli or tubulointerstitium presented with the worst prognoses [glomeruli: HR4/4 2.914 (95% CI 1.528, 5.557), P = 0.001; tubulointerstitium: HR4/4 3.059 (95% CI 1.602, 5.840), P = 0.001] (Table 1; Fig. 3D and E).
Table 1.
Association of mTORC1 activation pathway activation (p-RPS6, Ser235/236) with composite outcomes in LN patients
| Composite outcomes, no/no. at risk | Unadjusted risk |
Modela |
Modelb |
||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| mTORC1 pathway activation (glomeruli) (Reference: 1/2) | 22/94 | 1 | Reference | 1 | Reference | 1 | Reference |
| 2/2 | 36/93 | 2.519 (1.474, 4.304) | 0.001 | 2.376 (1.335, 4.228) | 0.003 | 2.262 (1.255, 4.078) | 0.007 |
| mTORC1 pathway activation (tubulointerstitium) (Reference: 1/2) | 24/94 | 1 | Reference | 1 | Reference | 1 | Reference |
| 2/2 | 34/93 | 2.241 (1.318, 3.810) | 0.003 | 1.956 (1.115, 3.432) | 0.019 | 1.844 (1.048, 3.246) | 0.034 |
| mTORC1 pathway activation (glomeruli) (Reference: 1/4) | 16/47 | 1 | Reference | 1 | Reference | 1 | Reference |
| 2/4 | 6/47 | 0.433 (0.169, 1.113) | 0.082 | 0.484 (0.186, 1.257) | 0.136 | 0.506 (0.194, 1.322) | 0.164 |
| 3/4 | 12/46 | 1.098 (0.517, 2.331) | 0.808 | 1.175 (0.544, 2.537) | 0.681 | 1.201 (0.555, 2.600) | 0.642 |
| 4/4 | 24/47 | 2.914 (1.528, 5.557) | 0.001 | 3.335 (1.552, 7.164) | 0.002 | 3.218 (1.481, 6.995) | 0.003 |
| mTORC1 pathway activation (tubulointerstitium) (Reference: 1/4) | 16/47 | 1 | Reference | 1 | Reference | 1 | Reference |
| 2/4 | 8/47 | 0.662 (0.283, 1.553) | 0.344 | 0.681 (0.290, 1.598) | 0.378 | 0.715 (0.304, 1.683) | 0.443 |
| 3/4 | 10/46 | 1.009 (0.454, 2.242) | 0.983 | 0.889 (0.392, 2.015) | 0.778 | 0.871 (0.383, 1.979) | 0.741 |
| 4/4 | 24/47 | 3.059 (1.602, 5.840) | 0.001 | 3.217 (1.539, 6.724) | 0.002 | 3.089 (1.474, 6.471) | 0.003 |
Model a was adjusted for age, sex, eGFR, proteinuria and hypertension (yes or no). bModel b was adjusted for age, sex, eGFR, proteinuria and chronicity index. mTOR: mammalian target of rapamycin; HR: hazard ratio; eGFR: estimated glomerular filtration rate.
Fig. 3.
Kaplan–Meier analysis of composite endpoints between the high and low mTORC1 activation groups
(A, B) Kaplan–Meier analysis of composite endpoints between the high and low mTORC1 activation groups in glomeruli (A) and tubulointerstitium (B) (dichotomy based on the mean optical density of mTORC1 activation). (C) Kaplan–Meier analysis of composite endpoints between the group with high mTORC1 pathway activation (both in glomeruli and tubulointerstitium) and low mTORC1 activation. (D, E) Kaplan–Meier analysis of composite endpoints between the different mTORC1 activation groups in the glomeruli (D) and tubulointerstitium (E) (quartering based on the mean optical density of mTORC1 activation). mTOR: mammalian target of rapamycin.
In a further multivariable Cox hazard analysis, mTORC1 activation was identified as an independent risk factor for composite outcomes after adjusting for traditional clinical markers [25, 26] (age, sex, eGFR, proteinuria and hypertension) (modela) [glomeruli: HR2/2 2.376 (95% CI 1.335, 4.228), P = 0.003; tubulointerstitium: HR2/2 1.956 (95% CI 1.115, 3.432), P = 0.019] [glomeruli: HR4/4 3.335 (1.552, 7.164), P = 0.002; tubulointerstitium: HR4/4 3.217 (95% CI 1.539, 6.724), P = 0.002] (Table 1). As chronicity indices have been reported to contribute significantly to prognosis prediction for LN patients (based on the univariate analysis), we conducted a multivariable Cox hazard analysis of mTORC1 activation after adjusting for age, sex, eGFR, proteinuria and chronicity indices (modelb). A similar result was also observed after the addition of mTORC1 activation (Table 1).
To evaluate the additive effect of mTORC1 activation on the baseline lesions for predicting the composite outcomes more appropriately, we conducted model discrimination and reclassification using the C-index, IDI and NRI. Compared with the base modela, adding mTORC1 activation in the glomeruli and tubulointerstitum improved the C-index from 0.643 (0.563–0.722) to 0.706 (0.633–0.778) (P < 0.001) and 0.694 (0.612–0.776) (P < 0.001), respectively. Significant improvements in IDI [glomeruli: 0.100 (0.026–0.225), P = 0.013; tubulointerstitium: 0.087 (0.015–0.184), P = 0.007] and NRI [glomeruli: 0.433 (0.106–0.600), P = 0.013; tubulointerstitium: 0.354 (0.089–0.526), P < 0.001] were discovered after the addition of mTORC1 activation. Compared with base modelb, a similar result was also observed after the addition of mTORC1 activation (Table 2).
Table 2.
Prediction performance of mTORC1 activation in LN patients
| Prediction model | C-index (95% CI) | P | IDI (95% CI) | P | NRI (95% CI) | P |
|---|---|---|---|---|---|---|
| Ma = age + sex + eGFR + proteinuria + hypertension | 0.643 (0.563, 0.722) | Reference | – | Reference | – | Reference |
| M1 = Ma+ mTORC1 activation in glomeruli | 0.706 (0.633, 0.778) | <0.001 | 0.100 (0.026, 0.225) | 0.013 | 0.433 (0.106, 0.600) | 0.013 |
| M2 = Ma + mTORC1 activation in tubulointerstitium | 0.694 (0.612, 0.776) | <0.001 | 0.087 (0.015, 0.184) | 0.007 | 0.354 (0.089, 0.526) | <0.001 |
| Mb = age + sex + eGFR + proteinuria + chronicity indices | 0.652 (0.570, 0.734) | Reference | – | Reference | – | Reference |
| M3 = Mb + mTORC1 activation in glomeruli | 0.716 (0.645, 0.786) | <0.001 | 0.096 (0.018, 0.227) | 0.007 | 0.305 (0.083, 0.536) | 0.007 |
| M4 = Mb + mTORC1 activation in tubulointerstitium | 0.715 (0.639, 0.791) | <0.001 | 0.081 (0.010, 0.183) | 0.007 | 0.349 (0.091, 0.567) | 0.007 |
Model (M)a was adjusted for age, sex, eGFR, proteinuria and hypertension (yes or no). bModel (M)b was adjusted for age, sex, eGFR, proteinuria and chronicity index. mTOR: mammalian target of rapamycin; IDI: integrated discrimination improvement index; NRI: net reclassification improvement index; eGFR: estimated glomerular filtration rate.
Differential proteome analysis between high and low mTORC1 activation in kidney biopsies from LN patients
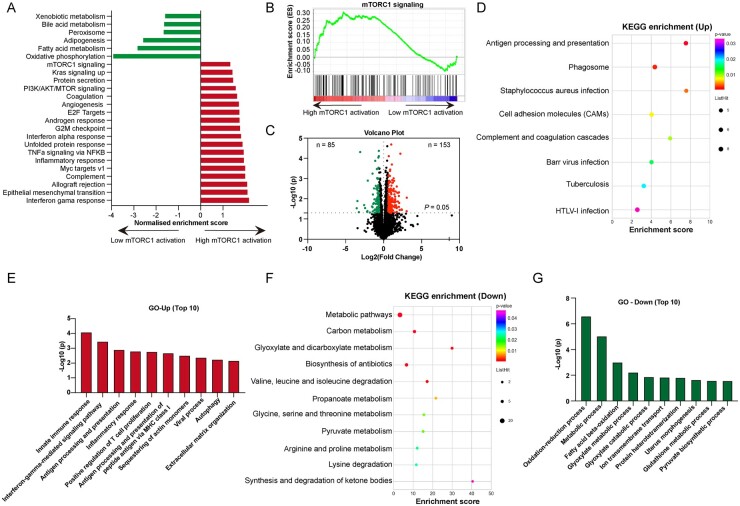
Furthermore, we applied mass spectrometric analyses to investigate the differential proteome between high and low mTORC1 activation in the kidney biopsies of 20 LN patients (10 with high activation and 10 with low activation; their baseline features are shown in supplementary Table S6, available at Rheumatology online). GSEA demonstrated a significant enrichment of the functional pathway of inflammation in the high mTORC1 activation group, and metabolic pathways were reduced in the high mTORC1 activation group, especially the fatty acid metabolism pathway (Fig. 4A). The mTORC1 signalling pathway was significantly enriched in the high activation group compared with in the low activation group (P = 0.033) (Fig. 4B).
Fig. 4.
Bioinformatic analysis of the high and low renal mTORC1 activation groups in the kidney of LN patients
The samples were stratified according to mTORC1 activation as high mTORC1 activation and low mTORC1 activation groups. (A) The enriched pathways identified by GSEA. (B) GSEA of mTORC1 signalling pathway between the high and low mTORC1 activation groups. (C) The volcano plot of differentially expressed proteins based on the threshold (fold-change ≥ 1.5, P < 0.05). (D, E) KEGG (D) and GO biological process analysis (E) were performed among the upregulated proteins [fold-change (high/low mTORC1 activation) ≥ 1.5, P < 0.05]. (F, G) KEGG (F) and GO biological process analysis (G) were performed among the downregulated proteins [fold-change (low/high mTORC1 activation) < 1.5, P < 0.05]. mTOR: mammalian target of rapamycin; GSEA: gene set enrichment analysis; KEGG; Kyoto Encyclopedia of Genes and Genomes; GO: Gene Ontology.
Proteins that met the inclusion criteria for pathway analysis had at least a 1.5-fold change in the high mTORC1 activation group compared with in the low mTORC1 activation group and P-values <0.05. A total of 153 proteins were overexpressed in the high activation group, and 85 proteins were overexpressed in the low activation group (Fig. 4C). We next subjected the 153 upregulated proteins to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis and revealed that the ‘antigen processing & presentation’, ‘phagosome pathway’ and ‘complement & coagulation cascades’ were significantly enriched (Fig. 4D). Upon Gene Ontology (GO) functional enrichment, the biological process was also enriched in ‘innate immune response’, ‘interferon-gamma-mediated signalling pathway’ and ‘antigen processing & presentation’ (Fig. 4E). The KEGG pathway and GO enrichment analysis of the downregulated proteins demonstrated that metabolic pathways were disrupted in the high mTORC1 activation group, especially the fatty acid beta-oxidation pathway (Fig. 4F and G). The functional protein association network demonstrated that the HLA family, C1q and MPO were key nodes among these proteins (supplementary Fig. S4, available at Rheumatology online). As shown in supplementary Fig. S5A–C (available at Rheumatology online), the proteins enriched in the three pathways mentioned above were upregulated in renal biopsies from LN patients with high mTORC1 activation. The immunofluorescence of MPO, C1q and HLA-DRB1 in the kidneys of LN patients validated the mass spectrometric results (supplementary Fig. S5D, available at Rheumatology online). Moreover, MPO was the most predominant protein based on the above mass spectrometric analysis (fold-change = 8.235, P = 0.009) and it was positively correlated with mTORC1 activation (glomeruli: r = 0.626, P = 0.003; tubulointerstitium: r = 0.430, P = 0.058).
According to the KEGG and GO analyses, antigen presentation and processing were enriched in the high mTORC1 activation group. Antigen-presenting cells process peptide epitopes and present them to CD4+/CD8+ T cells via MHC molecules [27, 28] (supplementary Fig. S6, available at Rheumatology online). Thus, we examined the infiltration of CD4+/CD8+ T cells in the kidneys of LN patients with high and low mTORC1 activation. As shown in supplementary Fig. S7 (available at Rheumatology online), the high mTORC1 activation group possessed significantly higher positive staining for CD4 and CD8 than the samples with low activation of mTORC1.
Discussion
Given the promising outcome from a few clinical trials that evaluated rapamycin for the treatment of SLE/LN patients, it is necessary to further elucidate the mTOR pathway activation pattern in the kidneys of LN patients and its clinicopathological significance [16, 19, 29]. In the current study, we calculated power and sample size requirements based on Cox regression via PASS software to provide more sufficient power to detect clinically important effects and better reflect the real-world situation. We detected the activation of the mTOR pathway in the kidneys of LN patients for the first time and comprehensively analysed the clinicopathological significance of mTOR activation in a well-defined patient cohort.
P-RPS6 (Ser235/236) and p-AKT (Ser473) are well-established readouts of mTORC1 and mTORC2 activation, respectively [30–32]. RICTOR/mTORC2 has been reported to directly phosphorylate Akt (Ser473) in vitro, and depletion of RICTOR/mTOR dramatically reduces p-Akt (Ser473) levels [33]. mTORC2 phosphorylates Akt at the hydrophobic motif site (Ser473), which is the best characterized readout of mTORC2 activity [34]. Knocking out Raptor to interrupt mTORC1 signalling and knocking out TSC1 to activate mTORC1 signalling significantly decreased and increased p-RPS6 (Ser235/236) in vivo, respectively [31, 32]. We identified that p-RPS6 (Ser235/236) and p-AKT (Ser473) were significantly elevated in LN patients, and our clinicopathological analysis revealed that mTORC1 activation was significantly correlated with the severity of renal involvement. Moreover, we found that mTORC1 activation could be an independent risk factor for prognosis by multivariable Cox hazard analysis. The combination of the C-index, IDI and NRI calculation further demonstrated that the addition of mTORC1 activation could significantly improve the ability to predict worse outcomes after adjusting for baseline lesions. This study provides initial evidence that detecting mTORC1 activation by tissue staining improves the prognostic value of renal biopsy in predicting clinical outcomes in our LN cohort.
Interestingly, the Kaplan–Meier analysis and Cox hazard analysis of mTORC1 demonstrated that the lowest level of activation of the mTORC1 pathway did not correspond to the best prognosis. Previous studies have also revealed that podocyte-specific deletion of mTORC1 can induce proteinuria and progressive glomerulosclerosis [32, 35], while tubule-specific deletion of mTORC1 can lead to defective concentrating mechanisms and polyuria [36].
The differential proteome analysis showed that high mTORC1 activation corresponds with serious disease conditions at the protein level, especially serious inflammatory infiltration. Consistent with this result, mTORC1 activation has been reported to regulate the proinflammatory response in RA and to be highly increased in lymphoproliferation and inflammatory lymphadenopathy [37, 38]. The proteomic analysis comprehensively supported the association between a higher level of mTORC1 activation and disease severity in LN patients at the protein level.
Our present study has some limitations. It is a retrospective study. The activation of mTORC1 in the peripheral circulation of LN patients was not tested, and a more precise value of mTORC1 activation in LN needs to be further clarified with deeper functional and animal experiments.
In conclusion, mTORC1 could be a biomarker to reveal disease activity and predict patient prognosis. Prospective validation studies are required to confirm its clinical utility.
Supplementary Material
Acknowledgements
The authors thank Jinwei Wang for the statistical consultation, Luoyi Wang for assistance with immunohistochemical staining and Zihan Li for the collection of normal kidney controls. Z.M. performed the experiments, analysed the statistics, and drafted the manuscript. Y.T. provided intellectual content of importance to this work and revised the manuscript. J.T. collected the general and follow-up data of the patients. L.L. conducted the proteomic assay. H.W. evaluated the pathology. F.Y. designed the study and revised the manuscript. A.P. and M.Z. reviewed and edited the manuscript. F.Y. had full access to all of the data and provided final approval of the submitted manuscript. All authors read and approved the manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (81870479), the Beijing Natural Science Foundation (7192207 and 7212114), the Chinese Academy of Medical Sciences Research Unit (2019RU023) and Clinical Medicine Plus X—Young Scholars Project of Peking University supported by ‘the Fundamental Research Funds for the Central Universities’.
Disclosure statement: The authors have declared no conflicts of interest.
Contributor Information
Zhaomin Mao, Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology; Key laboratory of Renal Disease, Ministry of Health of China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment, Ministry of Education of China; Peking-Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies, Peking University.
Ying Tan, Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology; Key laboratory of Renal Disease, Ministry of Health of China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment, Ministry of Education of China.
Juan Tao, Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology; Key laboratory of Renal Disease, Ministry of Health of China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment, Ministry of Education of China.
Linlin Li, Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology; Key laboratory of Renal Disease, Ministry of Health of China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment, Ministry of Education of China.
Hui Wang, Laboratory of Electron Microscopy, Department of Medicine, Peking University First Hospital.
Feng Yu, Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology; Key laboratory of Renal Disease, Ministry of Health of China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment, Ministry of Education of China; Department of Nephrology, Peking University International Hospital, Beijing, PR China.
Andras Perl, Departments of Medicine, Microbiology and Immunology, Biochemistry and Molecular Biology, State University of New York Upstate Medical University, New York, Syracuse, NY, USA.
Minghui Zhao, Renal Division, Department of Medicine, Peking University First Hospital; Peking University Institute of Nephrology; Key laboratory of Renal Disease, Ministry of Health of China; Key Laboratory of Chronic Kidney Disease Prevention and Treatment, Ministry of Education of China; Peking-Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies, Peking University.
Data availability statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Yu F, Haas M, Glassock R, Zhao MH.. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol 2017;13:483–95. [DOI] [PubMed] [Google Scholar]
- 2. Cameron JS. Lupus nephritis. J Am Soc Nephrol 1999;10:413–24. [DOI] [PubMed] [Google Scholar]
- 3. D’Cruz DP, Khamashta MA, Hughes GR.. Systemic lupus erythematosus. Lancet 2007;369:587–96. [DOI] [PubMed] [Google Scholar]
- 4. Ginzler EM, Dooley MA, Aranow C. et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 2005;353:2219–28. [DOI] [PubMed] [Google Scholar]
- 5. Anders HJ, Saxena R, Zhao MH. et al. Lupus nephritis. Nat Rev Dis Primers 2020;6:7. [DOI] [PubMed] [Google Scholar]
- 6. Laplante M, Sabatini DM.. mTOR signaling in growth control and disease. Cell 2012;149:274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann N Y Acad Sci 2015;1346:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez DR, Telarico T, Bonilla E. et al. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol 2009;182:2063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kato H, Perl A.. Mechanistic target of rapamycin complex 1 expands Th17 and IL-4+ CD4-CD8- double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J Immunol 2014;192:4134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Torigoe M, Iwata S, Nakayamada S. et al. Metabolic reprogramming commits differentiation of human CD27(+)IgD(+) B cells to plasmablasts or CD27(-)IgD(-) cells. J Immunol 2017;199:425–34. [DOI] [PubMed] [Google Scholar]
- 11. Wu C, Fu Q, Guo Q. et al. Lupus-associated atypical memory B cells are mTORC1-hyperactivated and functionally dysregulated. Ann Rheum Dis 2019;78:1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagai K, Tominaga T, Ueda S. et al. Mesangial cell mammalian target of rapamycin complex 1 activation results in mesangial expansion. J Am Soc Nephrol 2017;28:2879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang C, Chan CCY, Cheung KF. et al. Effect of mycophenolate and rapamycin on renal fibrosis in lupus nephritis. Clin Sci (Lond) 2019;133:1721–44. [DOI] [PubMed] [Google Scholar]
- 14. Stylianou K, Petrakis I, Mavroeidi V. et al. The PI3K/Akt/mTOR pathway is activated in murine lupus nephritis and downregulated by rapamycin. Nephrol Dial Transplant 2011;26:498–508. [DOI] [PubMed] [Google Scholar]
- 15. Oaks Z, Winans T, Caza T. et al. Mitochondrial dysfunction in the liver and antiphospholipid antibody production precede disease onset and respond to rapamycin in lupus-prone mice. Arthritis Rheumatol 2016;68:2728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lai ZW, Kelly R, Winans T. et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 2018;391:1186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandez DR, Crow MK.. CD8 T cells and mTOR: new concepts and targets for systemic lupus erythematosus. Lancet 2018;391:1126–7. [DOI] [PubMed] [Google Scholar]
- 18. Lui SL, Yung S, Tsang R. et al. Rapamycin prevents the development of nephritis in lupus-prone NZB/W F1 mice. Lupus 2008;17:305–13. [DOI] [PubMed] [Google Scholar]
- 19. Yap DYH, Tang C, Chan GCW. et al. Longterm data on sirolimus treatment in patients with lupus nephritis. J Rheumatol 2018;45:1663–70. [DOI] [PubMed] [Google Scholar]
- 20. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 21. Bajema IM, Wilhelmus S, Alpers CE. et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018;93:789–96. [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH. et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wiśniewski JR, Zougman A, Nagaraj N, Mann M.. Universal sample preparation method for proteome analysis. Nat Methods 2009;6:359–62. [DOI] [PubMed] [Google Scholar]
- 24. Tao Z, Meng X, Han Y-Q. et al. Therapeutic mechanistic studies of ShuFengJieDu capsule in an acute lung injury animal model using quantitative proteomics technology. J Proteome Res 2017;16:4009–19. [DOI] [PubMed] [Google Scholar]
- 25. Wu L, Li XQ, Chang DY. et al. Associations of urinary epidermal growth factor and monocyte chemotactic protein-1 with kidney involvement in patients with diabetic kidney disease. Nephrol Dial Transplant 2020;35:291–7. [DOI] [PubMed] [Google Scholar]
- 26. Barbour SJ, Coppo R, Zhang H. et al. ; International IgA Nephropathy Network. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med 2019;179:942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neefjes J, Jongsma ML, Paul P, Bakke O.. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol 2011;11:823–36. [DOI] [PubMed] [Google Scholar]
- 28. Kotsias F, Cebrian I, Alloatti A.. Antigen processing and presentation. Int Rev Cell Mol Biol 2019;348:69–121. [DOI] [PubMed] [Google Scholar]
- 29. Fernandez D, Bonilla E, Mirza N, Niland B, Perl A.. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum 2006;54:2983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM.. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005;307:1098–101. [DOI] [PubMed] [Google Scholar]
- 31. Inoki K, Mori H, Wang J. et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 2011;121:2181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gödel M, Hartleben B, Herbach N. et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 2011;121:2197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guertin DA, Sabatini DM.. Defining the role of mTOR in cancer. Cancer Cell 2007;12:9–22. [DOI] [PubMed] [Google Scholar]
- 34. Huang W, Zhu PJ, Zhang S. et al. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci 2013;16:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zschiedrich S, Bork T, Liang W. et al. Targeting mTOR signaling can prevent the progression of FSGS. J Am Soc Nephrol 2017;28:2144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grahammer F, Haenisch N, Steinhardt F. et al. mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc Natl Acad Sci USA 2014;111:E2817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karonitsch T, Kandasamy RK, Kartnig F. et al. mTOR senses environmental cues to shape the fibroblast-like synoviocyte response to inflammation. Cell Rep 2018;23:2157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arenas DJ, Floess K, Kobrin D. et al. Increased mTOR activation in idiopathic multicentric Castleman disease. Blood 2020;135:1673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.