Abstract
Objective
To assess predictors of subclinical RA-associated interstitial lung disease (RA-ILD) using quantitative lung densitometry (qLD).
Methods
RA patients underwent multi-detector row CT scanning at baseline and after an average of 39 months. Scans were analysed with qLD for the percentage of lung parenchyma with high attenuation areas (%HAA: the percentage of voxels of –600 to –250 Hounsfield units). Additionally, a pulmonary radiologist calculated an expert radiologist scoring (ERS) for RA-ILD features. Generalized linear models were used to identify indicators of baseline %HAA and predictors of %HAA change.
Results
Baseline %HAA was assessed in 193 RA patients and 106 had repeat qLD assessment. %HAA was correlated with ERS (Spearman’s rho = 0.261; P < 0.001). Significant indicators of high baseline %HAA (>10% of lung parenchyma with high attenuation) included female sex, higher pack-years of smoking, higher BMI and anti-CCP ≥200 units, collectively contributing an area under the receiver operator curve of 0.88 (95% CI 0.81, 0.95). Predictors of %HAA increase, occurring in 49% with repeat qLD, included higher baseline %HAA, presence of mucin 5B (MUC5B) minor allele and absence of HLA-DRB1 shared epitope (area under the receiver operator curve = 0.69; 95% CI 0.58, 0.79). The association of the MUC5B minor allele with %HAA change was higher among men and those with higher cumulative smoking. Within the group with increased %HAA, anti-CCP level was significantly associated with a greater increase in %HAA.
Conclusions
%HAA, assessed with qLD, was linked to several known risk factors for RA-ILD and may represent a more quantitative method to identify RA-ILD and track progression than expert radiologist interpretation.
Keywords: RA, interstitial lung disease, pulmonary fibrosis, rheumatoid lung, computed tomography
Rheumatology key messages.
RA-associated interstitial lung disease (RA-ILD) evaluation of CT scans by radiologists is flawed by inter- and intra-observer variability.
Quantitative lung densitometry is a computerized methodology that measures the extent of lung parenchyma with high attenuation areas (HAA).
HAA is a potential biomarker of interstitial lung abnormalities, which may aid in early diagnosis and management of RA-ILD.
Introduction
RA is a chronic systemic inflammatory disease associated with premature mortality [1]. RA-associated interstitial lung disease (RA-ILD), in particular, carries a markedly elevated burden of morbidity and mortality. For example, in a population-based cohort, median survival was only 6.6 years after RA-ILD diagnosis [2]. Approximately 10% of RA patients will develop clinically significant ILD over the disease course [3], but a much greater proportion, up to 20–50%, has radiographic evidence of subclinical ILD [4, 5]. Despite its high prevalence, the natural history and predictors of RA-ILD progression are poorly understood.
Semi-quantitative evaluation of chest CT scans by a radiologist, the current gold standard for detecting ILD, has led to the identification of older age, male sex, cigarette smoking, more severe RA, and high titres of RF and anti-CCP as risk factors for symptomatic RA-ILD [6, 7]. The gain-of-function mucin 5B (MUC5B) promoter variant rs35705950 was recently recognized as a genetic risk factor for the development of RA-ILD [8]. While shared epitope is the strongest genetic risk factor for the development of the articular disease in RA [9], the association between shared epitope and RA-ILD is not entirely clear.
A number of studies have shown significant inter- and intra- observer variability in the detection of ILD by radiologists [10]. The radiologists’ reading is, at best, semi-quantitative and there is also wide variation in interpretation of the pattern on CT scans between tertiary care centres with expertise in ILD compared with community radiologists [11]. This lack of reliability and quantification may have hampered the ability to identify predictors of RA-ILD and has therefore created the need for a more objective and accurate approach to the assessment of ILD.
Quantitative lung densitometry (qLD) is a methodology using a computerized algorithm to measure the density in Hounsfield units (HU) of each voxel of the lung parenchyma on a CT scan [12–15]. There are defined density thresholds in HU for normal lung and abnormal features, such as ILD or emphysema. These density thresholds are applied to determine the percent of lung that exhibits the feature of interest. The percentage of voxels with high attenuation [percentage high attenuation area (%HAA)] has been shown to be a quantitative CT biomarker of subclinical ILD; this technique has already been applied to quantify subclinical ILD in cardiac CT scans obtained as part of the Multi-Ethnic Study of Atherosclerosis (MESA) Lung study [14–17]. In addition, %HAA affecting at least 10% of the lung parenchyma was shown to correlate with restrictive lung physiology on pulmonary function testing and with interstitial lung abnormalities (ILA) on visual assessment by radiologists [18]. There are, to date, no studies that have examined the utility of qLD to detect RA-ILD against the visual CT interpretation by radiologists nor to assess predictors of RA-ILD.
In this retrospective analysis of prospectively collected data, %HAA was determined by qLD and was compared with expert radiologist scoring (ERS) among RA patients. We then sought to determine the association of demographic, genetic and RA-specific patient characteristics with high baseline %HAA, as well as %HAA progression in the follow-up period.
Methods
Study participants
Participants were enrolled in the Evaluation of Subclinical Cardiovascular disease and Predictors of Events in RA (ESCAPE-RA) study, a prospective cohort study investigating subclinical cardiovascular disease in RA. The included participants who met the 1987 RA classification criteria [19], were 45–84 years of age and did not have any prior cardiovascular events or procedures. The participants gave written informed consent. This cohort has been previously described in detail [20]. The study was approved by the Johns Hopkins institutional review board and further analyses were approved by the Institutional Review Board of Columbia University Medical Center.
At the baseline visit (October 2004 to May 2006), participants underwent cardiac CT scan. At visit 2 [occurring at 21 ± 3 months after baseline], participants underwent pulmonary function tests and completed a pulmonary symptom questionnaire. Finally at visit 3 [at 39 ± 4 months after baseline], participants underwent repeat cardiac CT scan.
Measurements
Cardiac CT
As described previously [20], cardiac multi-detector row CT scans were obtained using standard methods [21] with 3-mm thickness on a Toshiba Aquilion 64 scanner. Lung parenchyma from the level of the carina to the lung bases was included with truncation of the apical lung fields.
Quantitative lung densitometry
Densitometric evaluation of the lung fields of the cardiac CT scans was performed largely as described by Lederer et al. [14]. Each CT scan was evaluated for the extent (percentage) of voxels with HU that fell in the range between –600 and –250 HU. The percentage of parenchyma involved with HAA was calculated using the formula: (number of voxels between –600 and –250 HU/number of total voxels) × 100 [14].
Expert radiologist scoring of CT scans
The lung fields of the baseline visit 1 cardiac CT scans were scored by an expert radiologist using a previously described standardized method [22]. An ERS was determined by the presence and extent of lung involvement by ground glass opacities, reticulation, honeycombing and traction bronchiectasis using a semiquantitative scale (0 = none, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, 4 = 76–100%), with a maximum total score possible of 32. Twenty-five randomly selected CT scans were blindly read twice by the same radiologist to assess intra-observer variability. Twenty-five CT scans were read independently by a second radiologist to assess inter-observer variability.
Pulmonary function testing
Spirometry and diffusion capacity to carbon monoxide (DLCO) at visit 2 were performed according to the American Thoracic Society guidelines [23]. Obstructive pattern was defined as <70% for forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio, while restrictive pattern was defined as <80% of predicted value for FVC and ≥70% for FEV1/FVC ratio. Any impaired diffusion was defined as <80% of predicted value for DLCO.
Laboratory covariates
Fasting sera and plasma were separated by centrifugation, and were stored at –70°C. All assays (except RA autoantibodies) were performed at MESA-designated laboratories using MESA quality control procedures. CRP and IL-6 were measured as previously described [24]. RF was assessed by ELISA, with seropositivity defined at or above a level of 40 units. Anti-CCP antibody was assessed by ELISA, with seropositivity defined at or above a level of 60 units.
Genetics
Participants were assessed for the presence of the RA susceptibility alleles of HLA-DRB1 (i.e. shared epitope) as previously described [25]. The presence of the MUC5B rs35705950 promoter variant was also investigated in European American participants using a TaqMan SNP genotyping assay with primers and probes designed and synthesized by Applied Biosystems (Foster City, CA, USA). The major allele in all populations is guanine (G); the minor allele is thymine (T) [26]. The minor allele is the risk allele.
Other measures
Race was assessed by self-report. Smoking history was assessed using standardized questionnaires [27]. Participants were considered ever smokers if they smoked at least 100 cigarettes in their lifetime and current smokers if they smoked in the last 30 days.
Outcomes
The primary outcomes were: (i) high %HAA at baseline visit 1, which was defined as %HAA affecting at least 10% of the lung parenchyma; and (ii)% HAA increase in the follow-up study, which was defined as >0 for the percent change in %HAA [(%HAA at visit 3 – %HAA at baseline visit)/%HAA at baseline visit × 100].
Statistical analysis
The distribution of all variables was examined and transformation to normality was performed as required. Correlation between %HAA and ERS was calculated using Spearman correlation coefficients. Logistic regression was used to explore the associations of demographic, genetic and RA-specific characteristics with high baseline %HAA as well as %HAA increase in the follow-up period. Linear regression was used to explore the association of demographic, genetic and RA-specific characteristics with the log-transformed percent change in %HAA. The associated variables at P < 0.25 level were then carried into multivariable models. The assumptions of linear regression were checked as follows for final multivariable models: scatter plots were examined to ensure a linear relationship between independent and dependent variables and assess for heteroscedasticity. The Shapiro–Wilk test was used to ensure distributional normality of the residuals. Sensitivity analyses explored the effects of leveraging outliers after excluding observations with studentized residuals more extreme than –2 and +2. The final multivariable model met all of the above quality aspects and its results remained unchanged. All statistical calculations were performed using Stata 15 (StataCorp, College Station, TX, USA). A two-tailed α of 0.05 was used throughout.
Results
Characteristics of the study patients included in the cross-sectional analysis
Baseline characteristics of the 193 patients who underwent qLD at visit 1 are summarized in Table 1. The mean age was 59 years and median disease duration was 8 years. The majority of patients were female and White race, with a mean age of 59 years and a median RA duration of 8 years. Ever smoking (59%) and seropositivity for RF and/or anti-CCP (79%) were frequent. The majority of patients (94%) were treated with DMARDs, with 84% being treated with non-biologic DMARDs (the majority of whom were receiving MTX) and 46% being treated with biologic agents (the majority of whom were receiving TNF inhibitors).
Table 1.
Baseline characteristics of the 193 patients who underwent lung densitometry at visit 1
| Baseline % HAA |
||||
|---|---|---|---|---|
| Characteristic | Overall cohort (N = 193) | Below median, ≤4.86% (N = 97) | Above median, >4.86% (N = 96) | P-value |
| Demographics | ||||
| Age, years | 59 (8.7) | 59.7 (8.8) | 58.7 (8.6) | 0.4 |
| Male sex, n (%) | 76 (39) | 36 (37) | 40 (42) | 0.52 |
| Caucasian race, n (%) | 166 (86) | 80 (83) | 86 (90) | 0.16 |
| RA characteristic | ||||
| Smoking history | ||||
| Ever smoking, n (%) | 114 (59) | 50 (52) | 64 (67) | 0.033 |
| Current smoking, n (%) | 21 (11) | 7 (7) | 14 (15) | 0.1 |
| Pack-years smoking | 7.5 (0–30) | 1.4 (0–20.5) | 11.8 (0–38) | 0.02 |
| BMI, kg/m2 | 28.4 (15) | 26 (4) | 31 (5.4) | 0.0001 |
| RA duration, years | 8 (4–17) | 9 (4–17) | 8 (4–15.5) | 0.62 |
| RA serologies | ||||
| RF or anti-CCP positivity, n (%) | 151 (79) | 73 (76) | 78 (81) | 0.38 |
| RF and anti-CCP positivity, n (%) | 119 (62) | 60 (63) | 59 (62) | 0.88 |
| RF units | 105 (23–265) | 103 (21–252) | 120 (24–265) | 0.77 |
| CCP units | 116 (27–163) | 119 (27–156) | 108 (27–171) | 0.76 |
| Any shared epitope, n (%) | 132 (69) | 66 (69) | 66 (69) | 0.91 |
| MUC5B minor allele, n (%) | 35 (23) (N = 153) | 15 (19) (N = 80) | 20 (27) (N = 73) | 0.20 |
| DAS28-CRP | 3.7 (1.1) | 3.6 (1.1) | 3.7 (1.1) | 0.56 |
| Presence of RA nodules, n (%) | 27 (17) | 14 (17) | 13 (18) | 0.904 |
| CRP, mg/l | 2.5 (1.1–7.1) | 2.1 (1–6.7) | 3.2 (1.1–8.4) | 0.25 |
| IL-6, pg/ml | 3.7 (1.8–8.1) | 3.6 (1.7–6.2) | 4.3 (1.8–9.3) | 0.55 |
| Total SHS | 7.5 (0–41) | 8 (1–49) | 7 (0–32) | 0.37 |
| HAQ | 0.6 (0.1–1.3) | 0.6 (0–1.3) | 0.9 (0.3–1.4) | 0.057 |
| RA medication | ||||
| No DMARDs, n (%) | 11 (5.7) | 6 (6.2) | 5 (5.2) | 0.72 |
| Current non-biologic DMARDs, n (%) | 162 (84) | 82 (85) | 80 (83) | 0.69 |
| MTX, n (%) | 122 (63) | 64 (66) | 58 (60.4) | 0.42 |
| Cumulative dose of MTX, mg | 3480 (1500–6600) | 4143 (1600–6314) | 3300 (1500–7820) | 0.78 |
| Othera, n (%) | 86 (45) | 42 (43) | 44 (46) | 0.72 |
| Current biologic DMARDs, n (%) | 89 (46) | 39 (41) | 50 (52) | 0.11 |
| TNF inhibitors, n (%) | 85 (44) | 38 (39.6) | 47 (48.9) | 0.19 |
| Otherb, n (%) | 4 (2) | 1 (1) | 3 (3) | 0.30 |
| Cumulative prednisone for the last 10 years, g | 3.1 (0.1–9.5) | 2.8 (0–6.1) | 4.0 (0.7–11) | 0.04 |
| Respiratory symptoms at visit 2, n (%) | ||||
| SOB on exertion | 39 (21) | 14 (15) | 25 (27) | 0.039 |
| SOB on level ground | 22 (12) | 9 (9.6) | 13 (14) | 0.34 |
| Cough | 40 (22) | 22 (24) | 18 (20) | 0.52 |
| Phlegm | 26 (14) | 16 (17) | 10 (11) | 0.23 |
| Pulmonary function tests at visit 2 (N = 173), n (%) | ||||
| Obstructive pattern | 18 (10) | 14 (16) | 4 (5) | 0.016 |
| Restrictive pattern | 13 (8) | 3 (3.4) | 10 (12) | 0.037 |
| Impaired diffusion | 32 (19) | 16 (18) | 16 (19) | 0.91 |
| FVC % predicted | 102 (19) | 106 (20.4) | 97 (16.2) | 0.0069 |
| DLCO % predicted | 99 (85–111) | 99.5 (85–109) | 99 (84–113) | 0.88 |
| CT expert reading (Ν = 176) | ||||
| ILD score | 0 (0–2) | 0 (0–0) | 0 (0–3) | 0.019 |
| CT densitometry | ||||
| HAA %, range | 2.3–18.8 | 2.3–4.86 | 4.87–18.8 | – |
Results are presented as mean (s.d.), median (interquartile range) and frequency (percentage).
Other non-biologic DMARDs included SSZ, HCQ or LEF.
Other non-TNF biologics included abatacept, rituximab and tocilizumab. HAA: high attenuation areas; MUC5B: mucin 5B; SHS: Sharp–van der Heijde Score; SOB: shortness of breath; PFT: pulmonary function test; FVC: forced vital capacity; DLCO: diffusing lung capacity; ILD: interstitial lung disease.
Baseline %HAA for the cohort
The median baseline %HAA was 4.86% (score range was 2.3–18.8). Compared with subjects whose baseline %HAA fell below the median, subjects whose %HAA fell above the median had a greater number of pack-years of smoking, a greater proportion of ever smokers, higher BMI, more frequent shortness of breath on exertion, a restrictive pattern on pulmonary function tests, lower FVC % predicted and a higher ILD score based on expert evaluation (Table 1). There was weak positive correlation between baseline %HAA and ERS (Spearman’s rho = 0.261, P < 0.001). High baseline %HAA (affecting ≥10% of the lung parenchyma) was observed in 22 patients (11%).
Expert pulmonary radiologist interpretation
Of the 176 subjects whose baseline (visit 1) CT scans were evaluated by expert readers, the majority (68%) had no features of ILD (cohort median ERS = 0; Table 1). Among the 57 participants (32% of cohort) with evidence of ILD on expert read, the median ERS was 3 (score range was 1–10): 31 (54%) had scores of 1–3 (32 point maximum score), 23 (40%) had scores of 4–6 and 3 (5%) had scores of 7–10, indicating predominantly mild disease overall. Among studies with evidence of ILD, the expert readers identified specific patterns such as non-specific interstitial pneumonia, organizing pneumonia, bronchiolitis and ILD not otherwise specified. They did not identify any usual interstitial pneumonia (UIP) cases in this cohort. There were also mixed patterns, mostly reticulation and ground glass opacities. Ιntra-observer reliability was found to be 65%, while inter-observer reliability between expert readers was even lower (59%).
Cross-sectional associations of demographic and RA-specific characteristics with high baseline %HAA (defined as affecting ≥10% of the lung parenchyma)
Univariate and multivariable associations are summarized in Table 2. In univariate models, higher BMI, RF titre, CCP titre, DAS28-CRP score, log CRP and greater number of swollen joints were each significantly associated with increased likelihood of having a high baseline %HAA. In the extended and reduced multivariable models, female sex, greater number of pack-years of smoking, higher BMI and CCP titre at the highest decile were each significantly associated with high baseline %HAA. The area under the receiver operator characteristic curve for the extended and reduced multivariable models to predict high baseline %HAA was 0.87 and 0.88, respectively.
Table 2.
Cross-sectional associations of demographic and RA-specific characteristics with high baseline %HAA
| Univariate model |
Multivariable model (extended) |
Multivariable model (reduced) |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age per year | 1.003 (0.95, 1.05) | 0.91 | – | – | – | – |
| Female vs male | 2.44 (0.86, 6.92) | 0.094 | 4.25 (0.74, 24.57) | 0.106 | 5.34 (1.33, 21.43) | 0.018 |
| Caucasian vs other race | 0.74 (0.23, 2.38) | 0.62 | – | – | – | – |
| Ever smoker vs never smoker | 1.68 (0.66, 4.29) | 0.28 | – | – | – | – |
| Current smoking, yes vs no | 1.27 (0.34, 4.69) | 0.72 | – | – | – | – |
| Pack-years of smoking, per unit | 1.006 (0.99, 1.01) | 0.18 | 1.01 (0.99, 1.03) | 0.063 | 1.014 (1.003, 1.02) | 0.010 |
| BMI, per kg/m2 | 1.28 (1.16, 1.41) | <0.001 | 1.26 (1.09, 1.46) | 0.001 | 1.32 (1.17, 1.47) | <0.001 |
| Log RA duration, per year | 1.21 (0.74, 1.98) | 0.46 | – | – | – | – |
| RF or anti-CCP, yes vs no | 6.82 (0.89, 52.21) | 0.064 | – | – | – | – |
| Double RF and anti-CCP, yes vs no | 2.42 (0.86, 6.84) | 0.094 | – | – | – | – |
| RF, per unit | 1.001 (1.0001, 1.001) | 0.014 | – | – | – | – |
| RF titre above median value,a yes vs no | 4.24 (1.50, 11.96) | 0.006 | 3.01 (0.63, 14.41) | 0.168 | – | – |
| Anti-CCP, per unit | 1.006 (1.0003, 1.01) | 0.040 | – | – | – | – |
| Anti-CCP positive, yes vs no | 2.13 (0.60, 7.55) | 0.24 | – | – | – | – |
| Anti-CCP titre at the highest decile,b yes vs no | 4.18 (1.49, 11.68) | 0.006 | 8.49 (0.91, 79.19) | 0.060 | 11.34 (2.50, 51.22) | 0.002 |
| Any shared epitope vs none | 0.66 (0.27, 1.62) | 0.36 | – | – | – | – |
| 1 shared epitope allele vs none | 0.98 (0.39, 2.46) | 0.96 | 0.71 (0.17, 3.01) | 0.645 | – | – |
| 2 shared epitope alleles vs none | 0.13 (0.02, 1.04) | 0.054 | 0.11 (0.01, 1.54) | 0.101 | – | – |
| MUC5B minor allele vs none | 2.02 (0.63, 6.47) | 0.23 | – | – | – | – |
| 1 minor allele MUC5B vs none | 1.73 (0.49, 6.03) | 0.39 | 1.57 (0.32, 7.82) | 0.58 | – | – |
| 2 minor alleles MUC5B vs none | 6.06 (0.5, 73.4) | 0.157 | 1.19 (0.05, 28.98) | 0.914 | – | – |
| DAS28-CRP, per unit | 1.59 (1.06, 2.39) | 0.024 | – | – | – | – |
| DAS28-CRP >3.2, yes vs no | 4.33 (1.24, 15.10) | 0.022 | – | – | – | – |
| Log CDAI, per unit | 2.03 (0.79, 5.18) | 0.140 | 1.61 (0.28, 9.21) | 0.595 | – | – |
| CDAI >10 | 2.47 (0.31, 19.54) | 0.390 | – | – | – | – |
| Tender joints (44 joint count), per joint | 1.02 (0.98, 1.07) | 0.33 | – | – | – | – |
| Swollen joints (44 joint count), per joint | 1.09 (1.01, 1.19) | 0.028 | – | – | – | – |
| Log CRP, per mg/l | 1.61 (1.15, 2.27) | 0.006 | 0.99 (0.59, 1.67) | 0.967 | – | – |
| Log IL-6 level, per pg/ml | 1.44 (1.05, 1.96) | 0.023 | – | – | – | – |
| SHS, per unit | 0.99 (0.98, 1.01) | 0.58 | – | – | – | – |
| Use of non-biologic DMARDs, yes vs no | 1.27 (0.35, 4.57) | 0.72 | – | – | – | – |
| MTX, yes vs no | 0.89 (0.37, 2.18) | 0.80 | – | – | – | – |
| Cumulative dose of MTX (square root), per mg | 1.004 (0.99, 1.02) | 0.61 | – | – | – | – |
| Biologics, yes vs no | 1.30 (0.54, 3.12) | 0.55 | – | – | – | – |
| TNF inhibitors, yes vs no | 1.18 (0.49, 2.82) | 0.72 | – | – | – | – |
| Cumulative prednisone for the last 10 years | 0.98 (0.94, 1.04) | 0.59 | – | – | – | – |
| Baseline ILD score by expert read | 1.38 (1.13, 1.69) | 0.002 | ||||
| AUC | – | – | 0.87 (0.78, 0.97) | – | 0.88 (0.81, 0.95) | – |
OR represents the ratio of the likelihood of having high %HAA (affecting ≥10% of the lung parenchyma) vs not, per unit of the independent continuous variable of interest or for those with the independent dichotomous variable of interest vs those without.
Value is ≥100 units.
Value is ≥200 units. HAA: high attenuation areas; OR: odds ratio; MUC5B: mucin 5B; CDAI: Clinical Disease Activity Index; Log: logarithmic; SHS: Sharp–van der Heijde Score; AUC: area under the receiver operating characteristic curve; ILD: interstitial lung disease.
Characteristics of the study patients included in the longitudinal analysis
Of the 193 patients who underwent qLD at baseline, 106 had repeat qLD at visit 3 [39 ± 4 months post-baseline]. Those with repeat qLD had lower BMI, RF and anti-CCP titres and less frequent rheumatoid nodules compared with those without repeat qLD (supplementary Table S1, available at Rheumatology online). Otherwise, there were no other demographic, RA-specific or treatment differences between the two groups.
Change in %HAA between baseline (visit 1) and visit 3
The median absolute change in %HAA was –0.01% (score range was –6.1% to +13.3%). The median percent change in %HAA was –0.23% (score range was –69.3% to +297.3%). There was excellent correlation between absolute change and percent change in %HAA (Spearman’s rho = 0.99, P < 0.001). The percent change in %HAA increased in 52 (49%) participants (score range was +0.33 to +297.3%) and decreased in 54 (51%) participants (score range was –0.19% to –69%).
Associations of demographic and RA-specific characteristics with increased %HAA
Univariate and multivariable associations are presented in Table 3. In univariate models, shared epitope and log-transformed baseline %HAA were each inversely associated with %HAA increase. In addition, having the MUC5B minor allele trended toward having a positive association with %HAA increase, while the square root of baseline Sharp score trended toward having a negative association with the outcome. In the final reduced multivariable model, the inverse relationship between shared epitope and log-transformed baseline %HAA with %HAA increase remained significant (Fig. 1A). The MUC5B minor allele was more strongly associated with %HAA increase among patients with ≥3 pack-years of smoking compared with those with <3 pack-years of smoking (P = 0.07 for smoking interaction; Fig. 1B). In addition, the MUC5B minor allele was more strongly associated with %HAA increase among men compared with women (P = 0.09 for gender interaction; Fig. 1C).
Table 3.
Associations of demographic and RA-specific characteristics with %HAA increase
| Univariate model |
Multivariable model (extended) |
Multivariable model (reduced) |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age per year (V1) | 1.00 (0.95, 1.05) | 0.95 | – | – | – | – |
| Female vs male | 0.74 (0.33, 1.64) | 0.45 | – | – | – | – |
| Caucasians vs other race | 1.46 (0.51, 4.18) | 0.48 | – | – | – | – |
| Ever smoker vs never smoker (V1/V3) | 0.87 (0.40, 1.86) | 0.712 | – | – | – | – |
| Pack-years of smoking (V1) | 1.004 (0.99, 1.02) | 0.423 | – | – | – | – |
| BMI (V1), per kg/m2 | 0.98 (0.90, 1.08) | 0.79 | – | – | – | – |
| Log RA duration (V1), per year | 0.89 (0.59, 1.37) | 0.62 | – | – | – | – |
| RF or anti-CCP, yes vs no | 1.23 (0.50, 2.99) | 0.66 | – | – | – | – |
| Double RF and anti-CCP, yes vs no | 1.07 (0.49, 2.34) | 0.45 | – | – | – | – |
| RF, per unit | 1.001 (0.99, 1.007) | 0.58 | – | – | – | – |
| CCP, per unit | 1.001 (0.99, 1.006) | 0.63 | – | – | – | – |
| CCP positive, yes vs no | 0.78 (0.33, 1.86) | 0.58 | – | – | – | – |
| Any shared epitope vs none | 0.43 (0.19, 0.98) | 0.045 | 0.42 (0.17, 1.09) | 0.075 | 0.34 (0.14, 0.84) | 0.019 |
| MUC5B minor allele, yes vs no | 1.85 (0.71, 4.76) | 0.19 | 2.05 (0.75, 5.62) | 0.164 | 2.17 (0.80, 5.88) | 0.128 |
| DAS28-CRP (V1), per unit | 1.05 (0.72, 1.52) | 0.82 | – | – | – | – |
| Average DAS28-CRP (V1–V3), per unit | 0.85 (0.56, 1.30) | 0.45 | – | – | – | – |
| Log CDAI (V1), per unit | 0.87 (0.39, 1.93) | 0.74 | – | – | – | – |
| Log Average CDAI (V1–V3), per unit | 0.88 (0.31, 2.54) | 0.82 | – | – | – | – |
| Log CRP, per mg/l (V1) | 1.18 (0.88, 1.57) | 0.25 | – | – | – | – |
| Log average CRP (V1–V3) | 1.08 (0.79, 1.47) | 0.61 | – | – | – | – |
| Log IL-6 level (V1), per pg/ml | 1.10 (0.81, 1.49) | 0.54 | – | – | – | – |
| SHS (square root, V1), per unit | 0.91 (0.82, 1.01) | 0.055 | 0.93 (0.84, 1.04) | 0.25 | – | – |
| Use of non-biologic DMARDs (V1), yes vs no | 1.78 (0.59, 5.32) | 0.30 | – | – | – | – |
| MTX (V1), yes vs no | 1.52 (0.69, 3.37) | 0.29 | – | – | – | – |
| Cumulative dose of MTX (square root, V1), per mg | 1.01 (0.99, 1.02) | 0.51 | – | – | – | – |
| Cumulative dose of MTX (square root, V3), per mg | 1.003 (0.99, 1.01) | 0.34 | – | – | – | – |
| Biologics use (V1), yes vs no | 1.24 (0.57, 2.13) | 0.579 | – | – | – | – |
| TNF inhibitors (V1), yes vs no | 1.09 (0.5, 2.3) | 0.8 | – | – | – | – |
| Cumulative prednisone for the last 10 years (V1) | 1.003 (0.96, 1.04) | 0.57 | – | – | – | – |
| Log baseline %HAA | 0.35 (0.13, 0.88) | 0.027 | 0.34 (0.12, 0.94) | 0.037 | 0.35 (0.13, 0.97) | 0.043 |
| Baseline ILD score by expert read | 0.91 (0.70, 1.18) | 0.50 | – | – | – | – |
| Presence of GGO by expert read | 1.36 (0.38, 4.83) | 0.63 | – | – | – | – |
| Presence of reticulation, honeycombing, traction bronchiectasis by expert read | 0.96 (0.33, 2.77) | 0.947 | – | – | – | – |
| AUC | – | – | 0.69 (0.59, 0.79) | – | 0.69 (0.58, 0.79) | – |
OR represents the ratio of the likelihood of %HAA increase (defined as percent change in %HAA that had a positive value) vs not, per 1-unit increase of the independent continuous variable of interest or for those with the independent dichotomous variable of interest vs those without. HAA: high attenuation areas; OR: odds ratio; V1: baseline visit 1; V3: visit 3; MUC5B: mucin 5B; CDAI: Clinical Disease Activity Index; Log: logarithmic; SHS: Sharp–van der Heijde Score; ILD: interstitial lung disease; GGO: ground glass opacities; AUC: area under the receiver operating characteristic curve.
Fig. 1.
Adjusted probability of %HAA increase according to having a shared epitope or MUC5B allele
(A) Adjusted probability of %HAA increase, according to having a shared epitope allele. Model was adjusted for MUC5B minor allele status and log-transformed baseline %HAA, which were the only significant covariates retained in the multivariable modeling. Results are the mean (95% CI) in 106 RA patients. (B) Adjusted probability of %HAA increase, according to having a MUC5B minor allele and stratified by pack-years of smoking. P = 0.07 for smoking interaction. Model was adjusted for shared epitope status and log-transformed baseline %HAA, which were the only significant covariates retained in the multivariable modeling. Results are the mean (95% CI) in 106 RA patients. (C) Adjusted probability of %HAA increase, according to having a MUC5B minor allele and stratified by gender. P = 0.09 for gender interaction. Model was adjusted for shared epitope status and log-transformed baseline %HAA, which were the only significant covariates retained in the multivariable modeling. Results are the mean (95% CI) in 106 RA patients. HAA: high attenuation areas; MUC5B: mucin 5B.
Associations of demographic and RA-specific characteristics with the log-transformed percent change in %HAA among the subgroup of n = 52 patients whose %HAA increased
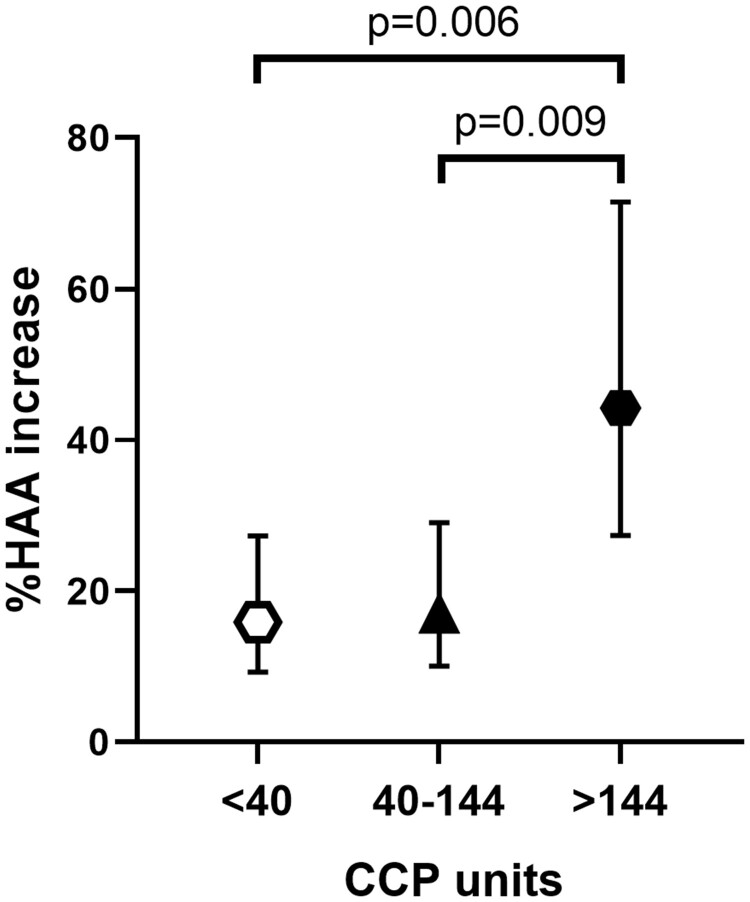
Univariate and multivariable associations are presented in Table 4. In univariate models, higher CCP units were significantly associated with the outcome. There were also trends toward significance of BMI, RF titre, shared epitope, average Sharp score, cumulative MTX dose and use of TNF inhibitors. However, after carrying these variables into the extended and reduced models, only higher CCP units remained significantly associated with higher log percent change in %HAA among %HAA progressors (P = 0.002; Fig. 2).
Table 4.
Associations of demographic and RA-specific characteristics with log-transformed percent change in %HAA among patients whose %HAA increased
| Univariate model |
Multivariable model (extended) |
Multivariable model (reduced) |
||||
|---|---|---|---|---|---|---|
| β | P-value | β | P-value | β | P-value | |
| Age per year (V1) | 0.006 | 0.772 | – | – | – | – |
| Female vs male | 0.25 | 0.504 | – | – | – | – |
| Caucasians vs other race | –0.18 | 0.73 | – | – | – | – |
| Ever smoker vs never smoker (V1/V3) | –0.25 | 0.49 | – | – | – | – |
| Pack-years of smoking (V1) | 0.0007 | 0.826 | – | – | – | – |
| BMI (V1), per kg/m2 | 0.055 | 0.24 | 0.069 | 0.149 | 0.048 | 0.22 |
| Log RA duration (V1), per year | 0.16 | 0.42 | – | – | – | – |
| RF or anti-CCP, yes vs no | 0.79 | 0.051 | – | – | – | – |
| Double RF and anti-CCP, yes vs no | 0.74 | 0.040 | – | – | – | – |
| RF, per unit | 0.00029 | 0.19 | 0.000052 | 0.83 | – | – |
| CCP, per unit | 0.0069 | 0.004 | 0.007 | 0.015 | 0.007 | 0.002 |
| CCP positive, yes vs no | 0.79 | 0.049 | – | – | – | – |
| Any shared epitope vs none | 0.69 | 0.061 | 0.35 | 0.405 | – | – |
| MUC5B minor allele, yes vs no | –0.16 | 0.69 | – | – | – | – |
| DAS28-CRP (V1), per unit | 0.081 | 0.63 | – | – | – | – |
| Average DAS28-CRP (V1–V3), per unit | –0.082 | 0.66 | – | – | – | – |
| Log CDAI (V1), per unit | 0.32 | 0.83 | – | – | – | – |
| Log average CDAI (V1–V3), per unit | –0.064 | 0.89 | – | – | – | – |
| Log CRP, per mg/l (V1) | –0.009 | 0.95 | – | – | – | – |
| Log Average CRP (V1–V3) | –0.046 | 0.75 | – | – | – | – |
| IL-6 level (V1), per pg/ml | –0.0028 | 0.74 | – | – | – | – |
| SHS (V1), per unit | 0.0047 | 0.27 | – | – | – | – |
| Average SHS (V1–V3), per unit | 0.0056 | 0.176 | –0.00031 | 0.95 | – | – |
| Use of non-biologic DMARDs (V1), yes vs no | –0.17 | 0.76 | – | – | – | – |
| MTX (V1), yes vs no | 0.16 | 0.67 | – | – | – | – |
| Cumulative dose of MTX (square root, V1), per mg | 0.0077 | 0.24 | – | – | – | – |
| Cumulative dose of MTX (square root, V3), per mg | 0.0057 | 0.20 | 0.002 | 0.625 | – | – |
| Biologics use (V1), yes vs no | 0.61 | 0.091 | – | – | – | – |
| TNF inhibitors (V1), yes vs no | 0.53 | 0.148 | 0.61 | 0.122 | 0.51 | 0.098 |
| Cumulative prednisone for the last 10 years (V1) | 0.021 | 0.20 | –0.0048 | 0.822 | – | – |
| Log baseline %HAA | 0.12 | 0.29 | – | – | – | – |
| Baseline ILD score by expert read | 0.11 | 0.447 | – | – | – | – |
| Presence of GGO by expert read | 0.89 | 0.13 | 0.16 | 0.831 | – | – |
| Presence of reticulation, honeycombing, traction bronchiectasis by expert read | 0.160 | 0.77 | – | – | – | – |
| Adjusted R2 | – | – | 0.176 | 0.035 | 0.183 | 0.006 |
Beta coefficients represent the average change in the log-transformed percent change in %HAA among %HAA progressors, per 1-unit increase of the independent continuous variable of interest or for those with the independent dichotomous variable of interest vs those without. HAA: high attenuation areas; OR: odds ratio; V1: baseline visit 1; V3: visit 3; MUC5B: mucin 5B; CDAI: Clinical Disease Activity Index; Log: logarithmic; SHS: Sharp–van der Heijde Score; ILD: interstitial lung disease; GGO: ground glass opacities.
Fig. 2.
Adjusted %HAA increase, according to anti-CCP titre (split into tertiles)
Model was adjusted for BMI and TNF inhibitor treatment at visit 1, which were the only significant covariates retained in the multivariable model. Results are the mean (95% CI) in 52 RA patients. HAA: high attenuation areas.
Discussion
This is the first study in RA patients to evaluate qLD and compare it with visual CT interpretation by expert radiologists as well as to assess predictors of progression. %HAA is a potential surrogate densitometric marker of subclinical ILD and mostly represents ground-glass opacities and interstitial thickening/reticulation [14, 28]. %HAA correlates with serum markers of both inflammation and extracellular matrix remodelling (serum IL-6 and MMP-7, correspondingly) [16]. In addition, %HAA correlates with respiratory clinical outcomes, such as lower FVC, lower 6-min walk distance, visually identifiable ILD, increased rate of ILD hospitalization and ILD-specific death, and higher all cause-mortality rate over a 12.2-year period [16, 18, 29]. Furthermore, %HAA correlates with cumulative cigarette smoking exposure among RA patients as well as subjects in the general population [30].
We found that among RA patients with ILA based on expert read, smoking, anti-CCP, BMI and female sex were each associated with high baseline %HAA. Even though %HAA is a potential surrogate densitometric marker for ILA, the higher baseline %HAA in obese patients and women may not reflect ILD, but image noise from chest wall soft tissue or atelectasis in these patient groups [31]. In the longitudinal analysis, the anti-CCP titre was associated with the percent increase in %HAA. Our findings on the effect of MUC5B minor allele on %HAA progression suggest that smoking and male sex are associated with increased risk for ILD among RA patients who carry the MUC5B minor allele. It is possible that smoking and hormonal factors cause epigenetic changes and alter the expression of MUC5B gene and therefore the risk for ILD. Interestingly, having a shared epitope allele was associated with a protective effect against %HAA increase in this study. This finding is in agreement with a previous Japanese cohort study in which HLA-DRB1*04 shared epitope was inversely associated with RA-ILD [32].
Lung densitometry has several advantages over CT evaluation by radiologists. It is quantitative, operator independent and easily applied to standard clinical CT scans. It could therefore be a more sensitive marker of ILA. To optimize consistency of expert reads in this study, all studies were read by a senior radiologist with nearly 40 years of experience and followed a systematic algorithm. Even with this methodology, the intra-observer and inter-observer correlation were suboptimal. By comparison, the intraclass correlation coefficient for %HAA is reported at 0.93 [14]. In addition, CT scans were not obtained due to suspected lung disease in this study. This approach reduces the risk of selection bias toward sampling the more severe cases. In contrast, previous observational studies have focused on clinically apparent ILD and their identified predictors may be a reflection of later-stage disease or could represent effects rather than causes of ILD [6–8]. Furthermore, ILA may be a precursor to symptomatic disease and could be more amenable to early treatment [29, 33]. Similar to previously published data, we found that the correlation between %HAA and expert reads was not strong [18], suggesting either that the human eye (expert radiologist) cannot recognize subtle high attenuation areas or that the features recognized by experts are more contextual than simply areas of high attenuation.
There are a number of potential limitations of this study resulting from embedding this analysis in a cohort study of subclinical cardiovascular disease in RA. The analysis of lung parenchyma was performed on cardiac CT scan images with 3-mm slice thickness, which provides inferior morphological detail compared with the 1–1.25 mm slice thickness used in dedicated high-resolution chest CTs. This could likely cause at least some loss of very early and subtle ILD changes. In addition, lung apices were not included in the cardiac CT scans. However, a good correlation has been shown between dedicated chest CT scans and cardiac CTs when evaluating %HAA (Spearman correlation coefficient = 0.87) [14]. Our RA cohort consisted of patients who had predominantly mild and subclinical ILD. Therefore, the risk factors identified in this study may not be predictors of more severe disease. In addition, pulmonary function tests were obtained at a single time point, which did not allow us to study longitudinal changes in pulmonary physiology. Also, a smaller group of patients of the original cohort underwent repeat densitometry in the follow-up period. The difference in BMI, RF, anti-CCP titre and presence of RA nodules between the subjects who had repeat densitometry and those who did not, may have led to underestimation of ILD changes over time. Finally, even though %HAA measurement can generally be affected by the presence of emphysema [18], this cohort had a median score of emphysema of 0 (interquartile range 0–0) on expert read.
Our study has several implications. Risk factors such as smoking, anti-CCP, absence of shared epitope and having the MUC5B minor allele could identify asymptomatic RA patients at risk for ILD who are appropriate for additional ILD screening. Our study provides further insight into the different genetic risk profiles of articular disease vs ILD in RA. Further, our study revealed that the MUC5B promoter variant, which was recently recognized as a strong risk factor for clinically evident RA-ILD [8], may play a role in progression of subclinical RA-ILD as well. Finally, qLD could represent an advance in the field of RA-ILD and a tool for therapeutic clinical trial outcomes. This will require validation in future prospective studies.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Stanley Siegelman (Department of Radiology, Johns Hopkins University School of Medicine) and Dr Jason Oaks for their expert reading of the lung fields of the CT scans. M.K.A., S.K.D., D.A.P., J.M.B. and J.T.G. were involved in the study conception, hypothesis, design of study, data analysis and writing of the manuscript. D.J.L., C.J., E.A.H. and E.J.B. were involved in data analysis and revision of the manuscript. All authors have read and approved the final manuscript.
Funding: ESCAPE was funded by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS) (NIH Grant Number AR‐050026‐01) and the quantitative densitometry analyses were funded by the Rheumatology Research Foundation.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The data from which this manuscript is derived are not publicly available, but may be available for limited and pre-specified use by contacting the senior author.
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
Michail K Alevizos, Division of Rheumatology, Columbia University Irving Medical Center, New York, NY.
Sonye K Danoff, Division of Pulmonary and Critical Care, Johns Hopkins University, Baltimore, MD.
Dimitrios A Pappas, Division of Rheumatology, Columbia University Irving Medical Center, New York, NY.
David J Lederer, Division of Pulmonary and Critical Care, Columbia University Irving Medical Center, New York, NY.
Cheilonda Johnson, Division of Pulmonary, Allergy, and Critical Care, University of Pennsylvania, Philadelphia, PA.
Eric A Hoffman, Department of Radiology, University of Iowa, Iowa City, IA, USA.
Elana J Bernstein, Division of Rheumatology, Columbia University Irving Medical Center, New York, NY.
Joan M Bathon, Division of Rheumatology, Columbia University Irving Medical Center, New York, NY.
Jon T Giles, Division of Rheumatology, Columbia University Irving Medical Center, New York, NY.
References
- 1. Gonzalez A, Maradit Kremers H, Crowson CS. et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum 2007;56:3583–7. [DOI] [PubMed] [Google Scholar]
- 2. Hyldgaard C, Hilberg O, Pedersen AB. et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 2017;76:1700–6. [DOI] [PubMed] [Google Scholar]
- 3. Olson AL, Swigris JJ, Sprunger DB. et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011;183:372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR.. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax 2001;56:622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fujii M, Adachi S, Shimizu T. et al. Interstitial lung disease in rheumatoid arthritis: assessment with high-resolution computed tomography. J Thorac Imaging 1993;8:54–62. [PubMed] [Google Scholar]
- 6. Kelly CA, Saravanan V, Nisar M. et al. ; British Rheumatoid Interstitial Lung (BRILL) Network. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatology (Oxford) 2014;53:1676–82. [DOI] [PubMed] [Google Scholar]
- 7. Sparks JA, He X, Huang J. et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis-associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol 2019;71:1472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Juge PA, Lee JS, Ebstein E. et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med 2018;379:2209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gregersen PK, Silver J, Winchester RJ.. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 1987;30:1205–13. [DOI] [PubMed] [Google Scholar]
- 10. Collins CD, Wells AU, Hansell DM. et al. Observer variation in pattern type and extent of disease in fibrosing alveolitis on thin section computed tomography and chest radiography. Clin Radiol 1994;49:236–40. [DOI] [PubMed] [Google Scholar]
- 11. Flaherty KR, Andrei AC, King TE Jr. et al. Idiopathic interstitial pneumonia: do community and academic physicians agree on diagnosis? Am J Respir Crit Care Med 2007;175:1054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu Y, van Beek EJ, Hwanjo Y. et al. Computer-aided classification of interstitial lung diseases via MDCT: 3D adaptive multiple feature method (3D AMFM). Acad Radiol 2006;13:969–78. [DOI] [PubMed] [Google Scholar]
- 13. Camiciottoli G, Orlandi I, Bartolucci M. et al. Lung CT densitometry in systemic sclerosis: correlation with lung function, exercise testing, and quality of life. Chest 2007;131:672–81. [DOI] [PubMed] [Google Scholar]
- 14. Lederer DJ, Enright PL, Kawut SM. et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med 2009;180:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoffman EA, Jiang R, Baumhauer H. et al. Reproducibility and validity of lung density measures from cardiac CT Scans–The Multi-Ethnic Study of Atherosclerosis (MESA) lung study. Acad Radiol 2009;16:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Podolanczuk AJ, Oelsner EC, Barr RG. et al. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J 2016;48:1442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bernstein EJ, Barr RG, Austin JHM. et al. Rheumatoid arthritis-associated autoantibodies and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis. Thorax 2016;71:1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kliment CR, Araki T, Doyle TJ. et al. A comparison of visual and quantitative methods to identify interstitial lung abnormalities. BMC Pulm Med 2015;15:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 20. Giles JT, Szklo M, Post W. et al. Coronary arterial calcification in rheumatoid arthritis: comparison with the Multi-Ethnic Study of Atherosclerosis. Arthritis Res Ther 2009;11:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carr JJ, Nelson JC, Wong ND. et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 22. Goldin JG, Lynch DA, Strollo DC. et al. ; Scleroderma Lung Study Research Group. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 2008;134:358–67. [DOI] [PubMed] [Google Scholar]
- 23. Miller MR, Crapo R, Hankinson J. et al. ; ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J 2005;26:153–61. [DOI] [PubMed] [Google Scholar]
- 24. Nettleton JA, Steffen LM, Mayer-Davis EJ. et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2006;83:1369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobayashi H, Giles JT, Polak JF. et al. Increased prevalence of carotid artery atherosclerosis in rheumatoid arthritis is artery-specific. J Rheumatol 2010;37:730–9. [DOI] [PubMed] [Google Scholar]
- 26. Sherry ST, Ward MH, Kholodov M. et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 2001;29:308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bild DE, Bluemke DA, Burke GL. et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 28. Do KH, Lee JS, Colby TV, Kitaichi M, Kim DS.. Nonspecific interstitial pneumonia versus usual interstitial pneumonia: differences in the density histogram of high-resolution CT. J Comput Assist Tomogr 2005;29:544–8. [DOI] [PubMed] [Google Scholar]
- 29. Podolanczuk AJ, Oelsner EC, Barr RG. et al. High-attenuation areas on chest computed tomography and clinical respiratory outcomes in community-dwelling adults. Am J Respir Crit Care Med 2017;196:1434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson C, Giles JT, Bathon J. et al. Smoking and subclinical ILD in RA versus the multi-ethnic study of atherosclerosis. PLoS One 2016;11:e0153024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paul NS, Kashani H, Odedra D. et al. The influence of chest wall tissue composition in determining image noise during cardiac CT. AJR Am J Roentgenol 2011;197:1328–34. [DOI] [PubMed] [Google Scholar]
- 32. Furukawa H, Oka S, Shimada K. et al. Association of human leukocyte antigen with interstitial lung disease in rheumatoid arthritis: a protective role for shared epitope. PLoS One 2012;7:e33133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gochuico BR, Avila NA, Chow CK. et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med 2008;168:159–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from which this manuscript is derived are not publicly available, but may be available for limited and pre-specified use by contacting the senior author.