Abstract
Antimicrobial peptides (AMPs) are produced by all living organisms exhibiting antimicrobial activities and representing the first line of innate defense against pathogens. In this context, AMPs are suggested as an alternative to classical antibiotics. However, several researchers reported their involvement in different processes defining them as Multifunctional AMPs (MF-AMPs). Interestingly, these agents act as the endogenous responses of the human organism against several dangerous stimuli. Still, they are identified in other organisms and evaluated for their anticancer therapy. Chromogranin A (CgA) is a glyco-phosphoprotein discovered for the first time in the adrenal medulla but also produced in several cells. CgA can generate different derived AMPs influencing numerous physiological processes. Dermaseptins (DRSs) are a family of α-helical-shaped polycationic peptides isolated from the skin secretions of several leaf frogs from the Phyllomedusidae family. Several DRSs were identified as AMPs and, until now, more than 65 DRSs have been classified. Recently, these exogenous molecules were characterized for their anticancer activity. In this review, we summarize the role of these two classes of MF-AMPs as an example of endogenous molecules for CgA-derived peptides, able to modulate inflammation but also as exogenous molecules for DRSs, exerting anticancer activities.
Keywords: antimicrobial peptides, multifunctional antimicrobial peptides, chromogranin A-derived peptides, immunomodulators, dermaseptins, anticancer activities
1. Introduction
The first line of response of mammalians against pathogenic invasion is innate immunity [1]. It consists of different molecules produced and released by various cell types belonging to the organs, immune or other systems [1]. Among these molecules, we find proteins with direct antimicrobial activity or those which activate the complement proteins [1]. Furthermore, small peptides play an essential role thanks to the presence of specific cationic sequences that interact with the membrane of the pathogens [1,2]. These molecules are called host defense peptides and are more widely known as antimicrobial peptides (AMPs) [2]. They can not only act through different antimicrobial mechanisms of action but [1,2] also with different intensities against an extensive collection of pathogens [1,2,3,4]. However, in light of the potential human clinical application, they can be classified as endogenous AMPs produced by the human organism, such as Defensins, Cathelicidins and Dermcidins [3,4]. On the other hand, exogenous AMPs are produced by microorganisms themselves, plants, insects, amphibians and fishes or mammals but not identified in humans, such as Thionins, Piscidins, Cecropins and Dermaseptins [5,6,7,8,9]. However, increasing data show that these AMPs are multifunctional peptides (MF-AMPs) with different roles in cardiovascular, nervous and renal systems [10,11,12]. They are also reported as chemokines, vaccine adjuvants, regulators of the innate defense, immunomodulators and anticancer agents [13,14]. All these properties of MF-AMPs confer to these molecules a broad spectrum of potential applications in the biomedical field. The 20th century will be remembered as the time when antibiotic resistance became a world health problem [15]. Different studies reported this issue and recommended limiting the use of antibiotics to contain the evolution of resistant bacteria [15,16]. However, to the present day, the problem is not solved. The World Health Assembly recognized antibiotic resistance as an alarming issue in human medicine and a leading cause of worldwide death [15,17]. In this context, the MF-AMPs appear to be a possible alternative to antibiotics and have acquired an increasingly clinical interest [18]. At the same time, prevention of nosocomial infection and inflammatory activation of the intervention site after the implantation of medical devices is a significant hospital challenge [19]. Additionally, in this case, MF-AMPs are promising, and several studies reported their functionalization of biomaterials, conferring antimicrobial and anti-inflammatory properties [20]. Finally, some of these agents can modulate systemic and tissue inflammation and immune cell activation [21], supporting future clinical applications as immunomodulators. At the same time, several studies showed the activities of these agents against different models of cancer cells, demonstrating a profile of MF-AMPs with potential clinical application also in cancer therapy [14]. Therefore, the principal aim of this review is to report AMPs as multifunctional agents for future clinical applications. More specifically, we will focus on endogenous Chromogranin A-derived peptides for immunomodulation and Dermaseptins as exogenous agents for cancer therapy.
2. General Features, Mechanism of Action and Possible Clinical Application of MF-AMPs
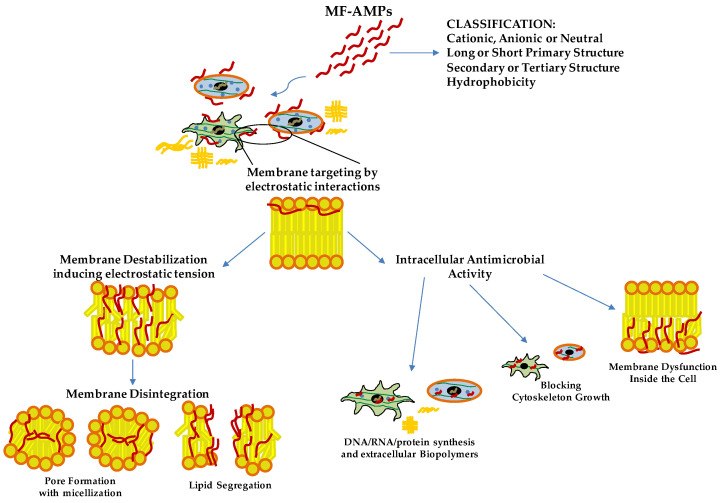
AMPs are effective agents for killing or blocking the growth of free microorganisms due to the self-endogenous origins and natural factors produced by the organisms [4,22]. In addition, the endogenous AMPs are not toxic at high concentrations, and some of these are reported as multifunctional agents with immunomodulatory activity without generating pathogen resistance [22]. Among these, cationic AMPs target bacterial cell membranes in a non-specific manner for pathogens. Still, they are not toxic for host cells due to the specific electrostatic interaction with microbial membrane compounds, such as negatively charged lipids or specific microbial ligands for antimicrobial peptides [23,24]. These agents belong to the host defense response of vertebrates’ innate immune system [23,24]. More than 2000 AMPs are known to be derived from several organisms and catalogued in the AMPs Database [25,26,27]. The peptides are classified based on four different biochemical characteristics: 1. net charge (anionic, cationic and neutral); 2. length of the primary structure (more or less than 100 amino acids); 3. typology of secondary or tertiary structure (linear with β-sheet, α-helix, extended loop and very complex structure, such as cyclic peptides); and 4. hydrophobicity (amphipathic, hydrophobic and hydrophilic) [23,28,29] (Figure 1).
Figure 1.
Antimicrobial Mechanisms of Action ofMultifunctional Antimicrobial Peptides (MF-AMPs). Based on their biochemical characteristics, MF-AMPs can interact with the membrane lipids, inducing the pathogens’ instability and rigidity of the cell membrane. Upon reaching the minimum inhibitory concentration (MIC) values, they cause pore formation and cell lysis. Some MF-AMPs exert a cell-penetrating peptides-like function. They can penetrate the membrane, blocking pathogens’ growth by internal membrane dysfunction, cytoskeleton interaction and interference of biomolecules production. Deoxyribonucleic acid (DNA); ribonucleic acid (RNA).
These molecules’ general mechanism of action consists of an initial phase with membrane targeting based on electrostatic interactions with lipids and membrane ligands [23,28]. After achieving a threshold concentration, the molecular interactions between the peptides and membrane induce a conformational phase transition of these molecules. This effect is characterized by a modification from the disordered structure of peptides, typical in aqueous environments, to α-helical or β-sheet conformation upon interaction with phospholipids in the pathogen membrane [23,28]. Then, the peptides can destabilize the membrane inducing electrostatic tension. Finally, membrane disintegration is obtained by pore formation with micellization or lipid segregation (Figure 1). This phenomenon is caused by different molecular mechanisms, such as self-association and multimerization, barrel-stave mechanism, toroid pore or wormhole mechanism and carpet mechanism [23,24,28]. In addition, due to their lipophilic profile, some AMPs do not degrade the membrane but may translocate across it. These peptides exert a cell-penetrating peptides (CPPs)-like function. They induce inhibition of intracellular functions by blocking the cytoskeleton growth, causing membrane dysfunction inside the cell or inhibiting extracellular biopolymers and DNA/RNA/protein synthesis [22,23,28] (Figure 1). In recent years, these agents have acquired an increasing interest in clinical applications. They may represent a novel and promising therapeutic alternative to conventional antibiotics for preventing and eradicating resistant pathogens. AMPs also prevent pathogens’ biofilm formation due to antimicrobial activity [20,29]. In addition, AMPs are elective to destabilize existing biofilms targeting matrix proteins or signaling pathways for growth and essential metabolic processes or compounds [20,29]. In these aspects, another therapeutic utilization of AMPs is preventing infection in the surgical sites and microbial biofilms on biomaterials often associated with the onset of nosocomial infections. Different biomaterials functionalized with peptides and peptidomimetics agents are available for clinical applications. As a few examples, in addition to medically implanted devices, they can be used orally and for wound sites in the surgical area [30,31]. Other vital interests have been reported for endogenous MF-AMPs based on their properties to modulate systemic and tissue inflammation and immune cell activation [21], supporting future clinical applications as immunomodulators. On the other hand, numerously exogenous MF-AMPs are reported as anti-proliferative against several cancer cells with potential clinical application in cancer therapy [14].
3. CgA-Derived Peptides as Inflammatory Modulator Molecules
3.1. CgA-Derived AMPs
Chromogranin A (CgA) is a glycoprotein with 431 residues and a molecular weight of 49 kDa belonging to the Granin family, discovered for the first time at the end of the 1960s in the granules of adrenomedullary chromaffin cells [32]. In the last few decades, CgA has been identified in immune cells [33,34,35], neurons [36], cardiomyocytes [37], keratinocytes and fibroblasts [38]. This protein may influence different physiological processes. Its role was reported in cardiac function and cardio-protection [39], catecholamine storage and feedback release [40] and the modulation of vascular function [41] but also in cellular recruitment and the modulation of immune response [32,35,42]. However, this prohormone produces, by proteolytic processing, active biological peptides, such as Vasostatins (Vs), Prochromacin, Chromacin, Pancreastatin, WE 14, Catestatin (Cts), Parastatin and Serpinin [43,44]. At the end of the 1990s and early 2000s, several CgA-derived peptides were discovered as AMPs acting against several bacteria, fungi and yeast. The CgA-derived peptides have been found in biological fluids involved in host-defense responses, such as serum, saliva and neutrophils secretions, or against pathogens in the first barrier of the human body, such as the skin [33,34,38,45,46]. The CgA-derived peptides act as antimicrobial agents in the micro-molar range [33,46,47,48]. These concentrations are also reported in the biological fluid after stimulation with pathogen toxins or during infection [33,34,42,46]. Among the CgA-derived peptides, the Vs-I and Cts were first identified as antimicrobial agents; however, their antimicrobial domains were rapidly reported. Vs-I was initially identified as a vasoinhibitory agent [49]. For Vs-I, Lugardon characterized this peptide’s antimicrobial activities against many pathogens [33,47,50]. However, after the incubation of Vs-I with endoproteinase Glu-C, a digested sequence CgA47-66, called Chromofungin (Chr), was identified and found highly active against several fungi and yeasts [47]. It has a global hydrophobicity and amphipathic character, allowing a strong interaction with the membrane. Specifically, Chr possesses a positive charge of +3.5, showing an amphipathic helix in the C-terminal part in the sequence CgA53-66 and at the N-terminal domain, a hydrophobic sequence corresponding to CgA48-51 and a hydrophilic structure CgA53-46, respectively [47,51]. The Vs-I and Chr antimicrobial mechanism of action is explained through the specific interaction of peptides with ergosterol, one of the main components of yeast and fungal membranes, inducing increased pressure and penetration into the membrane [47,51] (Figure 1). Other data demonstrated that Chr could inhibit Calcineurin activity by interacting with Calmodulin [47] (Figure 1). Within microbial cells, Vs-I and Chr may interfere with the Calcium/Calmodulin/Calcineurin signaling pathway by blocking the pathway implicated in virulence and skeleton development of cell walls [52]. Cts was identified as a catecholamine release-inhibitory peptide [53]. Cts is a small 21-amino-acid cationic peptide with a positive net charge of +5 within the bovine sequence (bCgA344-364) possessing a C-terminal hydrophobic sequence. Taylor and colleagues identified a smaller peptide (CgA344-358) derived from Cts with a more substantial inhibitory effect on catecholamine release [54]. This peptide was called Cateslytin (Ctl) by Briolat et al. and is also characterized by its antimicrobial activities with potent effects compared to Cts [46]. Ctl is also a positively charged (+5) arginine-rich antimicrobial peptide and, in an aqueous solution, is a linear peptide with a disordered structure. However, when interacting with the membrane, Ctl acquires an α-helical form [55]. Other studies with a system mimicking bacterial membrane demonstrated that Ctl could convert its structure into antiparallel β-sheets precipitating against the negatively charged part of the membranes [56]. Then, Ctl induced an increased rigidity, permeability gradient and membrane pore formation in the domains containing ergosterol [56,57,58] (Figure 1).
3.2. CgA-Derived Peptides and Immune Cells Activities and Inflammation
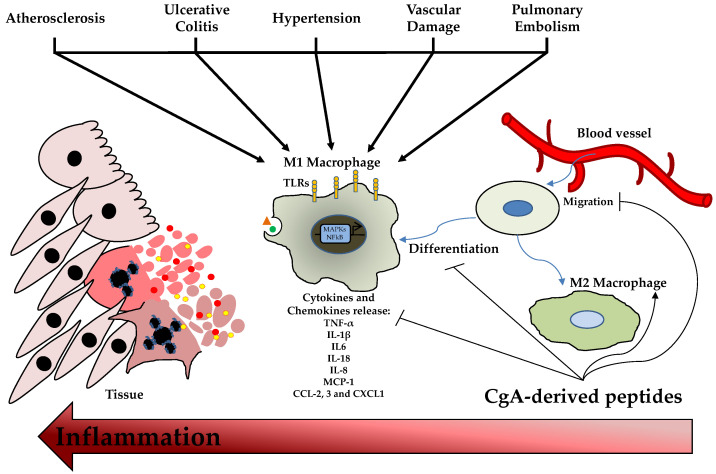
The involvement of CgA and its derived peptides in innate immunity is well known for its antimicrobial activity. In addition, the role of these MF-AMPs is also reported in immune cells, conferring them a complex profile of immunity modulators. This section analyzes this profile studied using in vitro and in vivo models. The role of CgA-derived peptides on immune cells was studied for the first time in 2009; in particular, the effects of Chr and Cts were evaluated on polymorphonuclear neutrophils. After the treatments with the peptides, Chr and Cts were observed inside the cells, demonstrating their ability to penetrate the mammalian membrane and the profile of CPPs [34]. Then, in the presence of extracellular calcium, the two peptides induced a transient calcium influx in the cells, binding Calmodulin-binding factors (W7 and CMZ) and activating iPLA2 [34]. In addition, the pharmacological block of these channels inhibited the calcium flux induced by Chr and Cts [34]. On the other hand, when extracellular calcium is absent, the peptides cannot induce calcium secretion [34]. Notably, the secretion of polymorphonuclear neutrophils treated with the two CgA-derived peptides induced the secretion of several important factors for innate immunity and inflammation, such as Lactotransferrin, Lysozyme, Neutrophil Gelatinase Associated Lipocalin and S100 calcium-binding protein A8/A9 [34]. Several studies reported the role of Vs-1 as an anti-atherogenesis and anti-inflammatory factor suppressing the adhesion of monocytes to endothelial cells by adhesion molecule down-regulation [59,60]. Xiong and coworkers reported the anti-inflammatory role of Vs-II (N-terminal fragment of CgA containing Vs-I; CgA1-113) in an apolipoprotein E-deficient (ApoE−/−) mice model fed with a high-fat diet developing atherosclerosis. In this study, Vs-II treatment reduced the occurrence of atherosclerotic plaque and attenuated lesions [59]. Furthermore, Vs-II significantly reduced the production of pro-inflammatory cytokines in aortic tissue, such as Tumor Necrosis Factor-α (TNF-α), Monocyte Chemoattractant Protein-1 (MCP-1) and Vascular Cell Adhesion Molecule-1 (VCAM-1) [59]. The same authors demonstrated, by several in vivo analyses, that these anti-inflammatory properties are based on the ability of Vs-II to reduce leukocytes adhesion on ApoE−/− mice arteries but also on the recruitment, transmigration and accumulation of M1 macrophages in the lesions [59]. In the same animal model, Sato et al. showed that Vs-I treatment reduces aortic atherosclerotic lesions development due to reductions in intra-plaque inflammation, macrophage infiltration and aortic smooth muscle cells proliferation and plasma glucose level [60]. From a cellular point of view, Vs-I suppressed the lipopolysaccharide (LPS)-induced production of chemokine MCP1 and vascular damage markers, such as VCAM-1 and E-selectin, in human endothelial cells [60]. At the same time, Vs-I was found to reduce M1 pro-inflammatory macrophages differentiation and IL6 release but also oxidized low-density lipoprotein (oxLDL)-induced foam cell formation of macrophages [60]. Of great clinical interest, Vs-I is expressed around Monckeberg’s medial calcific sclerosis in human radial arteries [60]. Additionally, the immunomodulatory role of Chr has been reported in a mice model of ulcerative colitis induced by dextran sulfate sodium administration [61,62]. This model decreased Chr expression [62]. Furthermore, Chr treatment, by intracolonic administration, significantly reduced the inflammation and severity of colitis. The anti-inflammatory effects were due to the differentiation of macrophages into M2 anti-inflammatory clones with the consequent reduction of released IL-18 and the increased expression of M2 markers [61,62]. Using the same animal model, Kapoor and colleagues demonstrated that intrarectal Chr treatment reduced colitis severity and inflammation [63]. In parallel, this was associated with a significant decrease in the expression of CD11c, CD40, CD80, CD86 IL6 and IL12p40 in the inflamed colonic mucosa, mesenteric lymph nodes and spleen [63]. In addition, Chr reduces in CD11c positive cells the expression of CD80, CD86 and NF-κB in the spleen and colon, respectively [63]. All these in vivo data demonstrated that Chr has protective properties against intestinal inflammation and exerts the role of immunomodulator for intestinal macrophages and dendritic cells (DCs). These effects were also demonstrated in vitro with macrophages showing that Chr increased the production of anti-inflammatory factors and the M2 differentiation [61]. At the same time, Chr treatment significantly reduced the expression of M1 macrophage markers and the activation of the NF-κB pathway [62]. Additionally, in this case, in vitro experiments with M1 macrophages demonstrated that this peptide could decrease cellular migration, proinflammatory cytokines production and release, and NF-κB phosphorylation [62]. Furthermore, treatment with Chr or a conditioned medium of Chr-treated macrophages M2 induced epithelial cell proliferation and migration but also decreased oxidative stress and pro-inflammatory cytokine production [61]. In addition, the Chr treatment of naïve bone marrow-derived CD11c positive DCs reduced the LPS-induced expression of CD40, CD80, CD86 IL-6 and IL-12p40 [63]. These results were also confirmed in intestinal tissue isolated from patients with ulcerative colitis, demonstrating that Chr expression was down-regulated in these patients compared to healthy controls [62]. Indeed, the mRNA levels of Chr were positively correlated with the mRNA expression of M2 macrophages activation markers and negatively to the expression of collagen, IL-8 and IL-18, but also with M1 activation markers (TLR-4 expression and NF-kB activation) and consequent pro-inflammatory cytokines production [61,62]. In these patients, the reduction of Chr level is also associated with a negative linear relationship with CD11c and CD86 [63]. Moreover, another study confirmed the anti-inflammatory effects of Chr on monocytes. Treatment with this peptide significantly inhibited the transcription of pro-inflammatory factors, such as NF-kB and AP-1, in these cells [64]. Furthermore, Rabbi and colleagues showed that Cts reduced intestinal inflammation and the onset of colitis lesions by a Stat-3 activation [65]. At the same time, the markers of M1 macrophage activation and the colonic levels of pro-inflammatory cytokines, such as IL-6, IL-1β and TNF-α, were significantly decreased by Cts treatment [66]. However, Cts did not influence M2 macrophage markers [66]. Furthermore, these anti-inflammatory effects were confirmed in cellular experiments using macrophages isolated from the peritoneal cavity and the bone marrow, demonstrating that in vitro treatment with Cts significantly decreased the production of the pro-inflammatory cytokines and phosphorylation of Stat-3 [65]. In addition, peritoneal macrophages isolated from naïve mice and treated with Cts and LPS displayed a reduction in the expression and production of pro-inflammatory cytokines blocking the activation of M1 macrophages [66]. In addition, the genetic deletion of Cts induces hypertensive conditions and left ventricular hypertrophy accompanied by significant macrophage infiltration in cardiac tissue and adrenal gland [67]. In this context, the absence of Cts induced an increased level of pro-inflammatory cytokines TNF-α, C-C motif chemokine ligand (CCL)-2, 3, C-X-C motif chemokine ligand (CXCL)-1 and catecholamines but also an elevated inflammation in heart with the up-regulation of cardiac genes, such as Tnfa, Ifng, Emr1, Itgam, Itgax, Nos2a, IL12b, Ccl2 and Cxcl1 [67]. It is of great interest that the intraperitoneal administration of Cts reversed this phenotype. In addition, macrophage depletion blocked the onset of hypertension in Cts-knockout (KO) mice [67]. Furthermore, bone-marrow transfer of KO animals in wild-type (WT) counterparts induced hypertension and cardiac inflammation, while opposite conditions showed the opposite phenotype [67]. All these data strongly suggest that the anti-hypertensive effects of Cts are partially mediated by an immunosuppressive action of this peptide on macrophages [67]. The role of Cts as an immunomodulator was also explored in the context of atherosclerosis and vascular injury. In fact, in vitro treatment with Cts on endothelial cells significantly reduces the release of TNF-α and vascular damage markers, such as ICAM-1 and VCAM-1, after LPS exposure [68]. At the same time, Cts treatment suppresses inflammatory responses and oxidizes the low-density lipoprotein-induced foam cell formation of human macrophages [68]. Kojima and colleagues demonstrated that Cts injection to ApoE−/− mice significantly reduces macrophage infiltration and the consequent atherosclerotic lesions onset in the aorta but also suppresses aortic smooth muscle cells proliferation and collagen deposition in atheromatous plaques [68]. Furthermore, in vitro experiments with human aortic smooth muscle cells showed that Cts treatment can block collagen-1 and fibronectin expression and migration, proliferation and apoptotic process [68]. Of significant clinical impact, coronary artery disease patients displayed a substantial reduction of plasmatic levels of Cts but an increased expression in coronary atheromatous plaques [68]. In an acute pulmonary embolism in vivo model and cellular experiments with human pulmonary artery endothelial cells, Cts treatment was found to abolish thrombin-induced inflammation blocking TLR-4 expression and p38 phosphorylation, decreasing the consequent acute pulmonary embolism [69]. In conclusion, Vs-I and Chr than Cts are key attenuators of inflammation in different tissue and pathological conditions by reducing immune cell infiltration and inflammatory activation (Figure 2).
Figure 2.
ChromgraninA (CgA)-derived Peptides and Inflammation. Damage or stress stimuli induce the migration, proliferation and activation of macrophages with consequent pro-inflammatory cytokines and chemokines release. This phenomenon generates cell death and inflammation. CgA-derived peptides can reduce M1 polarization and promote M2 anti-inflammatory macrophages differentiation. C-C motif chemokine ligand (CCL); C-X-C motif chemokine ligand (CXCL); interleukin (IL); monocyte chemoattractant protein (MCP); toll-like receptor (TLR); tumor necrosis factor (TNF).
4. Dermaseptins and Anticancer Therapy
4.1. Dermaseptins
Dermaseptins (DRSs) are a class of peptides identified in the skin secretions of several Amazonian tree frogs of the family Phyllomedusidae, in particular, the species Phyllomedusa [70,71]. The first member of DRS family was isolated from the skin secretion of P. sauvagei and named DRS-S1 [72]. This 34-residues-containing peptide has antimicrobial activity against Gram-positive and Gram-negative bacteria, yeast and protozoa without affecting mammalian cells. The second is DRS-B2, isolated from exudates of P. bicolor [73,74,75]. It is also known as adenoregulin due to its capacity to increase the affinity of the agonist toward the receptor of adenosine A1 [76]. DRS-B2, with its 33 amino-acid residues, is considered the most abundant member and the most active peptide of the B family (B for the frog species P. bicolor). To date, more than 65 DRSs, listed in the APD3 database (https://aps.unmc.edu/, accessed on 1 June 2022) [26], have been isolated primarily from the skin secretion of South American tree frogs of the 67-member family Phyllomedusidae (https://amphibiansoftheworld.amnh.org/, accessed on 1 June 2022). Multiple alignments of the 67 sequences of DRSs listed in APD3 clearly showed that these polycationic peptides, rich in Lys residue, share a signature consisting of a conserved Trp residue at position three and a consensus AA(A/G)KAAL(G/N)A motif in the middle region [70]. Their MIC values are in the low micromolar range for a large panel of microorganisms, comprising bacteria (S. aureus, E. coli), yeast (C. albicans), filamentous fungi (A. fumigatus) and protozoa, such as Leishmania Mexicana, and show no hemolytic activity against human and rabbit erythrocytes. The mode of action by which DRSs kill these microorganisms follows the “carpet” mechanism [77,78]. These polycationic peptides, destructured in aqueous media, adopt an alpha helix structure upon contact with the host cell plasma membranes and then interact with their negative charges [79,80,81,82]. Once bound, the peptide will disrupt membrane permeability and cause the death of the microorganism (Figure 1).
4.2. Dermaseptins and Anticancer Properties
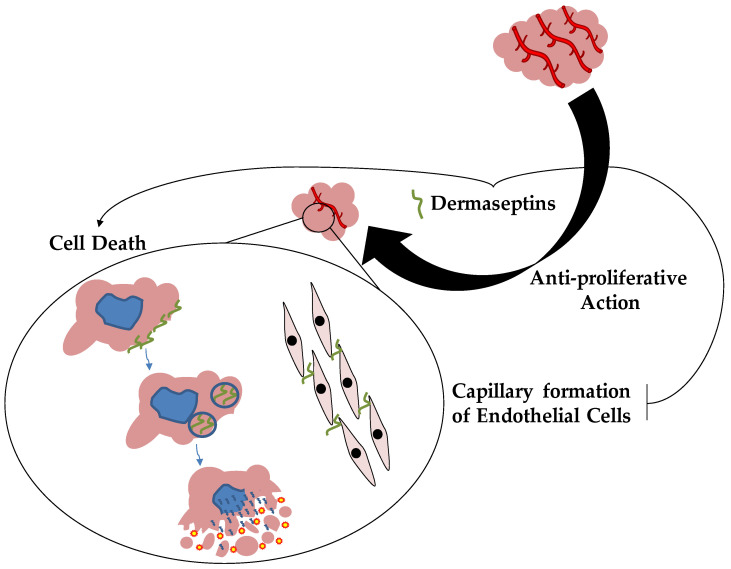
The first two anticancer DRSs peptides were isolated from the South American Amazonian tree frog, Phyllomedusa bicolor. These molecules, DRSs-B2 and DRS-B3, were tested in vitro against a human prostatic adenocarcinoma PC-3 cell line, showing an antiproliferative effect with an EC50 around 2–3 μM and demonstrating the inhibition of proliferation of more than 90% [83]. In addition, these two peptides also inhibited PC-3 cell colony formation in soft agar and the proliferation, differentiation and capillary formation of endothelial cells [83,84]. Furthermore, DRS-B2 blocks the proliferation and colony formation of several human tumor cell types, such as prostatic adenocarcinoma LNCAP, prostatic carcinoma DU145, mammary carcinoma (MDA-MB2318) cell lines and B-lymphoma lines [83]. These effects were also confirmed in vivo by a cell line PC3 murine xenograft model, showing that DRS-B2 inhibits tumor growth [83]. The anticancer mechanism of action of DRS-B2 was demonstrated by in vitro experiments with tumor PC3 cells. This peptide interacted with tumor cell surface, aggregating and penetrating the cells. Furthermore, it induced the release of cytosolic lactate dehydrogenase, a marker of cytotoxicity and necrosis, but no effects were observed on mitochondrial membrane potential and caspase 3 activations for apoptotic involvement [83]. Concerning the mechanisms of action of DRS-B2, confocal microscopy studies revealed that this peptide rapidly accumulates to cytoplasmic membranes, packed in vesicles and into the nucleus [85]. These effects were also partially mediated by glycosaminoglycans’ interaction with DRS-B2 and the consequent structural modification of the peptide with the α-helical domain [85]. Recently, a synthetic hormonotoxin molecule composed of dermaseptin-B2 associated with luteinizing hormone-releasing hormone (LHRH) was tested to improve the peptide’s antitumor activity, reducing its peripheral toxicity and lethality. This hormonotoxin displayed an anticancer effect very similar to DRS-B2 both in vitro and in vivo [86]. The LHRH addition to dermaseptin-B2 does not alter the peptide’s secondary structure and biological function [86]. On the other hand, double staining flow cytometry analysis showed that this hormonotoxin induced apoptosis instead of a necrotic process caused by DRS-B2 [86]. This different anticancer mechanism of action explains better tolerance and the lower toxicity of the hormonotoxin compared to dermaseptin-B2 [86]. In addition, other biochemical approaches have been used to increase the antitumor activity of DRS, delivering these agents in tumor cells, as seen in DRS-DStomo01 peptide [87]. DRS-DStomo01 was entrapped in chitosan nanoparticles, and the antitumor activity was tested in vitro against HeLa cells. The peptide induces DNA fragmentation and mitochondrial hyperpolarization with consequent cytotoxicity for cancer cells [87]. However, when used in chitosan nanoparticles, DRS-DStomo01 was more active than free peptides [87]. In 2016, two novel members of DRSs family were identified in the skin secretion of the frog Pachymedusa dacnicolor and called DRS-PD 1 and 2 [88]. Both peptides were reported to be active against many microorganisms, such as E. coli, S. aureus, P. aeruginosa and C. albicans, but with no lytic effects on mammalian red cells [88]. DRS-PD 2 displayed anti-proliferative effects against cancer cell lines, such as H157, PC-3 and U251-MG, within the concentration range of 10−9 to 10−4 M [88]. This property was also reported for DRS-PD 1 but only for human neuronal glioblastoma U251MG cell line [88]. In addition, these peptides could also inhibit the proliferation of human microvessel endothelial cells with the same concentration range for anticancer activity [88]. Other peptides from the South American orange-legged leaf frog (Phyllomedusa hypochondrialis) were identified and called DRS-PH. This peptide was active against several pathogens, such as E. coli, P. aeruginosa, S. aureus and its methicillin-resistant strain (MRSA), E. faecalis and C. albicans, in a concentration range from 1 μM to 512 μM [89]. Once again, this DRS displayed a broad spectrum of anticancer properties against different cancer cell lines, including MCF-7, H157, U251MG, MDA-MB-435S and PC-3 [89]. Very recently, different studies identified, from the skin of Phyllomedusa sauvagei, DRSs-PS type 1, 3 and 4, characterizing their anticancer properties [90,91,92]. In 2019, Long and coworkers demonstrated that DRS-PS1 has antimicrobial effects against S. aureus, E. coli and C. albicans [90]. Interestingly, DRS-PS 1 showed anti-proliferative effects on human glioblastoma U-251 MG, perturbing cell membrane integrity at the concentration of 0.1 μM [90]. Furthermore, the anticancer action with lower concentrations involves apoptosis activation by mitochondrial-related signal involvement [90]. DRS-PS 3 showed a broad spectrum of antimicrobial activities against several pathogens, such as S. aureus, E. coli and C. albicans, at high concentrations but with reduced cytotoxicity for erythrocytes [91]. However, the synthetic, more cationic and hydrophobic analogues created by replacing acidic amino acids D and E at 5 and 17, respectively, of the DRS-PS3 sequence by lysines (K5/D5, K17/E17-DRS-PS 3) or by replacing two neutral amino acids A10 and G11 with the hydrophobic amino acid leucine (L10/A10, L11/G11-DRS-PS 3) strongly increased their antimicrobial activities against the same pathogens with MIC values of 8 µM or less [91]. On the other hand, both artificial analogues exhibit a more significant hemolytic effect on red blood cells than DRS-PS 3 [91]. Furthermore, these peptides showed anticancer activities against H157, PC3 and HMEC-1 cell lines in the micromolar range but the most active was L10/A10, L11/G11-DRS-PS 3 [91]. Additionally, DRS-PS 4 displayed antimicrobial effects with many pathogens, such as S. aureus and MRSA, E. faecalis, E. coli, P. aeruginosa and C. albicans, in a range of concentrations from 1 µM to 32 µM, with biofilms eradicating properties of these microorganisms [92]. The antimicrobial mechanism of action is based on the ability of this peptide to permeabilize the bacterial cell membrane [92]. However, the hemolysis activity of DRS-PS 4 was tested using horse red blood cells showing slight effects at antimicrobial concentrations [92]. In addition, the anticancer activity of DRS-PS 4 was also evaluated on several human cell lines, including U251MG, MDA-MB-435S, H157, PC-3 and MCF-7, displaying a dose-dependent inhibitory activity with high cytotoxicity in a concentration range from 10−9 to 10−4 M [92]. On the other hand, it presents a slight suppressing effect on human microvascular endothelial cells [92]. Very recently, Dong et al. discovered the DRS-PP from frog Phyllomedusa palliata. This peptide was active at 2 µM against E. coli, S. aureus and MRSA, C. albicans, P. aeruginosa, E. faecalis and K. pneumoniae [93]. It is of great interest that DRS-PP showed anti-proliferative effects with cytotoxic activities on different cancer cells, such as H157, MCF-7, PC-3 and U251 MG, but no effects on human microvascular endothelial cells [93]. In vivo studies confirmed the anticancer property of this agent; in fact, DRS-PP was tested on a subcutaneous H157 tumor model of nude mice showing significant anti-tumor activity in a dose-dependent manner without hepatopulmonary and toxic side effects [93]. These effects are mediated via disruptive membrane action but exert pro-apoptotic effects induced by mitochondrial and death receptor pathways [93]. Finally, in 2021, DRS-TO was identified in the tiger-striped Leaf Frog, Phyllomedesa tomopterna, showing that this peptide was active against S. aureus and MRSA, E. faecalis, E. coli, and C. albicans [94]. Additionally, no hemolytic effect was observed on red blood cells, but DRS-TO showed anticancer activity against U251MG, H157 and PC-3 cancer cell lines at higher concentrations [94]. All these data report the great potential of DRSs as anticancer agents and their mechanism of action targeting membrane but also inducing pro-apoptotic effects by mitochondrial dysfunction and death receptor pathways (Figure 3).
Figure 3.
Dermaseptins and Cancer. Dermaseptins act as an anti-proliferative agent against several cancer cells in vitro and in vivo. The anticancer mechanism of action is based on the ability of these MF-AMPs to accumulate in cancer cells, inducing cell death and blocking tumor vascularization.
5. Conclusions and Future Perspectives
In conclusion, the role of MF-AMPs is reported to be crucial in preventing infection. These molecules are essential for the first response to infections and may represent an alternative approach and open future clinical applications for the resolution of antibiotic resistance. At the same time, the endogenous MF-AMPs displayed different effects on different organs and cell types. Among these, they can influence the immune system’s inflammatory process and cellular components. In this perspective, CgA-derived peptides, such as Chr and Cts, are the perfect examples of immunomodulation mediated by MF-AMPs. On the other hand, exogenous MF-AMPs produced by different species but not in humans showed several potential therapeutic approaches. As reported in this review, DRSs appear to be excellent anti-proliferative factors with varying models of cancer cells. From a clinical point of view, these peptides may represent elective candidates for future anticancer therapy.
Acknowledgments
F.S. thanks “Università Italo-Francese” for the financial support provided in the context of ‘Vinci Project 2014′ (no. C2-72) during the work realized for his Ph.D. thesis on MF-AMPs. The authors thank Marie-Hélène Metz-Boutigue for the stimulating discussions and her full support for all the above-mentioned research on CgA-derived peptides.
Author Contributions
Writing—initial draft preparation, F.S.; writing—review and editing, M.A. and J.-E.G. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This review was funded by Natural Sciences and Engineering Research Council of Canada (project number RGPIN-2022-03626) to J.-E.G.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riera Romo M., Perez-Martinez D., Castillo Ferrer C. Innate immunity in vertebrates: An overview. Immunology. 2016;148:125–139. doi: 10.1111/imm.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auvynet C., Rosenstein Y. Multifunctional host defense peptides: Antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J. 2009;276:6497–6508. doi: 10.1111/j.1742-4658.2009.07360.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z., Wang G. APD: The Antimicrobial Peptide Database. Nucleic Acids Res. 2004;32:D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandwein M., Bentwich Z., Steinberg D. Endogenous Antimicrobial Peptide Expression in Response to Bacterial Epidermal Colonization. Front. Immunol. 2017;8:1637. doi: 10.3389/fimmu.2017.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Mandal S., Panda A.K., Murugan C., Xu X., Senthil Kumar N., Jin F. Antimicrobial Peptides: Novel Source and Biological Function with a Special Focus on Entomopathogenic Nematode/Bacterium Symbiotic Complex. Front. Microbiol. 2021;12:555022. doi: 10.3389/fmicb.2021.555022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J., Hu S., Jian W., Xie C., Yang X. Plant antimicrobial peptides: Structures, functions, and applications. Bot. Stud. 2021;62:5. doi: 10.1186/s40529-021-00312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masso-Silva J.A., Diamond G. Antimicrobial peptides from fish. Pharmaceuticals. 2014;7:265–310. doi: 10.3390/ph7030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fasina Y.O., Obanla T., Dosu G., Muzquiz S. Significance of Endogenous Antimicrobial Peptides on the Health of Food Animals. Front. Vet. Sci. 2021;8:585266. doi: 10.3389/fvets.2021.585266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartels E.J.H., Dekker D., Amiche M. Dermaseptins, Multifunctional Antimicrobial Peptides: A Review of Their Pharmacology, Effectivity, Mechanism of Action, and Possible Future Directions. Front. Pharmacol. 2019;10:1421. doi: 10.3389/fphar.2019.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y. The role of antimicrobial peptides in cardiovascular physiology and disease. Biochem. Biophys. Res. Commun. 2009;390:363–367. doi: 10.1016/j.bbrc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Stuart B.A.R., Franitza A.L., Lezi E. Regulatory Roles of Antimicrobial Peptides in the Nervous System: Implications for Neuronal Aging. Front. Cell Neurosci. 2022;16:843790. doi: 10.3389/fncel.2022.843790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan L.L., Liang W., Ren Z., Li C., Chen Y., Niu W., Fang X., Liu Y., Zhang M., Diana J., et al. Cathelicidin-related antimicrobial peptide protects against ischaemia reperfusion-induced acute kidney injury in mice. Br. J. Pharmacol. 2020;177:2726–2742. doi: 10.1111/bph.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung A.T., Gellatly S.L., Hancock R.E. Multifunctional cationic host defence peptides and their clinical applications. Cell. Mol. Life Sci. 2011;68:2161–2176. doi: 10.1007/s00018-011-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafari A., Babajani A., Sarrami Forooshani R., Yazdani M., Rezaei-Tavirani M. Clinical Applications and Anticancer Effects of Antimicrobial Peptides: From Bench to Bedside. Front. Oncol. 2022;12:819563. doi: 10.3389/fonc.2022.819563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antimicrobial Resistance C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beovic B. The issue of antimicrobial resistance in human medicine. Int. J. Food Microbiol. 2006;112:280–287. doi: 10.1016/j.ijfoodmicro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Tagliabue A., Rappuoli R. Changing Priorities in Vaccinology: Antibiotic Resistance Moving to the Top. Front. Immunol. 2018;9:1068. doi: 10.3389/fimmu.2018.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rima M., Rima M., Fajloun Z., Sabatier J.M., Bechinger B., Naas T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics. 2021;10:1095. doi: 10.3390/antibiotics10091095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leaper D., Wilson P., Assadian O., Edmiston C., Kiernan M., Miller A., Bond-Smith G., Yap J. The role of antimicrobial sutures in preventing surgical site infection. Ann. R. Coll. Surg. Engl. 2017;99:439–443. doi: 10.1308/rcsann.2017.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Somma A., Moretta A., Cane C., Cirillo A., Duilio A. Antimicrobial and Antibiofilm Peptides. Biomolecules. 2020;10:652. doi: 10.3390/biom10040652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guryanova S.V., Ovchinnikova T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022;23:2499. doi: 10.3390/ijms23052499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sierra J.M., Fuste E., Rabanal F., Vinuesa T., Vinas M. An overview of antimicrobial peptides and the latest advances in their development. Expert. Opin. Biol. Ther. 2017;17:663–676. doi: 10.1080/14712598.2017.1315402. [DOI] [PubMed] [Google Scholar]
- 23.Yeaman M.R., Yount N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 24.Moellering R.C., Jr. Discovering new antimicrobial agents. Int. J. Antimicrob. Agents. 2011;37:2–9. doi: 10.1016/j.ijantimicag.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Wang G., Li X., Wang Z. APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37:D933–D937. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G., Li X., Wang Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung C.R., Jhong J.H., Wang Z., Chen S., Wan Y., Horng J.T., Lee T.Y. Characterization and Identification of Natural Antimicrobial Peptides on Different Organisms. Int. J. Mol. Sci. 2020;21:986. doi: 10.3390/ijms21030986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malmsten M. Antimicrobial peptides. Ups. J. Med. Sci. 2014;119:199–204. doi: 10.3109/03009734.2014.899278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribeiro S.M., Felicio M.R., Boas E.V., Goncalves S., Costa F.F., Samy R.P., Santos N.C., Franco O.L. New frontiers for anti-biofilm drug development. Pharmacol. Ther. 2016;160:133–144. doi: 10.1016/j.pharmthera.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 30.De la Fuente-Nunez C., Korolik V., Bains M., Nguyen U., Breidenstein E.B., Horsman S., Lewenza S., Burrows L., Hancock R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012;56:2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Gomez S., Martinez-de-Tejada G. Antimicrobial Peptides as Anti-biofilm Agents in Medical Implants. Curr. Top. Med. Chem. 2017;17:590–603. doi: 10.2174/1568026616666160713141439. [DOI] [PubMed] [Google Scholar]
- 32.Helle K.B., Metz-Boutigue M.H., Cerra M.C., Angelone T. Chromogranins: From discovery to current times. Pflugers Arch. 2018;470:143–154. doi: 10.1007/s00424-017-2027-6. [DOI] [PubMed] [Google Scholar]
- 33.Lugardon K., Raffner R., Goumon Y., Corti A., Delmas A., Bulet P., Aunis D., Metz-Boutigue M.H. Antibacterial and antifungal activities of vasostatin-1, the N-terminal fragment of chromogranin A. J. Biol. Chem. 2000;275:10745–10753. doi: 10.1074/jbc.275.15.10745. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D., Shooshtarizadeh P., Laventie B.J., Colin D.A., Chich J.F., Vidic J., de Barry J., Chasserot-Golaz S., Delalande F., Van Dorsselaer A., et al. Two chromogranin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS ONE. 2009;4:e4501. doi: 10.1371/journal.pone.0004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eissa N., Hussein H., Kermarrec L., Ali A.Y., Marshall A., Metz-Boutigue M.H., Hendy G.N., Bernstein C.N., Ghia J.E. Chromogranin-A Regulates Macrophage Function and the Apoptotic Pathway in Murine DSS colitis. J. Mol. Med. 2018;96:183–198. doi: 10.1007/s00109-017-1613-6. [DOI] [PubMed] [Google Scholar]
- 36.Laguerre F., Anouar Y., Montero-Hadjadje M. Chromogranin A in the early steps of the neurosecretory pathway. IUBMB Life. 2020;72:524–532. doi: 10.1002/iub.2218. [DOI] [PubMed] [Google Scholar]
- 37.Pasqua T., Corti A., Gentile S., Pochini L., Bianco M., Metz-Boutigue M.H., Cerra M.C., Tota B., Angelone T. Full-length human chromogranin-A cardioactivity: Myocardial, coronary, and stimulus-induced processing evidence in normotensive and hypertensive male rat hearts. Endocrinology. 2013;154:3353–3365. doi: 10.1210/en.2012-2210. [DOI] [PubMed] [Google Scholar]
- 38.Radek K.A., Lopez-Garcia B., Hupe M., Niesman I.R., Elias P.M., Taupenot L., Mahata S.K., O’Connor D.T., Gallo R.L. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J. Investig. Dermatol. 2008;128:1525–1534. doi: 10.1038/sj.jid.5701225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penna C., Tullio F., Perrelli M.G., Mancardi D., Pagliaro P. Cardioprotection against ischemia/reperfusion injury and chromogranin A-derived peptides. Curr. Med. Chem. 2012;19:4074–4085. doi: 10.2174/092986712802429966. [DOI] [PubMed] [Google Scholar]
- 40.Kim T., Loh Y.P. Chromogranin A: A surprising link between granule biogenesis and hypertension. J. Clin. Investig. 2005;115:1711–1713. doi: 10.1172/JCI25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasqua T., Rocca C., Spena A., Angelone T., Cerra M.C. Modulation of the coronary tone in the expanding scenario of Chromogranin-A and its derived peptides. Future Med. Chem. 2019;11:1501–1511. doi: 10.4155/fmc-2018-0585. [DOI] [PubMed] [Google Scholar]
- 42.Eissa N., Hussein H., Hendy G.N., Bernstein C.N., Ghia J.E. Chromogranin-A and its derived peptides and their pharmacological effects during intestinal inflammation. Biochem. Pharmacol. 2018;152:315–326. doi: 10.1016/j.bcp.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Helle K.B., Angeletti R.H. Chromogranin A: A multipurpose prohormone? Acta Physiol. Scand. 1994;152:1–10. doi: 10.1111/j.1748-1716.1994.tb09779.x. [DOI] [PubMed] [Google Scholar]
- 44.Koshimizu H., Cawley N.X., Kim T., Yergey A.L., Loh Y.P. Serpinin: A novel chromogranin A-derived, secreted peptide up-regulates protease nexin-1 expression and granule biogenesis in endocrine cells. Mol. Endocrinol. 2011;25:732–744. doi: 10.1210/me.2010-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizuhashi F., Koide K., Toya S., Takahashi M., Mizuhashi R., Shimomura H. Levels of the antimicrobial proteins lactoferrin and chromogranin in the saliva of individuals with oral dryness. J. Prosthet. Dent. 2015;113:35–38. doi: 10.1016/j.prosdent.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 46.Briolat J., Wu S.D., Mahata S.K., Gonthier B., Bagnard D., Chasserot-Golaz S., Helle K.B., Aunis D., Metz-Boutigue M.H. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell. Mol. Life Sci. 2005;62:377–385. doi: 10.1007/s00018-004-4461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lugardon K., Chasserot-Golaz S., Kieffer A.E., Maget-Dana R., Nullans G., Kieffer B., Aunis D., Metz-Boutigue M.H. Structural and biological characterization of chromofungin, the antifungal chromogranin A-(47-66)-derived peptide. J. Biol. Chem. 2001;276:35875–35882. doi: 10.1074/jbc.M104670200. [DOI] [PubMed] [Google Scholar]
- 48.Lugardon K., Chasserot-Golaz S., Kieffer A.E., Maget-Dana R., Nullans G., Kieffer B., Aunis D., Metz-Boutigue M.H. Structural and biological characterization of chromofungin, the antifungal chromogranin A (47-66)-derived peptide. Ann. N. Y. Acad. Sci. 2002;971:359–361. doi: 10.1111/j.1749-6632.2002.tb04496.x. [DOI] [PubMed] [Google Scholar]
- 49.Aardal S., Helle K.B., Elsayed S., Reed R.K., Serck-Hanssen G. Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J. Neuroendocrinol. 1993;5:405–412. doi: 10.1111/j.1365-2826.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 50.Metz-Boutigue M.H., Goumon Y., Strub J.M., Lugardon K., Aunis D. Antimicrobial chromogranins and proenkephalin-A-derived peptides: Antibacterial and antifungal activities of chromogranins and proenkephalin-A-derived peptides. Ann. N. Y. Acad. Sci. 2003;992:168–178. doi: 10.1111/j.1749-6632.2003.tb03147.x. [DOI] [PubMed] [Google Scholar]
- 51.Metz-Boutigue M.H., Kieffer A.E., Goumon Y., Aunis D. Innate immunity: Involvement of new neuropeptides. Trends Microbiol. 2003;11:585–592. doi: 10.1016/j.tim.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Park H.S., Lee S.C., Cardenas M.E., Heitman J. Calcium-Calmodulin-Calcineurin Signaling: A Globally Conserved Virulence Cascade in Eukaryotic Microbial Pathogens. Cell Host Microbe. 2019;26:453–462. doi: 10.1016/j.chom.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahata S.K., O’Connor D.T., Mahata M., Yoo S.H., Taupenot L., Wu H., Gill B.M., Parmer R.J. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J. Clin. Investig. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor C.V., Taupenot L., Mahata S.K., Mahata M., Wu H., Yasothornsrikul S., Toneff T., Caporale C., Jiang Q., Parmer R.J., et al. Formation of the catecholamine release-inhibitory peptide catestatin from chromogranin A. Determination of proteolytic cleavage sites in hormone storage granules. J. Biol. Chem. 2000;275:22905–22915. doi: 10.1074/jbc.M001232200. [DOI] [PubMed] [Google Scholar]
- 55.Jean-Francois F., Khemtemourian L., Odaert B., Castano S., Grelard A., Manigand C., Bathany K., Metz-Boutigue M.H., Dufourc E.J. Variability in secondary structure of the antimicrobial peptide Cateslytin in powder, solution, DPC micelles and at the air-water interface. Eur. Biophys. J. 2007;36:1019–1027. doi: 10.1007/s00249-007-0169-8. [DOI] [PubMed] [Google Scholar]
- 56.Jean-Francois F., Castano S., Desbat B., Odaert B., Roux M., Metz-Boutigue M.H., Dufourc E.J. Aggregation of cateslytin beta-sheets on negatively charged lipids promotes rigid membrane domains. A new mode of action for antimicrobial peptides? Biochemistry. 2008;47:6394–6402. doi: 10.1021/bi800448h. [DOI] [PubMed] [Google Scholar]
- 57.Jean-Francois F., Elezgaray J., Berson P., Vacher P., Dufourc E.J. Pore formation induced by an antimicrobial peptide: Electrostatic effects. Biophys. J. 2008;95:5748–5756. doi: 10.1529/biophysj.108.136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jean-Francois F., Desbat B., Dufourc E.J. Selectivity of cateslytin for fungi: The role of acidic lipid-ergosterol membrane fluidity in antimicrobial action. FASEB J. 2009;23:3692–3701. doi: 10.1096/fj.09-135574. [DOI] [PubMed] [Google Scholar]
- 59.Xiong W., Wang X., Dai D., Zhang B., Lu L., Tao R. The anti-inflammatory vasostatin-2 attenuates atherosclerosis in ApoE(−/−) mice and inhibits monocyte/macrophage recruitment. Thromb. Haemost. 2017;117:401–414. doi: 10.1160/TH16-06-0475. [DOI] [PubMed] [Google Scholar]
- 60.Sato Y., Watanabe R., Uchiyama N., Ozawa N., Takahashi Y., Shirai R., Sato K., Mori Y., Matsuyama T., Ishibashi-Ueda H., et al. Inhibitory effects of vasostatin-1 against atherogenesis. Clin. Sci. 2018;132:2493–2507. doi: 10.1042/CS20180451. [DOI] [PubMed] [Google Scholar]
- 61.Eissa N., Hussein H., Kermarrec L., Grover J., Metz-Boutigue M.E., Bernstein C.N., Ghia J.E. Chromofungin Ameliorates the Progression of Colitis by Regulating Alternatively Activated Macrophages. Front. Immunol. 2017;8:1131. doi: 10.3389/fimmu.2017.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eissa N., Hussein H., Kermarrec L., Elgazzar O., Metz-Boutigue M.H., Bernstein C.N., Ghia J.E. Chromofungin (CHR: CHGA47-66) is downregulated in persons with active ulcerative colitis and suppresses pro-inflammatory macrophage function through the inhibition of NF-kappaB signaling. Biochem. Pharmacol. 2017;145:102–113. doi: 10.1016/j.bcp.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Kapoor K., Eissa N., Tshikudi D., Bernstein C.N., Ghia J.E. Impact of intrarectal chromofungin treatment on dendritic cells-related markers in different immune compartments in colonic inflammatory conditions. World J. Gastroenterol. 2021;27:8138–8155. doi: 10.3748/wjg.v27.i47.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schneider F., Marban C., Ajob G., Helle S., Guillot M., Launoy A., Maestraggi Q., Scavello F., Rohr O., Metz-Boutigue M.H. In Trauma Patients, the Occurrence of Early-Onset Nosocomial Infections Is Associated with Increased Plasma Concentrations of Chromogranin A. Shock. 2018;49:522–528. doi: 10.1097/SHK.0000000000001000. [DOI] [PubMed] [Google Scholar]
- 65.Rabbi M.F., Labis B., Metz-Boutigue M.H., Bernstein C.N., Ghia J.E. Catestatin decreases macrophage function in two mouse models of experimental colitis. Biochem. Pharmacol. 2014;89:386–398. doi: 10.1016/j.bcp.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Rabbi M.F., Eissa N., Munyaka P.M., Kermarrec L., Elgazzar O., Khafipour E., Bernstein C.N., Ghia J.E. Reactivation of Intestinal Inflammation Is Suppressed by Catestatin in a Murine Model of Colitis via M1 Macrophages and Not the Gut Microbiota. Front. Immunol. 2017;8:985. doi: 10.3389/fimmu.2017.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ying W., Tang K., Avolio E., Schilling J.M., Pasqua T., Liu M.A., Cheng H., Gao H., Zhang J., Mahata S., et al. Immunosuppression of Macrophages Underlies the Cardioprotective Effects of CST (Catestatin) Hypertension. 2021;77:1670–1682. doi: 10.1161/HYPERTENSIONAHA.120.16809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kojima M., Ozawa N., Mori Y., Takahashi Y., Watanabe-Kominato K., Shirai R., Watanabe R., Sato K., Matsuyama T.A., Ishibashi-Ueda H., et al. Catestatin Prevents Macrophage-Driven Atherosclerosis but Not Arterial Injury-Induced Neointimal Hyperplasia. Thromb. Haemost. 2018;118:182–194. doi: 10.1160/TH17-05-0349. [DOI] [PubMed] [Google Scholar]
- 69.Chen H., Liu D., Ge L., Wang T., Ma Z., Han Y., Duan Y., Xu X., Liu W., Yuan J., et al. Catestatin prevents endothelial inflammation and promotes thrombus resolution in acute pulmonary embolism in mice. Biosci. Rep. 2019;39:BSR20192236. doi: 10.1042/BSR20192236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amiche M., Ladram A., Nicolas P. A consistent nomenclature of antimicrobial peptides isolated from frogs of the subfamily Phyllomedusinae. Peptides. 2008;29:2074–2082. doi: 10.1016/j.peptides.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 71.Nicolas P., El Amri C. The dermaseptin superfamily: A gene-based combinatorial library of antimicrobial peptides. Biochim. Biophys. Acta. 2009;1788:1537–1550. doi: 10.1016/j.bbamem.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Mor A., Nguyen V.H., Delfour A., Miglioresamour D., Nicolas P. Isolation, Amino-Acid-Sequence, and Synthesis of Dermaseptin, a Novel Antimicrobial Peptide of Amphibian Skin. Biochemistry. 1991;30:8824–8830. doi: 10.1021/bi00100a014. [DOI] [PubMed] [Google Scholar]
- 73.Amiche M., Ducancel F., Lajeunesse E., Boulain J.C., Menez A., Nicolas P. Molecular cloning of a cDNA encoding the precursor of adenoregulin from frog skin. Relationships with the vertebrate defensive peptides, dermaseptins. Biochem. Biophys. Res. Commun. 1993;191:983–990. doi: 10.1006/bbrc.1993.1314. [DOI] [PubMed] [Google Scholar]
- 74.Amiche M., Ducancel F., Mor A., Boulain J.C., Menez A., Nicolas P. Precursors of vertebrate peptide antibiotics dermaseptin b and adenoregulin have extensive sequence identities with precursors of opioid peptides dermorphin, dermenkephalin, and deltorphins. J. Biol. Chem. 1994;269:17847–17852. doi: 10.1016/S0021-9258(17)32386-4. [DOI] [PubMed] [Google Scholar]
- 75.Mor A., Amiche M., Nicolas P. Structure, synthesis, and activity of dermaseptin b, a novel vertebrate defensive peptide from frog skin: Relationship with adenoregulin. Biochemistry. 1994;33:6642–6650. doi: 10.1021/bi00187a034. [DOI] [PubMed] [Google Scholar]
- 76.Daly J.W., Caceres J., Moni R.W., Gusovsky F., Moos M., Seamon K.B., Milton K., Myers C.W. Frog Secretions and Hunting Magic in the Upper Amazon—Identification of a Peptide That Interacts with an Adenosine Receptor. Proc. Natl. Acad. Sci. USA. 1992;89:10960–10963. doi: 10.1073/pnas.89.22.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pouny Y., Rapaport D., Mor A., Nicolas P., Shai Y. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry. 1992;31:12416–12423. doi: 10.1021/bi00164a017. [DOI] [PubMed] [Google Scholar]
- 78.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta. 1999;1462:55–70. doi: 10.1016/S0005-2736(99)00200-X. [DOI] [PubMed] [Google Scholar]
- 79.Bechinger B. The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochim. Biophys. Acta. 1999;1462:157–183. doi: 10.1016/S0005-2736(99)00205-9. [DOI] [PubMed] [Google Scholar]
- 80.Zasloff M. Antimicrobial peptides in health and disease. N. Engl. J. Med. 2002;347:1199–1200. doi: 10.1056/NEJMe020106. [DOI] [PubMed] [Google Scholar]
- 81.Lequin O., Bruston F., Convert O., Chassaing G., Nicolas P. Helical structure of dermaseptin B2 in a membrane-mimetic environment. Biochemistry. 2003;42:10311–10323. doi: 10.1021/bi034401d. [DOI] [PubMed] [Google Scholar]
- 82.Amiche M., Galanth C. Dermaseptins as models for the elucidation of membrane-acting helical amphipathic antimicrobial peptides. Curr. Pharm. Biotechnol. 2011;12:1184–1193. doi: 10.2174/138920111796117319. [DOI] [PubMed] [Google Scholar]
- 83.Van Zoggel H., Carpentier G., Dos Santos C., Hamma-Kourbali Y., Courty J., Amiche M., Delbe J. Antitumor and angiostatic activities of the antimicrobial peptide dermaseptin B2. PLoS ONE. 2012;7:e44351. doi: 10.1371/journal.pone.0044351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Van Zoggel H., Hamma-Kourbali Y., Galanth C., Ladram A., Nicolas P., Courty J., Amiche M., Delbe J. Antitumor and angiostatic peptides from frog skin secretions. Amino Acids. 2012;42:385–395. doi: 10.1007/s00726-010-0815-9. [DOI] [PubMed] [Google Scholar]
- 85.Dos Santos C., Hamadat S., Le Saux K., Newton C., Mazouni M., Zargarian L., Miro-Padovani M., Zadigue P., Delbe J., Hamma-Kourbali Y., et al. Studies of the antitumor mechanism of action of dermaseptin B2, a multifunctional cationic antimicrobial peptide, reveal a partial implication of cell surface glycosaminoglycans. PLoS ONE. 2017;12:e0182926. doi: 10.1371/journal.pone.0182926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Couty M., Dusaud M., Miro-Padovani M., Zhang L., Zadigue P., Zargarian L., Lequin O., de la Taille A., Delbe J., Hamma-Kourbali Y., et al. Antitumor Activity and Mechanism of Action of Hormonotoxin, an LHRH Analog Conjugated to Dermaseptin-B2, a Multifunctional Antimicrobial Peptide. Int. J. Mol. Sci. 2021;22:11303. doi: 10.3390/ijms222111303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Medeiros K.A., Joanitti G.A., Silva L.P. Chitosan nanoparticles for dermaseptin peptide delivery toward tumor cells in vitro. Anticancer Drugs. 2014;25:323–331. doi: 10.1097/CAD.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 88.Shi D., Hou X., Wang L., Gao Y., Wu D., Xi X., Zhou M., Kwok H.F., Duan J., Chen T., et al. Two Novel Dermaseptin-Like Antimicrobial Peptides with Anticancer Activities from the Skin Secretion of Pachymedusa dacnicolor. Toxins. 2016;8:144. doi: 10.3390/toxins8050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang L., Chen D., Wang L., Lin C., Ma C., Xi X., Chen T., Shaw C., Zhou M. Dermaseptin-PH: A Novel Peptide with Antimicrobial and Anticancer Activities from the Skin Secretion of the South American Orange-Legged Leaf Frog, Pithecopus (Phyllomedusa) hypochondrialis. Molecules. 2017;22:1805. doi: 10.3390/molecules22101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Long Q., Li L., Wang H., Li M., Wang L., Zhou M., Su Q., Chen T., Wu Y. Novel peptide dermaseptin-PS1 exhibits anticancer activity via induction of intrinsic apoptosis signalling. J. Cell Mol. Med. 2019;23:1300–1312. doi: 10.1111/jcmm.14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan Y., Chen X., Ma C., Xi X., Wang L., Zhou M., Burrows J.F., Kwok H.F., Chen T. Biological Activities of Cationicity-Enhanced and Hydrophobicity-Optimized Analogues of an Antimicrobial Peptide, Dermaseptin-PS3, from the Skin Secretion of Phyllomedusa sauvagii. Toxins. 2018;10:320. doi: 10.3390/toxins10080320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen D., Zhou X., Chen X., Huang L., Xi X., Ma C., Zhou M., Wang L., Chen T. Evaluating the Bioactivity of a Novel Antimicrobial and Anticancer Peptide, Dermaseptin-PS4(Der-PS4), from the Skin Secretion of Phyllomedusa sauvagii. Molecules. 2019;24:2974. doi: 10.3390/molecules24162974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dong Z., Hu H., Yu X., Tan L., Ma C., Xi X., Li L., Wang L., Zhou M., Chen T., et al. Novel Frog Skin-Derived Peptide Dermaseptin-PP for Lung Cancer Treatment: In vitro/vivo Evaluation and Anti-tumor Mechanisms Study. Front. Chem. 2020;8:476. doi: 10.3389/fchem.2020.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Z., Xi X., Lu Y., Hu H., Dong Z., Ma C., Wang L., Zhou M., Chen T., Du S., et al. In vitro activities of a novel antimicrobial peptide isolated from phyllomedusa tomopterna. Microb. Pathog. 2021;153:104795. doi: 10.1016/j.micpath.2021.104795. [DOI] [PubMed] [Google Scholar]