Abstract
Periodontitis, a chronic, inflammatory disease, induces systemic inflammation and contributes to the development of neurodegenerative diseases. The precise etiology of the most common neurodegenerative disorders, such as sporadic Alzheimer’s, Parkinson’s diseases and multiple sclerosis (AD, PD, and MS, respectively), remains to be revealed. Chronic neuroinflammation is a well-recognized component of these disorders, and evidence suggests that systemic inflammation is a possible stimulus for neuroinflammation development. Systemic inflammation can lead to deleterious consequences on the brain if the inflammation is sufficiently severe or if the brain shows vulnerabilities due to genetic predisposition, aging, or neurodegenerative diseases. It has been proposed that periodontal disease can initiate or contribute to the AD pathogenesis through multiple pathways, including key periodontal pathogens. Dysbiotic oral bacteria can release bacterial products into the bloodstream and eventually cross the brain-blood barrier; these bacteria can also cause alterations to gut microbiota that enhance inflammation and potentially affect brain function via the gut–brain axis. The trigeminal nerve has been suggested as another route for connecting oral bacterial products to the brain. PD and MS are often preceded by gastrointestinal symptoms or aberrant gut microbiome composition, and alterations in the enteric nervous system accompany the disease. Clinical evidence has suggested that patients with periodontitis are at a higher risk of developing PD and MS. This nexus among the brain, periodontal disease, and systemic inflammation heralds new ways in which microglial cells, the main innate immune cells, and astrocytes, the crucial regulators of innate and adaptive immune responses in the brain, contribute to brain pathology. Currently, the lack of understanding of the pathogenesis of neurodegeneration is hindering treatment development. However, we may prevent this pathogenesis by tackling one of its possible contributors (periodontitis) for systemic inflammation through simple preventive oral hygiene measures.
Keywords: inflammation, dysbiosis, neurodegeneration, oral microbiome, periodontal disease, oral hygiene
Introduction
The incidence of periodontal disease increases with age, and in the United States, 70.1% of adults aged 65 y and older are diagnosed with some form of periodontal disease, making it the second most common oral ailment, after dental caries. Periodontitis is a severe form of periodontal disease that results in loss of tooth and damage of the jawbone structure if left untreated. The detrimental consequences of periodontitis are not limited to the oral cavity as an ever-growing number of studies show that both periodontal bacteria and inflammatory mediators induced by these bacteria can travel to distant organs and contribute to the development of various pathologies, including the brain. Periodontitis is triggered by certain Gram-negative anaerobic bacteria in dental plaque and their metabolites in addition to bacterial proteases (Kornman et al. 1997; Delima et al. 2002; Stathopoulou et al. 2010; Taguchi et al. 2015). The major periodontitis pathogens, including Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Prevotella intermedia, and Fusobacterium nucleatum, stimulate proinflammatory responses not only in oral cavity. The effects of these key periodontitis pathogens on inflammation and immune responses at systemic levels have been summarized in Table 1. Animals infected with T. forsythia or P. gingivalis exhibited significant elevations of specific IgG and IgM in the serum (Velsko et al. 2014; Chukkapalli et al. 2015). Importantly, oral epithelial cells exposed to repeated assaults by bacterial toxins, such as lipopolysaccharide (LPS) and gingipain (cysteine protease secreted by P. gingivalis), secrete proinflammatory cytokines, including tumor necrosis factor α (TNF-α), interleukins 1β (IL-1β) and 6 (IL-6), interferon γ (INF-γ), and prostaglandin E2 (PGE2), that trigger the cascade of molecular events eventually leading to gingival cell death. The cytokines can also be disseminated via the bloodstream, thus leading to systemic inflammation and triggering inflammatory responses in distant organs, including the brain. Many studies, whether epidemiological, postmortem, or those performed in in vivo models, have found an association between systemic inflammation and neurodegeneration (Perry et al. 2007; Pott Godoy et al. 2008; Londoño and Cadavid 2010).
Table 1.
Systemic Inflammation and Immune Responses Induced by Periodontitis Pathogens.
| Periodontitis Pathogen | Impacts beyond Oral Cavity | Diseases/Models |
|---|---|---|
| Aggregatibacter actinomycetemcomitans | Serum IFN-γ elevation associated with enhanced dental plaque load withA. actinomycetemcomitans; presence associated with increased population of CD3−/CD16+ (NK lymphocytes) in patients’ blood (Andrukhov et al. 2011) | Periodontitis patients |
| Porphyromonas gingivalis | Serum TNF-α elevation associated with enhanced dental plaque load with P. gingivalis (Andrukhov et al. 2011) P. gingivalis infection increased shedding of human umbilical vein endothelial cells; upregulation in proinflammatory cytokines TNF-α, IL-6, and IL-8 (Bugueno et al. 2020) Increased inflammation within the deep connective tissue (Delima et al. 2002) Significantly elevated P. gingivalis–specific IgG and IgM antibodies; P. gingivalis genomic data detected in hearts, aorta, spleens, and kidneys (Velsko et al. 2014) |
Periodontitis patients In vitro Nonhuman primate with periodontitis ApoE-null mice orally infected with P. gingivalis |
| Tannerella forsythia | Elevated levels of serum IgG and IgM antibodies; increased serum amyloid A and significantly reduced serum nitric oxide; significantly increased serum lipoproteins (Chukkapalli et al. 2015) | Mice orally infected withT. forsythia |
| Treponema denticola | Presence associated with increased CD3+/CD8+ cells (cytotoxic T lymphocytes) in patients’ blood (Andrukhov et al. 2011) AD patients had at least 1 Treponema species detected in the brain cortex than non-AD donors (χ2 = 11.99, P < 0.001) in PCR. Treponema was also detected in trigeminal ganglia of 3 AD and 2 control donors (Riviere et al. 2002). |
Periodontitis patients Frontal lobe cortex of human donors with AD and controls |
| Prevotella intermedia | Prevotella significantly increased in β-amyloid–positive subjects (Kamer et al. 2021) | Human subgingival periodontal bacteria and cerebrospinal fluid amyloid biomarker |
| Fusobacterium nucleatum | Ventriculitis and brain abscess due to F. nucleatum infection in a man with no significant predisposing factors (Kai et al. 2008) Caused the host to produce inflammatory factors and recruit inflammatory cells; induced immune suppression of gut mucosa by suppressing the function of immune cells (Wu et al. 2019) Promoted intestinal inflammation, increased immune cell infiltration, and depleted mucus layers (Engevik et al. 2021) |
Clinical case report Patients with colorectal cancers Mice harboring a human microbiome |
AD, Alzheimer’s disease; IL, interleukin; INF-γ, interferon γ; NK, natural killer; PCR, polymerase chain reaction; TNF-α, tumor necrosis factor α.
Alzheimer’s and Parkinson’s diseases (AD and PD, respectively) are the most prevalent neurodegenerative diseases, and their incidence is increasing over time. Despite decades of research and elucidated molecular pathological events and symptoms, the etiology of many neurodegenerative disorders remains unknown. At present, no effective disease-modifying drugs for these disorders exist, and once the neurodegenerative process begins, it will progress, leading to an increase in neuronal death and loss of synapses that are clinically manifested as cognitive or physical decline. Aducanumab, a new drug recently approved by the Food and Drug Administration (FDA) for AD targets β-amyloid (Aβ) aggregates, has provided ambiguous clinical results (Mullard 2021). Therefore, it is of utmost importance to identify more preventable risk factors to prevent, slow down, or delay neurodegeneration.
It is widely agreed that chronic neuroinflammation may be the initial molecular pathologies that lead to the neuronal demise in neurodegenerative disorders. The questions that remain to be answered are what starts and then perpetuates neuroinflammation and whether it can be prevented. In recent years, periodontitis has become the focus of research aimed at understanding the root cause of chronic neuroinflammation due to its known role in causing systemic inflammation and its proximity to the central nervous system (CNS). Theoretically, these factors can contribute to the development of inflammation in the brain and then possibly lead to neurodegeneration. In the current review, we present the newest findings regarding the connection and underlying mechanisms between periodontitis and neurodegenerative diseases linked through neuroinflammation in the hopes of understanding the mechanisms of these interactions.
Periodontitis and Neuroinflammation
Neuroinflammation is a result of an innate immune response in the brain with 2 forms: 1) acute form, characterized by a transient expression of the inflammatory mediators, and (2) chronic, during which the resolution phase of inflammation is significantly delayed. The latter process is a continuous, low-grade inflammation accomplished by prolonged secretion of proinflammatory cytokines by glial cells that over time contribute to neuronal cell death. When the systemic inflammatory stimulus is not resolved timely, it leads to a state in which constantly activated microglia become overly sensitive to a new immune trigger and respond in an exaggerated way (Perry and Holmes 2014). Studies have demonstrated that Toll-like receptor 4 (TLR4) activation by LPS in astrocytes and microglia induced a proinflammatory signal in the brain (Gorina et al. 2011; Chen et al. 2012).
Patients with periodontitis could have neuroinflammation due to sustained systemic inflammation. C-reactive protein (CRP), a marker of systemic inflammation, has also been shown to be significantly higher in the serum of periodontal disease patients (Goyal et al. 2014; Chang et al. 2020). Likewise, a study identified a correlation between higher proportions of certain periodontal bacteria in the dental plaques of patients with periodontitis and increased levels of specific proinflammatory cytokines and changes in the types of lymphocytes in their serum. Increases of A. actinomycetemcomitans were associated with significantly increased serum levels of IFN-γ while a high P. gingivalis load was associated with an increase in serum levels of TNF-α (Andrukhov et al. 2011). This finding suggests that predominance of certain periodontal bacteria is associated with different subsets of immune cells in the peripheral blood of the periodontitis patients since different immune cells produce different types of cytokines. The fact that periodontitis induces systemic inflammation and leads to increased levels of proinflammatory cytokines in the serum together with microglial priming make periodontitis a likely source of chronic neuroinflammation.
The CNS is protected by the blood–brain barrier (BBB), but extended exposure to noxious species can disrupt and increase the BBB permeability, allowing them to reach the brain through a systemic route or a cranial route (Table 2). In addition, the peripheral proinflammatory cytokines may activate the vagus nerve (Capuron and Miller 2011), which then relays the information to the CNS. Leptomeningeal cells, present in the innermost layer of the meninges, may transduce the peripheral inflammatory signals from macrophages to microglia (Liuet al. 2013; Wu et al. 2019). Various types of infectious agents, including the keystone periodontal pathogens, P. gingivalis and T. denticola, have been found in postmortem brain tissue obtained from AD patients, which provide a link between bacteremia and neurodegeneration (Riviere et al. 2002; Poole et al. 2013; Dominy et al. 2019).
The link between periodontitis and neuroinflammation observed in human subjects is supported by animal studies. Systemic inflammation induced by peripheral injection of bacterial LPS leads to expression of proinflammatory cytokines, such as TNF-α and IL-1β in the rodent brain. Animal studies also show that age exacerbates the level of neuroinflammation and behavioral deficits in mice after peripheral administration of LPS in a manner similar to elderly patients who have cognitive deficits in addition to frequent systemic infections (Godbout et al. 2005). In a murine model of periodontitis in which a ligature was placed around the second maxillary molar, increased expression of proinflammatory cytokines was detected in the gingival tissue and in the brain, indicating that the periodontitis-induced inflammation can trigger the immune response in the brain and cause neuroinflammation (Furutama et al. 2020). Importantly, it has been demonstrated that even without infection of periodontitis pathogens, ligature-induced experimental periodontitis can affect the microglia and the brain’s cytokine profile in wild-type mice and cause a significant decrease in plaque-associated microglia in 5×FAD mice, a well-established mouse model of AD with rapid Aβ accumulation (Kantarci et al. 2020).
Table 2.
Routes for Pathobiological Substance Entry into the Brain.
| Routes | Mechanisms | References |
|---|---|---|
| Direct bacterial invasion | Bacteria and bacterial lipopolysaccharides can reach the CNS and activate matrix metalloproteases, which disrupt the BBB, increasing its permeability and allowing bacteria to penetrate the brain parenchyma. | Systemic route (Wright et al. 2007; Frister et al. 2014) |
| Pathogenic bacteria can enter the brain through the peripheral nerve route such as the olfactory and trigeminal nerves. | Cranial route (Riviere et al. 2002; Olsen and Singhrao 2015) | |
| Invasion of proinflammatory cytokines | The peripheral proinflammatory cytokines can activate the vagus nerve, which then relays the information to the CNS. | Neural pathways (Capuron and Miller 2011) |
| The inflammatory mediators can enter the cerebral parenchyma and initiate the inflammatory response through the region of the brain that lacks the BBB, like the choroid plexus and circumventricular organs. | Humoral pathways (D’Mello and Swain 2017) | |
| Activated peripheral monocytes can be actively recruited by the chemokine system into the brain parenchyma, where they secrete proinflammatory cytokines, such as TNF-α, which activate microglia, leading to neuroinflammation. | Cellular pathways (D’Mello and Swain 2017) | |
| Indirect communication | Leptomeningeal cells, present in the innermost layer of the meninges, transduce the peripheral inflammatory signals from macrophages to microglia in the brain via Toll-like receptor 2. | Leptomeningeal cells (Wu et al. 2005; Liu et al. 2013) |
BBB, blood–brain barrier; CNS, central nervous system; TNF-α, tumor necrosis factor α.
Another possible mechanism linking periodontitis and systemic inflammation is by disturbing the gut microbiome. Oral microorganisms and bacteria can be found in fecal samples of participants. The gut microbiome is critical in regulating multiple neurochemical pathways through a highly complicated host-microbiome system, termed the gut–brain axis, and any disruption that occurs could upset this homeostasis. The BBB and blood cerebrospinal fluid (CSF) barriers are important in regulating neuroinflammation. The gut can affect the BBB by using gastrointestinal (GI)–derived hormonal secretion, metabolic cofactors, and production of small molecules or through cytokine or oxidative stress and other inflammatory mechanisms that can affect BBB permeability (Main and Minter 2017; Lanza et al. 2018). Braak and Del Tredici (2008) had presented a staging system for PD based on the specific pattern of α-synuclein spread and postulated that sporadic PD begins in 2 places—the neurons of the nasal cavity and the neurons of the gut—and the pathology spreads by the olfactory tract and vagal nerve, respectively, to and within the CNS. The concept of “leaky gut–leaky brain” suggests that with age and under certain pathological conditions, bacterial molecular metabolites from the gut epithelial barrier can translocate or diffuse systematically or disseminate to a distal site by passing through the BBB or CSF barriers. Consequently, they may contribute to disease or modulate health immunologically or biochemically by both direct and indirect means (Main and Minter 2017; Lanza et al. 2018). Chronic periodontitis caused by microbial dysbiosis can lead to neuroinflammation and cognitive impairment through partial activation of the TLR4/myeloid differentiation primary response 88/nuclear factor–κβ signaling pathway (Xue et al. 2020), suggesting the oral–gut–brain axis. A recent study found that oral administration of P. gingivalis induced cognitive impairment and gut dysbiosis in mice (Chi et al. 2021). Importantly, this study showed that the oral gavage of P. gingivalis decreased the solute clearance function of the glymphatic system. The glymphatic dysfunction could lead to accumulations of metabolic wastes, including Aβ, and contribute to AD.
Periodontitis and Neurodegenerative Diseases
Alzheimer’s Disease
On a molecular level, AD is characterized by deposition ofβ-amyloid plaques and neurofibrillary tangles (NFTs). These inclusions are associated with progressive synaptic and neuronal loss, especially in learning and memory storage areas, such as the hippocampus and entorhinal cortex. Continuous neuronal demise leads to brain atrophy and cognitive impairment that results from this continuous process. Clinically, AD symptoms include memory deficits, negative impact on judgment and decision-making, lack of orientation to physical surroundings, and decline in language processing, among others. Cross-sectional analysis of a population-based study found that an increase in peripheral inflammatory markers, such as CRP, IL-1β and IL-6, and TNF-α, in AD patients is associated with an increase in the incidence of dementia in elderly patients (Gorelick 2010; Metti and Cauley 2012). In addition, activated complement cascade factors were found bound to Aβ plaques in the brains of AD patients, again indicating the involvement of the immune responses (Yasojima et al. 1999). These studies implicate systemic inflammation as a key element that may cause or exacerbate AD-related cognitive decline.
It is plausible that systemic inflammation induced by periodontitis could affect the neuronal environment in the brain and contribute to neuroinflammation. The proinflammatory mediators produced by inflamed gingiva may disseminate from the periodontal pockets and reach the brain via the bloodstream or directly via the trigeminal nerve, and dysbiotic oral bacteria can cause an imbalance in CNS homeostasis indirectly. Supporting evidence has been provided by postmortem histopathological examinations that detected P. gingivalis and T. denticola in addition to bacterial proteases in the brains and trigeminal nerves of AD patients, suggesting that these virulent species could have contributed to the development of AD pathology (Riviere et al. 2002; Poole et al. 2013; Dominy et al. 2019). Dominy et al. (2019) provided compelling evidence with the detection of P. gingivalis and its proteases in postmortem brain tissues and trigeminal nerves from AD patients and correlated its presence with tau pathology.
Further links between periodontitis and AD have been identified using AD transgenic mouse models, such as human amyloid protein precursor transgenic (hAPP-tg) and 5×FAD. Oral application of P. gingivalis mimicking the effects of periodontitis revealed significant impairment in cognitive function and an increase in Aβ deposition (Ishida et al. 2017; Kantarci et al. 2020). In addition, the levels of proinflammatory cytokines, such as TNF-α and IL-1β, were higher in the brains of P. gingivalis–treated transgenic mice compared to untreated mice (Ishida et al. 2017; Kantarci et al. 2020). This result suggests that periodontitis may directly exacerbate the pathology, neuroinflammation, and cognitive functions in AD patients. Analogous results have been obtained with wild-type mice in which gingipain was orally applied over the course of 22 wk to mimic the chronic effects of periodontitis (Ilievski et al. 2018). At the end of the study, gingipain was detected in the mice brain tissue, including glia and neurons, in addition to extracellularly, confirming translocation of oral bacteria into the brain. The same study also showed a correlation between gingipain-induced chronic periodontitis and neurodegeneration as visualized by Fluoro-Jade staining in treated versus untreated mice. Astro- and microgliosis and extracellular deposition of Aβ42 and NFTs were also detected in the treated group but not in the control group, strongly indicating a causative effect between periodontitis and AD-like pathology in wild-type mice (Ilievski et al. 2018). Other studies recorded learning deficits and impaired memory storage in both mouse and rat models of periodontitis in addition to greater memory deficits in older animals compared to younger controls (Ding et al. 2018; Hu et al. 2020). Overall, as summarized in Table 3, periodontitis induces systemic inflammation and correlates with cognitive decline in human subjects and in animal models. A direct causal connection between periodontitis and AD is underscored by the detection of periodontal bacteria in the brains of AD patients and the presence of AD pathology in murine models of periodontitis.
Table 3.
Association between Periodontitis and Neurodegenerative Diseases.
| Types of Study | Finding |
|---|---|
| 8,640 patients with dementia without prior periodontal disease and 8,640 individuals without dementia history | Dementia and AD were associated with a higher risk of periodontal disease dependent of age and independent of systemic confounding factors (Ma et al. 2022). |
| 48 elderly cognitively normal subjects | Higher subgingival periodontal dysbiosis was significantly associated with reduced CSF amyloid β at both genera and species levels (Kamer et al. 2021). |
| 5,396 cases of newly diagnosed PID, 10,792 cases without PID | Patients with PID had a higher risk of developing PD (adjusted hazard ratio = 1.431; 95% CI, 1.141–1.794; P = 0.002) (Chen et al. 2017). |
| Human cerebral cortex lysates of 3 AD and 6 control brains; CSF from 10 patients diagnosed with probable AD with mild to moderate cognitive impairment | Gingipains load were significantly higher in AD patients than control brains (P < 0.0001) and significantly correlated with tau pathology (P < 0.0001) and ubiquitin load (P < 0.0001) (Dominy et al. 2019). |
| 4,765 newly diagnosed cases of PD, 19,060 without PD | Dental scaling decreases the development of PD (Chen et al. 2018). |
| 74 PD patients and 74 controls | PD patients had weakened oral health status and reduced oral hygiene care (van Stiphout et al. 2018). |
| 316 patients diagnosed with MS and 1,580 randomly selected controls | MS patients were 1.86 times (95% CI, 1.39–2.48) more likely to have previously diagnosed chronic periodontitis than controls after adjusting for variables (Sheu and Lin 2013). |
| 153,165 PD participants with prescription for anti-PD medication | Competent dental care and toothbrush frequency significantly reduced risk of development of new-onset PD (Woo et al. 2020). |
| Young and middle-aged mice (control or infected) with live Porphyromonas gingivalis ATCC 33277 by oral gavage | P. gingivalis can impair spatial learning and memory, with significant increased inflammatory cytokines in brain tissues of middle-aged mice but not in young mice (P < 0.01) (Ding et al. 2018). |
| FVB/N mice and mutant LRRK2 mice (transgenic R1441G PD model) with and without oral gavage of P. gingivalis | P. gingivalis increased gut inflammation and permeability in PD mice. α-Synuclein was higher in the myenteric plexus of colon in P. gingivalis–treated PD mice (Feng et al. 2020). |
| Wild-type C57/BL6 mice with ligature | Periodontal inflammation-induced IL-6 led to neuroinflammation and disrupted the BBB (Furutama et al. 2020). |
| Young male Sprague-Dawley rats periodontitis models | Periodontitis induced by P. gingivalis–LPS led to neuroinflammation and impaired learning and memory in rats (Hu et al. 2020). |
| Periodontitis mice induced by oral application of Pg/gingipain and control | P. gingivalis/gingipain led to brain inflammation, neurodegeneration, and amyloid β production in wild-type mice (Ilievski et al. 2018). |
| Oral inoculation of P. gingivalis in APP-Tg AD mice | Periodontitis induced by bacterial infection exacerbated features of AD in transgenic mice (Ishida et al. 2017; Hao et al. 2022). |
| Ligature-induced periodontal disease on 5×FAD mice and wild-type mice | Ligature-induced periodontitis increased neuroinflammation in wild-type mice and disrupted the neuroinflammatory response in 5×FAD mice (Kantarci et al. 2020). |
| Oral administration of P. gingivalis 3×/wk for 1 mo in mice | P. gingivalis impaired cognition associated with gut dysbiosis, neuroinflammation, and glymphatic dysfunction (Chi et al. 2021). |
| MS mouse model received control, subcutaneous, or oral gavage of P. gingivalis | Infection with P. gingivalis had significantly greater maximal clinical scores of autoimmune encephalomyelitis compared to control (Polak et al. 2018). |
| CP induced with ligature and control | CP mice exhibited significant neuronal loss in cortex, reduction of synaptophysin in hippocampus and cortex, increase of proinflammatory cytokine levels, disruption of the BBB, gut microbiota dysbiosis, and systemic inflammation (Xue et al. 2020). |
AD, Alzheimer’s disease; BBB, blood–brain barrier; CI, confidence interval; CP, chronic periodontitis; CSF, cerebrospinal fluid; IL-6, interleukin-6; LPS, lipopolysaccharide; MS, multiple sclerosis; PD, Parkinson’s disease; PID, periodontal inflammatory disease.
Parkinson’s Disease
PD is a progressive neurodegenerative disorder characterized by deterioration of dopaminergic neurons in the substantia nigra pars compacta that is associated with the presence of Lewy body inclusions composed mainly of α-synuclein. PD mostly affects motor neurons and results in adverse symptoms, such as tremor, rigidity, bradykinesia, or involuntary movement, among others, which are related to movement. PD patients can also suffer from cognitive impairment (Dickson 2012). Neuroinflammation has been long implicated in PD etiology in addition to a number of environmental and genetic factors. Neuroinflammation in PD has mostly been demonstrated by activated microglia, which are the main immune cells in the brain and produce cytokines; increased levels of cytokines have been detected in postmortem brains of PD patients. These cytokines include IL-1β, IL-6, IL-8, IL-10, IL-12, IL-15, and TNF-α. Activated microglia may lead to neurotoxicity by causing an increase in the levels of toxic reactive oxygen species (ROS) that interfere with the function of several proteins and affect cellular homeostasis.
PD patients with increased tremor or cognitive impairment experience difficulty maintaining oral hygiene and are thus at increased risk of oral dysbiosis and oral disease (van Stiphout et al. 2018). The influence of PD on periodontitis is well accepted, while studies that link periodontitis to PD have started to surface only recently. More direct evidence from a retrospective matched-cohort study by Chen et al. (2017) established that patients with periodontitis are at a higher risk of developing PD. A subsequent population-based cohort study from the same group noted that dental scaling over 5 consecutive years has a protective effect against development and progression of PD in patients with and without periodontal disease (Chen et al. 2018). Another group found a positive correlation between an increase in tooth loss and the development of new-onset PD in a longitudinal study (Woo et al. 2020). Studies reported by these groups showed for the first time that oral dysbiosis and poor oral health might predispose patients to developing PD. A further association between periodontal disease and PD has been provided by Adams et al. (2019), who detected that gingipain R1 (RgpA), a protease produced byP. gingivalis, was present in the clots from blood samples of PD patients to a significantly higher extent than in the clots obtained from healthy patients. The study proved the clotting ability of RgpA and LPS by showing that incubating recombinant gingipain with fibrinogen leads to hypercoagulation, confirming that the clots detected in PD patients are most likely induced by bacterial pathogens. The group also confirmed the presence of systemic inflammation by demonstrating an increase in the levels of inflammatory cytokines in PD patients (Adams et al. 2019).
One of the latest studies investigating the involvement of dysbiotic periodontal bacteria in PD used a known PD mouse model, the leucine-rich repeat kinase 2 (LRRK2) R1441G. The R1441G mutation in the LRRK2 gene results in late-onset PD. To mimic chronic periodontitis, P. gingivalis was administered to LRRK2 R1441G mice orally over the course of 1 mo. The treatment increased α-synuclein in the myenteric plexus of the colon, decreased the number of dopaminergic neurons in the substantia nigra, and increased the number of activated microglia (Feng et al. 2020). This study also revealed another possible mechanism by which periodontitis can lead to neuroinflammation. According to these data, periodontal bacteria can negatively affect the epithelial lining in the gut, causing the cells to secrete proinflammatory cytokines that can then enter the bloodstream and reach the brain, where they contribute to neuroinflammation. Recent studies just have begun to uncover the mechanisms behind this process and answer the question of whether there is any causality between periodontitis and PD, but as of now, more studies are needed to provide conclusive evidence.
Multiple Sclerosis
Multiple sclerosis (MS) is a progressive neurodegenerative disorder in which deterioration of myelin sheaths is followed by axonal injury in the CNS that then results in number of sensory, motor, and cognitive impairments. Although the trigger for MS relapse is unknown, it is considered an autoimmune inflammatory disease in which a combination of environmental and genetic factors can cause CNS antigens, such as myelin basic protein (MBP), to be targeted by the immune system, which then leads to demyelination, axonal loss, gliosis, and inflammation.
A study by Moreno et al. (2011) used Lewis rats to develop an experimental autoimmune encephalomyelitis (EAE) model in which the rats were injected intraperitoneally with LPS to investigate whether systemic inflammatory stimulus can lead to an enhanced axonal damage. The study recorded microglial activation, increased expression of inducible nitric oxide synthase (iNOS), and IL-1β in addition to an increase in axonal injury by which an association between peripheral inflammation and increased levels of circulating cytokines with deterioration of axons in the rodent model of MS was shown (Moreno et al. 2011). Polak et al. (2018) studied a mouse model of MS by exposure to myelin oligodentrocyte glycoprotein (MOG), in which either subcutaneous or oral infection with P. gingivalis aggravated MS pathology with an increase in proliferation of lymphocytes and clinical symptoms, such as weakness of the limbs and tail, palsy, and other signs of disease. Both experiments suggest that pathological MS symptoms might be related to peripheral inflammation due to the presence of periodontal bacteria.
A case control study conducted in a Taiwanese population found an association between chronic periodontitis and MS in women but not in men (Sheu and Lin 2013). A similar study conducted in the Norwegian population found no significant correlation between periodontitis and MS (Gustavsen et al. 2015). More population-based studies are required to answer whether an association between periodontitis and MS exists in general or whether the association is ethnicity and gender dependent.
Conclusions and Perspective
The incidence of these neurodegenerative diseases is on the rise, and no treatment that would prevent or reverse the pathology once it has already taken place has been found. Identifying preventable risk factors is the best and, most likely, the only strategy. Nevertheless, chronic neuroinflammation is an important factor contributing to the development and progression of neurodegenerative diseases that are not hereditary. It has also been elucidated that neuroinflammation is associated with peripheral inflammation as repeated systemic infections and inflammatory insults cause exacerbation of the existing pathology in addition to causing an increase in the incidence of developing the neuropathology and cognitive deficits in human subjects and animal models. We summarized the clinical and in vivo studies supporting the correlation between periodontitis and the 3 neurodegenerative diseases (Table 3). Current animal studies suggested that periodontitis may be a causal factor for the neurodegeneration. Of note, most of the studies were conducted in animal models using P. gingivalis, which is not part of murine normal flora. However, P. gingivalis ATCC 33277, a genetically identical strain originally isolated from an adult periodontitis patient strain, is the most commonly used strain in the mouse model and can induce alveolar bone loss in mice similar to humans. Moreover, P. gingivalis–induced periodontitis models are especially informative in examining downstream events related to the host immune reaction (Graves et al. 2008). More clinical studies using human oral and brain samples are warranted, and at the same time, a mouse periodontitis model with a humanized oral bacterial community will be of significant value.
Our current understanding of how systemic inflammation causes chronic neuroinflammation together with the newest data presented in this review allows us to elucidate 3 possible mechanisms by which oral pathogens can contribute to the development and progression of neurodegeneration (Fig.): 1) proinflammatory cytokines secreted by epithelial cells in the diseased periodontal pockets induced by the toxic products of dysbiotic oral bacteria, such as P. gingivalis, can reach the brain parenchyma via the bloodstream. Once in the brain, the cytokines activate the resident immune cells (microglia and astrocytes) in the brain by inducing them to obtain proinflammatory phenotypes and secrete their own proinflammatory mediators, such as TNF-α. These mediators activate the signal transduction pathways that lead to neuronal apoptosis. If this process proceeds for a long period of time, the extent of neuronal cell death manifests as neurodegeneration. 2) Pathogenic periodontal bacteria can induce dysbiosis in the gut, leading to its inflammation. Inflamed epithelial cells in the gut secrete proinflammatory mediators that travel through the bloodstream and induce neuroinflammation in the brain, as discussed above. 3) Bacteria can travel to the brain via the trigeminal nerve, and once in the brain parenchyma, the oral pathogens and their toxic products can directly induce neurotoxic effects. For example, the LPS or gingipain produced by the oral pathogens can interact with the microglia and astrocytes to initiate the cascade of events leading to neuronal cell death.
Figure.
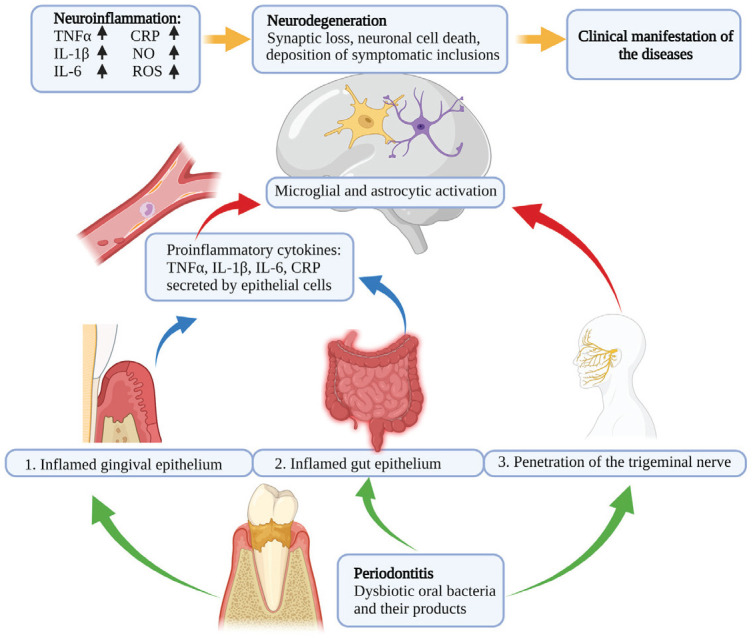
Periodontitis stimulates neuroinflammation, leading to neurodegenerative diseases. Oral pathogens contribute to manifestation of neurodegeneration through the following: (1) proinflammatory cytokines, which reach the brain parenchyma via the bloodstream; (2) oral–gut–brain axis; and (3) bacteria that travel to the brain via the trigeminal nerve.
These findings provide a strong background and warrant future studies of the links between chronic periodontitis and chronic neurodegenerative diseases, especially considering the high incidence and severe detrimental effects these conditions have on the general population. Revealing the mechanisms and types of pathogenic periodontal bacteria and bacterial products that activate astrocytes and microglial cells may provide new therapeutic targets for the prevention and treatment of neurodegenerative diseases. For now, the knowledge we gathered for this article should enable us to make vulnerable patients aware that adequate oral care can go beyond preventing disorders of the oral cavity and inspire more research on this topic. Prevention and treatment of periodontitis and its related inflammation could be a potential therapeutic strategy, at least in combination with other remedies, to reduce neuroinflammation and prevent and treat neurodegenerative diseases.
Author Contributions
X. Li, contributed to conception, drafted manuscript, critically revised the manuscript; M. Kiprowska, T. Kansara, P. Kansara, P. Li, contributed to data acquisition, drafted the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Dr. Deepak Saxena for his effort and time on proofreading this manuscript. The figure was created with BioRender.com.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: X. Li is the cofounder of Periomics Care LLC.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by National Institutes of Health grants R01DE02707401A1S1 and R01AG068857.
ORCID iDs: X. Li  https://orcid.org/0000-0002-7414-5734
https://orcid.org/0000-0002-7414-5734
P. Kansara  https://orcid.org/0000-0002-9268-2402
https://orcid.org/0000-0002-9268-2402
References
- Adams B, Nunes JM, Page MJ, Roberts T, Carr J, Nell TA, Kell DB, Pretorius E. 2019. Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front Aging Neurosci. 11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrukhov O, Ulm C, Reischl H, Nguyen PQ, Matejka M, Rausch-Fan X. 2011. Serum cytokine levels in periodontitis patients in relation to the bacterial load. J Periodontol. 82(6):885–892. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. 2008. Invited article: nervous system pathology in sporadic Parkinson disease. Neurology. 70(20):1916–1925. [DOI] [PubMed] [Google Scholar]
- Bugueno IM, Zobairi El-Ghazouani F, Batool F, El Itawi H, Anglès-Cano E, Benkirane-Jessel N, Toti F, Huck O. 2020. Porphyromonas gingivalis triggers the shedding of inflammatory endothelial microvesicles that act as autocrine effectors of endothelial dysfunction. Sci Rep. 10(1):1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Miller AH. 2011. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 130(2):226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Kim AR, Pi SH, You HK. 2020. A study on the correlation between C-reactive protein concentration and teeth with a ≥5 mm periodontal pocket in chronic periodontitis patients. Int J Dent. 2020:8832186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Huang JY, Wu YT, Chang YC. 2018. Dental scaling decreases the risk of Parkinson’s disease: a nationwide population-based nested case-control study. Int J Environ Res Public Health. 15(8):1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Wu YT, Chang YC. 2017. Periodontal inflammatory disease is associated with the risk of Parkinson’s disease: a population-based retrospective matched-cohort study. PeerJ. 5:e3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD. 2012. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 32(34):11706–11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Cheng X, Lin L, Yang T, Sun J, Feng Y, Liang F, Pei Z, Teng W. 2021. Porphyromonas gingivalis–induced cognitive impairment is associated with gut dysbiosis, neuroinflammation and glymphatic dysfunction. Front Cell Infect Microbiol. 11:755925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli SS, Rivera-Kweh MF, Velsko IM, Chen H, Zheng D, Bhattacharyya I, Gangula PR, Lucas AR, Kesavalu L. 2015. Chronic oral infection with major periodontal bacteria Tannerella forsythia modulates systemic atherosclerosis risk factors and inflammatory markers. Pathog Dis. 73(3):ftv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delima AJ, Karatzas S, Amar S, Graves DT. 2002. Inflammation and tissue loss caused by periodontal pathogens is reduced by interleukin-1 antagonists.J Infect Dis. 186(4):511–516. [DOI] [PubMed] [Google Scholar]
- Dickson DW. 2012. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med. 2(8):a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Ren J, Yu H, Yu W, Zhou Y. 2018. Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immun Ageing. 15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello C, Swain MG. 2017. Immune-to-brain communication pathways in inflammation-associated sickness and depression. Curr Top Behav Neurosci. 31:73–94. [DOI] [PubMed] [Google Scholar]
- Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, et al. 2019. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 5(1):eaau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engevik MA, Danhof HA, Ruan W, Engevik AC, Chang-Graham AL, Engevik KA, Shi Z, Zhao Y, Brand CK, Krystofiak ES, et al. 2021. Fusobacterium nucleatum secretes outer membrane vesicles and promotes intestinal inflammation. mBio. 12(2):e02706–e02720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YK, Wu QL, Peng YW, Liang FY, You HJ, Feng YW, Li G, Li XJ, Liu SH, Li YC, et al. 2020. Oral p. Gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice. J Neuroinflammation. 17(1):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frister A, Schmidt C, Schneble N, Brodhun M, Gonnert FA, Bauer M, Hirsch E, Müller JP, Wetzker R, Bauer R. 2014. Phosphoinositide 3-kinase γ affects LPS-induced disturbance of blood–brain barrier via lipid kinase-independent control of cAMP in microglial cells. Neuromolecular Med. 16(4):704–713. [DOI] [PubMed] [Google Scholar]
- Furutama D, Matsuda S, Yamawaki Y, Hatano S, Okanobu A, Memida T, Oue H, Fujita T, Ouhara K, Kajiya M, et al. 2020. IL-6 induced by periodontal inflammation causes neuroinflammation and disrupts the blood-brain barrier. Brain Sci. 10(10):679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. 2005. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 19(10):1329–1331. [DOI] [PubMed] [Google Scholar]
- Gorelick PB. 2010. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann N Y Acad Sci. 1207:155–162. [DOI] [PubMed] [Google Scholar]
- Gorina R, Font-Nieves M, Marquez-Kisinousky L, Santalucia T, Planas AM. 2011. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 59(2):242–255. [DOI] [PubMed] [Google Scholar]
- Goyal L, Bey A, Gupta ND, Sharma VK. 2014. Comparative evaluation of serum C-reactive protein levels in chronic and aggressive periodontitis patients and association with periodontal disease severity. Contemp Clin Dent. 5(4):484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. 2008. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 35(2):89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsen MW, Celius EG, Moen SM, Bjølgerud A, Berg-Hansen P, Nygaard GO, Sandvik L, Lie BA, Harbo HF. 2015. No association between multiple sclerosis and periodontitis after adjusting for smoking habits. Eur J Neurol. 22(3):588–590. [DOI] [PubMed] [Google Scholar]
- Hao X, Li Z, Li W, Katz J, Michalek SM, Barnum SR, Pozzo-Miller L, Saito T, Saido TC, Wang Q, et al. 2022. Periodontal infection aggravates C1q-mediated microglial activation and synapse pruning in Alzheimer’s mice. Front Immunol. 13:816640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li H, Zhang J, Zhang X, Xia X, Qiu C, Liao Y, Chen H, Song Z, Zhou W. 2020. Periodontitis induced by P. gingivalis-LPS is associated with neuroinflammation and learning and memory impairment in Sprague-Dawley rats. Front Neurosci. 14:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilievski V, Zuchowska PK, Green SJ, Toth PT, Ragozzino ME, Le K, Aljewari HW, O’Brien-Simpson NM, Reynolds EC, Watanabe K. 2018. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS One. 13(10):e0204941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N, Ishihara Y, Ishida K, Tada H, Funaki-Kato Y, Hagiwara M, Ferdous T, Abdullah M, Mitani A, Michikawa M, et al. 2017. Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. NPJ Aging Mech Dis. 3(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai A, Cooke F, Antoun N, Siddharthan C, Sule O. 2008. A rare presentation of ventriculitis and brain abscess caused by Fusobacterium nucleatum. J Med Microbiol. 57(Pt 5):668–671. [DOI] [PubMed] [Google Scholar]
- Kamer AR, Pushalkar S, Gulivindala D, Butler T, Li Y, Annam KRC, Glodzik L, Ballman KV, Corby PM, Blennow K, et al. 2021. Periodontal dysbiosis associates with reduced CSF Aβ42 in cognitively normal elderly. Alzheimers Dement (Amst). 13(1):e12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci A, Tognoni CM, Yaghmoor W, Marghalani A, Stephens D, Ahn J-Y, Carreras I, Dedeoglu A. 2020. Microglial response to experimental periodontitis in a murine model of Alzheimer’s disease. Sci Rep. 10(1):18561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornman KS, Page RC, Tonetti MS. 1997. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 14:33–53. [DOI] [PubMed] [Google Scholar]
- Lanza G, Bella R, Cantone M, Pennisi G, Ferri R, Pennisi M. 2018. Cognitive impairment and celiac disease: is transcranial magnetic stimulation a trait d’union between gut and brain? Int J Mol Sci. 19(8):2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wu Z, Zhang X, Ni J, Yu W, Zhou Y, Nakanishi H. 2013. Leptomeningeal cells transduce peripheral macrophages inflammatory signal to microglia in response to Porphyromonas gingivalis LPS. Mediators Inflamm. 2013:407562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londoño D, Cadavid D. 2010. Bacterial lipoproteins can disseminate from the periphery to inflame the brain. Am J Pathol. 176(6):2848–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma KS, Hasturk H, Carreras I, Dedeoglu A, Veeravalli JJ, Huang JY, Kantarci A, Wei JC. 2022. Dementia and the risk of periodontitis: a population-based cohort study. J Dent Res. 101(3):270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main BS, Minter MR. 2017. Microbial immuno-communication in neurodegenerative diseases. Front Neurosci. 11:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metti AL, Cauley JA. 2012. How predictive of dementia are peripheral inflammatory markers in the elderly? Neurodegener Dis Manag. 2(6):609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno B, Jukes JP, Vergara-Irigaray N, Errea O, Villoslada P, Perry VH, Newman TA. 2011. Systemic inflammation induces axon injury during brain inflammation. Ann Neurol. 70(6):932–942. [DOI] [PubMed] [Google Scholar]
- Mullard A. 2021. Controversial Alzheimer’s drug approval could affect other diseases. Nature. 595(7866):162–163. [DOI] [PubMed] [Google Scholar]
- Olsen I, Singhrao SK. 2015. Can oral infection be a risk factor for Alzheimer’s disease? J Oral Microbiol. 7:29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. 2007. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 7(2):161–167. [DOI] [PubMed] [Google Scholar]
- Perry VH, Holmes C. 2014. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 10(4):217–224. [DOI] [PubMed] [Google Scholar]
- Polak D, Shmueli A, Brenner T, Shapira L. 2018. Oral infection with P. gingivalis exacerbates autoimmune encephalomyelitis. J Periodontol. 89(12):1461–1466. [DOI] [PubMed] [Google Scholar]
- Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. 2013. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis. 36(4):665–677. [DOI] [PubMed] [Google Scholar]
- Pott Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. 2008. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain. 131(Pt 7):1880–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere GR, Riviere KH, Smith KS. 2002. Molecular and immunological evidence of oral treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol. 17(2):113–118. [DOI] [PubMed] [Google Scholar]
- Sheu JJ, Lin HC. 2013. Association between multiple sclerosis and chronic periodontitis: a population-based pilot study. Eur J Neurol. 20(7):1053–1059. [DOI] [PubMed] [Google Scholar]
- Stathopoulou PG, Benakanakere MR, Galicia JC, Kinane DF. 2010. Epithelial cell pro-inflammatory cytokine response differs across dental plaque bacterial species. J Clin Periodontol. 37(1):24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi H, Aono Y, Kawato T, Asano M, Shimizu N, Saigusa T. 2015. Intragingival injection of Porphyromonas gingivalis–derived lipopolysaccharide induces a transient increase in gingival tumour necrosis factor-α, but not interleukin-6, in anaesthetised rats. Int J Oral Sci. 7(3):155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stiphout MAE, Marinus J, van Hilten JJ, Lobbezoo F, de Baat C. 2018. Oral health of Parkinson’s disease patients: a case-control study. Parkinsons Dis. 2018:9315285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velsko IM, Chukkapalli SS, Rivera MF, Lee J-Y, Chen H, Zheng D, Bhattacharyya I, Gangula PR, Lucas AR, Kesavalu L. 2014. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One. 9(5):e97811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HG, Chang Y, Lee JS, Song TJ. 2020. Association of tooth loss with new-onset Parkinson’s disease: a nationwide population-based cohort study. Parkinsons Dis. 2020:4760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, Brooks HF, Mani AR, Harry D, Stadlbauer V, Zou Z, et al. 2007. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology. 45(6):1517–1526. [DOI] [PubMed] [Google Scholar]
- Wu J, Li Q, Fu X. 2019. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl Oncol. 12(6):846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Zhang J, Nakanishi H. 2005. Leptomeningeal cells activate microglia and astrocytes to induce IL-10 production by releasing pro-inflammatory cytokines during systemic inflammation. J Neuroimmunol. 167(1–2):90–98. [DOI] [PubMed] [Google Scholar]
- Xue L, Zou X, Yang XQ, Peng F, Yu DK, Du JR. 2020. Chronic periodontitis induces microbiota-gut-brain axis disorders and cognitive impairment in mice. Exp Neurol. 326:113176. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, McGeer PL. 1999. Up-regulated production and activation of the complement system in Alzheimer’s disease brain. Am J Pathol. 154(3):927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]


