Abstract
Mycobacterium avium subsp. paratuberculosis is an intracellular pathogen of macrophages that causes a chronic enteritis (Johne's disease) in ruminants. The purpose of this study was to determine whether M. avium subsp. paratuberculosis infection causes apoptosis in bovine monocytes. Using Hoechst 33342 staining, we observed increased numbers of apoptotic monocytes within 6 h of infection with M. avium subsp. paratuberculosis, and these numbers increased further at 24 and 48 h. This effect appeared to require viable bacilli, because monocytes infected with heat-killed M. avium subsp. paratuberculosis did not exhibit a significant increase in apoptosis. Preincubation of monocytes with bovine growth hormone prior to infection with M. avium subsp. paratuberculosis did not significantly alter the number of apoptotic cells.
Johne's disease is a chronic intestinal infection, caused by Mycobacterium avium subsp. paratuberculosis, that affects cattle, sheep, and other ruminants (1). The disease is characterized by granulomatous enteritis, which leads to chronic diarrhea and progressive emaciation (1). Young animals (less than 30 days old) (1) are at greatest risk of infection by this bacterium, which can persist in the environment for long periods. If left untreated, the infection can spread quietly within a herd of animals (1). Although any animal in a herd may become infected, infection occurs most commonly in young animals that ingest infected manure or consume infected milk (1). Once ingested, the bacilli persist and multiply within macrophages in the intestinal tract and other lymphoid tissues (10).
Relatively little is known about the host-pathogen interactions that regulate the pathogenesis of paratuberculosis. In two separate studies, the growth of M. avium subsp. paratuberculosis was reduced in bovine monocytes pretreated with crude interferon (IFN) or recombinant IFN-α (rIFN-α) (20) or rIFN-γ (19). Growth of M. avium subsp. paratuberculosis in the J774 murine macrophage cell line could be enhanced or decreased by prior exposure to various concentrations of tumor necrosis factor alpha (13). There is indirect evidence that hormones may contribute indirectly to the reported increase in the onset of clinical Johne's disease after parturition and during lactation (1). Increased cytokine expression by splenocytes was observed when the M. avium subsp. paratuberculosis-infected mice were infused with 1,25-vitamin D3, a steroid hormone with known immunomodulatory functions, and fed a low-calcium diet (14). Feola et al. demonstrated that bovine peripheral blood monocytes, when exposed to bovine growth hormone (BGH) at 10 ng/ml, supported enhanced intracellular growth of M. avium subsp. paratuberculosis (2).
One potential defense mechanism against intracellular pathogens is apoptosis of infected cells. There is growing evidence that monocytes and macrophages can control mycobacterial growth via this strategy. Infection of human monocytes with M. avium-intracellulare or with M. bovis BCG resulted in monocyte apoptosis and reduced mycobacterial viability (3, 7, 9). Infection of human monocytes or alveolar macrophages with M. tuberculosis resulted in increased mortality of macrophages at 6 days postinfection (4, 12), as measured by common indicators of apoptosis such as nuclear fragmentation and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays. A recent report demonstrated that more virulent strains of M. tuberculosis avoid causing apoptosis in human alveolar macrophages whereas less virulent mycobacteria, such as M. tuberculosis H37Ra, M. bovis BCG, and M. kansasii, cause macrophage apoptosis (5). Investigators have also observed higher levels of apoptosis when other mediators are present. For example, picolinic acid was shown to induce apoptosis in macrophages infected with M. avium, and this effect was increased by the addition of IFN-γ (11).
One possible explanation for our earlier observation that bovine growth hormone enhances the survival of M. avium subsp. paratuberculosis in bovine monocytes (2) is that growth hormone inhibits apoptosis of infected monocytes, perhaps via stimulating the release of insulinlike growth factor 1 (8). If this were true, it would result in greater numbers of viable monocytes able to support bacillary survival and multiplication. The overall purpose of the present study was to determine whether infection of bovine peripheral blood monocytes with M. avium subsp. paratuberculosis increases monocyte apoptosis. A second goal was to determine whether pretreatment with bovine growth hormone (BGH) affected apoptosis in infected monocytes.
To prepare monocytes, blood was collected from the tail veins of healthy adult donor cattle by veinipuncture, using sodium citrate (0.4% [vol/vol]) as anticoagulant. The blood was centrifuged for 30 min at 600 × g, and the platelet-rich plasma was removed. The buffy coat cells were resuspended in 35 ml of Hanks balanced salts solution (HBSS; Mediatech, Herndon, Va.), layered over 15 ml of Ficoll-Histopaque 1083 (Sigma Diagnostic, Inc., St. Louis, Mo.), and centrifuged for 40 min at 600 × g. The mononuclear cells were collected from the interface, washed once with HBSS, and resuspended in HBSS. The cells were then mixed by inversion with a hypotonic lysis buffer (13.2 mM phosphate without NaCl) to eliminate red blood cells. Isotonicity was restored by the addition of a second buffer solution (13.2 mM phosphate with 2.7% NaCl). The cells were then pelleted by centrifugation, washed with HBSS, resuspended in RPMI 1640 medium (Mediatech) supplemented with 0.5% fetal bovine serum (FBS) (Intergen, Purchase, N.Y.), and adjusted to a concentration of 5 × 106 cells/ml. The cells were distributed (0.1 ml per well) into wells, containing 10-mm-diameter sterile glass coverslips, in a 24-well tissue culture plate (Falcon, Franklin Lakes, N.J.). The monocytes were allowed to adhere for 1 h at 39°C, and the nonadherent cells were removed by washing with warm HBSS. Monocytes were then incubated in RPMI 1640 medium plus 5% FBS and supplemented with 50 U of penicillin (Sigma), 0.05 mg of streptomycin (Sigma), and 10 μg of polymyxin B sulfate (Sigma) per ml.
M. avium subsp. paratuberculosis BO45 was grown to a final concentration of 109 CFU/ml in tissue culture flasks containing Middlebrook 7H9 broth (Difco, Detroit, Mich.) supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-calatase (OADC; Difco), 0.5% (vol/vol) Tween 80 (Fischer, Fair Lawn, N.J.), and 2 μg of mycobactin J (Allied Monitor, Fayette, Mo.) per ml. The bacteria were harvested by centrifugation, washed twice with phosphate-buffered saline (PBS), and dispersed as a predominantly single-cell suspension using a motor-driven, overhead stirrer (Wheaton Instruments, Milville, N.J.) and glass-Teflon homogenizer in a biosafety cabinet. The bacteria were resuspended in PBS plus 10% glycerol, aliquoted, and stored at −70°C. Viable bacteria were counted by the BACTEC method, as previously described by Lambrecht et al. (6). In some cases, mycobacteria were also counted microscopically with the aid of a Petroff-Hauser chamber. Heat-killed bacteria were prepared by utilizing thermal death curves previously established by Sung and Collins (17).
Monocytes were incubated in medium with or without BGH (10 ng per ml) for approximately 24 h prior to infection. The monolayers were then washed to remove antibiotics, infected with a 10:1 ratio of M. avium subsp. paratuberculosis to monocytes in RPMI 1640 medium plus 5% FBS, and incubated at 39°C with 5% CO2. After approximately 3 h, the uningested bacilli were removed by three washes with warm HBSS. The appropriate medium (with or without BGH) was then added, and the cells were incubated at 39°C for an additional 3 to 48 h. The slides were then washed with HBSS, fixed, and stained with Hoechst 33342 to detect apoptosis as described below. Staurosporine (Sigma) was added at 500 nM to some wells as a positive control for apoptosis. We have previously demonstrated that a 6-h incubation period is sufficient for staurosporine to cause apoptosis in bovine leukocytes (16).
A cell that is undergoing apoptosis demonstrates nuclear condensation and DNA fragmentation, which can be detected by staining with Hoechst 33342 and fluorescence microscopy. Coverslips with adherent infected monocytes were collected at specified time points and washed, and the monocytes were fixed with 4% paraformaldehyde and stained with Hoechst 33342 (5 μg/ml) for 20 min at room temperature. The coverslips were washed, mounted on glass slides, and stored at 4°C until quantification by fluorescence microscopy could be performed. Three coverslips were used per experimental group, with at least 200 cells in four random fields being counted on each slide. Each experiment was repeated using cells from different donor cattle. Data were analyzed for statistical significance by a one-way analysis of variance using the Instat biostatistics package (GraphPad Software, Inc., San Diego, Calif.). If a significant F value was obtained (P < 0.05), the Tukey-Kramer test was performed to compare the means of treatment groups with those of controls. The level of significance for all comparisons was set at P < 0.05.
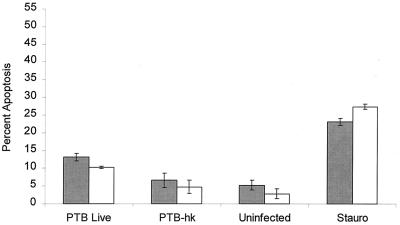
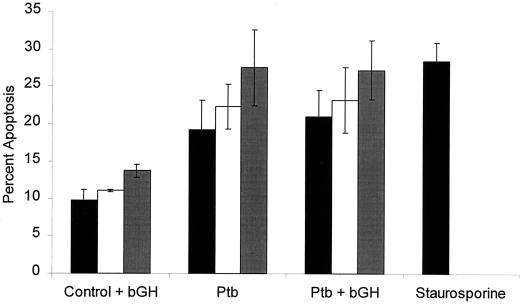
As illustrated in Fig. 1, bovine monocytes infected with live M. avium subsp. paratuberculosis for 6 h and stained with Hoechst 33342 exhibited numerous cells with fragmented nuclei. Microscopic examination of the monocyte monolayers revealed that monocyte infected with M. avium subsp. paratuberculosis had a more prominent nucleus and that the monolayers contained more cells in the later morphological stages of apoptosis (e.g., severe membrane blebbing) than did uninfected monocyte monolayers. When we counted the cells, we observed an increased percentage of apoptotic monocytes compared with uninfected monocytes (P < 0.01) (Fig. 2). This effect appeared to require viable bacilli, because monocytes that ingested heat-killed M. avium subsp. paratuberculosis did not cause a significant increase in apoptosis (P > 0.05) (Fig. 2). We had previously reported that pretreatment of bovine monocytes with BGH increased the intracellular growth of M. avium subsp. paratuberculosis. This raised the question whether BGH inhibition of monocyte apoptosis accelerated the enhanced multiplication of bacilli. This does not appear to be the case (Fig. 3), because preincubation of monocytes with BGH before infection with M. avium subsp. paratuberculosis did not significantly alter the numbers of apoptotic monocytes (P > 0.05) or significantly affect the level of apoptosis exhibited by staurosporine-treated or control uninfected monocytes (P > 0.05). Nor did longer incubation of infected monocytes demonstrate a protective effect of BGH-treated monocytes. Infected monocytes incubated in vitro for 24 or 48 h exhibited a slight increase in percentage of apoptotic cells (Fig. 3). This did not differ between BGH-treated and untreated monocytes.
FIG. 1.
Photomicrograph of apoptotic changes in control (A) or M. avium subsp. paratuberculosis-infected (B) bovine monocytes. Adherent cells were incubated in RPM 1640 medium plus 5% FBS for 6 h. The cells were then washed, fixed with 4% paraformaldehyde, and stained with Hoechst 33342 (5 μg/ml) for 20 min at 25°C. The slides were then examined by fluorescence microscopy and photographed. Cells with signs of apoptosis (fragmented nuclei) are enclosed within circles.
FIG. 2.
Quantitation of apoptosis in M. avium subsp. paratuberculosis-infected bovine monocytes stained with Hoechst 33342. Prior to infection, the monocytes were incubated overnight with 10 ng of BGH per ml (open bars) or with medium alone (negative control) (shaded bars). The monocytes were then infected with 106 CFU of live or heat-killed (hk) M. avium subsp. paratuberculosis (PTB) per ml. Additional monolayers of uninfected monocytes were treated with 500 nM staurosporine (Stauro) (positive control) or left untreated (negative control). The results are the mean and standard error of the mean of four experiments, using monocytes from four different donor cattle.
FIG. 3.
Quantitation of apoptosis in M. avium subsp. paratuberculosis-infected bovine monocytes incubated in vitro for up to 48 h. Prior to infection, the monocytes were allowed to incubate overnight with 10 ng of BGH per ml or with medium alone. Bovine monocytes were then infected with 106 CFU of live M. avium subsp. paratuberculosis (Ptb) per ml. Additional monolayers of uninfected monocytes were treated with 500 nM staurosporine (positive control) or left untreated (negative control). At 6 h (solid bars), 24 h (open bars), and 48 h (shaded bars) of incubation, triplicate monolayers were stained with Hoechst 33342 and the percentage of apoptotic cells was quantified by microscopy. The results are the mean and standard error of the mean of three experiments, using monocytes from different donor cattle.
We attempted to perform flow cytometry analysis of TUNEL-stained M. avium subsp. paratuberculosis-infected monocytes but found it to be an ineffective method. We presume that the infected cells were susceptible to the harsh chemical process needed to label the cells, resulting in a very small number of surviving cells for flow cytometric quantification. Uninfected cells did not demonstrate the same susceptibility to the staining method (data not shown). For this reason, the method was discontinued. Likewise, we could not isolate sufficient DNA from infected monocytes to detect internucleosomal DNA fragmentation (DNA ladder) by agarose gel electrophoresis.
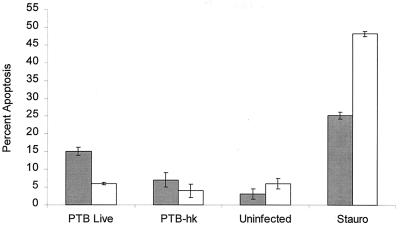
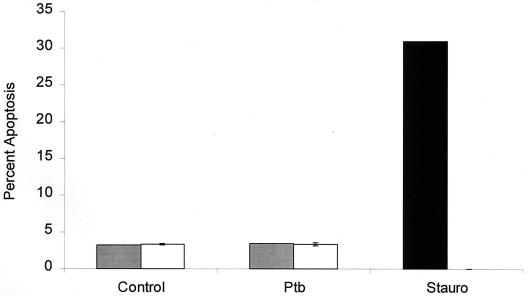
We also evaluated a bovine macrophage cell line, originally produced by simian virus 40 transformation of bovine peritoneal macrophages (generously provided by J. Stabel, Ames, Iowa) (15). It has been reported previously that this cell line can ingest and restrict the growth of M. avium subsp. paratuberculosis (13). As illustrated in Fig. 4, the cell line was resistant to apoptosis when infected with live M. avium subsp. paratuberculosis. Like bovine monocytes, the macrophage cell line underwent apoptosis when treated with staurosporine. Incubation of the infected macrophage cell line for up to 48 hours did not result in an increase in apoptotic cells (Fig. 5).
FIG. 4.
Comparison of apoptosis in M. avium subsp. paratuberculosis-infected bovine monocytes and a bovine macrophage cell line stained with Hoechst 33342. Bovine monocytes (shaded bars) and a bovine macrophage cell line (open bars) were infected with 106 CFU of live or heat-killed (hk) M. avium subsp. paratuberculosis (PTB) per ml. Some uninfected wells were treated with 500 nM staurosporine (Stauro) (positive control) or left untreated (negative control) for 6 h. The results are the mean and standard error of the mean of two experiments, using monocytes obtained from separate donors.
FIG. 5.
A M. avium subsp. paratuberculosis-infected bovine macrophage cell line is resistant to apoptosis when incubated in vitro for 24 (shaded bars) or 48 h (open bars). Monolayers of a bovine macrophage cell line (BoMac) were infected with 106 CFU of live M. avium subsp. paratuberculosis (Ptb) per ml and incubated in vitro for up to 48 h. Some uninfected wells were treated with 500 nM staurosporine (Stauro) (positive control) or left untreated (negative control). At the indicated item points, triplicate monolayers were stained with Hoechst 33342 and apoptotic cells were quantified by microscopy. The results are the mean and standard error of the mean of two experiments.
Some studies of the intracellular growth of Mycobacterium spp. have examined the effects of apoptosis on a monocyte or macrophage over a period of days. In the present study, we observed that a relatively brief (6-h) infection with M. avium subsp. paratuberculosis caused a significant level of apoptosis in bovine monocytes. This is somewhat similar to the results of Molloy et al. (9), who reported that apoptosis, but not necrosis, is associated with killing of intracellular M. bovis BCG in human monocytes. In that study, it was demonstrated that the apoptotic pathway was induced in a relatively short period (1 to 6 h) in infected human monocytes. The data in the present study demonstrate activation of the apoptotic pathway, within hours of bovine monocytes ingesting live M. avium subsp. paratuberculosis. Although microscopic examination of acid-fast-stained coverslips demonstrated a similar level of monocyte ingestion of both the live and heat-killed bacilli (data not shown), heat-killed M. avium subsp. paratuberculosis did not cause monocytes to undergo apoptosis. Although live and heat-killed M. avium subsp. paratuberculosis elicit similar cytokine responses from bovine peripheral blood mononuclear cells (18), the data presented here demonstrate a requirement for viable bacilli to stimulate the apoptotic pathway in bovine monocytes. This is consistent with observations of Keane et al. (4, 5), who found that human macrophage viability was not affected when cells were challenged with heat-killed M. tuberculosis during a 5-day incubation period. The data in the present study also demonstrate that exposure to BGH had no effect on the level of apoptosis in M. avium subsp. paratuberculosis infected bovine monocytes during a 6- to 48-h incubation period. This contrasts with a previous study, in which incubation with growth hormone enhanced intracellular bacillary growth over a longer period (6 to 12 days) (10). Although the two studies used different times of incubation, the results of the present study suggest that inhibition of monocyte apoptosis is unlikely to explain the effects of BGH on bacillary incubation observed in the previous study.
We also explored the possible use of a bovine macrophage cell line (simian virus 40-transformed peritoneal macrophages) to examine the interaction of M. avium subsp. paratuberculosis with its intracellular environment. Because the daily processing of bovine blood to obtain monocytes is tedious, time-consuming, and expensive, use of this cell line is an attractive alternative. Furthermore, a cell line might offer advantages in minimizing the variability that can be observed using cells from multiple donor cattle. However, unlike freshly obtained bovine monocytes, this macrophage cell line did not undergo apoptosis when infected with live M. avium subsp. paratuberculosis. This observation is reminiscent of the results of a previous study (3), which demonstrated differing levels of apoptosis in the THP-1 human monocyte cell line and human monocyte-derived macrophages. Caution must therefore be used when extrapolating from cell lines to primary cultures of mononuclear phagocytes during investigations of apoptosis caused by mycobacteria or mycobacterial products.
In summary, this study provides evidence that apoptosis occurs relatively quickly (6 h or less) in bovine monocytes infected with M. avium subsp. paratuberculosis. Perhaps apoptosis reflects a rapid attempt by bovine mononuclear phagocytes to rid themselves of M. avium subsp. paratuberculosis. It remains to be answered whether this would deny M. avium subsp. paratuberculosis its preferred intracellular niche and limit its multiplication.
Acknowledgments
This work was supported by funds from the Wisconsin Agricultural Experiment Station (WIS04171) and the USDA National Research Initiative (99-35204-7789).
REFERENCES
- 1.Chiodini R J, Van Kruiningen H J, Merkal R S. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 2.Feola R P, Collins M T, Czuprynski C J. Hormonal modulation of phagocytosis and intracellular growth of Mycobacterium avium ss. paratuberculosis in bovine peripheral blood monocytes. Microb Pathog. 1999;26:1–11. doi: 10.1006/mpat.1998.0246. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi T, Catanzaro A, Rao S P. Apoptosis of human monocytes and macrophages by Mycobacterium avium sonicate. Infect Immun. 1997;65:5262–5271. doi: 10.1128/iai.65.12.5262-5271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keane J, Balcewicz-Sablinska M K, Remold H G, Chupp G L, Meek B B, Fenton M J, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keane J, Romold H G, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000;164:2016–2020. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- 6.Lambrecht R S, Carriere J F, Collins M T. A model for analyzing growth kinetics of a slowly growing Mycobacterium sp. Appl Environ Microbiol. 1988;54:910–916. doi: 10.1128/aem.54.4.910-916.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laochumroonvorapong P, Paul S, Elkon K B, Kaplan G. H2O2 induces monocyte apoptosis and reduces viability of Mycobacterium avium-M. intracellulare within cultured human monocytes. Infect Immun. 1996;64:452–459. doi: 10.1128/iai.64.2.452-459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minshall C, Liu Q, Arkins S, Kelley K W. Growth hormone in immunology. In: Torosian M H, editor. Growth hormone in critical illness—research and clinical studies. R. G. New York, N.Y: Landes Co.; 1995. pp. 161–186. [Google Scholar]
- 9.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Momotani E, Whipple D L, Theirmann A B. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet Pathol. 1988;25:131–137. doi: 10.1177/030098588802500205. [DOI] [PubMed] [Google Scholar]
- 11.Pais T F, Appelberg R. Macrophage control of mycobacterial growth induced by picolinic acid is dependent on host cell apoptosis. J Immunol. 2000;164:389–397. doi: 10.4049/jimmunol.164.1.389. [DOI] [PubMed] [Google Scholar]
- 12.Placido R, Mancino G, Amendola A, Mariani F, Vendetti S, Piacentini M, Sanduzzi A, Bocchino M L, Zembala M, Colizzi V. Apoptosis of human monocytes/macrophages in Mycobacterium tuberculosis infection. J Pathol. 1997;181:31–38. doi: 10.1002/(SICI)1096-9896(199701)181:1<31::AID-PATH722>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Stabel J R. Temporal effects of tumor necrosis factor-alpha on intracellular survival of Mycobacterium paratuberculosis. Vet Immunol Immunopathol. 1995;45:211–220. doi: 10.1016/0165-2427(94)05342-p. [DOI] [PubMed] [Google Scholar]
- 14.Stabel J R, Goff J P. Influence of vitamin D3 and dietary calcium on secretion of interleukin 1, interleukin 6, and tumor necrosis factor in mice infected with Mycobacterium paratuberculosis. Am J Vet Res. 1996;57:825–829. [PubMed] [Google Scholar]
- 15.Stabel J R, Stabel T J. Immortalization and characterization of bovine peritoneal macrophages transfected with SV40 plasmid DNA. Vet Immunol Immunopathol. 1995;45:221–200. doi: 10.1016/0165-2427(94)05348-v. [DOI] [PubMed] [Google Scholar]
- 16.Stevens P K, Czuprynski C J. Pasteurella haemolytica leukotoxin induces bovine leukocytes to undergo morphologic changes consisted with apoptosis in vitro. Infect Immun. 1996;64:2687–2694. doi: 10.1128/iai.64.7.2687-2694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sung N, Collins M T. Thermal tolerance of Mycobacterium paratuberculosis. Appl Environ Microbiol. 1998;64:999–1005. doi: 10.1128/aem.64.3.999-1005.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney R W, Jones D E, Habecker P, Scott P. Interferon-gamma and interleukin-4 gene expression in cows infected with Mycobacterium paratuberculosis. Am J Vet Res. 1998;59:842–847. [PubMed] [Google Scholar]
- 19.Zhao B, Collins M T, Czuprynski C J. Effects of gamma interferon and nitric oxide on the interaction of Mycobacterium avium subsp. paratuberculosis with bovine monocytes. Infect Immun. 1997;65:1761–1766. doi: 10.1128/iai.65.5.1761-1766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zurbrick B, Follett D M, Czuprynski C J. Cytokine regulation of the intracellular growth of Mycobacterium paratuberculosis in bovine monocytes. Infect Immun. 1988;56:1692–1697. doi: 10.1128/iai.56.7.1692-1697.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]