Abstract
BACKGROUND
Treatment of infective endocarditis secondary to Pseudomonas aeruginosa can be challenging because of this organism’s ability to acquire antimicrobial resistance over time.
METHODS
We describe a patient with native aortic valve infective endocarditis due to P. aeruginosa who developed progressive multi-drug resistance while on therapy. The resistance mechanisms were characterized using whole-genome sequencing.
RESULTS
We identified two mutations in subsequent isolates (dacB and OprD) that conferred resistance to anti-pseudomonal penicillins, cephalosporins, and carbapenems. The patient was treated with combination high-dose continuous infusion meropenem and ciprofloxacin therapy, in addition to bioprosthetic aortic valve replacement and repair of ventricular septal wall defect. Antibiotics were continued for 6 weeks post–cardiac surgery and the patient remains infection free 18 months post-completion of antibiotic therapy.
CONCLUSION
Clinicians should be aware of the ability of P. aeruginosa to acquire resistance mechanisms in response to selective antibiotic pressures in high-inoculum infections such as infective endocarditis. The mutations identified in this case report correlated well with the evolving antimicrobial resistance profile observed.
Keywords: infective endocarditis, Pseudomonas aeruginosa
Mots-clés : endocardite infectieuse, Pseudomonas aeruginosa
Abstract
HISTORIQUE
Il peut être difficile de traiter une endocardite infectieuse causée par un Pseudomonas aeruginosa en raison de la capacité de cet organisme à acquérir une résistance aux antimicrobiens.
MÉTHODOLOGIE
Les chercheurs décrivent un patient atteint d’une endocardite infectieuse de la valve aortique d’origine, attribuable à un P. aeruginosa, qui a acquis une multirésistance progressive pendant son traitement. Le mécanisme de résistance était caractérisé par le séquençage du génome entier.
RÉSULTATS
Les auteurs ont dépisté deux mutations dans les isolats subséquents (dacB et OprD ), responsables d’une résistance aux pénicillines, aux céphalosporines et aux carbapénèmes antipseudomonaux. Le patient a reçu une polythérapie de perfusion continue de méropénem à forte dose et de ciprofloxacine, en plus du remplacement d’une valve aortique bioprothétique et de la réparation d’une communication interventriculaire. L’antibiothérapie s’est poursuivie six semaines après l’opération, et le patient n’avait pas d’infection 18 mois après la fin de l’antibiothérapie.
CONCLUSION
Les cliniciens devraient savoir que le P. aeruginosa peut acquérir des mécanismes de résistance en réponse aux pressions antibiotiques sélectives en cas d’infections comportant un titre élevé d’inoculum comme une endocardite infectieuse. Les mutations constatées dans le présent rapport de cas étaient bien corrélées avec l’évolution du profil de résistance antimicrobienne observé.
Case Presentation
A 64-year-old woman presented to hospital with a 2-day history of left leg erythema, pain, and fever. Her medical history was significant for recurrent lower extremity cellulitis secondary to chronic lymphedema and severe aortic stenosis (aortic valve area 0.68 cm2). Blood cultures drawn on admission grew Pseudomonas aeruginosa susceptible to all first-line agents (Table 1). She was treated empirically with piperacillin–tazobactam 4.5 g intravenously (IV) every 6 hours. Her clinical status improved, and as a result, blood cultures were not repeated to assess for clearance. She was subsequently transitioned to oral cephalexin and ciprofloxacin to complete a 14-day course of therapy for bacteremia and cellulitis.
Table 1:
Antimicrobial susceptibility results for sequential Pseudomonas aeruginosa isolates
| Antibiotics | Minimum inhibitory concentration (mg/L) and interpretation* | ||||
|---|---|---|---|---|---|
| Blood, first admission | Blood, day 0† | Blood, day 4 | Blood, day 8‡ | Aortic valve, day 13 | |
| Amikacin | ≤4 (S) | ≤4 (S) | ≤4 (S) | ≤4 (S) | 8 (S) |
| Aztreonam | 8 (S) | 8 (S) | >16 (R) | 4 (S) | 16 (I) |
| Cefepime | ≤2 (S) | ≤2 (S) | 16 (I) | ≤2 (S) | 16 (I) |
| Ceftazidime | 4 (S) | 4 (S) | >16 (R) | 2 (S) | >16 (R) |
| Ceftolozane–tazobactam | NC | ≤4/4 (S) | ≤4/4 (S) | NC | ≤4/4 (S) |
| Ciprofloxacin | ≤0.25 (S) | ≤0.25 (S) | ≤0.25 (S) | ≤0.25 (S) | ≤0.25 (S) |
| Colistin | 1 (S) | 1 (S) | 1 (S) | 1 (S) | 1 (S) |
| Doripenem | 0.25 (S) | 0.25 (S) | 0.5 (S) | 0.5 (S) | >2 (R) |
| Gentamicin | 2 (S) | 2 (S) | 2 (S) | 2 (S) | 4 (S) |
| Imipenem | 2 (S) | 2 (S) | 2 (S) | 2 (S) | >8 (R) |
| Levofloxacin | ≤1 (S) | ≤1 (S) | ≤1 (S) | ≤1 (S) | ≤1 (S) |
| Meropenem | ≤1 (S) | ≤1 (S) | ≤1 (S) | ≤1 (S) | 4 (I) |
| Piperacillin–tazobactam | 8/4 (S) | 8/4 (S) | 32/4 (I) | 8/4 (S) | ≥64/4 (R) |
| Polymyxin B | 1 (S) | 1 (S) | 1 (S) | 1 (S) | 1 (S) |
| Ticarcillin–clavulanic acid | 32 (I) | 32 (I) | 128 (R) | 32 (I) | 128 (R) |
| Tobramycin | ≤1 (S) | ≤1 (S) | ≤1 (S) | ≤1 (S) | 2 (S) |
* Interpretation of minimum inhibitory concentrations based on Clinical & Laboratory Standards Institute M100 Performance Standards
† Day number is relative to second hospitalization
‡Two different biotypes were isolated from day 8 blood cultures. Only one biotype (susceptibility results shown) was sequenced; the second biotype (not shown) demonstrated an identical susceptibility profile to the day 4 blood culture isolate
S = Susceptible, I = Intermediate, R = Resistant, NC = Not completed
Four days after completion of antibiotic therapy, she re-presented to hospital with fever, rigors, and night sweats. On physical examination, she was hypotensive and febrile (39°C) with a previously identified systolic ejection murmur at the right upper sternal border. There were no signs of cellulitis or new skin lesions during this second hospital presentation. She was re-started empirically on piperacillin–tazobactam 4.5 g IV every 6 hours. Admission blood cultures grew P. aeruginosa with the same susceptibility profile as the prior isolate (Table 1).
Despite appropriate antimicrobial therapy with piperacillin–tazobactam, she remained persistently febrile and bacteremic. Subsequent isolates of P. aeruginosa from blood cultures demonstrated progressive resistance to multiple antimicrobial agents (Table 1), prompting a change in therapy to meropenem and tobramycin on day 8 of admission.
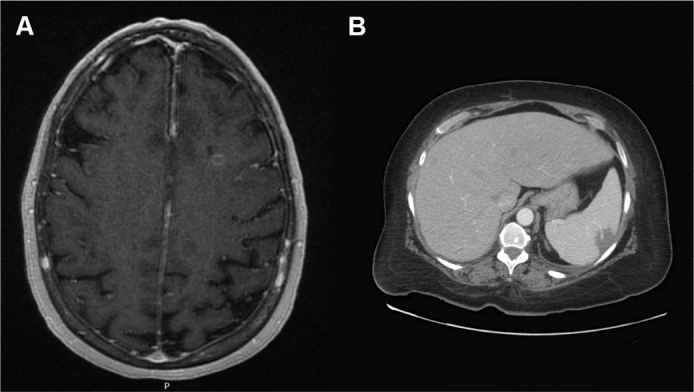
On day 9 of admission, the patient developed vertigo and nystagmus. MRI of the brain demonstrated multifocal cerebral and cerebellar infarcts with a left temporal lobe ring-enhancing lesion suspicious for abscess (Figure 1A). Computed tomography (CT) of the abdomen demonstrated multiple new splenic infarcts (Figure 1B). Given the suspicion of a cardioembolic source, a work-up for infective endocarditis was initiated. Transthoracic and transesophageal imaging did not demonstrate evidence of vegetation. CT angiogram of the head and chest did not identify any evidence of aneurysm.
Figure 1:
Brain MRI showing parietotemporal infarct and leptomeningeal enhancement (A) and abdomen CT demonstrating splenic infarcts (B)
CT = Computed tomography
Diagnosis
On day 11 of admission, the patient developed cardiogenic shock with evidence of cardiac tamponade. She was taken emergently to the operating room for exploration. Intra-operatively, the aortic valve was noted to be bicuspid with no clear evidence of vegetation, but resection of the valve revealed a deep aortic root abscess invading the septum. Evacuation of pericardial effusion, bioprosthetic aortic valve replacement, and repair of ventricular septal wall defect were performed. Bacterial cultures of the aortic valve and ventricular septum grew P. aeruginosa (Table 1).
All blood and operative culture isolates (including those from the first admission) were sent to the Public Health Ontario Laboratory for whole-genome sequencing. All isolates were found to be identical to each other (sequence type 252) with one to two single nucleotide polymorphism differences. Bacterial isolates from day 4 and 13 demonstrated a 12-nucleotide deletion in the dacB gene. In addition, insertion of a cytosine nucleotide causing a frameshift mutation in the OprD gene was noted from the day 13 (aortic valve) culture isolate.
Given the concerns about multi-drug resistance and significant toxicities associated with prolonged aminoglycoside use, the patient was transitioned to 1 g meropenem IV every 4 hours via continuous infusion (given the intermediate susceptibility result from the aortic valve culture) and 400 mg ciprofloxacin IV every 8 hours for 6 additional weeks post-operatively. Ceftolozane–tazobactam was avoided because of the findings of brain abscess and the lack of data supporting adequate central nervous system penetration of this antibiotic. The patient made a complete recovery and remains well 18 months after completion of antibiotic therapy.
Discussion
P. aeruginosa is an aerobic, non-spore-forming, gram-negative bacillus that is commonly found in freshwater environments. Infections resulting from P. aeruginosa typically occur among patients with underlying immunocompromise; however, infections among immunocompetent patients also occur, particularly in the nosocomial setting (1). Common clinical syndromes associated with P. aeruginosa include bacteremia, pneumonia, urinary tract infections, head and neck infections (particularly of otologic origin), and skin and soft tissue infections. Injection drug use is an important risk factor for P. aeruginosa bacteremia, specifically when water contaminated with this organism is used as a diluent (2).
In the absence of injection drug use, endocarditis due to P. aeruginosa is uncommon; approximately 3% of endocarditis cases overall are due to this organism (3). The predominant risk factors for pseudomonal endocarditis unrelated to injection drug use include presence of a prosthetic heart valve, intra-cardiac device, and central venous catheter, as well as underlying diabetes mellitus or receipt of immunosuppressive therapy (4,5). Among persons with nosocomial endocarditis, the proportion of cases due to P. aeruginosa may be higher (6). Mortality due to left-sided endocarditis caused by P. aeruginosa is high even with appropriate antimicrobial therapy (7). For this reason, clinical practice guidelines suggest surgical intervention in combination with antimicrobial therapy (8).
P. aeruginosa has the potential for multi-drug and high-level antimicrobial resistance as a result of various mechanisms. These mechanisms of resistance may be intrinsic or acquired and include enzymatic inactivation of antimicrobial agents, alterations in penicillin-binding proteins, porins, and efflux pumps. Initial case reports in the 1980s described the failure of piperacillin–tazobactam therapy among patients with P. aeruginosa endocarditis, with isolates demonstrating significant increases in piperacillin minimum inhibitory concentrations and beta-lactamase production post-therapy (9).
The availability of whole-genome sequencing has allowed for further characterization of these resistance mechanisms. Domitrovic and colleagues described the development of cephalosporin resistance in five sequential P. aeruginosa blood culture isolates over a 7-month period in a patient with relapsed prosthetic aortic valve endocarditis treated with beta-lactam therapy (10). All isolates also possessed a premature stop codon in OprD as well as mutations in gyrA, parC, and nalC, thereby conferring resistance to carbapenems and quinolones, respectively (10). Gürtler and colleagues reported a case of P. aeruginosa prosthetic aortic valve endocarditis and persistent bacteremia in which the responsible organism sequentially developed resistance to piperacillin–tazobactam, ceftazidime, and meropenem (11). Genetic sequencing demonstrated mutations in ampR, ampD, and OprD (11).
The findings of a mutation in dacB and OprD genes in our case correlate with the susceptibility profile observed. Resistance to anti-pseudomonal penicillins and cephalosporins from the day 4 and 13 isolates coincide with the mutation in the dacB gene. The inactivation of dacB (which encodes for penicillin-binding-protein 4) in experimental knockout strains of P. aeruginosa has been shown to significantly increase ampC expression, which likely explains the observed resistance pattern (12). To our knowledge, this is only the second case report of resistance development through this gene mutation during therapy (13). The finding of selective imipenem resistance in the aortic valve culture isolate is consistent with loss of OprD function as the underlying mechanism (14). Two different biotypes were isolated from day 8 blood cultures, one demonstrating an identical susceptibility to the day 0 isolate, whereas the second isolate possessed the same susceptibility pattern as the day 4 isolate, suggesting the presence of hetero-resistant subpopulations.
Guidelines from the Infectious Diseases Society of America suggest combination antimicrobial therapy for cases of endocarditis due to non-HACEK (Haemophilus spp, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae) gram-negative aerobic bacilli, particularly P. aeruginosa (8). However, studies examining the optimal approach to the medical management of pseudomonal endocarditis in humans are limited, and no randomized controlled trials have been performed with this population. In the largest prospective cohort study of 58 patients hospitalized with endocarditis due to non-HACEK gram-negative bacteria (of which approximately one-fifth of cases were due to P. aeruginosa), infection due to a multi-drug-resistant organism was independently associated with increased mortality (5). Almost all patients (86%) were treated with combination antibiotic therapy (5). Mortality was similar between those treated medically and those who received a combined medical–surgical approach; however, the subset of patients who developed complications (such as abscess or valve perforation) had higher mortality rates when treated with medical therapy alone (5).
Despite the lack of echocardiographic evidence of endocarditis in our case, clinical suspicion of endocarditis was high. The patient possessed multiple indications for early surgical intervention, including persistent bacteremia and recurrent embolic phenomena despite appropriate combination antimicrobial therapy. Ultimately, the diagnosis of infective endocarditis was made intra-operatively. Although there is growing evidence to support the use of positron emission tomography and CT in diagnosing select cases equivocal for infective endocarditis (15), this imaging modality may not be readily accessible at many centres and in this case would not have changed management given the high suspicion for endovascular infection and progressive deterioration necessitating surgical intervention.
We suspect that chronic skin breakdown from lymphedema and chronic dermatomycosis served as a portal of entry for P. aeruginosa to contribute to an initial skin and soft tissue infection, with subsequent bacteremia and seeding of a vulnerable bicuspid aortic valve. A high inoculum of bacteria within the vegetation (before cardiac surgery) likely allowed for evolution of resistance in response to selective antimicrobial pressure. The ability of P. aeruginosa to quickly develop resistance highlights the importance of repeating susceptibility testing on serial isolates when persistent bacteremia is encountered.
In conclusion, this case highlights the ability of P. aeruginosa to acquire antimicrobial resistance during therapy, particularly in high-inoculum infections such as infective endocarditis. Using whole-genome sequencing, we were able to identify mutations in OprD and dacB that corresponded to the development of progressive antibiotic resistance during the patient’s treatment course. Although the optimal approach to management of pseudomonal infective endocarditis remains unclear, combination antimicrobial therapy in addition to surgical intervention may be needed.
Ethics Approval:
N/A
Informed Consent:
Informed consent was obtained from the patient.
Registry and the Registration No. of the Study/Trial:
N/A
Funding:
N/A
Disclosures:
L Palmay has received consulting fees from Sanofi and Cubicin, received honoraria from the Canadian Society of Hospital Pharmacists, and has participated on a data safety monitoring board or advisory board for Sanofi and Cubicin. The other authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
References
- 1.Migiyama Y, Yanagihara K, Kaku N, et al. Pseudomonas aeruginosa bacteremia among immunocompetent and immunocompromised patients: relation to initial antibiotic therapy and survival. Jpn J Infect Dis. 2016;69(2):91–6. 10.7883/yoken.JJID.2014.573. Medline: [DOI] [PubMed] [Google Scholar]
- 2.Reyes MP, Ali A, Mendes RE, Biedenbach DJ. Resurgence of Pseudomonas endocarditis in Detroit, 2006-2008. Medicine (Baltimore). 2009;88(5):294–301. 10.1097/MD.0b013e3181b8bedc. Medline: [DOI] [PubMed] [Google Scholar]
- 3.Sandre RM, Shafran SD. Infective endocarditis: review of 135 cases over 9 years. Clin Infect Dis. 1996;22(2):276–86. 10.1093/clinids/22.2.276. Medline: [DOI] [PubMed] [Google Scholar]
- 4.Reyes MP, Palutke WA, Wylin RF. Pseudomonas endocarditis in the Detroit Medical Center. 1969-1972. Medicine (Baltimore). 1973;52(3):173–94. 10.1097/00005792-197305000-00001. Medline: [DOI] [PubMed] [Google Scholar]
- 5.Falcone M, Tiseo G, Durante-Mangoni E, et al. Risk factors and outcomes of endocarditis due to Non-HACEK Gram-negative bacilli: data from the prospective multicenter Italian Endocarditis Study cohort. Antimicrob Agents Chemother. 2018;62(4):e02208–17. 10.1128/AAC.02208-17. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouëllo JP, Asfar P, Brenet O, et al. Nosocomial endocarditis in the intensive care unit: an analysis of 22 cases. Crit Care Med. 2000;28(2):377–82. 10.1097/00003246-200002000-00015. Medline: [DOI] [PubMed] [Google Scholar]
- 7.Komshian SV, Tablan OC, Palutke W, Reyes MP. Characteristics of left-sided endocarditis due to Pseudomonas aeruginosa in the Detroit Medical Center. Rev Infect Dis. 1990;12(4):693–702. 10.1093/clinids/12.4.693. Medline: [DOI] [PubMed] [Google Scholar]
- 8.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435–86. 10.1161/CIR.0000000000000296. Medline: [DOI] [PubMed] [Google Scholar]
- 9.Letendre ED, Mantha R, Turgeon PL. Selection of resistance by piperacillin during Pseudomonas aeruginosa endocarditis. J Antimicrob Chemother. 1988;22(4):557–62. 10.1093/jac/22.4.557. Medline: [DOI] [PubMed] [Google Scholar]
- 10.Domitrovic TN, Hujer AM, Perez F, et al. Multidrug resistant Pseudomonas aeruginosa causing prosthetic valve endocarditis: a genetic-based chronicle of evolving antibiotic resistance. Open Forum Infect Dis. 2016;3(4):ofw188. 10.1093/ofid/ofw188. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gürtler N, Osthoff M, Rueter F, et al. Prosthetic valve endocarditis caused by Pseudomonas aeruginosa with variable antibacterial resistance profiles: a diagnostic challenge. BMC Infect Dis. 2019;19:530. 10.1186/s12879-019-4164-3. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moya B, Dötsch A, Juan C, et al. Beta-lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 2009;5(3):e1000353. 10.1371/journal.ppat.1000353. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbosa C, Gregg KS, Woods RJ. Variants in ampD and dacB lead to in vivo resistance evolution of Pseudomonas aeruginosa within the central nervous system. J Antimicrob Chemother. 2020;75(11):3405–8. 10.1093/jac/dkaa324. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Luo YF, Williams BJ, Blackwell TS, Xie CM. Structure and function of OprD protein in Pseudomonas aeruginosa: from antibiotic resistance to novel therapies. Int J Med Microbiol. 2012;302(2):63–8. 10.1016/j.ijmm.2011.10.001. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erba PA, Pizzi MN, Roque A, et al. Multimodality imaging in infective endocarditis: an imaging team within the endocarditis team. Circulation. 2019;140(21):1753–1765. 10.1161/CIRCULATIONAHA.119.040228. Medline: [DOI] [PubMed] [Google Scholar]


