Abstract
Differences between therapeutic effects of medical cannabis inflorescences and those of their extracts are generally attributed to the differences in administration form and in the resultant pharmacokinetics. We hypothesized that difference may further extend to the composition of the actually consumed drug. Cannabinoid and terpene contents were compared between commercial cannabis inflorescences (n = 19) and decarboxylated extracts (n = 12), and between inflorescences and decarboxylated extracts produced from them (n = 10). While cannabinoid content was preserved in the extracts, a significant loss of terpenes was evident, mainly in the more volatile monoterpenes and monoterpenoids (representing a loss of about 90%). This loss changes the total terpene content, the proportion of monoterpenes out of the total terpenes, and the monoterpene/cannabinoid ratio. Terpene deficiency might impair extracts’ pharmacological efficacy and might contribute to the patients’ preference to inflorescences-smoking. This argues against the validity of terms such as “whole plant” and “full spectrum” extracts and creates a misleading assumption that extracts represent the pharmacological profile of the sourced inflorescences. Furthermore, it reduces the diversity in extracts, such as loss of differences between sativa-type and indica-type. Enriching cannabis extracts with selected terpenes may provide a suitable solution, generating a safe, precise, and reproducible drug with tailored cannabinoid and terpene contents. Careful selection of terpenes to be added enables tailor-made extracts, adjusted for various medicinal aims and for different populations.
Keywords: terpenes, monoterpenes, cannabis extracts, full-spectrum, terpenes enrichment, entourage effect
1. Introduction
The number of medical cannabis users is increasing rapidly. Alongside this, the number and variety of authorized medical cannabis compositions and delivery methods available on the market continuously increases. Physicians and caregivers prescribing medical cannabis, as well as patients who use it regularly, face difficulties in selecting the most suitable preparation. While there might not be sufficient scientific data to support such selection, it is clear that the main objectives for the most suitable products include (i) an optimal composition (an optimal combination of active pharmaceutical ingredients (APIs)) for the specifically treated indication; (ii) precision—an exact knowledge and control of the administered dose of each important API; (iii) reproducibility—as-perfect-as-possible repeatability in the composition of the selected product, week after week and month after month; (iv) ability to adjust the composition for varying requirements, e.g., fitting to changes in day activity; and (v) ability to adjust the composition to varying population needs, for example women vs. men, and children vs. aging population.
A large fraction of the medical cannabis users smoke their cannabis drug (in Israel, more than 80% [1]). This preference seems unfortunate. Firstly, smoking is accompanied by known health-threatening issues, including the inhalation of ash particles and of toxic oxidation products. This is even worse when smoking cannabis mixed with tobacco, which is a common practice among many medical cannabis patients. Secondly, the short-onset time in smoking (beneficial in acute cases) is less important for most medical cannabis users, who suffer from chronic ailments, and who can benefit from the extended effect of the oils. Thirdly, smoking suffers badly from inaccurate analysis and poor reproducibility [2,3] making it unsuitable for a precise medicinal treatment. Impaired accuracy and reproducibility in smoking results from multiple reasons, including agricultural effects and post-harvest and industrial processing parameters, as well as from non-standardized consumption factors [4]. A seemingly preferred delivery form is based on cannabis extracts, also referred to as cannabis oils, which are produced by extracting cannabis inflorescences and diluting in a vegetable oil. Extracts are consumed sublingually, via spray into the oral cavity, or further formulated into tablets and capsules. Each one of these forms has its own pharmacokinetics and absorption rate. Compared to smoking, extracts-based products are safer for use, provide longer lasting effects, and enable better accuracy and reproducibility. Additionally, the majority of known clinical trials are based on administering extracts, rather than on smoking, e.g., [5,6,7,8,9].
The present study is directed at identifying a potential drawback of cannabis oils (in addition to undesired flavor in some formulations), and at potential means for resolving that drawback. It focuses on significant differences in the compositions of the consumed oils compared with those of the corresponding smoked inflorescence, differences which might reduce the efficacy of the former in some treatment cases. It suggests that, contrary to expectation by most caregivers and users, the content and composition of the APIs in the extracts differ markedly from those in the inflorescence used to produce them. The main difference is not in the cannabinoid content, but rather in the content of the terpenes, which are not reported in most commercially marketed cannabis products and in many clinical trials. To evaluate possible differences, the compositions and amounts of cannabinoids and terpenes in cannabis inflorescences and extracts, including inflorescences and decarboxylated extracts produced from them, were compared.
More than 200 different terpenes and terpenoids [10] have been identified in cannabis, out of which about 20 are most common, including myrcene, limonene, pinene, linalool, terpinolene, β-caryophyllene, and humulene. Most cannabis terpenes belong to one of the following groups, arranged here according to increasing boiling point order: monoterpenes (including myrcene, pinene, limonene, ocimene, and terpinolene), monoterpenoids (including linalool, terpineol, and geraniol), sesquiterpenes (including caryophyllene and humulene), and sesquiterpenoids (including nerolidol, guaiol, and bisabolol) [10,11,12,13], see Table 1. The total terpene concentration in cannabis inflorescences was commonly in the 1% range, however, due to selective breeding, this concentration has risen to about 3% in some inflorescences [11], reaching terpene-cannabinoid ratios of up to about 1:10. Despite these relatively low concentrations, it is argued that terpenes modulate the pharmacodynamic effects in cannabis, e.g., [14,15,16,17,18,19], as further discussed below.
Table 1.
Terpenes’ classification.
| Terpene Class | Terpenes | Boiling Points (°C) |
|---|---|---|
| Monoterpenes (C10H16) | α-Pinene | 155 |
| Camphene | 159 | |
| Sabinene | 163 | |
| β-Pinene | 166 | |
| Myrcene | 168 | |
| Carene | 171 | |
| Ocimene | 175 | |
| Limonene | 176 | |
| Terpinolene | 185 | |
| Monoterpenoids (C10H18O) | Linalool | 198 |
| Terpineol | 217 | |
| Geraniol | 230 | |
| Sesquiterpenes (C15H24) | β-Caryophyllene | 263 |
| Humulene | 276 | |
| Sesquiterpenoids (C15H26O) | Nerolidol | 276 |
| Guaiol | 290 | |
| Bisabolol | 314 |
Terpenes have been suggested, over thousands of years, to have various, broadranging therapeutic properties, which provide the basis for traditional and modern aromatherapy and herbal medicine. Different terpenes have been described as having various therapeutic properties, for instance, myrcene: analgesic, anti-inflammatory, sedative, and muscle relaxant; α-pinene: anti-oxidant, anti-inflammatory, and bronchodilator; linalool: anti-convulsant, analgesic, sedative and anti-depressant; limonene: ameliorates stress and depression; and β-caryophyllene: gastroprotective, analgesic, and antibiotic effect [15,17,20,21]. Within the endocannabinoid system, terpenes were demonstrated to activate or modulate cannabinoid receptor type 1 (CB1), cannabinoid receptor type 2 (CB2), transient receptor potential ankyrin 1 (TRPA1), transient receptor potential vanilloid 1 (TRPV1) and peroxisome proliferator-activated receptor (PPAR) receptors [18,22,23,24]. In addition to their own therapeutic properties, terpenes were suggested to affect the therapeutic activity of cannabinoids via synergic and/or entourage effects [25]. The role of terpenes in the entourage effect of cannabis was first suggested by Russo [15], and was subsequently supported by studies demonstrating the role of specific terpenes and their interaction with cannabinoids in increasing tetrahydrocannabinol (THC) antinociception [18], THC’s anxiolytic effect [19], THC and CBD (cannabinol) cytotoxic activity [16], and more [11,14,21,26,27,28].
2. Results and Discussion
Although the role of terpenes in cannabis’ pharmaceutical effect is gaining increased acceptance [11,15,16,17,18,19,21,28,29,30], most scientific reports and commercial products are provided with information regarding their cannabinoid content, but with no terpene data. Where terpene content is presented, it is typically in the form of concentration or in the form of the fraction of each terpene out of the total terpene content [12,27]. However, for medical applications and for the discussion of the entourage/synergistic effect, more important are the administered terpene doses and the relative proportion of terpenes to cannabinoids (See also [31]).
Cannabinoid and terpene data for inflorescences of nineteen different cannabis chemovars (or chemotypes, frequently related as “cannabis strains”) grown in Israel and marketed as medical cannabis inflorescences are presented as concentrations and as administered doses, i.e., as milligrams of each terpene per 100 mg total cannabinoid content (Table 2 and Figure 1).
Table 2.
Cannabinoid and terpene compositions in commercial medical cannabis inflorescences in Israel. Data is presented as absolute percentage 1 and in the form of milligrams of each terpene per 100 mg total cannabinoid content (in italic).
| Compound Name | Inf 1 | Inf 2 | Inf 3 | Inf 4 | Inf 5 | Inf 6 | Inf 7 | Inf 8 | Inf 9 | Inf 10 | Inf 11 | Inf 12 | Inf 13 | Inf 14 | Inf 15 | Inf 16 | Inf 17 | Inf 18 | Inf 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THCA | 10.7 | 14.5 | 18.8 | 21.4 | 22.8 | 24.4 | 17.9 | 13.1 | 20.8 | 21.9 | 19.4 | 19.2 | 19.7 | 16.5 | 14.5 | 16.1 | 20.4 | 7.0 | 0.6 |
| THC | 3.5 | 1.6 | 1.7 | 1.3 | 0.7 | 1.0 | 1.3 | 1.0 | 1.7 | 0.6 | 1.9 | 1.3 | 0.8 | 1.1 | 0.6 | 3.8 | 0.9 | 0.9 | 0 |
| Total THC | 12.9 | 14.3 | 18.2 | 20.1 | 20.7 | 22.4 | 17.1 | 12.5 | 20.0 | 19.8 | 18.9 | 18.1 | 18.1 | 15.6 | 13.3 | 17.9 | 18.8 | 7.0 | 0.5 |
| CBDA | 0.1 | 12 | 16.8 | ||||||||||||||||
| CBD | 1.1 | 0.7 | |||||||||||||||||
| Total CBD | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 0.1 | 11.6 | 15.4 |
| Total quantified Cannabinoids | 12.9 | 14.3 | 18.2 | 20.1 | 20.7 | 22.4 | 17.1 | 12.5 | 20.0 | 19.8 | 18.9 | 18.1 | 18.1 | 15.6 | 13.3 | 17.9 | 18.9 | 18.7 | 15.9 |
| α-Pinene | 0.18 1.39 |
0.03 0.19 |
0.31 1.71 |
0.03 0.17 |
0.41 1.94 |
0.52 2.31 |
0.05 0.30 |
0.48 3.87 |
0.36 1.76 |
0.37 1.86 |
0.40 2.12 |
0.20 1.10 |
0.03 0.17 |
0.20 1.46 |
0.29 1.61 |
0.03 0.15 |
0.04 0.28 |
||
| Camphene | 0.01 0.05 |
0.01 0.05 |
0.01 0.03 |
||||||||||||||||
| Sabinene | 0.03 0.13 |
0.03 0.14 |
|||||||||||||||||
| β-Pinene | 0.07 0.52 |
0.04 0.31 |
0.16 0.87 |
0.67 3.33 |
0.19 0.89 |
0.11 0.5 |
0.07 0.38 |
0.24 1.91 |
0.18 0.87 |
0.18 0.91 |
0.20 1.04 |
0.10 0.56 |
0.60 3.31 |
0.05 0.35 |
0.08 0.62 |
0.09 0.48 |
0.04 0.21 |
||
| Myrcene | 0.08 0.64 |
0.49 3.44 |
0.39 2.13 |
0.07 0.32 |
0.23 1.08 |
0.91 4.04 |
0.15 0.87 |
0.54 2.66 |
0.39 2.00 |
0.42 2.21 |
0.55 3.06 |
0.36 2.33 |
0.10 0.78 |
0.09 0.49 |
0.10 0.51 |
0.25 1.35 |
0.23 1.47 |
||
| Carene | |||||||||||||||||||
| Ocimene | 0.01 0.09 |
0.11 0.51 |
0.08 0.36 |
0.18 1.42 |
0.03 0.15 |
||||||||||||||
| Limonene | 0.06 0.47 |
0.25 1.76 |
0.14 0.78 |
0.26 1.30 |
0.12 0.57 |
0.26 1.53 |
0.37 2.93 |
0.15 0.76 |
0.15 0.75 |
0.18 0.95 |
0.08 0.45 |
0.24 1.34 |
0.24 1.54 |
0.07 0.52 |
0.08 0.44 |
0.02 0.11 |
0.11 0.61 |
0.04 0.25 |
|
| Terpinolene | |||||||||||||||||||
| Linalool | 0.02 0.14 |
0.09 0.61 |
0.04 0.23 |
0.20 1.00 |
0.08 0.36 |
0.09 0.53 |
0.04 0.17 |
0.05 0.25 |
0.06 0.29 |
0.03 0.16 |
0.18 1.00 |
0.07 0.44 |
0.02 0.18 |
0.03 0.15 |
0.06 0.32 |
0.02 0.12 |
|||
| Terpineol | |||||||||||||||||||
| Geraniol | |||||||||||||||||||
| β-Caryophyllene | 0.08 0.60 |
0.20 1.37 |
0.30 1.62 |
0.29 1.42 |
0.09 0.42 |
0.45 1.99 |
0.32 1.87 |
0.20 1.61 |
0.29 1.44 |
0.28 1.42 |
0.30 1.57 |
0.13 0.74 |
0.22 1.21 |
0.17 1.09 |
0.09 0.64 |
0.15 0.86 |
0.06 0.33 |
0.17 0.93 |
0.05 0.34 |
| Humulene | 0.03 0.24 |
0.05 0.37 |
0.13 0.72 |
0.13 0.62 |
0.03 0.13 |
0.12 0.53 |
0.08 0.45 |
0.06 0.44 |
0.12 0.61 |
0.12 0.62 |
0.13 0.69 |
0.05 0.25 |
0.10 0.54 |
0.05 0.30 |
0.03 0.21 |
0.05 0.29 |
0.07 0.36 |
0.02 0.13 |
|
| Nerolidol | 0.01 0.10 |
0.03 0.19 |
0.03 0.16 |
0.12 0.60 |
0.10 0.47 |
0.06 0.31 |
0.08 0.42 |
0.03 0.19 |
0.09 0.51 |
0.02 0.18 |
0.04 0.20 |
0.03 0.18 |
|||||||
| Guaiol | 0.05 0.37 |
0.07 0.49 |
0.10 0.45 |
0.13 0.74 |
0.04 0.24 |
0.05 0.34 |
0.05 0.36 |
0.07 0.40 |
0.02 0.12 |
0.05 0.28 |
0.04 0.24 |
||||||||
| Bisabolol | 0.17 1.34 |
0.15 1.05 |
0.19 1.02 |
0.03 0.13 |
0.10 0.48 |
0.08 0.38 |
0.22 1.27 |
0.18 0.88 |
0.15 0.76 |
0.15 0.79 |
0.08 0.42 |
0.02 0.09 |
0.11 0.70 |
0.15 1.15 |
0.26 1.47 |
0.06 0.32 |
0.05 0.29 |
0.03 0.18 |
|
| Total MONO 2 | 0.42 3.25 |
0.9 6.31 |
1.04 5.72 |
1.27 6.31 |
1.14 5.35 |
1.62 7.22 |
0.62 3.61 |
1.27 10.13 |
1.27 6.22 |
1.14 5.77 |
1.26 6.60 |
0.96 5.33 |
1.09 6.02 |
0.72 4.66 |
0.47 3.57 |
0.58 3.17 |
0.15 0.8 |
0.5 2.67 |
0.33 2.13 |
| Total SESQUI 3 | 0.34 2.65 |
0.5 3.47 |
0.65 3.52 |
0.56 2.78 |
0.32 1.48 |
0.65 2.90 |
0.75 4.32 |
0.26 2.05 |
0.69 3.40 |
0.61 3.11 |
0.66 3.47 |
0.33 1.83 |
0.43 2.36 |
0.38 2.44 |
0.34 2.54 |
0.57 3.22 |
0.17 0.96 |
0.34 1.86 |
0.14 0.89 |
| Total terpene content | 0.76 5.9 |
1.40 9.79 |
1.68 9.24 |
1.83 9.09 |
1.43 6.83 |
2.27 10.11 |
1.36 7.93 |
1.52 12.19 |
1.94 9.63 |
1.75 8.87 |
1.91 10.07 |
1.30 7.16 |
1.51 8.37 |
1.11 7.09 |
0.82 6.11 |
1.14 6.39 |
0.32 2.10 |
0.83 4.53 |
0.47 3.02 |
| MONO 1 out of total terpene content (%) | 55.1 | 64.5 | 61.9 | 69.5 | 78.3 | 71.4 | 45.5 | 83.1 | 64.7 | 65.0 | 65.5 | 74.4 | 71.9 | 65.6 | 58.4 | 49.6 | 54.5 | 58.9 | 70.5 |
1 % represents percent weight/weight. 2 MONO relates to the sum of monoterpenes and monoterpenoids. 3 SESQUI relates to the sum of sesquiterpenes and sesquiterpenoids. Inf = inflorescence, THC = tetrahydrocannabinol, THCA = tetrahydrocannabinolic acid, CBD = cannabidiol, CBDA = cannabidiol acid. Total cannabinoid content was calculated as if all of the cannabinoids were in their decarboxylated form. Blank cells indicate terpene levels which are under reporting limit. α-terpinene, p-cymene, γ-terpinene, iso-pulegol, eucalyptol, borneol and caryophyllene oxide were also assessed and were found to be under quantification limit.
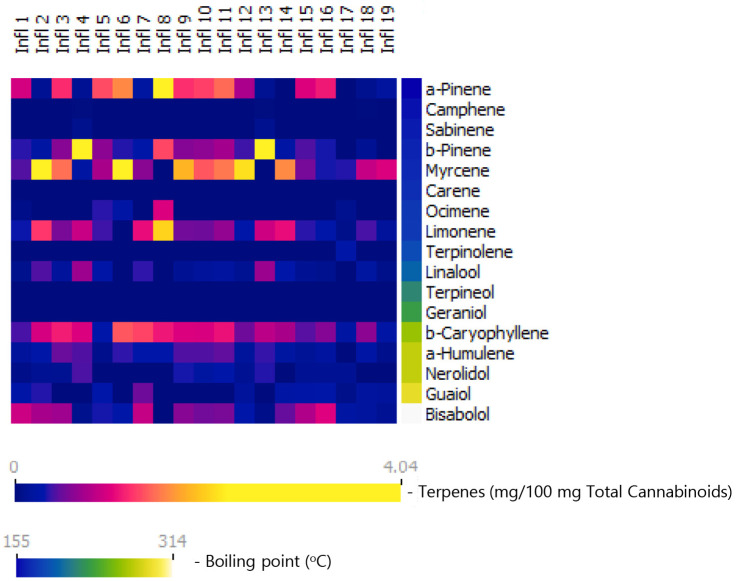
Figure 1.
Terpene content in nineteen commercial cannabis inflorescences marketed in Israel, presented as milligrams of each terpene per 100 mg total cannabinoid content. Terpenes are arranged according to their boiling points. As seen, monoterpenes comprise the largest terpene group in these inflorescences. Furthermore, the diversity between inflorescences is driven, to a large extent by monoterpenes.
Terpenes are arranged according to their boiling points. Total terpene contents in the cannabis inflorescences tested here range between 0.4% and 2.3%, and terpene–cannabinoid ratios range between 2.1 and 12.2 mg total terpenes per 100 mg total cannabinoids (Table 2). It is important to note that the inflorescences are rich in monoterpenes and monoterpenoids, forming together 46% to 83% of the total terpenes in the composition and up to 10.1 mg per 100 mg cannabinoids. The most prevalent monoterpenes are myrcene, alpha pinene, beta pinene, and limonene, reaching up to 4 mg each per 100 mg cannabinoids. These results are in agreement with prior reports [10,11,12,16]. Another important aspect to keep in mind is that, as shown in Figure 1, the diversity of the inflorescences is derived, to a large extent, from the monoterpene and monoterpenoid content.
On average, medical cannabis patients in Israel consume about 1 g of inflorescence per day [1], containing up to about 200 mg cannabinoids. The corresponding total terpene content ranges from about 4 to 24 mg. Note that these figures are true for the content of the consumed cigarettes, but not necessarily for the doses of actually consumed cannabinoids and terpenes, since active components are lost during smoking (via burning or evaporation prior to absorption within the airways [4]).
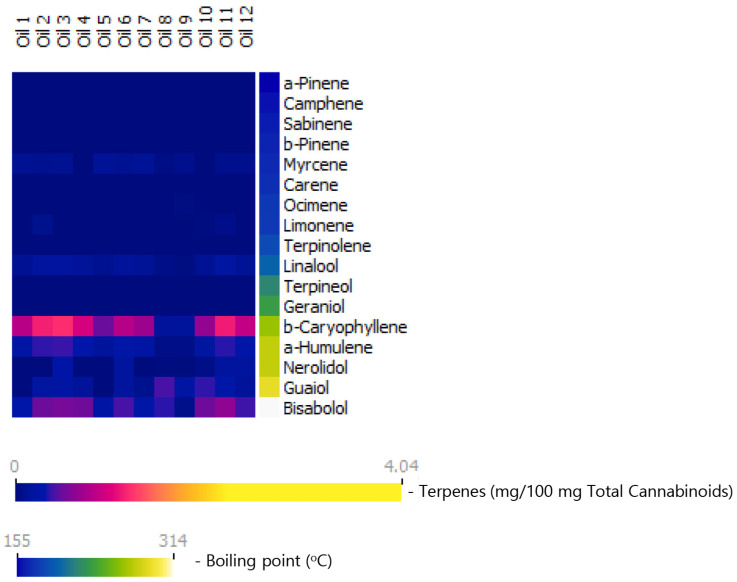
Cannabinoid and terpene data for commercial medical cannabis extracts (oils) in the Israeli market are shown in Table 3 and in Figure 2. In these oils, the total terpene contents (up to 0.93%) and the content of total terpenes per 100 mg total cannabinoids (up to 4.3 mg, Table 3) are low compared with those in the inflorescences. In oils, the combined contents of sesquiterpenes and sesquiterpenoids are similar to those in the inflorescences (up to 3.8 vs. up to 4.3 mg per 100 mg cannabinoids), but those of the monoterpene and monoterpenoids, combined, is notably lower (up to 0.55 mg vs. 10.1 mg). As a result, the proportions of monoterpenes and monoterpenoids, combined, drop from up to 83% in the inflorescence to up to 22% in the oils.
Table 3.
Cannabinoid and terpene compositions in commercial cannabis oil products in Israel. Data is presented as absolute percentage 1 and in the form of milligrams of each terpene per 100 mg total cannabinoid content (in italic).
| Compound Name | Oil 1 | Oil 2 | Oil 3 | Oil 4 | Oil 5 | Oil 6 | Oil 7 | Oil 8 | Oil 9 | Oil 10 | Oil 11 | Oil 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THCA | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | |||||||
| THC | 5.3 | 20.0 | 15.4 | 10.1 | 3.0 | 10.9 | 5.1 | 1.4 | 1.1 | 1.0 | 21.3 | 21.9 |
| Total THC | 5.3 | 20.0 | 15.6 | 10.3 | 3.0 | 11.1 | 5.1 | 1.4 | 1.1 | 1.0 | 21.6 | 22.2 |
| CBDA | ||||||||||||
| CBD | 10.4 | 4.4 | 3.3 | 2.2 | 15.0 | 10.8 | 10.4 | 28.0 | 28.1 | 20.3 | 4.5 | 3.3 |
| Total CBD | 10.4 | 4.4 | 3.3 | 2.2 | 15.0 | 10.8 | 10.4 | 28.0 | 28.1 | 20.3 | 4.5 | 3.3 |
| Total quantified cannabinoids | 15.7 | 24.4 | 18.9 | 12.5 | 18.0 | 21.9 | 15.5 | 29.4 | 29.2 | 21.3 | 26.1 | 25.7 |
| α-Pinene | ||||||||||||
| Camphene | ||||||||||||
| Sabinene | ||||||||||||
| β-Pinene | ||||||||||||
| Myrcene | 0.03 0.18 |
0.04 0.15 |
0.03 0.16 |
0.04 0.21 |
0.04 0.17 |
0.03 0.20 |
0.02 0.08 |
0.04 0.12 |
0.03 0.13 |
0.03 0.12 |
||
| Carene | ||||||||||||
| Ocimene | 0.01 0.04 |
|||||||||||
| Limonene | 0.03 0.13 |
0.01 0.03 |
0.03 0.10 |
|||||||||
| Terpinolene | ||||||||||||
| Linalool | 0.03 0.18 |
0.07 0.27 |
0.06 0.29 |
0.03 0.23 |
0.03 0.14 |
0.05 0.23 |
0.03 0.21 |
0.03 0.09 |
0.02 0.06 |
0.04 0.17 |
0.08 0.31 |
0.06 0.21 |
| Terpineol | ||||||||||||
| Geraniol | ||||||||||||
| β-Caryophyllene | 0.19 1.20 |
0.41 1.67 |
0.33 1.77 |
0.18 1.41 |
0.13 0.72 |
0.26 1.19 |
0.16 1.01 |
0.07 0.24 |
0.07 0.24 |
0.20 0.96 |
0.43 1.65 |
0.33 1.28 |
| α-Humulene | 0.05 0.35 |
0.13 0.53 |
0.11 0.56 |
0.06 0.46 |
0.04 0.24 |
0.08 0.38 |
0.05 0.34 |
0.02 0.09 |
0.03 0.09 |
0.06 0.31 |
0.14 0.52 |
0.11 0.41 |
| Nerolidol | 0.07 0.36 |
0.06 0.27 |
0.01 0.04 |
0.01 0.03 |
0.02 0.09 |
0.07 0.26 |
0.07 0.26 |
|||||
| Guaiol | 0.07 0.30 |
0.06 0.32 |
0.03 0.23 |
0.06 0.27 |
0.02 0.13 |
0.17 0.59 |
0.09 0.32 |
0.11 0.54 |
0.10 0.38 |
0.06 0.23 |
||
| Bisabolol | 0.06 0.41 |
0.18 0.74 |
0.15 0.79 |
0.09 0.76 |
0.06 0.34 |
0.13 0.60 |
0.06 0.39 |
0.15 0.52 |
0.03 0.11 |
0.16 0.75 |
0.24 0.93 |
0.15 0.57 |
| Total MONO 2 | 0.06 0.36 |
0.14 0.55 |
0.09 0.45 |
0.03 0.23 |
0.07 0.35 |
0.09 0.40 |
0.06 0.41 |
0.05 0.17 |
0.07 0.25 |
0.05 0.27 |
0.14 0.44 |
0.09 0.33 |
| Total SESQUI 3 | 0.3 1.96 |
0.79 3.24 |
0.72 3.80 |
0.36 2.86 |
0.23 1.30 |
0.59 2.71 |
0.29 1.87 |
0.42 1.48 |
0.23 0.79 |
0.55 2.65 |
0.98 3.74 |
0.72 2.75 |
| Total Terpene content | 0.36 2.32 |
0.93 3.79 |
0.81 4.25 |
0.39 3.09 |
0.30 1.65 |
0.68 3.11 |
0.35 2.28 |
0.47 1.65 |
0.30 1.04 |
0.60 2.92 |
1.12 4.18 |
0.81 3.08 |
| MONO 1 out of total terpene content (%) | 16.7 | 15.0 | 11.1 | 7.7 | 23.3 | 13.3 | 16.7 | 10.6 | 24.1 | 8.3 | 12.5 | 11.1 |
1 % represents percent weight/weight. 2 MONO relates to the sum of monoterpenes and monoterpenoids. 3 SESQUI relates to the sum of sesquiterpenes and sesquiterpenoids. THC = tetrahydrocannabinol, THCA = tetrahydrocannabinolic acid, CBD = cannabidiol, CBDA = cannabidiol acid. Total cannabinoid content was calculated as if all of the cannabinoids were in their decarboxylated form. Blank cells indicate terpene levels which are under reporting limit. α-terpinene, p-cymene, γ-terpinene, iso-pulegol, eucalyptol, borneol and caryophyllene oxide were also assessed and were found to be under quantification limit.
Figure 2.
Terpene content in commercial olive-oil-diluted cannabis extracts (cannabis oils) marketed in Israel, presented as milligrams of each terpene per 100 mg total cannabinoid content. Terpenes are arranged according to their boiling points. As seen, monoterpenes and monoterpenoids are majorly lost in these extracts. Monoterpene loss nearly eliminates the diversity in extracts with regard to terpene profile.
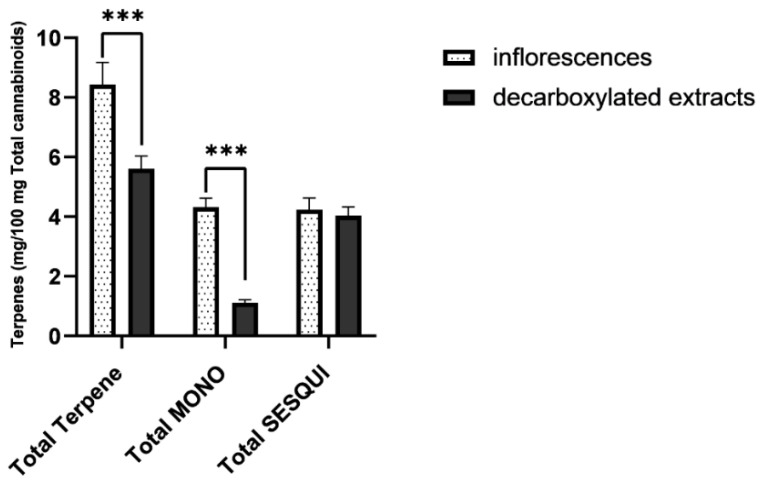
Comparisons between the inflorescence and oil samples are provided in Figure 3 [Total terpene content- inflorescences: M = 7.6, SD = 2.6; oils: M = 2.8, SD = 1.0 mg/ 100 mg cannabinoids, (t(25.768) = 7.3, p < 0.001, d = 2.3). Total monoterpene and monoterpenoid content: inflorescences: M = 5.0, SD = 2.1; oils: M = 0.3, SD = 0.1 mg/ 100 mg cannabinoids, (t(18.208) = 9.6, p < 0.001, d = 2.8). Total sesquiterpene and sesquiterpenoid content: inflorescences: M = 2.6, SD = 0.9); oils: (M = 2.4, SD = 0.9) mg/ 100 mg cannabinoids, (t(29) = 0.5, p > 0.05, d = 0.2). Independent sample t-test]. It is important to note that this marked reduction in the fraction of the monoterpenes nearly eliminates the diversity in the oils with regards to the terpenes profile, as seen in Figure 2.
Figure 3.
Averaged terpene/cannabinoid proportions of commercial cannabis inflorescences (n = 19) and commercial cannabis extracts (n = 12), presented as milligrams of each terpene per 100 mg total cannabinoid content. As seen, total terpene proportion in cannabis extracts is significantly reduced. This reduction derives from a significant loss of monoterpene and monoterpenoids. Total MONO = total monoterpenes and monoterpenoids. Total SESQUI = total sesquiterpenes and sesquiterpenoids. Asterisks denote significance level. *** p < 0.001. Error Bars denote SEM.
The lower monoterpene and monoterpenoid content of the oils was previously reported [12,32,33] and is typically attributed to their relative volatility, leading to evaporation during the extraction and during the thermal treatment steps of typical cannabis oil manufacturing processes. Those steps include inflorescences’ drying, grinding, extraction, and decarboxylating [4,33] the natural acid tetrahydrocannabinolic acid (THCA) and cannabidiol acid (CBDA) into their neutral THC and CBD form (the endocannabinoids receptors’ agonists form, [34,35,36,37]).
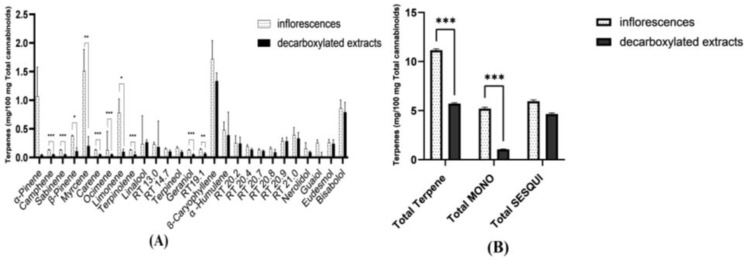
Since the oils in Figure 2 do not directly correspond to the inflorescences in Figure 1, and in order to further verify the selective loss of monoterpenes and monoterpenoids during cannabis oil production, a dedicated industrial trial was conducted, comparing the terpene contents of ten inflorescences to those of concentrated decarboxylated extracts produced from these inflorescences. The results, presented in Table 4 and in Figure 4, confirm the previous observations: total terpene content drops due to a drastic drop in the content of monoterpenes. Since there is no significant reduction in the content of the sesquiterpene and sesquiterpenoids, the fraction of monoterpenes out of the total terpene content is markedly reduced in the extracts. [Total terpene content: inflorescences: M = 11.2, SD = 0.4; oils: M = 5.7, SD = 0.3 mg/100 mg cannabinoids, ((t(18) = 2.9, p < 0.01, d = 1.3). Total monoterpene and monoterpenoid content: inflorescences: M = 5.2, SD = 0.5; oils: M = 1.1, SD = 0.1 mg/100 mg cannabinoids, (t(10.646) = 10.0, p < 0.001, d = 4.5). Total sesquiterpene and sesquiterpenoid content: inflorescences: M = 5.9, SD = 0.4; oils: M = 4.6, SD = 0.3 mg/100 mg cannabinoids, (t(18) = 0.414, p > 0.05, d = 0.185). Independent sample t-test]. See Supplementary data for further information).
Table 4.
Cannabinoid and terpene compositions in various inflorescences and in decarboxylated extracts produced from them. Data is presented as absolute percentage 1 and in the form of milligrams of each terpene per 100 mg total cannabinoid content (in italic).
| Inf 1 | Ext1 | Inf 2 | Ext 2 | Inf 3 | Ext 3 | Inf 4 | Ext 4 | Inf 5 | Ext 5 | Inf 6 | Ext 6 | Inf 7 | Ext 7 | Inf 8 | Ext 8 | Inf 9 | Ext 9 | Inf 10 | Ext 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THCA | 7.8 | 1.6 | 12.9 | 0.6 | 13.7 | 5.3 | 12.8 | 2.8 | 10.3 | 2.6 | 13.9 | 4.8 | 16.8 | 0.9 | 17.1 | 2.2 | 16.7 | 0.9 | 20.3 | 0.6 |
| THC | 1.2 | 58.4 | 1.6 | 61 | 1 | 54.2 | 0.6 | 57.3 | 1.1 | 59 | 0.7 | 56.1 | 0.8 | 62.3 | 0.6 | 57.2 | 0.6 | 69.9 | 0.7 | 62.4 |
| Total THC | 8 | 59.8 | 12.9 | 61.5 | 13 | 58.8 | 11.8 | 59.8 | 10.1 | 61.3 | 12.9 | 60.3 | 15.5 | 63 | 15.5 | 59.1 | 15.3 | 70.4 | 18.5 | 62.9 |
| CBDA | ||||||||||||||||||||
| CBD | ||||||||||||||||||||
| Total CBD | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Total quantified cannabinoids | 8 | 59.8 | 12.9 | 61.5 | 13 | 58.8 | 11.8 | 59.8 | 10.1 | 61.3 | 12.9 | 60.3 | 15.5 | 63.0 | 15.5 | 59.1 | 15.3 | 70.4 | 18.5 | 62.9 |
| α-Pinene | 0.1 1.25 |
0.15 1.12 |
0.07 0.57 |
0.02 0.16 |
0.18 1.4 |
0.03 0.15 |
0.02 0.14 |
0.83 5.43 |
0.07 0.36 |
|||||||||||
| Camphene | ||||||||||||||||||||
| Sabinene | ||||||||||||||||||||
| β-Pinene | 0.04 0.51 |
0.06 0.47 |
0.02 0.1 |
0.02 0.16 |
0.03 0.26 |
0.07 0.5 |
0.04 0.24 |
0.04 0.27 |
0.03 0.04 |
0.19 1.21 |
0.13 0.64 |
|||||||||
| Myrcene | 0.03 0.38 |
0.06 0.44 |
0.03 0.2 |
0.06 0.53 |
0.01 0.02 |
0.14 1.37 |
0.02 0.03 |
0.08 0.6 |
0.03 0.05 |
0.37 2.3 |
0.62 4.1 |
0.03 0.04 |
0.30 1.94 |
0.04 0.05 |
0.39 2.1 |
|||||
| Carene | ||||||||||||||||||||
| Ocimene | 0.01 0.1 |
|||||||||||||||||||
| Limonene | 0.03 0.36 |
0.05 0.38 |
0.04 0.3 |
0.03 0.06 |
0.02 0.04 |
0.11 1.13 |
0.03 0.06 |
0.06 0.5 |
0.05 0.09 |
0.11 0.7 |
0.14 0.93 |
0.02 0.03 |
0.10 0.66 |
0.05 0.07 |
0.52 2.78 |
0.10 0.14 |
||||
| Terpinolene | 0.05 0.08 |
|||||||||||||||||||
| Linalool | 0.12 0.2 |
0.15 0.25 |
0.02 0.2 |
0.12 0.21 |
0.02 0.15 |
0.1 0.16 |
0.06 0.55 |
0.32 0.52 |
0.02 0.1 |
0.1 0.17 |
0.06 0.39 |
0.14 0.21 |
0.05 0.35 |
0.12 0.21 |
0.04 0.24 |
0.12 0.16 |
0.16 0.84 |
0.35 0.56 |
||
| RT 13.0 * | 0.03 0.33 |
0.22 0.37 |
0.04 0.34 |
0.2 0.33 |
0.03 0.2 |
0.12 0.21 |
0.03 0.06 |
0.04 0.38 |
0.22 0.37 |
0.04 0.3 |
0.19 0.31 |
0.04 0.28 |
||||||||
| RT 14.7 * | 0.01 0.18 |
0.02 0.19 |
0.11 0.18 |
0.06 0.1 |
0.03 0.26 |
0.16 0.26 |
0.02 0.2 |
0.1 0.17 |
||||||||||||
| Terpineol | 0.06 0.1 |
0.05 0.08 |
0.06 0.1 |
0.02 0.15 |
0.07 0.10 |
0.02 0.14 |
0.06 0.10 |
0.02 0.15 |
0.01 0.01 |
0.07 0.4 |
0.19 0.31 |
|||||||||
| Geraniol | ||||||||||||||||||||
| RT19.1 * | 0.03 0.06 |
0.07 0.12 |
0.04 0.07 |
0.02 0.04 |
0.02 0.03 |
0.04 0.26 |
0.09 0.13 |
0.04 0.07 |
||||||||||||
| β-Caryophyllene | 0.06 0.77 |
0.57 0.98 |
0.11 0.82 |
0.67 1.09 |
0.14 1.1 |
0.64 1.09 |
0.13 1.04 |
0.6 1 |
0.13 1.27 |
0.68 1.11 |
0.08 0.6 |
0.37 0.61 |
0.45 3.0 |
0.96 1.48 |
0.44 2.88 |
0.99 1.66 |
0.40 2.62 |
1.26 1.71 |
0.57 3.0 |
1.08 1.73 |
| α -Humulene | 0.02 0.26 |
0.17 0.28 |
0.04 0.28 |
0.2 0.33 |
0.03 0.2 |
0.17 0.29 |
0.04 0.3 |
0.19 0.31 |
0.04 0.36 |
0.2 0.33 |
0.03 0.2 |
0.13 0.22 |
0.14 0.90 |
0.32 0.5 |
0.13 0.85 |
0.30 0.51 |
0.08 0.51 |
0.26 0.36 |
0.14 0.78 |
0.27 0.43 |
| RT 20.2 * | 0.09 0.15 |
0.1 0.16 |
0.1 0.3 |
0.02 0.04 |
0.04 0.07 |
0.01 0.1 |
0.04 0.07 |
0.22 1.4 |
0.49 0.75 |
0.05 0.07 |
0.03 0.08 |
|||||||||
| RT 20.4 * | 0.07 0.11 |
0.12 0.2 |
0.04 0.3 |
0.1 0.3 |
0.01 0.1 |
0.06 0.1 |
0.01 0.13 |
0.08 0.13 |
0.02 0.03 |
0.04 0.25 |
0.08 0.13 |
0.04 0.24 |
0.12 0.17 |
0.07 0.40 |
0.17 0.27 |
|||||
| RT 20.7 * | 0.01 0.15 |
0.08 0.14 |
0.02 0.16 |
0.1 0.16 |
0.06 0.1 |
0.02 0.14 |
0.09 0.15 |
0.01 0.09 |
0.06 0.09 |
0.07 0.12 |
0.02 0.16 |
0.06 0.1 |
0.02 0.13 |
0.06 0.08 |
0.01 0.10 |
0.06 0.09 |
||||
| RT 20.8 * | 0.03 0.06 |
0.04 0.06 |
0.07 0.12 |
0.01 0.08 |
0.05 0.08 |
0.01 0.09 |
0.05 0.08 |
0.01 |
0.02 0.04 |
0.03 0.21 |
0.02 0.16 |
0.12 0.20 |
0.01 0.07 |
0.06 0.09 |
0.02 0.10 |
0.06 0.09 |
||||
| RT 20.9 * | 0.02 0.31 |
0.23 0.38 |
0.04 0.34 |
0.26 0.42 |
0.04 0.3 |
0.18 0.3 |
0.06 0.48 |
0.34 0.57 |
0.06 0.6 |
0.36 0.58 |
0.04 0.3 |
0.16 0.2 |
0.05 0.32 |
0.05 0.33 |
0.18 0.24 |
0.02 0.14 |
||||
| RT 21.0 * | 0.02 0.29 |
0.19 0.32 |
0.04 0.29 |
0.24 0.39 |
0.03 0.3 |
0.12 0.21 |
0.06 0.48 |
0.35 0.59 |
0.05 0.52 |
0.31 0.51 |
0.04 0.3 |
0.15 0.25 |
0.23 1.49 |
|||||||
| Nerolidol | 0.05 0.09 |
0.08 0.12 |
0.02 0.03 |
0.02 0.19 |
0.12 0.2 |
0.01 0.1 |
0.06 0.1 |
0.03 0.23 |
0.04 0.24 |
|||||||||||
| Guaiol | 0.03 0.38 |
0.24 0.42 |
0.05 0.37 |
0.27 0.44 |
0.02 0.2 |
0.16 0.27 |
0.06 0.57 |
0.36 0.58 |
0.05 0.4 |
0.26 0.43 |
||||||||||
| Eudesmol * | 0.03 0.41 |
0.3 0.51 |
0.05 0.4 |
0.26 0.43 |
0.04 0.3 |
0.16 0.27 |
0.01 0.08 |
0.03 0.04 |
0.06 0.61 |
0.38 0.62 |
0.05 0.4 |
0.26 0.43 |
0.02 0.14 |
0.02 0.11 |
0.02 0.16 |
0.07 0.10 |
0.01 0.06 |
|||
| Bisabolol | 0.12 1.5 |
0.84 1.42 |
0.18 1.38 |
0.96 1.57 |
0.11 0.8 |
0.65 1.11 |
0.08 0.63 |
0.4 0.67 |
0.12 1.24 |
0.78 1.27 |
0.17 1.3 |
0.88 1.47 |
0.10 0.63 |
0.25 0.38 |
0.07 0.45 |
0.21 0.34 |
0.03 0.20 |
0.12 0.16 |
0.07 0.38 |
0.15 0.24 |
| Total Terpene content | 0.55 7.18 |
3.26 5.43 |
0.9 7.1 |
3.84 6.2 |
0.58 4.5 |
2.81 5.1 |
0.59 4.74 |
2.36 3.93 |
1.0 9.78 |
4.2 6.85 |
1.1 7.4 |
2.97 4.94 |
2.0 12.7 |
2.39 3.72 |
1.67 11.06 |
2.11 3.35 |
2.12 13.66 |
2.4 3.66 |
2.25 12.0 |
2.46 3.63 |
| Monoterpenes out of total terpenes content (%) | 43 | 12 | 42 | 14 | 23 | 14 | 29 | 9 | 42 | 18 | 50 | 19 | 34 | 13 | 53 | 16 | 69 | 9 | 59 | 20 |
1 % represents percent weight/weight. Inf = inflorescence, THC = tetrahydrocannabinol, THCA = tetrahydrocannabinolic acid, CBD = cannabidiol, CBDA = cannabidiol acid. Total cannabinoid content was calculated as if all of the cannabinoids were in their decarboxylated form. Blank cells indicate terpene levels which are under reporting limit. α-terpinene, p-cymene, γ-terpinene, iso-pulegol, eucalyptol, borneol and caryophyllene oxide were also assessed and were found to be under quantification limit. * Due to lack in analytical standards, content was estimated by calculating terpene’s area from α-humulene response factor. Retention Time (RT) of identified terpenes (min): α-Pinene (6.5); Camphene (7); Sabinene (7.3), Myrcene (7.4), β-Pinene (7.5), Carene (8.3), α-Terpinene (8.5), Ocimene (8.7), p-Cymene (8.9), Limonene (8.8), γ-Terpinene (9.7), Eucalyptol (9.2), Terpinolene (10.6), Linalool (11.5), Iso-Pulegol (13.2), Borneol (14), Terpineol (14.2), Geraniol (15.5), β-Caryophyllene (18.9), α-Humulene (19.6), Nerolidol (21.2), Guaiol (22.2), Caryophyllene oxide (22.3), Eudesmol (22.8), Bisabolol (23.3).
Figure 4.
Averaged terpene content of ten inflorescences and of the decarboxylated extracts produced from them, presented as milligrams of each terpene per 100 mg total cannabinoids content. (A) Averaged data per each terpene. (B) Averaged data for total terpene content, total monoterpene and monoterpenoid content (total MONO), and total sesquiterpene and sesquiterpenoid content (total SESQUI) (see Supplementary data for further details). As seen, while the content of sesquiterpenes and sesquiterpenoids is largely kept in the formed extracts, monoterpenes are completely or mostly lost. Asterisks denote significance level. * p < 0.05, ** p < 0.01, *** p < 0.001. Error Bars denote SEM.
To summarize these results, comparing commercial cannabis inflorescences to commercial cannabis oils consumed by medical cannabis patients in Israel, a significantly lower total amount of terpenes was found in the latter and a much-reduced fraction of the monoterpenes and monoterpenoids out of the total terpene content. The same observations were found when directly comparing inflorescences to decarboxylated extracts produced from them. Future studies should further address potential differences in bioactive components, other than terpenes, such as flavonoids.
This deficiency in terpenes of the oils, particularly in that of monoterpenes and monoterpenoids, may have major implications. This is since terpenes have therapeutic properties of their own and, furthermore, may modify the therapeutic activity of cannabinoids via a synergic and/or entourage effect [25], as discussed above.
Of particular importance here is the therapeutic role of monoterpenes, which form a major fraction of the terpenes in inflorescences, but are mostly lost in the oils manufactured from them [10,11,12,38]. As shown above, monoterpenes are also mainly responsible for the diversity of the chemovars. Previous reports have suggested that monoterpenes have a primary role in classifying a chemovar as having a dominant indica effect vs. a dominant sativa effect. The presence of myrcene has the strongest association with the Indica type, providing a sedative, effect, while chemotypes with low myrcene levels have a more energetic type [10,26,27]. The presence of terpinolene, another monoterpene, was found by Casano et al. [26] to be mostly associated with the sativa type.
Given the deficiency of oils in these important active pharmaceutical ingredients, there is no support for terms such as “whole plant extracts” or “full spectrum extracts”, when applied to cannabis extracts/oils. Most studies, clinical trials, and reviews using these terms, e.g., [39,40,41,42], lack important composition information and are not really conclusive without terpene content data. Furthermore, these terms are misleading, creating the expectation that these “whole plant/full spectrum” extracts represent the composition of the original plant. Similarly deficient are clinical trials of oils specified by the strain used to form them, assuming that this strain’s properties are retained in the formed extract [43,44,45]. As the composition of terpenes in extracts varies with cultivation conditions and depends on the extraction and thermal process steps used [4,33,42,46,47], no reproducibility is guaranteed for different extracts of the same plant source, unless directly analyzed and corrected. It is worth noting that Sativex® and Cannador®, the intensively studied and used cannabis extracts, are defined by their THC and CBD content, with no data on the other extracted ingredients [40,48]. The deficiency, or major reduction in monoterpene content in the oils, may support the hypothesis regarding the impaired efficacy of oils compared with smoking, which drives patients to prefer the latter. It also points out the directions to resolve this issue and to make oils the preferred formulations, as further detailed below.
3. Conclusions and Implications
In conclusion, while smoking has many disadvantages, there could be some justifications to patients’ claims indicating an inferior effect of the oils. Oils significantly lack in monoterpene content as compared with inflorescences, the relative proportion of the various terpenes changes, and the differentiation gained by the content of specific monoterpenes is lost. As an example, monoterpenes are responsible for the differences between the effects of sativa and indica type chemovars, but those distinguishing terpenes are lost in oil production, so that oils produced from sativa-type chemovars do not differ much from oils produced from indica-type chemovars of similar cannabinoid content.
Some publications suggest collecting the lost terpenes and adding them back to the produced oil [33,47]. Given the number of steps wherein terpenes evaporate and given the need to separate terpene vapors from other gaseous components (e.g., solvent vapors), such collection is practically infeasible in industrial production, processing dozens of kilograms of inflorescence per batch. However, there is a much simpler solution. The terpenes of interest are produced not only in cannabis and could be obtained from other sources, e.g., hops (myrcene), pine (pinene), and lavender (linalool). Those terpenes are commercially available, most times at high purity and at a relatively low price. Accordingly, terpene loss may be resolved by obtaining those terpenes from other plants and adding them to the generated cannabis extracts. This modified method has several important advantages, as detailed below.
First are accuracy and reproducibility. According to the modified method, the extracts formed are analyzed for their terpene content and then calculated amounts of each desired terpene are added to accurately reach the selected level. This way, the final concentration of each terpene would be the same in each preparation, independent of the extracted inflorescence or of the production parameters. Additionally, purified cannabinoid (“isolates”) may be used without worrying about the terpenes lost in the purification step [49]. Similarly, synthetically or bio-synthetically produced cannabinoids formed with no terpenes may be used for desired compositions.
An even more important advantage is, that one is not limited to terpene compositions replicating those of particular chemovars. It should be kept in mind that the terpene compositions of a “whole plant” or a “full spectrum” are not designed by nature for the benefit of the medical user, but rather for the needs of the cannabis plant, such as plant protection [12,50,51] (but see also Namdar et al. [16] with a different reasoning). Additionally, generating new compositions for improved efficacy and for new applications does not require years of costly breeding of new chemovars. Cannabinoids and terpenes can be simply added (or removed) to reach any desired composition. For example, the loss of the sativa/indica nature in extraction could be resolved by adding terpenes for recreating the original plant terpene composition. However, why stop there? Terpenes known to improve sleep quality can be added to various oils for night use, while terpenes improving awareness and functionality can be added to various oils designed for day use. Going even further, cannabis oil compositions can specifically be designed for various medical functions, e.g., pain management or anxiety relief. Suitable terpene compositions, based on terpenes’ pharmaceutical properties and their role in modulating the cannabinoid pharmaceutical effects [15,16,17,18,19,21], will be added to each designated product. Adjusting the composition to varying requirements (e.g., pain composition for day use vs. ones for night use) or to varying populations (e.g., children vs. adults or men vs. women) allows further tailoring the cannabis oils medicinal product to desired needs. This enrichment of oils with selected terpenes for selected needs is robustly doable in industrial settings and is already implemented in products available on Israeli market [52,53,54,55].
4. Materials and Methods
4.1. Materials
Commercial medical cannabis extracts (“cannabis oils”) and commercial inflorescences, produced by various Israeli licensed producers, were obtained from Bazelet Pharma (Or Akiva, Israel). Cannabinoid standards: cannabinol (CBN), cannabichromene (CBC), cannabichromenic acid (CBCA), cannabigerolic acid (CBGA), cannabigerol (CBG), cannabidiolic acid (CBDA), cannabidiol (CBD), delta-9-tetrahydrocannabinoic acid (THCA) and delta-9-tetrahydrocannabinol (THC) were purchased from Cerrilliant (Cerilliant Corporation, Round Rock, TX, USA). Cannabidivarin (CBDV), cannabidivarinic Acid (CBDVA), tetrahydrocannabivarin (THCV), and delta-8-tetrahydrocannabinol (Δ8-THC) were purchased from Restek (Bellefonte, PA, USA). All cannabinoid standards are Certified Reference Materials (CRM) standards, at 1000 µg/mL in methanol. Terpene standards were purchased from Restek (Bellefonte, PA, USA), Merck (Rosh-Ha’ayin, Israel), and Phyto Lab (Vestenbergsgreuth, Germany) (See Supplementary data for further details). Ethanol for standard solutions and samples preparation was HPLC grade (J.T. Baker, Phillipsburg, NJ, USA). All terpene standards are Certified Reference Materials (CRM) standards, at 2500 µg/mL in isopropanol.
4.2. Methods
In order to directly evaluate the terpene content changes during a typical industrial cannabis oil production, an industrial trial was performed in which inflorescences of ten different chemovars were analyzed and then extracted. Inflorescences of each chemovar went through the following steps. A 10 kg batch was dried at ambient temperature and at 50% relative humidity to reach a moisture content of 11–12%. The 10 kg of dried inflorescences were ground and then contacted at ambient temperature with 60 L ethanol, while mixing for a duration of 30 min. The formed ethanol-diluted extract was separated. The same extraction procedure was repeated for a second time with 40 more liters of fresh ethanol and the second formed ethanol-diluted extract was separated from the residual extracted plant material. Multiple previous tests have confirmed full cannabinoids extraction in this procedure. The two ethanol-diluted extracts were combined and the ethanol was evaporated out at 40 °C under reduced pressure, to form a concentrated extract (of about 60% THCA). This concentrated extract was held at 110 °C during an hour, for decarboxylation of the acid form THCA to THC. The same procedure was repeated for each one of the ten chemovars. Analysis and extract production were conducted at the IGMP Bazelet Medical Cannabis manufacturing plant in Or Akiva, Israel.
4.3. Analysis
Inflorescences and extracts were analyzed for their cannabinoid and terpene content, using high performance liquid chromatography (HPLC) and gas chromatography (GC), respectively. Inflorescences were prepared for analysis via a standardized procedure, comprising extraction of 0.5 gr inflorescences in 45 gr ethanol.
High Performance Liquid Chromatography (HPLC): The analysis of cannabinoids was carried out on HPLC Waters PDA 2996 (Waters Corporation, Milford, MA, USA), equipped with a pump, autosampler, column-oven, and a Photodiode Array detector (PDA) detector. The analytical balance was Mettler Toledo MS205DU (Mettler Toledo, Columbus, OH, USA). The HPLC column used was Phenomenex Luna Omega C18 column (Phenomenex, Torrance, CA, USA). The mobile phase was buffer (ammonium acetate): acetonitrile, at 1:1 ratio, at a constant flow of 0.1 mL/min. Detection used wavelength of 220 nm, injected volume: 10 µL. The method is fully validated for 12 cannabinoids in line with the requirements of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) [56] guidelines, Israeli Medical Cannabis Association (IMCA), European Pharmacopoeia (EP) [57], and United States Pharmacopeia (USP) [58]. The nominal working concertation is 100 µg/mL and the method range is 0.1–120.0% of that nominal working concentration, proved by linearity, precision, and accuracy studies. The limit of detection of the method is 0.1 µg/mL and the limit of quantitation −0.2 µg/mL. Uncertainties were within 5% of the reported value. Total cannabinoid content was calculated as if all of the cannabinoids were in their decarboxylated form (See also Supplementary data).
Gas Chromatography (GC): Terpene analysis was carried out on Agilent Technologies GC system model 6890 N (Agilent Technologies, Santa Clara, CA, USA) equipped with Flame Ionization Detector (FID). Identification was based on the retention times of the Certified Reference Materials (CRM) standards and was verified by GC MS (Gas chromatography–mass spectrometry) at Aminocann (Aminolab, Ness Ziona, Israel). A CTC autosampler (Pal RTC, CTC analytics, Zwingen, Switzerland) was used. The column used was Phenomenex ZB-624plus (Phenomenex, Torrance, CA, USA) with helium as carrier at 1.2 mL/min constant flow. The method is fully validated for 25 terpenes likely to be present in cannabis. The method is fully validated according to the requirements of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) [56] guidelines, Israeli Medical Cannabis Association (IMCA), European Pharmacopoeia (EP) [57], and United States Pharmacopeia (USP) [58]. The range of the method was between 200–4000 µg/mL, proved by linearity, precision, and accuracy studies. The limit of reporting was 200 µg/mL. Uncertainties were within 5% of the reported value. Terpenes lacking analytical standards are presented by their retention time and their content was estimated by calculating their area according to α-Humulene response factor. Retention times for identified terpenes are also provided (see Table 4) for reference.
Statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA) (See Supplementary data for further detail).
Acknowledgments
The authors thank Danielle Hen-Shoval, Daniel Schlesinger, Dana Berneman Zeitouni, and Iso Heller for their assistance with the manuscript. The authors thank Noam Huppert and Inna Perutski for HPLC and GC analyses.
Supplementary Materials
The following supporting information can be downloaded online: https://www.mdpi.com/article/10.3390/molecules27206920/s1; Figure S1: HPLC chromatogram—a mixture of cannabinoid standards; Figure S2: GC chromatogram-Terpene standards; Table S1: Comparisons between individual terpene content in ten inflorescences and decarboxylated extracts produced from them.
Author Contributions
Writing—original draft preparation, N.R.; writing—review and editing, A.M.E. and E.M.D. All authors have equally contributed to the work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
N.R. and A.M.E. are employers in Bazelet group, a medical cannabis manufacture in Israel. E.M.D. supervises trials supported by Bazelet group.
Sample Availability
Data of evaluated inflorescences and cannabis extracts are available from the authors.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ministry of Health The Israeli Medical Cannabis Agency (IMCA) [(accessed on 1 July 2022)]; Available online: https://www.health.gov.il/Subjects/cannabis/Documents/licenses-status-july-2021.pdf.
- 2.Namdar D., Mazuz M., Ion A., Koltai H. Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods. Ind. Crops Prod. 2018;113:376–382. doi: 10.1016/j.indcrop.2018.01.060. [DOI] [Google Scholar]
- 3.Hanuš L.O. The Study of Chemical Differences of Hashish from Different Sources Seized in Israel. 2014. [(accessed on 1 July 2022)]. Available online: https://www.researchgate.net/publication/291355863_The_study_of_chemical_differences_of_hashish_from_different_sources_seized_in_Israel.
- 4.Eyal A.M., Berneman Zeitouni D., Tal D., Schlesinger D., Davidson E.M., Raz N. Vapor Pressure, Vaping, and Corrections to Misconceptions Related to Medical Cannabis’ Active Pharmaceutical Ingredients’ Physical Properties and Compositions. Cannabis Cannabinoid Res. 2022 doi: 10.1089/can.2021.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haroutounian S., Arendt-Nielsen L., Belton J., Blyth F.M., Degenhardt L., Di Forti M., Eccleston C., Finn D.P., Finnerup N.B., Fisher E., et al. International Association for the Study of Pain Presidential Task Force on Cannabis and Cannabinoid Analgesia: Research Agenda on the Use of Cannabinoids, Cannabis, and Cannabis-Based Medicines for Pain Management. Pain. 2021;162:S117–S124. doi: 10.1097/j.pain.0000000000002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Hong P.J., May C., Rehman Y., Oparin Y., Hong C.J., Hong B.Y., AminiLari M., Gallo L., Kaushal A., et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: A systematic review and meta-analysis of randomised clinical trials. BMJ. 2021;374 doi: 10.1136/bmj.n1034. [DOI] [PubMed] [Google Scholar]
- 7.McKee K.A., Hmidan A., Crocker C.E., Lam R.W., Meyer J.H., Crockford D., Trépanier A., Aitchison K.J., Tibbo P.G. Potential therapeutic benefits of cannabinoid products in adult psychiatric disorders: A systematic review and meta-analysis of randomised controlled trials. J. Psychiatr. Res. 2021;140:267–281. doi: 10.1016/j.jpsychires.2021.05.044. [DOI] [PubMed] [Google Scholar]
- 8.Koppel B.S., Brust J.C., Fife T., Bronstein J., Youssof S., Gronseth G., Gloss D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556–1563. doi: 10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher E., Moore R.A., Fogarty A.E., Finn D.P., Finnerup N.B., Gilron I., Haroutounian S., Krane E., Rice A.S., Rowbotham M., et al. Cannabinoids, Cannabis, and Cannabis-Based Medicine for Pain Management: A Systematic Review of Randomised Controlled Trials. Pain. 2021;162:S45–S66. doi: 10.1097/j.pain.0000000000001929. [DOI] [PubMed] [Google Scholar]
- 10.Hanuš L.O., Hod Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids. 2020;3:61–73. doi: 10.1159/000509733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis M.A., Russo E.B., Smith K.M. Pharmacological Foundations of Cannabis Chemovars. Planta Med. 2018;84:225–233. doi: 10.1055/s-0043-122240. [DOI] [PubMed] [Google Scholar]
- 12.Shapira A., Berman P., Futoran K., Guberman O., Meiri D. Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections. Anal. Chem. 2019;91:11425–11432. doi: 10.1021/acs.analchem.9b02844. [DOI] [PubMed] [Google Scholar]
- 13.Fischedick J.T. Identification of Terpenoid Chemotypes Among High (−)-trans-Δ9- Tetrahydrocannabinol-Producing Cannabis sativa L. Cultivars. Cannabis Cannabinoid Res. 2017;2:34–47. doi: 10.1089/can.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aviram J., Lewitus G.M., Vysotski Y., Yellin B., Berman P., Shapira A., Meiri D. Prolonged Medical Cannabis Treatment is Associated With Quality of Life Improvement and Reduction of Analgesic Medication Consumption in Chronic Pain Patients. Front. Pharmacol. 2021;12:1199. doi: 10.3389/fphar.2021.613805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namdar D., Voet H., Ajjampura V., Nadarajan S., Mayzlish-Gati E., Mazuz M., Shalev N., Koltai H. Terpenoids and Phytocannabinoids Co-Produced in Cannabis Sativa Strains Show Specific Interaction for Ell Cytotoxic Activity. Molecules. 2019;24:3031. doi: 10.3390/molecules24173031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuutinen T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018;157:198–228. doi: 10.1016/j.ejmech.2018.07.076. [DOI] [PubMed] [Google Scholar]
- 18.LaVigne J.E., Hecksel R., Keresztes A., Streicher J.M. Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci. Rep. 2021;11:8232. doi: 10.1038/s41598-021-87740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamal B.S., Kamal F., Lantela D.E. Cannabis and the Anxiety of Fragmentation—A Systems Approach for Finding an Anxiolytic Cannabis Chemotype. Front. Neurosci. 2018;12:730. doi: 10.3389/fnins.2018.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McPartland J.M., Russo E.B. Cannabis and Cannabis Extracts: Greater than the Sum of Their Parts? J. Cannabis Ther. 2012;1:103–132. doi: 10.1300/J175v01n03_08. [DOI] [Google Scholar]
- 21.Russo E.B., Marcu J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In: Kendall D., Alexander S.P.H., editors. Advances in Pharmacology. Volume 80. Academic Press; Cambridge, MA, USA: 2017. pp. 67–134. [DOI] [PubMed] [Google Scholar]
- 22.Sharma C., Al Kaabi J.M., Nurulain S.M., Goyal S.N., Kamal M.A., Ojha S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016;22:3237–3264. doi: 10.2174/1381612822666160311115226. [DOI] [PubMed] [Google Scholar]
- 23.Goto T., Takahashi N., Hirai S., Kawada T. Various Terpenoids Derived from Herbal and Dietary Plants Function as PPAR Modulators and Regulate Carbohydrate and Lipid Metabolism. PPAR Res. 2010;2010 doi: 10.1155/2010/483958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meotti F.C., De Andrade E.L., Calixto J.B. TRP Modulation by Natural Compounds. Mamm. Transient Recept. Potential Cation Channels. 2014;223:1177–1238. doi: 10.1007/978-3-319-05161-1_19. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Shabat S., Fride E., Sheskin T., Tamiri T., Rhee M.-H., Vogel Z., Bisogno T., De Petrocellis L., Di Marzo V., Mechoulam R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998;353:23–31. doi: 10.1016/S0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- 26.Casano S., Grassi G., Martini V., Michelozzi M. Variations in Terpene Profiles of Different Strains of Cannabis sativa L. Acta Hortic. 2011;925:115–121. doi: 10.17660/ActaHortic.2011.925.15. [DOI] [Google Scholar]
- 27.Hazekamp A., Tejkalová K., Papadimitriou S. Cannabis: From Cultivar to Chemovar II—A Metabolomics Approach to Cannabis Classification. Cannabis Cannabinoid Res. 2016;1:202–215. doi: 10.1089/can.2016.0017. [DOI] [Google Scholar]
- 28.Koltai H., Namdar D. Cannabis Phytomolecule ‘Entourage’: From Domestication to Medical Use. Trends Plant Sci. 2020;25:976–984. doi: 10.1016/j.tplants.2020.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Ferber S.G., Namdar D., Hen-Shoval D., Eger G., Koltai H., Shoval G., Shbiro L., Weller A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2019;18:87–96. doi: 10.2174/1570159X17666190903103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallily R., Yekhtin Z., Hanuš L.O. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018;3:282–290. doi: 10.1089/can.2018.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jugl S., Sajdeya R., Morris E.J., Goodin A.J., Brown J.D. Much Ado about Dosing: The Needs and Challenges of Defining a Standardized Cannabis Unit. Med. Cannabis Cannabinoids. 2021;4:121–124. doi: 10.1159/000517154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milay L., Berman P., Shapira A., Guberman O., Meiri D. Metabolic Profiling of Cannabis Secondary Metabolites for Evaluation of Optimal Postharvest Storage Conditions. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.583605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ternelli M., Brighenti V., Anceschi L., Poto M., Bertelli D., Licata M., Pellati F. Innovative methods for the preparation of medical Cannabis oils with a high content of both cannabinoids and terpenes. J. Pharm. Biomed. Anal. 2020;186 doi: 10.1016/j.jpba.2020.113296. [DOI] [PubMed] [Google Scholar]
- 34.De Petrocellis L., Ligresti A., Moriello A.S., Allarà M., Bisogno T., Petrosino S., Stott C.G., Di Marzo V. Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J. Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M., Wang Y.-H., Avula B., Radwan M.M., Wanas A., Van Antwerp J., Parcher J.F., ElSohly M.A., Khan I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016;1:262–271. doi: 10.1089/can.2016.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McPartland J.M., Macdonald C., Young M., Grant P., Furkert D.P., Glass M. Affinity and Efficacy Studies of Tetrahydrocannabinolic Acid A at Cannabinoid Receptor Types One and Two. Cannabis Cannabinoid Res. 2017;2:87–95. doi: 10.1089/can.2016.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Citti C., Pacchetti B., Vandelli M.A., Forni F., Cannazza G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA) J. Pharm. Biomed. Anal. 2018;149:532–540. doi: 10.1016/j.jpba.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 38.Sexton M., Shelton K., Haley P., West M. Evaluation of Cannabinoid and Terpenoid Content: Cannabis Flower Compared to Supercritical CO2 Concentrate. Planta Med. 2018;84:234–241. doi: 10.1055/s-0043-119361. [DOI] [PubMed] [Google Scholar]
- 39.Marinotti O., Sarill M. Differentiating Full-Spectrum Hemp Extracts from CBD Isolates: Implications for Policy, Safety and Science. J. Diet. Suppl. 2020;17:517–526. doi: 10.1080/19390211.2020.1776806. [DOI] [PubMed] [Google Scholar]
- 40.Maayah Z.H., Takahara S., Ferdaoussi M., Dyck J.R.B. The anti-inflammatory and analgesic effects of formulated full-spectrum cannabis extract in the treatment of neuropathic pain associated with multiple sclerosis. Inflamm. Res. 2020;69:549–558. doi: 10.1007/s00011-020-01341-1. [DOI] [PubMed] [Google Scholar]
- 41.Maayah Z.H., Takahara S., Ferdaoussi M., Dyck J.R. The molecular mechanisms that underpin the biological benefits of full-spectrum cannabis extract in the treatment of neuropathic pain and inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165771. [DOI] [PubMed] [Google Scholar]
- 42.Nahler G., Jones T.M., Russo E.B. Cannabidiol and Contributions of Major Hemp Phytocompounds to the “Entourage Effect”; Possible Mechanisms. Altern. Complement. Integr. Med. 2019;5:1–16. doi: 10.24966/ACIM-7562/100066. [DOI] [Google Scholar]
- 43.Gallily R., Yekhtin Z. Avidekel Cannabis extracts and cannabidiol are as efficient as Copaxone in suppressing EAE in SJL/J mice. Inflammopharmacology. 2019;27:167–173. doi: 10.1007/s10787-018-0536-3. [DOI] [PubMed] [Google Scholar]
- 44.Baron E.P., Lucas P., Eades J., Hogue O. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J. Headache Pain. 2018;19:37. doi: 10.1186/s10194-018-0862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aran A., Harel M., Cassuto H., Polyansky L., Schnapp A., Wattad N., Shmueli D., Golan D., Castellanos F.X. Cannabinoid treatment for autism: A proof-of-concept randomized trial. Mol. Autism. 2021;12:6. doi: 10.1186/s13229-021-00420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarjani M.P., Young O., Kebede L., Seyfoddin A. Processing and extraction methods of medicinal cannabis: A narrative review. J. Cannabis Res. 2021;3:1–15. doi: 10.1186/s42238-021-00087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sommano S.R., Chittasupho C., Ruksiriwanich W., Jantrawut P. The Cannabis Terpenes. Molecules. 2020;25:5792. doi: 10.3390/molecules25245792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potter D.J. A Review of the Cultivation and Processing of Cannabis (Cannabis sativa L.) for Production of Prescription Medicines in the UK. Drug Test. Anal. 2014;6:31–38. doi: 10.1002/dta.1531. [DOI] [PubMed] [Google Scholar]
- 49.Russo E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 2019;9 doi: 10.3389/fpls.2018.01969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boncan D.A.T., Tsang S.S., Li C., Lee I.H., Lam H.-M., Chan T.-F., Hui J.H. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020;21:7382. doi: 10.3390/ijms21197382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang A.C., Osbourn A. Plant terpenes that mediate below-ground interactions: Prospects for bioengineering terpenoids for plant protection. Pest Manag. Sci. 2019;75:2368–2377. doi: 10.1002/ps.5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eyal A.M., Raz N. Terpene-Enriched Cannabinoid Composition. WO 2017/158539. [(accessed on 1 July 2022)]. Available online: https://www.lens.org/lens/patent/044-893-408-400-92X/frontpage.
- 53.Raz N., Eyal A.M. Terpene-Enriched Cannabinoid Product for Women Health. WO 2019/003163. [(accessed on 1 July 2022)]. Available online: https://www.lens.org/lens/patent/009-467-646-043-39X/frontpage?l=en.
- 54.Raz N., Eyal A.M. Terpene-Enriched Cannabinoid Compositions and Uses Thereof in the Treatment of Infectious Conditions. WO 2019/220324. [(accessed on 1 July 2022)]. Available online: https://www.lens.org/lens/patent/124-913-798-135-062/frontpage?l=en.
- 55.Raz N., Berneman Zeitouni D., Heller I., Eyal A.M. Terpene-Enriched Cannabinoid Composition and Method of Treatment for Treating Conditions And/or Symptoms Associated With Autism Spectrum Disorder. WO 2020/157639. [(accessed on 1 July 2022)]. Available online: https://www.lens.org/lens/patent/156-058-183-509-765/frontpage?l=en.
- 56.Guideline I.C.H. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmon. Guidel. Bioanal. Method Valid. M. 2019;10:2018. [Google Scholar]
- 57.European Pharmacopoeia Commission Strasbourg: Council OF Europe: European Directorate for the Quality of Medicines and Healthcare. Eur. Treaty Ser. 2010 [Google Scholar]
- 58.The United States Pharmacopeial Convention . The United States Pharmacopeia: The National Formulary. The United States Pharmacopeial Convention; Rockville, MD, USA: 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.