Abstract
The prevalence of neurodegenerative diseases is increasing globally, with an imperative need to identify and expand the availability of pharmaceutical treatment strategies. Alzheimer's disease is the most common neurodegenerative disease for which there is no cure and limited treatments. Rodent models are primarily used in Alzheimer's disease research to investigate causes, pathology, molecular mechanisms, and pharmaceutical therapies. However, there is a lack of a comprehensive understanding of Alzheimer's disease causes, pathogenesis, and optimal treatments due in part to some limitations of using rodents, including higher economic cost, which can influence sample size and ultimately statistical power. It is necessary to expand our animal model toolbox to provide alternative strategies in Alzheimer's disease research. The zebrafish application in neurodegenerative disease research and neuropharmacology is greatly expanding due to several vital strengths spanning lower economic costs, the smaller size of the organism, a sequenced characterized genome, and well described anatomical structures. These characteristics are coupled to the conserved molecular function and disease pathways in humans. The existence of orthologs for genes associated with Alzheimer's disease in zebrafish is also confirmed. While wild-type zebrafish appear to lack some of the neuropathological features of Alzheimer's disease, the advent of genetic editing technologies has expanded the evaluation of the amyloid and neurofibrillary tangle hypotheses using the zebrafish and exploration of pharmaceutical molecular targets. An overview of how genetic editing technologies are being used on the zebrafish to create models to investigate the causes, pathology, molecular mechanisms, and pharmaceutical targets of Alzheimer's disease is detailed.
Keywords: Alzheimer's disease, amyloid, brain, drug discovery, genetic models, neurofibrillary, tau, zebrafish
1. INTRODUCTION
As of 2020, there are over 50 million people worldwide with dementia, with approximately 10 million new cases appearing each year. Among those 50 million patients, 30-35 million have been diagnosed with Alzheimer's disease (AD). While dementia tends to impact an older population (>65 years), it should not be regarded as a normal part of aging. AD has a prevalence of 10-30% in individuals aged >65 years, with more than 95% having the sporadic form of AD known as late-onset Alzheimer's disease (LOAD) and less than 5% having early-onset AD (EOAD). Familial AD (FAD), most commonly associated with EOAD, has provided scientists with the vast knowledge of molecular events underlying the disease progression through studies identifying common genetic mutations in these patients. These genetic targets have then been interrogated in laboratory models and confirmed to produce pathologies similar to those observed in AD patients.
Clinical characteristics of AD include progressive memory loss, speech and motor impairments, depression,and delusion, eventually leading to a decrease in health standards. AD pathogenesis is characterized by the failure to clear the amyloid-β (Aβ) peptide from interstitial fluid in the brain.
Improper clearance of Aβ leads to accumulation of Aβ peptides and plaque formation. This toxic oligomerization of Aβ peptides is hypothesized to be the initiating factor for a cascade of neurotoxic events in AD, including synaptic damage or dysfunction, which leads to neuronal dysfunction, injury, and/or death. This chain of events is known as the amyloid cascade hypothesis. While this hypothesis is supported by observing patients with EOAD and LOAD, some observations from transgenic animal studies and therapeutic studies are not well supported by this hypothesis [1]. Discrepancies between animal studies and human studies, along with failures in human clinical trials aimed at reducing Aβ production (e.g., L-411,575 and LY-450,139), accumulation, and aggregation (i.e., AlzhemedTM), highlight a need to explore alternative explanations for AD pathogenesis.
An alternative hypothesis is the tau hypothesis. In the tau hypothesis of pathogenesis, tau is modified, leading to oligomerization. Oligomerization starts a cascade of events that produce neurofibrillary tangles (NFTs). These NFTs located inside the cell body cause neuronal cell death and trigger a cascade of molecular events, including inflammation that causes further neuronal degeneration [2]. While there are studies that imply that the peripheral nervous system is implicated in AD, most existing knowledge and research have been dedicated to effects on the central nervous system (CNS).
The CNS is composed of three parts: the spinal cord, brain stem, and brain. The spinal cord conveys sensory information to the brain and is responsible for transmitting signals from the brain to the trunk and limbs [3, 4]. The phosphorylated tau protein and the NFTs are found in AD patients' spinal cord [5]. In 2018, Yeh et al. found that AD cumulative incidence in spinal cord injury patients is higher than in non-spinal cord injury patients [6]. Another study by Lorenzi et al. found that spinal cord features related to the presence of spinal cord atrophy were significantly reduced in AD patients compared to healthy controls [7, 8]. While this area of AD research is of interest, it is not the main focus.
Similar to the spinal cord, the brain stem may also be a target of neurodegeneration and a source of the cognitive and behavioral symptoms of AD [9], but it is not a major current direction in AD research. Instead, currently, the main focus of AD research is on brain tissue. Brain tissue comprises three major components: white matter, grey matter, and cerebral vasculature. The gray matter, which contains glial cells (oligodendrocytes, microglia, astrocytes, and ependymal cells), is implicated in AD pathology. It is hypothesized that the oxidative stress, neuroinflammation, and excitotoxicity associated with phosphorylated tau and Aβ peptides promote loss of myelin in the CNS. There is also evidence that NG2-glia—oligodendrocyte progenitor cells (OPCs) are disrupted during the AD pathological cascade [10, 11]. This disruption alters the production of glial cells that myelinate the axons of neurons, which is an essential process needed for rapid synaptic signaling. The impact of AD is also observed on astrocytes [10, 12], microglia [13], and specific ependymal cells called tanycytes [14]. In AD, the synaptic terminal of neurons is also thought to be altered by AD pathology. The synaptic terminal, including pre-and post-synaptic terminals, appears to have impaired mitochondrial transport [15] and is related to decreased synaptic function and survival due to reduced ATP transport from the mitochondria [16]. The last foundational component of the brain directly damaged in the onset of AD is the cerebral vasculature and its interface with brain parenchyma, the blood-brain barrier (BBB). It has been shown in human brain endothelial cell lines [17, 18] and human post-mortem samples [19] that Aβ deposition in brain blood vessels leads to an inflammatory response and/or cytotoxicity, and this dysfunction of the BBB can contribute to the onset and progression of AD [20]. As it is not yet known if the amyloid or tau cascades are the driving mechanisms of AD, current research directed towards the mechanisms, pathology, and treatments focuses on the amyloid and tau hypotheses.
2. MOLECULAR BASIS AND CURRENT UNDERSTANDING OF THE PROGRESSION OF AD
As mentioned above, the two main pathological hallmarks of AD are Aβ plaques and neurofibrillary tangles (NFTs). The presence of Aβ plaques in the brain of AD patients gave life to the amyloid cascade hypothesis. It is now believed that the increased production or decreased clearance of Aβ begins decades before the onset of clinical symptoms. The cascade set off by the accumulation and eventual aggregation of the Aβ monomer promotes oligomerization of the peptide; eventually, these oligomers form Aβ fibrils. These Aβ fibrils are large and insoluble and further assemble into plaques. Aβ oligomers, to some degree, can still spread through the brain [21]. These initial plaques can mature to senile plaques and inhibit synaptic function. Initial synaptic dysfunction is believed to occur in a preclinical disease stage. The synaptic dysfunction progresses, and neuritic injury follows (severe neuronal dysfunction, degeneration, death) [22, 23]. The increase in homeostatic levels of molecules involved in cell death and inflammation activates neuronal ionic homeostasis, kinase and phosphatase activity. This change in kinase and phosphatase activity contributes to the destabilization of the microtubule protein tau and hyperphosphorylation of tau that leads to oligomerization and NFTs. Tau-mediated neuronal injury is considered the biomarker which indicates that a patient may move from preclinical symptoms to mild cognitive impairment (MCI). Neuronal cell death and inflammation continue as a patient with MCI progresses to the final disease stage, dementia [24].
While the amyloid cascade is widely accepted, numerous failures in clinical trials to improve disease state through the removal of Aβ plaques support the notion that Aβ plaques and their toxicity may be a pathology that appears later in disease progression. Alternatively, the tau hypothesis proposes that hyperphosphorylation of tau and subsequent oligomerization and NFT products cause neuronal death. As a result of the increased cell death and the release of oligomeric tau filaments, an anti-inflammatory response is activated. Activated microglial cells release molecules that cause inflammation, which induces neuronal degeneration in cells directly targeted by NFTs and oligomeric tau filaments [25].
While several leads into potential neuropharmacological treatments have been uncovered, the approved drugs can only ameliorate some of the AD symptoms. Unfortunately, no current intervention alters the underlying disease mechanism. With a void still present in our understanding of how AD pathology progresses and the underlying cascade of molecular events, the need for additional robust biological models is evident. Over the past 20 years, most preclinical studies have used transgenic mice to study the efficacy and toxicity of potential therapeutics. In most preclinical studies preceding the safety and efficacy testing in early stages of phase 1 clinical trials, transgenic mice are used to screen and offer pertinent information regarding the potential mechanism of drug activity in vivo. The two most common transgenic mice used are the 3xTg-AD and 5xFAD mice. In order to have significant pathology associated with neurodegeneration including synaptic and neuronal loss, transgenic mice have to express three or more familial AD / AD-associated gene mutations. The 3xTg-AD mouse genome houses mutant alleles of PSEN1, APP, and MAPT. The 5xFAD mouse bears three APP and two PSEN1 mutant alleles. Transgenic mice models without multiple mutations fail to support the amyloid cascade hypothesis as observations indicate no changes in neurodegeneration. While these transgenic mice models do support the amyloid cascade hypothesis, there are discrepancies between behavior improvements in these transgenic mice lines and AD patients in clinical trials. These differences and high costs associated with rodent model testing have slowed down progress towards developing AD therapeutics, supporting the need for complementary economic whole animal models with high-throughput assaying potential.
3. THE ZEBRAFISH AS A COMPLEMENTARY MODEL FOR AD RESEARCH
The zebrafish (Danio rerio) is an in vivo whole animal model with increasing popularity in all areas of biological, disease, and drug discovery research due to several strengths, including the smaller size of the organism, lower economic costs associated with husbandry and maintenance, ex vivo development, and shorter life periods. Furthermore, the zebrafish possesses high biological, structural, functional, and genetic conservations with mammals, including mice and humans. As such, the zebrafish is being widely applied in neurobiology and neurological disease research to define molecular mechanisms of neuropathology.
The zebrafish brain is functionally and molecularly similar to mammals. Brain endothelial cells (BECs) from adult zebrafish exhibit restrictive barrier properties to both large (44 kDa) and small (443 Da) tracers, such as horseradish peroxidase (HRP), sulfo-NHS-biotin (N-hydroxysulfo-succinimidobiotin), and small biotin adducts. Tight junction molecules are expressed at cerebral endothelial cell junctions, including claudin-5 (cldn5a) and tight-junction protein-a (tjpa). Tight junction molecule gene expression has been observed as early as three days post-fertilization (dpf). Central artery (CtA), metencephalic artery (MtA), and middle cerebral vein (MCeV) exhibit restrictive properties to Rhodamine-dextran (10 kDa), DAPI (350 Da), and FITC-dextran (2000 kDa) by 3 dpf [26]. The near-transparent chorion of zebrafish embryos allows for the use of non-invasive real-time tracing of fluorescent molecules for evaluating the permeability of BECs and also eases genetic manipulation. The early appearance of myelinated axons and the regulation and communication of myelination cells are also conserved in zebrafish [27]. Moreover, promising research studying underlying mechanisms of spinal cord injury in the zebrafish highlights potential application in AD research [28, 29]. Furthermore, key molecular targets associated with AD are conserved in the zebrafish genome, including the presenilins, amyloid precursor protein and amyloid-beta, beta- and gamma secretases, and MAPT (Table 1).
Table 1.
Zebrafish orthologs of human genes mutated in FAD.
| Protein | Human Gene and Protein Length | Zebrafish Gene and Protein Length | Amino Acid Similarity | References |
|---|---|---|---|---|
| Amyloid Precursor Protein | APP | appa & appb | [36] | |
| APP Protein: 695 a.a. | Appa Protein: 738 a.a. | 74% | ||
| Appb Protein: 694 a.a. | 77% | |||
| β-secretase | BACE1 & BACE2 | bace1 & bace2 | [42] | |
| BACE1 Protein: 501 a.a. | Bace1 Protein: 505 a.a. | 82% | ||
| BACE2 Protein: 518 a.a. | Bace2 Protein: 462 a.a. | - | [43] | |
| γ-secretase | PSENEN | psenen | [44, 45] | |
| PSENEN Protein: 101 a.a. | Psenen Protein: 101 a.a. | 91% | ||
| NCSTN | ncstn | [46] | ||
| NCSTN Protein: 709 a.a. | Ncstn Protein: 707 a.a. | 56% | ||
| APH1b | aph1b | |||
| APH1b Protein: 257 a.a. | Aph1p Protein: 258 a.a. | - | ||
| Prensenilin-1 | PSEN1 | psen1 | [47] | |
| PSEN1 Protein: 456 a.a. | Psen1 Protein: 456 a.a. | 75% | ||
| Prensenilin-2 | PSEN2 | psen2 | [30] | |
| PSEN2 Protein: 456 a.a. | Psen2 Protein: 441 a.a. | 76% | ||
| Apolipoprotein E | APOE | apoea | [48, 49] | |
| APOE Protein: 317 a.a. | Apoea Protein: 269 a.a. | 49% | ||
| apoeb | ||||
| Apoeb Protein: 281 a.a. | 51% | |||
| Sortilin related receptor 1 | SORL1 | sorl1 | [50] | |
| SORL1 Protein: 2214 a.a. | Sorl1 Protein: 2213 a.a. | 64% | ||
3.1. The Presenilins
PSEN genes in humans play a normal role in development. In zebrafish, psen1 and psen2 are orthologs of PSEN. These zebrafish orthologs are expressed at higher levels in zebrafish during development [30]. Knockdown psen1 zebrafish with inhibited psen1 function display conserved phenotypes with presenilin knockout (KO) mice, implying functional importance during somite formation and notch signaling [26, 31, 32]. To study the brain's histaminergic system [26], researchers use mutant zebrafish lacking psen1 activity. Sundvik et al. found that not only is psen1 a regulator for histaminergic neuronal development, but indirectly, psen1 mediates cognitive functions in usually affected AD patients. Interestingly, the loss of expression of the psen2 ortholog appears to have the most significant effect on Notch signaling [33], a phenotype not usually observed in Psen2 KO mice [34]. The impact of Notch signaling loss during zebrafish development decreases the production of interneurons in the spinal cord, which is an area disturbed in AD patients [5, 35].
3.2. APP Co-orthologs
The amyloid-beta precursor protein (APP) in the zebrafish is present as a set of orthologs: appa and appb. These orthologs have a widespread expression in the CNS during development [36]. The near-transparent embryonic chorion and complete genome sequence allowed researchers to develop zebrafish genetic models fusing green fluorescent protein (GFP) to an appb regulatory sequence for visualization of the developmental stage-specific location of appb gene expression in real-time [36, 37]. Liao et al. found that both appa and another closely related APP gene, amyloid-beta precursor-like protein 2 (aplp2), accumulated in the vasculature of the mutant zebrafish [38]. Expression inhibition of appa or appb during development led to two different results with appa inhibition having little effect and inhibition of appb resulting in a body length reduction. Treatment with human APP mRNA ameliorated these pathological changes in the zebrafish larvae [39]. Furthermore, the treatment of appb inhibiting zebrafish larvae with human APP mRNA was “more effective” than treatment with mRNA encoding human mutant forms of APP, including the Swedish double mutation K595N and M596L [39]. These findings support the conservation and similarity of APP in zebrafish.
3.3. Amyloid-beta (Aβ)
While amyloid-beta (Aβ) has not been observed to accumulate in the brain tissue of zebrafish naturally, Aβ functional and toxicity assays are possible with both the wild-type and genetically modified lines of zebrafish [25, 40, 41]. Genetically modified lines allow investigations into perturbations that influence Aβ accumulation and treatments that ameliorate Aβ.
3.4. β-secretase & γ-secretase
Both β-secretase and γ-secretase are attractive AD targets for drug development [42-44]. Studies characterizing the molecular function and expression of bace1 and bace2 have used wild-type zebrafish and zebrafish with their genomes edited with zinc finger nucleases (ZFNs) [45]. These studies support the use of zebrafish as a tool for screening β-secretase inhibitors for selective and specific therapeutics.
3.5. MAPT
The zebrafish genome contains the co-orthologs, mapta and maptb, for human MAPT. Both mapta and maptb have been manipulated to further understand the function and role their ratios play in AD pathogenesis. The use of transgenic zebrafish expressing human MAPT showed biochemical changes consistent with those observed in human tauopathies supporting similarities among zebrafish Mapta and Maptb and human MAPT [46].
3.6. Potential Limitations and Areas of Continued Research
Overall, research shows that many parts of the CNS that are implicated in AD disease progression and pathology are conserved in zebrafish. In addition, there is also a similarity in molecular expression and function of AD genetic targets, supporting the use of the zebrafish to address AD-related research questions. While the zebrafish is an advantageous model to use for molecular analysis, some limitations have been noted. For example, zebrafish have been indicated to lack some of the complexity needed for advanced cognitive behavior observations, but research on zebrafish cognitive behavior is continuing and providing a better understanding of similarities and differences [51-54]. These studies include those focused on the histaminergic system using psen1 -/- zebrafish, in which these genetically modified zebrafish larvae were less responsive to light compared to wild-type zebrafish [55, 56]. This behavioral observation is supported by previous studies that histamine is a “wakefulness promoting factor” and mediator of vigilance and cognitive performance [57]. Further studies are expanding our understanding of additional behavioral traits in larval and adult zebrafish. Expansion in this research area and others will continue to provide an overall better understanding of similarities and differences between the zebrafish and mammals, and ultimately permit improved interpretation and translation potential.
4. EDITING THE ZEBRAFISH GENOME
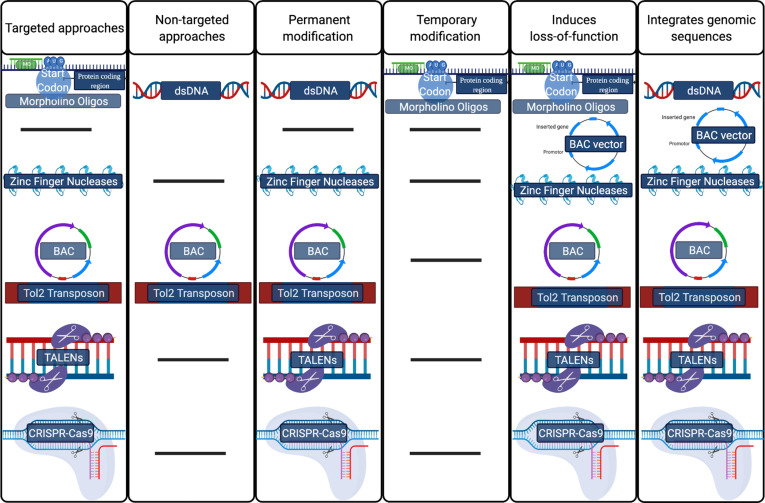
Zebrafish embryos are easy to manipulate using various gene-editing technologies. The zebrafish possess both a vertebrate neural structure and a homologous and fully sequenced genome with several gene orthologs of genes mutated in EOAD (Table 1), as discussed previously. Genetic modifiers, such as morpholino oligonucleotide (MO) injections, are often used to demonstrate the conservation of gene and protein function in zebrafish. However, a limitation of studies that only briefly modify gene expression is that these approaches can only block translation or create alternative splicing events for a short time window and do not allow researchers to observe the late onset of phenotypes. The ease in genetic manipulation and sequenced genome allows scientists to use zebrafish for analyzing the function of genes and molecular pathways using additional genomic editing systems. Systems developed to modify an organism's DNA range from targeted to non-targeted, specific to selective, resource-consuming to economical, and user-friendly to nearly inaccessible (Fig. 1). Non-targeted methods include those using microinjection or electroporation with naked DNA and bacterial artificial chromosomes (BACs) for transgenesis. The use of naked DNA for BAC transgenesis results in approximately 1-3% germline transmission [58].
Fig. (1).
Popular methods in the gene-editing toolbox organized by important functional characteristics. [BAC: bacterial artificial chromosome; CRISPR-Cas9: clustered regularly interspaced short palindromic repeats–associated nuclease 9; TALENs: transcription activator-like effector nucleases].
Stable germline transmission can be achieved with ~15% transmission rate using Tol2 transposons with BACs (transposon-mediated BAC transgenesis). Naked DNA alone is an example of a non-targeted system; however, this method for transgenesis is not preferred as transgene integration occurs at a much lower rate. Targeted genomic editing systems that can produce and introduce mutations in DNA include DNA transposons, zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats–associated nucleases (CRISPR-Cas). There are many ways to edit the zebrafish genome, and the editing technology selected is based on desired outcomes in the research. For example, for research geared at characterizing a gene's location, non-targeted transgenesis methods like transposon-mediated BAC transgenesis or BAC transgenesis are quick and easy systems to use. Alternatively, CRISPR-Cas technologies make a double-stranded break (DSB) in predicted protein-coding and non-coding regions of DNA and are often used to determine protein-coding areas, promoters, enhancers, silencers, and insulators. CRISPR-Cas systems can also be used to determine if a protein function is conserved in zebrafish. Determining the location and time of expression or gene translation is achieved using non-targeted systems and targeted editing systems like CRISPR-Cas9. When multiple systems are an option, efficiency, germline transmission rates, type of edit, size of insert, and ease-of-use are then considered. Some of the most common and efficient methods are detailed below in the subsequent sections.
4.1. Transposable Elements
DNA transposons are a useful tool for generating transgenic zebrafish. Many transposable elements can be used for practical chromosomal engineering; however, the more common and effective transposable element is the Tol2 transposon system. This system via transposase (TP)-dependent cut-and-paste mechanism, a Tol2 element placed on either side of a DNA sequence, is reliable for integrating a transgene or a mutated DNA. This Tol2 transposase can mediate BAC transgenesis. Combining a BAC plasmid and an iTol2 cassette increases the observed rate of integration of BACs into the zebrafish genome. The Tol2-compatible BAC plasmid is constructed in E. coli by recombineering. A reporter gene (such as GFP) is introduced in the BAC and integrated by homologous recombination. This BAC containing the reporter gene, the transgene, and iTol2 cassette, is microinjected into a transparent zebrafish embryo at the single-cell stage. Integration rates of edit are based on application and construct sizes. This method has a large carrying capacity greater than 160 kb.
4.2. TALENs
Transcription activator-like effector nucleases (TALENs) employ custom-designed and loci-specific proteins fused to sequence-independent nuclease domain, similar to zinc-finger nucleases, to generate targeted mutations. As a protein-based system, TALENs use sequence-specific binding motifs to create targeted DSBs. TALENs are composed of two molecular parts: (1) transcription activator-like effectors (TALEs) and (2) nucleases (e.g., Fok I). TALENs are considered a system with high gene-editing specificity as a product of the required homodimerization of the core DNA binding motifs attached to the TALE nuclease. Off-target effects are relatively rare using TALENs attributing to the TALE's high DNA binding specificity and length of the target sequence (15-19 bp) and the regulation of endonuclease activity by nuclease homodimerization. TALENs create a specific DSB that is efficiently transmitted in the germline [59].
4.3. CRISPR-Cas9
The CRISPR-Cas9 system is a relatively new gene-editing technology composed of two fundamental molecules: a cluster of short repeat sequences separated by unique sequence spacers and directed by guide RNA and a CAS protein with nuclease activity. CRISPR-Cas9 is used to create transgenic and mutant zebrafish lines via induction of a DSB, point mutation, or insertion of a genomic sequence. CRISPR-based screens of knockout zebrafish, such as genome-wide CRISPR screens, facilitate the discovery of functional non-coding elements. Knockouts are created when the CRISPR-Cas9 induced DSB makes a frameshift mutation via one of two gene repair pathways. This CRISPR-induced frameshift changes a codon and subsequently silences translation of the modified gene. A knock-in using CRISPR-Cas9 is achieved using both homology-dependent and independent recombination. Homology-dependent recombination (HDR) requires the genomic sequence inserted to be 20 bp or less and to have homologous flanking arms. CRISPR-Cas9 knock-in using larger inserts or exogenous DNA typically utilizes the non-homologous end-joining (NHEJ) pathway. The integration rates of mutations and transgenesis are much higher than any other gene-editing system, and technologies are expanding into other CRISPR-Cas-based editing systems (e.g., Cas12, Cas13, among others) [60].
5. GENETICALLY ENGINEERED ZEBRAFISH ALZHEIMER'S DISEASE MODELS
One of the earliest factors identified by researchers as being imperative to consider during the development and assessment of AD therapeutics is the need for increased specificity of drugs. Some drugs approved for clinical trials are selective and target Aβ or tau peptides or other APP and tau-associated proteins; however, in doing so, physiologically relevant peptides that did not contribute to the formation of senile Aβ plaques or the presence of oligemic tau were eliminated. While preclinical data was promising, phase one and phase two trials of many studies were discontinued due to discrepancies between animal data and the adverse events recorded during safety and efficacy clinical trials. One reason may be the lack of specificity for the pathologic forms of Aβ and tau protein. There is still a need to understand which molecules are and are not associated with the amyloidogenic pathway. There is always a need to identify the sequence of events and which pathologies are symptoms of the critical event leading to neurodegeneration in AD patients. As such, several genetically engineered zebrafish AD models have been developed to address mechanistic, progression, and therapeutic AD research challenges.
5.1. Zebrafish App Models
Several strategies have been employed in the development of zebrafish App models to address AD research questions. For example, using a transgenic zebrafish that expresses green fluorescent protein (GFP) in vascular endothelial cells, Cameron et al. determined that exposure to monomeric Aβ peptides can induce angiogenesis in developing zebrafish [37]. This transgenic zebrafish model, Tg(kdr:eGPF)s843, was conceived from microinjection of a linearized construct consisting of a zebrafish gene, eGFP tag, and promoter. This model is important as it can be used to determine the long-term effects of Aβ exposure in the cerebral vasculature and consequences of hypervascularization in adult transgenic zebrafish. Research with this model highlighted a conserved physiological role of Aβ and APP in the zebrafish [37].
Another transgenic zebrafish expressing GFP, Tg(zAppb-i1-eGFP), was developed and used to analyze the expression of the zebrafish APP ortholog appb [39]. Research with this model showed Appb expression to be localized in the brain. Furthermore, researchers observed Appb expression in the developing vasculature. The ease of use of genomic editing systems in zebrafish allows researchers to control and verify human APP expression in the zebrafish CNS. These simple models are useful to screen potential molecules that can decrease the presence of Aβ or App by measuring their fluorescence signal in both qualitative and quantitative assays.
Morpholino oligonucleotide (MO) injections are a less permanent genomic modification used to inhibit the translation of a zebrafish protein temporarily. MO injections of APPa-MO and APPb-MO at the one-cell stage produced a defective convergent-extension movement during gastrulation. This observed change in phenotype was rescued by human APP695 mRNA, but not human APP containing the fAD Swedish mutation (APPswe) [61]. This result not only shows the physiological conservation of APP in zebrafish but demonstrates that specific MOs can be used to elucidate molecular targets.
More permanent genomic integration was achieved using the Tol2 gene trap with a red fluorescent protein (RFP). Researchers integrated Tol2 gene traps with RFP into APP orthologs and amyloid precursor-like protein 2 (aplp2). The Tol2 gene trap localized expression of appa and aplp2 and presented no RFP protein expression signal in brain endothelial cells [62]. This observation is consistent with the hypothesis that appa and aplp2 are secreted by neurons [62].
Using CRISPR-Cas9 to generate permanent genetic edits is more efficient than the system used to study APP and APLP2 orthologs' expression. For example, a CRISPR-Cas9 system was used to efficiently introduce a homozygous mutation that manufactured a premature stop codon in exon 2 of appb. This loss-of-function mutation, confirmed by western blot, also underlined the presence of compensatory mechanisms when protein translation was inhibited in the APP pathway [42, 43].
Another research group used the Tol2 gene-editing system to insert the human APP gene (APPswe) in the zebrafish genome under the control of the zebrafish appb promoter [63]. The Tol2 plasmid used to generate this transgenic zebrafish contained a zebrafish promoter, exogenous human cDNA, and a CMV promotor and eGFP tag. eGFP expression was used to examine the time and location of expression of the transgene. The zebrafish telencephalon, which is comparable to the mammalian hippocampus, was morphologically altered in this transgenic model. Both histopathological and ultrastructural shifts were detected in the cerebral microvasculature and in neurons, which was supported by results of previous studies [25, 41, 54, 64, 65]. A novel outcome of this integration was the visualization of Aβ deposition in the brain of the transgenic adult zebrafish. Increased optimization of this genetic modification to the zebrafish genome is a promising future direction as an AD disease model with Aβ pathology in the zebrafish brain, increasing the ease and pace of screening of potential small molecule therapeutics and immunotherapies.
Genetically modified zebrafish APP models also include those that allow scientists to attain a simplified representation of Bace1 and Bace2 protein function. Bace1 and Bace2 are proteins produced by bace1 and bace2, orthologs of human BACE1. Again, BACE1 is responsible for 1 of 2 enzymes that cleave APP into Aβ peptides 40 and 42. Considering the role of the β-secretase, many researchers have attempted to target BACE1 and BACE1 protein to treat AD symptoms and progression. The zebrafish bace1 ortholog is highly homologous to human BACE1. This orthology is supported by evidence that bace1 possesses transmembrane domain characteristics. Comparison of protein sequences revealed that Bace1 is 74-82% identical to BACE1 and shares both protease active sites and the transmembrane domain [42, 66]. Researchers interested in the phenotype of bace1 -/- loss-of-function used zinc finger nuclease mRNA to generate a loss of function bace1 -/- mutant alleles to further investigate this genetic target.
The zebrafish has a co-ortholog of bace1 and bace2. A loss-of-function bace2 -/- mutant has also been generated using N-ethyl-N-nitrourea (ENU) mutagenesis to further understand similarities and differences to bace1. In the bace2 mutant, a point mutation changing nucleic acid C to A leads to a premature in-frame stop codon. The resulting truncated Bace2 protein did not have protease active sites. Upon confirmation of mutation and loss-of-function, the bace1 -/- mutant was then crossed with transgenic zebrafish lines expressing GFP. The transgenic lines used included Tg(foxd3:GFP) and the double transgenic line, Tg(claudink:Gal4); Tg(14xUAS:GFP). The Tg(foxd3:GFP) line drives the expression of GFP under the control of the forkhead box D3 promoter (foxd3). This transgenic line can be used to analyze Schwann cells. The double transgenic line using [Tg(claudink:Gal4); Tg(14xUAS:GFP)] drives membrane bound GFP expression under the control of claudin k promoter and is used to evaluate myelination. The zebrafish that were a cross between bace1 -/- mutants and the transgenic line expressing GFP under the control of claudin k promoter had a consistent myelination phenotype similar to Bace1-KO mice, including a severe reduction in Schwann cell myelination in the PNS [67, 68]. Evaluation of the effect of no bace1 activity on myelination of oligodendrocytes was possible with a cross of the bace1 -/- mutated line and the Tg(foxd3:GFP) line. This homozygous bace1 -/- mutant can be used as a positive control for screening β-secretase inhibitors (BSIs) and for evaluating that these inhibitors do not affect CNS myelination at a molecular level. In addition, using the claudin k:GFP transgenic zebrafish to screen other BSIs allows researchers to visualize the effects of BSIs on Schwann cell myelination.
While CRISPR systems are still relatively new to the genomic editing tool kit, new nucleases with different affinities are already being used to edit the zebrafish genome to create App models. For example, the CRISPR-Cpf1 system was applied to generate a null mutation in sorl1 and then TALEN pair was used to introduce an early-onset familial AD-like frameshift mutation in sorl1 [69]. The application of models being developed indicates that the zebrafish genome can be modified to study familial mutations and how these familial mutations behave mechanistically [68].
5.2. Zebrafish Tau Models
There are several models of tauopathy in zebrafish. These tauopathy models include two models with transient expression of human tau fused to GFP under either a gata2 promoter or neuronal HuC/D promoter [69]. The expression of human tau under both promoters showed tau phosphorylation. Eventually, a stable model generated by expressing TAU0N4R, controlled by enolase promoter, was prepared [70]. The transgenic zebrafish generated with FTDP-17 mutation led to a disruption in the cytoskeleton, which is a pathology observed in human NFTs. This model allowed researchers to study the functional consequence of human tau mutations on sites associated with hereditary dementias (e.g., G272V, K280, P301S, S305N, and R406W) and those mutated at selected post-translational modification sites.
Zebrafish tauopathy models are used to evaluate signaling factors and drugs that can alleviate neuronal death induced by pathogenic tau oligomers [71]. Studies with zebrafish tauopathy models support the hypothesis that tau oligomers are more toxic to neurons than aggregates of hyperphosphorylated tau and that truncated forms of Tau protein are neurotoxic. Tauopathy models were used to evaluate the effect of Nrf2 overexpression and distal acquired demyelinating symmetric neuropathy (DADs) treatment.
As mentioned above, certain zebrafish lines expressing human tau are not stable. Using the zebrafish eno2 promoter, Bai et al. generated a stable tauopathy model [72]. A BAC strategy was used to develop two models: one to confirm the utility of the eno2 gene and promoter and a second to observe the expression of human tau under the control of the enolase promoter. These previous tauopathy models laid the path for an optimized model that could overcome their shortcomings. For instance, using a medaka Tol2 transposable element mRNA and bidirectional expression system, Paquet et al. generated a transgenic zebrafish with stable expression of human tau-Proline(P) 301 Leucine (L) [73]. Both human TAUP301L and DsRed were integrated into the zebrafish genome to permit the identification of the tau-expressing cells in real-time by the co-expression of DsRed fluorescence. It is important to note that at five weeks, this mutant zebrafish line displayed NFTs. After the successful and efficient generation of zebrafish expressing human TAUP301L, characterization of tau hyperphosphorylation in adult zebrafish brains was possible, and studies found that human TAUP301L expression resulted in hyperphosphorylated tau, but no NFTs [74]. Future studies could use this tauopathy model to evaluate the role of tau phosphorylation in neurodegeneration and to screen therapeutics for decreasing Tau phosphorylation following environmental perturbations.
5.3. Zebrafish Presenilin Models
Presenilin activity is a significant component of the aspartyl protease complexes responsible for γ-secretase cleavage of APP [75]. Presenilin is the catalytic component of γ-secretase, a complex fabricated from four different integral membrane proteins [76]. There are also early reports of presenilin 1 participation in tau phosphorylation. Previous studies have shown that tau and GSK-3β bind to the same regions of PSEN1 [77], and that PSEN1 mutation can reduce cytoskeletal association and eventually potentiate tau phosphorylation [45]. This evidence of presenilin proteins interacting with both Aβ and tau suggests a molecular link between Aβ production and tau phosphorylation in patients with AD. PSEN1 and PSEN2 are two of four loci focused for studying AD pathological etiology. Presenilins could become a target in therapeutics for patients with early-onset AD. It is estimated that 200+ PSEN1 mutations and ~20 PSEN2 mutations are directly linked to the onset of familial AD [78].
Several genetic editing strategies have been employed to study the presenilins in zebrafish. MO injection was used to inhibit protein translation of Psen1 and Psen2 in zebrafish embryos and induced changes in melanocytes, leading to an increase in dorsal longitudinal ascending (DoLA) interneurons [79]. Psen2 inhibition reduced Notch signaling and changed the expression of neurogenin1 (neurog1) in the spinal cord of zebrafish. While the genomic modification here is temporary, using MOs to block Psen2 protein expression in the zebrafish creates a sensitive bioassay to evaluate the non-apoptotic activity of Psen2 protein.
Another study assessed the regulatory protein, presenilin enhancer 2 (Pen-2), using MO injection [26]. This study demonstrated that knockdown of Pen-2 directly induced a p53-dependent apoptotic pathway. Islet-1 (a LIM homeodomain protein), acetylated tubulin, trigeminal (a complex of cranial neurons), and Rohon-Beard (RB) neurons were evaluated for survival after knockdown of Pen-2, Psen1, or Aph-1. Researchers found that knockdown of Psenen (Pen-2) resulted in the loss of axons and neurons positive for acetylated tubulin and islet-1. It was also discovered that the knockdown of p53 could rescue the resulting massive neuronal apoptosis.
To examine accelerated brain aging, Hin and colleagues used the zebrafish to model the K115fs mutation, a frameshift mutation in PSEN2 leading to a “premature” stop codon [31]. This premature stop in sequence leads to a truncated ORF and is another early-onset familial AD mutation of PSEN2. Several pathological alterations were inspected in the mutant zebrafish brain and were found to be comparable to alterations in human AD brains. The mutants produced via TALENs provided evidence of increased oxidative stress and that altered energy metabolism preceded AD. This model and the same genome editing concept, but more efficient technology, can be used to evaluate early alterations in the brain of fAD-mutation carriers. For example, since oxidative stress and energy metabolism were altered, these and other endpoints can be assessed as these outcomes are thought to be early events in AD [80].
One area that remains a therapeutic challenge is the restoration of cognitive function. Many drugs have shown promise in preclinical trials and were deemed safe in phase 1 of clinical trials but failed to meet primary behavioral improvements (e.g., those tested in the mini-mental state examination, call detail record, and free and cued selective reminding test) in phases 2 and 3. The histaminergic system is a promising target for therapeutics aimed at improving cognitive function scores and psen1 is confirmed to regulate histamine neuron development and behavior in zebrafish [81]. In a psen1 -/- zebrafish (PS1 hu2547) model prepared by targeting-induced local lesions in genomes (TILLING), it was determined that this mutation resulted in significant alterations in the histaminergic system during development and that these changes persisted into adulthood. There was also evidence that changes in the histaminergic system may be explained by dysfunction in the gamma-secretase complex that regulates the development of histaminergic neurons via Notch signaling. This finding filled a significant knowledge gap that could not be addressed in knockout mice since the absence of PS1 is lethal and causes significant defects to the skeleton and CNS [82]. psen1 expression influencing the histaminergic system was further supported by evidence that other neurotransmitter systems implicated in AD were not impaired in the psen1 -/- zebrafish.
6. AD THERAPEUTICS
There is a great need for improved AD therapeutic strategies. Acetylcholinesterase inhibitors (AChEIs) were among the first drugs approved for AD treatment and included drugs, such as rivastigmine, galantamine, and donepezil. As of 2017, only five medications developed to improve AD symptoms, including cognitive function, were approved by the US Food and Drug Administration (FDA). Aside from the AChEIs, another drug memantine (an NMDA receptor antagonist) is used to treat moderate-to-severe AD symptoms, unlike donepezil, which is approved for use at all stages of AD [83]. Currently, individuals are either affected by early-onset AD [5% of total AD cases in the US, including familial (>5%) and sporadic (>~1%)] or late-onset AD (95% of total AD cases in the US). Patients who begin to experience mild cognitive decline and other symptoms described to occur during the end of the preclinical stage can undergo specialized injection of radiolabeled tracer agent and PET scan to detect deposition of Aβ. While this method accurately diagnoses the disease, it is still a limited used diagnostic with most AD cases confirmed post-mortem (with 96% sensitivity and 100% specificity). Aside from assessing degrees of increasing cognitive dysfunction, other AD clinical markers include a semi-invasive evaluation of cerebral spinal fluid (CSF) for Aβ1-42, hyperphosphorylated human tau protein, and total tau protein content. Regardless of if a patient is suffering from cognitive impairment or moderate or progressive AD-like dementia, current treatment is limited to the two previously mentioned classes of pharmacological therapeutics [i.e., AChEIs or the NMDA receptor antagonist memantine (or a mixture of both)] [84]. Currently, approved AD therapeutics are void of treatments that target etiological pathologies of AD or improvement in cognitive scores. However, neuropharmacological research is focused on determining the keystone event that, if targeted, can reverse and or prevent clinical symptoms of AD, including cognitive decline and neurodegeneration via Aβ plaques and NFTs.
6.1. APP Therapeutics
The toxic Aβ plaques observed in AD patients are classified as senile, diffuse, and neuritic [85]. Aβ is formed via proteolytic cleavage by β- and γ-secretases of APP, known as the amyloidogenic pathway. Aβ1-42 (a product of the amyloidogenic pathway) and Aβ1-40 (a physiologically relevant molecule in the brain) are the two primary forms for Aβ oligomers. To reduce plaques, researchers have targeted small molecules, secretases, and modulators in the amyloidogenic pathway. Small molecules of interest include Aβ peptides in both soluble and insoluble forms. Inhibition of small molecules via therapeutics focuses on inhibiting accumulation of Aβ and/ or inhibiting the formation of plaques. Elayta and PRI-002 are small molecules currently in clinical trials. Elayta, which has been declared safe for phase 3 clinical trials, is a small molecule antagonist of sigma-2 receptors. Inhibition of sigma-2 receptors reduces oligomer binding. The mechanism of action of this small molecule is still unknown but assumed to compete with oligomeric Aβ. This reduction of Aβ at synapses reduces the toxicity induced by subsequent Aβ oligomers. PRI-002 is an enantiomeric peptide designed to interfere with Aβ's oligomerization, and this occurs through binding of this small molecule to Aβ42 monomers and stabilizing them. Due to the promising data produced in preclinical studies with transgenic mice, PRI-002 is approved for phase 1 clinical trials. A small molecule anti-inflammatory drug, thalidomide, has entered the list of potential therapeutics. A drug known for its controversial arrival to the pharmaceutical scene in the early 1960s, thalidomide, has found a new application in therapeutics based on its immunosuppressive and anti-angiogenic activity. A more refined, more potent, and less toxic agent, lenalidomide, has been enrolled as a potential small-molecule therapeutic to reduce amyloid pathology and gliosis. This derivative of thalidomide has been shown to have reduced amyloid pathology and gliosis in vitro and preclinical studies as an anti-inflammatory drug. These and many other small molecule inhibitors are currently under review in clinical trials, most of which were set to end in 2020 or early 2021.
Another area of therapeutic interest for researchers is anti-aggregation agents. These agents can be combined for combination therapy (e.g., ALZT-OP1) or modified amino acid residues (e.g., ALZ-801). ALZT-OP1 is considered a combination therapy as it contains cromolyn and ibuprofen. Ibuprofen acts as a typical anti-inflammatory compound by suppressing cytokine release, while cromolyn sodium binds to Aβ peptides and inhibits polymerization into an oligomer [86]. ALZ-801, similar to ALZT-OP1, inhibits Aβ oligomer formation [87, 88]. This modified amino acid ALZ-801 is a prodrug of homotaurine and is metabolized to a compound typically found in the brain that inhibits Aβ aggregation [89]. Both of these anti-aggregation agents are under review and in different stages of clinical trials.
Beta- and gamma-secretase are two target molecules of interest that have produced mixed results in using targeted approaches to treat AD. BACE1 is an essential enzyme for producing Aβ peptides. Inhibitors of BACE1 that have made it through clinical trials with FDA approval are few. One example is citalopram, a small molecule of interest as it has been shown to reduce Aβ in the CSF by 25% through increased stimulation of the non-amyloidogenic pathway [90]. While there are attractive candidates for both small molecules observed in AD pathology and secretases directly involved in producing the proteins that lead to toxic effects observed in AD patients, a portion of the molecular cascade and the pathological events are still unclear.
Another area of interest for AD treatment is passive and active immunotherapy using monoclonal antibodies. In transgenic mice, immunotherapy decreased the amount of Aβ in the brain and improved cognitive function associated behavior [91]. In 2012, the safety, tolerability, and antibody response of CAD106, an active Aβ immunotherapy, were determined [92]. The findings prompted a subsequent study two years later to assess the long-term safety, tolerability, and antibody response to CAD106. The results indicated that CAD106 might be a valuable therapeutic [93]. Like CAD106, another potential immunotherapeutic, CNP520, was under clinical investigation as an intervention/ treatment [94], but as of 2019, this CNP520 arm of a combination study was discontinued as it presented worsened cognitive function in patients. Phase 3 trials evaluating immunotherapeutic lecanemab (BAN2041) are also underway; it is one of the more promising potential drugs showing an association with slowed cognitive decline in human trials. Another monoclonal antibody with ongoing clinical trials is donanemab, LY3002813. This passive immunotherapeutic binds to large oligomers, like lecanemab, but with higher affinity. This plaque-binding antibody does not cause microhemorrhages or other issues reported with others [95, 96]. While there seem to be a few candidates for treating AD via monoclonal antibodies, there are also vaccines in development to target AD pathologies. A few currently under clinical evaluation include ABvac 40 and UB-311. UB-311 is a synthetic peptide vaccine that couples a helper T-cell epitope, which stimulates a type 2 regulatory immune response [97]. UB-311 and ABvac40 were both found safe for phase 2 clinical trials and are still ongoing. ABvac40 is different from UB-311 and other vaccines developed for AD as ABvac40 targets the C terminus of Aβ40 [98].
Unfortunately, almost seven years after the first initially developed monoclonal antibodies, immunotherapy has yet to improve patients' cognitive scores during clinical trials. As with many newly developed therapeutics influenced by the amyloid cascade hypothesis, the ongoing development of immunotherapies proceeds with the intent to improve and/ or preserve cognition in patients with MCI due to AD. To some extent, the lack of success in correlating cognitive improvement with decreases in levels of Aβ in all forms supports the notion that insoluble forms of Aβ are a result of the actual initiator of neurodegeneration and not the initiator itself. The scientists who believe that Aβ oligomers and plaques are a clinical sign that the disease has set in, tend to overlap with those who think hyperphosphorylated tau and NFTs may be more closely related to providing insight on the progression and treatment of the disease. While many researchers disagree on the time of arrival of tau protein, many agree on the toxicity of hyperphosphorylated tau in the nervous system.
6.2. Tauopathy Therapeutics
Detachment, hyperphosphorylation, and aggregation eventually lead to neuronal death. Kinases, including glycogen synthase kinase 3β (GSK3β), cyclin-dependent kinase 5 (CDK5), extracellular signal-regulated protein kinase 2 (ERK2), and microtubule affinity-regulating kinase (MARK), are all directly involved in phosphorylating tau (a molecular event believed to initiate detachment of normal tau from microtubules). The goal of inhibiting hyperphosphorylation is to inhibit or slow down further destabilization of microtubules by the detachment of normal tau from the microtubules. There are no known CDK5 inhibitor therapeutics in development. In general, CDK inhibitors as a therapeutic treatment in clinical trials are associated with multiple myeloma and lymphocytic leukemia [99]. Like CDK5 inhibitors described above, ERK2 inhibitors are also not of interest as a target for therapy. In the long-term, the goal of therapeutics aimed at inhibiting hyperphosphorylation is to prevent the subsequent oligomerization and aggregation of the available and hyperphosphorylated tau. However, inhibiting these molecules is not the main direction of research. Instead, removing hyperphosphorylated tau and inhibiting the aggregation and/or oligomerization is the main focus of tau therapeutic development and research. A few small molecule inhibitors of tau aggregation are in phase 1 (e.g., LY3372689) and phase 3 (e.g., LMTM) clinical trials. LY3372689 is an inhibitor of the O-GlcNAcase (OGA) enzyme, a post-translational modifier that regulates proteins like tau. N-GlcNAcylation (the addition of N-acetylglucosamine to serine and threonine residues) of tau reduces the formation of the subsequent toxic aggregates. LMTM (or TRx0237), a purified version of methylene blue, is the second generation of Rember®. This second generation is said to prevent tau aggregation or remove existing aggregates and to possess better pharmacokinetics.
Tau immunotherapy is a growing area of therapeutic research and development. Similar to APP-based immunotherapies, researchers have developed both vaccines (active immunotherapy) and antibodies (passive immunotherapies). The molecular nature of the AADvac1 vaccine has not been disclosed; however, this active vaccine elicits an immune response to pathologically modified forms of tau. This vaccine finished phase 1 trial, and while no data has been posted, it is evident that further trials are needed to establish proof of clinical efficacy [100]. ACI-35 is a liposome-based vaccine designed to mount an immune response to certain pathological conformers of phosphorylated tau without eliciting an autoimmune response against its physiological relevant forms. In preclinical rodent studies, ACI-35 injection preferentially induced more polyclonal IgG antibodies specifically targeted for phosphorylated tau than non-phosphorylated tau [101]. Unlike AADvac1, ACI-35 has moved into phase 2 of clinical trials.
Active clinical trials for passive tau immunotherapies are numerous. BIIB076 is a human recombinant monoclonal anti-tau IgG1 antibody. In vitro, this antibody targets the mid-domain of tau and blocks tau from aggregating. This antibody also blocks the uptake of tau into neuronal cells and the propagation of tau in interneurons [102]. Phase 1 of BIIB076 clinical trials ended in March 2020, and the results are anxiously anticipated. Bepranemab is a humanized monoclonal IgG4 antibody; however, unlike BIIB076, this antibody binds to tau near the microtubule-binding domain. Researchers believe that antibodies that bind to central regions may interfere with cell-to-cell propagation of pathogenic aggregated tau than antibodies that target the N-terminal. In January of 2021, phase 2 clinical trials for JNJ-63733657 (a humanized IgG1 monoclonal antibody) began. This passive therapy is designed to interfere with the cell-to-cell propagation of pathogenic, aggregated tau. While this antibody has some of the same design specifications, this potential therapeutic is supposedly more potent and has a higher affinity for phosphorylated tau [60]. Gosuranemab (or BIIB-092) is another humanized IgG4 monoclonal anti-tau antibody. Phase 2 trials of BIIB-092 are still ongoing, but results presented at the CTAD conference indicated no adverse events that could be attributed to the antibody and a 100% decrease in unbound N-terminal tau fragments in the CSF occurred [103].
Monoclonal antibodies, such as E2814 and Lu AF87908, target phosphorylated tau and have at least begun the first clinical trial phase. Semorinemab (or RO7105705), an anti-tau IgG4 antibody, is designed to target extracellular tau to limit microglial activation and inflammatory responses. Small molecules, antibodies, and vaccines make up the most potential therapeutics, but another investigational therapeutic has arrived on the scene: DNA/RNA-based IONIS-MAPTRx, or BIIB080. BIIB080 is the first antisense oligonucleotide (ASO) developed to target tau expression and has entered clinical trial in 2019.
6.3. Challenges in Identifying Optimal AD Therapeutics in Mammalian Models
Many studies of tau vaccines have demonstrated safety and efficacy in animal models. However, most face the common challenge of translating the animal study results to human clinical trials. The discrepancies between pathological and cognitive improvements in preclinical studies and early phases of human clinical trials for Aβ- and tau-related therapeutics highlight a need for further scientific mechanistic investigations. For example, Aβ accumulation and deposition of Aβ-plaques are not significantly correlated to neuronal loss and cognitive decline. This is likely because there are still key pieces of information missing. The debate about which pathological abnormality is best to target to reduce progression or directly inhibit further neurological decline is a major barrier in current and upcoming research for effective treatments. Unknown physiological roles of APP, Aβ, tau, and other fragments involved in AD pathology impede success in therapeutic development, especially in the beginning. Researchers also share a knowledge deficiency in understanding the functional roles of many targets of therapeutics.
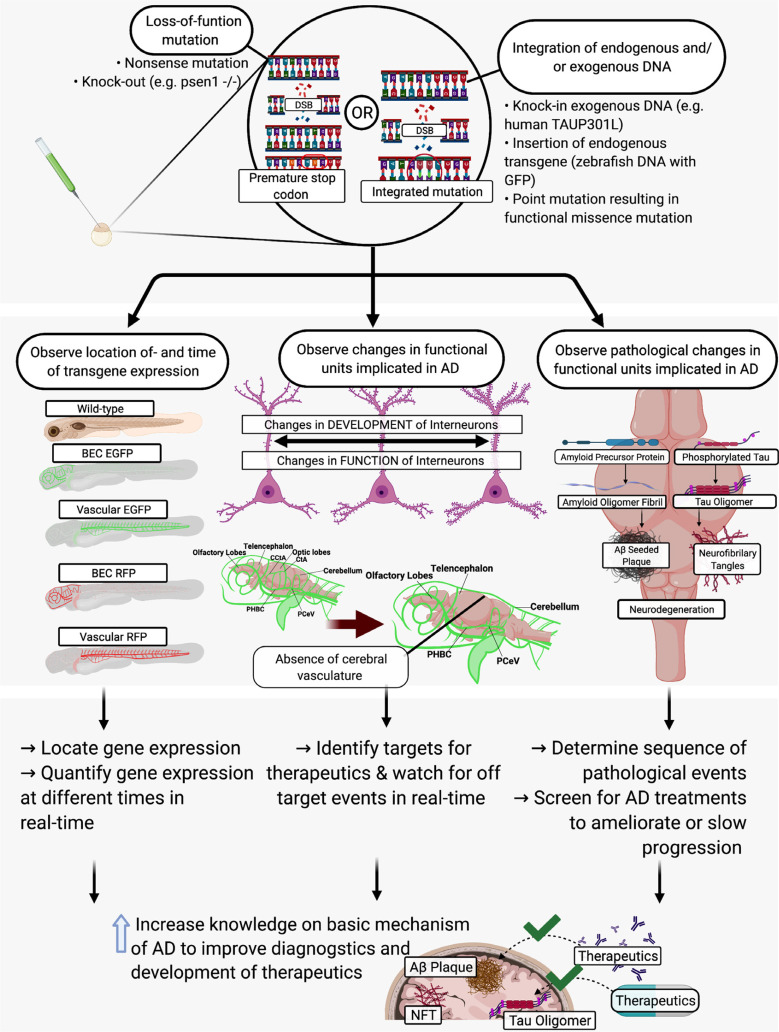
A new challenge faced by scientists developing therapeutics for AD includes targeting intrinsic factors that signal hormetic-based mechanisms. These hormetic-based mechanisms can regulate adaptive cellular stress response pathways. The treatment carried out by Spilman et al. of APP mutant mice with rapamycin inhibitor stimulated neuronal autophagy and reduced amyloid beta pathology [104]. Mattson et al. found that modifying environmental factors could activate intrinsic mechanisms and that at a low dose led to amelioration of cognitive decline in 3xTg AD mice [105]. Brose et al. proposed another example of potential therapeutics with the purpose to activate hormetic intrinsic factors in order to pro-actively treat rat hippocampal neurons [106]. In this study, Brose et al. proactively treated hippocampal neurons with hydroxyurea (HU) and observed protective effects against oxidative, metabolic, and excitotoxic stress. HU is a ribonuclease inhibitor, and in the study conducted by Brose et al. [106], HU improved cellular metabolic defects and activated adaptive cellular stress responses in vivo (Fig. 2).
Fig. (2).
The use of gene-editing technology to study AD and screen potential therapeutics. Abbreviations: AD: Alzheimer’s disease; BEC: brain endothelial cell; CCtA: common cerebral artery; CtA: Cerebral artery; DSB: double strand break; EGFP: enhanced green fluorescent protein; GFP: green fluorescent protein; NFT: neurofibrillary tangle; PCeV: posterior cerebral vein; PHBC: posterior hindbrain channel; RFP: red fluorescent protein].
Other challenges include the timeline required and overall cost for large-scale preclinical animal and human clinical trials. The list of factors that contribute to impeding the success of past and current potential therapeutics grows.
6.4. Zebrafish in Drug Discovery
The use of wild-type and genetically modified zebrafish can overcome many of the challenges faced with mammalian models in many aspects of AD research. For example, AD progression studies are stressed by the increased consumption of resources for essential maintenance of mammalian models. The shorter life span and smaller size of the zebrafish provide scientists with the ability to economically maintain groups and note the progression of diseases with late onset. Scientific findings support genetic and structural conservation of the zebrafish CNS and an overall need to find a drug that stops and potentially ameliorates cognitive deterioration. While the zebrafish is being used in many aspects of drug discovery, much of the AD research completed using zebrafish is still heavily focused on elucidating the mechanism of AD progression. However, some zebrafish models have been put to use to screen potential therapeutics, including surfen and oxalyl surfen, Lithium, and Lanthionine ketamine-5-ethyl ester as well chaperone-gold nanoparticles [103, 107-109]. Lanthionine ketamine-5-ethyl ester (LKE) is a small molecule administered to zebrafish with okadaic acid (OKA)-induced AD to elucidate the neuroprotective properties of LKE [107]. Koehler et al. utilized these adult zebrafish with OKA-induced AD and found that treatment with LKE significantly reduced the number of apoptotic brain cells, increased phosphor-activation of important pro-survival factors, and increased brain-derived neurotrophic factor (BDNF) in the zebrafish. Lithium is another molecule of interest in AD therapeutics that has been studied using the zebrafish model. Lithium treatment in zebrafish reversed impaired cognition and increased tau phosphorylation [108]. After intraventricular injection of Aβ into the brain of wild-type zebrafish embryos, Nery and colleagues found significant increases in tau phosphorylation via Ser202 and Thr205 phosphorylation levels (two amino acids targeted by GSK-3β) [108].
Two small molecules, surfen and oxalyl surfen, were applied to a zebrafish model of tauopathy [103]. Surfen and its derivative oxalyl surfen possess heparan sulfate antagonist properties, which are characteristics that could assist in mitigating both tau hyperphosphorylation and neuronal degradation. The in vivo treatment of these small molecules in the transgenic zebrafish, Tg[HuC::hTauP301L; DsRed], significantly reduced the accumulation of pThr181, a tau phosphor-epitope. While small molecule screening using wild-type and modified zebrafish is one promising tool for AD researchers, the use of a different type of therapeutic, chaperone-gold nanoparticles, is on the horizon as a favorable inhibitor of amyloidosis. Javed et al. engineered βCas chaperones onto gold nanoparticles. These nanoparticles were then delivered via intracardial injection, and mitigation of Aβ42-induced toxicity in the brain was evaluated [109]. The intracardiac injection of βCas-gold nanoparticles mitigated the toxicity of the Aβ42 injected into the cerebrovascular region.
6.5. Future Directions and Applications of Zebrafish Genetic Models in AD Therapeutics
As described above, zebrafish are highly responsive to genetic modification. The use of these genetic editing technologies in conjunction with the zebrafish model is an efficient and expanding area for AD therapeutics research (Fig. 2). The zebrafish model allows scientists to observe the appearance of pathological phenotypes rapidly and with ease after genetic modification due to the transparency during embryogenesis and rapid development. Many potential therapeutics require specific regimens to evaluate efficacy. The length of time needed to administer these “long-term” therapeutics and the required amount hamper the haste required to assess all potential therapeutics. There is a need for a homogeneous in vivo model with high-throughput and high-content screening capacity that can rapidly and efficiently identify more active and specific compounds with therapeutic potential. Using zebrafish, it is possible to study the molecular and pathological sequences of events in a compressed timescale, with increased confidence and with great statistical power.
CONCLUSION
After many decades of intensive AD research, uncertainty remains, especially with regard to the fundamental mechanisms underlying AD pathology. The use of many current disease models is followed by considerable doubt, as there are discrepancies between preclinical animal results and phase 1 safety and phase 2 efficacy results in human trials. Unfortunately, our lack of understanding of the fundamental mechanisms of AD has impeded the success of effective therapeutic developments. An alternative, economic, and efficient pathway that researchers can use to investigate aspects of AD development and treatment involves the use of zebrafish, and specifically, the use of genetically modified zebrafish. Zebrafish present as a complementary model system to the more traditional rodent models when the aim is to discover neuropharmacological solutions for AD.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
Funding has been provided by the National Institutes of Health, National Institute of Environmental Health Sciences Diversity Supplement (R01ES027078-03S1), and from the Purdue University Institute for Drug Discovery and Institute for Integrative Neuroscience.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Mohandas E., Rajmohan V., Raghunath B. Neurobiology of Alzheimer’s disease. Indian J. Psychiatry. 2009;51(1):55–61. doi: 10.4103/0019-5545.44908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raudino F. Involvement of the spinal cord in the Alzheimer’s disease: A literature review. Arch. Neurosci. 2016;3(4) doi: 10.5812/archneurosci.33834]. [DOI] [Google Scholar]
- 3.Dugger B.N., Hidalgo J.A., Chiarolanza G., Mariner M., Henry-Watson J., Sue L.I., Beach T.G. The distribution of phosphorylated tau in spinal cords of Alzheimer’s disease and non-demented individuals. J. Alzheimers Dis. 2013;34(2):529–536. doi: 10.3233/JAD-121864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito Y., Murayama S. Expression of tau immunoreactivity in the spinal motor neurons of Alzheimer’s disease. Neurology. 2000;55(11):1727–1729. doi: 10.1212/WNL.55.11.1727. [DOI] [PubMed] [Google Scholar]
- 5.Yeh T.S., Ho Y.C., Hsu C.L., Pan S.L. Spinal cord injury and Alzheimer’s disease risk: a population-based, retrospective cohort study. Spinal Cord. 2018;56(2):151–157. doi: 10.1038/s41393-017-0009-3. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzi R.M., Palesi F., Castellazzi G., Vitali P., Anzalone N., Bernini S., Cotta Ramusino M., Sinforiani E., Micieli G., Costa A., D’Angelo E., Gandini Wheeler-Kingshott C.A.M. Unsuspected involvement of spinal cord in Alzheimer disease. Front. Cell. Neurosci. 2020;14:6. doi: 10.3389/fncel.2020.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J.H., Ryan J., Andreescu C., Aizenstein H., Lim H.K. Brainstem morphological changes in Alzheimer’s disease. Neuroreport. 2015;26(7):411–415. doi: 10.1097/WNR.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grinberg L.T., Rueb U., Heinsen H. Brainstem: neglected locus in neurodegenerative diseases. Front. Neurol. 2011;2:42. doi: 10.3389/fneur.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vajn K., Plunkett J.A., Tapanes-Castillo A., Oudega M. Axonal regeneration after spinal cord injury in zebrafish and mammals: differences, similarities, translation. Neurosci. Bull. 2013;29(4):402–410. doi: 10.1007/s12264-013-1361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fakhoury M. Microglia and astrocytes in Alzheimer’s disease: implications for therapy. Curr. Neuropharmacol. 2018;16(5):508–518. doi: 10.2174/1570159X15666170720095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost G.R., Li Y.M. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017;7(12):170228. doi: 10.1098/rsob.170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer’s Disease. J. Cell Biol. Rockefeller University Press; 2018. pp. 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raikwar S. P., Bhagavan S. M., Beladakere Ramaswamy S., Thangavel R., Dubova I., Pushpavathi Selvakumar G., Ejaz Ahmed M., Kempuraj D., Iyer S., Zaheer S., Zaheer A. Are Tanycytes the Missing Link between Type 2 Diabetes and Alzheimer’s Disease? [DOI] [PMC free article] [PubMed]
- 14.Babin P.J., Thisse C., Durliat M., Andre M., Akimenko M.A., Thisse B. Both apolipoprotein E and A-I genes are present in a nonmammalian vertebrate and are highly expressed during embryonic development. Proc. Natl. Acad. Sci. USA. 1997;94(16):8622–8627. doi: 10.1073/pnas.94.16.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholls D.G., Budd S.L. Mitochondria and Neuronal Survival. Physiological Reviews. American Physiological Society; 2000. pp. 315–360. [DOI] [PubMed] [Google Scholar]
- 16.Carrano A., Hoozemans J.J.M., van der Vies S.M., Rozemuller A.J.M., van Horssen J., de Vries H.E. Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid. Redox Signal. 2011;15(5):1167–1178. doi: 10.1089/ars.2011.3895. [DOI] [PubMed] [Google Scholar]
- 17.Erickson M.A., Banks W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cerebral Blood Flow Metabolism. SAGE Publications; 2013. pp. 1500–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roher A.E., Kuo Y.M., Esh C., Knebel C., Weiss N., Kalback W., Luehrs D.C., Childress J.L., Beach T.G., Weller R.O., Kokjohn T.A. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol. Med. 2003;9(3-4):112–122. doi: 10.1007/BF03402043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zenaro E., Piacentino G., Constantin G. The Blood-Brain Barrier in Alzheimer’s Disease. Neurobiology of Disease. Academic Press Inc.; 2017. pp. 41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong J-Y., Kwon H-B., Ahn J-C., Kang D., Kwon S-H., Park J.A., Kim K-W. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res. Bull. 2008;75(5):619–628. doi: 10.1016/j.brainresbull.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Verdile G., Fuller S., Atwood C.S., Laws S.M., Gandy S.E., Martins R.N. The role of beta amyloid in Alzheimer’s disease: still a cause of everything or the only one who got caught? Pharmacol. Res. 2004;50(4):397–409. doi: 10.1016/j.phrs.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Hardy J., Selkoe D. J. ?The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science (80-. ) 2002;297(19):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 23.Newman M., Verdile G., Martins R.N., Lardelli M. Zebrafish as a tool in Alzheimer’s disease research. Biochim. Biophys. Acta. 2011;1812(3):346–352. doi: 10.1016/j.bbadis.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Maccioni R.B., Farías G., Morales I., Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Arch. Med. Res. 2010;41(3):226–231. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Cameron D.J., Galvin C., Alkam T., Sidhu H., Ellison J., Luna S., Ethell D.W. Alzheimer’s-related peptide amyloid-β plays a conserved role in angiogenesis. PLoS One. 2012;7(7):e39598. doi: 10.1371/journal.pone.0039598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundvik M., Chen Y.C., Panula P. Presenilin1 regulates histamine neuron development and behavior in zebrafish, Danio rerio. J. Neurosci. 2013;33(4):1589–1597. doi: 10.1523/JNEUROSCI.1802-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butt A.M., De La Rocha I.C., Rivera A. Oligodendroglial Cells in Alzheimer’s Disease.Advances in Experimental Medicine and Biology. Springer New York LLC. 2019;Vol. 1175:325–333. doi: 10.1007/978-981-13-9913-8_12. [DOI] [PubMed] [Google Scholar]
- 28.Preston M.A., Macklin W.B. Zebrafish as a Model to Investigate CNS Myelination.GLIA. John Wiley and Sons Inc.; 2015. pp. 177–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brösamle C., Halpern M.E. Characterization of myelination in the developing zebrafish. Glia. 2002;39(1):47–57. doi: 10.1002/glia.10088. [DOI] [PubMed] [Google Scholar]
- 30.Groth C., Nornes S., McCarty R., Tamme R., Lardelli M. Identification of a second presenilin gene in zebrafish with similarity to the human Alzheimer’s disease gene presenilin2. Dev. Genes Evol. 2002;212(10):486–490. doi: 10.1007/s00427-002-0269-5. [DOI] [PubMed] [Google Scholar]
- 31.Shen J., Bronson R.T., Chen D.F., Xia W., Selkoe D.J., Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89(4):629–639. doi: 10.1016/S0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 32.Wong P.C., Zheng H., Chen H., Becher M.W., Sirinathsinghji D.J.S., Trumbauer M.E., Chen H.Y., Price D.L., Van der Ploeg L.H.T., Sisodia S.S. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387(6630):288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 33.Nornes S., Newman M., Wells S., Verdile G., Martins R.N., Lardelli M. Independent and cooperative action of Psen2 with Psen1 in zebrafish embryos. Exp. Cell Res. 2009;315(16):2791–2801. doi: 10.1016/j.yexcr.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 34.Herreman A., Hartmann D., Annaert W., Saftig P., Craessaerts K., Serneels L., Umans L., Schrijvers V., Checler F., Vanderstichele H., Baekelandt V., Dressel R., Cupers P., Huylebroeck D., Zwijsen A., Van Leuven F., De Strooper B. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc. Natl. Acad. Sci. USA. 1999;96(21):11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Q., Zhao W.J., Ou G.Y., Xue W.K. An overview of experimental and clinical spinal cord findings in Alzheimer’s disease. Brain Sci. 2019;9(7):E168. doi: 10.3390/brainsci9070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musa A., Lehrach H., Russo V.A. Distinct expression patterns of two zebrafish homologues of the human APP gene during embryonic development. Dev. Genes Evol. 2001;211(11):563–567. doi: 10.1007/s00427-001-0189-9. [DOI] [PubMed] [Google Scholar]
- 37.Lee J-A., Cole G.J. Generation of transgenic zebrafish expressing green fluorescent protein under control of zebrafish amyloid precursor protein gene regulatory elements. Zebrafish. 2007;4(4):277–286. doi: 10.1089/zeb.2007.0516. [DOI] [PubMed] [Google Scholar]
- 38.Liao H-K., Wang Y., Noack Watt K.E., Wen Q., Breitbach J., Kemmet C.K., Clark K.J., Ekker S.C., Essner J.J., McGrail M. Tol2 gene trap integrations in the zebrafish amyloid precursor protein genes appa and aplp2 reveal accumulation of secreted APP at the embryonic veins. Dev. Dyn. 2012;241(2):415–425. doi: 10.1002/dvdy.23725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joshi P., Liang J.O., DiMonte K., Sullivan J., Pimplikar S.W. Amyloid precursor protein is required for convergent-extension movements during Zebrafish development. Dev. Biol. 2009;335(1):1–11. doi: 10.1016/j.ydbio.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 40.Luna S., Cameron D.J., Ethell D.W. Amyloid-β and APP deficiencies cause severe cerebrovascular defects: important work for an old villain. PLoS One. 2013;8(9):e75052. doi: 10.1371/journal.pone.0075052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunvong K., Huffmire D., Ethell D.W., Cameron D.J. Amyloid-β increases capillary bed density in the adult zebrafish retina. Invest. Ophthalmol. Vis. Sci. 2013;54(2):1516–1521. doi: 10.1167/iovs.12-10821. [DOI] [PubMed] [Google Scholar]
- 42.Moussavi Nik S.H., Wilson L., Newman M., Croft K., Mori T.A., Musgrave I., Lardelli M. The BACE1-PSEN-AβPP regulatory axis has an ancient role in response to low oxygen/oxidative stress. J. Alzheimers Dis. 2012;28(3):515–530. doi: 10.3233/JAD-2011-110533. [DOI] [PubMed] [Google Scholar]
- 43.van Bebber F., Hruscha A., Willem M., Schmid B., Haass C. Loss of Bace2 in zebrafish affects melanocyte migration and is distinct from Bace1 knock out phenotypes. J. Neurochem. 2013;127(4):471–481. doi: 10.1111/jnc.12198. [DOI] [PubMed] [Google Scholar]
- 44.Francis R., McGrath G., Zhang J., Ruddy D.A., Sym M., Apfeld J., Nicoll M., Maxwell M., Hai B., Ellis M.C., Parks A.L., Xu W., Li J., Gurney M., Myers R.L., Himes C.S., Hiebsch R., Ruble C., Nye J.S., Curtis D. aph-1 and pen-2 are required for Notch pathway signaling, γ-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev. Cell. 2002;3(1):85–97. doi: 10.1016/S1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 45.Campbell W.A., Yang H., Zetterberg H., Baulac S., Sears J.A., Liu T., Wong S.T.C., Zhong T.P., Xia W. Zebrafish lacking Alzheimer presenilin enhancer 2 (Pen-2) demonstrate excessive p53-dependent apoptosis and neuronal loss. J. Neurochem. 2006;96(5):1423–1440. doi: 10.1111/j.1471-4159.2006.03648.x. [DOI] [PubMed] [Google Scholar]
- 46.Lim A., Moussavi Nik S.H., Ebrahimie E., Lardelli M. Analysis of nicastrin gene phylogeny and expression in zebrafish. Dev. Genes Evol. 2015;225(3):171–178. doi: 10.1007/s00427-015-0500-9. [DOI] [PubMed] [Google Scholar]
- 47.Leimer U., Lun K., Romig H., Walter J., Grünberg J., Brand M., Haass C. Zebrafish (Danio rerio) presenilin promotes aberrant amyloid β-peptide production and requires a critical aspartate residue for its function in amyloidogenesis. Biochemistry. 1999;38(41):13602–13609. doi: 10.1021/bi991453n. [DOI] [PubMed] [Google Scholar]
- 48.Durliat M., André M., Babin P.J. Conserved protein motifs and structural organization of a fish gene homologous to mammalian apolipoprotein E. Eur. J. Biochem. 2000;267(2):549–559. doi: 10.1046/j.1432-1327.2000.01033.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee J., Peterson S.M., Freeman J.L. Alzheimer’s disease risk genes in wild-type adult zebrafish exhibit gender-specific expression changes during aging. Neurogenetics. 2016;17(3):197–199. doi: 10.1007/s10048-016-0485-1. [DOI] [PubMed] [Google Scholar]
- 50.Lee J., Peterson S.M., Freeman J.L. Sex-specific characterization and evaluation of the Alzheimer’s disease genetic risk factor sorl1 in zebrafish during aging and in the adult brain following a 100 ppb embryonic lead exposure. J. Appl. Toxicol. 2017;37(4):400–407. doi: 10.1002/jat.3372. [DOI] [PubMed] [Google Scholar]
- 51.Coburn C.A., Stachel S.J., Li Y.M., Rush D.M., Steele T.G., Chen-Dodson E., Holloway M.K., Xu M., Huang Q., Lai M.T., DiMuzio J., Crouthamel M.C., Shi X.P., Sardana V., Chen Z., Munshi S., Kuo L., Makara G.M., Annis D.A., Tadikonda P.K., Nash H.M., Vacca J.P., Wang T. Identification of a small molecule nonpeptide active site β-secretase inhibitor that displays a nontraditional binding mode for aspartyl proteases. J. Med. Chem. 2004;47(25):6117–6119. doi: 10.1021/jm049388p. [DOI] [PubMed] [Google Scholar]
- 52.Dockens R., Wang J-S., Castaneda L., Sverdlov O., Huang S-P., Slemmon R., Gu H., Wong O., Li H., Berman R.M., Smith C., Albright C.F., Tong G. A placebo-controlled, multiple ascending dose study to evaluate the safety, pharmacokinetics and pharmacodynamics of avagacestat (BMS-708163) in healthy young and elderly subjects. Clin. Pharmacokinet. 2012;51(10):681–693. doi: 10.1007/s40262-012-0005-x. [DOI] [PubMed] [Google Scholar]
- 53.Siemers E.R., Quinn J.F., Kaye J., Farlow M.R., Porsteinsson A., Tariot P., Zoulnouni P., Galvin J.E., Holtzman D.M., Knopman D.S., Satterwhite J., Gonzales C., Dean R.A., May P.C. Effects of a γ-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006;66(4):602–604. doi: 10.1212/01.WNL.0000198762.41312.E1. [DOI] [PubMed] [Google Scholar]
- 54.McGowan E., Pickford F., Kim J., Onstead L., Eriksen J., Yu C., Skipper L., Murphy M.P., Beard J., Das P., Jansen K., DeLucia M., Lin W.L., Dolios G., Wang R., Eckman C.B., Dickson D.W., Hutton M., Hardy J., Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47(2):191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haas H., Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 2003;4(2):121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 56.Sundvik M., Panula P. Organization of the histaminergic system in adult zebrafish (Danio rerio) brain: neuron number, location, and cotransmitters. J. Comp. Neurol. 2012;520(17):3827–3845. doi: 10.1002/cne.23126. [DOI] [PubMed] [Google Scholar]
- 57.Suster M.L., Abe G., Schouw A., Kawakami K. Transposon-mediated BAC transgenesis in zebrafish. Nat. Protoc. 2011;6(12):1998–2021. doi: 10.1038/nprot.2011.416. [DOI] [PubMed] [Google Scholar]
- 58.Bedell V.M., Ekker S.C. Using engineered endonucleases to create knockout and knockin Zebrafi Sh models. Methods in Molecular Biology; Pruett-Miller, S.M., Ed.; 2015;Vol. 1239:291–305. doi: 10.1007/978-1-4939-1862-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Irion U., Krauss J., Nüsslein-Volhard C. Precise and efficient genome editing in zebrafish using the CRISPR/Cas9 system. Development. 2014;141(24):4827–4830. doi: 10.1242/dev.115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saleem S., Kannan R.R. Zebrafish: an emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery. Cell Death Discov. 2018;4(45):45. doi: 10.1038/s41420-018-0109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ring S., Weyer S.W., Kilian S.B., Waldron E., Pietrzik C.U., Filippov M.A., Herms J., Buchholz C., Eckman C.B., Korte M., Wolfer D.P., Müller U.C. The secreted β-amyloid precursor protein ectodomain APPs α is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J. Neurosci. 2007;27(29):7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banote R.K., Chebli J., Şatır T.M., Varshney G.K., Camacho R., Ledin J., Burgess S.M., Abramsson A., Zetterberg H. Amyloid precursor protein-b facilitates cell adhesion during early development in zebrafish. Sci. Rep. 2020;10(1):10127. doi: 10.1038/s41598-020-66584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pu Y-Z., Liang L., Fu A-L., Liu Y., Sun L., Li Q., Wu D., Sun M-J., Zhang Y-G., Zhao B-Q. Generation of Alzheimer’s disease transgenic zebrafish expressing human APP mutation under control of zebrafish appb promotor. Curr. Alzheimer Res. 2017;14(6):668–679. doi: 10.2174/1567205013666161201202000. [DOI] [PubMed] [Google Scholar]
- 64.Meyer E.P., Ulmann-Schuler A., Staufenbiel M., Krucker T. Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2008;105(9):3587–3592. doi: 10.1073/pnas.0709788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soontornniyomkij V., Lynch M.D., Mermash S., Pomakian J., Badkoobehi H., Clare R., Vinters H.V. Cerebral microinfarcts associated with severe cerebral β-amyloid angiopathy. Brain Pathol. 2010;20(2):459–467. doi: 10.1111/j.1750-3639.2009.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barthelson K., Pederson S.M., Newman M., Lardelli M. Brain transcriptome analysis reveals subtle effects on mitochondrial function and iron homeostasis of mutations in the SORL1 gene implicated in early onset familial Alzheimer’s disease. Mol. Brain. 2020;13(1):142. doi: 10.1186/s13041-020-00681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomasiewicz H.G., Flaherty D.B., Soria J.P., Wood J.G. Transgenic zebrafish model of neurodegeneration. J. Neurosci. Res. 2002;70(6):734–745. doi: 10.1002/jnr.10451. [DOI] [PubMed] [Google Scholar]
- 68.Wu B.K., Yuan R.Y., Lien H.W., Hung C.C., Hwang P.P., Chen R.P.Y., Chang C.C., Liao Y.F., Huang C.J. Multiple signaling factors and drugs alleviate neuronal death induced by expression of human and zebrafish tau proteins in vivo. J. Biomed. Sci. 2016;23(1):25. doi: 10.1186/s12929-016-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bai Q., Garver J.A., Hukriede N.A., Burton E.A. Generation of a transgenic zebrafish model of Tauopathy using a novel promoter element derived from the zebrafish eno2 gene. Nucleic Acids Res. 2007;35(19):6501–6516. doi: 10.1093/nar/gkm608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paquet D., Bhat R., Sydow A., Mandelkow E.M., Berg S., Hellberg S., Fälting J., Distel M., Köster R.W., Schmid B., Haass C. A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J. Clin. Invest. 2009;119(5):1382–1395. doi: 10.1172/JCI37537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cosacak M.I., Bhattarai P., Bocova L., Dzewas T., Mashkaryan V., Papadimitriou C., Brandt K., Hollak H., Antos C.L., Kizil C. Human TAUP301L overexpression results in TAU hyperphosphorylation without neurofibrillary tangles in adult zebrafish brain. Sci. Rep. 2017;7(1):12959. doi: 10.1038/s41598-017-13311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391(6665):387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 73.De Strooper B., Iwatsubo T., Wolfe M.S. Presenilins and γ-secretase: structure, function, and role in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012;2(1):a006304. doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takashima A., Murayama M., Murayama O., Kohno T., Honda T., Yasutake K., Nihonmatsu N., Mercken M., Yamaguchi H., Sugihara S., Wolozin B. Presenilin 1 associates with glycogen synthase kinase-3β and its substrate tau. Proc. Natl. Acad. Sci. USA. 1998;95(16):9637–9641. doi: 10.1073/pnas.95.16.9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nornes S., Groth C., Camp E., Ey P., Lardelli M. Developmental control of Presenilin1 expression, endoproteolysis, and interaction in zebrafish embryos. Exp. Cell Res. 2003;289(1):124–132. doi: 10.1016/S0014-4827(03)00257-X. [DOI] [PubMed] [Google Scholar]
- 76. “PSEN-1 | ALZFORUM.” Available from: . https://www.alzforum. org/mutations/psen-1(accessed Jan. 22, 2021).
- 77.Ochalek A., Mihalik B., Avci H.X., Chandrasekaran A., Téglási A., Bock I., Giudice M.L., Táncos Z., Molnár K., László L., Nielsen J.E., Holst B., Freude K., Hyttel P., Kobolák J., Dinnyés A. Neurons derived from sporadic Alzheimer’s disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation. Alzheimers Res. Ther. 2017;9(1):90. doi: 10.1186/s13195-017-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hin N., Newman M., Kaslin J., Douek A.M., Lumsden A., Zhou X-F., Ludington A., Adelson D.L., Pederson S., Lardelli M. Accelerated Brain Aging towards Transcriptional Inversion in a Zebrafish Model of Familial Alzheimer’s Disease. PLoS One. doi: 10.1101/262162]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pigino G., Pelsman A., Mori H., Busciglio J. Presenilin-1 mutations reduce cytoskeletal association, deregulate neurite growth, and potentiate neuronal dystrophy and tau phosphorylation. J. Neurosci. 2001;21(3):834–842. doi: 10.1523/JNEUROSCI.21-03-00834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zon L.I., Peterson R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005;4(1):35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 81."FDA-approved treatments for Alzheimer's," Aug. 2019. Accessed: Jan. 22, 2021. Available from: . https://www.alz.org/media/documents/fda-approved-treatments-alzheimers-ts.pdf
- 82.Weller J., Budson A. Current Understanding of Alzheimer’s Disease Diagnosis and Treatment.F1000Research; F1000 Research Ltd, 2018. [DOI] [PMC free article] [PubMed]
- 83.Elmaleh D. R. Combination Therapies for the Treatment of Alzheimer’s Disease and Related Disorders - Google Patents. WO2014066318A1, 2021.
- 84.Kocis P., Tolar M., Yu J., Sinko W., Ray S., Blennow K., Fillit H., Hey J.A. Elucidating the Aβ42 Anti-Aggregation Mechanism of Action of Tramiprosate in Alzheimer’s Disease: Integrating Molecular Analytical Methods, Pharmacokinetic and Clinical Data. CNS Drugs. 2017;31(6):495–509. doi: 10.1007/s40263-017-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hey J.A., Yu J.Y., Versavel M., Abushakra S., Kocis P., Power A., Kaplan P.L., Amedio J., Tolar M. Clinical pharmacokinetics and safety of ALZ-801, a novel prodrug of tramiprosate in development for the treatment of Alzheimer’s disease. Clin. Pharmacokinet. 2018;57(3):315–333. doi: 10.1007/s40262-017-0608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cirrito J. R., Wallace C. E., Yan P., Davis T. A., Gardiner W. D., Doherty B. M., King D., Yuede C. M., Lee J.-M., Sheline Y. I. Effect of Escitalopram on Aβ Levels and Plaque Load in an Alzheimer Mouse Model. 2020. [DOI] [PMC free article] [PubMed]
- 87.Buttini M., Masliah E., Barbour R., Grajeda H., Motter R., Johnson-Wood K., Khan K., Seubert P., Freedman S., Schenk D., Games D. β-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer’s disease. J. Neurosci. 2005;25(40):9096–9101. doi: 10.1523/JNEUROSCI.1697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bard F., Cannon C., Barbour R., Burke R.L., Games D., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., Kholodenko D., Lee M., Lieberburg I., Motter R., Nguyen M., Soriano F., Vasquez N., Weiss K., Welch B., Seubert P., Schenk D., Yednock T. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6(8):916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 89.Farlow M.R., Andreasen N., Riviere M.E., Vostiar I., Vitaliti A., Sovago J., Caputo A., Winblad B., Graf A. Long-term treatment with active Aβ immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimers Res. Ther. 2015;7(1):23. doi: 10.1186/s13195-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Winblad B., Andreasen N., Minthon L., Floesser A., Imbert G., Dumortier T., Maguire R.P., Blennow K., Lundmark J., Staufenbiel M., Orgogozo J.M., Graf A. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11(7):597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 91.] Novartis Pharmaceuticals. A Randomized, Double-blind, Placebocontrolled, Two-cohort, Parallel Group Study to Evaluate the Efficacy of CAD106 and CNP520 in Participants at Risk for the Onset of Clinical Symptoms of Alzheimer’s Disease. Available from: https://clinicaltrials.gov/ct2/show/NCT02565511(accessed Jan 25, 2021)
- 92.Donanemab ALZFORUM." Available from: https://www. alzforum.org/therapeuticsdonanemab(accessed Jan. 25, 2021)/
- 93..“UB-311 | ALZFORUM.” Available from: . https://www. alzforum. org/therapeutics/ub-311(accessed Jan. 25, 2021)
- 94.Lacosta A.M., Pascual-Lucas M., Pesini P., Casabona D., Pérez-Grijalba V., Marcos-Campos I., Sarasa L., Canudas J., Badi H., Monleón I., San-José I., Munuera J., Rodríguez-Gómez O., Abdelnour C., Lafuente A., Buendía M., Boada M., Tárraga L., Ruiz A., Sarasa M. Safety, tolerability and immunogenicity of an active anti-Aβ40 vaccine (ABvac40) in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase I trial. Alzheimers Res. Ther. 2018;10(1):12. doi: 10.1186/s13195-018-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fabre C., Gobbi M., Ezzili C., Zoubir M., Sablin M.P., Small K. Im, E.; Shinwari, N.; Zhang, D.; Zhou, H.; Le Tourneau, C. Clinical Study of the Novel Cyclin-Dependent Kinase Inhibitor Dinaciclib in Combination with Rituximab in Relapsed/Refractory Chronic Lymphocytic Leukemia Patients. Cancer Chemother. Pharmacol. 2014;74(5):1057–1064. doi: 10.1007/s00280-014-2583-9. [DOI] [PubMed] [Google Scholar]
- 96.Kumar S.K., LaPlant B., Chng W.J., Zonder J., Callander N., Fonseca R., Fruth B., Roy V., Erlichman C., Stewart A.K. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood. 2015;125(3):443–448. doi: 10.1182/blood-2014-05-573741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Novak P., Schmidt R., Kontsekova E., Kovacech B., Smolek T., Katina S., Fialova L., Prcina M., Parrak V., Dal-Bianco P., Brunner M., Staffen W., Rainer M., Ondrus M., Ropele S., Smisek M., Sivak R., Zilka N., Winblad B., Novak M. FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res. Ther. 2018;10(1):108. doi: 10.1186/s13195-018-0436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Theunis C., Crespo-Biel N., Gafner V., Pihlgren M., López-Deber M.P., Reis P., Hickman D.T., Adolfsson O., Chuard N., Ndao D.M., Borghgraef P., Devijver H., Van Leuven F., Pfeifer A., Muhs A. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8(8):e72301. doi: 10.1371/journal.pone.0072301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nobuhara C.K., DeVos S.L., Commins C., Wegmann S., Moore B.D., Roe A.D., Costantino I., Frosch M.P., Pitstick R., Carlson G.A., Hock C., Nitsch R.M., Montrasio F., Grimm J., Cheung A.E., Dunah A.W., Wittmann M., Bussiere T., Weinreb P.H., Hyman B.T., Takeda S. Tau Antibody Targeting Pathological Species Blocks Neuronal Uptake and Interneuron Propagation of Tau in Vitro. Am. J. Pathol. 2017;187(6):1399–1412. doi: 10.1016/j.ajpath.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.“JNJ-63733657 ALZFORUM.” Available from: . https://www. alzforum.org/therapeutics/jnj-63733657accessed Jan. 26, 2021].
- 101."Gosuranemab ALZFORUM." Available from: https://www. alzforum.org/therapeutics/gosuranemab[accessed Jan. 26, 2021]
- 102.Zambusi A., Ninkovic J. Regeneration of the Central Nervous System-Principles from Brain Regeneration in Adult Zebrafish. World J. Stem Cells. Baishideng Publishing Group Co; 2020. pp. 8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naini S.M.A., Yanicostas C., Hassan-Abdi R., Blondeel S., Bennis M., Weiss R.J., Tor Y., Esko J.D., Soussi-Yanicostas N. Surfen and Oxalyl Surfen Decrease Tau Hyperphosphorylation and Mitigate Neuron Deficits in vivo in a Zebrafish Model of Tauopathy. Transl. Neurodegener. 2018;7(6) doi: 10.1186/s40035-018-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spilman P., Podlutskaya N., Hart M.J., Debnath J., Gorostiza O., Bredesen D., Richardson A., Strong R., Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mattson M.P., Moehl K., Ghena N., Schmaedick M., Cheng A. Intermittent Metabolic Switching, Neuroplasticity and Brain Health. Nature Rev. Neurosci. Nature Publishing Group; 2018. pp. 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deering Brose R., Lehrmann E., Zhang Y., Reeves R. H., Smith K. D., Mattson M. P. Hydroxyurea attenuates oxidative, metabolic, and excitotoxic stress in rat hippocampal neurons and improves spatial memory in a mouse model of Alzheimer’s disease. 2018. [DOI] [PMC free article] [PubMed]
- 107.Koehler D., Shah Z.A., Hensley K., Williams F.E. Lanthionine ketimine-5-ethyl ester provides neuroprotection in a zebrafish model of okadaic acid-induced Alzheimer’s disease. Neurochem. Int. 2018;115:61–68. doi: 10.1016/j.neuint.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nery L.R., Eltz N.S., Hackman C., Fonseca R., Altenhofen S., Guerra H.N., Freitas V.M., Bonan C.D., Vianna M.R.M.R. Brain intraventricular injection of amyloid-β in zebrafish embryo impairs cognition and increases tau phosphorylation, effects reversed by lithium. PLoS One. 2014;9(9):e105862. doi: 10.1371/journal.pone.0105862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Javed I., Peng G., Xing Y., Yu T., Zhao M., Kakinen A., Faridi A., Parish C.L., Ding F., Davis T.P., Ke P.C., Lin S. Inhibition of amyloid beta toxicity in zebrafish with a chaperone-gold nanoparticle dual strategy. Nat. Commun. 2019;10(1):3780. doi: 10.1038/s41467-019-11762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]