Abstract
Introduction:
Social jetlag (SJL), the discrepancy in sleep timing between weekdays and weekends, is associated with higher BMI and cardiometabolic risk and is common in young adults. We examined whether chronic SJL impacts weight gain in young adults participating in a weight gain prevention trial.
Methods:
Young adults (n=599, age 18-35; BMI: 21.0-30.9 kg/m2) completed assessments at 0, 4, 12, and 24 months. Multilevel mixed growth models were used to examine 1) associations between demographics and longitudinal SJL and 2) longitudinal SJL as a predictor of weight change and cardiometabolic outcomes. SJL was assessed as a continuous and clinically-significant dichotomous (< vs. ≥2 hours) variable.
Results:
Thirty-eight percent of participants had clinically-significant SJL at ≥ 1 timepoints (Baseline M±SD=1.3±0.89). Younger (b=−0.05, p<0.001), female (b=0.18, p=0.037) and Black (compared to white, b=0.23, p=0.045) participants were more likely to have greater SJL. Individuals with high SJL (≥2 hours; between-person effect) were more likely to have greater weight gain over 2 years (b=0.05, p=0.028). High SJL did not affect the rate of change in waist circumference or cardiometabolic markers over time.
Conclusions:
High SJL is associated with greater weight gain over time. Reducing SJL may positively impact weight status in young adults.
Keywords: Social jetlag, sleep, weight gain prevention, young adults
Introduction
Social jetlag (SJL) signifies a potential mismatch between circadian and social sleep clocks and is typically measured as the difference in mid-point of sleep between weekdays, when sleep schedules are altered to meet job demands, and weekends, when sleep schedules theoretically follow natural circadian rhythms (Wittmann, Dinich, Merrow, & Roenneberg, 2006). This circadian misalignment has been associated with a variety of medical and mental health conditions, including greater adiposity, type 2 diabetes, metabolic syndrome and other cardiometabolic risk factors, and depression (Levandovski et al., 2011; Wong, Hasler, Kamarck, Muldoon, & Manuck, 2015; Zuraikat et al., 2020). In particular, data indicate that typically experiencing SJL of 2 hours or greater may be especially detrimental to(Kim, Lyu, & Kim, 2020; Koopman et al., 2017; Levandovski et al., 2011; Rutters et al., 2014).
Mechanisms thought to underlie these processes relate back to the central circadian clock, which is housed in the suprachiasmatic nucleus (SCN) and entrained primarily by the day/night cycle (Mohawk, Green, & Takahashi, 2012). This central clock then helps to regulate peripheral clocks that are housed in nearly all tissues in the body and regulate local physiological processes, including metabolism (Bass & Takahashi, 2010; Mohawk et al., 2012). Circadian disruption, such as SJL, can interfere with the natural synchrony of these clocks and has been shown to negatively impact glucose homeostasis, lipogenesis, sterol turnover, and oxidative metabolism, among other processes (Bass & Takahashi, 2010; Morris et al., 2015). Behaviorally, greater SJL also relates to poorer diet quality (Zerón-Rugerio, Cambras, & Izquierdo-Pulido, 2019) and less physical activity (Rutters et al., 2014). These processes combined likely contribute to higher weights and poorer cardiovascular and metabolic health documented in individuals with high levels of SJL.
Young adulthood (18-35 years) is a particularly high risk period for sleep disturbance (Gaultney, 2010; Gradisar et al., 2013). Prior research indicates clinically-significant SJL (≥2 hours) occurs in up to 30% of young adults and can be chronic for a subset (Lanoye & LaRose, 2020; McMahon et al., 2018). Causes may relate to chronotypes, with preference for the latest sleep times/wake times occurring around 20 years old, making early social demands particularly detrimental (Fischer, Lombardi, Marucci-Wellman, & Roenneberg, 2017). Young adulthood is also a period of rapid weight gain and high obesity incidence (Dutton et al., 2016; Ellison-Barnes, Johnson, & Gudzune, 2021; Hales, Carroll, Fryar, & Ogden, 2020; Mulye et al., 2009).
Although sleep problems are commonly linked to higher weights in the general adult population, studies of the association between SJL and BMI in young adults have had mixed results (McMahon et al., 2019; Zerón-Rugerio et al., 2019). McMahon and colleagues examined a group of 390 healthy young adults and found no association between SJL and anthropometric or blood pressure outcomes (McMahon et al., 2019). However, Zerón-Rugerio and colleagues examined a group of 534 young adults and found that greater SJL was associated with greater BMI (Zerón-Rugerio et al., 2019). Study differences may explain the contrasting outcomes. The sample in Zerón-Rugerio et al. was younger (21 vs. 27 years old) and had higher average SJL (1.6 vs. 1.1 hrs) than McMahon et al. It may be that SJL needs to be substantial to affect weight. SJL was also measured using usual bedtimes and wake times in Zerón-Rugerio et al. compared to a 6-10 day measurement period in McMahon et al.. Greater SJL may also need to be habitual to affect weight. Thus, questions remain about the association between SJL and weight/cardiovascular health in young adulthood.
Treatments to help young adults manage their weight can be effective when adapted for this population, although treatment outcomes are heterogeneous (Jakicic et al., 2016; LaRose et al., 2019; Wing, Russell, et al., 2020). It is unclear how sleep health, specifically SJL, may contribute to varied outcomes. One prior study examined SJL in emerging adults (18-25 years) with overweight or obesity who were participating in a behavioral weight loss program and found that ≥2 SJL at baseline was not related to outcomes at 6 months (Lanoye & LaRose, 2020), but this has not been assessed in programs designed to prevent weight gain in individuals at risk for obesity. Moreover, chronic sleep issues may plausibly be more related to long-term health effects than acute issues (Kervezee, Kosmadopoulos, & Boivin, 2020).
The Study of Novel Approaches for Weight Gain Prevention (SNAP), a randomized trial comparing two self-regulation behavioral interventions and a control group for weight gain prevention in young adults, provided a unique opportunity to examine the longitudinal association of high SJL with weight change and cardiometabolic outcomes over 2 years. The current study fills a gap in the literature by assessing the association between changes in SJL and changes in weight and cardiovascular risk factors longitudinally in young adults. We tested the hypothesis that high SJL over time would be associated with greater weight gains as well as increases in waist circumference and cardiovascular risk factors.
Methods
The Study of Novel Approaches for Weight Gain Prevention (SNAP) was a 3-arm randomized controlled trial testing the relative efficacy of two self-regulation interventions for weight gain prevention – a large change (LC) and a small change (SC) approach – and a self-guided control condition (Wing et al., 2013; Wing et al., 2016). The trial protocol and outcomes have been previously detailed (Wing et al., 2013; Wing et al., 2016), but key elements are presented here in brief. Participants (n=599) were 18-35 years old with a body mass index (BMI) of 21.0 to 30.9 kg/m2. Inclusion criteria required participants to be English speaking with Internet access and to be able to safely participate fully with intervention recommendations (e.g., engage in physical activity). Exclusion criteria included a recent weight loss of >10 pounds in the prior 6 months, pregnancy or plans to become pregnant, current use of weight-affecting medications, or a history of anorexia or bulimia nervosa, substance abuse disorder, severe psychiatric disorders, or bariatric surgery (Wing et al., 2013).
The LC and SC interventions in SNAP were based on self-regulation skills and aimed to help participants stay at or below their weight at randomization using behavioral skills training (Wing et al., 2013). The contact schedule included 4 months of in-person sessions (1x/week for first 2 months, 1x/month for subsequent months). The LC intervention was designed to create a 5-10 pound weight loss buffer during the first 4 months through daily caloric restriction of 500-1000 kilocalories and an increase in activity to 250 minutes/week, whereas the SC intervention encouraged two 100-calories changes each day (one to diet and one to physical activity) to counteract small caloric excesses over time. The self-guided control arm received one group session during which they were introduced to both the LC and SC approaches. They were encouraged to select and follow their preferred method with no further training or support, aside from provision of publicly available websites. Sleep was not an intervention target; no psychoeducation, messaging or behavioral strategies regarding sleep were included in any of the arms. One exception was a newsletter sent to participants enrolled in winter 2012 regarding the importance of sufficient sleep duration and basic sleep hygiene recommendations.
The primary outcome, defined as the average weight change over an average of 3 years (similar to area under the curve), differed significantly among all three arms of the trial (LC: −2.37 (0.22) kg, SC: −0.56 (0.22) kg, and control: 0.26 (0.22) kg) (Wing et al., 2016). Mean weight changes at 2 years indicated LC (−1.5(0.34)%) and SC (−0.77(0.33)%) were superior to control (0.54(0.33)%), but not different from each other.
Measures
All measures were completed by assessors masked to study condition.
Social Jetlag Chronicity.
To assess bedtime and waketime, participants were asked “During the past month, what time did you usually go to bed in the evening (turn out the lights to go to sleep)?” and “During the past month, what time did you usually get out of bed in the morning?”. Times were self-reported in hours and minutes at each of the assessments (BL, 4 months, 1 year and 2 years); AM or PM was selected by ticking a box. This question was used to assess sleep in the EARLY trial, which was a consortium focused on weight management interventions in young adults (Lytle et al., 2014). The midpoint of sleep was then calculated and a difference score was created from the midpoint of weekday and weekend sleep (Wittmann et al., 2006). The absolute value of the SJL score was dichotomized into ≥2 hours or <2 hours. This cut-off was selected as prior studies have indicated individuals with this level of SJL have higher cortisol levels, are more physically inactive, have a higher resting heart rate, and are more likely to have metabolic syndrome (Koopman et al., 2017; Rutters et al., 2014). In adults 45-61, it was also associated with a higher prevalence of diabetes/prediabetes (Koopman et al., 2017).
Anthropometrics.
Weight was measured in kilograms using a digital calibrated scale to the nearest 0.1 kg and height was measured using a wall-mounted stadiometer to the nearest 0.5 cm. Waist circumference was measured at the midpoint between the top of the iliac crest and the bottom of the costal margin in the mid-axillary line in cm to the closest 0.1 cm using specialized measured tape with 4 ounces of tension. Two measures were taken for weight, height, and waist circumference and averaged. If two measures were different by >0.1 kg (weight), >0.5cm (height), or >1.0 cm (waist circumference) a third measure was taken and averaged with the first two.
Cardiometabolic Measures.
Diastolic and systolic blood pressure (DBP, SBP) was assessed with a Dinamap Monitor Pro 100. Participant was seated and cuff size was determined by arm circumference. Following a 5-min rest, three measures were taken 30 seconds apart and averaged. Fasting venous blood samples were collected and analyzed and HOMA-IR [(mg/dl) * (uU/mL)] was calculated.
Statistical Analysis
Prior to analysis, data were examined for AM/PM reporting errors. Individuals with time in bed <4 and >12 were flagged (3% of data) and likely reporting errors (e.g., 12 AM reported as 12 PM) were identified and agreed upon by two members of the authorship team. Following calculation of social jetlag variable, values in the top 1% and bottom 1% for each time point were winsorized to limit the influence of outliers.
Multilevel mixed growth models nested within person were used to analyze the data. To examine how SJL changed over time, fixed intercept effects of linear and quadratic time (months) were tested and the superior model, based on a X2 difference test (linear vs. linear and quadratic), was maintained. A binomial multilevel growth model also examined changes in clinically-significant SJL over time (≥2 hrs vs. <2 hrs). To examine the association that demographics and treatment assignment had with SJL, each variable was included with time in a separate model and tested as an individual predictor (e.g., are there consistent differences in SJL by gender over time) and as a moderator (e.g., does the change in SJL over time differ by gender).
Multilevel mixed growth models were also used to test the effects of both continuous and clinically-significant (dichotomous) SJL on weight, waist circumference, and cardiometabolic outcomes. Each outcome was tested with a separate model. To begin model building, the linear and quadratic (when relevant – glucose, insulin, and HOMA only had two observations) effects of time (months) on outcomes were examined. Again, the superior model, based on a X2 difference test, was maintained. Next, covariates (age, sex, race, and treatment assignment) alone and their interaction with linear time were added to models to control for their influence on outcomes. Finally, both within- and between-person SJL effects were included in the models. Within-person SJL effects show how variability in SJL within an individual at a certain time point accounts for the variability in the outcome at that time point. Between-person SJL effects show differences in variability on the outcome over time between individuals. Between-person SJL was included as an individual predictor and also in an interaction with linear time. A significant between-person SJL by time interaction would indicate that the change in the outcome (i.e., weight, waist circumference, cardiometabolic outcome) differed depending on an individual’s average SJL compared to other individuals. Within-person SJL was person-centered and between-person SJL was grand mean centered. Missing data was handled with Restricted Maximum Likelihood estimations.
Results
Demographics and Social Jetlag Chronicity
Out of the 599 participants, a total of 451 participants had complete sleep data at all 4 time points (BL, 4months, 1year, 2 years). Of these, 447 had weight data, 415 had waist circumference data, 414 had BP data, and 399 had data for glucose, insulin, and HOMA-IR at 2 years. As shown in Table 1, at baseline, participants went to sleep a little after 11 pm on weekdays and 12 am on weekends and woke up around 7 am on weekdays and 8:45 am on weekends. The average level of SJL was 1 hour and 20 minutes and 21.2% had ≥2 hours SJL. Across the study, 38% of participants experienced ≥2 hours of SJL at one timepoint or more.
Table 1.
Baseline Sample Characteristics (n=599)
| Variable | M±SDa |
|---|---|
| Gender (n(%)) | |
| Female | 469 (78%) |
| Male | 130 (22%) |
| Age | 28.16±4.40 |
| Race/Ethnicity (n(%)) | |
| White | 438 (73%) |
| African American | 66 (11%) |
| Hispanic | 46 (8%) |
| Other Race/Ethnicity | 49 (8%) |
| BMI (km/m2) | 25.44±2.59 |
| Waist Circumference (cm) | 82.22±8.58 |
| Systolic Blood Pressure (mmHg) | 110.39±11.05 |
| Diastolic Blood Pressure (mmHg) | 70.52±8.97 |
| Glucose (mg/dL) | 89.79±6.66 |
| Insulin (uU/mL) | 8.11±4.22 |
| HOMA ((mg/dL)*(uU/mL)) | 1.81±0.97 |
| Weekday Bedtime (h:min) | 23:10±1:25 |
| Weekday Wake time (h:min) | 7:08±1:33 |
| Weekend Bedtime (h:min) | 24:10±1:25 |
| Weekend Wake time (h:min) | 8:43±1:28 |
| Social Jetlag (h:min) | 1:20±0:53 |
| Clinically-significant SJL (≥2 hours) | 127 (21.2%) |
Unless otherwise noted
SJL showed a linear decrease over time (b=−0.004, SEb=0.001, t(1646) = −2.69, p=0.007). Linear models were superior to models with a quadratic effect, indicating the rate of change was constant. The estimated marginal mean change in SJL was a reduction of 6.36 minutes at 24 months. The proportion of individuals meeting the ≥2 hour cut-off showed a trend level reduction over time (OR=0.98, SEb=0.007, z = −1.92, p=0.055). Age and gender showed a fixed effect on continuous SJL, with younger individuals (b=−0.05, SEb=0.007, t(882.4) = −6.20, p<0.001), females (b=0.18, SEb=0.086, t(862.1) = 2.09, p=0.037), and Black participants (b=0.23, SEb=0.114, t(856.9) = 2.01, p=0.045) showing greater SJL over time compared to older individuals, males, and white participants, respectively. Only age was significant when using the dichotomous SJL outcome. Treatment condition was not associated with SJL. Neither demographics nor treatment were found to influence the rate of change in SJL over time and were therefore not examined as potential moderators of SJL on anthropometric and cardiometabolic outcomes.
Social Jetlag and Changes in Anthropometrics and Cardiometabolic Outcomes
Over two years, participants on average lost 1.18±6.54% body weight, decreased waist circumference by 1.37±5.77cm, and lowered SBP and DBP by 2.74±8.96 mmHg and 1.24±6.69 mmHg, respectively. Glucose increased by 0.50±6.00 mg/dL, insulin decreased by 0.76±3.99 uU/mL, and HOMA decreased by −0.15±0.94 mg/dL*uU/mL. All models showed significant linear effects over time. Weight, waist circumference, and BP also showed positive quadratic effects, indicating that the rate of change slowed over the study period (glucose, insulin, and HOMA were only measured at two time points and a quadratic effect could not be tested).
In models adjusting for time, treatment arm, race/ethnicity, gender, and age, continuous SJL at the within- or between-person level was not associated with change over time for any physical or cardiometabolic outcome. When SJL was treated dichotomously (≥2 hrs vs. <2 hrs), between-person SJL showed an interaction with time on weight (b=0.05, SEb=0.024, t(1566) = 2.19, p=0.028) such that that individuals who were more likely to experience clinically-significant SJL over the course of participation had greater weight gain over time (Table 2; Figure 2). The interaction with waist circumference (b=0.05, SEb=0.032, t(1537) = 1.69, p=0.090) only approached statistical significance. Within-person dichotomous SJL had no effects on weight or waist circumference. A within-person SJL effect was found on glucose. Time points in which participants met clinically-significant SJL were associated with lower glucose measurements than no clinically-significant SJL (b=−1.51, SEb=0.56, t(585.8) = −2.78, p=0.006). No within- or between-person SJL effects were found on SBP, DBP, insulin, or HOMA. [see Table 2].
Table 2.
Model results estimating the effects of clinically-significant social jetlag (≥ 2 hrs) on weight, waist circumference, and cardiometabolic outcomes in participants (n=599) across 2 years
| Weight | Waist Circumference | SBP | DBP | Glucose | Insulin | HOMA | |
|---|---|---|---|---|---|---|---|
| Fixed Effect (Estimate (SE)) | |||||||
| Intercept | 75.2 (2.70)* | 0.82 (2.20)* | 0.01 (3.00)* | 0.67 (2.64)* | 87.8 (2.00)* | 12.6 (1.24)* | 2.79 (0.29) |
| Slope | −0.17 (0.01)* | −0.15 (0.07) | −0.17 (0.13) | −0.27 (0.09)* | 0.14 (0.09) | −0.01 (0.06) | 0.0002 (0.01) |
| Curvature | 0.01 (0.001)* | 0.01 (0.001)* | 0.01 (0.002)* | 0.01 (0.002)* | - | - | - |
| Age | 0.23 (0.01)* | 0.27 (0.07)* | 0.09 (0.09) | 0.23 (0.08)* | 1.52 (0.64)* | −0.14 (0.04)* | −0.03 (0.01) |
| Gender | −0.15 (0.88)* | −9.5 (0.74)* | −10.67 (0.98)* | −3.59 (0.86)* | −4.06 (0.65)* | −0.19 (0.40) | −0.14 (0.01) |
| Minority Status | 1.80 (0.81)* | 1.44 (0.68)* | −1.31 (0.91) | −1.16 (0.80) | 1.51 (0.60)* | −0.72 (0.37) | −0.12 (0.09) |
| Large Change | −1.70 (0.88) | −1.35 (0.74) | −0.44 (0.98) | 0.15 (0.86) | 0.48 (0.65) | 0.47 (0.40) | 0.12 (0.01) |
| Small Change | 0.23 (0.88) | 0.10 (0.75) | 1.05 (0.97) | −0.03 (0.86) | −0.11 (0.65) | 0.31 (0.40) | 0.07 (0.01) |
| Age x Time | −0.002 (0.002) | −0.002 (0.002) | −0.01 (0.04)* | −0.005 (0.003)* | −0.003 (0.003) | 4.4e−4(0.002) | 4.0e−5(4.0e−4) |
| Gender x Time | 0.01 (0.01) | −0.02 (0.02) | 0.11 (0.04)* | 0.06 (0.03)* | 0.02 (0.03) | −0.02 (0.02) | −0.003 (0.004) |
| Minority x Time | −0.003 (0.02) | −0.02 (0.02) | 0.02 (0.04) | 0.01 (0.03) | −0.03 (0.03) | −0.004 (0.02) | −0.002 (0.004) |
| Large Change x Time | −0.05 (0.02)* | −0.07 (0.02)* | 0.01 (0.04) | −0.01 (0.03) | −0.07 (0.03)* | −0.04 (0.02)* | −0.01 (0.004) |
| Small Change x Time | −0.06 (0.02)* | −0.05 (0.02)* | −0.04 (0.04) | −0.03 (0.03) | −0.03 (0.03) | −0.01 (0.02) | −0.004 (0.004) |
| Within-Person SJL | 0.0002 (0.22) | 0.09 (0.13) | 0.30 (0.51) | 0.46 (0.37) | −1.56 (0.60)* | 0.06 (0.04) | −0.02 (0.09) |
| Between-Person SJL | 0.60 (1.22) | −0.44 (0.39) | 1.82 (1.45) | −0.36 (1.24) | 3.74 (1.05)* | 0.46 (0.67) | 0.17 (0.16) |
| Between-Person SJL x Time | 0.05 (0.02)* | 0.01 (0.01) | −0.05 (0.06) | 0.04 (0.04) | −0.05 (0.04) | 0.02 (0.03) | 0.004 (0.007) |
| Random Effect (Variance (SD)) | |||||||
| Variance of Intercept | 72.66 (8.52) | 46.46 (6.82) | 72.51 (8.52) | 61.63 (7.85) | 24.12 (4.91) | 8.24 (2.87) | 0.43 (0.66) |
| Residual Variance | 7.47 (2.73) | 12.46 (3.53) | 38.21 (6.18) | 20.25 (4.50) | 17.49 (4.18) | 7.79 (2.79) | 0.44 (0.66) |
p<0.05;
Only 2 time points were measured for glucose, insulin, and HOMA so curvature was not assessed.
Figure 2.
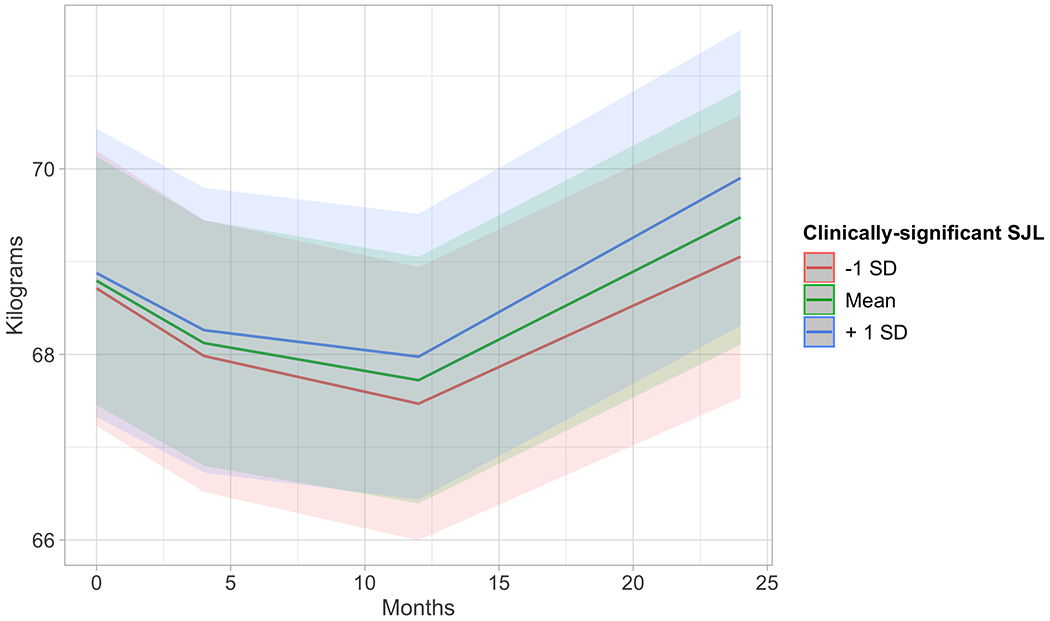
Estimated marginal means from multilevel mixed growth models with average levels of clinically-significant social jetlag predicting weight change over 2 years. Results indicate that greater average levels of clinically-significant social jetlag predict greater weight gain (p=0.028) over 2 years.
Discussion
Clinically-significant SJL was experienced by 38% of young adults participating in the SNAP weight gain prevention trial at one or more time points, and results showed more chronic levels of high SJL may hinder weight management success during this high-risk developmental period. Women, younger individuals, and Black individuals may be particularly at risk, given current findings suggesting greater SJL in these subpopulations.
The finding that high levels of SJL over time were associated with poorer weight outcomes with an observed trend toward associations with waist outcomes in SNAP is consistent with previous cross-sectional literature suggesting SJL is related to greater adiposity (Wong et al., 2015; Zerón-Rugerio et al., 2019; Zuraikat et al., 2020). Findings are in contrast to those from Lanoye and LaRose (2020), who found that participants with ≥ 2 hours of SJL at study entry did not manifest smaller reductions in weight or adiposity at 6 months. Discrepancies in findings likely highlight that it is not just high SJL, but the persistence of high SJL over time that is relevant to treatment success in this age group. Chronic circadian disruption likely exacerbates the associated dysfunctional behavioral and metabolic processes and provides a longer time course in which to affect body weight (Bass & Takahashi, 2010; Caliandro, Streng, van Kerkhof, van der Horst, & Chaves, 2021; Mohawk et al., 2012). The fact that no within-person fluctuations in SJL were associated with weight and waist circumference support this assertion.
The study populations and intervention goals also differed from Lanoye and LaRose (2020). SNAP had a broader age range (18-35 yrs. vs. 18-25 yrs.) and participants had BMIs 21.0-30.9 instead of 25-45 kg/m2, with goals of weight gain prevention instead of weight loss. Notably, when using continuous measures of SJL, there were no significant differences found between individuals on weight or waist circumference. There may be a threshold at which SJL becomes detrimental to weight management. This is supported by other literature that has found ≥ 2 hours of SJL to be associated with greater endocrine, behavioral, and cardiovascular risk factors (Koopman et al., 2017; Rutters et al., 2014).
A 38% prevalence of SJL ≥ 2 hours at one timepoint or more indicates likely circadian misalignment was a common experience over 2 years; however, only 18 participants who provided full data had ≥ 2 hours SJL at all time points, suggesting transience of significant SJL over time. Young adults experience frequent and significant life transitions that may impact their sleep-wake schedules (Frech, 2014), potentially accounting for the variability. Of note, an epidemiological study by McMahon and colleagues (2018) found latent classes of low and high SJL to be stable over a 2-year period in young adults. The high SJL class had an average of 1.4 hours of SJL, which is markedly lower than the ≥ 2 hour cut-off in the current study. It may be that individuals are more likely to shift in and out of very high SJL, but may still show general individual differences for relatively consistent high versus low SJL.
Findings also showed a statistically-significant, albeit minor (6.5 minute), decrease in mean SJL over time, which was also found in the study by McMahon and colleagues (2018). As individuals age, chronotypes start to shift earlier and individuals are more likely to establish responsibilities (e.g., children) that beget earlier bedtimes and wake times on weekends, perhaps helping to equalize weekday and weekend sleep (Fischer et al., 2017). Similar rationale may explain why older participants were less likely to experience enduring clinically-significant SJL than younger participants. Indeed, other cross-sectional research has found SJL decreases with age (Kelly et al., 2020; Koopman et al., 2017). Obesity incidence also decreases throughout adulthood (Pan, Freedman, Gillespie, Park, & Sherry, 2011) and in the control group in the current study, older individuals (25-35 yrs) only gained 2.97 kg over 6 years while younger individuals (18-24.9 yrs) gained 7.3 kg (Pan et al., 2011; Wing, Espeland, et al., 2020). Future work may consider the combined role of age and SJL on weight outcomes.
Additionally, female participants were more likely to experience higher levels of SJL than males across the study. Research on gender differences in SJL have been mixed, with some showing males experience greater SJL than females (Chandrakar, 2017; Lanoye & LaRose, 2020), others showing no relationship to gender (Lang et al., 2018; Randler, Vollmer, Kalb, & Itzek-Greulich, 2019), and at least one suggesting females have greater SJL (Diaz). Biologically, males have more evening-oriented chronotypes compared to females (Randler & Engelke, 2019), which may help explain previous findings showing greater SJL in males, although gender differences decrease over time. On the other hand, females generally report greater sleep disturbance (Mong & Cusmano, 2016), likely due in part to sex hormones (Mong & Cusmano, 2016) as well as cultural factors such as caregiving (Chen, Kawachi, Subramanian, Acevedo-Garcia, & Lee, 2005). As noted, young adulthood is a common time to “settle down” and begin a family, which may help explain the current findings that females had higher SJL levels than males.
Individuals self-identifying as Black or African American were also more likely to experience higher levels of SJL than individuals self-identifying as white. Findings regarding race/ethnicity and sleep disturbances have been equivocal, although work in young adults suggests African Americans may be more prone to poor sleep health than whites (Billings & Berg-Cross, 2014; Walsemann, Ailshire, Fisk, & Brown, 2017). Black individuals in this age group are more likely to be unemployed, are more likely to have children in the home, and are more likely to live in neighborhoods where they feel unsafe, all of which may impact sleep timing (Walsemann et al., 2017). Moreover, Black individuals experience chronic stress related to discrimination, which may also impair sleep processes (Goosby, Straley, & Cheadle, 2017). Neither McMahon and colleagues nor Lanoye and LaRose found SJL to relate to race/ethnicity; however, results from this study suggest that when SJL is consistently higher in Black young adults over time. This finding is particularly relevant given Black participants tend to have greater cardiovascular risk and do not always benefit from lifestyle interventions to the same extent as white participants (Lewis, Edwards-Hampton, & Ard, 2016). Future studies may consider testing the utility of including sleep interventions to improve racial disparities in outcomes.
Neither high SJL over time nor individual fluctuations in SJL were related to cardiometabolic outcomes in young adults, with the exception of glucose. Within-person, when SJL was 2 hours or greater, individuals were more likely to have lower glucose than when glucose was less than 2 hours. This relationship is the opposite of what would be expected; however, the mean differences were small (~1.5 mg/dL) and well within healthy levels (<100 mg/dL). Other variables not included in the current models (e.g, weight) may also contribute to observed differences. Relatedly, the study sample was young and generally healthy with average scores on all cardiometabolic outcomes in the clinically normal range, thus changes in SJL may not be impactful enough to have a significant effect. Also, while sleep variability has consistently been implicated in poorer cardiometabolic risk factors, a recent review suggests that blood pressure in particular may not be linked to SJL specifically (Makarem, Zuraikat, Aggarwal, Jelic, & St-Onge, 2020).
While this study has numerous strengths including a large sample size and measurement of a variety of cardiometabolic outcomes, a primary limitation to the current study is the self-report of typical sleep times. More detailed self-reported sleep measurements (e.g., a sleep diary) or objective sleep measurements (e.g., actigraph) are preferable to capture day-to-day variability and reduce potentially inaccurate reporting. Reporting on sleep/wake times on weekdays and weekends may also not account for unique schedules of subsets of young adults (e.g., college students, individuals who work weekends), although previous research has found number of work hours was related to SJL assessed with a similar measurement (Lanoye & LaRose, 2020). Moreover, the analysis did not account for individuals who experience successive time points of SJL. This additional detail may provide more insight regarding differences in time course of SJL and their effects on weight.
Results from this study provide an initial indication that chronic high SJL in young adults at risk for obesity may limit the benefit of weight gain prevention interventions that do not address sleep. Behavioral and metabolic processes that have been shown to be disrupted by high levels of SJL, such as poorer diet, decreased physical activity, or disruption to glucose homeostasis, may have contributed to this effect (Bass & Takahashi, 2010; Caliandro et al., 2021; Mohawk et al., 2012). Integrating education and skills training around sleep in weight management programs for young adults may improve intervention outcomes. Intervention may need to be specifically geared toward maintaining consistent sleep schedules across the week (i.e., targeting SJL), as more general sleep intervention has not been found to effectively improve sleep in the context of other weight management trials in young adults (Laska et al., 2016). Future studies may consider more sensitive and objective measures of sleep and variability over time to confirm this relationship and determine the additive benefit of incorporating sleep interventions into weight gain prevention programs.
Figure 1.
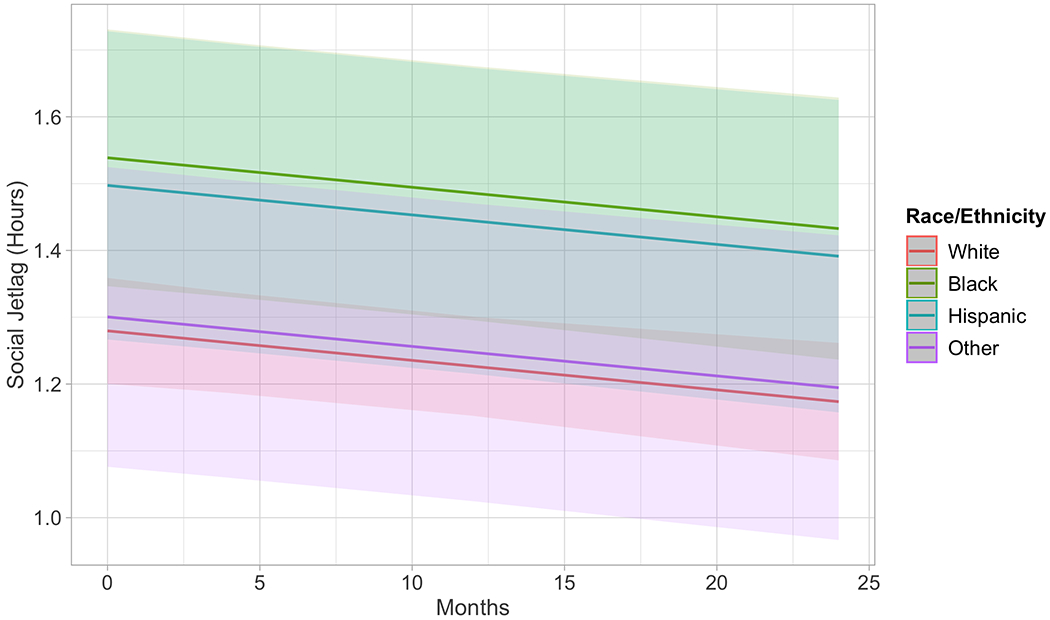
Multilevel model results with race/ethnicity predicting social jetlag over 24 months. Black participants began the study with significantly more social jetlag than white participants (p=0.045) and stay consistently different over time (interaction of race/ethnicity with time was non-significant).
Funding:
This work was supported by the following grants from the National Institutes of Health: NHLBI T32HL076134, NIDDK K23DK128561, and NHLBI U01HL090864
Conflicts of Interest:
DFT serves on the Scientific Advisory board and receives grant funding from WW. JGL receives grant funding from WW. RRW is on the Scientific Advisory Board of Noom. The other authors declared no conflict of interest.
Footnotes
Ethics Approval: This study was approved by the Institutional Review Board at The Miriam Hospital and University of North Carolina and was conducted in accordance with the Helsinki Declaration as revised in 2013.
Code Availability: Not applicable
Clinical Trial Registration: ClinicalTrials.gov Identifier NCT01183689
Availability of Data Material:
Data are available at NIH/NHLBI BioLINCC
Bibliography/References Cited
- Bass J, & Takahashi JS (2010). Circadian integration of metabolism and energetics. Science, 330(6009), 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings T, & Berg-Cross L (2014). Sleep competing activities and sleep problems in minority college students. Journal of Racial and Ethnic Health Disparities, 1(4), 300–308. [Google Scholar]
- Caliandro R, Streng AA, van Kerkhof LW, van der Horst GT, & Chaves I (2021). Social jetlag and related risks for human health: A timely review. Nutrients, 13(12), 4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrakar P (2017). Social jetlag in school students: evidence to suggest that sleep deprivation during work days is common. Biological Rhythm Research, 48(1), 99–112. [Google Scholar]
- Chen Y-Y, Kawachi I, Subramanian S, Acevedo-Garcia D, & Lee Y-J (2005). Can social factors explain sex differences in insomnia? Findings from a national survey in Taiwan. Journal of Epidemiology & Community Health, 59(6), 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton GR, Kim Y, Jacobs DR Jr, Li X, Loria CM, Reis JP, … Shikany JM (2016). 25-year weight gain in a racially balanced sample of US adults: the CARDIA study. Obesity, 24(9), 1962–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Barnes A, Johnson S, & Gudzune K (2021). Trends in Obesity Prevalence Among Adults Aged 18 Through 25 Years, 1976-2018. JAMA, 326(20), 2073–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Lombardi DA, Marucci-Wellman H, & Roenneberg T (2017). Chronotypes in the US–influence of age and sex. PLoS One, 12(6), e0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech A (2014). Pathways to adulthood and changes in health-promoting behaviors. Advances in life course research, 19, 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaultney JF (2010). The prevalence of sleep disorders in college students: impact on academic performance. Journal of American College Health, 59(2), 91–97. [DOI] [PubMed] [Google Scholar]
- Goosby BJ, Straley E, & Cheadle JE (2017). Discrimination, sleep, and stress reactivity: Pathways to African American-White cardiometabolic risk inequities. Population Research and Policy Review, 36(5), 699–716. [Google Scholar]
- Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, & Czeisler CA (2013). The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America poll. Journal of Clinical Sleep Medicine, 9(12), 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C, Carroll M, Fryar C, & Ogden C (2020). Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020; 360. In. [PubMed] [Google Scholar]
- Jakicic JM, Davis KK, Rogers RJ, King WC, Marcus MD, Helsel D, … Belle SH (2016). Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: the IDEA randomized clinical trial. JAMA, 316(11), 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Finn J, Healy U, Gallen D, Sreenan S, McDermott JH, & Coogan AN (2020). Greater social jetlag associates with higher HbA1c in adults with type 2 diabetes: a cross sectional study. Sleep medicine, 66, 1–9. [DOI] [PubMed] [Google Scholar]
- Kervezee L, Kosmadopoulos A, & Boivin DB (2020). Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. European Journal of Neuroscience, 51(1), 396–412. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lyu YS, & Kim SY (2020). Impact of Social Jetlag on Weight Change in Adults: Korean National Health and Nutrition Examination Survey 2016–2017. International journal of environmental research and public health, 17(12), 4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman AD, Rauh SP, van ‘t Riet E, Groeneveld L, Van Der Heijden AA, Elders PJ, … Rutters F (2017). The association between social jetlag, the metabolic syndrome, and type 2 diabetes mellitus in the general population: the new Hoorn study. Journal of biological rhythms, 32(4), 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CJ, Reynolds AC, Appleton SL, Taylor AW, Gill TK, McEvoy RD, … Adams RA (2018). Sociodemographic and behavioural correlates of social jetlag in Australian adults: results from the 2016 National Sleep Health Foundation Study. Sleep medicine, 51, 133–139. [DOI] [PubMed] [Google Scholar]
- Lanoye A, & LaRose JG (2020). Social Jetlag and Emerging Adults’ Performance in a Behavioral Weight Loss Trial. Emerging Adulthood, 2167696820982439. [Google Scholar]
- LaRose JG, Tate DF, Lanoye A, Fava JL, Jelalian E, Blumenthal M, … Wing RR (2019). Adapting evidence-based behavioral weight loss programs for emerging adults: A pilot randomized controlled trial. Journal of health psychology, 24(7), 870–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laska MN, Lytle LA, Nanney MS, Moe SG, Linde JA, & Hannan PJ (2016). Results of a 2-year randomized, controlled obesity prevention trial: Effects on diet, activity and sleep behaviors in an at-risk young adult population. Preventive medicine, 89, 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, … Allebrandt KV (2011). Depression scores associate with chronotype and social jetlag in a rural population. Chronobiology international, 28(9), 771–778. [DOI] [PubMed] [Google Scholar]
- Lewis KH, Edwards-Hampton SA, & Ard JD (2016). Disparities in treatment uptake and outcomes of patients with obesity in the USA. Curr Obes Rep, 5(2), 282–290. [DOI] [PubMed] [Google Scholar]
- Lytle LA, Svetkey LP, Patrick K, Belle SH, Fernandez ID, Jakicic JM, … Wing R (2014). The EARLY trials: a consortium of studies targeting weight control in young adults. Translational behavioral medicine, 4(3), 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarem N, Zuraikat FM, Aggarwal B, Jelic S, & St-Onge M-P (2020). Variability in sleep patterns: an emerging risk factor for hypertension. Current hypertension reports, 22(2), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DM, Burch JB, Wirth MD, Youngstedt SD, Hardin JW, Hurley TG, … Drenowatz C (2018). Persistence of social jetlag and sleep disruption in healthy young adults. Chronobiology international, 35(3), 312–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DM, Burch JB, Youngstedt SD, Wirth MD, Hardin JW, Hurley TG, … Drenowatz C (2019). Relationships between chronotype, social jetlag, sleep, obesity and blood pressure in healthy young adults. Chronobiology international, 36(4), 493–509. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, & Takahashi JS (2012). Central and peripheral circadian clocks in mammals. Annual review of neuroscience, 35, 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, & Cusmano DM (2016). Sex differences in sleep: impact of biological sex and sex steroids. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1688), 20150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, … Scheer FA (2015). Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proceedings of the National Academy of Sciences, 112(17), E2225–E2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulye TP, Park MJ, Nelson CD, Adams SH, Irwin CE Jr, & Brindis CD (2009). Trends in adolescent and young adult health in the United States. Journal of Adolescent Health, 45(1), 8–24. [DOI] [PubMed] [Google Scholar]
- Pan L, Freedman DS, Gillespie C, Park S, & Sherry B (2011). Incidences of obesity and extreme obesity among US adults: findings from the 2009 Behavioral Risk Factor Surveillance System. Population health metrics, 9(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randler C, & Engelke J (2019). Gender differences in chronotype diminish with age: a meta-analysis based on morningness/chronotype questionnaires. Chronobiology international, 36(7), 888–905. [DOI] [PubMed] [Google Scholar]
- Randler C, Vollmer C, Kalb N, & Itzek-Greulich H (2019). Breakpoints of time in bed, midpoint of sleep, and social jetlag from infancy to early adulthood. Sleep medicine, 57, 80–86. [DOI] [PubMed] [Google Scholar]
- Rutters F, Lemmens SG, Adam TC, Bremmer MA, Elders PJ, Nijpels G, & Dekker JM (2014). Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? Journal of biological rhythms, 29(5), 377–383. [DOI] [PubMed] [Google Scholar]
- Walsemann KM, Ailshire JA, Fisk CE, & Brown LL (2017). Do gender and racial/ethnic disparities in sleep duration emerge in early adulthood? Evidence from a longitudinal study of US adults. Sleep medicine, 36, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Espeland MA, Tate DF, Perdue LH, Bahnson J, Polzien K, … Lewis CE (2020). Weight Gain Over 6 Years in Young Adults: The Study of Novel Approaches to Weight Gain Prevention Randomized Trial. Obesity, 28(1), 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Russell GB, Tate DF, Espeland MA, LaRose JG, Gorin AA, … Bahnson J (2020). Examining Heterogeneity of Outcomes in a Weight Gain Prevention Program for Young Adults. Obesity, 28(3), 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Tate D, Espeland M, Gorin A, LaRose JG, Robichaud EF, … Lewis CE (2013). Weight gain prevention in young adults: design of the study of novel approaches to weight gain prevention (SNAP) randomized controlled trial. BMC Public Health, 13(1), 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Tate DF, Espeland MA, Lewis CE, LaRose JG, Gorin AA, … Ferguson E (2016). Innovative self-regulation strategies to reduce weight gain in young adults: the study of novel approaches to weight gain prevention (SNAP) randomized clinical trial. JAMA Intern Med, 176(6), 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, & Roenneberg T (2006). Social jetlag: misalignment of biological and social time. Chronobiology international, 23(1-2), 497–509. [DOI] [PubMed] [Google Scholar]
- Wong PM, Hasler BP, Kamarck TW, Muldoon MF, & Manuck SB (2015). Social jetlag, chronotype, and cardiometabolic risk. The Journal of Clinical Endocrinology & Metabolism, 100(12), 4612–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerón-Rugerio MF, Cambras T, & Izquierdo-Pulido M (2019). Social jet lag associates negatively with the adherence to the mediterranean diet and body mass index among young adults. Nutrients, 11(8), 1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuraikat FM, Makarem N, Redline S, Aggarwal B, Jelic S, & St-Onge M-P (2020). Sleep Regularity and Cardiometabolic Heath: Is Variability in Sleep Patterns a Risk Factor for Excess Adiposity and Glycemic Dysregulation? Current diabetes reports, 20(8), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at NIH/NHLBI BioLINCC


