Abstract

Majority of protein structure studies use Escherichia coli (E. coli) and other model organisms as expression systems for other species’ genes. However, protein folding depends on cellular environment factors, such as chaperone proteins, cytoplasmic pH, temperature, and ionic concentrations. Because of differences in these factors, especially temperature and chaperones, native proteins in organisms such as extremophiles may fold improperly when they are expressed in mesophilic model organisms. Here we present a methodology of assessing the effects of using E. coli as the expression system on protein structures. We compare these effects between eight mesophilic bacteria and Thermus thermophilus (T. thermophilus), a thermophile, and found that differences are significantly larger for T. thermophilus. More specifically, helical secondary structures in T. thermophilus proteins are often replaced by coil structures in E. coli. Our results show unique directionality in misfolding when proteins in thermophiles are expressed in mesophiles. This indicates that extremophiles, such as thermophiles, require unique protein expression systems in protein folding studies.
1. Introduction
Identification of protein structure is a major requirement in studies on protein functions1,2 and interactions3−5 and in the design of novel enzymes.6−8 As of July 2022, there were more than 190000 records in Protein Data Bank (PDB),9 the largest database of protein structures,10 of which almost 180000 were protein structure entries. The deposited structures can be used in various fields of research. For example, PDB data had been recently used in research related to COVID-19,11−14 protein evolution,15−17 computational enzyme,18 and drug design,19 as well as for training computational protein structure prediction algorithms.20−24
While PDB holds a relatively large variety of protein types and source species, diversity is much lower for expression systems used in structure determination experiments. Protein source species are the species that the protein-coding sequences were taken from, and protein expression systems are the species that these proteins were grown in (Figure 1). A majority of PDB experiments use Escherichia coli (E. coli) as the expression system. For example, out of over 1800 PDB entries with Bacillus subtilis (B. subtilis) as the source organism, more than 1700 were grown in E. coli. For most other species, the proportion of proteins that were grown in E. coli is even higher.
Figure 1.

Example of protein structures in different expression systems. TT_EC file is formed using proteins that have T. thermophilus as source organism and E. coli as expression system. TT_TT is formed using proteins that have T. thermophilus as both source and expression system (sometimes referred to as “native” expression system in this research).
While using recombinant model organisms—those with genetically recombined genes—for protein production is generally effective, lower protein activity and solubility can be observed in many cases, including the formation of inclusion bodies.25−27 Various protocols and methodologies have been developed in an effort to improve recombinant protein production quality; however, their effectiveness is not uniform across different protein source species. For many thermophilic species, production of recombinant protein in E. coli in active form is still a major challenge. For example, multiple studies show that a large fraction of Thermus thermophilus (T. thermophilus) proteins are formed in insoluble and/or in inactive form when grown in recombinant E. coli,28−31 potentially because T. thermophilus has optimal growth temperatures around 70–80 °C, which are much higher than that of E. coli (37 °C). It has been previously reported that soybean Late Embryogenesis Abundant proteins became more hydrated upon heating,32 suggesting that protein solubility is influenced by the cellular environment; therefore what is not soluble in mesophilic E. coli may well be soluble in T. thermophilus. In fact, induction temperature is one of the most critical growing conditions to produce soluble protein.33
These findings suggest that proteins may misfold in recombinant expression systems having dissimilar cellular environments. Nevertheless, expression system is not always considered important or even reported in structural studies. Moreover, protein structure prediction models, including the most advanced ones, such as AlphaFold2,24 do not use expression system and cellular environment as factors in training and validation. This disparity highlights the need for a methodology to assess the effect of different expression systems on protein structure determination.
Here we present a metric to evaluate protein secondary structure (SS) differences and analyze how changing the expression system from “native” to E. coli affects protein SS. We used PDB data to create AA/SS data sets for T. thermophilus (TT) and seven nonthermophilic bacteria species (where AA means amino acid and SS means secondary structure). For each species (say XX), there are two sets of protein structure data, one obtained with XX as both the protein source and the expression system and the other with XX as the source species but E. coli as the expression system. These two sets of protein structures are represented as XX_XX and XX_EC (Figure 1, where XX is TT). We then processed the data into probability matrices and calculated Jensen–Shannon divergence (JSD) between the XX_XX matrix and the XX_EC matrix. This JSD measured the difference in protein structure between XX_XX and XX_EC. We found that JSD was significantly higher between TT_TT and TT_EC than between XX_XX and XX_EC (where XX is a mesophilic bacterial species). This implies that T. thermophilus proteins in nonthermophilic species do not fold in the same way as in their “native” thermophilic expression system, while for the other species the expression system did not affect protein folding.
2. Materials and Methods
For a fair comparison, we need the same protein from a source species but expressed in different expression systems, one being the source system (native) and the other being E. coli. An overview of our methodology generating such data is summarized in Figures 2 and 3.
Figure 2.

Overview of methodology (part 1). Relevant structure IDs were found through a search on PDBe (A) and processed into AA.fasta files (B). The found structures with the same protein origin species and different expression species were paired on the basis of AA sequences using BLASTp (C), and the paired AA/SS were saved as datafiles (D).
Figure 3.
Overview of methodology (part 2). Datafiles were used to construct AA/SS count (A) and proportion matrices (B). For each studied species, JSD was calculated between the “native” proportion matrix and the recombinant E. coli proportion matrix (D). The differences between the matrices were also visualized using heat maps (C).
2.1. Data Collection
Bacterial species from which we collected the data are listed in Table 1 and were selected because they have sufficient data available on PDB both as protein source and expression systems. The PDB online advanced searching tool was used to create separate ID lists for each source/expression pair. More specifically, PDB Europe (PDBe) was used because of its more advanced filtering interface; however, its data are the same as on PDB. Advanced search can be accessed through this link: https://www.ebi.ac.uk/pdbe/entry/search/index/?advancedSearch:true=. We used several filters to obtain protein ID lists: (1) organism name, (2) expression host name, (3) resolution, and (4) molecule type. Protein source and expression system were set in accordance with Table 1, molecular type was set to “protein” and experimental method was set to X-ray diffraction only with resolution between 0 and 2.5 Å. Note that proteins are purified in their naturally folded state in the expression system, ideally in their functional forms, before crystallization and X-ray diffraction.
Table 1. Species Used in the Analysisa.
| protein source species | expression system | structure IDs list | entries before BLASTp | entries after BLASTp |
|---|---|---|---|---|
| Bacillus subtilis | B. subtilis | BS_BS | 8 | 4 |
| Bacillus subtilis | E. coli | BS_EC | 1145 | 17 |
| Desulfovibrio vulgaris | D. vulgaris | DV_DV | 26 | 4 |
| Desulfovibrio vulgaris | E. coli | DV_EC | 67 | 20 |
| Lactococcus lactis | L. lactis | LL_LL | 25 | 5 |
| Lactococcus lactis | E. coli | LL_EC | 166 | 6 |
| Pseudomonas fluorescens | P. fluorescens | PF_PF | 13 | 4 |
| Pseudomonas fluorescens | E. coli | PF_EC | 275 | 2 |
| Pseudomonas putida | P. putida | PP_PP | 3 | 1 |
| Pseudomonas putida | E. coli | PP_EC | 542 | 37 |
| Salmonella enterica | S. enterica | SE_SE | 34 | 29 |
| Salmonella enterica | E. coli | SE_EC | 860 | 17 |
| Streptomyces rubiginosus | St. rubiginosus | SR_SR | 17 | 17 |
| Streptomyces rubiginosus | E. coli | SR_EC | 11 | 11 |
| Thermus thermophilus | T. thermophilus | TT_TT | 19 | 7 |
| Thermus thermophilus | E. coli | TT_EC | 1091 | 3 |
For each protein source species there are two expression systems used—that of the source species (“native” expression system) and E. coli. Lists of structure IDs were created for each source/expression pair. For example, BS_BS contains IDs of structures with B. subtilis as both protein source organism and protein expression system, while BS_EC has IDs of structures with B. subtilis as protein source organism and E. coli as protein expression system.
Structure IDs were taken for each protein origin/expression system pair found using the online search and used to obtain the corresponding SS and AA sequences. We used a PDB Secondary Structure file in FASTA format (latest version is accessible at https://cdn.rcsb.org/etl/kabschSander/ss.txt.gz—sometimes multiple refreshes of the page are required to obtain the file; alternatively, a copy of the file is available at https://github.com/alibekk93/project-protein_folding_distances/ss.txt.gz) to collect SS and AA. Each structure in this file is represented by AA and SS strings, where each single AA corresponds to a single SS at the same position. The numbers of structure IDs identified for each ID list are shown on Table 1. Identical AA sequences from the same expression systems were allowed as their corresponding SS sequences could differ and provide relevant data.
2.2. Filtering the Data Using BLASTp
To identify proteins whose structure has been determined when they were expressed in both the source species (native environment) and in E. coli, we processed the AA sequences obtained from the previous step into BLASTp database files using DAMBE.34 For each pair of compared protein databases a BLASTp search was performed using AA sequences of the proteins, with the recombinant E. coli expression system database as query and the native database as BLASTp database.
We used ungapped BLASTp with three-letter words to match proteins from different databases. Minimal matching accuracy was set to 95% to allow for small variations of AA sequence due to point mutations and random errors in experiment without allowing different proteins to be matched. Minimal matching length was set to 50 to remove short matching sequences, and the maximal E-value was set to 0.01. BLASTp parameters were set so that only proteins with very similar AA chains and those likely to be the same protein were kept in both databases and so that only matching parts could contribute to the analysis. For each pair obtained, sequence start and end were used to cut out the matching parts of the sequences and not include the nonmatching parts.
Filtering protein databases using BLASTp removed most of the proteins from the original Protein ID lists. This happened because most entries on PDB are only available with one expression system (grown in E. coli). On the other hand, those entries available in the native expression system are also not always available with the E. coli expression system. For example, the original BS_BS database contained eight entries and the original BS_EC database had 1145 entries; however, after BLASTp only four entries from BS_BS matched with 17 entries from BS_EC. The full list of numbers of entries in the databases before and after BLASTp is shown in Table 1.
2.3. Construction of Probability Matrices
First, tabular BLASTp results were used to create data files in FASTA format for each protein source species/expression system combination, each containing the AA and SS sequences. Structure IDs were used to obtain AA and SS sequences from the PDB file. Query and database start and end positions were used to cut the matching parts from the obtained sequences. This way only matching parts of the proteins would be left.
In many cases a single-query structure would match more than one database structure and vice versa; therefore, it was necessary to multiply AA and SS sequences in those cases to make sure that matching parts are the same length. In this way we obtained chains from the BLAST database with identical AA sequences, but possibly different SS sequences, and had each variation of SS in correct proportions. AA and SS sequences were concatenated for each of the databases so that all AA sequences were in one line and all SS sequences were in another line of the resulting file.
Data files were processed into count matrices to count each AA/SS combination. We converted count matrices into probability matrices by simple division of each count value by count matrix sum. Protein SS can be described with three SS types: H (helix), E (sheet), and C (coil) or with eight SS types: H (α-helix), I (π-helix), G (310-helix), E (β-sheet), B (β-bridge), C (coil), S (bend), and T (turn).. We refer to the two classification systems as 3-SS (three types of SS) and 8-SS (eight types of SS) in this work. While many models and studies, especially the earlier ones, use 3-SS, using 8-SS can give more details. In order to work with both 8-SS and 3-SS, we transformed the original 8-SS matrices to 3-SS by adding up the matrix values.
2.4. Jensen–Shannon Divergence Calculation and Statistical Analysis
After the matrices were created, they were compared to each other, so that for each protein source species the two matrices compared were with E. coli and “native” expression systems. Jensen–Shannon divergence (JSD) was used to evaluate differences between matrices. A common measure of probability distribution differences is Kullback–Leibler divergence (KLD). It is nonsymmetric, which means that KLD of distribution P from distribution Q does not have to be equal to KLD of distribution Q from distribution K. KLD is defined as
where P(x) and Q(x) are discrete probability distributions.
JSD is a metric similar to KLD, and it could be called a symmetrized and smoothed version of it. It is defined as
where P(x) and Q(x) are discrete probability
distributions, M = 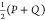 , and D is KLD.
, and D is KLD.
In our case JSD is a more suitable measure than KLD due to the large number of zeros in our data. KLD requires a division of one probability by another, and when the denominator probability is zero (which is very often the case in our data), calculation results in infinity. Standard practice is to drop zeros completely, but in our case that would be dropping a very significant amount of our data, because probability matrices contain a lot of zeros. Contrary to KLD, JSD does not have this problem thanks to its symmetric nature. Probability distributions are compared not with each other but with their average distribution, which means that only positions where both probability matrices are zero need to be dropped—those positions are exact in any case and therefore dropping them is not an issue.
Larger JSD would mean that changing the protein expression system from “native” to E. coli had more effects and that a recombinant protein SS is a worse representative of native SS. Moreover, in order to deduce possible mechanisms of how the change of the expression system affects protein folding, we calculated difference between the matrices by subtracting recombinant proportion matrix values from native proportion matrix. Subtraction results would not be a good evaluation of matrix divergence but can show at which AA/SS positions the differences between matrices are large.
We estimated the statistical significance of calculated JSD using resampling techniques. Bootstrapping was used to calculate 95% confidence intervals of JSD—AA/SS positions were randomly resampled with replacement 1000 times from the original data files and JSD were calculated for them. In addition, we used permutation to test the significance of differences between species’ JSD values by combining all data files into a uniform distribution and resampling positions from it 10000 times to form data of 1000 residues in length.
3. Results
3.1. Magnitude of Expression System Effect
Calculated results are summarized on Table 2. In addition, we visualize bootstrapping results on box plots (Figures 4 and 5) and permutation results on histograms (Figure 6). The largest JSD was found between T. thermophilus matrices, and this was the case using both 8-SS and 3-SS. Moreover, bootstrapping results show that T. thermophilus JSD is the only one significantly higher than other species’ JSD for both 8-SS and 3-SS.
Table 2. Mean Jensen–Shannon Divergences between Native and Recombinant AA/SS Matricesa.
| protein source species | JSD8 | JSD8 p-value | JSD3 | JSD3 p-value |
|---|---|---|---|---|
| Bacillus subtilis | 0.010 | 0.082 | 0.001 | 0.697 |
| Desulfovibrio vulgaris | 0.008 | 0.343 | 0.003 | 0.004 |
| Lactococcus lactis | 0.007 | 0.594 | 0.003 | 0.012 |
| Pseudomonas fluorescens | 0.009 | 0.270 | 0.002 | 0.101 |
| Pseudomonas putida | 0.002 | 1.000 | 0.000 | 1.000 |
| Salmonella enterica | 0.003 | 0.999 | 0.000 | 1.000 |
| Streptomyces rubiginosus | 0.004 | 0.995 | 0.001 | 0.992 |
| Thermus thermophilus | 0.033 | 0.000 | 0.014 | 0.000 |
p-values are from bootstrapping analysis testing the null hypothesis that different expression systems have no effect on protein structure. JSD8 and JSD3 are the calculated JSD using 8 or 3 types of SS. Both JSD8 and JSD3 are the greatest for T. thermophilus matrices, and that is the only species where both metrics are significantly different from bootstrapped distributions. This indicates that switching the protein expression system from “native” to E. coli affects the folding of T. thermophilus proteins more than other species’ proteins.
Figure 4.
Box plots of bootstrapped JSD (8 SS types). High JSD indicates larger differences between “native” and E. coli expression systems. T. thermophilus JSD are much higher than those of other bacteria.
Figure 5.
Box plots of bootstrapped JSD (3 SS types). High JSD indicates larger differences between “native” and E. coli expression systems. T. thermophilus JSD are much higher than those of other bacteria.
Figure 6.
Distributions of permuted JSD results. T. thermophilus JSD (red line) is much larger than JSDs of the other species (gray lines) and the nonspecific JSD (gray histogram).
3.2. Directionality of Expression System Effect
Heat maps in Figures 7 and 8 show differences between proportion matrices used in JSD calculations. These figures help in identifying which particular elements of matrices were most different and display potential directionality of the differences. In line with JSD results, T. thermophilus matrices show more differences than any other species’ matrix pair. It can be seen that using E. coli as an expression system for thermophilic proteins leads to lower frequencies in helices and higher frequencies of coils. The 8-SS heat map shows that this effect is particularly strong on 310-helices (structure G). Other protein expression systems considered in this study show smaller differences from that of E. coli as there are no large JSD values detected for them.
Figure 7.
Heat maps of proportion matrices differences with 3 SS types showing directionality of the effect induced by using E. coli as the expression system. More negative values (red) indicate larger proportions in E. coli as the expression system; more positive values (green) indicate larger proportions in “native” expression systems. The effects were most visible in T. thermophilus, where helices (H) were observed more frequently when proteins were expressed in T. thermophilus and coils (C) were instead more abundant when proteins were expressed in E. coli. No such effect nor directionality of differences could be seen in other species. The three SS types are H (helix), E (sheet), and C (coil).
Figure 8.
Heat maps of proportion matrices differences with 8 SS types showing directionality of effect induced by using E. coli as the expression system. More negative values (red) indicate larger proportions in E. coli as the expression system; more positive values (green) indicate larger proportions in “native” expression systems. Strong effects could be observed in T. thermophilus, where α-helices (H) and 310-helices (G) were observed more frequently when proteins were expressed in T. thermophilus and coils (C) were instead more abundant when proteins were expressed in E. coli. No such effect nor directionality of differences can be seen in other species. The eight SS types are H (α-helix), I (π-helix), G (310-helix), E (β-sheet), B (β-bridge), C (coil), S (bend), and T (turn).
No strong patterns have been discovered in terms of variability of secondary structures between different amino acids (Figures 7 and 8). While distances between matrices of T. thermophilus proteins seem to be high with hydrophobic alanine, valine, and glycine, that is also the case for histidine (charged) and threonine (polar and uncharged).
4. Discussion
4.1. Lack of Required Chaperones
There are several possible explanations for variations in JSD for different species. Because T. thermophilus is a thermophile, it is adapted to protein denaturation, partially through chaperone-dependent protein folding. It is possible that when E. coli is used as an expression system, certain helices are not formed or repaired due to a lack of these chaperones and coils are formed instead. For example, DnaK chaperone expression requires less ATPase activity in T. thermophilus than in E. coli and it participates in protein folding mediation.35 Trigger factor proteins also show differences in structure and activity between the species.36 Such effects are likely to be particularly important for any intrinsically unstructured proteins that require chaperone activity for correct folding and structural stability.37 While a common way around this problem is to co-express required folding chaperones together with the studied protein, it is not always clear whether that was done on PDB because not all structures there have publications and, even if they do, it is not always clearly explained whether co-expression of chaperones was performed. Moreover, co-expression of chaperones does not always provide the desired effects as differences in other cell-specific factors may lead to chaperones losing their activity or even becoming toxic for the host.38
4.2. Suboptimal Cellular Environment
Due to thermophilic adaptations of T. thermophilus, it is possible that the tendency toward coil structures instead of helical structures in E. coli as an expression system is a result of differences in cellular conditions of the expression systems, namely, nonoptimal folding temperatures. This would explain why this effect is more apparent for 310-helices than α-helices, as the latter are more stable.39 Regardless, future research directions may prompt researchers to study varying environmental conditions of expression systems and their effect on protein folding using data with varying temperature, pH, and salinity.
Differences in protein solubility due to temperatures could have affected protein crystallization and thus structure identification.40−42 To assess this possibility, an analysis of structures with different crystallization techniques performed might be necessary. That kind of analysis would help to determine whether effects observed in this experiment are due to differences in cellular environments and chaperones or due to experimental design.
4.3. Codon Optimization
Protein folding can be affected by the rate of protein synthesis, which can be controlled by codon usage.43,44 Assuming equal translation initiation rates, nonoptimal codon usage in the recombinant expression system will lead to slower rates of protein production, which in turn may lead to protein misfolding and aggregation.45 Unfortunately, the PDB itself has no information about codon optimization in experiments and nucleotide sequences are not provided. Moreover, not all records have corresponding publications with full description of experimental and even the ones that had been published often do not have information on whether codons were optimal. This means that lack of codon optimization is a potential factor that caused high differences between E. coli and T. thermophilus expression systems in our analysis.
4.4. Protein Crowding
Macromolecular crowding is another potential explanation of our results. As concentrations of proteins and other macromolecules inside the cells increase, the volume available for new proteins being produced falls.46−48 Crowding leads to an increase in protein thermodynamic activity, which affects folding, among other processes.49−51 In prokaryotes this effect is more profound than in eukaryotes.46
Naturally, protein crowding may occur in both E. coli and T. thermophilus expression systems, as well as other systems, but recombinant expression systems are more likely to have this effect.52,53E. coli natural cellular concentration could be unsuitable for T. thermophilus protein folding and may lead to increased crowding and inclusion body formation.26 In addition, chaperone-assisted misassemble prevention mechanisms may be compromised in recombinants as they would lack the required chaperones.52,54
4.5. Significance
The connection between protein structure and protein function is established, as structure directly dictates function.55 Improperly folded proteins often lose their initial functions and can even gain novel toxic functions in their place. For example, many misfolded proteins related to Parkinson’s and Alzheimer’s diseases have neurotoxic functions.56 It is possible that thermophilic proteins grown in E. coli lose their functions entirely or partially. Previous studies identified that T. thermophilus enzyme activity is reduced when using mesophilic recombinant hosts, such as E. coli.28,31,57,58 Additionally, E. coli had been previously shown to be an inadequate expression system for thermophilic proteins in functional metagenomic59−61 and directed evolution studies.62−64 Our results are in line with the previous findings; we also expand on them, showing how a mesophilic expression system can affect secondary structures of thermophile proteins and that the change has directionality toward less helices and more coils.
Additionally, helical structures have been shown to be more common in thermostable proteins and are associated with thermostability.65,66 The higher tendency for helix formation for T. thermophilus proteins when grown in “native” expression system could be a mechanism of protein stabilization under higher temperatures. Using E. coli as an expression system led to higher proportions of coils and therefore might have reduced thermostability adaptation.
In some cases, thermophile protein misfolding can be removed by subunit rearrangement caused by heating of the protein.58 However, that is not very common and more often the problem of misfolding can be solved by using thermophiles as expression systems for thermophilic proteins can be a solution to the problem of misfolding.28,57 These facts suggest that the protein expression system cellular environments need to be matched with those of protein source organisms in order to facilitate correct folding.
4.6. Study Limitations
Our study has multiple limitations which we should address here as well. First, it is evident that the number of protein structures which remain after all filtering procedures is very small for many of the species, including T. thermophilus. While we attempt to lower the impact of the low number of structures with resampling, it is still possible that the differences that we observe are related to specific protein structures or even by some errors in PDB experiments. In addition to the main results, described previously, we calculated JSD between matrices without BLASTp filtering. The rest of the procedures were kept the same as before. This way we could greatly increase the data size; however, the drawback is that proteomes now consisted of very different proteins and therefore these results cannot be fully conclusive either. Nevertheless, we found that JSD between T. thermophilus matrices is much higher than for all other species, the same as with the main results. We provide these additional results in the Supporting Information (Figures S1 and S2).
Second, our approach of using matrices in calculating JSD is double-edged. On one hand, using matrices allows us to compare entire proteomes rather than single proteins in a simple and computationally efficient way. This way we can compare proteomes which consist of very different proteins. On the other hand, only a pairwise comparison would show what effect protein type has on differences in folding. Ideally, all proteomes in our study should consist of the same proteins and in that case a pairwise comparison would be highly advantageous. Additionally, for the sake of simplicity and easier interpretation, our matrices were computed using one-to-one AA/SS pairing. This approach neglects potential effects that neighbor AA has on SS. It may be beneficial to use windows of several AA/SS to compute matrices in future research.
As we allowed AA sequences to differ by 5% during our BLASTp filtering step, the datafile AA sequences had some level of variation and this could have an effect on SS and JSD8/JSD3. We looked at how AA similarity affected JSD8 and JSD3, and there seems to be no relationship (Figures S3 and S4). T. thermophilus large JSD8 and JSD3 results are highly unlikely to be explained by AA differences; however, this is still possible due to the complex nature of the AA/SS relationship and this possibility should not be ignored completely.
We believe that obtaining more structural data would be essential in order to design a study which would not have the limitations that we discussed. This is especially the case with data of expression systems other than E. coli. Often predicted structures could be used when PDB does not have sufficient data; however, to our knowledge, no protein structure prediction model has an expression system as a feature.
5. Conclusion
In conclusion, our results show that thermophilic protein folding in mesophilic E. coli introduces significant changes on the structure level. Misfolding of thermophilic proteins grown using mesophilic hosts can lead to loss or change of protein functions which will harm both research and industrial applications. While there can be many possible explanations for the reasons of misfolding, it is important to study T. thermophilus and other extremophiles protein expression with protein source species as protein expression systems in order to minimize expression system effects. It is also evident that a much higher diversity of expression systems on PDB is essential for more thorough understanding of expression system effects on protein folding.
Acknowledgments
This research was funded by a Discovery Grant from the Natural Science and Engineering Research Council (NSERC, RGPIN/2018-03878) of Canada to X.X.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04786.
Figures for additional analysis of proteomes without BLASTp filtering and links to raw secondary structure data and our code (PDF)
Author Contributions
A.K. carried out the experiment and wrote the manuscript with support from Y.W. and X.X.; X.X. supervised the project.
The authors declare no competing financial interest.
Supplementary Material
References
- Yang J.; Yan R.; Roy A.; Xu D.; Poisson J.; Zhang Y. The I-TASSER Suite: Protein Structure and Function Prediction. Nat. Methods 2015, 12 (1), 7. 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisstock J. C.; Lesk A. M. Prediction of Protein Function from Protein Sequence and Structure. Q. Rev. Biophys. 2003, 36 (3), 307–340. 10.1017/S0033583503003901. [DOI] [PubMed] [Google Scholar]
- Brady G. P.; Sharp K. A. Entropy in Protein Folding and in Protein—Protein Interactions. Curr. Opin. Struct. Biol. 1997, 7 (2), 215–221. 10.1016/S0959-440X(97)80028-0. [DOI] [PubMed] [Google Scholar]
- Gohlke H.; Hendlich M.; Klebe G. Knowledge-Based Scoring Function to Predict Protein-Ligand Interactions. J. Mol. Biol. 2000, 295 (2), 337–356. 10.1006/jmbi.1999.3371. [DOI] [PubMed] [Google Scholar]
- Zhang Q. C.; Petrey D.; Deng L.; Qiang L.; Shi Y.; Thu C. A.; Bisikirska B.; Lefebvre C.; Accili D.; Hunter T.; et al. Structure-Based Prediction of Protein-Protein Interactions on a Genome-Wide Scale. Nature 2012, 490 (7421), 556–560. 10.1038/nature11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M.; Maibaum J.; Rahuel J.; Grütter M. G.; Cohen N.-C.; Rasetti V.; Rüger H.; Göschke R.; Stutz S.; Fuhrer W.; et al. Structure-Based Design of Aliskiren, a Novel Orally Effective Renin Inhibitor. Biochem. Biophys. Res. Commun. 2003, 308 (4), 698–705. 10.1016/S0006-291X(03)01451-7. [DOI] [PubMed] [Google Scholar]
- Koga N.; Tatsumi-Koga R.; Liu G.; Xiao R.; Acton T. B.; Montelione G. T.; Baker D. Principles for Designing Ideal Protein Structures. Nature 2012, 491 (7423), 222. 10.1038/nature11600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss G.; Çelebi-Ölçüm N.; Moretti R.; Baker D.; Houk K. Computational Enzyme Design. Angew. Chem., Int. Ed. 2013, 52 (22), 5700–5725. 10.1002/anie.201204077. [DOI] [PubMed] [Google Scholar]
- Berman H. M.; Westbrook J.; Feng Z.; Gilliland G.; Bhat T. N.; Weissig H.; Shindyalov I. N.; Bourne P. E. The Protein Data Bank. Nucleic Acids Res. 2000, 28 (1), 235–242. 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley S. K.; Berman H. M.; Kleywegt G. J.; Markley J. L.; Nakamura H.; Velankar S.. Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. In Protein Crystallography; Springer, 2017; pp 627–641. 10.1007/978-1-4939-7000-1_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibmer C. K.; Ayres F.; Hermanus T.; Madzivhandila M.; Kgagudi P.; Oosthuysen B.; Lambson B. E.; de Oliveira T.; Vermeulen M.; van der Berg K.; Rossouw T.; Boswell M.; Ueckermann V.; Meiring S.; von Gottberg A.; Cohen C.; Morris L.; Bhiman J. N.; Moore P. L. SARS-CoV-2 501Y.V2 Escapes Neutralization by South African COVID-19 Donor Plasma. Nat. Med. 2021, 27 (4), 622–625. 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- Khan S. A.; Zia K.; Ashraf S.; Uddin R.; Ul-Haq Z. Identification of Chymotrypsin-like Protease Inhibitors of SARS-CoV-2 via Integrated Computational Approach. J. Biomol. Struct. Dyn. 2021, 39 (7), 2607–2616. 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- Khan R. J.; Jha R. K.; Amera G. M.; Jain M.; Singh E.; Pathak A.; Singh R. P.; Muthukumaran J.; Singh A. K. Targeting SARS-CoV-2: A Systematic Drug Repurposing Approach to Identify Promising Inhibitors against 3C-like Proteinase and 2′-O-Ribose Methyltransferase. J. Biomol. Struct. Dyn. 2021, 39 (8), 2679–2692. 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B.; Valle C.; de Lamballerie X.; Canard B.; Seidah N. G.; Decroly E. The Spike Glycoprotein of the New Coronavirus 2019-NCoV Contains a Furin-like Cleavage Site Absent in CoV of the Same Clade. Antiviral Res. 2020, 176, 104742. 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüler A.; Bornberg-Bauer E. Evolution of Protein Domain Repeats in Metazoa. Mol. Biol. Evol. 2016, 33 (12), 3170–3182. 10.1093/molbev/msw194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konaté M. M.; Plata G.; Park J.; Usmanova D. R.; Wang H.; Vitkup D. Molecular Function Limits Divergent Protein Evolution on Planetary Timescales. eLife 2019, 8, e39705 10.7554/eLife.39705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharir-Ivry A.; Xia Y. The Impact of Native State Switching on Protein Sequence Evolution. Mol. Biol. Evol. 2017, 34 (6), 1378–1390. 10.1093/molbev/msx071. [DOI] [PubMed] [Google Scholar]
- Harrington L. B.; Jha R. K.; Kern T. L.; Schmidt E. N.; Canales G. M.; Finney K. B.; Koppisch A. T.; Strauss C. E. M.; Fox D. T. Rapid Thermostabilization of Bacillus Thuringiensis Serovar Konkukian 97–27 Dehydroshikimate Dehydratase through a Structure-Based Enzyme Design and Whole Cell Activity Assay. ACS Synth. Biol. 2017, 6 (1), 120–129. 10.1021/acssynbio.6b00159. [DOI] [PubMed] [Google Scholar]
- Durairaj D. R.; Shanmughavel P. In Silico Drug Design of Thiolactomycin Derivatives Against Mtb-KasA Enzyme to Inhibit Multidrug Resistance Of Mycobacterium Tuberculosis. Interdiscip. Sci.: Comput. Life Sci. 2019, 11 (2), 215–225. 10.1007/s12539-017-0257-0. [DOI] [PubMed] [Google Scholar]
- Senior A. W.; Evans R.; Jumper J.; Kirkpatrick J.; Sifre L.; Green T.; Qin C.; Žídek A.; Nelson A. W. R.; Bridgland A.; Penedones H.; Petersen S.; Simonyan K.; Crossan S.; Kohli P.; Jones D. T.; Silver D.; Kavukcuoglu K.; Hassabis D. Improved Protein Structure Prediction Using Potentials from Deep Learning. Nature 2020, 577 (7792), 706–710. 10.1038/s41586-019-1923-7. [DOI] [PubMed] [Google Scholar]
- Waterhouse A.; Bertoni M.; Bienert S.; Studer G.; Tauriello G.; Gumienny R.; Heer F. T.; de Beer T. A. P.; Rempfer C.; Bordoli L.; Lepore R.; Schwede T. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46 (W1), W296–W303. 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivov G. G.; Shapovalov M. V.; Dunbrack R. L. Improved Prediction of Protein Side-Chain Conformations with SCWRL4. Proteins: Struct., Funct., Bioinf. 2009, 77 (4), 778–795. 10.1002/prot.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Anishchenko I.; Park H.; Peng Z.; Ovchinnikov S.; Baker D. Improved Protein Structure Prediction Using Predicted Interresidue Orientations. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (3), 1496–1503. 10.1073/pnas.1914677117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J.; Evans R.; Pritzel A.; Green T.; Figurnov M.; Ronneberger O.; Tunyasuvunakool K.; Bates R.; Žídek A.; Potapenko A.; Bridgland A.; Meyer C.; Kohl S. A. A.; Ballard A. J.; Cowie A.; Romera-Paredes B.; Nikolov S.; Jain R.; Adler J.; Back T.; Petersen S.; Reiman D.; Clancy E.; Zielinski M.; Steinegger M.; Pacholska M.; Berghammer T.; Bodenstein S.; Silver D.; Vinyals O.; Senior A. W.; Kavukcuoglu K.; Kohli P.; Hassabis D. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596 (7873), 583–589. 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano G. L.; Ceccarelli E. A. Recombinant Protein Expression in Escherichia Coli: Advances and Challenges. Front. Microbiol. 2014, 5, 172. 10.3389/fmicb.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen H. P.; Mortensen K. K. Advanced Genetic Strategies for Recombinant Protein Expression in Escherichia Coli. J. Biotechnol. 2005, 115 (2), 113–128. 10.1016/j.jbiotec.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Kaur J.; Kumar A.; Kaur J. Strategies for Optimization of Heterologous Protein Expression in E. Coli: Roadblocks and Reinforcements. Int. J. Biol. Macromol. 2018, 106, 803–822. 10.1016/j.ijbiomac.2017.08.080. [DOI] [PubMed] [Google Scholar]
- Hidalgo A.; Betancor L.; Moreno R.; Zafra O.; Cava F.; Fernández-Lafuente R.; Guisán J. M.; Berenguer J. Thermus Thermophilus as a Cell Factory for the Production of a Thermophilic Mn-Dependent Catalase Which Fails To Be Synthesized in an Active Form in Escherichia Coli. Appl. Environ. Microbiol. 2004, 70 (7), 3839–3844. 10.1128/AEM.70.7.3839-3844.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López O.; Cerdán M.-E.; González-Siso M.-I. Thermus Thermophilus as a Source of Thermostable Lipolytic Enzymes. Microorganisms 2015, 3 (4), 792–808. 10.3390/microorganisms3040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus F.; Bertoldo C.; Kähler M.; Antranikian G. Extremophiles as a Source of Novel Enzymes for Industrial Application. Appl. Microbiol. Biotechnol. 1999, 51 (6), 711–729. 10.1007/s002530051456. [DOI] [PubMed] [Google Scholar]
- Krefft D.; Papkov A.; Zylicz-Stachula A.; Skowron P. M. Thermostable Proteins Bioprocesses: The Activity of Restriction Endonuclease-Methyltransferase from Thermus Thermophilus (RM.TthHB27I) Cloned in Escherichia Coli Is Critically Affected by the Codon Composition of the Synthetic Gene. PLoS One 2017, 12 (10), e0186633. 10.1371/journal.pone.0186633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulages J. L.; Kim K.; Walters C.; Cushman J. C. Temperature-Induced Extended Helix/Random Coil Transitions in a Group 1 Late Embryogenesis-Abundant Protein from Soybean. Plant Physiol. 2002, 128 (3), 822–832. 10.1104/pp.010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.; Bigelow L.; Borovilos M.; Dementieva I.; Duggan E.; eschenfeldt W.; Hatzos C.; Joachimiak G.; Li H.; Maltseva N.; Mulligan R.; Quartey P.; Sather A.; Stols L.; Volkart L.; Wu R.; Zhou M.; Joachimiak A.. High-Throughput Protein Purification for X-Ray Crystallography and NMR. In Advances in Protein Chemistry and Structural Biology; Joachimiak A., Ed.; Structural Genomics, Part A; Academic Press, 2008; Vol. 75, pp 85–105. 10.1016/S0065-3233(07)75003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X. DAMBE7: New and Improved Tools for Data Analysis in Molecular Biology and Evolution. Mol. Biol. Evol. 2018, 35 (6), 1550–1552. 10.1093/molbev/msy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee S.; Reinstein J. The DnaK/ClpB Chaperone System from Thermus Thermophilus. Cell. Mol. Life Sci. 2002, 59 (10), 1598–1606. 10.1007/PL00012486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin-Roulling A.; Schmidpeter P. A.; Schmid F. X.; Feller G. Functional Adaptations of the Bacterial Chaperone Trigger Factor to Extreme Environmental Temperatures. Environ. Microbiol. 2015, 17 (7), 2407–2420. 10.1111/1462-2920.12707. [DOI] [PubMed] [Google Scholar]
- Gsponer J.; Futschik M. E.; Teichmann S. A.; Babu M. M. Tight Regulation of Unstructured Proteins: From Transcript Synthesis to Protein Degradation. Science 2008, 322 (5906), 1365–1368. 10.1126/science.1163581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahdev S.; Khattar S. K.; Saini K. S. Production of Active Eukaryotic Proteins through Bacterial Expression Systems: A Review of the Existing Biotechnology Strategies. Mol. Cell. Biochem. 2007, 307 (1), 249–264. 10.1007/s11010-007-9603-6. [DOI] [PubMed] [Google Scholar]
- Rohl C. A.; Doig A. J. Models for the 310-helix/Coil, Π-helix/Coil, and Α-helix/310-helix/Coil Transitions in Isolated Peptides. Protein Sci. 1996, 5 (8), 1687–1696. 10.1002/pro.5560050822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikol V.; Giegé R. Phase Diagram of a Crystalline Protein: Determination of the Solubility of Concanavalin A by a Microquantitation Assay. J. Cryst. Growth 1989, 97 (2), 324–332. 10.1016/0022-0248(89)90213-3. [DOI] [Google Scholar]
- Chayen N.; Akins J.; Campbell-Smith S.; Blow D. M. Solubility of Glucose Isomerase in Ammonium Sulphate Solutions. J. Cryst. Growth 1988, 90 (1–3), 112–116. 10.1016/0022-0248(88)90305-3. [DOI] [Google Scholar]
- McPherson A.[7] Crystallization of Proteins by Variation of PH or Temperature. In Methods in Enzymology; Elsevier, 1985; Vol. 114, pp 125–127. 10.1016/0076-6879(85)14009-7. [DOI] [PubMed] [Google Scholar]
- Plotkin J. B.; Kudla G. Synonymous but Not the Same: The Causes and Consequences of Codon Bias. Nat. Rev. Genet. 2011, 12 (1), 32–42. 10.1038/nrg2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz-Stachula A.; Zolnierkiewicz O.; Sliwinska K.; Jezewska-Frackowiak J.; Skowron P. M. Modified ‘One Amino Acid-One Codon’ Engineering of High GC Content TaqII-Coding Gene from Thermophilic Thermus Aquaticus Results in Radical Expression Increase. Microb. Cell Fact. 2014, 13 (1), 7. 10.1186/1475-2859-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedialkova D. D.; Leidel S. A. Optimization of Codon Translation Rates via TRNA Modifications Maintains Proteome Integrity. Cell 2015, 161 (7), 1606–1618. 10.1016/j.cell.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J. Macromolecular Crowding: An Important but Neglected Aspect of the Intracellular Environment. Curr. Opin. Struct. Biol. 2001, 11 (4), 500. 10.1016/S0959-440X(00)00239-6. [DOI] [PubMed] [Google Scholar]
- Kinjo A. R.; Takada S. Effects of Macromolecular Crowding on Protein Folding and Aggregation Studied by Density Functional Theory: Dynamics. Phys. Rev. E 2002, 66 (5), 051902. 10.1103/PhysRevE.66.051902. [DOI] [PubMed] [Google Scholar]
- Miklos A. C.; Sarkar M.; Wang Y.; Pielak G. J. Protein Crowding Tunes Protein Stability. J. Am. Chem. Soc. 2011, 133 (18), 7116–7120. 10.1021/ja200067p. [DOI] [PubMed] [Google Scholar]
- Kim J. S.; Yethiraj A. Crowding Effects on Protein Association: Effect of Interactions between Crowding Agents. J. Phys. Chem. B 2011, 115 (2), 347–353. 10.1021/jp107123y. [DOI] [PubMed] [Google Scholar]
- Samiotakis A.; Cheung M. S. Folding Dynamics of Trp-Cage in the Presence of Chemical Interference and Macromolecular Crowding. I. J. Chem. Phys. 2011, 135 (17), 175101. 10.1063/1.3656691. [DOI] [PubMed] [Google Scholar]
- Kuznetsova I. M.; Turoverov K. K.; Uversky V. N. What Macromolecular Crowding Can Do to a Protein. Int. J. Mol. Sci. 2014, 15 (12), 23090–23140. 10.3390/ijms151223090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal A. H.; Geerke-Volmer A. A.; van Mierlo C. P. M.; van Berkel W. J. H. Chaotropic Heat Treatment Resolves Native-like Aggregation of a Heterologously Produced Hyperthermostable Laminarinase. Biotechnol. J. 2017, 12 (6), 1700007. 10.1002/biot.201700007. [DOI] [PubMed] [Google Scholar]
- Ninh P. H.; Honda K.; Sakai T.; Okano K.; Ohtake H. Assembly and Multiple Gene Expression of Thermophilic Enzymes in Escherichia Coli for in Vitro Metabolic Engineering. Biotechnol. Bioeng. 2015, 112 (1), 189–196. 10.1002/bit.25338. [DOI] [PubMed] [Google Scholar]
- Hartl F. U.; Bracher A.; Hayer-Hartl M. Molecular Chaperones in Protein Folding and Proteostasis. Nature 2011, 475 (7356), 324–332. 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Berg J. M.; Tymoczko J. L.; Stryer L.. Protein Structure and Function. Biochemistry, 5th ed.; W. H. Freeman, 2002. [Google Scholar]
- Winklhofer K. F.; Tatzelt J.; Haass C. The Two Faces of Protein Misfolding: Gain- and Loss-of-Function in Neurodegenerative Diseases. EMBO J. 2008, 27 (2), 336–349. 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino Y.; Goda S.; Suematsu Y.; Doi K. Development of a New Gene Expression Vector for Thermus Thermophilus Using a Silica-Inducible Promoter. Microb. Cell Fact. 2020, 19 (1), 126. 10.1186/s12934-020-01385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda S.; Kojima M.; Nishikawa Y.; Kujo C.; Kawakami R.; Kuramitsu S.; Sakuraba H.; Hiragi Y.; Ohshima T. Intersubunit Interaction Induced by Subunit Rearrangement Is Essential for the Catalytic Activity of the Hyperthermophilic Glutamate Dehydrogenase from Pyrobaculum Islandicum. Biochemistry 2005, 44 (46), 15304–15313. 10.1021/bi050478l. [DOI] [PubMed] [Google Scholar]
- Angelov A.; Mientus M.; Liebl S.; Liebl W. A Two-Host Fosmid System for Functional Screening of (Meta)Genomic Libraries from Extreme Thermophiles. Syst. Appl. Microbiol. 2009, 32 (3), 177–185. 10.1016/j.syapm.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Angelov A.; Loderer C.; Pompei S.; Liebl W. Novel Family of Carbohydrate-Binding Modules Revealed by the Genome Sequence of Spirochaeta Thermophila DSM 6192. Appl. Environ. Microbiol. 2011, 77 (15), 5483–5489. 10.1128/AEM.00523-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis B.; Angelov A.; Mientus M.; Li H.; Pham V. T. T.; Lauinger B.; Bongen P.; Pietruszka J.; Gonçalves L. G.; Santos H.; Liebl W. Identification of Novel Esterase-Active Enzymes from Hot Environments by Use of the Host Bacterium Thermus Thermophilus. Front. Microbiol. 2015, 6, 275. 10.3389/fmicb.2015.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chautard H.; Blas-Galindo E.; Menguy T.; Grand'Moursel L.; Cava F.; Berenguer J.; Delcourt M. An Activity-Independent Selection System of Thermostable Protein Variants. Nat. Methods 2007, 4 (11), 919–922. 10.1038/nmeth1090. [DOI] [PubMed] [Google Scholar]
- Mate D. M.; Rivera N. R.; Sanchez-Freire E.; Ayala J. A.; Berenguer J.; Hidalgo A. Thermostability Enhancement of the Pseudomonas Fluorescens Esterase I by in Vivo Folding Selection in Thermus Thermophilus. Biotechnol. Bioeng. 2020, 117 (1), 30–38. 10.1002/bit.27170. [DOI] [PubMed] [Google Scholar]
- Bosch S.; Sanchez-Freire E.; del Pozo M. L.; Ćesnik M.; Quesada J.; Mate D. M.; Hernández K.; Qi Y.; Clapés P.; Vasić-Rački Đ.; Findrik Blažević Z.; Berenguer J.; Hidalgo A. Thermostability Engineering of a Class II Pyruvate Aldolase from Escherichia Coli by in Vivo Folding Interference. ACS Sustainable Chem. Eng. 2021, 9 (15), 5430–5436. 10.1021/acssuschemeng.1c00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto M.; Olimpieri P. P.; Di Rienzo L.; Ambrosetti F.; Corsi P.; Lepore R.; Tartaglia G. G.; Milanetti E. Insights on Protein Thermal Stability: A Graph Representation of Molecular Interactions. Bioinformatics 2019, 35 (15), 2569–2577. 10.1093/bioinformatics/bty1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt G.; Argos P. Protein Thermal Stability: Hydrogen Bonds or Internal Packing?. Folding Des. 1997, 2, S40–S46. 10.1016/S1359-0278(97)00062-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








