Abstract
Background
Rapid recurrence, defined as gross tumor recurrence after primary operation but prior to initiating postoperative radiation therapy (PORT), is underappreciated in head and neck cancer (HNC).
Methods
CT simulation images in patients with HNC managed surgically with adjuvant therapy at a single center between 2010 and 2017 were retrospectively reviewed.
Results
A total of 194 HNC patients were included. Rapid recurrence occurred in 39 patients (20%) with a median time from operation to CT simulation of 37 days. On MVA, extranodal extension (ENE) was the only predictor of rapid recurrence (p= .03). While rapid recurrence, ENE, and perineural invasion were all associated with poor OS on MVA, rapid recurrence was the strongest predictor (HR 5.47).
Conclusion
Rapid recurrence occurs at an underappreciated rate and is associated with poor survival outcomes. Patients with ENE are at highest risk and may benefit from diagnostic imaging evaluations immediately prior to PORT.
Keywords: Recurrence, extranodal extension (ENE), head and neck cancer (HNC), post operative radiation (PORT), rapid recurrence
Introduction
Head and neck cancer (HNC) is often first managed with an operation.1 Select patients receive postoperative radiation therapy (PORT) with or without concurrent chemotherapy to improve locoregional control and survival.2–4 Despite multimodality therapy, 25–30 % of patients ultimately experience locoregional failure.5,6 Treatment outcomes after locoregional failure are poor due to the aggressive behavior of recurrent HNC and reduction is available therapeutic options, so initial treatment is best focused on cure.7
The NCCN (National Comprehensive Cancer Network) recommends that post-operative (chemo)radiation commence ≤ 6 weeks after resection.8 Modern prospective National Clinical Trials Network (NCTN) trials recommend that adjuvant therapy begin 4–8 weeks after resection (e.g. NCT02734537). This is because many experiences report locoregional control and/or survival improvement with a shortened treatment ‘package time’ (defined as date from operation to date of completion of radiation).9–11. Although rarely directly reported, one rationale for shortening package time is that some tumors produce structurally detectable recurrence in a relatively short interval.
Rapid recurrence, defined as detectable tumor recrudescence after an oncologically sound primary operation but prior to initiating timely PORT, is a poorly understood phenomenon. Many treatment teams have appreciated isolated rapid recurrences in the past but there is a prevailing notion that this primarily reflects adequacy of resection rather than tumor biology. Recently a large series from an institution that regularly obtains diagnostic imaging [computed tomography (CT) and/or magnetic resonance imaging (MRI)] in the interval between surgery and radiation12 reported that 15% of patients treated with primary surgery had recurrence prior to the initiation of adjuvant therapy. While post-operative imaging patterns of care are subject to institutional bias, it seems that most patients in the U.S.A. do not undergo diagnostic imaging prior to initiating PORT, thus resulting in potential miss of rapid recurrence in a sizable proportion of patients. It is possible that the high recurrence rate after multi-modality therapy is in part referable to an under appreciation of prompt reappearance of adequately resected tumor. Better characterization of rapid recurrence of HNC is needed.
Materials and Methods
Patients with primary HNC managed surgically with planned adjuvant therapy at a single center with retrievable CT simulations between 2010 and 2017 were identified. Patients who received an operation at an outside site were eligible for inclusion. Patients who received radiation at an outside facility and did not have post-operative, pre-radiation imaging available were excluded. All patients received definitive resection of all gross disease (at the primary site and neck) with curative intent; patients with gross residual disease were excluded. Patients managed with a tonsillectomy or neck dissection prior to the initiation of definitive (chemo)radiation were ineligible as were any patients managed with an incomplete operation due to intra-operative complications. Recurrent disease managed with salvage surgery was excluded. Therapeutic neck dissection was performed in patients with clinically positive node(s). Elective neck dissection(s) were performed based on primary tumor location and for tumors ≥ T2 or with clinical thickness ≥ 2 mm. Indications for PORT include microscopic close or positive surgical margin, selected T3, T4, pN2-N3, perineural invasion (PNI), lymphovascular invasion (LVI), and any extra nodal extension (ENE)4.
Radiation planning CT simulation images were obtained with a 2.5mm slice thickness and performed with IV contrast unless there was a medical contraindication or patient refusal of contrast administration. All patients were immobilized with a thermoplastic mask. All CT simulation images were reviewed by the treating radiation oncologist (TJG) but not by a diagnostic radiologist prior to the initiation of therapy. All patients received curative intent radiation, with dose to the high risk region receiving at least 60 Gy. The surgical bed and involved side of the neck were typically treated with 60 Gy in 30 fractions, and the elective side of the neck with 54 Gy in 30 fractions. When patients present with microscopic involved surgical margin, ENE or multiple high risk factors, the dose to high risk regions is typically escalated to 66 Gy in 30–33 fractions with concurrent systemic therapy. Patients appreciated to have rapid recurrence at the time of CT simulation by the treating radiation oncologist were treated with dose escalated radiation to 66 – 70 Gy.
All patients were staged according to the American Joint Committee on Cancer 7th edition. Initial post PORT imaging evaluation was performed at 3 months and then as clinically indicated. Patients are assessed clinically no less often than every 3 months by a head and neck surgeon and radiation oncologist for the first 2 years, every 6 months the third to fifth year, then no less frequently than annually after.
CT simulation images were retrospectively reviewed by a neuroradiologist blinded to patient outcome but provided with preoperative imaging, pre-operative physical examination reports, and the surgical pathology report. The modality of preoperative imaging varied according to disease site and stage, and consisted of CT, MRI and/or positron emission tomography (PET). The primary site, ipsilateral side of the neck and contralateral side of the neck at the time of simulation were separately evaluated. Images interpreted as either suspected or definite recurrence were categorized as “rapid recurrence”. At the primary site operative bed abnormally enhancing soft tissue with evidence of hyper-enhancement in comparison to the surrounding soft tissue was interpreted as at least suspicious for rapid recurrence. In the neck, lymph nodes measuring greater than 1.0 cm in its short axis were thought to represent abnormal lymph nodes with possible metastatic disease. In addition lymph nodes measuring less than 1 cm in short axis, but located in the expected drainage site of the primary tumor were also interpreted as rapid recurrence. Finally, loss of the normal uniform shape of the lymph node and/or loss of the central fatty hila, presence of central low attenuation, calcification and hyper-enhancement were also considered to be abnormal. The margin of the lymph node was closely scrutinized for any irregularities in contour and for extra capsular spread of tumor.
Standard descriptive statistics were used to characterize the patient population. Fisher’s exact tests and Wilcoxon rank sum tests were used to assess the relationships between presence/absence of rapid recurrence at the primary site, ipsilateral neck, contralateral neck or any location with categorical or continuous variables, respectively. Patient-related variables included age, race and sex. Tumor-related variables included tumor sub-site, histologic grade, clinical stage, pathologic stage, tumor size, margin status, ENE, PNI, LVI, radiation dose (60 Gy vs > 60 Gy), and time between operation and CT simulation. Multivariable logistic regression was used to simultaneously model the relationships between rapid recurrence at any location with factors that were significantly associated with rapid recurrence at the primary site, ipsilateral neck or contralateral neck in univariate analyses using a p value of 0.05.
Log-rank tests and univariate Cox proportional hazards models were used to identify factors associated with the time from the end of radiation therapy until local, regional, distant metastasis or death from any cause. Individuals who did not experienced the outcome of interest at or before the last contact date were censored at that time. Multivariable Cox models were also fit to each of these failure time outcomes. These models included established prognosis factors and the presence/absence of rapid recurrence as covariates. Kaplan-Meier plots were generated to display time to local/regional recurrence and overall survival time distributions. The relationship between rapid recurrence and dose was assessed by Fisher’s exact test.
All statistical tests were two-sided and used a 5% type I error. Statistically analyses were conducted using SAS statistical software V9.4 (Cary, NC).
Results
We identified 194 HNC patients with a median time from operation to CT simulation of 37 (range 12 – 91) days. Oral cavity and squamous cell carcinoma were the most common site (58%) and histology (85%), respectively. Patients were primarily Caucasian (87%) and male (61%), with median age of 63 (range 25–90). A majority of the patients were managed with primary site surgery and an ipsilateral neck dissection (n= 133, 69%); a minority were managed with bilateral neck dissection (n= 29, 15%) or primary site surgery alone (n= 32, 16%). The mean lymph node yield of ipsilateral and contralateral neck dissection was 38.5 and 26.3, respectively. Of the 32 patients treated without neck dissection, a majority (n=23, 72%) had either primary salivary gland or paranasal sinus cancer. Sixty-two percent of patients were AJCC 7th edition pathologic stage IV, 23% had ENE, 24% had LVI, 45% had PNI, and the mean pathologic tumor size was 2.8 cm. Microscopic involved surgical margin occurred in 20 (10%) patients (Table 1), the majority of whom had salivary or sinus cancers.
Table 1.
Patient and tumor characteristics
| Rapid Recurrence | No Recurrence | |||
|---|---|---|---|---|
|
| ||||
| Covariate | N= 194 | 39 | 155 | p value |
|
| ||||
| Age, median (range) in years | 63 (25–90) | 63 (25–90) | 63 (38–84) | 0.88 |
| Male sex | 118 (61%) | 21 (54%) | 97 (63%) | 0.36 |
| Caucasian race | 168 (87%) | 34 (87%) | 134 (87%) | 1 |
| Tumor sub-site | 1 | |||
| Oral Cavity | 114 (59%) | 23 (59%) | 91 (59%) | |
| Oropharynx/Larynx/Hypopharynx | 48 (25%) | 10 (26%) | 38 (25%) | |
| Other | 32 (16%) | 6 (15%) | 26 (16%) | |
| Histologic grade | 0.47 | |||
| Grade 1–2 | 95 (51%) | 17 (44%) | 78 (50%) | |
| Grade 3–4 | 91 (49%) | 21 (54%) | 70 (45%) | |
| Clinical stage | 0.15 | |||
| Stage I-III | 97 (50%) | 15 (38%) | 82 (53%) | |
| Stage IV | 97 (50%) | 24 (62%) | 73 (47%) | |
| Pathologic stage | 0.2 | |||
| Stage I-III | 74 (38%) | 11 (28%) | 63 (41%) | |
| Stage IV | 120 (62%) | 28 (72%) | 92 (59%) | |
| Tumor size, mean | 2.8 cm | 3.3 cm | 2.7 cm | 0.04 |
| Margin status | 1 | |||
| Negative | 174 (90%) | 35 (90%) | 139 (90%) | |
| Positive | 20 (10%) | 4 (10%) | 16 (10%) | |
| ENE | 0.005 | |||
| No | 150 (77%) | 25 (64%) | 122 (79%) | |
| Yes | 44 (23%) | 14 (36%) | 33 (21%) | |
| PNI | 0.003 | |||
| No | 107 (55%) | 17 (44%) | 90 (58%) | |
| Yes | 87 (45%) | 22 (56%) | 65 (42%) | |
| LVI | ||||
| No | 147 (76%) | 23 (59%) | 127 (82%) | 0.06 |
| Yes | 47 (24%) | 16 (41%) | 28 (18%) | |
| Time between operation and CT simulation, median (range) in days | 37 (12–91) | 37 (12–79) | 37 (12–91) | 0.42 |
| Radiation Dose | 0.005 | |||
| 60 Gy | 150 (77%) | 23 (59%) | 127 (82%) | |
| >60 Gy | 44 (23%) | 16 (41%) | 28 (18%) | |
bold indicates p <0.05
Predictors of Rapid Recurrence
Rapid recurrence in an operated field and progression in observed necks was appreciated in 39 patients (20%), all of whom received curative intent radiation with at least 60 Gy to the high-risk region. Given these rapid recurrences were retrospectively identified, 23 patients were not recognized to have recurrence at the time of CT simulation and received 60 Gy while the rest received escalated dose of 66–70 Gy. The most common site of rapid recurrence was at the primary site (n=22, 56%) although progression in both the ipsilateral (n=17, 44%) and contralateral (n=13, 33%) sides of the neck were not uncommon. A proportion of ipsilateral neck failures developed in necks that were observed (4 of 17) – all had salivary gland cancer and none had a regional recurrence after radiation with a median follow-up of 2.1 years. All but two of the contralateral neck failures ensued in a setting of observation rather than neck dissection. A majority of patients with contralateral neck failures also had recurrence at either the primary site or dissected ipsilateral neck. Multiple patients had rapid recurrence at multiple sites (n=13, 33%).
In univariate analyses, LVI was a significant risk factor of ipsilateral neck rapid recurrence while pathologic stage IV and PNI were risk factors of contralateral neck rapid recurrence (p < 0.05). Increased tumor size was associated with both ipsilateral and contralateral rapid recurrence. ENE was associated with recurrence at the primary site. Neither positive surgical margin nor interval between resection and CT simulation were associated with rapid recurrence at any location (Table 2).
Table 2.
Univariable and multivariable analysis for predictors of rapid recurrence
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
|
|
|||||
| Primary Site (n=22) | Ipsilateral Neck (n=17) | Contralateral Neck (n=13) | Recurrence (any site) | Recurrence (any site) | |
|
|
|||||
| Covariate | Odds Ratio (95% Confidence Interval) | ||||
|
| |||||
| Male sex | 0.61 (0.25, 1.48) | 1.20 (0.42, 3.39) | 0.53 (0.17, 1.64) | 0.70 (0.34, 1.42) | - |
| Caucasian race | 3.57 (0.46, 27.8) | 1.18 (0.25, 5.47) | 0.49 (0.12, 1.90) | 1.07 (0.37, 3.03) | - |
| Oral cavity sub-site (reference: oropharynx/hypopharynx/larynx) | 1.80 (0.57, 5.68) | 0.83 (0.24, 2.90) | 0.72 (0.20, 2.58) | 0.96 (0.42, 2.21) | - |
| Histologic grade 3–4 (reference: grade 1–2) | 1.45 (0.58, 3.63) | 1.38 (0.49, 3.87) | 0.63 (0.20, 2.01) | 1.38 (0.67, 2.82) | - |
| Clinical stage 4 (reference: stage 1–3) | 1.51 (0.61, 3.73) | 1.94 (0.69, 5.47) | 1.65 (0.52, 5.25) | 1.80 (0.88, 3.69) | - |
| Pathologic stage 4 (reference: stage 1–3) | 0.88 (0.36, 2.17) | 2.12 (0.67, 6.79) | 8.11 (1.03, 63.7) * | 1.74 (0.81, 3.76) | 0.980 (0.40, 2.43) |
| Positive margin | 0.86 (0.18, 3.96) | 2.02 (0.53, 7.73) | 0.0 ^ | 0.99 (0.31, 3.16) | - |
| ENE | 2.71 (1.07, 6.85) * | 2.65 (0.94, 7.43) | 2.28 (0.70, 7.35) | 3.16 (1.48, 6.73) ** | 2.63 (1.05, 6.62 ) * |
| PNI | 1.02 (0.42, 2.51) | 1.43 (0.53, 3.87) | 7.60 (1.64, 35.3) ** | 1.79 (0.88, 3.64) | 1.21 (0.55, 2.66) |
| LVI | 1.95 (0.76, 4.98) | 3.15 (1.14, 8.69) * | 2.07 (0.64, 6.66) | 2.07 (0.97, 4.42) | 1.28 (0.55, 3.00) |
| Time between operation and CT simulation in days ^^ | p= 0.81 | p= 0.65 | p= 0.76 | p= 0.42 | - |
| Age in years ^^ | p= 0.68 | p= 0.10 | p= 0.90 | p= 0.88 | - |
| Tumor size ^^ | p= 0.68 | p= 0.006 | p= 0.03 | p = 0.04 | 1.15 (0.93, 1.42) |
-p<.05
-p<.01
No patient with positive margin had contralateral neck rapid recurrence
Odds ratio not performed for continous variables
A multivariable logistic regression model for “recurrence at any site” group of patients (n=39, patients counted once regardless of site(s) of failure) demonstrated that ENE was the sole statically significant independent risk factor for rapid recurrence (odds ratio= 2.63 95% CI 1.05 – 6.62, p= 0.04). Tumor size was no longer a risk factor for rapid recurrence on MVA.
Clinical impact of rapid recurrence
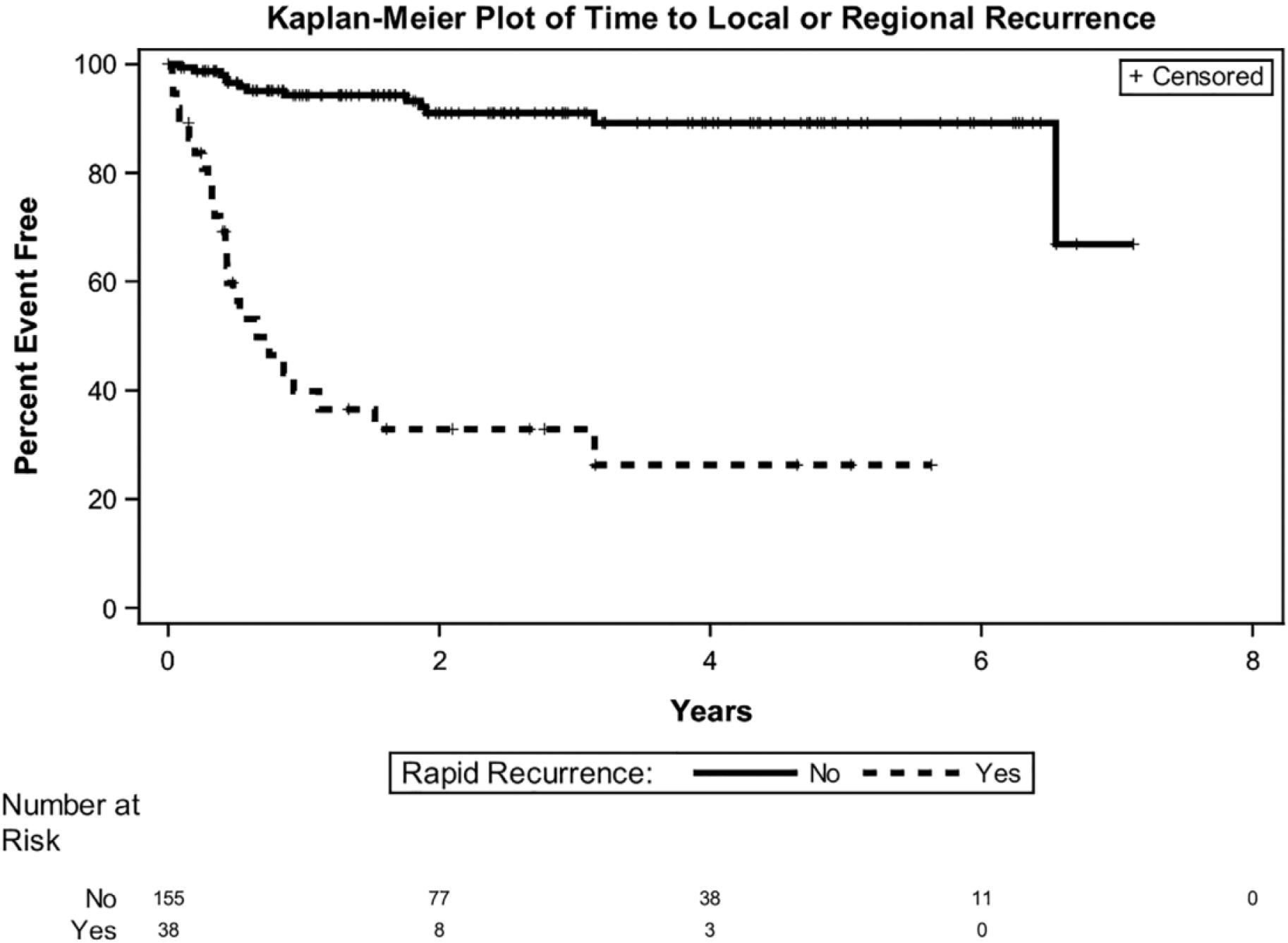
Rapid recurrence at any site was significantly associated with local, regional and distant recurrence (p < .0001 for all) (Table 3). Regional recurrence was additionally associated with ENE and PNI (p <0.05 for all). The 2 year locoregional control rates for patients with rapid recurrence and without it were 33% and 91%, respectively (Figure 1a).
Table 3.
Univariable and multivariable analysis for predictors of local recurrence, regional recurrence, distant recurrence, and overall survival
| Local Recurrence | Regional Recurrence | Distant Recurrence | Overall Survival | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | |
|
|
||||||||
| Covariate | Hazard Ratio (95% CI), p-value | |||||||
|
| ||||||||
| Pathologic Stage | ||||||||
| Stage I-III | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Stage IV | 1.08 (.49–2.38), p= 0.86 | 0.54 (0.20, 1.41), p = 0.21 | 1.43 (0.58–3.51) p= 0.44 | 0.54 (0.17–1.69), p= 0.29 | 1.73 (0.76–3.90), p= 0.19 | 0.85 (0.33–2.21), p=0.73 | 2.33 (1.15–4.71), p< 0.01 | 1.19 (0.53–2.65), p=0.68 |
| Tumor size, mean | 1.19 (0.98–1.45), p= 0.09 | 1.24 (0.97, 1.60) p= 0.09 | 1.15 (0.92–1.44), p= 0.23 | 1.31 (0.99–1.72), p= 0.06 | 1.25 (1.04–1.50), p< 0.01 | 1.2 (0.96–1.49), p= 0.12 | 1.22 (1.06–1.42), p< 0.01 | 1.18 (0.99–1.41), p= 0.07 |
| Rapid Recurrence | 13.9 (5.96–32.5), p< 0.01 | 13.1 (5.55–31.1), p< 0.01 | 16.6 (6.47–42.8), p< 0.01 | 15.4 (5.77–41.2), p< 0.01 | 4.67 (2.23–9.78), p< 0.01 | 3.71 (1.75–7.88), p< 0.01 | 6.16 (3.41–11.1) p< 0.01 | 5.47 (3.01–9.97), p< 0.01 |
| ENE | ||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 2.77 (1.25–6.15), p= 0.01 | 2.01 (0.77–5.26), p= 0.15 | 4.57 (1.97–10.6), p< 0.01 | 3.73 (1.32–10.6), p< 0.01 | 3.90 (1.86–8.19), p< 0.01 | 2.05 (0.83–5.07), p= 0.12 | 4.78 (2.64–8.67), p< 0.01 | 2.75 (1.36–5.54), p< 0.01 |
| PNI | ||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 2.11 (0.97–4.61), p= 0.06 | 1.70 (0.75–3.82), p= 0.20 | 4.15 (1.62–10.6) p< 0.01 | 3.82 (1.43–10.2), p< 0.01 | 2.96 (1.38–6.39), p< 0.01 | 1.86 (0.84–4.15), p= 0.13 | 3.19 (1.68–5.80), p< 0.01 | 2.07 (1.09–3.93), p< 0.05 |
| LVI | ||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 1.87 (0.84–4.21) p= 0.13 | 1.03 (0.41–2.56), p= 0.95 | 1.05 (0.39–2.85) p= 0.92 | 0.43 (0.15–1.23), p= 0.12 | 3.93 (1.89–8.14) p< 0.01 | 1.89 (0.81–4.38), p= 0.14 | 2.80 (1.55–5.03) p< 0.01 | 1.15 (0.59–2.23), p=0.68 |
bold indicates p <0.05
FIGURE 1.

Locoregional control of rapid recurrence
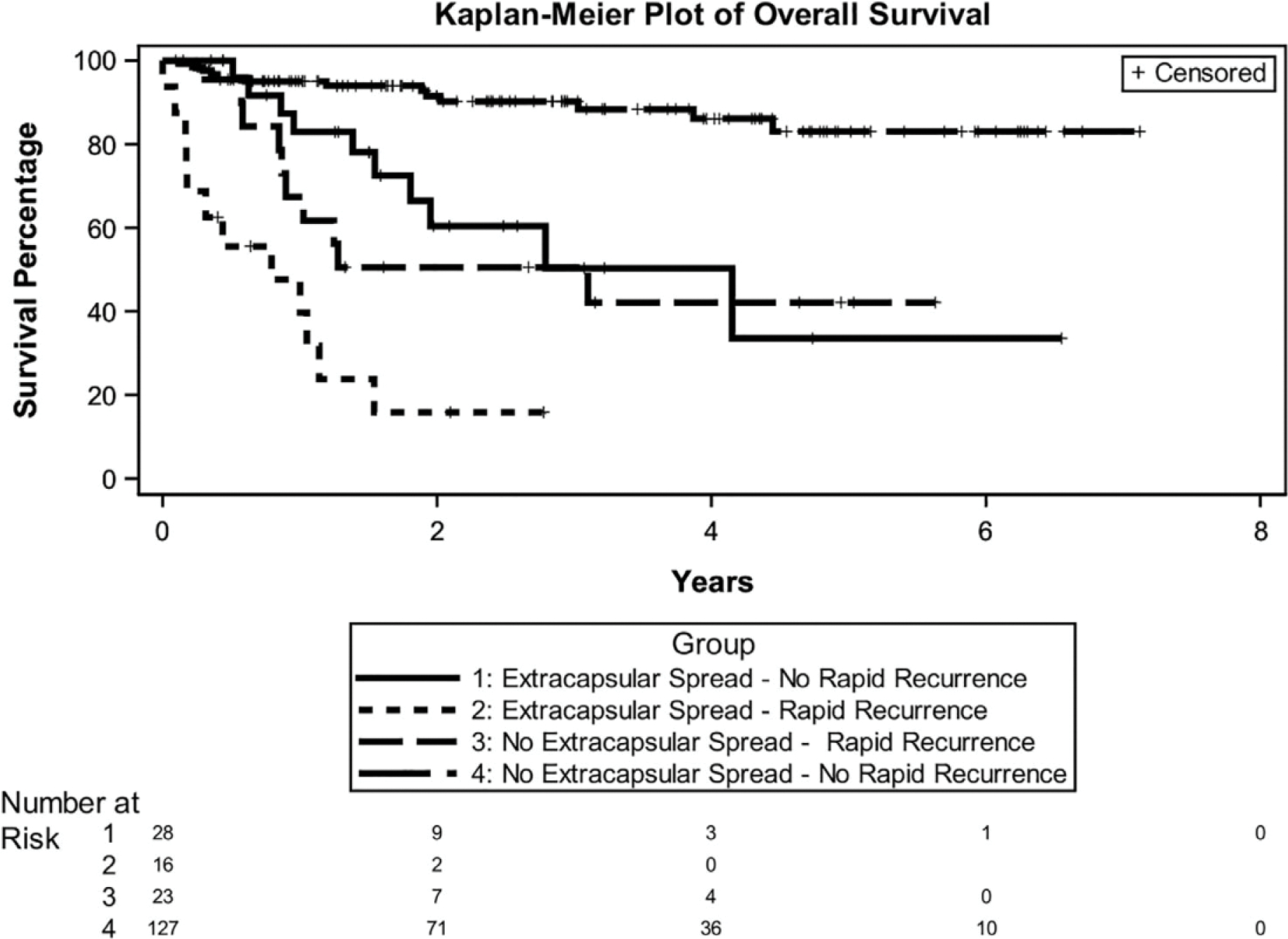
While rapid recurrence, ENE, and PNI were all associated with poor overall survival on MVA, rapid recurrence was the strongest predictor (HR 5.47, 95% CI 3.01– 9.97) followed by ENE (HR 2.75, 95% CI 1.36–5.54). The 2-year overall survival (OS) for patients with and without rapid recurrence were 36% and 86% (p < 0.001), respectively (Figure 1b). Since ENE is the most common adjuvant high risk factor of HNC2 and because it was predictive of rapid recurrence, an additional analysis of rapid recurrence and ENE was performed (Figure 2). This demonstrated an extraordinarily favorable outcome among patients with neither ENE nor rapid recurrence, regardless of the presence/absence of other risk factors.
FIGURE 2.
Overall survival of rapid recurrence
Given biologic differences amongst different etiologies of HNC, a sensitivity analysis was then performed to exclude patients with salivary gland cancer and all oropharynx cancer, given that 90% (34/38) of oropharynx cancer in our series with known p16 status was p16 positive (Table 4). This showed consistent results, with rapid recurrence (HR 7.32, p< 0.01) and ENE (HR 3.36, p< 0.01) remaining as strong predictors of poor overall survival.
Table 4.
Multivariable analysis for predictors of local recurrence, regional recurrence, distant recurrence, and overall survival (excluding oropharynx and salivary gland)
| Covariate | Local Recurrence | Regional Recurrence | Distant Recurrene | Overall Survival |
|---|---|---|---|---|
|
|
||||
| Pathologic Stage | ||||
| Stage I-III | Reference | Reference | Reference | Reference |
| Stage IV | 0.79, p= 0.67 | 1.32, p= 0.67 | 1.49, p= 0.54 | 1.52, p= 0.38 |
| Tumor size, mean | 1.15, p= 0.38 | 1.06, p= 0.76 | 1.17, p= 0.28 | 1.14, p= 0.25 |
| Rapid Recurrence | 14.7, p< 0.01 | 20.9, p< 0.01 | 6.30, p< 0.01 | 7.32, p< 0.01 |
| ENE | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 2.47, p= 0.08 | 6.48, p< 0.01 | 3.22, p= 0.02 | 3.36, p< 0.01 |
| PNI | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 1.59, p= 0.32 | 6.29, p< 0.01 | 1.60, p= 0.35 | 2.08, p= 0.06 |
| LVI | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 0.95, p= 0.93 | 0.25, p= 0.02 | 1.13, p= 0.82 | 0.75, p= 0.48 |
bold indicates p <0.05
Patients retrospectively appreciated to have rapid recurrence were more likely to have received escalated doses exceeding 60Gy (p= 0.001). 2-year OS in those treated with 60 Gy (n= 23) and > 60 Gy (n= 16) were 25% and 56%, respectively (p = 0.19) (Figure 3). These patients were also more likely to have received concurrent chemotherapy. There was no difference in 2-year OS (p=0.9) in those treated with (n=19) and without (n=20).
FIGURE 3.
Overall survival stratified by extranodal extension (ENE) and rapid recurrence
Discussion
Timely initiation of adjuvant therapy for HNC is challenging. While many patients recover uneventfully, some require procedures associated with long hospitalizations (such as free tissue transfer), unexpected complications, and/or depression stemming from lack of function. Initiating inconvenient, daily, side-effect laden adjuvant therapy some 6 weeks after resection in these setting can be difficult to achieve. However, similar to analyses evaluating the impact of treatment package time, this report documents the ability of HNC to recur quickly and the need to expedite therapy. The methodology used also illustrates important factors pertinent to adjuvant HNC management.
A majority of patients in this series had thin slice CT scans with IV contrast (86%) performed relatively expeditiously after resection (median 5.3 weeks). Almost all patients (98%) in this series were managed by a subspecialist head and neck radiation oncologist (TJG) with an interest in identifying rapid recurrence. Despite adequate imaging and a treatment philosophy promoting reliable identification of rapid recurrence, more than half of the patients retrospectively diagnosed with rapid recurrence (n = 23) were not recognized to have recurred at the time of treatment planning and thus were treated with the usual adjuvant dosing (60 Gy). While inclined to assume that this phenomenon is common only at ‘other’ institutions and potentially referable to poor surgical technique and/or radiation simulation delays, the reported data are striking; fifteen to 30 percent of patients experience radiographically detectable rapid recurrence.
An early prospective analysis of 91 consecutive patients evaluated with PET/CT after head and neck resection but prior to the initiation of PORT demonstrated a 15% rate of treatment change as a result of imaging PET (all suspicious PET findings were biopsied when technically possible).13 Despite this, regular application of PET/CT after head and neck resection was not instituted at most centers in view of cost, the high reported false positive rate (>50%) and an association of rapid recurrence with known risk factors for progression (e.g. recurrent disease). Three subsequent reports focused exclusively on oral cavity cancer; two smaller studies considered PET/CT simulations14,15 and a large analysis from a center employed diagnostic imaging (either CT or MRI neck) regularly obtained prior to the initiation of PORT.12 All reported a 15–30% rate of rapid recurrence – similar to the current series (Table 5).
Table 5.
Comparison of studies with rapid recurrence
| Study | Disease Site | Number of patients | Imaging consistent with rapid recurrence | Biopsy confirmed rapid recurrence | Modality | Significant factors for rapid recurrence on MVA | Local control of patients with rapid recurrence |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Shitani et al. | All head & neck + skin | 91 | 29.7% (n=27) | 41% (11 of 27) | PET/CT | 2 yr LRC 70% | |
| Liao et al. | Oral cavity | 29 | 24% (n=7) | - | PET/CT | 2 yr DFS 29% | |
| Dutta et al. | Oral cavity | 44 | 31.8% (n=14) | 21% (3 of 14) | PET/CT | 2 yr LC 77% | |
| Hosni et al. | Oral cavity | 601 | 15% (n=88) | 32% (22 of 88) | CT or MRI | Oral tongue,pT3–4, pN2–3, positive margin | 3 yr LC 69% (70 Gy) |
| Current series | All head & neck | 194 | 21% (n=40) | - | CT Simulation | ENE | 2 yr LC 56% (>60Gy) |
The current series showed that rapid recurrence occurs not only in oral cavity but all HNC. While there are biologic differences amongst HNC, postoperative therapy is guided using relatively standard risk factors2,3 with standard dosing of radiation or chemoradiation. We performed a sensitivity analysis excluding oropharynx and salivary gland subsite to demonstrate that the results are consistent and rapid recurrence is not driven by any specific HNC subsite. Although results are similar when salivary gland cancer is included in the model and the concept seems sound (cancer recurrence within weeks of an operation is associated with worse survival regardless of histology), the number of salivary gland tumors in this series is too small to comment on the impact of histology.
The identification of rapid recurrence can be difficult. In the current series the most common locations of rapid recurrence were at the primary site and in the ipsilateral side of the neck. At a median of five weeks after the operation the primary site and operated neck(s) are still evolving, even in the absence of free tissue transfer. Hence, it can be extraordinarily difficult for those not trained in diagnostic radiology to appreciate subtle early recurrences in the dissected beds (by contrast, appreciating a rapid recurrence in an undissected neck is not as demanding). Head and neck programs not specifically evaluating patients for rapid recurrence may be missing them.
Perhaps it is not noteworthy that the 2-year survival of patients with rapid recurrence was poor. However, it is surprising that treatment with gross cancer dosing (66 Gy in 30 fractions or 70 Gy in 35 fractions) may have produced a clinically significant improvement. Although the numbers are small, increased dose “salvage” (chemo)radiation may be effective. Accurate identification of rapid recurrence to trigger increased radiation dose (and likely volume) could potentially improve outcomes. Similarly the addition of concurrent systemic therapy in situations where it would not otherwise already be indicated represents another potential avenue to intensify therapy that may be effective. Our series showed similar survival but again the numbers may be too small to detect a difference.
Rapid recurrence is primarily referable to poor prognosis tumors, not to inadequate surgery or delayed radiation
It is tempting to suspect that involved surgical margins and a protracted interval between resection and adjuvant therapy are significant factors predictive of rapid recurrence. Similar to modern database series16, positive margin was relatively uncommon in our series (10%). A majority (65%, n=13) of our cases with involved margins occurred with primary salivary gland cancer or paranasal sinus cancer. Thus mucosal positive margins occurred in less than five percent of patients in this series and interpretation of the impact of margins is therefore limited. The mean lymph node yield of 38.5 and 26.3 in ipsilateral and contralateral neck dissection also reflect the adequacy of surgery. Nonetheless our series demonstrated a rapid recurrence rate similar to that of other analyses suggesting that the attribution of rapid recurrence to inadequate clearance of the primary tumor is not justified. Instead, ENE is the prognostic factor independently predictive of rapid recurrence.
ENE is an established high-risk factor in the management of HNC2,3. While frozen section assessment may limit positive margins in the care of a subspecialist head and neck surgeon, ENE is not influenced by surgical technique and is instead referable to the cancer itself. While the scoring of ENE is debated17, it is an unquestioned harbinger of poor prognosis that triggers a recommendation for concurrent chemotherapy in the adjuvant setting. This report suggests that the poor prognosis of ENE at least in part reflects rapid recurrence. Tumors that display ENE are more prone to recurrence not just regionally in the neck but also at the primary site. ENE leads to rapid recurrence not simply as a failure of node dissection but rather as an indicator of the overall biologic aggressiveness of the tumor itself. It is possible that tumors rapidly progressing pre-operatively may similarly be correlated with post-surgical rapid recurrence, however quantifying tumor growth is difficult and this dataset cannot determine whether a subjective assessment of pre-operative growth is an independent risk factor for rapid recurrence.
Rapid recurrence as an underappreciated poor prognostic factor
There are many relative indications for adjuvant therapy of HNC. Advanced T-stage designation (T3–4), advanced N-stage designation (more than a single node smaller than 3 cm), PNI and LVI all often engender recommendations of adjuvant radiation alone. Despite this, the 3-year locoregional control of patients managed with so-called ‘intermediate’ risk HNC is 70%. To that end, a major NCTN trial (NCT02734537) is currently accruing in order to test molecular markers18 to better assess patients currently recommended adjuvant radiation without chemotherapy and to evaluate the impact of addition of cisplatin. ENE and involved surgical margins prompt a recommendation of adjuvant chemoradiation. The 3-year locoregional control of patients managed with trimodality therapy for high-risk HNC is only about 50%.
This analysis perhaps provides insight into an aspect of poor results from standard therapy. There is a subset of patients who recur so rapidly that radiographically detectable structural disease is seen within five weeks from resection. If diagnostic scans interpreted by a radiologist with head and neck expertise or a PET/CT are not obtained it is likely that some of these recurrences are missed and standard radiation is administered (rather than apparently efficacious increased-dose “salvage” chemoradiation). This probably occurs both in intermediate and high-risk patients but appears more likely in the high-risk setting of ENE.
Detailed imaging and sophisticated interpretation warrant serious consideration prior to the initiation of PORT for patients with ENE. Even when a patient will receive concurrent chemoradiation, prompt identification of a rapid recurrence to allow targeting of increased doses of “salvage” radiation is of potential benefit. Is the best post resection management adjuvant chemoradiation in 6 weeks? Or should patients with ENE be managed according to pathways designed to prevent early repopulation and recurrence? Should patients with gross clinical ENE receive neoadjuvant systemic therapy? Should systemic therapy be in the early adjuvant period?19 Currently most (if not all) adjuvant head and neck treatment protocols are written to exclude patients with gross disease present at the time of CT simulation. If they truly represent 15–30% of adjuvant HNC patients, more resources need to be dedicated to this underappreciated patient population.
The limitation of this analysis is its retrospective nature. As a consequence the blinded radiologic assessment was conducted on thin slice CT simulation images. Perhaps true diagnostic studies would be superior in detecting early recurrence. While there was no pathologic confirmation of rapid recurrence, the associated prognostic implications suggest that the phenomenon is real.
FIGURE 4.
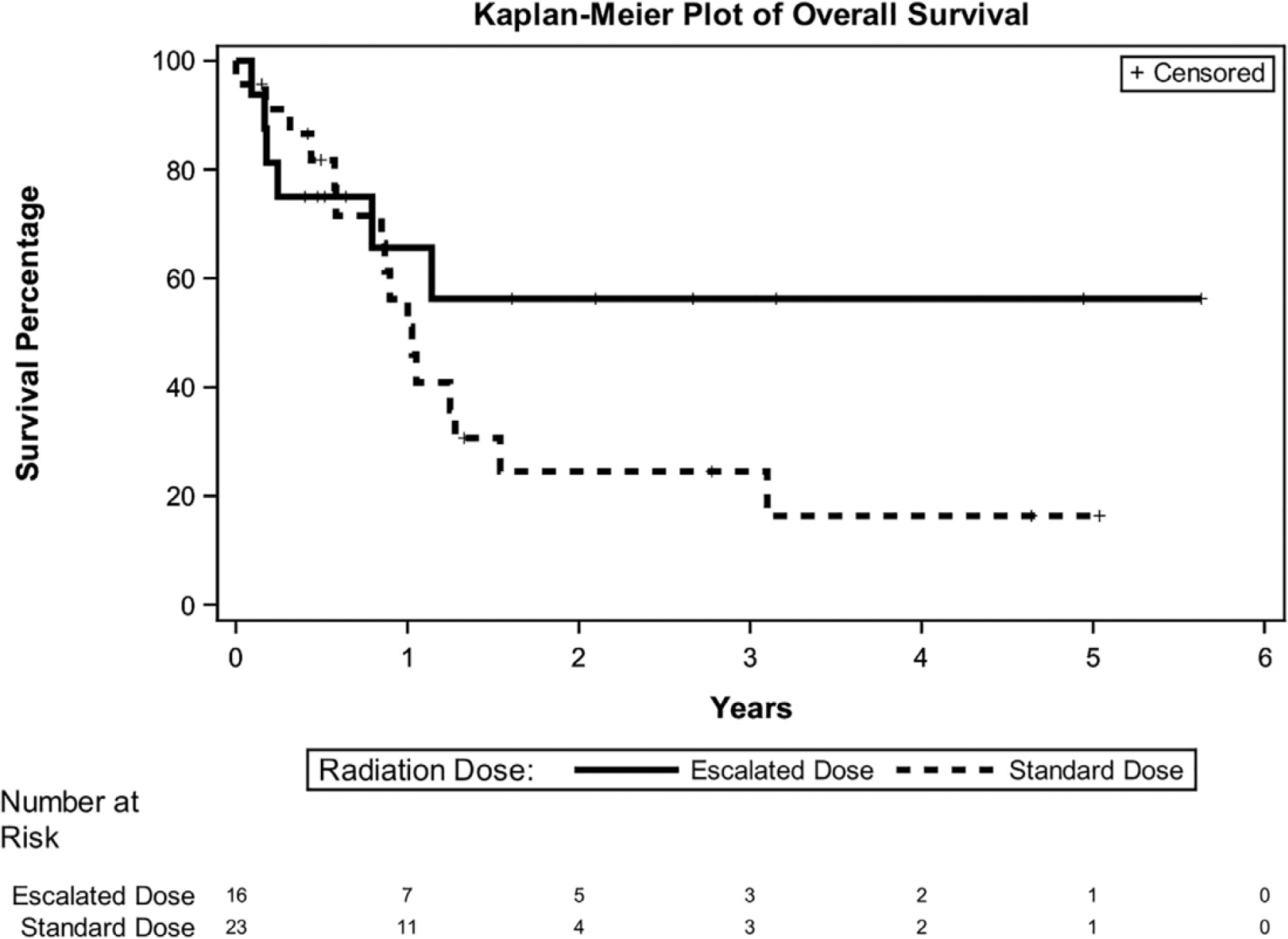
Overall survival stratified by dose escalation
Footnotes
Prior Presentation: Presented as a poster at American Society for Radiation Oncology (ASTRO) 2019 in Chicago, IL.
References
- 1.Murphy CT et al. Increasing time to treatment initiation for head and neck cancer: an analysis of the National Cancer Database. Cancer 121, 1204–1213 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Cooper JS et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 350, 1937–1944 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Bernier J et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. The New England Journal of Medicine 8 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Hinerman RW et al. Postoperative irradiation for squamous cell carcinoma of the oral cavity: 35-year experience. Head & Neck 26, 984–994 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Cooper JS et al. Long-term Follow-up of the RTOG 9501/Intergroup Phase III Trial: Postoperative Concurrent Radiation Therapy and Chemotherapy in High-Risk Squamous Cell Carcinoma of the Head and Neck. International Journal of Radiation Oncology*Biology*Physics 84, 1198–1205 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langendijk JA et al. Risk-group definition by recursive partitioning analysis of patients with squamous cell head and neck carcinoma treated with surgery and postoperative radiotherapy. Cancer 104, 1408–1417 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Peters LJ et al. Evaluation of the dose for postoperative radiation therapy of head and neck cancer: First report of a prospective randomized trial. International Journal of Radiation Oncology*Biology*Physics 26, 3–11 (1993). [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. Head and Neck Cancers (Version 2.2019). Available at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. (Accessed: 17th May 2019)
- 9.Ang KK et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. International Journal of Radiation Oncology*Biology*Physics 51, 571–578 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Harris JP et al. Association of Survival With Shorter Time to Radiation Therapy After Surgery for US Patients With Head and Neck Cancer. JAMA Otolaryngol Head Neck Surg 144, 349–359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumati V et al. Association between treatment delays and oncologic outcome in patients treated with surgery and radiotherapy for head and neck cancer. Head & Neck 41, 315–321 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Hosni A et al. Predictors of Early Recurrence Prior to Planned Postoperative Radiation Therapy for Oral Cavity Squamous Cell Carcinoma and Outcomes Following Salvage Intensified Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 103, 363–373 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Shintani SA et al. Utility of PET/CT Imaging Performed Early After Surgical Resection in the Adjuvant Treatment Planning for Head and Neck Cancer. International Journal of Radiation Oncology*Biology*Physics 70, 322–329 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Liao C-T et al. Impact of a second FDG PET scan before adjuvant therapy for the early detection of residual/relapsing tumours in high-risk patients with oral cavity cancer and pathological extracapsular spread. European Journal of Nuclear Medicine and Molecular Imaging 39, 944–955 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Dutta PR et al. Postoperative PET/CT and target delineation before adjuvant radiotherapy in patients with oral cavity squamous cell carcinoma. Head Neck 38, E1285–E1293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avkshtol V et al. Examining adjuvant radiation dose in head and neck squamous cell carcinoma. Head & Neck 41, 2133–2142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferlito A et al. Prognostic significance of microscopic and macroscopic extracapsular spread from metastatic tumor in the cervical lymph nodes. Oral Oncology 38, 747–751 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Poeta ML et al. TP53 Mutations and Survival in Squamous-Cell Carcinoma of the Head and Neck. 10.1056/NEJMoa073770 (2009). doi: 10.1056/NEJMoa073770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal DI et al. Early Postoperative Paclitaxel Followed by Concurrent Paclitaxel and Cisplatin With Radiation Therapy for Patients With Resected High-Risk Head and Neck Squamous Cell Carcinoma: Report of the Phase II Trial RTOG 0024. Journal of Clinical Oncology (2009). doi: 10.1200/JCO.2008.21.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]


