Abstract
Background: Hydrazides play a vital role in making biologically active compounds in various fields of chemistry. These determine antioxidant, antidepressant, antimalarial, anti-inflammatory, antiglycating, and antimicrobial activity. In the present study, twenty-three new N′ benzylidene-4-(tert-butyl)benzohydrazide derivatives (4–26) were synthesized by the condensation of aromatic aldehydes and commercially available 4-(tert-butyl)benzoic acid. All the target compounds were successfully synthesized from good to excellent yield; all synthesized derivatives were characterized via spectroscopic techniques such as HREI-MS and 1H-NMR. Synthesized compounds were evaluated for in vitro urease inhibition. All synthesized derivatives demonstrated good inhibitory activities in the range of IC50 = 13.33 ± 0.58–251.74 ± 6.82 µM as compared with standard thiourea having IC50 = 21.14 ± 0.425 µM. Two compounds, 6 and 25, were found to be more active than standard. SAR revealed that electron donating groups in phenyl ring have more influence on enzyme inhibition. However, to gain insight into the participation of different substituents in synthesized derivatives on the binding interactions with urease enzyme, in silico (computer simulation) molecular modeling analysis was carried out.
Keywords: synthesis, urease inhibition, 4-(tert-butyl)benzohydrazide, crystal structure, in vitro, SAR, molecular docking
1. Introduction
Urease (EC 3.5.1.5) belongs to the family of amidohydrolase enzymes that possesses two nickel atoms in its core structure. The conversion of urea into ammonia and carbamate is catalyzed by the action of this enzyme. The excessive amount of ammonia released due to the hyperactivity of the urease enzyme leads to the alkalinity of the stomach, which in turn increases the gastric mucosa permeability [1]. The nitrogen metabolism of cattle and various other animals is controlled by the action of the urease enzyme [2]. The elevated levels of these enzymes lead to several pathogenic conditions, such as it helps in the survival of some bacterial pathogens, thus leading to some severe side effects [3]. In humans, the low pH of the stomach facilitates the survival of Helicobacter pylori (HP) which leads to the development of gastric and peptic ulcers, which may eventually cause cancer [4]. The increased level of ammonia is also responsible for several metabolic disorders and destroys the GIT epithelium. A number of compounds belonging to different classes are reported as urease inhibitors, such as thiolates that bind with the nickel atom of the enzyme. Hydroxamic acid and its derivatives act as competitive inhibitors and compete with urea, phosphorodiamidates, and a few peptide chains having a ligand that may chelate with nickel of urease. Unfortunately, these molecules have adverse side effects associated with them. Therefore, it is of crucial importance to identify more urease inhibitors having significant stability, bioavailability, and low toxicity [5].
Day by day, the chemistry of carbon–nitrogen double bond of hydrazone is fastly becoming the backbone of condensation reaction in benzo-fused N-heterocycles. Hydrazides are an important class of functional groups in organic chemistry possessing -NH-N=CH- groups with the availability of proton that aids in their pharmaceutical importance. The remedial possibilities of acid hydrazides gained momentum after the innovation of isonicotinic acid hydrazide (INH). The remarkable clinical value of INH [6,7] stimulated the study of other heterocyclic hydrazides possessing mono-cyclic nuclei such as furan, pyrrole, thiophene, and dicyclic nuclei such as quinoline and isoquinoline.
Benzohydrazides are reported to possess a wide variety of biological activities such as antiglycation [8,9], antioxidant [10,11,12], antileishmanial [13], antibacterial [14], antifungal [15], antitumor [16], and anticonvulsant [17]. Benzothiazole Schiff bases have also been reported with various biological activities [18,19,20]. In search of potentially active compounds, we synthesized N′-benzylidene-4-(tert-butyl)benzohydrazide derivatives (4–26) and evaluated their in vitro urease inhibitory activity, and found good results. Further modifications and studies on these compounds might be helpful in bringing a number of leading compounds that may serve as potential drug candidates.
2. Result and Discussion
2.1. Chemistry
Twenty-three different N′-benzylidene-4-(t-Bu)benzohydrazide derivatives, (4–26) were synthesized by a routes outlined in Scheme 1. In the first step, methyl 4-tert-butylbenzoate (2) was synthesized from 4-(t-Bu)benzoic acid (1) by refluxing in methanol for two hours in the presence of concentrated H2SO4. Methyl 4-tert-butylbenzoate (2) was then reacted with hydrazine hydrate in methanol to give the corresponding hydrazide 3. The diversely substituted hydrazones (4–26) were obtained by refluxing different substituted aromatic aldehydes with 4-(tBu)benzohydrazide in the presence of methanol and glacial acetic acid with continuous stirring to get N′-benzylidene-4-(tert-butyl)benzohydrazide derivatives 4–26. TLC technique was used to monitor the reaction progress periodically. For the confirmation of newly synthesized benzohydrazide derivatives 4–26 (Table 1) [21], spectroscopic techniques such as 1H-, 13C-NMR, and HREI-MS were performed.
Scheme 1.
General scheme for the synthesis of N′-benzylidene-4-(t-Bu)benzohydrazide derivatives. (a) MeOH, H2SO4, reflux, 2 h, (b) NH2NH2, H2O, MeOH, reflux, 3–4 h, (c) Aromatic aldehydes, AcOH, MeOH, reflux, 4–6 h.
Table 1.
Ar groups of synthesized N′-benzylidene-4-(t-Bu)benzohydrazide derivatives (4–26).
| Compds. | Ar | Compds. | Ar | Compds. | Ar |
|---|---|---|---|---|---|
| 4 |
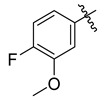
|
12 |
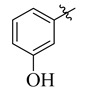
|
20 |
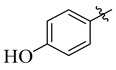
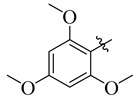
|
| 5 |
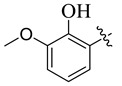
|
13 |
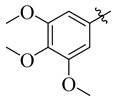
|
21 |
|
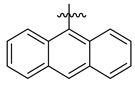
| 6 |
|
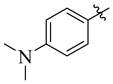
14 |
|
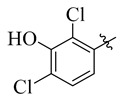
22 |
|
| 7 |
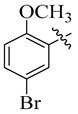
|
15 |
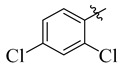
|
23 |
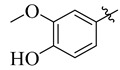
|
| 8 |
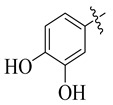
|
16 |
|
24 |
|
| 9 |
|
17 |
|
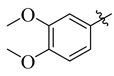
25 |
|
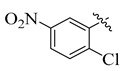
| 10 |
|
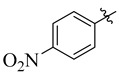
18 |
|
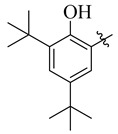
26 |
|
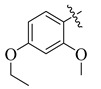
| 11 |
|
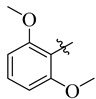
19 |
|
- | - |
2.2. Crystal Structure Description
The crystal structure of 4-tert-butyl-N′-[(E)-(4-fluoro-3-methoxyphenyl)methylidene]benzohydrazide (4+) has been reported previously [22].
2.2.1. Crystal Structure of 4-(tBu)-N′-(2-Hydroxy-3-methoxybenzylidene)benzohydrazide (5)
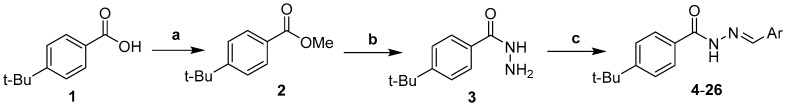
The 4-(tBu)-N′-(2-hydroxy-3-methoxybenzylidene)benzohydrazide (5) crystallized in the monoclinic, Cc space group. The asymmetric unit of 5 is shown in Figure 1. The crystal data of 5 is shown in Table 2, whereas the selected bond lengths and angles are shown in Table 3. The O3-C8 bond length is negligibly shorter than a similar bond length in compound 14. Similarly, the N-N bond length in compound 5 is 1.364 Å, comparably shorter than a similar bond length in 14. The reason coined for this effect is the substitution of hydroxyl and methoxy groups at ortho and meta (C1 and C2) positions of the benzene ring. Similar behavior is observed in bond lengths as well. Therefore, we will restrict the crystal description to 14 only.
Figure 1.
Molecular structure of 4-(t-Bu)-N′-(2-hydroxy-3-methoxybenzylidene)benzohydrazide (5).
Table 2.
Crystal and refinement data of complexes 5 and 14.
| Complex | 5 | 14 |
|---|---|---|
| Empirical formula | C19H24N2O4 | C20H25N3O |
| Formula mass | 344.40 | 323.43 |
| Temperature (K) | 170 K | 170 K |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system, space group | Monoclinic, Cc | Monoclinic, P21/n |
| Unit cell dimensions (Å) | a = 7.1944 (14) Å, b = 41.282 (8) Å, c = 6.4595 (13) Å, β = 104.83 (3)° | a = 12.408 (3) Å, b = 8.5968 (17) Å, c = 35.223 (7) Å |
| Z, Volume (Å3) | 1854.6 (7) | 3757.2 (14) |
| Crystal size (mm) | 0.48 × 0.47 × 0.10 | 0.48 × 0.17 × 0.12 |
| Calculated density (Mg m−3) | 1.233 | 1.144 |
| Absorption coefficient (mm−1 ) |
0.09 | 0.07 |
| F(000) | 736 | 1392 |
| Θ range for data collection | 6.5–58.9° | 6.2–59.1° |
| Limiting indices |
h = −9→9, k = −56→47, l = −8→8 |
h = −13→13, k = −9→9, l = −38→38 |
| Measured reflections | 10166 | 20065 |
| Independent reflections | 4887 | 2509 |
| Reflections with I > 2σ(I) | 3476 | 1888 |
| Rint | 0.105 | 0.247 |
| Refinement method | Full-matrix least-squares on F2 |
Full-matrix least-squares on F2 |
| Data/restraints/ parameters | 4887/246/3 | 2509/0/230 |
| R[F2 > 2σ(F2)] | 0.072 | 0.079 |
Table 3.
Selected bond lengths (Å) and bond angles (°) of 4-(t-Bu)-N′-(2-hydroxy-3-methoxybenzylidene)benzohydrazide (5).
| Moiety | Bond Length, Å | Moiety | Bond Length, Å |
| O1—C1 | 1.356 (4) | N1—C7 | 1.284 (5) |
| O2—C2 | 1.368 (4) | N1—N2 | 1.364 (4) |
| O2—C19 | 1.413 (5) | N2—C8 | 1.349 (5) |
| O3—C8 | 1.230 (5) | C8—C9 | 1.492 (6) |
| C6—C7 | 1.451 (5) | C12—C15 | 1.528 (6) |
| Moiety | Bond Angle, ° | Moiety | Bond Angle, ° |
| C2—O2—C19 | 116.6 (3) | N1—C7—C6 | 119.9 (3) |
| C7—N1—N2 | 117.0 (3) | O3—C8—N2 | 121.6 (4) |
| C8—N2—N1 | 118.4 (3) | O3—C8—C9 | 122.1 (4) |
| O1—C1—C6 | 123.1 (3) | N2—C8—C9 | 116.3 (3) |
| O1—C1—C2 | 117.4 (3) | C14—C9—C10 | 117.4 (4) |
| O2—C2—C3 | 125.2 (4) | C14—C9—C10 | 117.4 (4) |
| O2—C2—C1 | 114.1 (3) | C1—C6—C7 | 121.2 (3) |
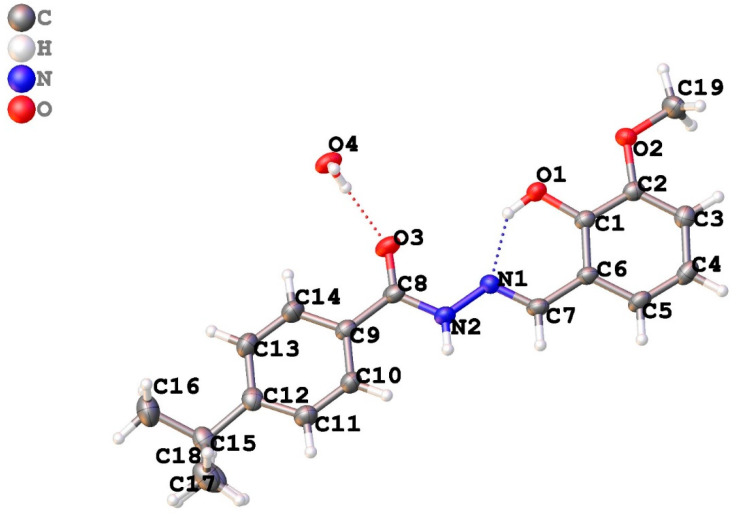
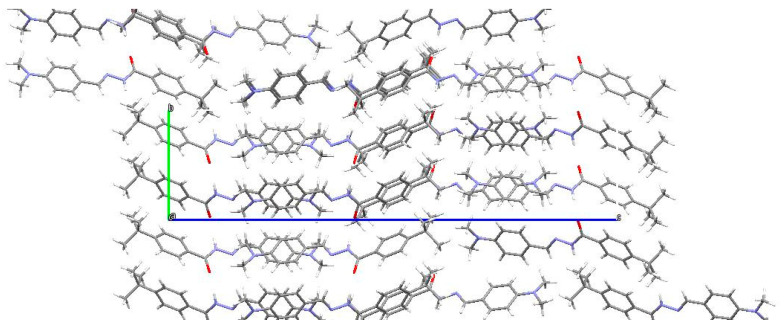
The hydrogen bonding is unique in 5, as shown in Table 4. Overall, there is a single intramolecular hydrogen bonding interaction shown by O1—H1O···N1 with a length of 2.581 (4) Å showing strong interaction. Similarly, there are intermolecular hydrogen bonds as well, which lead to the accumulation of a co-crystallized water molecule in the crystal lattice. The hydrogen bonding interaction is shown in Table 4. The crystal packing diagram of 5 is shown in Figure 2.
Table 4.
Hydrogen-bond geometry with selected bond lengths (Å) and bond angles (°).
| D—H···A | d(D—H) | d(H···A) | d(D···A) | ∠ (D—H···A) |
|---|---|---|---|---|
| O1—H1O···N1 | 0.87 (6) | 1.84 (6) | 2.581 (4) | 142 (6) |
| N2—H2N···O4 i | 0.85 (7) | 2.00 (7) | 2.808 (4) | 160 (6) |
| C7—H7···O4 i | 0.95 | 2.47 | 3.250 (5) | 139 |
| C19—H19A···O2 ii | 0.98 | 2.57 | 3.259 (6) | 127 |
| O4—H4O···O3 | 0.81 (5) | 1.89 (5) | 2.696 (4) | 174 (6) |
| O4—H4P···O1 iii | 0.84 (5) | 2.19 (6) | 2.967 (4) | 154 (6) |
| O4—H4P···O2 iii | 0.84 (5) | 2.42 (6) | 3.025 (4) | 130 (6) |
Symmetry codes: (i) x + 1, y, z; (ii) x, −y + 1, z−1/2; (iii) x, y, z + 1.
Figure 2.
Crystal packing diagram of 4-(t-Bu)-N′-(2-hydroxy-3-methoxybenzylidene)benzohydrazide (5).
2.2.2. Crystal Structure of 4-Tert-butyl-N′-{(E)-[4-(dimethylamino)phenyl]methylidene}benzohydrazide (14)
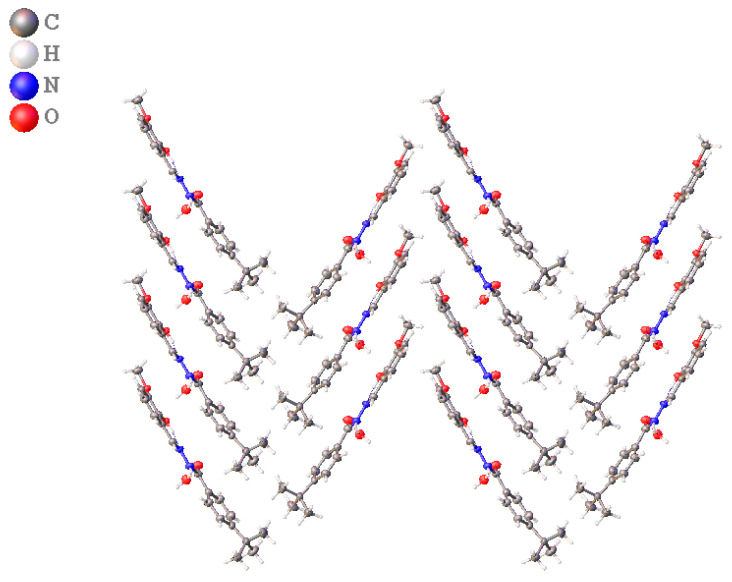
The resulting hydrazone was crystallized in a monoclinic Pbca space group with only one molecule in the asymmetric unit, as shown in Figure 3. The bond lengths and bond angles of the molecule fall within the expected range for similar molecules [22,23,24,25,26,27,28,29,30,31,32]. The crystal data for the compound is given in Table 2, whereas the bond lengths and the reported ranges are given in Table 5. It is apparent from the table that the bond length for the hydrazide moiety N2–N3 is 1.395 (3) Å, for the Schiff base linkage N2–C9 is 1.260 (4) Å, and for the N3–C10 is 1.332 (4) [24,25,26,27,28,29,30,31,32,33,34]. The two phenyl rings connected by hydrazide moiety are rather twisted in comparison with other related examples. The twist is identified from the 15.46 torsion between the two phenyl planes. The value is lying on the coplanar side of the range (4.74°) reported earlier for similar compounds. The connecting hydrazide moiety along the Schiff base imine group (O1–C10–N3–N2–C9) produces a plane lying at the 11.13° and 23.21° to the planes produced by C5–C4–C3–C8–C7–C6 and C14–C13–C12–C11–C16–C15, respectively. The torsion in planes is apparently influenced by the crystal packing effects and hydrogen bonding. The crystal lattice of the hydrazide is marked by the strong hydrogen bonding (Table 6) of D···A = 2.939 (3)Å {N3—H1N3···O1i} and D···A = 3.322 (4) Å { C9—H9···O1i}. The crystal packing diagram of the compound is shown in Figure 4.
Figure 3.
Molecular Structure of compound 14.
Table 5.
Selected bond lengths (Å) and bond angles (°) of compound 14.
| Moiety | Bond Length, Å | Moiety | Bond Length, Å |
| N1—C3 | 1.386 (4) | N2—N3 | 1.395 (3) |
| N1—C2 | 1.434 (5) | N3—C10 | 1.332 (4) |
| N1—C1 | 1.437 (6) | N3—H1N3 | 0.83 (3) |
| N2—C9 | 1.260 (4) | O1—C10 | 1.237 (3) |
| Moiety | Bond Angle, ° | Moiety | Bond Angle, ° |
| C3—N1—C2 | 119.2 (4) | N1—C3—C4 | 121.9 (3) |
| C3—N1—C1 | 119.9 (3) | C8—C3—N1 | 120.4 (3) |
| C2—N1—C1 | 118.3 (3) | N2—C9—C6 | 122.9 (3) |
| C9—N2—N3 | 114.4 (2) | N2—C9—H9 | 123.3 (19) |
| C10—N3—N2 | 120.7 (2) | O1—C10—N3 | 122.9 (2) |
| C10—N3—H1N3 | 127 (2) | O1—C10—C11 | 120.7 (2) |
| N2—N3—H1N3 | 113 (2) | N3—C10—C11 | 116.4 (2) |
| N1—C1—H1A | 109.5 | N1—C1—H1B | 109.5 |
Table 6.
Hydrogen-bond geometry with bond lengths (Å) and bond angles (°).
| D—H···A | d(D—H) | d(H···A) | d(D···A) | ∠(D—H···A) |
|---|---|---|---|---|
| N3—H1N3···O1 i | 0.83 (3) | 2.12 (3) | 2.939 (3) | 169 (3) |
| C9—H9···O1 i | 0.90 (3) | 2.57 (3) | 3.322 (4) | 142 (2) |
Symmetry code: (i) −x + 3/2, y − 1/2, z.
Figure 4.
Crystal packing diagram of compound 14.
2.3. In Vitro Urease Inhibitory Activity
In vitro urease, inhibition was detected for the synthesized N′-benzylidene-4-(t-Bu) benzohydrazide derivatives (4–26), which indicated inhibition in the range of IC50 = 13.33 ± 0.58 µM to IC50 = 251.74 ± 6.82 µM as compared with standard thiourea IC50 = 21.14 ± 0.425 µM (Table 7).
Table 7.
In vitro urease inhibitory activity of synthesized N′-benzylidene-4-(t-Bu)benzohydrazide derivatives (4–26).
| Compounds | IC50 ± SEM (µM) | Compounds | IC50 ± SEM (µM) |
|---|---|---|---|
| 4 | 88.75 ± 7.71 | 16 | 79.01 ± 7.53 |
| 5 | 56.57 ± 3.18 | 17 | 27.45 ± 1.65 |
| 6 | 13.33 ± 0.58 | 18 | 66.14 ± 1.79 |
| 7 | 64.50 ± 3.97 | 19 | 67.48 ± 4.16 |
| 8 | 80.01 ± 7.43 | 20 | 51.41 ± 11.50 |
| 9 | 63.50 ± 3.97 | 21 | 71.27 ± 2.47 |
| 10 | 63.54 ± 3.97 | 22 | 38.57 ± 11.54 |
| 11 | 63.52 ± 2.97 | 23 | 251.74 ± 6.82 |
| 12 | 39.57 ± 11.54 | 24 | 37.47 ± 12.54 |
| 13 | 80.93 ± 7.43 | 25 | 13.42 ± 0.33 |
| 14 | 81.21 ± 7.4 | 26 | 49.47 ± 12.74 |
| 15 | 82.21 ± 7.43 | Thiourea | 21.14 ± 0.42 |
SEM is the standard error of the mean, thiourea = standard for urease inhibition activity.
2.4. Structure-Activity Relationship (SAR)
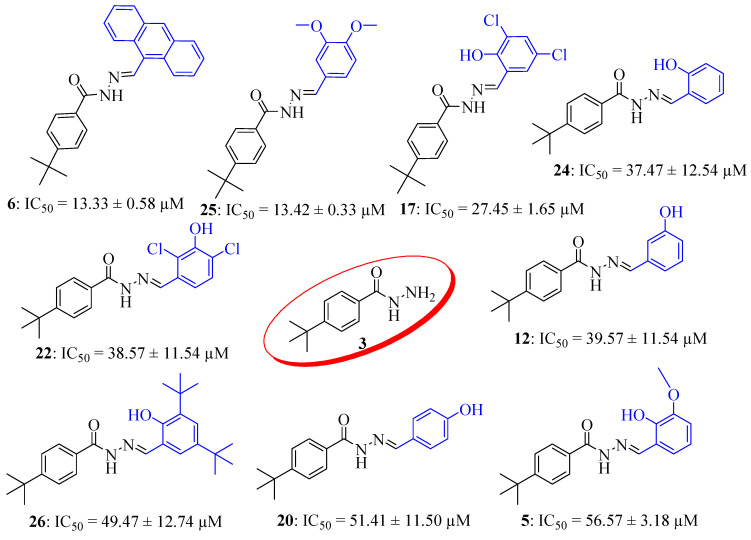
To explain the structure–activity relationship (SAR) of these analogs, we have classified the top eight compounds of the series into two groups according to their nature of substituents. Limited SAR study was established for synthesized derivatives 4–26. The most active compounds among the series were compounds 6 and 25, with IC50 values of 13.33 ± 0.58 and 13.42 ± 0.33 µM when compared with standard thiourea (IC50 = 21.14 ± 0.425 µM) (Table 7). The potency of compounds 6 and 25 might be due to the presence of anthracene and di-methoxy moiety, respectively. In both cases, the substituents are electron rich in nature. Compounds 17, 24, 22, 12, 26, 20, and 5 are the second group of active compounds having hydroxyl groups as substituents. The activity of these analogs might be due to the hydroxyl group (Figure 5). It seems that the remaining compounds showed much lower activity than the standard. Results of the enzyme inhibition showed that the nature of the substituents played an important role in enhancing the inhibition potential of the core structure. Apart from it, both compounds 5 and 14 studied crystallographically also reveal activity. Compound 5 with IC50 = 56.57 ± 3.18 µM is revealing better activity than the compound 14 (IC50 = 81.21 ± 7.4 µM). The possible explanation for this behavior may be linked to the presence and absence of methoxy moiety. Compound 5 revealed almost identical activity to other similar analogs such as 20. Both the derivatives possess ortho-substituted hydroxyl group, which is involved in hydrogen bonding with hydrazide moiety, as may be seen in the crystal structure of compound 5.
Figure 5.
Active inhibitors of the synthesized series.
2.5. Molecular Docking, Interactions Report
To predict the inhibition mechanism of isolated compounds shown by the kinetics study, molecular docking of the active compounds was carried out with the crystal structure of the urease enzyme. The most favorable docking conformations of all compounds were observed inside the active site with proper orientation. The active site consists of both the hydrophobic and hydrophilic amino acids.
The hydrophilic amino acids included Glu166, 223, Arg339, His323, 324, Asp224, 363, and Asp 494, while the hydrophobic part was composed of Lys169, Ala170, 366, Leu319, Cys322, and Met 637. The two Ni ions also played a vital role by linking the key amino acid and ligands. It has been observed that almost all the conformations of all the ligands showed interactions with key residues inside the pocket. The docked poses were ranked by the scores from the GBVI/WSA binding free energy calculation. The most promising docked conformation of each compound was further evaluated for binding mode. Detailed docking results are listed in Table 8.
Table 8.
Docking scores (S) and Interactions Detail of the selected active compounds (9–11, 16, 18, 19, 22, 24, and 25).
| Compds. | Docking Score | Interactions Details | ||||
|---|---|---|---|---|---|---|
| Ligand | Receptor | Interaction | Distance (Å) | E (kcal/mol) | ||
| 9 | −3.05 | C23 | 5-ring-HIS324 | π-H | 3.83 | −0.8 |
| 6-ring | NZ-LYS169 | π-cation | 4.01 | −1.4 | ||
| 10 | −5.18 | N9 | SG-CYS322 | HBD | 3.13 | −3.5 |
| O24 | NI-NI798 | metal | 1.63 | −2.0 | ||
| 6-ring | CB-MET367 | π-H | 4.38 | −0.6 | ||
| 11 | −4.14 | 6-ring | CD-LYS169 | π-H | 4.32 | −0.8 |
| 16 | −4.71 | CL9 | NE2-HIS137 | HBD | 3.65 | −1.3 |
| 6-ring | CD-LYS169 | π-H | 4.14 | −0.9 | ||
| 18 | −12.09 | C3 | OD2-ASP363 | HBD | 3.30 | −1.7 |
| O23 | NI-NI798 | metal | 1.92 | −2.9 | ||
| O24 | NI-NI799 | metal | 1.89 | −2.4 | ||
| 19 | −3.03 | N8 | SG-CYS322 | HBD | 4.09 | −0.8 |
| 22 | −2.81 | CL7 | OD2-ASP363 | HBD | 2.73 | 1.2 |
| 24 | −8.65 | O7 | OD2-ASP363 | HBD | 2.45 | 0.3 |
| 6-ring | CD-LYS169 | π-H | 4.11 | −0.7 | ||
| 25 | −10.07 | N8 | O-CYS322 | HBD | 2.68 | −2.1 |
| O24 | NI-NI 798 | metal | 1.79 | −1.8 | ||
| 6-ring | CD-LYS169 | π-H | 4.23 | −0.8 | ||
| 6-ring | NE2-HIS222 | π-H | 4.18 | −0.6 | ||
The binding mode of the active compounds with the active site residues showed a healthy assignation with the backbone of the enzyme using hydrogen bonds, polar bonds, pi-pi, and pi-H interactions. Three-dimensional interactions of some favorable inhibitors are shown in Figure 6. This showed that ligands occupy the active site residues, using hydrogen bonds, polar bonds, arene-cation, and metal ion interactions to engage the backbone of the enzyme tightly.
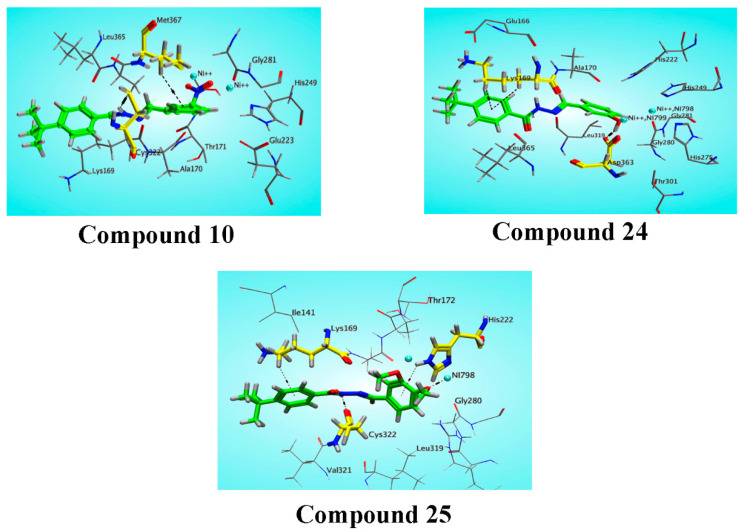
Figure 6.
Three dimensional interaction of compounds 10, 24, 25, with active site residues of urease. Ligands are shown in green, key residues of the active site are shown in yellow stick mode, hydrogen bonding and other interaction is shown in dark color dotted lines.
In the case of compound 10, the chloronitrotoluene part, NO2 and NH are found actively interacting with the active site residues Cys 322, Met 367, and Ni 798 through Hydrogen bonding, π-Hydrogen, and metal ion interactions, respectively. The phenolic substituted derivative 24 is observed with lesser interactions compared with the chloro-nitrobenzene derivative 10 by performing two interactions with Lys 169 and Asp 363. Asp 363 established a Hydrogen donor interaction with the OH group of the ligand while Lys 169 formed the H-π interaction with the aromatic π system of the ligand. The hydrazine moiety presented an inert behavior that might be attributed to the negative inductive effect of these groups.
The dimethoxy substituted derivative, compound 25, showed better interactions and docking scores in accordance with the biological activity. Figure 5 reflects that hydrazine moiety in compound 25 performed Hydrogen bonding with Cys 322, and the substituted methoxy group established metal/ionic contact with Ni 798 of the target protein. Moreover, the two aromatic rings pi system interact through π-H bonding to Lys 169 and His 222.
The docking study complemented the experimental results based on the multiple interactions of ligands with key residues of the urease enzyme. The docking pose of the most active compounds, 10, 24, and 25, inhibited the catalytic activities of the urease by binding firmly through strong hydrogen bonding, hydrophobic, polar interactions with key residues, and metal/ion contact.
The molecular docking study of these compounds revealed that the ligands with polar, light, and electron-rich groups such as hydroxyl, alkoxy, and nitro groups showed better interaction mode and high docking scores against the target protein and therefore had good inhibitory activities. On the other hand, ligands with electron-withdrawing groups, non-polar such as methyl or benzene, and bulky groups have shown poor interactions and low docking scores.
3. Experimental
3.1. Materials and Methods
NMR experiments were performed on Avance-Bruker (400.15 MHz for 1H). The mass spectra were recorded by HRESI-MS spectrometer (LCQ-DECA XP Plus, Thermo-Finnigan, San Diego, CA, USA). Thin layer chromatography (TLC) was performed on pre-coated silica gel aluminum plates (Kieselgel 60, 254, E. Merck, Darmstadt, Germany). Chromatograms were visualized by UV visible light at 254 nm or iodine vapors.
Highly pure analytical grade chemicals and solvents were procured from (Darmstadt, Germany) and used as received. Aryl benzaldehyde derivatives and 4-(t-Bu)benzoic acid were purchased from Sigma (St Louis, MO, USA). Sodium hydroxide, soluble starch, maltose, and other chemicals were obtained from Merck (Darmstadt, Germany).
3.1.1. General Procedure for the Synthesis of Methyl-4-(t-Bu)benzoate (2) and 4-(Tert-butyl) benzohydrazide (3)
4-(tert-butyl)benzoic acid 1 (2.5 g, 0.014 mmol) was suspended in methanol (25 mL), and concentrated sulphuric acid (H2SO4) (1.2 mL) was added to the flask, and refluxed for 2 h. Upon completion, the reaction mixture was poured into a beaker containing ice-cold distilled water and neutralized the mixture with sodium bicarbonate. Extracted with organic solvent (chloroform) to obtain 4-(tert-butyl)benzoic acid 2. It was then recrystallized with methanol. Methyl 4-tert-butylbenzoate 2 was refluxed with hydrazine hydrate (1.5 eqv.) in methanol (10 mL) for 3–4 h to obtain the 4-tert-butylbenzohydrazide 3. After the reaction, precipitates were formed, filtered, washed with distilled water, dried under a vacuum, and collected.
3.1.2. General Procedure for the Synthesis of N-Acylhydrazones 4-(t-But)benzohydrazide (4–26)
Twenty-three different novel hydrazones (4–26) of 4-(t-But)benzohydrazide 3 were synthesized in good to excellent yields. In a typical reaction, 4-(tert-butyl)benzohydrazide 3 (0.192 g, 0.001 mmol) was dissolved in methanol (10 mL) containing a catalytic amount of acetic acid, then various substituted aromatic aldehydes (0.001 mmol) were added to the flask and refluxed it for 4–6 h. The reaction mixture was decanted into a beaker containing crushed ice. Precipitates were formed, filtered, and washed with an excess of water and hot n-hexane, and the products were dried and collected [21]. All the synthesized derivatives were characterized by HRESI-MS and 1H-NMR spectroscopy. Furthermore, we have already reported the crystal structure of compound 4 [22].
3.2. Urease Inhibition Assay
The measurement of urease inhibitory activity was carried out according to the literature method [33]. The assay mixture containing 75 μL of Jack bean urease and 75 μL of tested compounds with various concentrations (dissolved in DMSO) was pre-incubated for 15 min on a 96-well assay plate. Acetohydroxamic acid was used as a reference. Then 75 μL of phosphate buffer at pH 6.8 containing phenol red (0.18 mmol·L−1) and urea (400 mmol·L−1) were added and incubated at room temperature. The reaction time required for enough ammonium carbonate to form to raise the pH phosphate buffer from 6.8 to 7.7 was measured by a microplate reader (560 nm), with the end-point being determined by the color change of the phenol-red indicator.
3.3. Docking Methodology
A molecular docking study was performed according to the reported methodology [34] to predict the binding mode of the active synthesized compounds in the active site of the urease enzyme using MOE (Molecular Operating Environment). The three-dimensional structures of the isolated compounds were built using the builder tool implemented in MOE software. The generated compounds were 3D protonated, and energy minimized using the default parameters of the MOE (gradient: 0.05, Force Field: MMFF94X). 3D structure of the target protein was retrieved from the protein databank (https://www.rcsb.org/ accessed on 4 August 2022) (PDB ID 4ubp), the solvent molecules were removed, and 3D protonation was carried out. To get a stable conformation of the protein molecule, 3D protonation of the protein was energy minimized using the default parameters of MOE. For docking studies, the parameters of MOE used were Placement: Triangle Matcher, Rescoring 1: London dG, Refinement: Forcefield, Rescoring 2: GBVI/WSA. For each ligand, 10 conformations were allowed to be formed, and the top-ranked conformations on the basis of docking score were selected for further analysis.
3.4. Crystal Structure Determination
Crystals 1, 5, and 14 were mounted on a glass fiber in inert paraffin oil. Data were recorded at 170 K on an STOE-IPDS 2T diffractometer with graphite-monochromated Mo-Kα-radiation (λ = 0.71073 Å). The program XArea was used to integrate diffraction profiles; numerical absorption corrections were carried out with the programs X-Shape and X-Red32, all from STOE© version 2010. The structures were solved by dual space methods (SHELXT-2016) [35] and refined by full-matrix least-squares techniques using the WingX GUI [36] and SHELXL-2018 [37]. All non-hydrogen atoms were refined with anisotropic displacement parameters. The hydrogen atoms were refined isotropically on calculated positions using a riding model with their Uiso values constrained to 1.5 Ueq of their pivot atoms for terminal sp3 carbon atoms and 1.2 times for all other carbon atoms.
Crystallographic data were deposited with the Cambridge Crystallographic Data Centre, CCDC, 12 Union Road, Cambridge CB21EZ, UK. These data can be obtained free of charge by quoting the depository numbers CCDC 2166390 (compound 5) and 1861240 (compound 14) by FAX (+44-1223-336-033), email (deposit@ccdc.cam.ac.uk) or their web interface (http://www.ccdc.cam.ac.uk accessed on 4 August 2022).
3.5. Analytical Physical and Spectroscopic Data of the Synthesized Compounds
3.5.1. 4-(Tert-butyl)-N′-(4-fluoro-3-methoxybenzylidene)benzohydrazide (4)
Yield: (72%), Crystal; Rf: 0.51 (n-hexane/ethyl acetate (2.5:7.5)); 1H NMR (400 MHz DMSO-d6) δ 1.39 (s, 9H), 3.89 (s, 3H), 6.98 (d, J = 7.5 Hz, 1H), 7.27 (d, J = 7.5 Hz, 1H), 7.47 (d, J = 7.0 Hz, 2H), 7.87 (d, J = 7.0 Hz, 2H), 8.12 (m, 1H), 8.58 (s, 1H), 11.68 (s, 1H); HRESI-MS [M + H]+ calculated for C19H21FN2O2, 328.1587, found 328.1598.
3.5.2. 4-(t-But)-N′-(2-Hydroxy-3-methoxybenzylidene)benzohydrazide (5)
Yield: (82%), Cream color; Rf: 0.52 (n-hexane/ethyl acetate (2.0:8.0)); 1HNMR (400 MHz DMSO-d6) δ 1.21 (s, 9H), 3.84 (s, 3H), 6.96 (d, J = 7.2 Hz, 1H), 7.19 (t, J = 7.2 Hz, 1H), 7.25 (d, J = 7.2 Hz, 1H), 7.43 (d, J = 7.0 Hz, 2H), 7.82 (d, J = 7.0 Hz, 2H), 8.53 (s, 1H), 10.55 (br.s, 1H), 11.58 (s, 1H); HRESI-MS [M + H]+ calculated for C19H22N2O3, 326.1630, found 326.1643.
3.5.3. N′-(Anthracen-9-ylmethylene)-4-(tert-butyl)benzohydrazide (6)
Yield: (78%), Color: Light blue; Rf: 0.50 (n-hexane/ethyl acetate (2.5:7.5)); 1HNMR (400 MHz DMSO-d6) δ 1.28 (s, 9H), 7.34 (t, J = 7.5 Hz, 4H), 7.49 (d, J = 7.2 Hz, 2H), 7.68–7.70 (m, 4H), 7.81 (d, J = 7.2 Hz, 2H), 8.07 (s, 1H), 8.53 (s, 1H), 11.61 (s, 1H); HRESI-MS [M + H]+ calculated for C26H24N2O, 380.1889, found 380.1897.
3.5.4. N′-(5-Bromo-2-methoxybenzylidene)-4-(t-But)benzohydrazide (7)
Yield: (85%), Light brown color; Rf: 0.50 (n-hexane/ethyl acetate (2.5:7.5)); 1HNMR (400 MHz DMSO-d6) δ 1.25 (s, 9H), 3.81 (s, 3H), 6.89 (d, J = 7.0 Hz, 1H), 7.24 (d, J = 7.0 Hz, 1H), 7.41 (d, J = 7.5 Hz, 2H), 7.84 (d, J = 7.5 Hz, 2H), 7.96 (s, 1H), 8.50 (s, 1H), 11.54 (s, 1H); HRESI-MS [M + H]+ calculated for C19H21BrN2O2, 388.0786 found 388.0793.
3.5.5. N′-(3,4-Dihydroxybenzylidene)-4-tert-butylbenzohydrazide (8)
Yield: (70%), Cream color; Rf: 0.50 (n-hexane/ethyl acetate (2.0:8.0)); 1HNMR (400 MHz DMSO-d6) δ 1.26 (s, 9H), 6.80 (d, J = 7.1 Hz, 1H), 7.11 (d, J = 7.1 Hz, 1H), 7.48 (d, J = 7.5 Hz, 2H), 7.89 (d, J = 7.5 Hz, 2H), 8.48 (s, 1H), 10.54 (br.s, 1H), 10.82 (br.s, 1H), 11.62 (s, 1H); HRESI-MS [M + H]+ calculated for C18H20N2O3, 312.1474 found 312.1490.
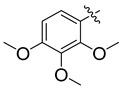
3.5.6. N′-(2,3,4-Trimethoxybenzylidene)-4-tert-butylbenzohydrazide (9)
Yield: (75%), Cream color; Rf: 0.54 (n-hexane/ethyl acetate (2.5:7.5)); 1H NMR (400 MHz DMSO-d6) δ 1.39 (s, 9H), 3.63 (s, 9H), 6.85 (d, J = 7.0 Hz, 1H), 7.17 (d, J = 7.0 Hz, 1H), 7.44 (d, J = 7.0 Hz, 2H), 7.81 (d, J = 7.0 Hz, 2H), 8.50 (s, 1H), 11.68 (s, 1H); HRESI-MS [M + H]+ calculated for C21H26N2O4, 370.1942, found 370.1967.
3.5.7. 4-(Tert-butyl)-N′-(2-chloro-5-nitrobenzylidene)benzohydrazide (10)
Yield: (88%), Cream color; Rf: 0.50 (n-hexane/ethyl acetate (2.5:7.5)); 1H NMR (400 MHz DMSO-d6) δ 1.26 (s, 9H), 7.48 (d, J = 7.5 Hz, 2H), 7.58 (d, J = 7.5 Hz, 2H), 7.86 (d, J = 9.0 Hz, 1H), 8.48 (s, 1H), 8.61 (d, J = 9.0 Hz, 1H), 8.74 (d, J = 1.5 Hz, 1H), 11.62 (s, 1H); HRESI-MS [M + H]+ calculated for C18H18ClN3O3, 359.1037, found 359.1087.
3.5.8. 4-(Tert-butyl)-N′-(4-ethoxy-2-methoxybenzylidene)benzohydrazide (11)
Yield: (78%), Cream color; (n-hexane/ethyl acetate (2.5:7.5)); Rf: 0.53 (N-Hexane/Ethylacetate 25:75); 1HNMR (400 MHz DMSO-d6) δ 1.41 (s, 9H), 2.01 (t, J = 6.8 Hz, 3H), 3.86 (q, J = 6.8 Hz, 2H), 3.89 (s, 3H), 6.68 (d, J = 1.0 Hz, 1H), 6.97 (d, J = 7.0 Hz, 1H), 7.28 (d, J = 7.0 Hz, 1H), 7.44 (d, J = 7.5 Hz, 2H), 7.87 (d, J = 7.5 Hz, 2H), 8.45 (s, 1H), 11.63 (s, 1H); HRESI-MS [M + H]+ calculated for C21H26N2O3, 354.1943, found 354.1952.
3.5.9. 4-(Tert-butyl)-N′-(3-hydroxybenzylidene)benzohydrazide (12)
Yield: (88%), Light brownish color; Rf: 0.52 (n-hexane/ethyl acetate (2.5:7.5)); 1HNMR (400 MHz DMSO-d6) δ 1.38 (s, 9H), 6.68 (d, J = 1.0 Hz, 1H), 6.92 (d, J = 7.2 Hz, 1H), 6.99 (t, J = 7.2 Hz, 1H), 7.18 (d, J = 7.2 Hz, 1H), 7.42 (d, J = 8.0 Hz, 2H), 7.88 (d, J = 8.0 Hz, 2H), 8.41 (s, 1H), 10.57 (s, 1H), 11.59 (s, 1H); HRESI-MS [M + H]+ calculated for C18H20N2O2, 296.1525, found 296.1576.
3.5.10. 4-(Tert-butyl)-N′-(3,4,5-trimethoxybenzylidene)benzohydrazide (13)
Yield: (80%), Light yellow color; Rf: 049 (n-hexane/ethyl acetate (2.0:8.0)); 1HNMR (400 MHz DMSO-d6) δ 1.31 (s, 9H), 3.76 (s, 9H), 6.62 (d, J = 1.5 Hz, 2H), 7.40 (d, J = 7.5 Hz, 2H), 7.83 (d, J = 7.5 Hz, 2H), 8.48 (s, 1H), 11.64 (s, 1H); HRESI-MS [M + H]+ calculated for C21H26N2O4, 370.1893, found 370.1923.
3.5.11. 4-(Tert-butyl)-N′-(4-(dimethylamino)benzylidene)benzohydrazide (14)
Yield: (85%), Cream color; Rf: 0.50 (n-hexane/ethyl acetate (2.0:8.0)); 1HNMR (400 MHz DMSO-d6) δ 1.34 (s, 9H), 2.76 (s, 6H), 6.94 (d, J = 7.0 Hz, 2H), 7.38 (d, J = 7.6 Hz, 2H), 7.52 (d, J = 7.6 Hz, 2H), 7.89 (d, J = 7.0 Hz, 2H), 8.38 (s, 1H), 11.36 (s, 1H); HRESI-MS [M + H]+ calculated for C20H25N3O, 323.1998, found 323.2022.
3.5.12. 4-(Tert-butyl)-N′-(2,4-dichlorobenzylidene)benzohydrazide (15)
Yield: (90%), Cream color; Rf: 0.54 (n-hexane/ethyl acetate (2.5:7.5)); 1HNMR (400 MHz DMSO-d6) δ 1.42 (s, 9H), 6.68 (d, J = 1.0 Hz, 1H), 7.12 (d, J = 7.1 Hz, 1H), 7.25 (d, J = 1.2 Hz, 1H), 7.41 (d, J = 7.5 Hz, 2H), 7.57 (d, J = 7.1 Hz, 1H), 7.86 (d, J = 7.5 Hz, 2H), 8.39 (s, 1H), 11.53 (s, 1H); HRESI-MS [M + H]+ calculated for C18H18Cl2N2O, 348.0796, found 348.0875.
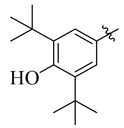
3.5.13. 4-(Tert-butyl)-N′-(3,5-di-tert-butyl-4-hydroxybenzylidene)benzohydrazide (16)
Yield: (78%), Cream color; Rf: 0.52 (n-hexane/ethyl acetate (2.5:7.5)); 1HNMR (400 MHz DMSO-d6) δ 1.37 (s, 9H), 1.38 (s, 18H), 7.21 (d, J = 1.5 Hz, 2H), 7.47 (d, J = 7.5 Hz, 2H), 7.82 (d, J = 7.5 Hz, 2H), 8.54 (s, 1H), 10.62 (s, 1H), 11.55 (s, 1H); HRESI-MS [M + H]+ calculated for C26H36N2O2, 408.2777, found 408.2794.
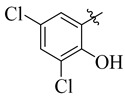
3.5.14. N′-(3,5-Dichloro-2-hydroxybenzylidene)-4-tert-butylbenzohydrazide (17)
Yield: (80%), Light yellow color; Rf: 0.52 (n-hexane/ethyl acetate (2.5:7.5)); 1HNMR (400 MHz DMSO-d6) δ 1.31 (s, 9H), 7.16 (d, J = 1.0 Hz, 1H), 7.23 (d, J = 1.0 Hz, 1H), 7.42 (d, J = 7.5 Hz, 2H), 7.88 (d, J = 7.5 Hz, 2H), 8.50 (s, 1H), 10.60 (s, 1H), 11.51 (s, 1H); HRESI-MS [M + H]+ calculated for C18H18Cl2N2O2, 364.1058, found 364.1069.
3.5.15. N′-(4-Nitrobenzylidene)-4-tert-butylbenzohydrazide (18)
Yield: (88%), Cream color; Rf: 0.53 (n-Hexane/ethyl acetate (3.0:7.0); 1HNMR (400 MHz DMSO-d6) ) δ 1.34 (s, 9H), 7.44 (d, J = 7.5 Hz, 2H), 7.82 (d, J = 7.5 Hz, 2H), 8.01 (d, J = 9.0 Hz, 2H), 8.52 (s, 1H), 8.56 (d, J = 9.0 Hz, 2H), 11.39 (s, 1H); HRESI-MS [M + H]+ calculated for C18H19N3O3, 325.1739, found 325.1752.
3.5.16. 4-(Tert-butyl)-N′-(2,6-dimethoxybenzylidene)benzohydrazide (19)
Yield: (65%), Cream color; Rf: 0.50 (n-hexane/ethyl acetate (3.0:7.0)); 1HNMR (400 MHz DMSO-d6) δ 1.31 (s, 9H), 3.58 (s, 6H), 6.87 (d, J = 6.8 Hz, 2H), 7.23 (t, J = 6.8 Hz, 1H), 7.48 (d, J = 8.0 Hz, 2H), 7.85 (d, J = 8.0 Hz, 2H), 8.52 (s, 1H), 11.63 (s, 1H); HRESI-MS [M + H]+ calculated for C20H24N2O3, 340.1787, found 340.1805.
3.5.17. 4-(t-But)-N′-(4-Hydroxybenzylidene)benzohydrazide (20)
Yield: (70%), Cream color; Rf: 0.52 (n-hexane/ethyl acetate (3.0:7.0)); 1HNMR (400 MHz DMSO-d6) δ 1.32 (s, 9H), 6.93 (d, J = 7.0 Hz, 2H), 7.22 (d, J = 7.0 Hz, 2H), 7.45 (d, J = 8.0 Hz, 2H), 7.91(d, J = 8.0 Hz, 2H), 8.58 (s, 1H), 10.76 (s, 1H), 11.63 (s, 1H); HRESI-MS [M + H]+ calculated for C18H20N2O2, 296.1538, found 296.1548.
3.5.18. N′-(2,4,6-Trimethoxybenzylidene)-4-tert-butylbenzohydrazide (21)
Yield: (82%), White color; Rf: 0.52 (n-hexane/ethyl acetate (2.5:7.5)); 1HNMR (400 MHz DMSO-d6) δ 1.35 (s, 9H), 3.63 (s, 6H), 3.71 (s, 3H), 6.68 (d, J = 1.0 Hz, 2H), 7.41 (d, J = 7.5 Hz, 2H), 7.85 (d, J = 7.5 Hz, 2H), 8.59 (s, 1H), 11.66 (s, 1H); HRESI-MS [M + H]+ calculated for C21H26N2O4, 370.1893, found 370.1923.
3.5.19. 4-(Tert-butyl))-N′-(2,4-dichloro-3-hydroxybenzylidene)benzohydrazide (22)
Yield: (90%), Cream color; Rf: 0.51 (n-hexane/ethyl acetate (3.0:7.0)); 1HNMR (400 MHz DMSO-d6) δ 1.24 (s, 9H), 7.16 (d, J = 7.2 Hz, 1H), 7.29 (d, J = 1.5 Hz, 1H), 7.49 (d, J = 7.0 Hz, 2H), 7.83 (d, J = 7.0 Hz, 2H), 10.46 (s, 1H), 11.60 (s, 1H); HRESI-MS [M + H]+ calculated for C18H18Cl2N2O2, 364.0745, found 365.0755.
3.5.20. 4-(Tert-butyl))-N′-(3-methoxy-4-hydroxybenzylidene)benzohydrazide (23)
Yield: (85%), Color: Cream; Rf: 0.52 (n-hexane/ethyl acetate (3.0:7.0)); 1HNMR (400 MHz DMSO-d6) δ 1.35 (s, 9H), 3.75 (s, 3H), 6.88 (d, J = 7.2 Hz, 1H), 7.06 (s, 1H), 7.35 (d, J = 7.2 Hz, 1H),7.44 (d, J = 8.0 Hz, 2H), 7.89 (d, J = 8.0 Hz, 2H), 8.58 (s, 1H), 10.61 (s, 1H), 11.65 (s, 1H); HRESI-MS [M + H]+ calculated for C19H22N2O3, 326.1630, found 326.1646.
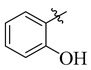
3.5.21. 4-(t-But)-N′-(2-Hydroxybenzylidene)benzohydrazide (24)
Yield: (80%), Color: Cream; Rf: 0.51 (n-hexane/ethyl acetate (2.0:8.0)); 1HNMR (400 MHz DMSO-d6) δ 1.24 (s, 9H), 7.01 (d, J = 7.2 Hz, 1H), 7.29 (t, J = 7.2 Hz, 1H), 7.49 (t, J = 7.2 Hz, 2H), 7.58 (d, J = 7.2 Hz, 1H), 7.63 (d, J = 7.2 Hz, 2H), 7.83 (d, J = 7.0 Hz, 2H), 8.52 (s, 1H), 10.74 (s, 1H), 11.60 (s, 1H); HRESI-MS [M + H]+ calculated for C18H20N2O2, 296.1525, found 296.1535.
3.5.22. 4-(t-But)-N′-(3,4-Dimethoxybenzylidene)benzohydrazide (25)
Yield: (70%), Color: Cream; Rf: 0.52 (n-hexane/ethyl acetate (2.0:8.0)); 1HNMR (400 MHz DMSO-d6) δ 1.35 (s, 9H), 3.75 (s, 3H), 3.76 (s, 3H), 6.88 (d, J = 7.2 Hz, 1H), 7.06 (s, 1H), 7.35 (d, J = 7.2 Hz, 1H),7.44 (d, J = 7.6 Hz, 2H), 7.89 (d, J = 7.6 Hz, 2H), 8.53 (s, 1H), 11.69 (s, 1H); HRESI-MS [M + H]+ calculated for C20H24N2O3, 340.1787, found 340.1802.
3.5.23. 4-(t-But)-N′-(3,5-Di-tert-butyl-2-hydroxybenzylidene)benzohydrazide (26)
Yield: (90%), Color: Cream; Rf: 0.52 (n-hexane/ethyl acetate (2.5:7.5)); 1HNMR (400 MHz DMSO-d6) δ 1.28 (s, 9H), 1.33 (s, 9H), 1.37 (s, 9H), 7.21 (s, 1H), 7.32 (s, 1H), 7.48 (d, J = 8.0 Hz, 2H), 7.81 (d, J = 8.0 Hz, 2H), 8.50 (s, 1H), 10.58 (s, 1H), 11.69 (s, 1H); HRESI-MS [M + H]+ calculated for C26H36N2O2, 408.2777, found 408.2783.
4. Conclusions
Twenty-three (4–26) different N-acylhydrazones of 4-(tert-butyl)benzohydrazide were synthesized in good to excellent yields, and these compounds were structurally deduced with the help of HRESI-MS and 1H-NMR spectroscopy. Finally, all the derivatives were screened for their in vitro urease inhibitory potential. Eight compounds showed good to excellent inhibitory potentials for the urease enzyme. Among the series, compound 6 (IC50 = 13.33 ± 0.58 µM) and 25 (IC50 = 13.42 ± 0.33 µM) were found as lead compounds with better inhibition then the standard thiourea (IC50 = 27.45 ± 1.65 µM). Similarly, the remaining compounds showed good to moderate activity with IC50 values from 27.45 ± 1.65 µM to 251.74 ± 6.82 µM. The potency of compounds 6 and 25 possibly will be due to the presence of anthracene moiety and dimethoxy at 3 and 4 positions (meta, para) on the benzene ring, respectively. SAR suggested that the nature of substituent played a vital role. On the above results, these compounds deserve further research as a novel class of urease enzyme inhibitors.
Acknowledgments
The authors extend their appreciation to The Research Council (BFP/RGP/CBS/21/002) for funding this project. The authors are also thankful to the Deanship of Scientific Research at King Khalid University for funding this work through the Large research groups program (RGP.2/64/43).
Author Contributions
S.A. and M.A. synthesized the compounds. S.R. did the crystallography of the crystal compounds. M.I., A.A. wrote the original draft of the manuscript. M.K. and N.U.R. performed structural elucidation. J.U. and A.K. conducted urease inhibition of the compounds. M.K., M.I., N.U.R., and A.A.-H. supervised the project and assisted in writing, reviewing, and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the authors on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
Funding will be provided by The Research Council (TRC) through the Research Grant Program (BFP/RGP/CBS/21/002).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmed M., Qadir M.A., Hameed A., Arshad M.N., Asiri A.M., Muddassar M. Azomethines, isoxazole, N-substituted pyrazoles and pyrimidine containing curcumin derivatives: Urease inhibition and molecular modeling studies. Biochem. Biophys. Res. Commun. 2017;490:434–440. doi: 10.1016/j.bbrc.2017.06.059. [DOI] [PubMed] [Google Scholar]
- 2.Sudkolai S.T., Nourbakhsh F. Urease activity as an index for assessing the maturity of cow manure and wheat residue vermicomposts. Waste Manag. 2017;64:63–66. doi: 10.1016/j.wasman.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Taha M., Shah S.A.A., Khan A., Arshad F., Ismail N.H., Afifi M., Imran S., Choudhary M.I. Synthesis of 3,4,5-trihydroxybenzohydrazone and evaluation of their urease inhibition potential. Arab. J. Chem. 2019;12:2973–2982. doi: 10.1016/j.arabjc.2015.06.036. [DOI] [Google Scholar]
- 4.Peek R.M., Blaser M.J. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 5.Upadhyay V., Poroyko V., Kim T.-J., Devkota S., Fu S., Liu D., Tumanov A.V., Koroleva E.P., Deng L., Nagler C., et al. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat. Immunol. 2012;13:947–953. doi: 10.1038/ni.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shamim S., Khan K.M., Salar U., Ali F., Lodhi M.A., Taha M., Khan F.A., Ashraf S., Ul-Haq Z., Ali M., et al. 5-Acetyl-6-methyl-4-aryl-3,4-dihydropyrimidin-2(1 H )-ones: As potent urease inhibitors; synthesis, in vitro screening, and molecular modeling study. Bioorganic Chem. 2017;76:37–52. doi: 10.1016/j.bioorg.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Judge V., Narasimhan B., Ahuja M., Sriram D., Yogeeswari P., De Clercq E., Pannecouque C., Balzarini J. Isonicotinic acid hydrazide derivatives: Synthesis, antimicrobial activity, and QSAR studies. Med. Chem. Res. 2011;21:1451–1470. doi: 10.1007/s00044-011-9662-9. [DOI] [PubMed] [Google Scholar]
- 8.Taha M., Ismail N.H., Lalani S., Fatmi M.Q., Wahab A.T., Siddiqui S., Khan K.M., Imran S., Choudhary M.I. Synthesis of novel inhibitors of α-glucosidase based on the benzothiazole skeleton containing benzohydrazide moiety and their molecular docking studies. Eur. J. Med. Chem. 2015;92:387–400. doi: 10.1016/j.ejmech.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Taha M., Ismail N.H., Jamil W., Rashwan H., Kashif S.M., Sain A.A., Adenan M.I., Anouar E.H., Ali M., Rahim F., et al. Synthesis of novel derivatives of 4-methylbenzimidazole and evaluation of their biological activities. Eur. J. Med. Chem. 2014;84:731–738. doi: 10.1016/j.ejmech.2014.07.078. [DOI] [PubMed] [Google Scholar]
- 10.Liu L., Alam M.S., Lee D.-U. Synthesis, Antioxidant Activity and Fluorescence Properties of Novel Europium Complexes with (E)-2- or 4-hydroxy-N’-[(2-hydroxynaphthalen-1-yl)methylene]benzohydrazide Schiff Base. Bull. Korean Chem. Soc. 2012;33:3361–3367. doi: 10.5012/bkcs.2012.33.10.3361. [DOI] [Google Scholar]
- 11.Prachumrat P., Kobkeatthawin T., Ruanwas P., Boonnak N., Laphookhieo S., Kassim M.B., Chantrapromma S. Synthesis, Crystal Structure, Antioxidant, and α-Glucosidase Inhibitory Activities of Methoxy-substituted Benzohydrazide Derivatives. Crystallogr. Rep. 2018;63:405–411. doi: 10.1134/S1063774518030227. [DOI] [Google Scholar]
- 12.Zhen-Gao S., Fang L., Jian-Wei Z., Jin-Long S., Ying G., Ning Z., Yun-Peng D. Synthesis, Crystal structure and antioxidant of 3, 5-dihydroxy-N′-(5-bro-2-hydroxybenzylidene) benzohydrazide hydrate. Struct. Chem. 2012;31:1309–1314. [Google Scholar]
- 13.Rando D.G., Avery M.A., Tekwani B.L., Khan S.I., Ferreira E.I. Antileishmanial activity screening of 5-nitro-2-heterocyclic benzylidene hydrazides. Bioorganic Med. Chem. 2008;16:6724–6731. doi: 10.1016/j.bmc.2008.05.076. [DOI] [PubMed] [Google Scholar]
- 14.Yele V., Mohammed A.A., Wadhwani A.D. Synthesis and Evaluation of Aryl/Heteroaryl Benzohydrazide and Phenylacetamide Derivatives as Broad-Spectrum Antibacterial Agents. ChemistrySelect. 2020;5:10581–10587. doi: 10.1002/slct.202002178. [DOI] [Google Scholar]
- 15.Reino J.L., Saiz-Urra L., Hernández-Galán R., Arán V.J., Hitchcock P.B., Hanson J.R., Gonzalez M.P., Collado I.G. Quantitative Structure−Antifungal Activity Relationships of Some Benzohydrazides against Botrytis cinerea. J. Agric. Food Chem. 2007;55:5171–5179. doi: 10.1021/jf0704211. [DOI] [PubMed] [Google Scholar]
- 16.Wayua C., Roy J., Putt K.S., Low P.S. Selective Tumor Targeting of Desacetyl Vinblastine Hydrazide and Tubulysin B via Conjugation to a Cholecystokinin 2 Receptor (CCK2R) Ligand. Mol. Pharm. 2015;12:2477–2483. doi: 10.1021/acs.molpharmaceut.5b00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhira S., Mandava K., Manda S., Prasanna S., Vijayalaxmi T. Synthesis and Evaluation of Some Novel N, N-Dialkylaminoalkoxy-2-Oxo-Indole-3-Ylidene Benzohydrazides as Anticonvulsant Agents. IOSR J. Pharm. Biol. Sci. 2017;12:84–93. doi: 10.9790/3008-1202028493. [DOI] [Google Scholar]
- 18.Mukhtar S.S., Hassan A.S., Morsy N.M., Hafez T.S., Hassaneen H.M., Saleh F.M. Overview on Synthesis, Reactions, Applications, and Biological Activities of Schiff Bases. Egypt. J. Chem. 2021 doi: 10.21608/ejchem.2021.79736.3920. [DOI] [Google Scholar]
- 19.Shanthalakshmi K., Mahesh B., Belagali S. Synthesis of Benzothiazole Schiff’s Bases and screening for the Antioxidant Activity. J. Chem. Pharm. Res. 2016;8:240–243. [Google Scholar]
- 20.Tariq S., Kamboj P., Amir M. Therapeutic advancement of benzothiazole derivatives in the last decennial period. Arch. der Pharm. 2018;352:e1800170. doi: 10.1002/ardp.201800170. [DOI] [PubMed] [Google Scholar]
- 21.Shah S., Khan M., Ali M., Wadood A., Rehman A.U., Shah Z., Yousaf M., Salar U., Khan K.M. Bis-1,3,4-Oxadiazole Derivatives as Novel and Potential Urease Inhibitors; Synthesis, In Vitro, and In Silico Studies. Med. Chem. 2022 doi: 10.2174/1573406418666220301161934. [DOI] [PubMed] [Google Scholar]
- 22.Ikram M., Khan M., Ahmad S., Rehman S., Schulzke C. The crystal structure of 4-tert-butyl-N′-[(E)-(4-fluoro-3-methoxyphenyl)methylidene]benzohydrazide, C19H21F1N2O2. Z. Kristallogr. NCS. 2018;233:643–645. doi: 10.1515/ncrs-2017-0408. [DOI] [Google Scholar]
- 23.Zhang Y., Zhang S.-P., Wu Y.-Y., Shao S.-C. 4-Fluorobenzaldehyde salicylhydrazone. Acta Crystallogr. Sect. E Struct. Rep. Online. 2005;62:o119–o120. doi: 10.1107/S1600536805040420. [DOI] [Google Scholar]
- 24.Dutkiewicz G., Narayana B., Samshuddin S., Yathirajan H.S., Kubicki M. Synthesis and Crystal Structures of Two New Schiff Base Hydrazones Derived from Biphenyl-4-Carbohydrazide. J. Chem. Crystallogr. 2011;41:1442–1446. doi: 10.1007/s10870-011-0118-3. [DOI] [Google Scholar]
- 25.Maheswari R., Manjula J., Gunasekaran B., Bakiyaraj G. (E)-4-Methoxy-N′-(2,4,5-trifluorobenzylidene)benzohydrazide monohydrate. IUCrData. 2016;1:x160846. doi: 10.1107/S2414314616008464. [DOI] [Google Scholar]
- 26.Cui Y.-M., Yang D., Guo W., Wang Q., Zhang P. Substituent group effects on the self-assembly of oxovanadium(V) complexes with hydrazone ligands bearing benzoic acid (1-methyl-3-oxobutylidene)hydrazide backbones. J. Struct. Chem. 2016;57:840–844. doi: 10.1134/S002247661604034X. [DOI] [Google Scholar]
- 27.Narayana B., Sunil K., Yathirajan H.S., Sarojini B.K., Bolte M. 2-Bromo-N′-[(E)-(4-fluorophenyl)methylene]-5-methoxybenzohydrazide monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online. 2007;63:o2948. doi: 10.1107/S1600536807024348. [DOI] [Google Scholar]
- 28.Wardell S.M.S.V., Ferreira M.D.L., De Souza M.V.N., Wardell J.L., Low J.N., Glidewell C. 2,4-Difluorobenzaldehyde benzoylhydrazone and 2,4-dichlorobenzaldehyde benzoylhydrazone are isostructural at 120 K withZ′ = 2: Complex sheets built from N—H...O, C—H...O and C—H...π(arene) hydrogen bonds. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006;62:o118–o121. doi: 10.1107/S0108270106001995. [DOI] [PubMed] [Google Scholar]
- 29.Seifullina I.I., Martsinko E.E., Chebanenko E.A., D’Yakonenko V.V., Shishkina S.V., Pirozhok O.V. Structure of the {[Cu2Ge(μ-Cit)2(μ-INH)2]·4H2O} n Coordination Polymer Where H4Cit is Citric Acid, INH is Isonicotinic Acid Hydrazide. J. Struct. Chem. 2018;59:154–159. doi: 10.1134/S0022476618010237. [DOI] [Google Scholar]
- 30.He D.-H., Zhu Y.-C., Yang Z.-R. N′-(4-Fluorobenzylidene)-3,4,5-trimethoxybenzohydrazide. Acta Crystallogr. Sect. E Struct. Rep. Online. 2008;64:o1648. doi: 10.1107/S1600536808023702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maheswari R., Manjula J., Gunasekaran B. (E)-4-Chloro- N′ -(2,4,5-trifluorobenzylidene)benzohydrazide. IUCrData. 2016;1 doi: 10.1107/S2414314616003047. [DOI] [Google Scholar]
- 32.Liu M., Duan Y., Wang Y., Zhang W.-X., Liu S. (E)-2-[2-(4-Fluorobenzylidene)hydrazinocarbonyl]-N-isopropylbenzamide. Acta Crystallogr. Sect. E Struct. Rep. Online. 2009;65:o1599. doi: 10.1107/S1600536809022181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao W.-J., Lv P.-C., Shi L., Li H.-Q., Zhu H.-L. Synthesis, molecular docking and biological evaluation of metronidazole derivatives as potent Helicobacter pylori urease inhibitors. Bioorganic Med. Chem. 2009;17:7531–7536. doi: 10.1016/j.bmc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 34.CCG, Molecular Operating Environment (MOE) Chem Comput Gr Inc.; Montreal, QC, Canada: 2022. [(accessed on 4 August 2022)]. Available online: https://www.chemcomp.com/ [Google Scholar]
- 35.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015;C71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrugia L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012;45:849–854. doi: 10.1107/S0021889812029111. [DOI] [Google Scholar]
- 37.Sheldrick G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015;71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available from the authors on reasonable request.