Abstract
Background
Stroke-associated pneumonia (SAP) commonly complicates acute ischemic stroke (AIS) and significantly worsens outcomes. Type 2 diabetes mellitus (T2DM) may contribute to malnutrition, impair innate immunity function, and increase the probability of SAP occurrence in AIS patients. We aimed to determine early predictors of SAP in AIS patients with T2DM and to construct a nomogram specifically for predicting SAP in this population by combining the A2DS2 score with available nutrition-related parameters.
Methods
A total of 1,330 consecutive AIS patients with T2DM were retrospectively recruited. The patients were randomly allocated to the training (n = 887) and validation groups (n = 443). Univariate and multivariate binary logistic regression analyses were applied to determine the predictors of SAP in the training group. A nomogram was established according to the identified predictors. The areas under the receiver operating characteristic curve (AUROC) and calibration plots were performed to access the predictive values of the nomogram. The decision curve was applied to evaluate the net benefits of the nomogram.
Results
The incidence of SAP was 9% and 9.7% in the training and validation groups, respectively. The results revealed that the A2DS2 score, stroke classification, Geriatric Nutritional Risk Index, hemoglobin, and fast blood glucose were independent predictors for SAP. A novel nomogram, A2DS2-Nutrition, was constructed based on these five predictors. The AUROC for A2DS2-Nutrition (0.820, 95% CI: 0.794–0.845) was higher than the A2DS2 score (0.691, 95% CI: 0.660–0.722) in the training group. Similarly, it showed a better predictive performance than the A2DS2 score [AUROC = 0.864 (95% CI: 0.828–0.894) vs. AUROC = 0.763 (95% CI: 0.720–0.801)] in the validation group. These results were well calibrated in the two groups. Moreover, the decision curve revealed that the A2DS2-Nutrition provided an additional net benefit to the AIS patients with T2DM compared to the A2DS2 score in both groups.
Conclusion
The A2DS2 score, stroke classification, Geriatric Nutritional Risk Index, hemoglobin, and fast blood glucose were independent predictors for SAP in AIS patients with T2DM. Thus, the proposed A2DS2-Nutrition may be a simple and reliable prediction model for SAP occurrence in AIS patients with T2DM.
Keywords: stroke-associated pneumonia, nutritional status, A2DS2 score, acute ischemic stroke, type 2 diabetes mellitus
Introduction
Stroke-associated pneumonia (SAP) is a spectrum of pneumonia complications that develop in non-ventilated patients following a stroke (1). The incidence of SAP was estimated to be 12.3% [95% confidential interval (CI), 11%–13.6%] (2). SAP significantly lengthens hospitalization and raises hospital costs (3–5). Furthermore, it is independently associated with worse functional outcomes and increased mortality rates (5, 6).
Diabetes was discovered to be related to SAP (7). Moreover, diabetes was found in approximately 33% of ischemic stroke patients and was closely associated with poor outcomes in this population (8). Patients with type 2 diabetes mellitus (T2DM) were more susceptible to community-acquired pneumonia (relative risk = 1.64, 95% CI, 1.55–1.73) than the non-T2DM in a meta-analysis incorporating about 15 million people (9). A plausible explanation is that hyperglycemia impair immunological function in T2DM patients (10). Furthermore, a significant malnutrition prevalence was found in hospitalized older patients with T2DM (11), due to gastroparesis, diet changes, and chronic kidney disease (12). Malnutrition is an issue commonly related to secondary immune deficiency and infection risk (13). Therefore, it is reasonable to pay more attention to the risk of SAP in acute ischemic stroke (AIS) patients with T2DM.
SAP is considered a potentially modifiable complication affecting stroke prognosis. Thus, identifying vulnerable patients is crucial to tailor monitoring and prophylactic strategies. Efforts have been made to establish prediction SAP scales, including the A2DS2, the PANTHERS, the ISAN, and the AIS-APS score (14). The A2DS2 score is the most extensively utilized risk scale in practice. It has had a moderate predive accuracy for SAP (AUROC = 0.85, 95% CI, 0.82–0.88) (15). In addition, some simple nutrition-related biomarkers, such as hyperglycemia (16) and serum album (17), had good predictive value for SAP. However, no existing research has been made to specifically predict SAP in patients with comorbidity of AIS and T2DM. There is also no prediction scale that takes nutritional risk status into account as an important factor.
In the present study, we retrospectively evaluated the relevance of the A2DS2 score with SAP in AIS patients with T2DM. Furthermore, we attempted to establish an individualized nomogram for discerning SAP in these patients by combining the A2DS2 score with simple nutrition-related biomarkers.
Materials and methods
Patients selection
This was a retrospective study conducted at the Peking University People’s Hospital (PKUPH) between January 2015 and September 2021. Consecutive AIS patients with T2DM who were admitted within 24 h of symptom onset were enrolled in our cohort. All participants met the WHO criteria for the diagnosis of AIS (18). The diagnosis of T2DM was based on the American Diabetes Association diagnostic criteria (19) or clear T2DM history. The exclusion criteria included the following: (1) age < 18 years; (2) diagnosis of transient ischemic attacks (TIA) or cerebral hemorrhage; (3) active pulmonary or other sites infection within the last 14 days; (4) history of severe hepatic diseases, hematological malignancy, or immunosuppressant treatment; (5) recent history of major trauma or surgery, and (6) incomplete medical records. This study was approved by the ethics committee at PKUPH and conducted in accordance with the Declaration of Helsinki.
Data collection
The demographic and clinical data were recorded at the time of admission, including age, sex, height, weight, stroke classification, National Institutes of Health Stroke Scale (NIHSS) score at admission, hypertension, dyslipidemia hyperuricemia, atrial fibrillation, previous myocardial infarction, chronic heart failure, previous stroke, dementia, dysphasia, chronic obstructive pulmonary disease, chronic kidney disease, malignancy, smoking status, and drinking status. AIS was classified into four subtypes, including total anterior circulation infarct, partial anterior circulation infarct, posterior circulation infarct, and lacunar infarct (LACI), according to the Oxfordshire Community Stroke Project (OCSP) classification system (20). Body mass index (BMI) was defined as the weight (kg) divided by height square (m2). As previously reported, the A2DS2 score was determined by summing up the points of the corresponding risk factors: 3 for age ≥ 75 years, 1 for atrial fibrillation, 2 for dysphasia, 1 for male, 0 for NHISS 0–4, 3 for NHISS 5–15, and 5 for NHISS ≥ 16 (21). The following laboratory parameters were collected within 24 h of admission: hemoglobin, neutrophils, lymphocyte, platelet, fast blood glucose (FBG), glycosylated hemoglobin, serum creatinine, estimated glomerular filtration rate, serum album (ALB), total cholesterol, total glyceride, high- density lipoprotein, and low-density lipoprotein. Neutrophil to lymphocyte ratio (NLR) was defined as the neutrophil count (109/L) divided by lymphocyte count (109/L). The Geriatric Nutritional Risk Index (GNRI) formula was as follows: GNRI = [1.489 × ALB (g/L)] + [41.7 × current weight (kg)/ideal body weight (kg)] (22). Ideal weight was calculated based on the Lorentz equations: for men: ideal body weight = height (cm) – 100 – [height (cm) - 150]/4; for women: ideal body weight = height (cm) – 100 – [height (cm) - 150]/2. GNRI scores > 98, 92–98, 82–91, and < 82 were considered to indicate absent, low, moderate, and severe malnutrition risk, respectively (22).
Outcome definition
SAP was defined as pneumonia complicating the first week after AIS onset in non-ventilated patients (1). In our study, clinically suspected SAP was diagnosed based on the modified Centers for Disease Control and Prevention criteria, according to the clinical symptoms and laboratory tests (23). Definite SAP was confirmed by typical chest X-ray (CXR) changes within 48 h of antibiotic initiation (1). However, only 36% of clinically suspected SAP manifested typical pneumonia findings on an initial CXR (1). Hence, in clinical practice, pulmonary computed tomography was undertaken within 48 h after consent following the suspicion of pneumonia, albeit with a with normal CXR appearance (24).
Statistical analysis
IBM SPSS Statistics version 22.0 (IBM Corp.) and R project for Statistical Computer version 4.1.2 were applied for the statistical analyses. A 2-sided P-value < 0.05 was considered statistically significant. Continuous variables were presented as mean with the standard deviation if normally distributed and median with interquartile range otherwise. The t-test and Mann–Whitney U test were performed for comparison among the groups in continuous variables. The Fisher’s exact test and Pearson’s Chi-square test were employed to explore differences in the categorical variables.
Random number generator of SPSS was applied for random grouping of the participants. Two-thirds of the patients were randomly allocated to the training group to establish a nomogram, and the remaining were allocated to the validation group. Univariate logistic regression was applied to identify the potential variables associated with SAP. Then, the variables were included in the multivariate logistic regression when the P-value < 0.05. The results of multivariate regression were visualized as a forest plot. A nomogram was constructed based on the multivariate logistic regression. The nomogram was compared with the A2DS2 score by performing the area under the receiver operating characteristic curve (AUROC). The calibration plots were used to access the performance of the nomogram with 1,000 bootstraps resamples. Moreover, the decision curve was applied to evaluate the net benefits of the nomogram.
Results
Clinical characteristics of the study cohort
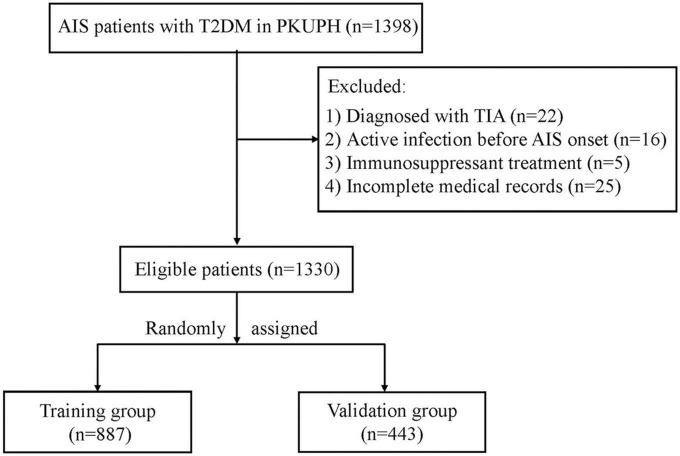
As shown in the flow chart of Figure 1, 1,398 T2DM patients with AIS attended PKUPH between January 2015 and September 2021. 22 patients diagnosed with TIA, 16 with the active infection before stroke onset (3 with sepsis and 13 with urinary tract infections), 5 receiving immunosuppressant treatment, and 25 with incomplete medical records were excluded from the cohort. Two-thirds of the patients (n = 887) were allocated to the training group, with the remaining (n = 443) allocated to the validation group. In addition to age, there was no significant difference in baseline demographic and clinical variables between the two groups (Table 1). SAP incidence rates were similar in both groups (9% vs. 9.7%, P = 0.683).
FIGURE 1.
Study flow diagram. AIS, acute ischemic stroke; T2DM, type 2 diabetes mellitus; PKUPH, Peking University People’s Hospital; TIA, transient ischemic attack.
TABLE 1.
Demographic and clinical variables of patients.
| Variable |
All cases (n = 1330) |
Training Group (n = 887) |
Validation Group (n = 443) | P-value |
| Male, n (%) | 868 (65.3) | 589 (66.4) | 279 (63.0) | 0.216 |
| Age (years), mean (SD) | 67.7 (12.0) | 67.1 (11.9) | 68.8 (12.2) | 0.016 |
| SAP, n (%) | 123 (9.2) | 80 (9.0) | 43 (9.7) | 0.683 |
| BMI (kg/m2), mean (SD) | 23.3 (4.6) | 23.4 (4.6) | 23.2 (4.7) | 0.439 |
| OCSP | ||||
| TACI, n (%) | 105 (7.9) | 70 (7.9) | 35 (7.9) | 0.995 |
| PACI, n (%) | 295 (22.2) | 190 (21.4) | 105 (23.7) | 0.345 |
| POCI, n (%) | 261 (19.6) | 177 (20.0) | 84 (19.0) | 0.667 |
| LACI, n (%) | 669 (50.3) | 450 (50.7) | 219 (49.4) | 0.656 |
| NIHSS at admission, median (IQR) | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 0.497 |
| A2DS2, median (IQR) | 1.0 (1.0–4.0) | 1.0 (1.0–4.0) | 1.0 (1.0–4.0) | 0.913 |
| GNRI, mean (SD) | 100.6 (11.5) | 100.7 (11.4) | 100.3 (11.8) | 0.522 |
| Smoking, n (%) | 511 (38.4) | 354 (39.9) | 157 (35.4) | 0.114 |
| Drinking, n (%) | 322 (24.2) | 210 (23.7) | 112 (25.3) | 0.519 |
| Hypertension, n (%) | 1,019 (78.4) | 688 (77.6) | 331 (74.7) | 0.248 |
| Dyslipidemia, n (%) | 914 (68.7) | 620 (69.9) | 294 (66.3) | 0.190 |
| Hyperuricemia, n (%) | 94 (7.1) | 65 (7.3) | 29 (6.5) | 0.600 |
| Atrial fibrillation, n (%) | 97 (7.3) | 63 (7.1) | 34 (7.7) | 0.705 |
| Previous MI, n (%) | 85 (6.4) | 61 (6.9) | 24 (5.4) | 0.305 |
| CHF, n (%) | 16 (1.2) | 8 (0.9) | 8 (1.8) | 0.154 |
| Previous stroke, n (%) | 112 (8.4) | 76 (8.6) | 36 (8.1) | 0.785 |
| COPD, n (%) | 17 (12.8) | 12 (1.4) | 5 (1.1) | 0.732 |
| CKD, n (%) | 45 (3.4) | 33 (3.7) | 12 (2.7) | 0.336 |
| Malignancy, n (%) | 69 (5.2) | 43 (4.8) | 26 (5.9) | 0.429 |
| Dementia, n (%) | 51 (3.8) | 35 (3.9) | 16 (3.6) | 0.765 |
| Dysphagia, n (%) | 175 (13.2) | 120 (13.5) | 55 (12.4) | 0.571 |
| Hemoglobin (g/L), mean (SD) | 133.4 (19.4) | 133.7 (19.4) | 132.8 (19.3) | 0.413 |
| Neutrophils (× 109/L), median (IQR) | 4.1 (3.3–5.4) | 4.1 (3.3–5.4) | 4.3 (3.4–5.4) | 0.199 |
| NLR, median (IQR) | 2.5 (1.9–3.7) | 2.5 (1.9–3.7) | 2.7 (2.0–3.9) | 0.087 |
| Lymphocyte (× 109/L), median (IQR) | 1.6 (1.2–2) | 1.6 (1.2–2.0) | 1.6 (1.2–1.9) | 0.517 |
| Platelet (× 109/L), median (IQR) | 195.0 (159.3–232.0) | 195.0 (159.0–232.0) | 195.0 (161.0–232.0 | 0.729 |
| FBG (mmol/L), median (IQR) | 7.7 (6.2–10.3) | 7.6 (6.2–10.4) | 7.8 (6.2–9.8) | 0.550 |
| HbA1c (%), median (IQR) | 7.7 (6.8–9.0) | 7.7 (6.8–9.0) | 7.6 (6.8–9.0) | 0.832 |
| SCR (μmol/L), median (IQR) | 70.0 (58.3–86) | 71.0 (59.0–87.0) | 69.0 (58.0–86.0) | 0.850 |
| eGFR (ml/min*1.73m2), median (IQR) | 88.3 (71.4–98.4) | 88.6 (72.1–98.3) | 87.4 (69.6–98.6) | 0.313 |
| ALB (g/L), median (IQR) | 38.7 (35.9–40.9) | 38.8 (36.0–40.9) | 38.5 (35.6–41.1) | 0.547 |
| TC (mmol/L), median (IQR) | 4.2 (3.4–5.1) | 4.2 (3.4–5.1) | 4.3 (3.5–5.2) | 0.537 |
| TG (mmol/L), median (IQR) | 1.5 (1.1–2.1) | 1.5 (1.2–2.1) | 1.5 (1.1–2.0) | 0.344 |
| HDL (mmol/L), median (IQR) | 1.0 (0.8–1.2) | 1.0 (0.8–1.1) | 1.0 (0.8–1.2) | 0.720 |
| LDL (mmol/L), median (IQR), | 2.6 (2.0–3.3) | 2.6 (2.0–3.3) | 2.6 (2.0–3.4) | 0.483 |
SAP, stroke-associated pneumonia; SD, standard deviation; IQR, interquartile range; BMI, body mass index, OCSP, Oxfordshire Community Stroke Project; TACI, total anterior circulation infarction; PACI, partial anterior circulation infarction; POCI, posterior circulation infarction; LACI, lacunar circulation infarction; NIHSS, National Institutes of Health Stroke Scale; GNRI, Geriatric Nutritional Risk Index; MI, myocardial infarction; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; NLR, neutrophil-to-lymphocyte ratio; FBG, fast blood glucose; HbA1c, glycosylated hemoglobin; SCR, serum creatinine clearance; eGFR, estimated glomerular filtration rate; ALB, album; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol.
Bold values mean P-value < 0.05.
Baseline characteristics of patients in the training group stratified by SAP
Patients with SAP (n = 80) were older (71.3 ± 12.2 vs. 66.7 ± 11.8 years) and had higher NHISS [5.5 (3.0–11.3) vs. 3.0 (2.0–5.0)], A2DS2 scores [4.0 (1.0–6.0) vs. 1.0 (1.0–4.0)], and GNRI (91.7 ± 11.4 vs. 101.6 ± 11.0) than those without SAP (n = 807). Patients diagnosed with SAP showed lower BMI (21.1 ± 3.5 vs. 23.6 ± 4.6 kg/m2) and had higher incidence of non-LACI (76.3% vs. 46.6%), atrial fibrillation (13.8% vs. 6.4%), previous myocardial infarction (16.3% vs. 5.9%), and dysphagia (11.6% vs. 32.5%) than its counterparts. For laboratory features, the levels of hemoglobin (117.9 ± 29.0 vs. 135.3 ± 17.4 g/L), lymphocyte [1.1 (0.7–1.5) vs. 1.6 (1.2–2.0) × 109/L], and ALB [35.3 (31.8–38.2) vs. 39.0 (36.5–41.0) g/L] were lower in the SAP group than in the non-SAP group. In addition, the levels of neutrophils [5.3 (3.8–7.0) vs. 4.0 (3.2–5.1) × 109/L], NLR [4.4 (2.0–6.9) vs. 2.5 (1.9–3.5) × 109/L], and FBG [9.8 (8.0–15.6) vs. 7.4 (6.1–10.0) × 109/L] were higher in the SAP group when compared with its counterparts (Table 2).
TABLE 2.
Univariate regression analysis of SAP in the training group.
| Variable | Non-SAP group (N = 807) | SAP group (N = 80) | P-value | OR (95% CI) | P-value |
| Male, n (%) | 540 (66.9) | 49 (61.3) | 0.306 | 0.782 (0.4870–1.254) | 0.307 |
| Age (years), mean (SD) | 66.7 (11.8) | 71.3 (12.2) | 0.001 | 1.035 (1.014–1.056) | 0.001 |
| BMI (kg/m2), mean (SD) | 23.6 (4.6) | 21.1 (3.5) | < 0.001 | 0.857 (0.804–0.911) | < 0.001 |
| OCSP | |||||
| LACI, n (%) | 431 (53.4) | 19 (23.8) | Reference | ||
| Non-LACI, n (%) | 376 (46.6) | 61 (76.3) | < 0.001 | 3.680 (2.159–6.272) | < 0.001 |
| NIHSS at admission, median (IQR) | 3.0 (2.0–5.0) | 5.5 (3.0–11.3) | < 0.001 | 1.072 (1.045–1.099) | < 0.001 |
| A2DS2, median (IQR) | 1.0 (1.0–4.0) | 4.0 (1.0–6.0) | < 0.001 | 1.368 (1.242–1.507) | < 0.001 |
| GNRI, mean (SD) | 101.6 (11.0) | 91.7 (11.4) | < 0.001 | 0.919 (0.897–0.940) | < 0.001 |
| Absent malnutrition risk, n (%) | 524 (64.9) | 24 (30.0) | Reference | ||
| Low malnutrition risk, n (%) | 138 (17.1) | 17 (21.3) | 2.812 (1.462–5.410) | 0.002 | |
| Moderate malnutrition risk, n (%) | 117 (14.5) | 25 (31.3) | 4.877 (2.675–8.893) | < 0.001 | |
| High malnutrition risk, n (%) | 27 (3.3) | 15 (18.8) | 12.681 (5.950–27.029) | < 0.001 | |
| Smoking, n (%) | 323 (40.0) | 31 (38.8) | 0.824 | 0.948 (0.592–1.519) | 0.824 |
| Drinking, n (%) | 190 (23.5) | 20 (25.0) | 0.770 | 1.083 (0.636–1.842) | 0.770 |
| Hypertension, n (%) | 628 (77.8) | 60 (75.0) | 0.564 | 0.855 (0.502–1.457) | 0.565 |
| Dyslipidemia, n (%) | 560 (69.4) | 60 (75.0) | 0.297 | 1.323 (0.781–2.243) | 0.298 |
| Hyperuricemia, n (%) | 61 (7.6) | 4 (5.0) | 0.402 | 0.644 (0.228–1.819) | 0.406 |
| Atrial fibrillation, n (%) | 52 (6.4) | 11 (13.8) | 0.015 | 2.315 (1.155–4.644) | 0.018 |
| Previous MI, n (%) | 48 (5.9) | 13 (16.3) | 0.001 | 3.068 (1.583–5.947) | < 0.001 |
| CHF, n (%) | 7 (0.9) | 1 (1.3) | 0.730 | 1.447 (0.176–11.91) | 0.731 |
| Previous stroke, n (%) | 70 (8.7) | 6 (7.5) | 0.720 | 0.854 (0.359–2.032) | 0.721 |
| COPD, n (%) | 9 (1.1) | 3 (3.8) | 0.086 | 3.455 (0.916–13.028) | 0.067 |
| CKD, n (%) | 27 (3.3) | 6 (7.5) | 0.110 | 2.342 (0.937–5.855) | 0.069 |
| Malignancy, n (%) | 36 (4.4) | 7 (8.8) | 0.099 | 2.054 (0.883–4.778) | 0.095 |
| Dementia, n (%) | 30 (3.7) | 5 (6.3) | 0.235 | 1.727 (0.651–4.582) | 0.273 |
| Dysphagia, n (%) | 94 (11.6) | 26 (32.5) | < 0.001 | 3.652 (2.182–6.112) | < 0.001 |
| Hemoglobin (g/L), mean (SD) | 135.3 (17.4) | 117.9 (29.0) | < 0.001 | 0.963 (0.953–0.973) | < 0.001 |
| Neutrophils (× 109/L), median (IQR) | 4.0 (3.2–5.1) | 5.3 (3.8–7.0) | < 0.001 | 1.137 (1.052–1.229) | 0.001 |
| NLR, median (IQR) | 2.5 (1.9–3.5) | 4.4 (2.0–6.9) | < 0.001 | 1.089 (1.047–1.132) | < 0.001 |
| Lymphocyte (× 109/L), median (IQR) | 1.6 (1.2–2.0) | 1.1 (0.7–1.5) | < 0.001 | 0.293 (0.189–0.454) | < 0.001 |
| Platelet (× 109/L), median (IQR) | 195.0 (160.0–229.0) | 192.0 (129.8–241.8) | 0.390 | 0.999 (0.996–1.002) | 0.5732 |
| FBG (mmol/L), median (IQR) | 7.4 (6.1–10.0) | 9.8 (8.0–15.6) | < 0.001 | 1.098 (1.048–1.151) | < 0.001 |
| HbA1c (%), median (IQR) | 7.6 (6.8–9.0) | 8.0 (6.9–10.2) | 0.148 | 1.122 (0.997–1.262) | 0.057 |
| SCR (μmol/L), median (IQR) | 71.0 (60.0–86.0) | 73.0 (47.8–101.3) | 0.677 | 1.001 (0.998–1.003) | 0.506 |
| eGFR (ml/min*1.73m2), median (IQR) | 88.8 (73.8–98.2) | 86.8 (63.0–104.3) | 0.467 | 0.993 (0.984–1.003) | 0.175 |
| ALB (g/L), median (IQR) | 39.0 (36.5–41) | 35.3 (31.8–38.2) | < 0.001 | 0.861 (0.822–0.902) | < 0.001 |
| TC (mmol/L), median (IQR) | 4.2 (3.4–5.1) | 4.4 (3.0–5.5) | 0.939 | 0.964 (0.818–1.136) | 0.659 |
| TG (mmol/L), median (IQR) | 1.5 (1.2–2.1) | 1.4 (1.2–2.2) | 0.834 | 1.010 (0.887–1.150) | 0.883 |
| HDL (mmol/L), median (IQR) | 1.0 (0.8–1.2) | 1.0 (0.7–1.1) | 0.243 | 0.605 (0.240–1.529) | 0.228 |
| LDL (mmol/L), median (IQR), | 2.6 (2.0–3.3) | 2.8 (1.7–3.6) | 0.889 | 0.985 (0.787–1.234) | 0.898 |
SAP, stroke-associated pneumonia; OR, odds ratios; SD, standard deviation; IQR, interquartile range; BMI, body mass index, OCSP, Oxfordshire Community Stroke Project; LACI, lacunar circulation infarction; NIHSS, National Institutes of Health Stroke Scale; GNRI, Geriatric Nutritional Risk Index; MI, myocardial infarction; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; NLR, neutrophil-to-lymphocyte ratio; FBG, fast blood glucose; HbA1c, glycosylated hemoglobin; SCR, serum creatinine clearance; eGFR, estimated glomerular filtration rate; ALB, album; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol.
Bold values mean P-value < 0.05.
Development of a predictive nomogram for SAP
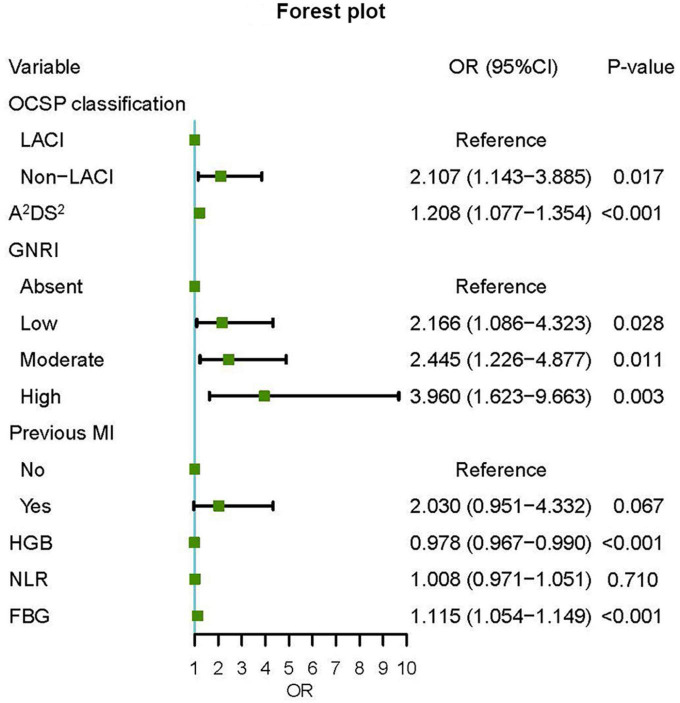
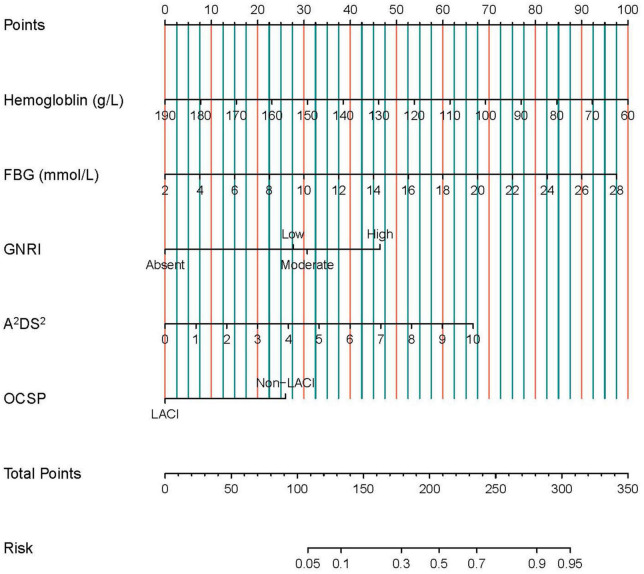
According to the univariate logistic regression analysis, fifteen factors were significantly correlated with SAP. They were age, BMI, OCSP classification, NIHSS score, A2DS2 score, Geriatric Nutritional Risk Index (GNRI), atrial fibrillation, previous myocardial infarction, dysphagia, hemoglobin, neutrophils, lymphocyte, NLR, FBG, and ALB (Table 2). Age, atrial fibrillation, dysphagia, and NIHSS were not included in the multivariate regression analysis since they were parts of the A2DS2 score. In addition, neutrophils and lymphocytes, and BMI and ALB, were excluded from subsequent analysis because they were the main elements of the NLR and GNRI formula separately. Finally, the remaining seven factors were incorporated into the multivariate logistic regression. The results revealed that A2DS2 score, OCSP classification (non-LACI), GNRI, hemoglobin, and FBG were independent predictors for SAP (Figure 2). All VIF values were ≤ 1.15, indicating low collinearity among the variables. Based on these independent predictors, a nomogram, A2DS2-Nutrition, was constructed to predict the probability of SAP in AIS patients with T2DM (Figure 3). The A2DS2-Nutrition assigned a probability (5%–95%) of SAP occurrence. Each risk factor was assigned a point based on the nomogram. The total points were calculated by adding the corresponding scores on each variable’s scale point. The probability of SAP could be obtained by locating the total points on the “Total Points” axis and drawing a vertical line down to the “SAP Risk” axis. For example, the A2DS2-Nutrition assigns a 50% probability of SAP in a LACI patient (0 points) with A2DS2 = 6 (40 points), glucose = 14 mmol/L (45 points), low malnutrition risk (27.5 points), and hemoglobin = 70 g/L (92.5 points), with a total score of 205 points.
FIGURE 2.
Forest plot of odds ratios for SAP. An OR (odds ratios) > 1 meant an increased risk of SAP (stroke-associated pneumonia); an OR < 1 meant the opposite. OCSP, Oxfordshire Community Stroke Project; LACI, lacunar circulation infarction; GNRI, Geriatric Nutritional Risk Index; MI, myocardial infarction; NLR, neutrophil-to-lymphocyte ratio; FBG, fast blood glucose.
FIGURE 3.
The nomogram for predicting the occurrence of SAP in AIS patients with T2DM. Each risk factor is given a point based on the nomogram. The total points are calculated by summing up the corresponding scores on the points scales of each variable. The probability of SAP can be obtained by locating the total points on the “Total Points” axis and drawing a vertical line down to the “SAP Risk” axis. SAP, stroke-associated pneumonia; GNRI, Geriatric Nutritional Risk Index; OCSP, Oxfordshire Community Stroke Project; LACI, lacunar circulation infarction; FBG, fast blood glucose.
Internal and external validation of the nomogram
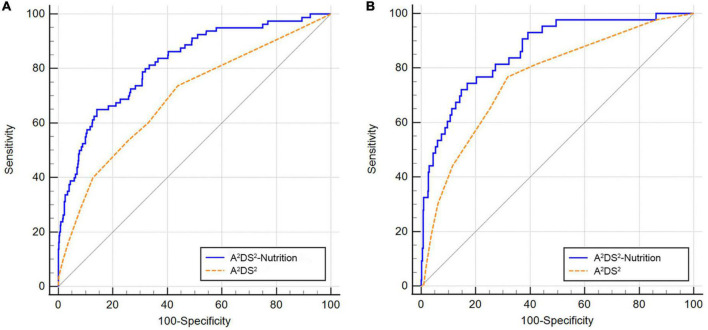
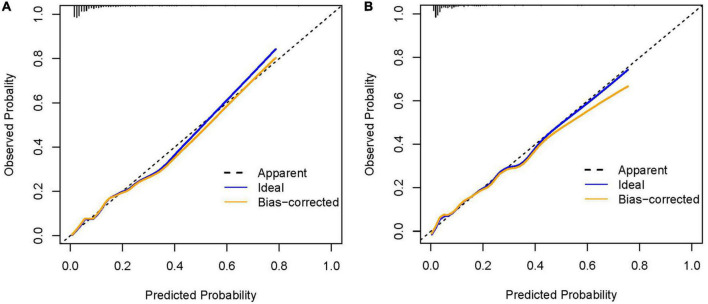
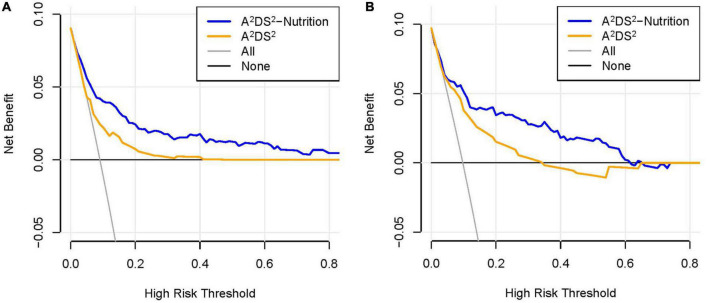
The AUROC for this nomogram in the training and validation groups was 0.820 (95% CI: 0.794–0.845) and 0.864 (95% CI: 0.828–0.894), respectively, which were significantly higher than those for A2DS2 [trainings group, 0.691 (95% CI: 0.660–0.722); validation group, 0.763 (95% CI: 0.720–0.801)] (Figures 4A,B). In the training group, the calibration plot for SAP probability showed good accordance between the predicted probability and the actual observation (Figure 5A); the mean absolute error was 0.009. Similarly, the calibration plot of the observed against the predicted possibility of SAP demonstrated satisfactory concordance in the validation group; the mean absolute error was 0.015 (Figure 5B). In addition, for a threshold probability range of 3% to 97%, using the A2DS2-Nutrition would yield a greater net benefit than applying the A2DS2 in the training group (Figure 6A). This means that our nomogram is superior to the A2DS2 sore for treatment decisions (e.g., prophylactic antibiotic therapy) in such a wide range of pre-defined risk thresholds. For a threshold probability range of 2% to 66%, the A2DS2-Nutrition would provide more net benefit to AIS patients with T2DM than the A2DS2 in the validation group (Figure 6B).
FIGURE 4.
Comparison of the AUROC values between the A2DS2-Nutrition and A2DS2 score. (A) In the training group, the A2DS2-Nutrition had a larger AUROC than the A2DS2 [0.820 (95% CI, 0.794–0.845) vs. 0.691 (95% CI, 0.660–0.722), P < 0.001]; (B) In the validation group, the AUROC of A2DS2-Nutrition was larger than the A2DS2 [0.864 (95% CI, 0.828–0.894) vs. 0.763 (95% CI, 0.720–0.801), P = 0.002].
FIGURE 5.
The calibration curve of the A2DS2-Nutrition. (A) Calibration curve in the training group; (B) Calibration curve in the validation group.
FIGURE 6.
Comparison of the decision curves between the A2DS2-Nutrition and A2DS2 score. (A) The decision curve in the training group; (B) the decision curve in the validation group.
Discussion
The A2DS2 score, stroke classification (non-LACI), GNRI, hemoglobin, and FBG, were independent predictors of SAP in this research. A nomogram was established according to these factors for predicting SAP risk in AIS patients with T2DM. It would assist neurologists in identifying vulnerable patients, making early decisions, and tailoring preventive strategies.
SAP is usually ascribed to stroke-induced immunodepression (5, 6). In addition, pneumonia is more frequent in T2DM patients since hyperglycemia predominantly affects innate immunity (10, 25). Moreover, diabetes is a critical factor for malnutrition in stroke patients [odds ratio (OR) = 2.60, 95% CI: 2.24–3.03] (26), resulting in compromised immunity and increased infection rates (13). Diabetes may increase the risk of pneumonia in people suffering from an ischemic stroke (27, 28). Furthermore, T2DM patients always have poorer clinical outcomes after infection, as evidenced by increased hospitalization incidence, length of stay, and complications (29). Nevertheless, no clinical tool exists to explicitly predict SAP occurrence in AIS patients with T2DM. Although the SAP scoring systems, such as A2DS2, have been established for stroke, they have not been specifically validated in the T2DM population. In the present study, the A2DS2 score had an insufficient accuracy in predicting SAP occurrence in the training group [AUROC = 0.691 (95% CI: 0.660–0.722)]. Furthermore, our nomogram had a higher AUROC for discriminating SAP compared with the A2DS2 score. This suggests that the A2DS2-Nutrition has a more reliable prediction ability. More importantly, the nomogram assesses the individual risk based on the A2DS2 score and nutritional status, which are easily accessible on admission.
The A2DS2 score was constructed using data from a large cohort of 15,335 stroke patients in Germany. This research revealed that the score had a high sensitivity (83%) and specificity (72%) (21). The A2DS2 score considers age, male sex, atrial fibrillation, dysphagia, and NIHSS, all of which are the mainly elements in other well-recognized SAP scales (30, 31). However, other research reported inconsistencies in A2DS2 sensitivities and specificities, probably due to different designs and study populations (15). Therefore, we modified the A2DS2 score by in our cohort combining it with stroke classification and nutritional parameters. The GNRI score is a simple scale system initially designed to assess malnutrition risk among elderly patients (22). Subsequently, the GNRI score was applied to predict sepsis mortality (32) and the probability of surgical site infections (33, 34). Malnutrition risk was found in 5.3% of 8,698 Chinese patients with AIS according to the GNRI score (35). In contrast, 22.1% (294/1330) of our cohort had moderate or severe malnutrition risk defined by GNRI score. A possible reason may be that T2DM patients often have an improper diet due to poor diabetes-related nutrition knowledge and barriers to dietary adherence (36). In line with this, the malnutrition risk of older COVID-19 patients with diabetes was 2.12 times higher than in those without diabetes in China (37). Lower GNRI scores were associated with an increased risk of long-term death and significant disability in patients with AIS (35). In addition, the GNRI score was employed as a biomarker for SAP risk classification (38). Patients with SAP showed lower GNRI scores than without SAP (96.88 ± 9.36 vs. 100.88 ± 8.25), indicating its predictive values in AIS patients (38). Considering the high malnutrition risk rate in elderly diabetic patients (11), the GNRI score was included in our prediction model as a critical element.
Hyperglycemia significantly contributed to SAP occurrence in our cohort. This is evidenced by the results of several studies, which demonstrated that glucose level has satisfactory values for predicting SAP (16, 39). Noteworthy, hyperglycemia reduces the neutrophil’s bactericidal ability by inhibiting migration, phagocytosis, and superoxide production (40). Furthermore, it may increase the circulation cytokine concentrations, such as tumor necrosis factor-α and interleukin-6, significantly contributing to post-stroke infection (16). In our prediction model, decreased hemoglobin was another independent factor for SAP. Anemia is evaluated to be present in 30% of AIS patients (41). Anemia on admission not only increased the mortality rate (OR = 1.97, 95% CI 1.57–2.47) (41), but also contributed to in-hospital pneumonia incidence (OR = 1.71, 95% CI 1.35–2.17) in stroke patients (42). A reason might be that the anemic patients may be nutritionally imbalanced and immune-compromised. In our study, decreased hemoglobin possibly indirectly reflected the malnutritional risk status of the AIS patients with T2DM.
To our best knowledge, A2DS2-Nutrition is the first nomogram for specifically predicting SAP in AIS patients with T2DM based on routine data. SAP is notorious for increased care dependency, 30-day mortality, and 1-year mortality (5, 6). Hence, our prediction model may facilitate physicians in making individualized decisions, and implementing early empirical antibiotic therapy, thereby benefiting patients in the long run. Furthermore, the nomogram highlights the need for glycemic control and nutritional support for T2DM patients. A recent study has shown that almost 50% of the patients were non-adherent to their T2DM treatment regimens (43). Thus, hyperglycemia should be strictly monitored and controlled upon admission. Meanwhile, timely nutritional supplements for malnourished individuals may reduce SAP risk.
There remain some limitations in this study. First, to make the nomogram more user-friendly, we did not include infecting-related data (e.g., C-reactive protein and procalcitonin), which were routinely unavailable upon admission in this research. This may affect the accuracy of the prediction model. Second, all the patients were from the same PKUPH center, and the nomogram needs external cohorts to be validated. Finally, our prediction model was developed in the Chinese cohort, limiting the extrapolation of the findings to regions outside our country. However, the model was entirely based on available data, providing the foreign researchers with a new applicable way in the future. Hence, multicenter studies with large sample sizes are required to corroborate the findings of our study.
Conclusion
The A2DS2 score, stroke classification, Geriatric Nutritional Risk Index, hemoglobin, and fast blood glucose were independent predictors for SAP in AIS patients with T2DM. The A2DS2-Nutrition might be a simple and reliable tool for predicting SAP occurrence in this population. It may assist physicians in identifying high-risk patients, making individual decisions, and initiating prophylactic therapies early, thus benefiting the patients in the long run.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee at Peking University People’s Hospital. The ethics committee waived the requirement of written informed consent for participation.
Author contributions
JZ and JB designed the study and interpreted the results of this work. YH and XS participated in the data collection. XS and JB analyzed the data and wrote the manuscript. All authors contributed to the discussion and approved the submitted version of the manuscript.
Acknowledgments
We would like to thank all the study participants for their support and contribution to this research.
Abbreviations
- SAP
stroke-associated pneumonia
- AIS
acute ischemic stroke
- T2DM
type 2 diabetes mellitus
- AUROC
areas under the receiver operating characteristic curve
- CI
confidence interval
- OR
odds ratio
- PKUPH
Peking University People’s Hospital
- TIA
transient ischemic attack
- NIHSS
National Institutes of Health Stroke Scale
- LACI
lacunar infarct
- OCSP
Oxfordshire Community Stroke Project
- BMI
body mass index
- FBG
fast blood glucose
- ALB
album
- NLR
neutrophil to lymphocyte ratio
- GNRI
Geriatric Nutritional Risk Index
- CXR
chest X-ray.
Funding
This study was supported by Peking University People’s Hospital Research and Development Fund (Grant No. RDB2013-16).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. (2015) 46:2335–40. 10.1161/strokeaha.115.009617 [DOI] [PubMed] [Google Scholar]
- 2.Badve MS, Zhou Z, Beek DVD, Anderson CS, Hackett ML. Frequency of post-stroke pneumonia: systematic review and meta-analysis of observational studies. Int J Stroke. (2019) 14:125–36. 10.1177/1747493018806196 [DOI] [PubMed] [Google Scholar]
- 3.Wilson RD. Mortality and cost of pneumonia after stroke for different risk groups. J Stroke Cerebrovasc Dis. (2012) 21:61–7. 10.1016/j.jstrokecerebrovasdis.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali AN, Howe J, Majid A, Redgrave J, Pownall S, Abdelhafiz AH. The economic cost of stroke-associated pneumonia in a UK setting. Top Stroke Rehabil. (2018) 25:214–23. 10.1080/10749357.2017.1398482 [DOI] [PubMed] [Google Scholar]
- 5.Teh WH, Smith CJ, Barlas RS, Wood AD, Bettencourt-Silva JH, Clark AB, et al. Impact of stroke-associated pneumonia on mortality, length of hospitalization, and functional outcome. Acta Neurol Scand. (2018) 138:293–300. 10.1111/ane.12956 [DOI] [PubMed] [Google Scholar]
- 6.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. (2011) 77:1338–45. 10.1212/WNL.0b013e31823152b1 [DOI] [PubMed] [Google Scholar]
- 7.Chapman C, Morgan P, Cadilhac DA, Purvis T, Andrew NE. Risk factors for the development of chest infections in acute stroke: a systematic review. Top Stroke Rehabil. (2018) 25:445–58. 10.1080/10749357.2018.1481567 [DOI] [PubMed] [Google Scholar]
- 8.Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. Prevalence of diabetes and its effects on stroke outcomes: a meta-analysis and literature review. J Diabetes Investig. (2019) 10:780–92. 10.1111/jdi.12932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunetti VC, Ayele HT, Yu OHY, Ernst P, Filion KB. Type 2 diabetes mellitus and risk of community-acquired pneumonia: a systematic review and meta-analysis of observational studies. CMAJ Open. (2021) 9:E62–70. 10.9778/cmajo.20200013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol. (2020) 11:1582. 10.3389/fimmu.2020.01582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanz París A, García JM, Gómez-Candela C, Burgos R, Martín Á, Matía P. Malnutrition prevalence in hospitalized elderly diabetic patients. Nutr Hosp. (2013) 28:592–9. 10.3305/nh.2013.28.3.6472 [DOI] [PubMed] [Google Scholar]
- 12.Vural Keskinler M, FeyÝzoglu G, Yildiz K, Oguz A. The frequency of malnutrition in patients with type 2 diabetes. Med Med J. (2021) 36:117–22. 10.5222/mmj.2021.44270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. (2007) 4:e115. 10.1371/journal.pmed.0040115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni J, Shou W, Wu X, Sun J. Prediction of stroke-associated pneumonia by the A2DS2, AIS-APS, and ISAN scores: a systematic review and meta-analysis. Expert Rev Respir Med. (2021) 15:1461–72. 10.1080/17476348.2021.1923482 [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Liu M, He W, Liu F, Cheng J, Wang H. Use of the A2DS2 scale to predict morbidity in stroke-associated pneumonia: a systematic review and meta-analysis. BMC Neurol. (2021) 21:33. 10.1186/s12883-021-02060-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao J, Hu Z, Lou F, Wu J, Wu Z, Yang S, et al. Higher stress hyperglycemia ratio is associated with a higher risk of stroke-associated pneumonia. Front Nutr. (2022) 9:784114. 10.3389/fnut.2022.784114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Wu T, Tian X, Lyu P, Wang J, Cao Y. High neutrophil percentage-to-albumin ratio can predict occurrence of stroke-associated infection. Front Neurol. (2021) 12:705790. 10.3389/fneur.2021.705790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO task force on stroke and other cerebrovascular disorders. Stroke. (1989) 20:1407–31. 10.1161/01.str.20.10.1407 [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. (2015) 38(Suppl):S8–16. 10.2337/dc15-S005 [DOI] [PubMed] [Google Scholar]
- 20.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. (1991) 337:1521–6. 10.1016/0140-6736(91)93206-o [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann S, Malzahn U, Harms H, Koennecke HC, Berger K, Kalic M, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. (2012) 43:2617–23. 10.1161/strokeaha.112.653055 [DOI] [PubMed] [Google Scholar]
- 22.Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. (2005) 82:777–83. 10.1093/ajcn/82.4.777 [DOI] [PubMed] [Google Scholar]
- 23.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. (1988) 16:128–40. 10.1016/0196-6553(88)90053-3 [DOI] [PubMed] [Google Scholar]
- 24.Kishore AK, Devaraj A, Vail A, Ward K, Thomas PG, Sen D, et al. Use of pulmonary computed tomography for evaluating suspected stroke-associated pneumonia. J Stroke Cerebrovasc Dis. (2021) 30:105757. 10.1016/j.jstrokecerebrovasdis.2021.105757 [DOI] [PubMed] [Google Scholar]
- 25.Knapp S. Diabetes and infection: is there a link?–A mini-review. Gerontology. (2013) 59:99–104. 10.1159/000345107 [DOI] [PubMed] [Google Scholar]
- 26.Chen N, Li Y, Fang J, Lu Q, He L. Risk factors for malnutrition in stroke patients: a meta-analysis. Clin Nutr. (2019) 38:127–35. 10.1016/j.clnu.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Li X. Correlation between inflammatory factors and post-stroke pneumonia in diabetic patients. Exp Therapeut Med. (2013) 6:105–8. 10.3892/etm.2013.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sui R, Zhang L. Risk factors of stroke-associated pneumonia in Chinese patients. Neurol Res. (2011) 33:508–13. 10.1179/016164111x13007856084205 [DOI] [PubMed] [Google Scholar]
- 29.Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. (2016) 4:148–58. 10.1016/s2213-8587(15)00379-4 [DOI] [PubMed] [Google Scholar]
- 30.Ji R, Shen H, Pan Y, Wang P, Liu G, Wang Y, et al. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. (2013) 44:1303–9. 10.1161/strokeaha.111.000598 [DOI] [PubMed] [Google Scholar]
- 31.Smith CJ, Bray BD, Hoffman A, Meisel A, Heuschmann PU, Wolfe CD, et al. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J Am Heart Assoc. (2015) 4:e001307. 10.1161/jaha.114.001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JS, Choi HS, Ko YG, Yun DH. Performance of the Geriatric Nutritional Risk Index in predicting 28-day hospital mortality in older adult patients with sepsis. Clin Nutr. (2013) 32:843–8. 10.1016/j.clnu.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 33.Funamizu N, Omura K, Ozaki T, Honda M, Mishima K, Igarashi K, et al. Geriatric nutritional risk index serves as risk factor of surgical site infection after pancreatoduodenectomy: a validation cohort Ageo study. Gland Surg. (2020) 9:1982–8. 10.21037/gs-20-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, Zhong M, Xu Z, Ye Q, Xie W, Gao S, et al. Development and validation of a nomogram based on geriatric nutritional risk index to predict surgical site infection among gynecologic oncology patients. Front Nutr. (2022) 9:864761. 10.3389/fnut.2022.864761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang G, Pan Y, Zhang R, Wang M, Meng X, Li Z, et al. Prevalence and prognostic significance of malnutrition risk in patients with acute ischemic stroke: results from the third china national stroke registry. Stroke. (2022) 53:111–9. 10.1161/strokeaha.121.034366 [DOI] [PubMed] [Google Scholar]
- 36.Han CY, Chan CGB, Lim SL, Zheng XM, Woon ZW, Chan YT, et al. Diabetes-related nutrition knowledge and dietary adherence in patients with Type 2 diabetes mellitus: a mixed-methods exploratory study. Proc Singapore Healthcare. (2020) 29:81–90. 10.1177/2010105820901742 [DOI] [Google Scholar]
- 37.Li T, Zhang Y, Gong C, Wang J, Liu B, Shi L, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. (2020) 74:871–5. 10.1038/s41430-020-0642-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai C, Yan D, Xu M, Huang Q, Ren W. Geriatric Nutritional Risk Index is related to the risk of stroke-associated pneumonia. Brain Behav. (2022) 12:e2718. 10.1002/brb3.2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Zhang Y, Ma L, Niu X, Chang J. Risk of stroke-associated pneumonia during hospitalization: predictive ability of combined A(2)DS(2) score and hyperglycemia. BMC Neurol. (2019) 19:298. 10.1186/s12883-019-1497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci. (2016) 351:201–11. 10.1016/j.amjms.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 41.Barlas RS, Honney K, Loke YK, McCall SJ, Bettencourt-Silva JH, Clark AB, et al. Impact of hemoglobin levels and anemia on mortality in acute stroke: analysis of uk regional registry data, systematic review, and meta-analysis. J Am Heart Assoc. (2016) 5:e003019. 10.1161/jaha.115.003019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei CC, Zhang ST, Tan G, Zhang SH, Liu M. Impact of anemia on in-hospital complications after ischemic stroke. Eur J Neurol. (2018) 25:768–74. 10.1111/ene.13595 [DOI] [PubMed] [Google Scholar]
- 43.QiMuge N, Fang X, Chang B, Li DM, Li Y. Predicting population: development and validation of a new predictive nomogram for evaluating medication nonadherence risk in a type 2 diabetes. PeerJ. (2022) 10:e13102. 10.7717/peerj.13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.