Abstract
Downregulation of MHC class I (MHCI) molecules on tumor cells is recognized as a resistance mechanism of cancer immunotherapy. Given that MHCI molecules are potent regulators of immune responses, we postulated that the expression of MHCI by tumor cells influences systemic immune responses. Accordingly, mice-bearing MHCI-deficient tumor cells showed reduced tumor-associated extramedullary myelopoiesis in the spleen. Depletion of natural killer (NK) cells abrogated these differences, suggesting an integral role of immune-regulatory NK cells during tumor progression. Cytokine-profiling revealed an upregulation of TNF-α by NK cells in tumors and spleen in mice-bearing MHCI expressing tumors, and inhibition of TNF-α enhanced host myelopoiesis in mice receiving adoptive transfer of tumor-experienced NK cells. Our study highlights a critical role of NK cells beyond its identity as a killer lymphocyte and more importantly, the potential host responses to a localized tumor as determined by its MHCI expression.
Keywords: Adaptive Immunity; Myeloid-Derived Suppressor Cells; Lymphocyte Activation; Killer Cells, Natural
Key messages.
Enhanced extramedullary myelopoiesis in the spleen is prominent in mice-bearing MHC class I-expressing tumors.
Tumor-experienced natural killer cells contribute to myelopoiesis via the production of TNF-α.
Introduction
Cancer is an inflammatory disease that affects systemic hematopoietic responses. Tumor-derived factors can drive myelopoiesis resulting in the accumulation of immature myeloid cells to support tumor growth and interference with antitumor immune responses.1 2 Moreover, the frequency of peripheral blood granulocyte–monocyte progenitors (GMPs) correlates with tumor progression and predict worse survival.3
Natural killer (NK) cells are not only implicated in the surveillance and eradication of cancer but also in the cross-talk with myeloid cells, including dendritic cells, to favor antitumor immune responses.4 As means of immune evasion, the downregulation of tumor MHC class I (MHCI) can result in resistance to immune checkpoint inhibition, but at the same time, potentially implicate susceptibility to NK cell-mediated killing.5–7 NK cells have demonstrated therapeutic efficacy in the setting of adoptive transfer in acute myeloid leukemia, with clinical responses correlating to the persistence of infused NK cells in peripheral blood and in the bone marrow.8–10
Given the apparent role of MHCI in shaping host NK cell responses, this study sought to explore the influence of tumor MHCI expression not only on NK cell functions but also on the immunological and hematological responses in tumor-bearing hosts.
Methods
Detailed methods are listed in online supplemental information.
jitc-2022-005308supp001.pdf (555.3KB, pdf)
In vivo experiments
At day 0, 0.2×106 wild-type (WT) or B2M-knockout (KO) 4T1 cells were injected into the mammary fat pad of Balb/cAnNCrl mice. For RMA/S, RMA, and B16F10, tumor cells were injected subcutaneously on day 0 into the right flank of C57BL/6 mice at 1×106, 0.1×106 and 0.1×106 cells, respectively. For NK cell depletion in C57BL/6 mice, anti-NK1.1 was administered intraperitoneally on day −1 and once per week post tumor inoculation. Tumors and spleen were collected and analyzed on day 14 for B16F10 and day 21 for 4T1, respectively. In vivo studies were approved by the Swedish board of Agriculture (8525-2020, 6197-2019).
Colony formation assay and myeloid output assay
Colony formation unit (CFU) with splenocytes were performed accordingly to a previously reported study.11 Cells from colony cultures were harvested for phenotypic analysis by flow cytometry.
Statistics
Unless stated otherwise, data were analyzed using Graphpad Prism software by either Student’s t-test (two groups) or one-way ANOVA with multiple comparisons.
Results and discussion
Tumor MHCI expression and NK cells drive extramedullary myelopoiesis in the spleen
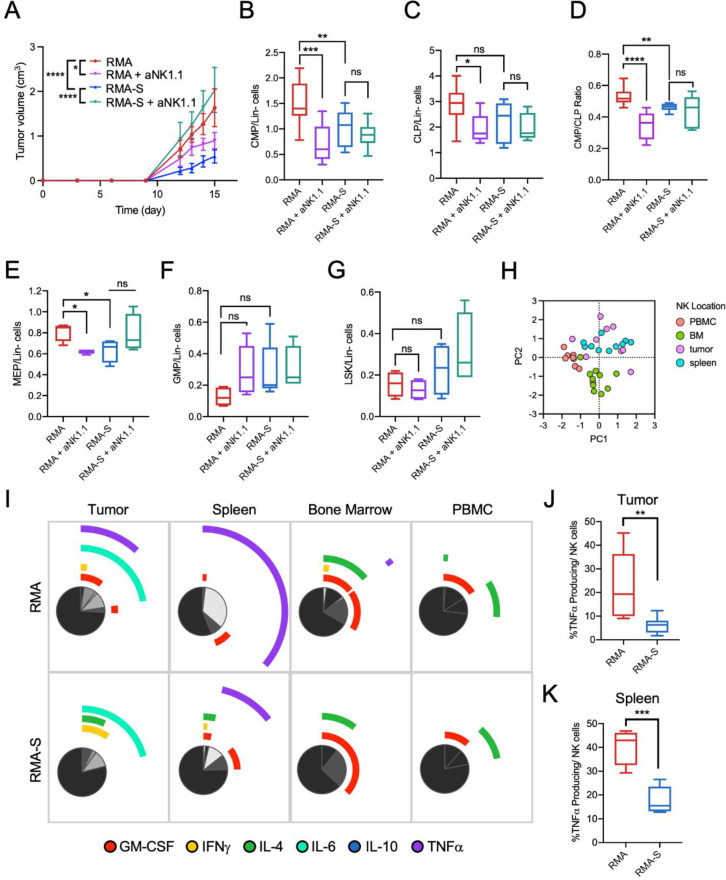
Recent studies demonstrated that tumor clones with high MHCI expression display low oncogenicity but high metastatic potential.12 13 Given that tumor MHCI expression affects immunogenicity and the immune microenvironment,14 this study sought to investigate if tumor MHCI expression influence hematopoietic responses in tumor-bearing hosts. The RMA and TAP-deficient RMA-S lymphoma models are widely used to study tumor MHCI and NK cells interactions.15 The progression of RMA/S tumors was significantly higher in the absence of host NK cells. In contrast, depletion of NK cells in RMA-bearing mice resulted in reduced tumor progression, suggesting a potential role of NK cells to support the progression of MHCI-positive tumors (figure 1A).
Figure 1.
Tumor MHCI expression and NK cells drive extramedullary myelopoiesis in the spleen. Tumor and spleen tissues were harvested from RMA-bearing and RMA/S-bearing C57BL/6 mice 15 days post tumor inoculation. (A) Progression of RMA and RMA/S (MHCI-deficient) syngeneic tumors in untreated mice or NK cell-depleted mice. One-way ANOVA with multiple comparisons at individual time points was used to test for significance. Frequencies of (B) common myeloid progenitor (CMP) and (C) common lymphoid progenitor (CLP) within lineage-negative live cells (Lin-cells) in spleen of RMA and RMA/S tumor-bearing mice. (D) CMP to CLP ratio in spleen of RMA and RMA/S tumor-bearing mice with or without NK cell depletion. Frequencies of (E) megakaryocytic-erythroid progenitor (MEP), (F) granulocyte-monocyte progenitor (GMP) and (G) LSK (lineage-, Sca1+, cKIT+) cells within Lin-cells in spleen of RMA and RMA/S tumor-bearing mice (A–G), n=4 per group. (H) PCA analysis of NK cell cytokine production profile based on flow cytometry of samples isolated from RMA and RMA/S-bearing mice. (I) Annotated pie charts for cytokine production profiles of NK cells residing in different locations of RMA and RMA/S-bearing mice. Frequencies of <5% are not shown. (J) frequencies of TNFα-producing NK cells within RMA and RMA/S tumors. (K) Frequencies of TNFα-producing NK cells in the spleen of RMA-bearing and RMA/S-bearing mice (D–G), n=5 per group. All data are presented in tukey’s boxplots except for (A, H, I). *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001. ANOVA, analysis of variance; MHCI, Major Histocompatibility Complex (MHC) class I; ns, non-significant; NK, natural killer.
Given that tumor progression drives extramedullary myelopoiesis in the spleen,1 frequencies of hematopoietic progenitors were analyzed. Comparing mice-bearing RMA to mice-bearing RMA/S tumors, the frequencies of CMP (common myeloid progenitor) and CMP:CLP (common lymphoid progenitor) ratio was significantly higher in mice-bearing RMA tumors even though the frequencies of CLP between these two groups were not significantly different. Notably, the differences in myelopoiesis between RMA-bearing and RMA/S-bearing mice were abrogated in NK cell-depleted mice (figure 1B, C, D). Furthermore, RMA-bearing mice show a higher frequency of MEP (megakaryocytic-erythroid progenitors) than RMA-bearing NK depleted-mice or RMA/S-bearing mice (figure 1E). Notably, since erythroid progenitors have considerable immune-suppressive capacities of T cells,16 17 NK cell depletion may impair antitumor T cell responses through enrichment of MEPs. No significant changes were observed in the frequencies of GMP and LSK (Lineage-, Sca-1+, cKIT+ cells) (figure 1F, G). To the best of our knowledge, this study is the first to report an association between tumor MHCI expression and cancer-associated myelopoiesis.
Exposure to tumor cells alters the composition of cytokines produced by host NK cells and several of these cytokines are recognized to influence hematopoiesis.18–20 We; therefore, hypothesized that the cytokine-profile of NK cells is involved in the observed hematopoietic alterations. Principal component analysis of the intracellular cytokine FACS (Fluorescence-Activated Cell Sorting) staining revealed that splenic NK cells share greater homology to tumorous NK cells as compared with those in bone marrow and peripheral blood (figure 1H). Compared with mice-bearing RMA/S tumors, higher frequencies of TNF-α producing splenic and tumorous NK cells was observed in mice-bearing RMA tumors (figure 1I, J, K). Since TNF-α produced by activated CD4 T cells has been shown to induce myelopoiesis,21 it is plausible that also NK cells follow a similar underlying mechanism to contribute to myelopoiesis.
Tumor-experienced NK cells enhance systemic myelopoiesis
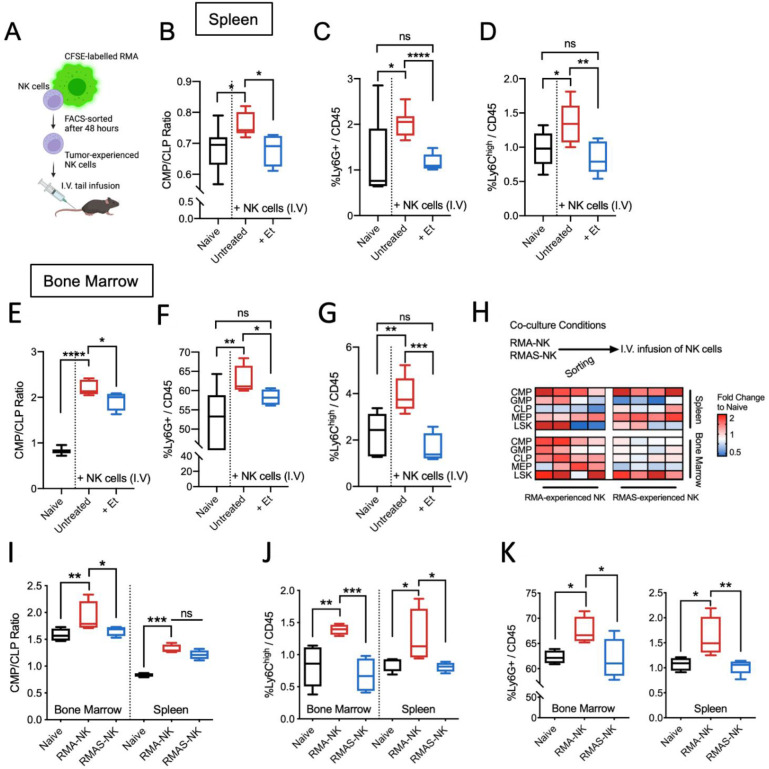
To investigate if tumor-experienced NK cells contribute to myelopoiesis, non-tumor-bearing mice were infused with RMA tumor-experienced NK cells (figure 2A). Similar to results obtained in RMA-bearing mice, the splenic ratio of CMP:CLP was significantly higher in mice receiving NK cells accompanied with increased proportions of immature monocytic (Ly6Chigh) cells and polymorphonuclear (Ly6G+) cells (figure 2B, C, D). To address if TNF levels influence myelopoiesis, NK cells were coadministered with the TNF inhibitor etanercept. In spleen, a significant reduction in CMP:CLP ratio, Ly6G+ and Ly6Chigh myeloid cells were observed compared with mice treated with NK cells alone (figure 2B, C, D). Likewise, CMP:CLP ratio within the bone marrow was elevated in mice infused with tumor-experienced NK cells which was significantly reduced in etanercept-treated mice (figure 2E). Similarly, NK cell infusion alone increased the frequency of Ly6G+ and Ly6Chigh myeloid cells and etanercept-treated mice had similar frequencies of these myeloid cells compared with control mice (figure 2F, G). In a separate experiment, etanercept treatment alone did not significantly change CMP and CLP frequencies. However, frequencies of LSK cells, GMP, and MEP in spleen and bone marrow was altered (online supplemental figure 1A–J). Nonetheless, etanercept did not significantly influence myelopoiesis with minor differences on Ly6G+and Ly6Chigh myeloid cells within both the bone marrow and spleen (online supplemental figure 1K to N).
Figure 2.
Infusion of tumor-experienced NK cells enhances systemic myelopoiesis without the presence of a tumor. (A) Experimental design of transfer of tumor-experienced NK cells into naïve C57BL/6 mice. Bone marrow and spleen tissues were analyzed 7 days after NK cell infusion. (B) CMP:CLP ratio, (C) frequencies of Ly6G+myeloid cells and (D) Ly6Chigh myeloid cells within the spleen of mice 7 days after receiving NK cell infusion and etanercept (et) treatment. (E) CMP:CLP ratio, (F) frequencies of Ly6G+myeloid cells and (G) Ly6Chigh myeloid cells within the bone marrow of mice 7 days after receiving NK cell infusion and etanercept (et) treatment. (B–G) n=5 naïve, 8 untreated with NK cell infusion, 5 with NK cell infusion and etanercept treatment given at 3 mg/kg body weight on days 0, 3, and 6. (H) Experimental design and heatmap overview of fold changes in various hmatopoietic progenitors compared with naïve mice. (I) CMP/CLP ratio within bone marrow and spleen of mice infused with RMA-experienced NK cells (RMA-NK) or RMA/S-experienced NK cells (RMA/S-NK) compared with untreated C57BL/6 mice. (J) Frequency of Ly6Chigh myeloid cells of total CD45+ cells within bone marrow and spleen of mice infused with RMA-NK or RMA/S-NK compared with untreated C57BL/6 mice. (K) frequency of Ly6G+ myeloid cells of total CD45+ cells within bone marrow (left panel) and spleen (right panel) of mice either infused with RMA-NK or RMA/S-NK compared with untreated C57BL/6 mice. (I–K) One-way ANOVA with multiple comparisons was used to test for significance with sample size of n=4 per group. All data are presented in tukey’s boxplots except for figure A and H. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001. ANOVA, analysis of variance; CMP:CLP, common myeloid progenitor:common lymphoid progenitor; NK, natural killer; ns, non-significant.
To address if NK cell-induced myelopoiesis is mediated by NK cells pre-exposed to MHCI-expressing RMA but not to MHCI-deficient RMA/S cells, a separate experiment was conducted in which differential hematopoietic responses were observed (figure 2H). A significant reduction of CMP/CLP ratio was observed in bone marrow but not in spleen of mice infused with RMA/S-experienced NK cells compared with mice infused with RMA-experienced NK cells (figure 2I). Nonetheless, frequencies of Ly6Chigh and Ly6G+myeloid cells were reduced in both spleen and bone marrow on infusion of RMA/S-experienced NK cells compared with mice infused with RMA-experienced NK cells (figure 2J and K). Collectively, differentially stimulated NK cells influence systemic hematopoiesis, where infusion of NK cells pre-exposed to MHCI-expressing target cells favor the generation of immature myeloid cells.
Melanoma and breast cancer show strong association between tumor MHCI expression and enhanced splenic myelopoiesis
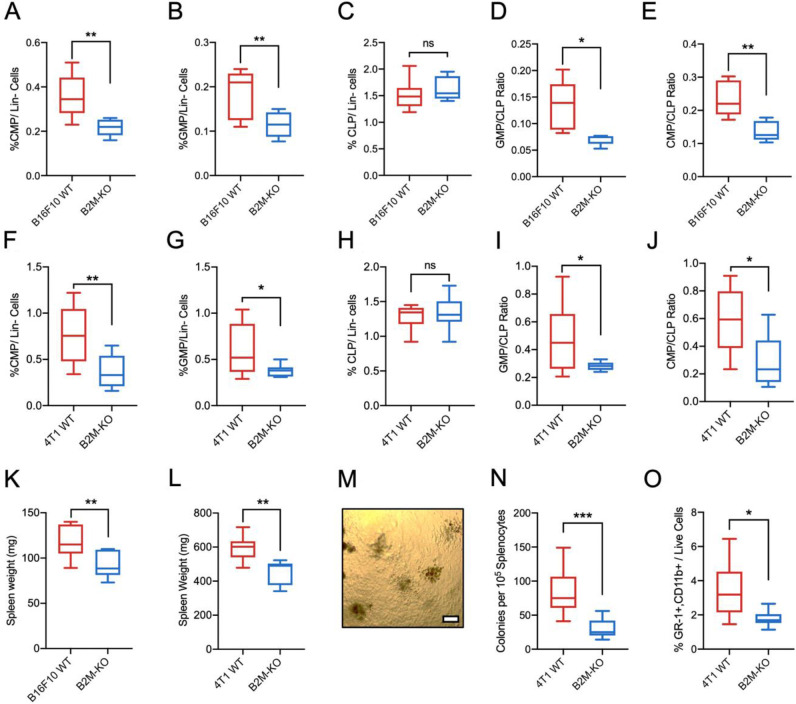
To corroborate our findings, we further investigated if NK cells influence myelopoiesis in other solid tumors. Even though tumor progression was not significantly different, CMP/CLP ratios, frequencies of Ly6G+and Ly6Chigh myeloid cells were significantly reduced in the spleen of NK cell-depleted mice-bearing B16F10 tumors (online supplemental figure 2A–D) and EO771 tumors (online supplemental figure 2E–H). To investigate if the observed effects on myelopoiesis by tumor MHCI expression extend beyond lymphoma into other cancers, CRISPR was used to KO B2M in B16F10 melanoma and 4T1 mammary carcinoma cells prior to tumor inoculation. In both models, an initial delay in tumor progression was observed by B2M-KO tumors but no significant differences in tumor size was observed after 14 days (online supplemental figure 3). Compared with mice-bearing B2M-KO tumors, mice-bearing WT tumors showed higher splenic myelopoiesis as evidenced by higher frequencies of GMP and CMP progenitors accompanied with high GMP:CLP and CMP:CLP ratios in both B16F10 (figure 3A, B, D, E) and 4T1 models (figure 3F, G, I, J). Similar to the RMA model, no significant changes were observed in the frequencies of CLP within the spleen (figure 3C,H). Unlike the RMA model (data not shown), spleen weights were prominently lower in mice-bearing B2M-KO compared with mice-bearing WT tumors (figure 3K,L) which is anticipated since cancer-driven myelopoiesis affects splenomegaly.22 In a CFU, splenocytes isolated from mice-bearing WT tumors formed significantly more colonies and contained higher frequency of immature myeloid cells than those isolated from mice-bearing B2M-KO tumors (figure 3M, N, O). Taken together, we demonstrate in different tumor models in both mice of C57BL/6 and BALB/c strains that tumor MHCI expression is associated with the host extramedullary myelopoiesis which is dependent partly on tumor progression and NK cells.
Figure 3.
B16F10 and 4T1 tumor models show stronger association between tumor MHCI expression and enhanced myelopoiesis with splenomegaly. Frequencies of (A) CMP, (B) GMP and (C) CLP within lineage-negative live cells (Lin- Cells) in spleen of B16F10 tumor-bearing mice. (D) Ratio of GMP to CLP and (E) ratio of CMP to CLP in spleen of B16F10 tumor-bearing mice. Frequencies of (F) common myeloid progenitor CMP, (G) GMP and (H) CLP within lineage-negative live cells (Lin- cells) in spleen of 4T1 tumor-bearing mice. (I) Ratio of GMP to CLP and (J) ratio of CMP to CLP in spleen of 4T1 tumor-bearing mice. (K) Weight of spleen isolated from B16F10 tumor-bearing mice. (L) weight of spleen isolated from 4T1 tumor-bearing mice (A–E and K). Bone marrow and spleen tissues were harvested from B16F10 WT and B16F10 B2M-KO-bearing C57BL/6 mice 14 days post-tumor inoculation, n=6 per group (F–J and L). Bone marrow and spleen tissues were harvested from 4T1 WT and 4T1 B2M-KO-bearing BALB/c mice 21 days post tumor inoculation, n=7 per group. (M) Representative image obtained from colon formation assay under 10X objective. Scale bar denotes 100 µm. (N) Number of colonies formed by splenocytes isolated from WT or B2M-KO tumors after 10 days of in vitro culture. (O) Percentage of GR-1+myeloid cells from cells harvested from colony formation assay. (J–L) n=9 per group. *p<0.05, **p<0.01 and ***p<0.001. CLP, common lymphoid progenitor; CMP, common myeloid progenitor; GMPs, granulocyte–monocyte progenitors; KO, knockout; ns, non-significant; MHCI, MHC class I; NK, natural killer; WT, wild-type.
Evidently, the expression of MHCI enhances tumor immunogenicity and responsiveness to immune checkpoint therapy.7 23 Other factors such as tumor mutational burden and the tumor microenvironment are also considered important for objective therapeutic response.24 While cancer-associated myelopoiesis is predominantly driven by a variety of tumor-derived growth factors such GM-CSF, G-CSF, IL-6, and IL-1β,2 25 26 this study demonstrates that the sensitivity of tumors to NK cell-mediated rejection alters the production of immune-regulatory cytokines produced by NK cells contributing to changes in myelopoiesis. Moreover, in the context of CMV infection, differential stimulation of NK cells contributes to the extramedullary expansion of TER119+ erythroid progenitors.27 While this study uncovers a novel regulatory role of NK cells, other tumor-derived factors may also contribute in part to the differential myelopoiesis responses associated with tumor MHCI expression. From a translational perspective, strategies to increase tumor MHCI expression could potentially influence NK cell-dependent myelopoiesis that may be worthwhile investigating in future studies. Further investigating NK cell-dependent myelopoiesis in patients with hematological malignancies could be highly relevant for the development of improved therapeutics and identification of potential biomarkers. Such findings could possibly extend to other pathological disorders beyond cancer.
Acknowledgments
We thank the animal facility (KM-F) and the flow cytometry core of Karolinska Institute for the assistance throughout the study. RMA and RMA/S were provided by Stina Wickström. We would also like to thank Apple Tay Huimin and Cui Weiyingqi for the technical assistance.
Footnotes
Twitter: @DrNeosy, @LundqvistLab
SYN and XJ contributed equally.
Contributors: Conceptualization: SN and AL. Methodology: SN, XJ, LT, DT, JG, ZC, MCDLS, NB, SDSF and AKW. Investigation and analysis: SN, XJ, LT, DT, JG, AKW, EA, CR, YC and AL. Writing-original Draft: SN. Writing-review and editing: SN, XJ, CR and AL. Supervision and project administration: SN, AL, EA, CR and YC. Funding acquisition: AL, EA and CR.
Funding: This works was supported by grants from The Swedish Cancer Society (#CAN2018/451 and #21 1524 Pj), The Cancer Research Funds of Radiumhemmet (181183 # and #211253), Stiftelsen Tornspiran, and Karolinska Institutet.
Competing interests: No, there are no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Wu C, Hua Q, Zheng L. Generation of myeloid cells in cancer: the spleen matters. Front Immunol 2020;11:1126. 10.3389/fimmu.2020.01126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Innamarato P, Pilon-Thomas S. Reactive myelopoiesis and the onset of myeloid-mediated immune suppression: implications for adoptive cell therapy. Cell Immunol 2021;361:104277. 10.1016/j.cellimm.2020.104277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu W-C, Sun H-W, Chen H-T, et al. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc Natl Acad Sci U S A 2014;111:4221–6. 10.1073/pnas.1320753111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huntington ND, Cursons J, Rautela J. The cancer–natural killer cell immunity cycle. Nat Rev Cancer 2020;20:437–54. 10.1038/s41568-020-0272-z [DOI] [PubMed] [Google Scholar]
- 5.Ljunggren H-G, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 1990;11:237–44. 10.1016/0167-5699(90)90097-S [DOI] [PubMed] [Google Scholar]
- 6.Tu MM, Mahmoud AB, Makrigiannis AP. Licensed and unlicensed NK cells: differential roles in cancer and viral control. Front Immunol 2016;7:166. 10.3389/fimmu.2016.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montesion M, Murugesan K, Jin DX, et al. Somatic HLA class I loss is a widespread mechanism of immune evasion which refines the use of tumor mutational burden as a biomarker of checkpoint inhibitor response. Cancer Discov 2021;11:282–92. 10.1158/2159-8290.CD-20-0672 [DOI] [PubMed] [Google Scholar]
- 8.Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016;8:357ra123. 10.1126/scitranslmed.aaf2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolstra H, Roeven MWH, Spanholtz J, et al. Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clinical Cancer Research 2017;23:4107–18. 10.1158/1078-0432.CCR-16-2981 [DOI] [PubMed] [Google Scholar]
- 10.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105:3051–7. 10.1182/blood-2004-07-2974 [DOI] [PubMed] [Google Scholar]
- 11.Regan-Komito D, Swann JW, Demetriou P, et al. GM-CSF drives dysregulated hematopoietic stem cell activity and pathogenic extramedullary myelopoiesis in experimental spondyloarthritis. Nat Commun 2020;11:155. 10.1038/s41467-019-13853-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrido C, Romero I, Berruguilla E, et al. Immunotherapy eradicates metastases with reversible defects in MHC class I expression. Cancer Immunol Immunother 2011;60:1257–68. 10.1007/s00262-011-1027-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero I, Garrido C, Algarra I, et al. MHC intratumoral heterogeneity may predict cancer progression and response to immunotherapy. Front Immunol 2018;9:102. 10.3389/fimmu.2018.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Algarra I, Garrido F, Garcia-Lora AM. MHC heterogeneity and response of metastases to immunotherapy. Cancer Metastasis Rev 2021;40:501–17. 10.1007/s10555-021-09964-4 [DOI] [PubMed] [Google Scholar]
- 15.Ljunggren HG, Kärre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med 1985;162:1745–59. 10.1084/jem.162.6.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namdar A, Koleva P, Shahbaz S, et al. CD71+ erythroid suppressor cells impair adaptive immunity against bordetella pertussis. Sci Rep 2017;7:7728. 10.1038/s41598-017-07938-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long H, Jia Q, Wang L, et al. Tumor-induced erythroid precursor-differentiated myeloid cells mediate immunosuppression and curtail anti-PD-1/PD-L1 treatment efficacy. Cancer Cell 2022;40:674–93. 10.1016/j.ccell.2022.04.018 [DOI] [PubMed] [Google Scholar]
- 18.Najafi S, Ghanavat M, Shahrabi S, et al. The effect of inflammatory factors and their inhibitors on the hematopoietic stem cells fate. Cell Biol Int 2021;45:900–12. 10.1002/cbin.11545 [DOI] [PubMed] [Google Scholar]
- 19.Cardoso A, Martins AC, Maceiras AR, et al. Interleukin-10 induces interferon-γ-dependent emergency myelopoiesis. Cell Rep 2021;37:109887. 10.1016/j.celrep.2021.109887 [DOI] [PubMed] [Google Scholar]
- 20.Chiba Y, Mizoguchi I, Hasegawa H, et al. Regulation of myelopoiesis by proinflammatory cytokines in infectious diseases. Cell. Mol. Life Sci. 2018;75:1363–76. 10.1007/s00018-017-2724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Sayed MF, Amrein MA, Bührer ED, et al. T-cell–secreted TNFα induces emergency myelopoiesis and myeloid-derived suppressor cell differentiation in cancer. Cancer Res 2019;79:346–59. 10.1158/0008-5472.CAN-17-3026 [DOI] [PubMed] [Google Scholar]
- 22.Wu C, Ning H, Liu M, et al. Spleen mediates a distinct hematopoietic progenitor response supporting tumor-promoting myelopoiesis. J Clin Invest 2018;128:3425–38. 10.1172/JCI97973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrido F, Aptsiauri N, Doorduijn EM, et al. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol 2016;39:44–51. 10.1016/j.coi.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JS, Ruppin E. Multiomics prediction of response rates to therapies to inhibit programmed cell death 1 and programmed cell death 1 ligand 1. JAMA Oncol 2019;5:1614–8. 10.1001/jamaoncol.2019.2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity 2010;32:790–802. 10.1016/j.immuni.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 26.Meyer C, Sevko A, Ramacher M, et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A 2011;108:17111–6. 10.1073/pnas.1108121108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan S, Ruzsics Z, Mitrović M, et al. Natural killer cells are required for extramedullary hematopoiesis following murine cytomegalovirus infection. Cell Host Microbe 2013;13:535–45. 10.1016/j.chom.2013.04.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005308supp001.pdf (555.3KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.