Abstract
N´-Nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) – which always occur together and are present exclusively in tobacco products – are classified as “carcinogenic to humans” (Group 1) by the International Agency for Research on Cancer. While 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) serves as an excellent biomarker for NNK exposure, the currently available biomarker for NNN exposure is urinary “total NNN” (free NNN plus its N-glucuronide). Quantitation of urinary NNN requires extensive precautions to prevent artifactual formation of NNN resulting from nitrosation of nornicotine during analysis. NNN itself can also be formed endogenously by the same nitrosation reaction which may sometimes cause overestimation of exposure to preformed NNN. It is thus important to develop an alternative biomarker to specifically reflect NNN metabolic fate and facilitate relevant cancer etiology studies. In this study, we report the first detection of N´-nitrosonornicotine-1N-oxide (NNN-N-oxide) in human urine. Using a highly specific and sensitive MS3 transition-based method, NNN-N-oxide was quantified with a mean level of 8.40 ± 6.04 fmol/mL in the urine of 10 out of 32 cigarette smokers. It occurred in a substantially higher level in the urine of 13 out of 14 smokeless tobacco users, amounting to a mean concentration of 85.2 ± 96.3 fmol/mL urine. No NNN-N-oxide was detected in any of the non-smoker urine samples analyzed (n = 20). The possible artifactual formation of NNN-N-oxide during sample preparation steps was excluded by experiments using added ammonium sulfamate. The low levels of NNN-N-oxide in the urine of tobacco users indicate that the pyridine N-oxidation pathway represents a minor detoxification pathway of NNN, which further supports the importance of the α-hydroxylation pathway of NNN metabolic activation in humans.
Graphical Abstract

INTRODUCTION
N´-Nitrosonornicotine (1, NNN, Scheme 1) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (7, NNK) are two strongly carcinogenic N-nitrosamines which always occur together and are present exclusively in tobacco products.1 The levels of NNN and NNK remain unacceptably high in some types of tobacco filler and tobacco smoke.2, 3 In some smokeless tobacco products in South-East Asia, NNN concentrations reached >50 μg/g tobacco powder.4 The carcinogenic activities of NNN and NNK have been well documented in laboratory animals including mice, rats, Syrian golden hamsters and mink.5, 6 NNN caused tumors predominantly in the tissues of esophagus, oral mucosa, nasal mucosa, lung and trachea, while NNK specifically induced lung carcinogenesis independent of the routes of administration. Based on the carcinogenicity data and an understanding of their carcinogenic mechanisms, the International Agency for Research on Cancer has classified NNN together with NNK as Group 1 carcinogens (“carcinogenic to humans”).7
Scheme 1.

Major urinary metabolites formed by NNN and NNK metabolism and their formation from the minor metabolic pathways of nicotine metabolism.
Both NNN and NNK require metabolic activation – mainly mediated by cytochrome P450s – to exert their carcinogenicity.5, 6 The major metabolite of NNK is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL, 10), which has been validated as an excellent exposure biomarker for NNK.8 However, the major metabolites of NNN – hydroxy acid 13 (accounting for 37.1 – 53.3% of total dose) and keto acid 12 (12.8 – 31.1%) in rats9 or hydroxy acid 13 (43.8 ± 4.0% of total radioactivity) and norcotinine 3 (13.1%) and its derivatives norcotinine-1N-oxide (4, 16.5%), 3′-hydroxynorcotinine (5, 16.9%) and 3′-(O-β-d-glucopyranuronosyl)hydroxynorcotinine (6, 5.4%) in a patas monkey10 – are not specific to NNN metabolism. As shown in Scheme 1, hydroxy acid 13 and keto acid 12 are also formed in NNK and nicotine metabolism.5, 6 Similarly, norcotinine 3 also represents 1 – 2% of the nicotine dose in the urine of smokers.11 Levels of nicotine in tobacco products are more than 1,000 times greater than those of NNN, so minor metabolites of nicotine are not suitable as NNN biomarkers.
The currently used biomarker for monitoring NNN exposure is urinary “total NNN” (free NNN plus its N-glucuronide).12 This biomarker has been successfully applied in the Shanghai Cohort study, providing strong evidence of its potential in predicting future esophageal cancer incidence in cigarette smokers.13 However, quantitation of urinary NNN requires extensive precautions to prevent artifactual formation.14, 15 Endogenous formation of NNN through facile nitrosation of nornicotine 17 – the metabolite of nicotine and also present in tobacco products – may also cause potential overestimation issues.16–19 It is thus important to develop a new biomarker which can specifically reflect NNN metabolic fate and facilitate tobacco-associated cancer etiology studies.
N´-nitrosonornicotine-1N-oxide (2, NNN-N-oxide, Scheme 1) is an NNN-specific metabolite which has been identified in the urine of NNN-treated mice, rats and hamsters, representing 6.7 – 10.7% of total radioactivity of NNN doses in rats.9, 20, 21 However, this metabolite was not detected in the urine and serum of a patas monkey administered [5-3H]NNN, which raises some questions about its likely presence in humans.10 In this study, we used a highly specific and sensitive liquid chromatography-nanoelectrospray ionization-high resolution tandem mass spectrometry (LC-NSI-HRMS/MS) method to analyze and quantify NNN-N-oxide in the urine of cigarette smokers and smokeless tobacco users.
EXPERIMENTAL SECTION
Caution:
NNN is strongly carcinogenic. It should be handled in a well-ventilated fume hood with extreme caution and with appropriate protective equipment.
Chemicals and supplies:
Racemic [pyridine-d4]NNN (catalog # N535002, 98% purity) was purchased from Toronto Research Chemicals (Ontario, Canada). StrataTM-X 33 μm Polymeric Reversed Phase cartridges (30 mg/1 mL, 100/pk, catalog # 8B-S100-TAK) were obtained from Phenomenex (Torrance, CA). Oasis® MCX 3 cc Vac Cartridges (60 mg Sorbent per Cartridge, 60 μm, 100/pk, Part # 186000253) and Oasis® PRiME HLB 3 cc Vac Cartridges (60 mg Sorbent per Cartridge, 100/pk, Part # 186008056) were procured from Waters (Milford, MA). Titan syringe filters (PTFE, 0.2 μm, 4 mm, 100/pk, catalog # 42204-NP) were obtained from Thermo Fisher Scientific (Waltham, MA). Needles (PTFE, Luer-Lock, 10/pack, part # 5188–5253) were purchased from Agilent Technologies (Santa Clara, CA). The 4 mL siliconized glass vials (catalog # CTV-7472) and 300 μL fused insert siliconized glass vials (catalog # CP-0952–03SIL) were procured from Chrom Tech (Apple Valley, MN). All other chemicals and supplies were obtained from Sigma-Aldrich (St. Louis, MO) or Thermo Fisher Scientific. Milli-Q water (Millipore) was routinely used unless otherwise mentioned.
NNN-N-oxide:
NNN-N-oxide was synthesized as reported previously.22 The synthesized compound was purified by reverse phase HPLC (>99% purity) for this study. HPLC purification was conducted using Waters Associates (Milford, MA) systems equipped with a Shimadzu SPD-10A 0.2 mm Prep UV-vis detector (254 nm). A Luna 5 μm C18(2) 100A 250 × 10 mm column purchased from Phenomenex (Torrance, CA) was used for the separation. The flow rate was 4 mL/min. The LC gradient started from 5% MeOH in H2O for 10 min, followed by a linear increase to 90% MeOH in H2O over 15 min. After holding at 90% MeOH in H2O for 1 min, the gradient was returned to the initial conditions of 5% MeOH in H2O over 2 min. The instrument was equilibrated for 2 min before the next injection. The desired product eluted at a retention time of 17.0 min. The NMR (Figure S1) and HRMS data agreed with the reported values. 1H NMR (500 MHz, DMSO-d6) δ 8.30 (t, J = 1.7 Hz, 0.6H, pyridine-H2, (E)-isomer), 8.18 (ddd, J = 6.4, 1.8, 0.9 Hz, 0.6H, pyridine-H6, (E)-isomer), 8.08 (dt, J = 8.0, 1.3 Hz, 0.8H, pyridine-H2 and -H6, (Z)-isomer), 7.43 (dd, J = 8.0, 6.4 Hz, 0.6H, pyridine-H5, (E)-isomer), 7.36 – 7.31 (m, 0.4H, pyridine-H5, (Z)-isomer), 7.28 (ddd, J = 7.9, 1.7, 0.8 Hz, 0.6H, pyridine-H4, (E)-isomer), 7.09 (dt, J = 8.0, 1.3 Hz, 0.4H, pyridine-H4, (Z)-isomer), 5.65 (t, J = 6.9 Hz, 0.6H, pyrrolidine-H2′, (E)-isomer), 5.06 (t, J = 7.4 Hz, 0.4H, pyrrolidine-H2′, (Z)-isomer), 4.56 (ddd, J = 12.3, 7.4, 5.2 Hz, 0.4H, pyrrolidine-H5′a, (Z)-isomer), 4.49 (dddd, J = 11.6, 8.1, 7.0, 1.3 Hz, 0.4H, pyrrolidine-H5′b, (Z)-isomer), 3.79 – 3.60 (m, 1.2H, pyrrolidine-H5′, (E)-isomer), 2.53 – 2.38 (m, 1H, pyrrolidine-H3′a, (Z)- and (E)-isomer), 2.15 – 2.02 (m, 1H, pyrrolidine-H3′b, (E)-isomer and pyrrolidine-H4′a, (Z)-isomer), 2.02 – 1.89 (m, 1.6H, pyrrolidine-H4′a, (E)-isomer and pyrrolidine-H4′b, (Z)- and (E)-isomer), 1.84 (ddt, J = 13.0, 8.0, 6.7 Hz, 0.4H, pyrrolidine-H3′b, (Z)-isomer). 13C NMR (126 MHz, DMSO-d6) δ 140.9 (pyridine-C3, (E)-isomer), 139.8 (pyridine-C3, (Z)-isomer), 137.9 (pyridine-C6, (E)-isomer), 137.5 (pyridine-C2, (E)-isomer), 137.3 (pyridine-C6, (Z)-isomer), 136.4 (pyridine-C2, (Z)-isomer), 126.6 (pyridine-C5, (E)-isomer), 126.3 (pyridine-C5, (Z)-isomer), 123.7 (pyridine-C4, (E)-isomer), 122.5 (pyridine-C4, (Z)-isomer), 61.2 (pyrrolidine-C2′, (E)-isomer), 57.6 (pyrrolidine-C2′, (Z)-isomer), 50.5 (pyrrolidine-C5′, (Z)-isomer), 46.3 (pyrrolidine-C5′, (E)-isomer), 32.7 (pyrrolidine-C3′, (Z) or (E)-isomer), 32.4 (pyrrolidine-C3′, (E) or (Z)-isomer), 22.4 (pyrrolidine-C4′, (Z) or (E)-isomer), 20.6 (pyrrolidine-C4′, (E) or (Z)-isomer). HRMS (Orbitrap): [M+H]+ calc’d 194.0924; found 194.0925.
[pyridine-d4]NNN-N-oxide:
Using the same synthetic approach,22 [pyridine-d4]NNN-N-oxide was synthesized starting from [pyridine-d4]NNN. To a solution of [pyridine-d4]NNN (2.5 mg) in CH2Cl2 (0.5 mL) was added excess m-chloroperoxybenzoic acid (~50 mg). The reaction mixture was stirred at room temperature overnight. Then the solvent was evaporated and the resulting residue was reconstituted in MeOH and subjected to reverse phase HPLC for purification. The fraction at 17.0 min was collected using the conditions described above. The desired product was obtained (2.7 mg, 99%) after concentrating to dryness and was characterized by NMR (Figure S2) and HRMS. 1H NMR (500 MHz, DMSO-d6) δ 5.77 – 5.46 (m, 0.6H, pyrrolidine-H2′, (E)-isomer), 5.06 (t, J = 7.4 Hz, 0.4H, pyrrolidine-H2′, (Z)-isomer), 4.74 – 4.34 (m, 0.8H, pyrrolidine-H5′, (Z)-isomer), 3.87 – 3.59 (m, 1.2H, pyrrolidine-H5′, (E)-isomer), 2.49 – 2.38 (m, 1H, pyrrolidine-H3′a, (Z)- and (E)-isomer), 2.17 – 2.03 (m, 1H, pyrrolidine-H3′b, (E)-isomer and pyrrolidine-H4′a, (Z)-isomer), 2.03 – 1.90 (m, 1.6H, pyrrolidine-H4′a, (E)-isomer and pyrrolidine-H4′b, (Z)- and (E)-isomer), 1.88 – 1.81 (m, 0.4H, pyrrolidine-H4′b, (E)-isomer). HRMS (Orbitrap): [M+H]+ calc’d 198.1175; found 198.1175.
Urine samples:
Urine samples from cigarette smokers (20 subjects) and nonsmokers (20 subjects) were retrieved from the Biorepository of the University of Minnesota Tobacco Research Programs. All urine collection procedures were approved by the University of Minnesota Institutional Review Board. The 20 cigarette smokers smoked an average (± SD) of 17.4 ± 10.2 cigarettes per day. The smoking status of 15 of the smokers and 15 of the nonsmokers was verified by their alveolar carbon monoxide (CO) level. Their demographics are summarized in Table S1. Pooled smokers’ urine used as the positive control and pooled non-smokers’ urine used as the negative control were all from the Biorepository. Urine of another 12 cigarette smokers were from an ongoing study comparing biomarkers of cigarette smoking with those of e-cigarette use or no use of any tobacco or nicotine product, approved by the University of Minnesota Institutional Review Board.23 Their demographics are summarized in Table S1; their smoking status was verified by the urinary biomarkers total nicotine equivalents (TNE) and total NNAL as shown in Table S2.
Urine samples from smokeless tobacco users (14 subjects) were retrieved from a previous study of toxicant exposure across different brands of smokeless tobacco products.24 They used an average of 16.1 g wet weight of the smokeless tobacco products/day with NNN occurring at a median level of 2.17 μg/g wet weight.24 Thus, the daily exposure of NNN to the smokeless tobacco users included in this study was estimated to be 34.9 μg (197 nmol).
Urine sample preparation:
Urine samples were partially purified to enrich NNN-N-oxide based on the protocol used for analysis of 4-(methylnitrosoamino)-1-(1-oxido-3-pyridinyl)-1-butanol (NNAL-N-oxide) with some modifications.25 Urine (0.5 mL) spiked with 25 fmol [pyridine-d4]NNN-N-oxide was added to a solution of CH3CN/MeOH/acetone (v/v/v, 1:1:1, 1.0 mL). The resulting mixture was vortexed and placed on ice for 15 min followed by centrifuging at 4 °C, 14000 r.p.m. for 10 min. The supernatant was transferred to a 4 mL siliconized glass vial and dried with a SpeedVac.
The dried sample was reconstituted in 1 mL H2O. It was loaded on an Oasis HLB cartridge (3 cc) that was preconditioned with 3 mL each of MeOH and H2O. The cartridge was washed with 3 mL H2O and the analyte was eluted with 3 mL 50% MeOH. The 50% MeOH fractions were dried with a SpeedVac. The dried sample was dissolved in 590 μL H2O and 500 μL 6% formic acid in H2O. The resulting solution was loaded on an Oasis MCX cartridge (3 cc) that was preconditioned with 3 mL each of 1% NH4OH in MeOH, MeOH, 2% formic acid in H2O and H2O. The cartridge was washed with 3 mL each of 2% formic acid in H2O, H2O, 2% formic acid in MeOH and MeOH. The analyte was eluted with 1.8 mL 1% NH4OH in MeOH which was collected in a 4 mL glass vial prefilled with 200 μL 6% formic acid in H2O. The fractions were dried with a SpeedVac. The dried sample was reconstituted in 500 μL H2O and loaded on a Strata-X cartridge (1 mL) that was preconditioned with 1 mL each of MeOH and H2O. The cartridge was washed with 1 mL each of H2O and 5% MeOH. The analyte was eluted with 1 mL 50% MeOH and dried with a SpeedVac. The residue was reconstituted in 100 μL 10% CH3CN (Optima®) in H2O (Optima®) and transferred to a 300 μL fused insert silanized glass vial. The sample was dried with a SpeedVac and re-dissolved in 50 μL of 5 mM NH4OAc in H2O (unadjusted pH, ~6.0) (Optima®). The solution was filtered with a Titan syringe filter (Thermo Fisher) to a new 300 μL fused insert silanized glass vial prior to mass spectrometry analysis.
Urine treated with ammonium sulfamate:
To investigate possible artifactual formation of NNN-N-oxide resulting from nitrosation of its potential precursor compounds such as nornicotine-1N-oxide during sample preparation, ammonium sulfamate (~5 mg) was added to the urine sample (0.5 mL)14, 15 with the same mixed solution of CH3CN/MeOH/acetone (1.0 mL) and processed as described above. The dried sample was dissolved in 1 mL H2O with addition of another ~5 mg ammonium sulfamate. It was partially purified by the same method as described above.
LC-NSI-HRMS/MS method:
A 2 μL aliquot of worked up urine was analyzed by the liquid chromatography-nanoelectrospray ionization-high resolution tandem mass spectrometry (LC-NSI-HRMS/MS) method. A Dionex UltiMate 3000 RSLCnano UPLC system equipped with a 5 μL autosampler injection loop was used by coupling to the Nanospray Flex ion source (Thermo Fisher Scientific, San Jose, CA). Chromatographic separation was performed using a custom-packed capillary column (75 μm i.d., ~20 cm length, 10 μm orifice) containing a fused-silica emitter (New Objective, Woburn, MA) with 5 μm particle size Luna C18 stationary phase (Phenomenex, Torrance, CA). The mobile phase consisted of (A) 5 mM NH4OAc in H2O (unadjusted pH, ~6.0) (Optima®) and (B) MeOH (Optima®). A 20-min program was used with a gradient starting from 5% B at a flow rate of 1 μL/min for 5.5 min, then holding at 5% B while decreasing the flow rate to 0.3 μL/min over 0.5 min. While running at the flow rate of 0.3 μL/min, the gradient increased linearly to 30% B over 10 min, followed by ramping to 90% B in 1 min. The gradient was held at 90% B for 0.5 min, then the flow rate was returned to 1 μL/min, and the gradient was returned to 5% B in 1 min. The instrument was equilibrated for 1.5 min at the initial conditions before the next injection. The injection valve was switched at 5.5 min to remove the sample loop from the flow path during the gradient. A temperature control of 5.0 °C was applied to the autosampler during the MS analysis. The needle height was set to 1 mm.
The samples were analyzed using a Thermo Scientific Orbitrap Fusion Lumos Tribrid™ mass spectrometer (San Jose, CA) in the positive ion profile mode. Levels of urinary NNN-N-oxide were quantified by the targeted MS3 scan. The first-generation (MS2) ions were isolated in the quadrupole mode with an isolation window of m/z 1.5. The MS activation for the MS2 fragmentation was collision-induced dissociation (CID) with a collision energy of 30%. The activation time was 10 ms. The second-generation (MS3) ions were isolated and analyzed by the Orbitrap detector with an isolation window of m/z 2.0. The MS3 activation type was higher-energy collision dissociation (HCD) with a collision energy of 10%. The Orbitrap detector was set with a resolution of 60000 and a defined scan range of m/z 100 – m/z 200. The maximum injection time was 118 ms. Two transitions were monitored for the MS3 quantitation of NNN-N-oxide in the urine: m/z 194.0924 [M+H]+ → 164.0944 [M - NO] + → 147.0917 [M-NO2]+ and m/z 198.1175 [pyridine-d4-M+H]+ → 168.1195 [pyridine-d4-M-NO]+ → 151.1168 [pyridine-d4-M-NO2]+. The normalized automatic gain control (AGC) target (%) setting was chosen to be “standard” (5 × 104) and the radio frequency (RF) lens (%) setting was 60. The number of micro-scans was set at 1. The spray voltage was 2.5 kV. The ion transfer tube temperature was 300 °C. The EASY-IC™ internal mass calibration feature was used to ensure maximum mass accuracy. Extraction of precursor ion signals and product ion signals was performed with an accurate mass tolerance of 5 ppm. Thermo Xcalibur Qual Browser (version 4.3) was used to process the MS data.
Calibration standard and quantitation:
The concentrations of calibration standards in H2O were chosen to cover the range of NNN-N-oxide levels expected in the human urine samples. Two calibration curves were established to determine the low and high concentrations of NNN-N-oxide in urine. For the low concentration range determination, the calibration standards were prepared with a constant concentration (0.5 fmol/μL) of [pyridine-d4]NNN-N-oxide and a varying concentration (0.01, 0.025, 0.05, 0.25 and 0.5 fmol/μL) of NNN-N-oxide. For the high concentration range determination, the varying concentrations of NNN-N-oxide were 0.5, 1.0, 2.5 and 5.0 fmol/μL while the concentration of [pyridine-d4]NNN-N-oxide was constant at 0.5 fmol/μL.
A linear calibration curve was constructed by plotting the MS3 peak ratios of NNN-N-oxide versus [pyridine-d4]NNN-N-oxide against the corresponding concentrations of the two standards. The determined levels of NNN-N-oxide in the urine were calculated using the same quantitative ratios of MS3 peak areas of the two analytes. The limit of quantitation (LOQ) in urine was assessed by the lowest spiked level of NNN-N-oxide in pooled non-smokers’ urine that produced a coefficient of variation (CV) of less than 20%. The recovery and the ion suppression rate were assessed by comparing the MS3 peak areas of [pyridine-d4]NNN-N-oxide in the blank urine versus the blank urine or H2O spiked with [pyridine-d4]NNN-N-oxide after drying. Mann-Whitney U test was used to compare the mean of total NNN and NNN-N-oxide in the urine of smokeless tobacco users (Table S3); the p value was <0.00001 at a 95% confidence interval.
RESULTS AND DISCUSSION
Characterization of NNN-N-oxide and [pyridine-d4]NNN-N-oxide
NNN-N-oxide and [pyridine-d4]NNN-N-oxide were synthesized in high yields by a one-step oxidation reaction starting from NNN and [pyridine-d4]NNN, respectively. Both synthesized compounds contained 60% (E)-isomer and 40% (Z)-isomer (Figures S1 and S2), which agreed with the NMR data reported previously.22, 26 The two isomers however were not separable under the HPLC conditions applied for compound purification and the MS analysis in this study.
As shown in Figure 1 and Scheme S1, the MS2 and MS3 fragmentation patterns of NNN-N-oxide and [pyridine-d4]NNN-N-oxide agree with the proposed patterns. Under the current MS conditions, the two most abundant MS2 ions for NNN-N-oxide were m/z 164.0939 and 147.0912, which correspond to protonated nornicotine-1N-oxide and iso-myosmine, respectively. The same pattern of the MS2 ions of m/z 168.1190 and 151.1163 was observed for [pyridine-d4]NNN-N-oxide. It is noteworthy that when using a different collision mode (e.g., HCD versus the current CID), the abundances of the MS2 ions of NNN-N-oxide and [pyridine-d4]NNN-N-oxide were different, with no formation of the ions of m/z 164.0944 and 168.1195 but the ions of m/z 147.0916 and 151.1167 predominated (Figure S3). This is probably due to the relative instability of the nornicotine-1N-oxide ion in the MS source at a higher collision energy condition. However, this product ion appears to be more structurally specific to NNN-N-oxide, which has been proven to be critical for developing a highly specific quantitation method for this analyte (vide infra).
Figure 1.
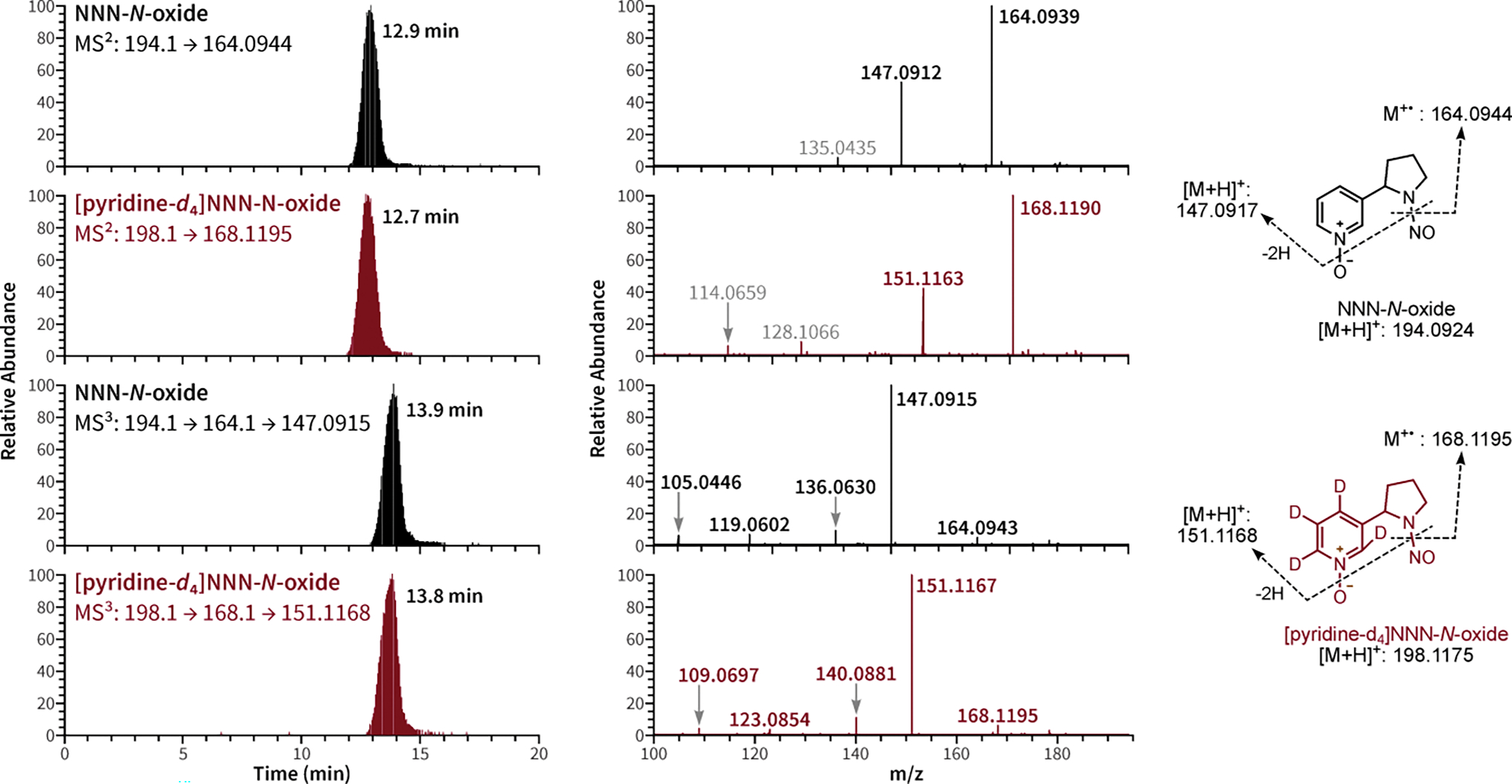
Chromatograms and the MS2 and MS3 fragmentation patterns of NNN-N-oxide and [pyridine-d4]NNN-N-oxide under CID conditions.
Enrichment of urinary NNN-N-oxide
NNN-N-oxide has been quantified in NNN-treated tissue culture mixtures27, 28 and the urine of NNN-treated rats21, 22 using a radioflow HPLC-based method. The analog of NNN-N-oxide – NNAL-N-oxide – was first detected and quantified in the urine of cigarette smokers and smokeless tobacco users by converting NNAL-N-oxide to NNAL by treatment with Proteus mirabilis.29 No previous study has reported the detection and quantitation of NNN-N-oxide in human urine.
To develop a method for urinary NNN-N-oxide purification, we first tried an off-line HPLC system equipped with a pentafluorophenylpropyl (PFP) column. However, NNN-N-oxide did not retain well in that HPLC system (data not shown). We then tested different combinations of solid phase extraction (SPE) cartridges to provide a robust protocol for analyte enrichment and purification. Using a modified protocol adapted from a metabolic profiling study with NNK,25 a suitable combination of SPE cartridges for NNN-N-oxide purification appears to be an HLB cartridge followed by an MCX cartridge and a Strata-X cartridge. The cartridge loading capacity needs to accommodate the required amount of urine for the analysis. A higher amount of urine (1.0 mL) did not provide a better MS signal compared to 0.5 mL, possibly due to a similarly increased level of ion suppression. Using the current protocol, the assay nominal recovery (recovery plus ion suppression) was 29 ± 8% (n = 4) by spiking [pyridine-d4]NNN-N-oxide into the pooled non-smokers’ urine compared to that in H2O.
MS Method Development
Based on our previous LC-NSI-HRMS/MS methods for DNA adduct quantitation,30, 31 an optimized method was developed for the urinary NNN-N-oxide assay. It is common to use MS2 transitions for quantitation with the Orbitrap detector since high specificity can be readily achieved by using a tight mass tolerance (5 ppm) filter. We first tried to use the most sensitive MS2 transition of m/z 194.1 → 147.0913 (at the collision energy of HCD30%) for the quantitation of NNN-N-oxide in urine. However, the assay accuracy and precision were unacceptable due to interference of background peaks. A similar result was obtained when switching to another MS2 transition of m/z 194.1 → 164.0944 (at the collision energy of CID30%) which was considered to be more structurally specific to NNN-N-oxide. After optimizing the collision energies for MS2 and MS3 fragmentation (Figure S4), the new MS3 transition of m/z 194.1 → 164.1 → 147.0913 (at the collision energies of CID30% and HCD10% for the MS2 and MS3 fragmentation, respectively) was found to be highly specific with good sensitivity.
The limit of detection (LOD) of the calibration standard of the MS3 quantitation method was 10 attomole (amol) on column; the limit of quantitation (LOQ) of the calibration standard was 50 amol on column. By spiking NNN-N-oxide into pooled non-smokers’ urine, the LOQ of the assay was 4 fmol/mL urine. The accuracy and precision of the assay were also good with a wide range of linearity (Table 1 and Figure 2). At the low concentration range (0.01 – 1 fmol on column; ratios of 0.01 – 1), the calibration curve y = 1.0977x – 0.0052 (R2 = 1) was used; at the high concentration range (1 – 10 fmol on column; ratios of 1 – 10), the calibration curve y = 1.0430x - 0.0029 (R2 = 0.9999) was used to provide better accuracy and precision (Figure 2). Using the pooled smokers’ urine as the positive control, the assay was highly reproducible with a mean (± SD) value of NNN-N-oxide of 5.1 ± 1.4 fmol/mL (n = 5).
Table 1.
Accuracy and precision data of the assay of NNN-N-oxide in urine.
|
|
|
|||||
|---|---|---|---|---|---|---|
| Spiked amount (fmol) | Intra-day |
Inter-day |
||||
| Measured amount (fmol; mean ± SD) | Accuracy (%) | Precision (CV%) | Measured amount (fmol; mean ± SD) | Accuracy (%) | Precision (CV%) | |
|
|
|
|||||
| 2 | 1.6 ± 0.1 | 78 | 8.1 | 1.7 ± 0.2 | 84 | 12.7 |
| 5 | 4.2 ± 0.4 | 84 | 9.8 | 4.5 ± 0.3 | 90 | 5.8 |
| 10 | 9.7 ± 0.5 | 96 | 5.6 | 9.7 ± 0.3 | 97 | 2.6 |
| 25 | 26.0 ± 1.0 | 104 | 3.7 | 1.7 ± 0.2 | 101 | 1.9 |
| 50 | 50.3 ± 3.3 | 101 | 6.6 | 51.4 ± 4.2 | 103 | 8.1 |
| 100 | 96.2 ± 1.4 | 96 | 1.5 | 96.2 ± 1.9 | 96 | 2.0 |
| 200 | 183.0 ± 8.8 | 92 | 4.8 | 192.9 ± 2.1 | 96 | 1.1 |
|
|
|
|||||
Figure 2.
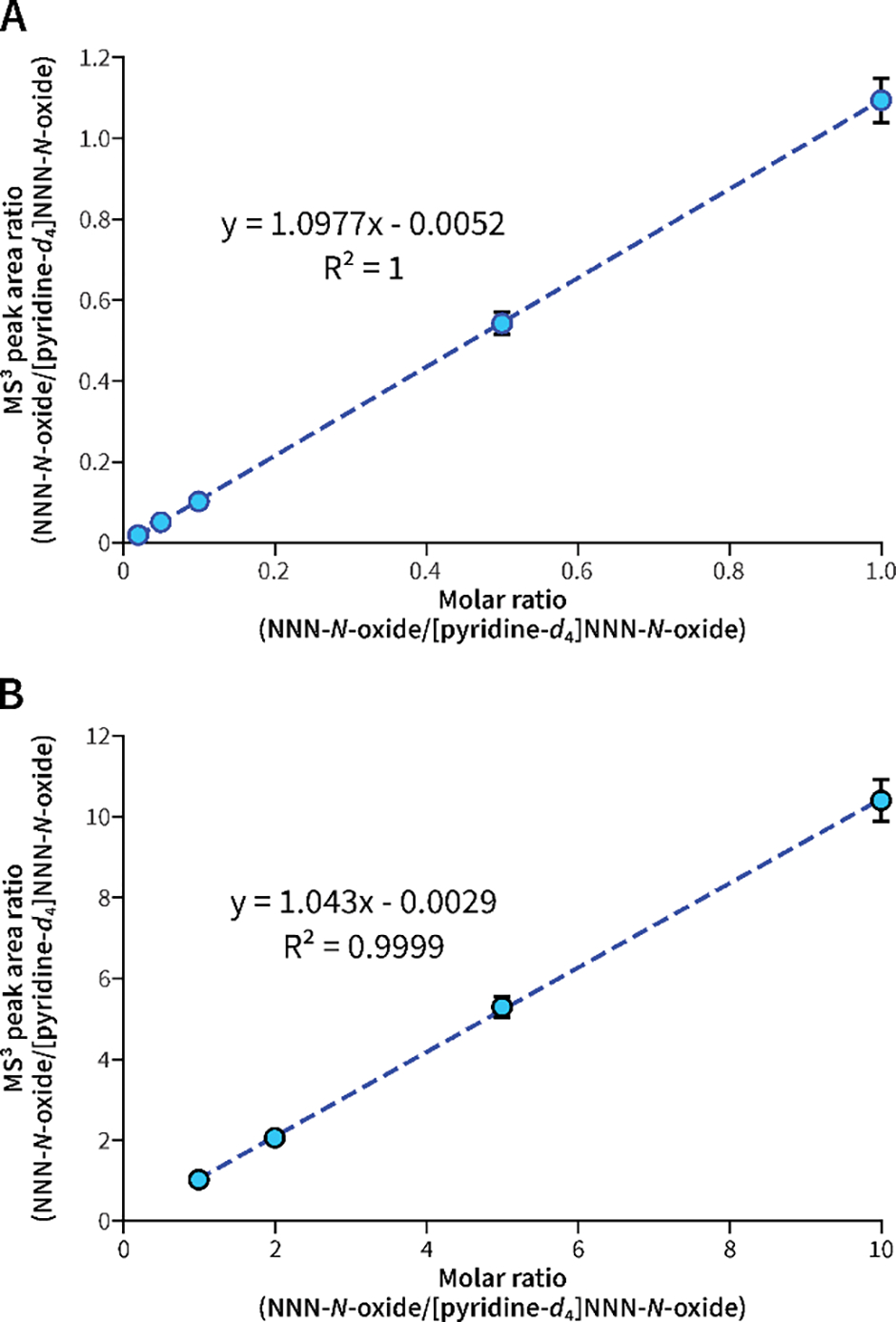
Calibration curves of NNN-N-oxide at (A) low and (B) high concentration ranges. A mean value of 3 replicates was used to establish each calibration curve with the standard error shown in bars.
Typical MS traces of NNN-N-oxide in urine are shown in Figure 3. The presence of NNN-N-oxide was clearly observed in the urine of cigarette smokers and smokeless tobacco users, with a higher occurrence in the latter group. No such peak was observed in non-smokers’ urine. It was noted that the peak shape was generally somewhat broad under various types of mobile phases (Figure S5). We hypothesized that this was mainly due to the nature of the analyte that contains the N-oxide moiety. The urine matrix also had an effect on the peak shape as observed among different subjects (Figure S6). However, with no interfering peaks from the background, the broad peak shape of NNN-N-oxide did not cause problems for its quantitation.
Figure 3.

Typical MS traces of NNN-N-oxide in the urine of pooled non-smokers, pooled cigarette smokers and smokeless tobacco users.
Levels of NNN-N-oxide in the urine of cigarette smokers and smokeless tobacco users
Using the MS3 quantitation method, we quantified the levels of NNN-N-oxide in the urine of 32 cigarette smokers, 14 smokeless tobacco users and 20 non-smokers. The demographics of 32 cigarette smokers and 20 non-smokers are summarized in Table S1; the urinary biomarkers including total nicotine equivalents (TNE) and total NNAL to verify the smoking status of 12 of the cigarette smokers can be found in Table S2. The levels of urinary biomarkers (TNE, total NNAL, total NNN) in the 14 smokeless tobacco users are presented in Table S3. It is noteworthy that the 14 subjects were selected based on their urinary total NNN levels exceeding 0.39 pmol/mg creatinine.
As shown in Table 2, the mean (± SD) level of NNN-N-oxide in the urine of 10 out of 32 cigarette smokers with positive detection was relatively low, amounting to 8.40 ± 6.04 fmol/mL urine. The total mean level was 3.25 ± 4.87 fmol/mL urine by assigning 2.00 (50% LOQ) and 0 fmol/mL to the samples with detectable but not quantifiable peaks and no detectable peaks of NNN-N-oxide, respectively. The relationship of urinary NNN-N-oxide levels with the smoking status of the positive smokers can also be found in Tables S1 and S2. The level of NNN-N-oxide can be compared to that of urinary total NNN in cigarette smokers from our two previous studies (60 ± 35 fmol/mL, n = 38;15 20.5 ± 27.1 fmol/mL, n = 20).14 Since NNN-N-oxide has been shown to be less tumorigenic than NNN in rats and Syrian golden hamsters, these results indicate that pyridine-N-oxidation is a minor detoxification pathway in NNN metabolism.32
Table 2.
Levels of NNN-N-oxide in the urine of cigarette smokers, non-smokers and smokeless tobacco users.
| No. | Cigarette smokers a | Non-smokers b | Smokeless tobacco users c | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Subject ID | NNN-N-oxide (fmol/mL) | Subject ID | NNN-N-oxide (fmol/mL) | Subject ID | NNN-N-oxide (fmol/mL) | |
|
| ||||||
| 1 | 252 | 9.92 | 253 | nd | 022 | 18.3 |
| 2 | 333 | 7.00 | 258 | nd | 326 | 40.8 |
| 3 | 335 | 24.8 | 260 | nd | 336 | 348 |
| 4 | 336 | 5.75 | 262 | nd | 408 | 9.31 |
| 5 | 338 | <LOQ | 264 | nd | 431 | 81.1 |
| 6 | 428 | nd | 422 | nd | 451 | 12.7 |
| 7 | 433 | <LOQ | 512 | nd | 452 | 75.6 |
| 8 | 442 | <LOQ | 514 | nd | 453 | 91.8 |
| 9 | 450 | <LOQ | 515 | nd | 459 | 51.2 |
| 10 | 455 | <LOQ | 517 | nd | 461 | 202 |
| 11 | 458 | 4.77 | 518 | nd | 478 | 129 |
| 12 | 459 | <LOQ | 520 | nd | 491 | 44.5 |
| 13 | 460 | nd | 524 | nd | 529 | 4.53 |
| 14 | 461 | <LOQ | 526 | nd | 1317 | <LOQ |
| 15 | 466 | <LOQ | 534 | nd | ||
| 16 | 468 | 4.57 | 538 | nd | ||
| 17 | 469 | nd | 539 | nd | ||
| 18 | 473 | nd | 544 | nd | ||
| 19 | 493 | <LOQ | 545 | nd | ||
| 20 | 496 | 5.96 | 546 | nd | ||
| 21 | 499 | nd | ||||
| 22 | 501 | 8.06 | ||||
| 23 | 502 | nd | ||||
| 24 | 503 | nd | ||||
| 25 | 508 | nd | ||||
| 26 | 513 | 8.55 | ||||
| 27 | 525 | nd | ||||
| 28 | 531 | nd | ||||
| 29 | 533 | <LOQ | ||||
| 30 | 536 | 4.66 | ||||
| 31 | 537 | nd | ||||
| 32 | 541 | nd | ||||
| Pos. Rate | 31.2% | Pos. Rate | 0% | Pos. Rate | 92.8% | |
| Mean ± SD (only positives; n = 10) | 8.40 ± 6.04 | Mean ± SD (only positives; n = 13) | 85.2 ± 96.3 | |||
| Mean ± SD (all) d | 3.25 ± 4.87 | Mean ± SD (all) d | 79.3 ± 95.2 | |||
Cigarette smokers were from the Biorepository of the University of Minnesota Tobacco Research Programs and a study of biomarkers of cigarette smoking, e-cigarette use or no use of any tobacco or nicotine product.23 Most of the subjects’ smoking status were verified either by exhaled CO level (Table S1) or by urinary biomarkers such as total nicotine equivalents (TNE) and total NNAL (Table S2).
Non-smokers were from the Biorepository of the University of Minnesota Tobacco Research Programs. Their demographics are summarized in Table S1.
Smokeless tobacco users were selected with the urinary total NNN levels of >0.39 pmol/mg creatinine from a previous study cohort.24
For the urine samples with detectable peaks of NNN-N-oxide but below the LOQ (labeled as <LOQ), a value of 2.00 fmol/mL (50% of LOQ) was assigned for statistical analysis; for the urine samples with no detection of NNN-N-oxide (labeled as nd), a value of 0 fmol/mL was assigned for statistical analysis.
The levels of NNN-N-oxide in the 14 smokeless tobacco users were substantially higher, occurring at a mean (± SD) level of 79.30 ± 95.17 fmol/mL (0.075 ± 0.054 pmol/mg creatinine). Similar to the smokers’ data, it was also significantly lower than total NNN (0.668 ± 0.335 pmol/mg creatinine; p < 0.00001) in the urine of the same smokeless tobacco users (Table S3). A weak linear correlation was noted between the levels of urinary NNN-N-oxide and urinary NNN in the smokeless tobacco users with a correlation coefficient of 0.48. Considering the higher exposure levels to nicotine and NNK (as represented by urinary total TNE and total NNAL) in the smokeless tobacco users than in the cigarette smokers (Table S3 versus Table S2), the higher amount of NNN-N-oxide in the urine of the smokeless tobacco users probably reflects the relatively higher levels of NNN uptake from smokeless tobacco products.33 It may also be possible that NNN-N-oxide is present in smokeless tobacco products and can be transferred to the users and excreted unchanged in urine. However, it has not been reported in any tobacco product, and warrants further investigation.
Similar to the cigarette smoker data, NNN-N-oxide represented a very small percentage of the estimated NNN dose in smokeless tobacco users. Based on our previous study, the estimated exposure to NNN in the smokeless tobacco users included in this study was 34.9 μg (197 nmol) per day.24 Using 1.081 L as the average 24 h urine output volume for adults,34 the level of NNN-N-oxide was 0.082 nmol per day in the smokeless tobacco users. It only accounted for approximately 0.04% of the estimated NNN dose on a daily basis. This data is also in line with the absence of detection of NNN-N-oxide in the urine of an NNN-treated patas monkey.10
The low levels of NNN-N-oxide detected in the urine of cigarette smokers and smokeless tobacco users agreed with the findings of the low occurrence of the pyridine N-oxidation products of NNK and NNAL.29 In the urine of 18 smokers and 11 smokeless tobacco users, NNK-N-oxide was not detected while NNAL-N-oxide was formed with a mean (± SD) value of 0.53 ± 0.36 and 0.41 ± 0.35 pmol/mg creatinine, respectively. However, NNAL-N-oxide only accounted for 13.2 and 10.5% of total NNAL (free NNAL plus its glucuronides) in the urine of smokers and smokeless tobacco users.29 As shown in Table S3, NNN-N-oxide accounted to a similar extent of 11.2% of total NNN in the urine of the 14 smokeless tobacco users included in this study. These data together provide evidence that pyridine N-oxidation of NNN represents a minor detoxification pathway in NNN metabolism in tobacco users.
Possible Artifactual Formation of NNN-N-oxide in Urine
Since artifactual formation of NNN can result from nitrosation of urinary nornicotine during analysis, this is a major concern for NNN quantitation in urine.14, 15 Thus, we considered the possible artifactual formation of NNN-N-oxide in our analyses. We added ammonium sulfamate, a known inhibitor of nitrosation,35 to the urine solutions and quantified levels of NNN-N-oxide compared to those in untreated urine. As shown in Table S4, no significant decrease in NNN-N-oxide was observed in the ammonium sulfamate treated urine of 3 cigarette smokers. This appears to agree with the apparent lack of nornicotine-1N-oxide – the most likely precursor to form NNN-N-oxide via nitrosation – in human urine.11
CONCLUSIONS
We for the first time detected and quantified NNN-N-oxide in the urine of cigarette smokers and smokeless tobacco users, using a high-resolution mass spectrometry-based method featuring an MS3 transition to provide high specificity for the analyte. NNN-N-oxide was not observed in any of the 20 non-smokers’ urine. The mean level of NNN-N-oxide in the urine of 10 out of 32 cigarette smokers was 8.40 ± 6.04 fmol/mL with a positive detection rate of 31.2%. It was relatively higher, reaching 85.2 ± 96.3 fmol/mL in the urine of 13 out of 14 smokeless tobacco users with a positive detection rate of 92.8%. Overall, the levels of NNN-N-oxide in tobacco users were quite low, indicating its role as a minor detoxification metabolite of NNN, which further supports the important role of NNN α-hydroxylation in its metabolic activation.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grant CA-81301 from the National Cancer Institute. Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, University of Minnesota, supported in part by Cancer Center Support Grant CA-077598. The authors thank Dr. Laura Maertens for the biorepository management, Nicole Thomson, Steven Carmella and Mei-Kuen Tang for their help with locating the urine samples and providing the urinary biomarker data. We thank Dr. Jiehong Guo for her help with statistical analysis. We also thank Drs. Linda von Weymarn, Yingchun Zhao and Peter Villalta for help with the operation of the mass spectrometer. Editorial assistance from Robert (Bob) Carlson is greatly appreciated.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at www.acs.org:
NMR spectra of NNN-N-oxide and [pyridine-d4]NNN-N-oxide (Figures S1 and S2); MS fragmentation pattern of NNN-N-oxide (Figure S3), collision energy optimization (Figure S4), LC mobile phase optimization (Figure S5), MS traces and fragmentation patterns of NNN-N-oxide in the urine of cigarette smokers and smokeless tobacco users (Figures S6); proposed fragmentation patterns (Scheme S1); demographics of 32 cigarette smokers and 20 non-smokers (Table S1), levels of urinary TNE and NNAL in 12 cigarette smokers (Table S2), levels of urinary TNE, NNAL and NNN in 14 smokeless tobacco users (Table S3), and artifactual formation study with ammonium sulfamate (Table S4).
REFERENCES
- (1).Hecht SS; Stepanov I; Carmella SG Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc. Chem. Res. 2016, 49 (1), 106–114. DOI: 10.1021/acs.accounts.5b00472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Edwards SH; Rossiter LM; Taylor KM; Holman MR; Zhang L; Ding YS; Watson CH Tobacco-specific nitrosamines in the tobacco and mainstream smoke of U.S. commercial cigarettes. Chem. Res. Toxicol. 2017, 30 (2), 540–551. DOI: 10.1021/acs.chemrestox.6b00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Edwards SH; Hassink MD; Taylor KM; Watson CH; Kuklenyik P; Kimbrell B; Wang L; Chen P; Valentín-Blasini L Tobacco-specific nitrosamines in the tobacco and mainstream smoke of commercial little cigars. Chem. Res. Toxicol. 2021, 34 (4), 1034–1045. DOI: 10.1021/acs.chemrestox.0c00367 [DOI] [PubMed] [Google Scholar]
- (4).Nasrin S; Chen G; Watson CJW; Lazarus P Comparison of tobacco-specific nitrosamine levels in smokeless tobacco products: High levels in products from Bangladesh. PLoS One 2020, 15 (5), e0233111. DOI: 10.1371/journal.pone.0233111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hecht SS Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998, 11, 559–603. [DOI] [PubMed] [Google Scholar]
- (6).Li Y; Hecht SS Metabolism and DNA adduct formation of tobacco-specific N-nitrosamines. Int. J. Mol. Sci. 2022, 23 (9), 5109. DOI: 10.3390/ijms23095109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).International Agency for Research on Cancer. Smokeless tobacco and some tobacco-specific N-nitrosamines. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 89; IARC, 2007; pp 1–592. [PMC free article] [PubMed] [Google Scholar]
- (8).Benowitz NL; Bernert JT; Foulds J; Hecht SS; Jacob P; Jarvis MJ; Joseph A; Oncken C; Piper ME Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob. Res. 2020, 22 (7), 1086–1097. DOI: 10.1093/ntr/ntz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Hecht SS; Lin D; Chen CB Comprehensive analysis of urinary metabolites of N’-nitrosonornicotine. Carcinogenesis 1981, 2 (9), 833–838. [DOI] [PubMed] [Google Scholar]
- (10).Upadhyaya P; Zimmerman CL; Hecht SS Metabolism and pharmacokinetics of N’-nitrosonornicotine in the patas monkey. Drug Metab. Dispos. 2002, 30 (10), 1115–1122. DOI: 10.1124/dmd.30.10.1115 [DOI] [PubMed] [Google Scholar]
- (11).Murphy SE Biochemistry of nicotine metabolism and its relevance to lung cancer. J. Biol. Chem. 2021, 296, 100722. DOI: 10.1016/j.jbc.2021.100722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Stepanov I; Hecht SS Tobacco-specific nitrosamines and their pyridine-N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol. Biomarkers Prev. 2005, 14 (4), 885–891. DOI: 10.1158/1055-9965.EPI-04-0753 [DOI] [PubMed] [Google Scholar]
- (13).Yuan JM; Knezevich AD; Wang R; Gao YT; Hecht SS; Stepanov I Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis 2011, 32, 1366–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Yang J; Carmella SG; Hecht SS Analysis of N’-nitrosonornicotine enantiomers in human urine by chiral stationary phase liquid chromatography-nanoelectrospray ionization-high resolution tandem mass spectrometry. J. Chromatogr. B 2017, 1044–1045, 127–131. DOI: 10.1016/j.jchromb.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kotandeniya D; Carmella SG; Pillsbury ME; Hecht SS Combined analysis of N’-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in the urine of cigarette smokers and e-cigarette users. J. Chromatogr. B 2015, 1007, 121–126. DOI: 10.1016/j.jchromb.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Knezevich A; Muzic J; Hatsukami DK; Hecht SS; Stepanov I Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N’-nitrosonornicotine in humans. Nicotine Tob. Res. 2013, 15 (2), 591–595. DOI: 10.1093/ntr/nts172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Stepanov I; Carmella SG; Briggs A; Hertsgaard L; Lindgren B; Hatsukami D; Hecht SS Presence of the carcinogen N’-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res. 2009, 69 (21), 8236–8240. DOI: 10.1158/0008-5472.CAN-09-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Stepanov I; Carmella SG; Han S; Pinto A; Strasser AA; Lerman C; Hecht SS Evidence for endogenous formation of N’-nitrosonornicotine in some long-term nicotine patch users. Nicotine Tob. Res. 2009, 11 (1), 99–105. DOI: 10.1093/ntr/ntn004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Bustamante G; Ma B; Yakovlev G; Yershova K; Le C; Jensen J; Hatsukami DK; Stepanov I Presence of the carcinogen N’-nitrosonornicotine in saliva of e-cigarette users. Chem. Res. Toxicol. 2018, 31 (8), 731–738. DOI: 10.1021/acs.chemrestox.8b00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hoffmann D; Castonguay A; Rivenson A; Hecht SS Comparative carcinogenicity and metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N’-nitrosonornicotine in Syrian golden hamsters. Cancer Res. 1981, 41 (6), 2386–2393. [PubMed] [Google Scholar]
- (21).Hecht SS; Young R Regiospecificity in the metabolism of the homologous cyclic nitrosamines, N’-nitrosonornicotine and N’-nitrosoanabasine. Carcinogenesis 1982, 3 (10), 1195–1199. [DOI] [PubMed] [Google Scholar]
- (22).Hecht SS; Chen CB; Hoffmann D Metabolic beta-hydroxylation and N-oxidation of N’-nitrosonornicotine. J. Med. Chem. 1980, 23 (11), 1175–1178. DOI: 10.1021/jm00185a005 [DOI] [PubMed] [Google Scholar]
- (23).Cheng G; Guo J; Carmella SG; Lindgren B; Ikuemonisan J; Niesen B; Jensen J; Hatsukami DK; Balbo S; Hecht SS Increased acrolein-DNA adducts in buccal brushings of e-cigarette users. Carcinogenesis 2022, 43 (5), 437–444. DOI: 10.1093/carcin/bgac026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Hatsukami DK; Stepanov I; Severson H; Jensen JA; Lindgren BR; Horn K; Khariwala SS; Martin J; Carmella SG; Murphy SE; et al. Evidence supporting product standards for carcinogens in smokeless tobacco products. Cancer Prev. Res. 2015, 8 (1), 20–26. DOI: 10.1158/1940-6207.CAPR-14-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Dator R; von Weymarn LB; Villalta PW; Hooyman CJ; Maertens LA; Upadhyaya P; Murphy SE; Balbo S In vivo stable-isotope labeling and mass-spectrometry-based metabolic profiling of a potent tobacco-specific carcinogen in rats. Anal Chem 2018, 90 (20), 11863–11872. DOI: 10.1021/acs.analchem.8b01881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hu MW; Bondinell WE; Hoffmann D Chemical studies on tobacco smoke XXIII. Synthesis of carbon-14 labelled myosmine, nornicotine and N’-nitrosonornicotine. J. Label. Compd. Radiopharm. 1974, 10 (1), 79–88. DOI: 10.1002/jlcr.2590100110 [DOI] [Google Scholar]
- (27).McIntee EJ; Hecht SS Metabolism of N’-nitrosonornicotine enantiomers by cultured rat esophagus and in vivo in rats. Chem. Res. Toxicol. 2000, 13 (3), 192–199. DOI: 10.1021/tx990171l [DOI] [PubMed] [Google Scholar]
- (28).Castonguay A; Stoner GD; Schut HA; Hecht SS Metabolism of tobacco-specific N-nitrosamines by cultured human tissues. Proc. Natl. Acad. Sci. U.S.A. 1983, 80 (21), 6694–6697. DOI: 10.1073/pnas.80.21.6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Carmella SG; Borukhova A; Akerkar SA; Hecht SS Analysis of human urine for pyridine-N-oxide metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco-specific lung carcinogen. Cancer Epidemiol. Biomarkers Prev. 1997, 6 (2), 113–120. [PubMed] [Google Scholar]
- (30).Li Y; Carlson ES; Zarth AT; Upadhyaya P; Hecht SS Investigation of 2’-deoxyadenosine-derived adducts specifically formed in rat liver and lung DNA by N’-nitrosonornicotine metabolism. Chem. Res. Toxicol. 2021, 34 (4), 1004–1015. DOI: 10.1021/acs.chemrestox.1c00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Li Y; Hecht SS Identification of an N’-nitrosonornicotine-specific deoxyadenosine adduct in rat liver and lung DNA. Chem. Res. Toxicol. 2021, 34 (4), 992–1003. DOI: 10.1021/acs.chemrestox.1c00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hecht SS; Young R; Maeura Y Comparative carcinogenicity in F344 rats and Syrian golden hamsters of N’-nitrosonornicotine and N’-nitrosonornicotine-1-N-oxide. Cancer Lett. 1983, 20 (3), 333–340. DOI: 10.1016/0304-3835(83)90032-0 [DOI] [PubMed] [Google Scholar]
- (33).Li Y; Hecht SS Carcinogenic components of tobacco and tobacco smoke: A 2022 update. Food Chem. Toxicol. 2022, 165, 113179. DOI: 10.1016/j.fct.2022.113179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Murakami K; Sasaki S; Takahashi Y; Uenishi K; Watanabe T; Kohri T; Yamasaki M; Watanabe R; Baba K; Shibata K; et al. Sensitivity and specificity of published strategies using urinary creatinine to identify incomplete 24-h urine collection. Nutrition 2008, 24 (1), 16–22. DOI: 10.1016/j.nut.2007.09.001 [DOI] [PubMed] [Google Scholar]
- (35).Douglass ML; Kabacoff BL; Anderson GA; Cheng MC The chemistry of nitrosamine formation inhibition and destruction. J. Soc. Cosmet. Chem. 1978, 29 (9), 581–606. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


