Abstract
Background
Immune checkpoint inhibitors (ICIs) have become a mainstay of cancer treatment. Their immune-boosting quality has one major drawback, their proclivity to induce a broad array of immune-related adverse events (irAEs) affecting, among others, the liver and sharing some similarities with classic autoimmune liver diseases (AILD). We aimed to compare clinical, laboratory and histological features of patients with liver-related irAEs and AILD.
Methods
We systematically compared liver irAEs with AILD, namely autoimmune hepatitis (AIH) and primary biliary cholangitis, regarding their clinical, laboratory, and histological features.
Results
Twenty-seven patients with liver irAEs (ICI group) and 14 patients with AILD were identified. We observed three distinct ICI-induced histological liver injury patterns: hepatitic (52%), cholangitic (19%), and mixed (29%). When comparing the ICI and AILD groups, centrilobular injury as well as granuloma formation were more prevalent in the former (p=0.067 and 0.002, respectively). CD4+/CD8+ T cell ratios were heterogeneous between the two groups, without statistically significant difference but with a trend toward increased CD8+ T cells among hepatitic irAEs as compared with AIH. Pattern of liver function test alteration was predictive for the type of irAEs but did not correlate with histological severity.
Conclusions
Liver irAEs have broad clinical, laboratory and histological presentations. Histological features of irAEs and AILD are distinct, likely underpinning their different immunological mechanisms.
Keywords: immunotherapy; cytotoxicity, immunologic; programmed cell death 1 receptor; CTLA-4 antigen; autoimmunity
WHAT IS ALREADY KNOWN ON THIS TOPIC
Immune checkpoint inhibitors have become a leading option for the treatment of advanced cancers. In parallel to their increasing indications, the incidence of liver immune-related adverse events (irAEs) is growing. Three distinct histological patterns of liver injury have been described to date: hepatitic, cholangitic and mixed cholangiohepatitic. However, few studies exist extensively characterizing these injuries and even fewer comparing them to classic autoimmune liver diseases (AILD).
WHAT THIS STUDY ADDS
Understanding the clinical and histological landscape of these toxicities is essential to improve their management. This study characterizes the largest cohort of liver irAEs to date and systematically compares them clinically, biologically and histologically to classic AILD, underpinning their different immunological mechanisms.
In addition, it provides insight into the place of liver biopsy in the diagnosis and management of liver irAEs, highlighting in particular the possible discordance between clinical and histological severity.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study substantiates early descriptions of liver toxicities following immune checkpoint inhibitors, describing patient characteristics and management in depth. For future research, it contributes to a better characterization and understanding of pathogenesis of the broad spectrum of liver irAEs.
Introduction
Immune checkpoint inhibitors (ICIs) have profoundly changed the therapeutic landscape of cancer. These drugs are unique in that they enhance the body’s immune response so as to target and destroy tumor cells and thus produce a favorable outcome in a wide range of malignancies.1 2
ICIs exploit the physiological control mechanisms used to downregulate autoreactive T-cells. They target cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) and programmed death receptor 1 (PD-1), which can be found on the T-cell surface, as well as PD-1 ligand (PD-L1) on tumor cells. Increased and durable anti-tumor immunity is obtained by promoting the production of protective anti-tumoral T-cell responses and overcoming immunosuppression in the tumor bed.3 4
Ipilimumab, a human anti-CTLA-4 IgG1 monoclonal antibody, was the first of these therapies to reach clinical application in 2011.1 Anti-PD-1 and anti-PD-L1 therapies have shown significant activity in a wide range of solid tumors across stages and histological subtypes, and have transformed standards of care.2 5
One major issue of ICIs is the probability of inducing autoimmune reactions because of their immune-boosting properties. These reactions are named immune-related adverse events (irAEs). Although their pathogenesis is only partially understood, they appear to be the consequence of activated resident memory CD8 cells invading healthy tissues and inducing inflammation.6–8 ICIs are associated with a broad array of irAEs affecting a variety of organs and possibly correlated with the efficacy of immunotherapy.9 Major locations include the skin, gastrointestinal tract, liver, pituitary gland, pancreas, lungs, kidneys and heart.10 As immunotherapy is increasingly prescribed, it is likely that the incidence of irAEs will increase accordingly.11
ICI-related hepatitis (irH) has been growingly identified in the past few years, ranging from asymptomatic laboratory abnormalities to life-threatening conditions associated with liver dysfunction.12 The diagnosis of irH is one of exclusion and, therefore, other possible causes of hepatitis must be ruled out, including liver injury induced by other drugs.13 Hepatotoxicity may occur in 1%–7% and 1%–6% of patients treated with anti-CTLA-4 and anti-PD-1, respectively.14 Importantly, anti-CTLA-4 and anti-PD-1 combination therapies are associated with significantly higher rates of severe toxicities.14–16 In fact, hepatitis was reported to be the most common grade 3 or 4 irAE to occur on the course of ICI therapies.15 These adverse events may have devastating consequences for patients, such as the withdrawal of immunotherapy and the introduction of immunosuppressive treatment.17
Important efforts were initiated to characterize histological changes induced by ICIs in the hepatobiliary system.18–20 Assessment of liver histology is helpful for the diagnosis of liver damage and the evaluation of its severity, as well as excluding hepatic tumor infiltration. In addition, an important differential diagnosis of irH is previously asymptomatic or subclinical liver disease unmasked by cancer immunotherapy.21
Different histological patterns of liver damage have been described, ranging from lobular hepatitis to cholangitis (irC).20 22 23 With regards to immunostaining, the CD4+/CD8+ T cell ratio has been found to favor CD8+ cells.18 20 21
We explored the spectrum of histological lesions, together with clinical and laboratory presentations of liver irAEs and systematically compared them to classic autoimmune liver disease (AILD).
Patients and methods
Data collection and patient involvement
All living patients included in the study provided written informed consent for reutilization of their medical and histopathological data. Information regarding the aim of the study and how it may benefit future patients in similar situations was discussed. Patients were not involved in the study design. Data from deceased patients could be included without written consent according to the protocol approved by our Ethical Committee.
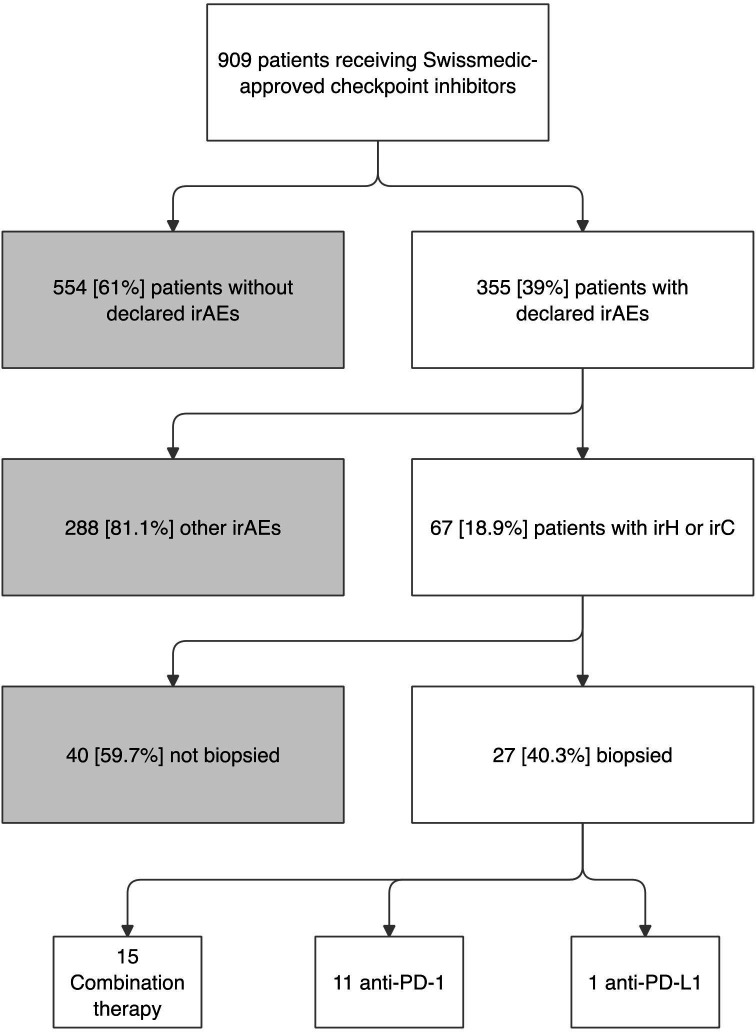
Two different groups of patients were retrospectively studied at Lausanne University Hospital, ie, patients with liver irAEs and patients with AILD. All patients treated with ICIs between January 2015 and September 2020 who developed grade ≥2 liver irAEs according to the Common Terminology Criteria for Adverse Events V. 5.016 were identified using a multiparametric clinical data warehouse search engine. Only patients who had a liver biopsy as part of their clinical workup were included in the study (figure 1).
Figure 1.
Identification of patients assigned to the immune checkpoint inhibitor group. Flow chart describing the identification and selection process of the 27 patients assigned to the immune checkpoint inhibitor (ICI) group. Swissmedic is the Swiss authority responsible for the approval as well as the surveillance of the efficacy and safety of drugs. IrAE, immune-related adverse event; IrC, immune-related cholangitis; IrH, immune-related hepatitis; PD-1, programmed cell death 1, PD-L1, programmed cell death ligand 1.
The second group included patients diagnosed with autoimmune hepatitis (AIH) or primary biliary cholangitis (PBC) between September 2009 and November 2018 according to European Association for the Study of the Liver Clinical Practice Guidelines,24 25 whenever histology was available to confirm the diagnosis. Liver irAEs being acute toxicities, we excluded AILD patients from the control group if they had a fibrosis score of F3 or F4 according to the Metavir classification, as advanced fibrosis is generally encountered in the setting of chronic disease.26 In addition, patients with AIH-PBC or AIH-PSC (primary sclerosing cholangitis) overlap syndrome were excluded.
Demographic, clinical, laboratory, and histological data were retrieved manually from electronic medical records and medical archives. All data were anonymized before analysis.
Demographic data included sex and age. Clinical data for patients with liver irAEs included nature and stage of the primary tumor, type of immunotherapy and potential concomitant anticancer treatments, oncological outcomes (progressive disease, stable disease, partial or complete response, according to RECIT V.1.1), underlying chronic liver disease, autoimmune diseases or other irAEs. Laboratory parameters included alanine transaminase (ALT), aspartate transaminase (AST), AST/ALT ratio, alkaline phosphatase (ALP), γ-glutamyl transferase, total bilirubin and international normalized ratio. For each item, the peak value as well as the time between the onset of immunotherapy and the peak value were collected. Based on liver function tests (LFTs), we assigned all patients to three grades of laboratory severity, that is, mild (ALT <400 U/L and total bilirubin <40 µmol/L), moderate (ALT >400 U/L and ˂1000 U/L and total bilirubin ˂40 µmol/L) or severe (ALT >1000 U/L and/or total bilirubin >40 µmol/L).
Acute or chronic infections by hepatitis A, B, C and E viruses, respectively, Epstein-Barr and cytomegalovirus were systematically ruled out.
The presence of AILD markers was assessed and included total serum IgG levels, antinuclear antibodies (ANA), anti-smooth muscle antibodies (SMA), anti-actin antibodies, and anti-mitochondrial antibodies (AMA). Total IgG >15 g/L, ANA and SMA ≥1/160, anti-actin antibodies >20 U, and AMA ≥1/40 were considered as significantly increased.
Information on immunosuppressive treatments (intravenous or oral corticosteroids (CSs), mycophenolate mofetil (MMF), anti-interleukin (IL)−6 receptor and others) was retrieved. The subsequent hepatic outcomes in terms of response to treatment and relapse after immunosuppressive therapy withdrawal were collected.
Histopathology and immunohistochemistry
All liver biopsy specimens had been previously fixed in 10% buffered formalin, embedded in paraffin and processed according to standard histological methods. Stains performed in all cases included H&E and a connective tissue stain (Masson’s trichrome) as well as immunostaining for cytokeratin 7 (CK7). Histological slides from all but one AIH patient were independently re-evaluated by two expert liver pathologists (FF and CS) who were blinded to clinical history.
For all biopsies, the following histological features were recorded and, if appropriate, graded: portal inflammation (0–3), interface hepatitis (0–3), bile duct injury (0–1), ductular reaction, biliary metaplasia (0–3, on CK7 immunostaining), lobular inflammation (0–3), lobular necrosis (0–3), emperipolesis (0–1), rosettes (0–1), granulomas (0–2), bilirubinostasis (0–1), centrilobular injury (0–3), and the type of inflammatory infiltrate: plasma cells, neutrophils, and eosinophils (0–2). The fibrosis stage was assessed by the METAVIR score on Masson’s trichrome.26 Additional findings such as steatosis were mentioned if present. The two independent pathologists determined the predominant pattern of liver injury (irH, irC or mixed, ie, cholangiohepatitic (irCH)). A score to categorize the severity of the histological damage was created, by adding the scores of portal, periportal, and lobular inflammation, centrilobular injury and lobular necrosis. Mild (or absent), moderate and severe cases were defined as having a total score of ≤4, 5–7 and ≥8, respectively.
Immunostaining for CD4 and CD8 was performed on a Ventana Benchmark (Ventana Medical Systems, Tucson, Arizona, USA) in the 23 patients of the ICI group and the 8 patients of the AILD group for whom unstained slides were available. Deparaffinized sections were submitted to antigen retrieval mediated by the CC1 standard protocol from the manufacturer (Ventana) and incubated with antibodies SP35 against CD4 (Ventana) and C8/144B against CD8 (Dako). CD4+/CD8+ T cells ratios were calculated in each case and registered as lower than, equal to or higher than 1.
Statistical analyses
Descriptive statistics included median and range for continuous variables, and frequencies and percentages for categorical variables. Categorical data were compared by Χ2 or Fischer’s exact test and continuous variables by t-test or non-parametric testing (Mann-Whitney U test), as appropriate. Statistical analyses were performed by using SPSS package V.26.
Results
Clinical and laboratory features of the ICI group
We identified 27 patients meeting the inclusion criteria for the ICI group (figure 1). Their demographic, clinical and laboratory characteristics are summarized in table 1 and online supplemental table 1. Fifteen patients (56%) were male, with a median age of 65 (range, 20–80s) years with skin melanoma as the most represented tumor (11 patients, 40%).
Table 1.
Demographic and clinical characteristics of patients included in the ICI group
| Characteristics | n=27 |
| Female, n (%) | 12 (44) |
| Age (years), median (range) | 65 (20–80s) |
| Cancer types, n (%) | |
| Melanoma | 11 (40) |
| Squamous cell lung carcinoma | 3 (11) |
| Uveal melanoma | 3 (11) |
| Lung adenocarcinoma | 2 (7) |
| Prostate adenocarcinoma | 2 (7) |
| Cholangiocarcinoma | 1 (4) |
| Mesothelioma | 1 (4) |
| Renal cell carcinoma | 1 (4) |
| Small cell lung carcinoma | 1 (4) |
| Serous-type ovarian carcinoma | 1 (4) |
| Urothelial carcinoma | 1 (4) |
| Immunotherapy regimen, n (%) | |
| Anti-PD-1* | 11 (40) |
| Anti-PD-L1* | 1 (4) |
| Combined therapy† | 15 (56) |
| Patients with previous cancer treatments‡ | 19/27 (70) |
| Profile of liver injury, n (%) | |
| Immune-related hepatitis | 14 (52) |
| Immune-related cholangitis | 5 (19) |
| Immune-related cholangiohepatitis | 8 (29) |
| Clinical irAE grade, n (%) | |
| 1 | 0/27 (0) |
| 2 | 1/27 (4) |
| 3 | 18/27 (67) |
| 4 | 8/27 (29) |
| Latency of toxicity§, median (range) | 8 (1–72) |
| Peak laboratory values, median (range) | |
| ALT (U/L) | 456 (125–1630) |
| AST/ALT ratio | 0.67 (0.18–2.05) |
| ALP (U/L) | 371 (45–1395) |
| GGT (U/L) | 461 (27–3475) |
| INR | 1.1 (1–2) |
| Total bilirubin (µmol/L) | 18 (7–289) |
| Patients with other irAEs¶, n (%) | 16/27 (59) |
*Nine patients treated with anti-PD-1 received pembrolizumab and two nivolumab. The only anti-PD-L1 used was durvalumab.
†Combined therapies included an association of ipilimumab (anti-CTLA-4) and an anti-PD-1.
‡Other previous oncological treatments included bevacizumab, carboplatin, cisplatin, cyclophosphamide, dabrafenib, etoposide, gemcitabine, olaparib, paclitaxel, pemetrexed, trametinib, vinorelbine.
§First ALT increase since first ICI dose (weeks).
¶Other irAEs included thyroiditis (9/27), rash (4/27), colitis (3/27), adrenalitis (2/27), hypophysitis (2/27), nephritis (2/27), vitiligo (2/27), neuropathy (1/27), pleuritis (1/27) and pneumonitis (1/27).
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTLA-4, cytotoxic T-lymphocyte associated antigen 4; GGT, y-glutamyl transferase; ICI, immune checkpoint inhibitor; INR, international normalized ratio; irAE, immune-related adverse event; irC, immune-related cholangitis; irH, immune-related hepatitis; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
jitc-2022-005635supp001.pdf (184.7KB, pdf)
Four different ICIs were used: ipilimumab (anti-CTLA-4 antibody), nivolumab and pembrolizumab (both anti-PD-1 antibodies) as well as durvalumab (anti-PD-L1 antibody). Fifteen patients (56%) received the combination of ipilimumab and nivolumab, which was the most frequently prescribed regimen in our study. One, 18 and 8 patients presented a grade 2, 3, and 4 liver irAE, respectively.16 In the one patient with grade 2, the decision to perform a liver biopsy was motivated by a complex manifestation with markedly increased serum ferritin.
Sixteen patients (59%) presented other associated irAEs during the clinical course. Associated irAEs were seen in 9/15 (60%) patients with a combination therapy and in 7/11 (64%) receiving anti-PD-1 alone. Of note, 2 out of 27 (7%) ICI patients presented pre-existing extrahepatic autoimmune diseases (Hashimoto’s thyroiditis and celiac disease).
Following the first ICI dose, ALT increase above the upper limit of normal was detected after a median of 8 (1-72) weeks. Anti-PD-1 and anti-PD-L1 used as single ICI tended to have a longer median time to onset of toxicity as compared with a combined ICI treatment (median: 14.5 (3–30) vs 8.0 (1–72) weeks, respectively, p=0.323). Peak LFTs are summarized in table 1.
Based on their LFTs, 11/27 (41%) patients were assigned to a mild, 7/27 (26%) to a moderate, and 9/27 (33%) to a severe clinical picture.
At the time of the liver biopsy, all patients were characterized in terms of serological markers for AILD. Four (15%) patients were found to present a serological constellation suggestive of AILD, as previously defined, although none had been diagnosed with AILD prior to the events (online supplemental table 2).
All patients with primary cholestasis underwent an MR cholangiography before liver biopsy. These MRs were all reviewed by experts in digestive radiology. None of the patients had a cholangiogram suspicious of a so-called primary sclerosing cholangitis pattern.
Oncological outcomes were assessed after a median follow-up period of 15 (1-63) months. Five (19%) patients presented complete remission, 6 (22%) stable disease and 8 (30%) disease progression. Seven (26%) patients died and one was lost to follow-up.
Various immunosuppressive regimens were used to treat liver irAEs. Initial treatment included oral or intravenous CSs in 24 (89%) patients. CSs were then replaced by other immunosuppressive drugs, including MMF, infliximab, or tocilizumab, in 15 (56%) difficult-to-treat patients. Infliximab was prescribed in patients with associated immune-related colitis. Nine (33%) patients required MMF for maintenance therapy, three (11%) received infliximab (one with MMF), and five (19%) received tocilizumab (one along with MMF). Seven of 14 (50%) patients with irH, 3 of 4 (75%) with irC and 5 of 8 (63%) with irCH required immunosuppressive treatment other than CS. Of the patients treated with tocilizumab, 4/5 (80%) had a cholangitic phenotype. Three patients achieved complete normalization of LFTs without the use of immunosuppression, of whom two stopped ICI treatment.
The median time to ALT normalization following immunosuppressive drug treatment institution was 6 (1–18) weeks.
Clinical and laboratory features of the AILD group
Fourteen patients were retrospectively identified for inclusion in the AILD group, 11 with type 1 AIH and three with PBC. Only three patients with PBC could be identified and included as a liver biopsy is not required for the diagnosis unless there is a suspicion of overlap with AIH or advanced fibrosis. These two situations were excluded in this study. None of these patients were treated at the time of biopsy. Thirteen (93%) patients were females with a median age of 49 (20s to 70s) years. Table 2 summarizes demographic, clinical, and laboratory characteristics of the patients, with additional details in online supplemental table 3.
Table 2.
Description of the autoimmune liver disease group
| Autoimmune hepatitis (AIH) | n=11 |
| Female, n (%) | 10 (91) |
| Age (years), median (range) | 50 (20–70s) |
| Peak LFTs at liver biopsy, median (range) | |
| ALT (U/l) | 757 (165–3301) |
| AST/ALT | 0.7 (0.2–1.1) |
| Total bilirubin (µmol/l) | 54 (10–388) |
| Serological markers of AIH | |
| Increased IgG, n (%) | 9 (82) |
| ANA >1/80, n (%) | 10 (91) |
| SMA >1/80 (%), n (%) | 10 (91) |
| Positive anti-actin antibodies, n (%) | 10 (91) |
| Patients with a history of extra-hepatic autoimmunity* | 6 (55) |
| Primary biliary cholangitis (PBC) | n=3 |
| Female, n (%) | 3 (100) |
| Age (years), median (range) | 46 (40–60s) |
| Peak LFT at liver biopsy, median (range) | |
| Alkaline phosphatase (U/l) | 250 (240–330) |
| Gamma-GT (U/l) | 167 (101–339) |
| ALT (U/l) | 74 (49–80) |
| AST/ALT | 0.6 (0.6–0.8) |
| Total bilirubin (µmol/l) | 7 (5–11) |
| Serological markers of PBC | |
| Anti-mitochondria antibodies, n (%) | 3 (100) |
| Anti-M2 antibodies, n (%) | 3 (100) |
| Patients with a history of extrahepatic autoimmunity† | 2 (66) |
*Extrahepatic autoimmunity: Graves’ disease (2/11), autoimmune thyroiditis (1/11), DRESS syndrome (1/11), lymphocytic colitis (1/11), rheumatoid arthritis (1/11), type 1 diabetes mellitus (1/11).
†Extrahepatic autoimmunity: Rheumatoid arthritis (2/3).
AIH, autoimmune hepatitis; ALT, alanine aminotransferase; ANA, anti-nuclear antibody; AST, aspartate aminotransferase; Gamma-GT, gamma-glutamyl transferase; irAE, immune-related adverse event; LFT, liver function tests; SMA, smooth muscle antibody.
Histological and immunohistochemical characterization of the ICI group
The median length of biopsy cores was 2.9 (1.3–6.0) centimeters, containing a median of 13 (10–30) portal tracts. Two patients from the ICI group were removed from the histological evaluation, as they had been treated with selective internal radiation therapy of the liver for metastatic disease. Hence, their liver biopsy presented predominant signs of ischemic postembolization hepatitis.
Of note, 15/25 (60%) patients had already started their immunosuppressive treatment at the time of biopsy. Median time from the start of immunosuppressive therapy to the liver biopsy in these patients was 9 (1–75) days. Four of these 15 patients had received only one dose of intravenous CS before the liver biopsy.
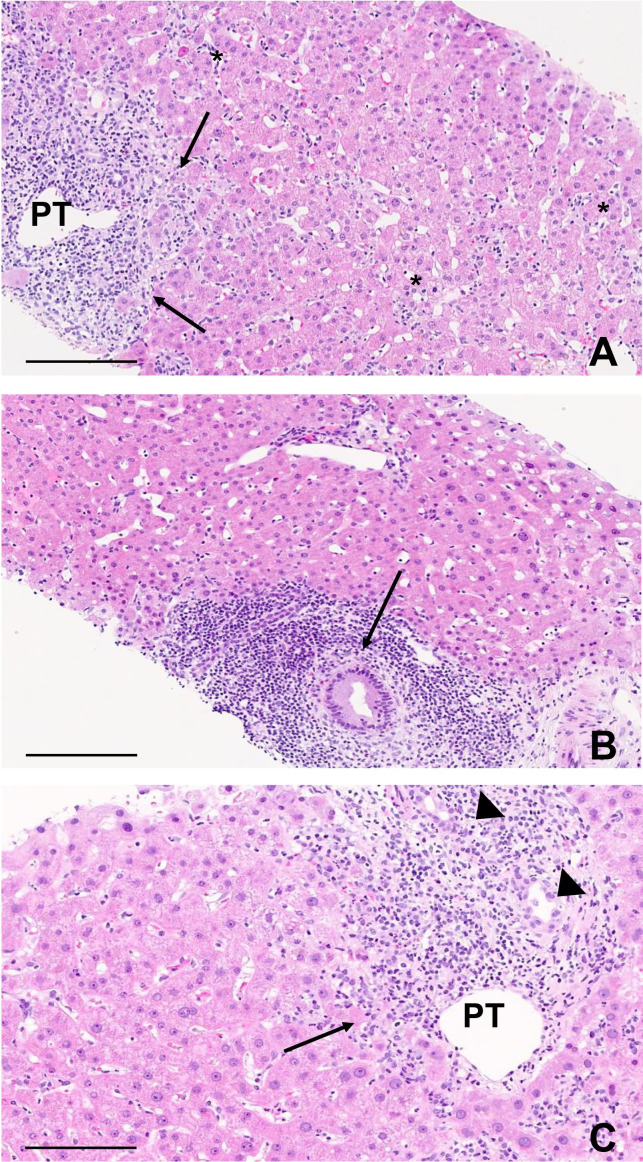
On histological assessment, 14 (56%) of the 25 ICI group biopsies showed a predominantly hepatitic liver injury pattern whereas 4 (16%) showed a cholangitic and 6 (24%) showed a mixed pattern, displaying between them bile duct injury (10/10), ductular reaction (10/10) and biliary metaplasia (7/10) (figure 2). In one patient, macrovesicular steatosis was the only lesion found in the liver biopsy. Steroids had been started 2 days prior to the biopsy in this patient. Detailed histological information is provided in table 3 and online supplemental table 4.
Figure 2.
Characteristic histological findings from patients with liver-related irAE. (A) Patient 8. ICI-related hepatitis. Prominent inflammation is seen in the portal tract (PT) with interface hepatitis (arrows), together with multiple foci of inflammation and apoptotic bodies in the lobule (*). Scale bar, 200 µm. (B) Patient 20. ICI-related cholangitis. A florid inflammatory duct lesion is found in a large PT (arrow) with no inflammation or apoptosis within the lobule. Scale bar, 200 µm. (C) Patient 22. ICI-related cholangiohepatitis. Both portal inflammation with interface hepatitis (arrow) and an irregular bile duct with ductular reaction (arrowheads) are observed. Scale bar, 200 µm. ICI, immune checkpoint inhibitor; irAE, immune-related adverse events.
Table 3.
Histological characteristics of 25 patients with liver-related irAEs
| Histological parameters | Hepatitic (n=14) | Cholangio-hepatitic (n=6) | Cholangitic (n=4) |
| Lobular inflammation | |||
| 0 | 2 (14%) | 1 (17%) | 0 (0%) |
| 1 | 3 (21%) | 5 (83%) | 4 (100%) |
| 2 | 7 (50%) | 0 (0%) | 0 (0%) |
| 3 | 2 (14%) | 0 (0%) | 0 (0%) |
| Lobular necrosis | |||
| 0 | 2 (14%) | 2 (33%) | 0 (0%) |
| 1 | 6 (43%) | 4 (67%) | 4 (100%) |
| 2 | 6 (43%) | 0 (0%) | 0 (0%) |
| 3 | 0 (0%) | 0 (0%) | 0 (0%) |
| Centrilobular injury | |||
| 0 | 6 (43%) | 5 (83%) | 4 (100%) |
| 1 | 5 (36%) | 1 (17%) | 0 (0%) |
| 2 | 2 (14%) | 0 (0%) | 0 (0%) |
| 3 | 1 (7%) | 0 (0%) | 0 (0%) |
| Portal inflammation | |||
| 0 | 0 (0%) | 0 (0%) | 0 (0%) |
| 1 | 7 (50%) | 1 (17%) | 1 (25%) |
| 2 | 4 (29%) | 4 (66%) | 2 (50%) |
| 3 | 3 (21%) | 1 (17%) | 1 (25%) |
| Interface hepatitis | |||
| 0 | 4 (29%) | 2 (33%) | 0 (0%) |
| 1 | 6 (43%) | 2 (33%) | 4 (100%) |
| 2 | 1 (7%) | 2 (33%) | 0 (0%) |
| 3 | 3 (21%) | 0 (0%) | 0 (0%) |
| Specific cell subpopulations Plasma cells | |||
| 0 | 5 (36%) | 2 (33%) | 1 (25%) |
| 1 | 8 (57%) | 4 (66%) | 3 (75%) |
| 2 | 1 (7%) | 0 (0%) | 0 (0%) |
| 3 | 0 (0%) | 0 (0%) | 0 (0%) |
| Eosinophils | |||
| 0 | 5 (36%) | 1 (17%) | 1 (25%) |
| 1 | 4 (29%) | 4 (66%) | 2 (50%) |
| 2 | 5 (36%) | 1 (17%) | 1 (25%) |
| Neutrophils | |||
| 0 | 3 (21%) | 0 (0%) | 0 (0%) |
| 1 | 9 (64%) | 2 (33%) | 1 (25%) |
| 2 | 2 (14%) | 4 (67%) | 3 (75%) |
| Biliary abnormalities Bile duct injury | |||
| 0 | 8 (57%) | 0 (0%) | 0 (0%) |
| 1 | 6 (43%) | 6 (100%) | 4 (100%) |
| Ductular reaction | |||
| 0 | 3 (21%) | 0 (0%) | 0 (0%) |
| 1 | 8 (57%) | 0 (0%) | 0 (0%) |
| 2 | 3 (21%) | 5 (83%) | 3 (75%) |
| 3 | 0 (0%) | 1 (17%) | 1 (25%) |
| Biliary metaplasia | |||
| 0 | 11 (79%) | 1 (17%) | 2 (50%) |
| 1 | 3 (21%) | 3 (50%) | 0 (0%) |
| 2 | 0 (0%) | 1 (17%) | 1 (25%) |
| 3 | 0 (0%) | 1 (17%) | 1 (25%) |
| Granulomas | |||
| 0 | 4 (29%) | 6 (100%) | 1 (25%) |
| 1 | 7 (50%) | 0 (0%) | 3 (75%) |
| 2 | 3 (21%) | 0 (0%) | 0 (0%) |
| Fibrosis | |||
| F0 | 4 (29%) | 0 (0%) | 0 (0%) |
| F1 | 9 (64%) | 3 (50%) | 3 (75%) |
| F2 | 1 (7%) | 2 (33%) | 0 (0%) |
| F2/F3 | 0 (0%) | 1 (17%) | 1 (25%) |
| CD4+/CD8+ ratio | 12 | 6 | 4 |
| ≤1 | 10 (83%) | 4 (66%) | 2 (50%) |
| >1 | 2 (17%) | 2 (33%) | 2 (50%) |
One patient had only macrovesicular steatosis at the biopsy and could not be included in either of the immune-related hepatitis, cholangitis or cholangiohepatitis classes. Classification is based on the description of histological features in online supplemental table 4.
irAEs, immune-related adverse events.
Portal and lobular inflammatory infiltrates were mainly lymphocytic, with the additional presence of eosinophils, neutrophils and only few scattered plasma cells. Lobular necrosis was present in 20 (80%) patients and was classified as mild to moderate. Regional distribution of inflammation, either in the portal tracts, at the interface (periportal) or within the lobules, was variable. In 10 cases (40%), the inflammation was predominantly located in the portal and periportal areas. In five cases (20%), inflammation was predominantly lobular, reaching the centrilobular region, and accompanied by centrilobular injury. Thirteen (52%) cases had granulomas. Neutrophilic infiltrates were present in all but four patients and were numerous in nine patients. Eosinophils were absent in eight patients and numerous in seven.
Rosette formation was observed only in one case and emperipolesis was absent. The four patients presenting with significantly increased autoantibodies did not present distinctive histological features.
Immunohistochemistry for CD4+ and CD8+ T cells was performed in 22/25 patients. CD4+/CD8+ ratios were heterogeneous: 12/22 (55%) patients had a CD4+/CD8+ ratio equal to 1, 6 (27%) and 4 (18%) a ratio greater and lower than one, respectively (table 3).
The severity of the histopathological picture was scored as mild (or none) in 7/25 (28%), moderate in 12/25 (48%) and severe in 6/25 (24%) cases.
Histological and immunohistochemical characterization of the AILD group
Histopathological material for 13 out of 14 patients in the AILD group was available for reassessment with 10 AIH and three PBC. In AIH, the inflammatory infiltrate was predominantly plasmocytic, mixed with rare eosinophils and lymphocytes and located in the portal areas (13/13; 100%), with periportal and lobular extension in 8/10 (80%). Lobular necroinflammation was present in 9/10 (90%) of AIH patients but not in a single PBC patient. No granulomas were observed, while rosette formation and emperipolesis were observed in 4/10 (40%) and 5/10 (50%) AIH patients, respectively. Key features of the PBC liver biopsies were portal based inflammatory infiltrate, with predominant plasma cells, associated to florid biliary lesions, ductopenia and biliary metaplasia. Online supplemental table 5 provides more detailed information.
Neutrophils and eosinophils were present in all but two patients with AIH. They were numerous in four and one patient, respectively. They were not found in any of the three PBC patients. CD4+ and CD8+ T cell infiltrates were evaluated in 8/13 (62%) patients with AILD. Five (63%) had a CD4+/CD8+ ratio greater than one and 3/8 (37%) a ratio equal to one. None had a ratio lower than one.
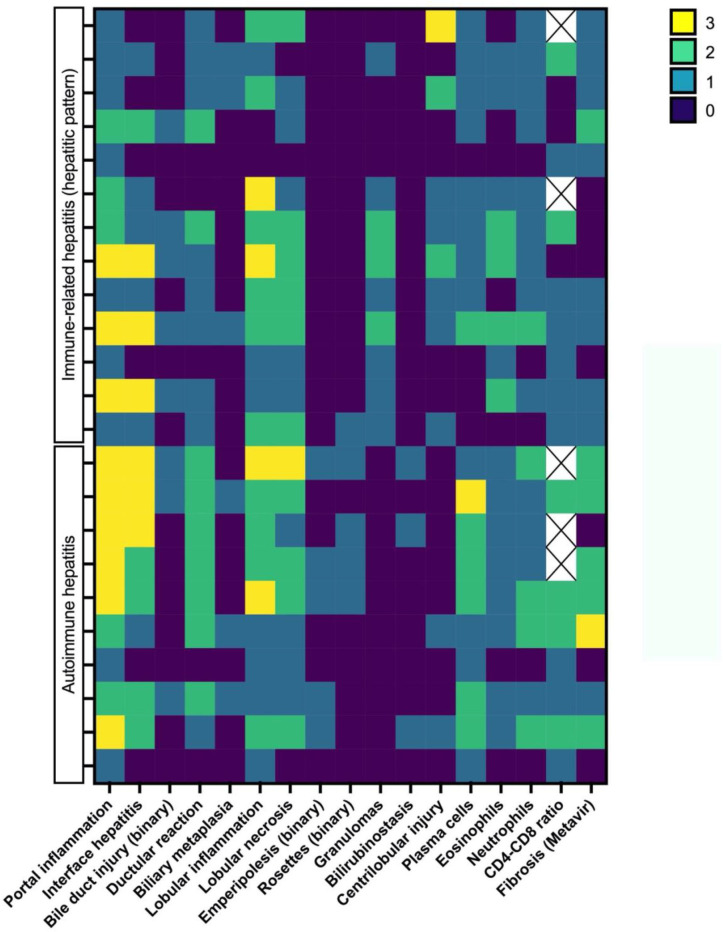
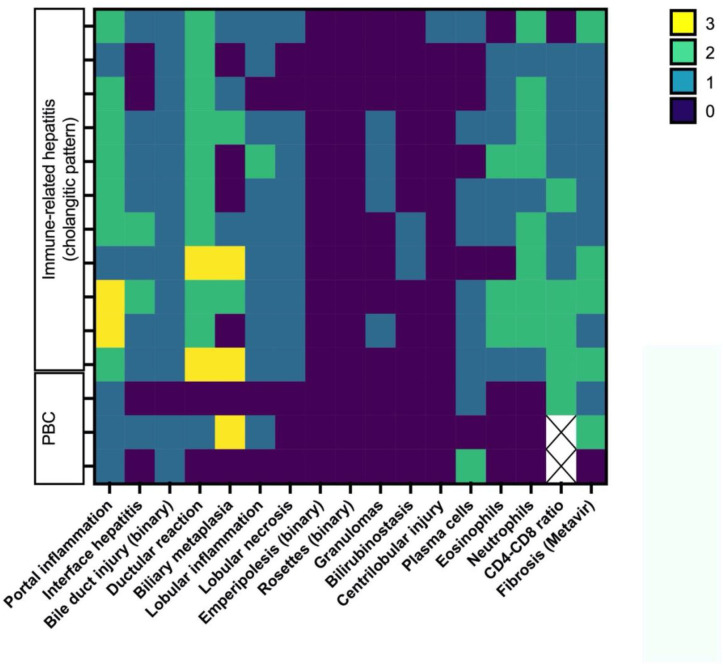
Comparison of the ICI and AILD groups
Figures 3 and 4 show, in a heatmap format, histological differences in injury patterns when comparing irH with AIH and irC/irCH with PBC. The following findings were relevant: a tendency for increased centrilobular injury and granulomas in irH and more frequent plasma cells, emperipolesis and rosette formation in AILD.
Figure 3.
Heatmap of the different histological features and their distribution between the immune-related hepatitis and autoimmune hepatitis groups. Histopathological variables of interest are represented on the x axis and graded according to online supplemental tables 4 and 5 using a colorimetric scale for each individual represented on the y axis.
Figure 4.
Heatmap of the different histological features and their distribution between the immune-related cholangitis or cholangiohepatitis and primary biliary cholangitis (PBC) groups. Histopathological variables of interest are represented on the x axis and graded according to online supplemental tables 4 and 5 using a colorimetric scale for each individual represented on the y axis.
Statistical analysis of histological results from the ICI group compared with the AILD group yielded several significant correlations and confirmed the findings displayed in the heatmap. Plasma cells were more abundant in inflammatory infiltrates of the AILD group than the ICI group (p=0.006). Granulomas were present in 13/25 patients (52%) from the ICI group but absent (0/13 patients) in the AILD group (p=0.002). Centrilobular injury tended to be more frequent in the ICI group compared with AILD (p=0.067). Rosette formations tended to be associated with AIH (4/10 vs 1/25 ICI patients, p=0.127) as did emperipolesis, present in 5/10 patients (50%) with AIH and absent (0/13 patients) in patients with irH (p=0.007). There was no statistically significant difference in CD4+/CD8+ ratios when comparing the ICI group to the AILD group (p=0.095).
Portal inflammatory lesions were more severe in irC patients than in PBC patients (p=0.038). Similarly, ductular reaction (p=0.005) and lobular necrosis (p=0.027) were more marked in the irC patients. Although no histological parameter was pathognomonic for either biliary injury, the severity of the picture was greater in irC. All other compared variables were not statistically significant (data not shown).
Correlation of LFTs with histopathological severity and pattern of the ICI group
We analyzed the correlation between LFTs values and the three histological liver injury patterns of the ICI group, namely irH, irC and irCH. Peak ALT values were significantly higher in irH compared with irC patients (median range: 559 (132–1630) vs 222 (125–331), p=0.035) with a good discriminative capacity to classify patients as having irH.
Peak ALP values were significantly lower in the irH patients compared with irC and irCH patients (median range: 196 (45–504) vs 702 (299–1395) vs 610 (266–969); p=0.0003) with a good discriminative capacity to classify patients as having irC or irCH.
Finally, we analyzed the correlation between the severity of LFT alterations at the time of liver biopsy and histological severity of inflammatory lesions, graded as mild, moderate and severe. There was no statistically significant correlation between clinical and histological severity, either considering clinical and histological severity as a binary or categorical variable. This observation was further confirmed when only considering patients who had not received any immunosuppressive treatment prior to liver biopsy. Clinical severity was graded as mild, whereas liver histology was severe, in 12% of the cases (3/25, patients 14, 18, and 25). Conversely, in one case (4%, patient 24) the clinical picture was considered severe whereas histology was described as mild, without having received immunosuppressive therapy.
Discussion
ICIs have become a leading option for the treatment of advanced cancers.10 27 28 In parallel to their increasing indications, the incidence of irAEs is expected to follow a similar trend.11 29 Among them, immune-related hepatotoxicity is of particular interest, as its occurrence may cause the ICI treatment to be halted or have fatal consequences.7 30–32 Moreover, irH is not uncommon, especially in combination therapies, and its incidence may be under-reported.14 15 33–35 To date, few case series have been reported with the scope of characterizing irH and irC patients from both a clinical and a histopathological perspective.17 21 36 37
Here, we describe 27 patients with liver irAEs and provide a thorough clinical, laboratory and histological characterization, as well as a comparison with 14 patients with AILD. By comparing classic AILD to ICI-related hepatic toxicities we show that these two diseases, although sharing some common aspects, are clinically, biologically, and histologically distinct.
From a clinical point of view, an important lesson that can be drawn is that the latency of toxicity is ICI-regimen dependent. In line with previous studies,17 38 anti-PD-1 or anti-PD-L1 treatments alone were found to be associated with a longer median time to onset of toxicity than combination therapies comprizing ipilimumab, an anti-CTLA-4 antibody (14.5 vs 8.0 weeks, respectively). Hence, clinicians should be aware of potential delayed toxicities associated with ICI and particularly regimens consisting of anti-PD-1 or anti-PD-L1 monotherapies.
Our experience also underlines the importance of a multidisciplinary approach to patients with ICI toxicities. Indeed, 16 (59%) patients presented extrahepatic irAEs during the clinical course.16
Liver irAEs have been infrequently associated with serological markers of autoimmunity.12 21 33 In our study, only 4 of the 27 (15%) ICI patients presented with a serological constellation suggestive of AILD. This raises the question of underlying subclinical liver autoimmunity unmasked by ICIs. Studies have suggested that immunotherapy could exacerbate or reveal latent autoimmune diseases.39–41 Notably, patients 20 and 26 showed histopathological lesions suggestive of typical PBC, raising the question of whether the ICI therapy unmasked an underlying PBC. Based on this observation, we suggest, in future studies, to prospectively assess the clinical utility of AILD autoantibodies in defining the individual risk of developing liver irAEs.
As recently described by Cohen et al, we confirm three distinct histological patterns of liver irAE, namely hepatitic, cholangitic and cholangiohepatitic.37 Indeed, liver irAEs may have variable histological presentations, with some studies even demonstrating a difference in histological phenotypes between anti-CTLA-4 and anti-PD-1 or anti-PD-L1 ICIs.17 In this context, fibrin ring granulomas and central vein endothelitis were more commonly observed in anti-CTLA-4 antibody therapies as compared with anti-PD-1 or anti-PD-L1 therapies.17 In line with this observation, we identified granulomas in 10/14 (71%) of patients treated with a combination of anti-CTLA-4 and anti-PD-1 or anti-PD-L1 as compared with only 3/11 (27%) of patients treated with anti-PD-1 or anti-PD-L1.
Histopathology of classic AIH is characterized by interface hepatitis, a plasma cell-rich portal infiltrate, rosette formation, and emperipolesis.42 By contrast, irH has a predominantly lymphocytic infiltrate with reportedly low CD4+/CD8+ ratios, associated with eosinophils, neutrophils and scarce plasma cells.17 19 21 37 Reports also highlight centrilobular hepatitis with associated endothelitis as an important hallmark of regional distribution in irH. Our results confirm these findings. First, centrilobular hepatitis with concomitant endothelitis was more frequent in our irH patients as compared with AIH. Second, in the ICI group, inflammatory infiltrates were composed primarily of lymphocytes, neutrophils and eosinophils with rare plasma cells, while plasma cells were the predominant type of inflammatory cells in classic AILD. Although not explored in the AILD patients, and as recently reported, neutrophils were very common in liver irAE infiltrates.43 As neutrophilic infiltrates are not typical of AILD, their appearance may further help identify and elucidate ICI liver toxicity mechanisms.
In a previous study on a smaller group of patients, Zen et al compared 7 cases of irH with 10 cases of AIH. In their experience, liver injury caused by cancer immunotherapy shared some features with AIH. However, there were obvious differences between the two conditions, including less zone-selective inflammation, as in our study, and fewer CD4+ compared with to CD8+ cells in irH.21 In thist study, CD4+/CD8+ ratios in the ICI group were very heterogenous. Sixteen out of 22 (73%) patients showed ratios ≤1, suggesting an important role of CD8 T cells in the infiltrate and pathophysiology of toxicity, even if there was no statistical difference with the AILD group.
Kupffer cells may also be found in ICI-induced inflammation, offering an explanation as to why many cases of irH present with granuloma formation, which is unrepresentative of AIH.17 33 37
In four and six patients with irC and irCH, respectively, biliary abnormalities were prominent, including florid bile duct inflammatory injury, ductular reaction and biliary metaplasia, with inflammation predominating in the portal tract. These histological observations were correlated with pronounced increases in parameters of cholestasis, as represented by higher ALP values and more frequent occurrence of jaundice. Of note, three patients with irCH (patients 22, 24 and 26) were recently reported by Moi et al.44 In this brief report, the authors described forms of irCH that were refractory to CS therapy, motivating the introduction of targeted IL-6 receptor blockade by tocilizumab, with favorable outcomes. In total, four out of our five patients receiving tocilizumab for CS-refractory irAEs had a biliary phenotype. Hence, patients with irC or irCH may more often have a CS-refractory course and require tailored therapy.45–47
Liver biopsy is particularly important in the diagnosis of delayed-onset toxicities.15 30 31 35 In our experience, one patient developed irC 72 weeks after the first ICI dose. In this setting, histology was crucial for a definitive diagnosis. Histopathological assessment is also useful in providing additional information on underlying liver disease and excluding differential diagnoses, such as tumor cell infiltration or other types of liver injury, which would substantially influence patient care and guide immunosuppressive treatment management.15 33 In our series, one patient had advanced fibrosis (METAVIR F3) in the setting of an underlying alcohol-related liver disease, another patient had a background of nonalcoholic steatohepatitis and two patients had ischemic hepatitis linked to SIRT. These elements were of importance when considering CS therapy and follow-up of the patients.
Based on these elements, from our perspective, liver biopsy played an important role in diagnosing and managing liver irAEs. We acknowledge that other authors concluded in their series that, in ICI-related grade 3 liver injury, performing a liver biopsy was associated with a delay in starting CSs and did not result in a faster resolution of the hepatitis.48 Importantly, in this report by Li et al, 12 of 107 biopsied patients (11%) did not actually have ICI hepatitis. In these cases, liver biopsy was therefore certainly of great help in avoiding potentially deleterious immunosuppressive therapy in an oncological context. In addition, in a liver biopsy performed after starting CS, the histology might be modified by the treatment. In conclusion, while routine biopsy may, depending on the context, postpone the initiation of therapy, it may also be of great clinical value to refine the diagnosis and guide or avoid potentially deleterious CS therapy. We, therefore, propose to maintain the biopsy in the diagnostic approach of severe forms of liver irAEs, which will be discussed on a case-by-case basis.
Immunosuppressive treatment may affect oncological responses to immunotherapy, while exposing cancer patients to opportunistic infections as well as a range of additional specific toxicities. Therefore, situations in which such treatments may be limited are important to identify.49 50 In our experience, the pattern of LFTs alterations was predictive for the type of irAEs, namely cholangitic and hepatitis forms. However, clinical severity, based on LFT’s and total bilirubin values at time of biopsy, did not statistically correlate with histopathological severity, with some situations in which clinical and histological findings were contrary. There was also no association between histopathological severity and improved hepatic or oncological outcomes. Further studies are needed to explore the prognostic value of liver biopsies regarding choice of immunosuppressive therapy and the utility of re-exposure to ICIs after treatment discontinuation.
We recognize some limitations to our study. First, its retrospective nature and the fact that some patients had already received immunosuppressive drugs at the time of liver biopsy may limit the strength of our findings. Further prospective studies systematically characterizing the liver toxicity of ICIs, also including patients with less severe grades of toxicity, are therefore needed. Second, we acknowledge the relatively small number of patients included in our study. However, only moderate to severe grades of toxicities qualify for liver biopsy and urgent immunosuppressive management is often difficult to postpone, making early liver biopsies rare.16 However, our series comparing liver irAEs with classic forms of AILD is to our knowledge the largest reported to date.
In conclusion, this study confirms that there are three different histological patterns in liver irAEs (irH, irC and irCH) and shows some specific features for this disease, different from the classical AILD. Liver biopsy represents a key element to document liver irAEs, and to explore the physiopathology of liver toxicities following ICI treatment. Histological severity does not always reflect clinical and biological parameters and is therefore a useful element for guiding the clinical management of liver irAEs.
Footnotes
Contributors: AC, DM and MF designed the study; AC, MO, AW, FF, CS and MF acquired data; JV and MF performed statistical analyses; AC, JV, DM, CS and MF wrote the manuscript; all authors revised the manuscript. MF acted as the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: No, there are no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: CS and MF share last authorship.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the ‘Commission cantonale d’éthique de la recherche sur l’être humain’ (CER-VD) on December 9, 2019 (protocol number 2019-01725). Informed consent was obtained from all living patients included in the study.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–9. 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39:98–106. 10.1097/COC.0000000000000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 2019;30:582–8. 10.1093/annonc/mdz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luoma AM, Suo S, Williams HL, et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 2020;182:655–71. 10.1016/j.cell.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 2019;16:563–80. 10.1038/s41571-019-0218-0 [DOI] [PubMed] [Google Scholar]
- 8.Sasson SC, Slevin SM, Cheung VTF, et al. Interferon-Gamma-Producing CD8+ Tissue Resident Memory T Cells Are a Targetable Hallmark of Immune Checkpoint Inhibitor-Colitis. Gastroenterology 2021;161:1229–44. 10.1053/j.gastro.2021.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing P, Zhang F, Wang G, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer 2019;7:341. 10.1186/s40425-019-0779-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 11.Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 2022;19:254–67. 10.1038/s41571-022-00600-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzman DL, Pelosof L, Rosenberg A, et al. Hepatotoxicity of immune checkpoint inhibitors: an evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int 2018;38:976–87. 10.1111/liv.13746 [DOI] [PubMed] [Google Scholar]
- 13.Remash D, Prince DS, McKenzie C, et al. Immune checkpoint inhibitor-related hepatotoxicity: a review. World J Gastroenterol 2021;27:5376–91. 10.3748/wjg.v27.i32.5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44:51–60. 10.1016/j.ctrv.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Sznol M, Ferrucci PF, Hogg D, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol 2017;35:3815–22. 10.1200/JCO.2016.72.1167 [DOI] [PubMed] [Google Scholar]
- 16.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 17.Martins F, Sykiotis GP, Maillard M, et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol 2019;20:e54–64. 10.1016/S1470-2045(18)30828-3 [DOI] [PubMed] [Google Scholar]
- 18.De Martin E, Michot J-M, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181–90. 10.1016/j.jhep.2018.01.033 [DOI] [PubMed] [Google Scholar]
- 19.Zen Y, Chen Y-Y, Jeng Y-M, et al. Immune-related adverse reactions in the hepatobiliary system: second-generation check-point inhibitors highlight diverse histological changes. Histopathology 2020;76:470–80. 10.1111/his.14000 [DOI] [PubMed] [Google Scholar]
- 20.Zen Y, Yeh MM. Checkpoint inhibitor-induced liver injury: a novel form of liver disease emerging in the era of cancer immunotherapy. Semin Diagn Pathol 2019;36:434–40. 10.1053/j.semdp.2019.07.009 [DOI] [PubMed] [Google Scholar]
- 21.Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol 2018;31:965–73. 10.1038/s41379-018-0013-y [DOI] [PubMed] [Google Scholar]
- 22.Doherty GJ, Duckworth AM, Davies SE, et al. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open 2017;2:e000268. 10.1136/esmoopen-2017-000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izumi H, Kodani M, Kurai J, et al. Nivolumab-induced cholangitis in patients with non-small cell lung cancer: case series and a review of literature. Mol Clin Oncol 2019;11:439–46. 10.3892/mco.2019.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European association for the study of the liver clinical practice guidelines: autoimmune hepatitis. J Hepatol 2015;63:971–1004. [DOI] [PubMed] [Google Scholar]
- 25.European association for the study of the liver clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145–72. [DOI] [PubMed] [Google Scholar]
- 26.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR cooperative Study Group. Hepatology 1996;24:289–93. 10.1002/hep.510240201 [DOI] [PubMed] [Google Scholar]
- 27.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:1270–1. 10.1056/NEJMc1509660 [DOI] [PubMed] [Google Scholar]
- 28.Wilky BA. Immune checkpoint inhibitors: the linchpins of modern immunotherapy. Immunol Rev 2019;290:6–23. 10.1111/imr.12766 [DOI] [PubMed] [Google Scholar]
- 29.Vaddepally RK, Kharel P, Pandey R, et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers 2020;12:738. 10.3390/cancers12030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dougan M, Blidner AG, Choi J, et al. Multinational association of supportive care in cancer (MASCC) 2020 clinical practice recommendations for the management of severe gastrointestinal and hepatic toxicities from checkpoint inhibitors. Support Care Cancer 2020;28:6129–43. 10.1007/s00520-020-05707-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Martin E, Michot J-M, Rosmorduc O, et al. Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep 2020;2:100170. 10.1016/j.jhepr.2020.100170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo T, Sugawara T, Shinkawa T. Fatal fulminant hepatitis induced by combined ipilimumab and nivolumab therapy despite favorable histologic response and confirmed by autopsy in a patient with clear cell renal cell carcinoma. Immunol Med 2020:1–6. [DOI] [PubMed] [Google Scholar]
- 33.Jennings JJ, Mandaliya R, Nakshabandi A, et al. Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol 2019;15:231–44. 10.1080/17425255.2019.1574744 [DOI] [PubMed] [Google Scholar]
- 34.Shoushtari AN, Friedman CF, Navid-Azarbaijani P, et al. Measuring toxic effects and time to treatment failure for nivolumab plus ipilimumab in melanoma. JAMA Oncol 2018;4:98–101. 10.1001/jamaoncol.2017.2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanaoka K, Moriizumi K, Okada H, et al. Pembrolizumab-induced delayed-onset hepatitis. Case Rep Gastroenterol 2020;14:586–92. 10.1159/000509953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawakami H, Tanizaki J, Tanaka K, et al. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs 2017;35:529–36. 10.1007/s10637-017-0453-0 [DOI] [PubMed] [Google Scholar]
- 37.Cohen JV, Dougan M, Zubiri L, et al. Liver biopsy findings in patients on immune checkpoint inhibitors. Mod Pathol 2021;34:426–37. 10.1038/s41379-020-00653-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 39.Danlos F-X, Voisin A-L, Dyevre V, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer 2018;91:21–9. 10.1016/j.ejca.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 40.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017;28:368–76. 10.1093/annonc/mdw443 [DOI] [PubMed] [Google Scholar]
- 41.Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016;2:234–40. 10.1001/jamaoncol.2015.4368 [DOI] [PubMed] [Google Scholar]
- 42.Okano N, Yamamoto K, Sakaguchi K, et al. Clinicopathological features of acute-onset autoimmune hepatitis. Hepatol Res 2003;25:263–70. 10.1016/s1386-6346(02)00274-7 [DOI] [PubMed] [Google Scholar]
- 43.Siwicki M, Gort-Freitas NA, Messemaker M, et al. Resident Kupffer cells and neutrophils drive liver toxicity in cancer immunotherapy. Sci Immunol 2021;6:eabi7083. 10.1126/sciimmunol.abi7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moi L, Bouchaab H, Mederos N, et al. Personalized cytokine-directed therapy with tocilizumab for refractory immune checkpoint inhibitor-related cholangiohepatitis. J Thorac Oncol 2021;16:318–26. 10.1016/j.jtho.2020.09.007 [DOI] [PubMed] [Google Scholar]
- 45.Haanen J, Ernstoff M, Wang Y, et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J Immunother Cancer 2020;8:e000604. 10.1136/jitc-2020-000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 2019;25:551–7. 10.1177/1078155217745144 [DOI] [PubMed] [Google Scholar]
- 47.Reddy CA, Schneider BJ, Brackett LM, et al. Nivolumab-induced large-duct cholangiopathy treated with ursodeoxycholic acid and tocilizumab. Immunotherapy 2019;11:1527–31. 10.2217/imt-2019-0121 [DOI] [PubMed] [Google Scholar]
- 48.Li M, Sack JS, Bell P, et al. Utility of liver biopsy in diagnosis and management of high-grade immune checkpoint inhibitor hepatitis in patients with cancer. JAMA Oncol 2021;7:1711–4. 10.1001/jamaoncol.2021.4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–14. 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 50.Pauken KE, Dougan M, Rose NR, et al. Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol 2019;40:511–23. 10.1016/j.it.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005635supp001.pdf (184.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.