Abstract
Background
Surveillance of the appropriateness of antimicrobial prescribing can identify targets for quality improvement in antimicrobial stewardship. Our objective was to measure antibiotic prescription prevalence, indication, and appropriateness at three rural community hospitals in a 1-day point prevalence study.
Methods
Inpatient antibiotic prescriptions given at three community hospitals on April 24, 2019 were provided by the hospital pharmacies. These prescriptions were analyzed using the Australian National Antimicrobial Prescribing Survey (NAPS) tool. Prescriptions were assessed by an infectious diseases physician and analyzed per prescription.
Results
Eighty prescriptions given to 58 inpatients were included. Antibiotic treatment prevalence was 58/120 beds (48.3%), and overall appropriateness was 37/80 prescriptions (46.3%). The most prescribed antibiotics were ceftriaxone (17 [21.3%]; 47.1% appropriate), piperacillin–tazobactam (10 [12.5%]; 10.0% appropriate), and moxifloxacin (9 [11.3%]; 0% appropriate). The most common indications were respiratory tract infections (36 [45.0%]; 36.1% appropriate), skin and soft tissue infections (14 [17.5%]; 78.6% appropriate), and urinary tract infections (9 [11.3%]; 11.1% appropriate). Of the 80 prescriptions, 50 (62.5%) documented an indication, and 71 (88.8%) documented a stop or review date.
Conclusions
We observed a high treatment prevalence and low appropriateness. Overall appropriateness was lower than in urban hospitals.
Key words: antimicrobial stewardship, antimicrobial appropriateness, inpatient care
Abstract
Historique
La surveillance de la pertinence des prescriptions d’antimicrobiens peut permettre de cibler des mesures d’amélioration de la qualité de la gestion antimicrobienne. Les chercheurs se sont donné l’objectif de mesurer la prévalence des prescriptions d’antibiotiques, leur indication et leur pertinence dans trois hôpitaux généraux ruraux au moyen d’une étude de prévalence ponctuelle d’un jour.
Méthodologie
Tous les antibiotiques prescrits aux patients hospitalisés ont été remis aux chercheurs par la pharmacie de trois hôpitaux généraux le 24 avril 2019. Les chercheurs ont analysé ces prescriptions au moyen de l’outil du sondage australien de prescriptions nationales d’antimicrobiens (NAPS, selon l’acronyme anglais). Un infectiologue les a évaluées, et chacune a été analysée.
Résultats
Les chercheurs ont inclus 80 prescriptions remises à 58 patients hospitalisés. La prévalence de traitement antibiotique s’élevait à 58 lits sur 120 (48,3 %), et la pertinence globale, à 37 prescriptions sur 80 (46,3 %). Les antibiotiques les plus prescrits étaient la ceftriaxone (17; 21,3 %), dont 47,1 % étaient appropriés; la pipéracilline-tazobactam (dix; 12,5 %), dont 10,0 % étaient appropriés; et la moxifloxacine (neuf; 11,3 %), dont 0 % était appropriés. Les indications les plus courantes étaient les infections respiratoires : 36 (45,0 %), dont 36,1 % étaient appropriées; les infections de la peau et des tissus mous : 14 (17,5 %), dont 78,6 % étaient appropriées; et les infections urinaires : neuf (11,3 %), dont 11,1 % étaient appropriées. Des 80 prescriptions, 50 (62,5 %) comportaient une indication, et 71 (88,8 %) comportaient une date d’arrêt ou de révision.
Conclusion
Les chercheurs ont observé une forte prévalence de traitements et une pertinence basse. La pertinence globale était plus basse que dans les hôpitaux urbains.
Mots-clés : gestion antimicrobienne, pertinence antimicrobienne, soins aux patients hospitalisés
Introduction
Antimicrobial use is associated with the development of antimicrobial resistance (1), and the goal of antimicrobial stewardship is reduction in antimicrobial use. Newfoundland and Labrador has the highest rate of antimicrobial usage in Canada at 9,857 defined daily doses per 1,000 inhabitants in 2018 (2).
Antimicrobial stewardship optimizes agent, dosage, route, and duration to limit the selection of antimicrobial resistance among bacteria and reduce adverse effects of antibiotics, such as Clostridiodes difficile–associated diarrhea (3). Antimicrobial resistance is an emerging threat to global health (4) and was associated with 5,400 deaths and a loss of $1.4 billion in Canada in 2018 (5).
Besides reduction in use rate, increase in appropriateness of use may improve antimicrobial stewardship (6). Appropriateness of use is subjective, but it includes avoidance of treatment when a bacterial infection is not present, selection of empiric treatment, transition of targeted treatment, matching of spectrum of activity with culture results, and dosing according to patient variables (7). In 10 long-term-care facilities in Newfoundland and Labrador’s Eastern Health region, 57.8% of antimicrobial prescriptions were inappropriate (8).
Rural hospitals may not have internal antimicrobial stewardship programs, but they may benefit from remote audit and feedback (9). A study from Australia found that inappropriate antimicrobial prescribing was more common in rural (24.0%) than in urban (22.1%) centres (10).
Our objective was to assess the appropriateness of antibiotic usage at three rural Newfoundland and Labrador hospitals, according to published Australian National Antimicrobial Prescribing Survey (NAPS) guidelines (11), and identify targets for improvement. Antibiotics are a subgroup of antimicrobials. Appropriateness of antibiotic prescriptions in rural hospitals has not been studied in Canada.
Methods
This study is a hospital-wide 1-day point prevalence survey of antibiotic appropriateness at three community hospitals in Newfoundland and Labrador, Canada. It is based on the secondary use of patient information and the procedures outlined by the Australian NAPS (11). This survey is being piloted in Canada. This method has previously been used to study two urban hospitals in Newfoundland and Labrador (Peter K Daley, manuscript in review). The 1-day point prevalence survey is feasible for data collection, with the acknowledgement that 1-day surveys may produce biased conclusions because they do not represent all prescriptions given over time.
All patients who were admitted to hospital 1 (72 beds), hospital 2 (41 beds), and hospital 3 (35 beds) as of 8:00 a.m. on the day of survey were included (the denominator). All three hospitals were located in the Eastern Health region of Newfoundland and Labrador. Patients receiving antibiotics on the day of the survey were included as cases (the numerator).
Data on all systemic antibiotics that were prescribed on April 24, 2019, at the three community hospitals were accessed from pharmacy records. Oral and enteral routes were included, and topical or local antibiotics were excluded. Antiviral and antifungal treatments were also excluded because they were not a focus of this survey. Patient identifiers were used to access clinical information, but data were anonymized before analysis.
Data collected from electronic chart review included demographics, prescription, documented or presumed indication, review or stop date, allergies, microbiology results, and surgical procedures. All data were entered into a standardized NAPS form (11) by a medical student investigator, and appropriateness was assessed by an infectious disease physician (PKD), according to local clinical guidelines available in the Spectrum™ app and microbiology results.
Antibiotic use was categorized as optimal, adequate, suboptimal, inadequate, or not assessable (11). Optimal and adequate categories were considered appropriate, and suboptimal and inadequate were considered inappropriate. The categories are defined by NAPS as follows:
Optimal: The prescription follows locally, regionally, or provincially endorsed guidelines, which includes antibiotic choice, dose, route, and duration. Alternatively, the prescription was reviewed and endorsed by an infectious disease clinician, and there is not a narrower spectrum or better antibiotic choice.
Adequate: The prescription does not follow the locally, regionally, or provincially endorsed guidelines, but it is a reasonable alternative choice for the likely causative pathogen. This also includes surgical prophylaxis when the duration is less than 24 hours post-operatively.
Suboptimal: The prescription presents a mild non– life-threatening allergy mismatch, or the prescription (antibiotic choice, dose, route, duration) is unreasonable for the causative pathogen. This could include situations in which the spectrum is excessively broad, there is unnecessary overlap in the spectrum, or the dose and duration are excessively large and long. It could also include the failure to de-escalate treatment after microbiology results.
Inadequate: The prescription is unlikely to treat the causative pathogen, the documented indication does not require antibiotics, there is a severe and life-threatening allergy mismatch, or the duration is greater than 24 hours for surgical prophylaxis.
Not assessable: The indication for the prescription is not documented or unclear, the appropriateness cannot be determined from the non-comprehensive notes, or the patient is too complex as a result of other comorbidities, allergies, or results.
The primary outcome was percentage appropriateness. We used IBM SPSS Statistics (IBM Corp., Armonk, New York) to compare appropriateness between groups, using χ2 analysis (two sided).
The study received full approval for the secondary use of data from the local Health Research Ethics Board (Ref. 2019.062, approved April 15, 2019).
Results
Antibiotic treatment prevalence was 58 (48.3%) of 120 occupied beds. A total of 101 prescriptions were given to 58 patients. Of these prescriptions, 21 (20.8%) were excluded because they were either topical or local antibiotics or antivirals. Figure 1 summarizes the prevalence and rate of appropriateness for the most commonly prescribed antibiotics, for the most common indications, and by facility.
Figure 1:
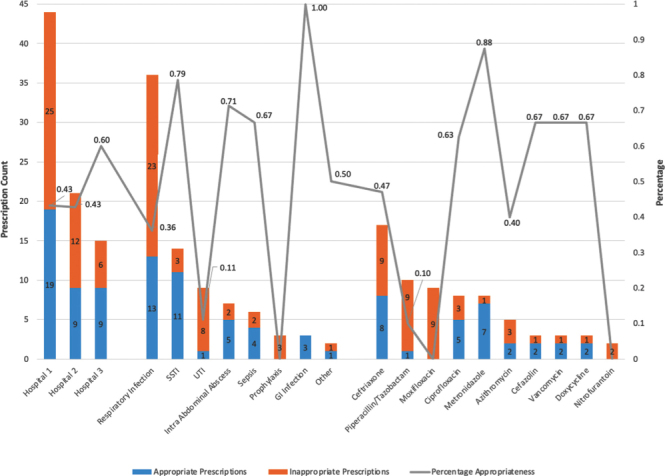
Prevalence and percentage appropriateness of antimicrobial prescription by facility, indication for prescription, and antimicrobial prescribed
Overall appropriateness was 46.3% (37 of 80 prescriptions). On the basis of the NAPS five-point system, 20 (25.0%) prescriptions were optimal, 17 (21.3%) were adequate, 21 (26.3%) were suboptimal, 22 (27.5%) were inadequate, and 0 (0%) were not assessable. The majority of prescriptions were delivered intravenously (50; 62.5%). Only 50 (62.5%) antibiotic prescriptions had an indication documented, and 71 (88.8%) prescriptions had a stop or review date indicated.
Ceftriaxone was the most prescribed antibiotic (17 [21.3%]; 47.1% appropriate), followed by piperacillin–tazobactam (10 [12.5%]; 10.0% appropriate), moxifloxacin (9 [11.3%]; 0% appropriate), metronidazole (8 [10.0%]; 87.5% appropriate), ciprofloxacin (8 [10.0%]; 62.5% appropriate), and azithromycin (5 [6.3%]; 40.0% appropriate).
The most common indication for antibiotic use was respiratory tract infections (36 [45.0%]; 36.1% appropriate), followed by skin and soft tissue infections (14 [17.5%]; 78.6% appropriate), and urinary tract infections (9 [11.3%]; 11.1% appropriate).
Hospital 1 demonstrated an appropriateness rate of 19/44 (43.2%), compared with 9/21 (42.9%) for hospital 2, and 9/15 (60.0%) for hospital 3.
Overall appropriateness in rural hospitals in this study (46.3%) was lower than that in urban hospitals in St. John’s, Newfoundland and Labrador (55.1%), studied in February 2018. This difference was statistically significant (p < 0.001).
Discussion
NAPS tool
The NAPS tool provides definitions to guide the assessment of appropriateness, which may improve accuracy compared with subjective assessment. It has been used since 2011 in Australia to disseminate a national report on the appropriateness of hospital use, and its use has expanded into surgical care, long-term care, and general practice (https://www.ncas-australia.org/naps). Further research will be required to determine the validity of this tool in rural hospitals and in Canada.
Appropriateness
This retrospective cross-sectional observational study of the appropriateness of antibiotic prescribing in rural hospitals is the first to be completed in Newfoundland and Labrador. Antibiotic treatment prevalence in rural hospitals (48.3%) was higher than that in two urban acute care facilities (31.2%). Overall antibiotic appropriateness in rural hospitals (46.3%) was slightly lower than in urban hospitals (55.1%; p < 0.001). This lower appropriateness may be due to a lack of internal antibiotic stewardship programs at these rural hospitals or to other factors that impede antimicrobial stewardship in rural areas, including lack of resources, geographical isolation, lack of access to an infectious diseases specialist, and rural physicians’ culture of self-reliance and independence (12).
The most commonly prescribed antibiotic in this study was ceftriaxone (21.3%, 47.1% appropriate), compared with piperacillin–tazobactam (14.6%, 51.9% appropriate) in the urban hospital study (Peter K Daley, manuscript in review). Appropriateness of piperacillin–tazobactam (10.0%) and moxifloxacin (0%) prescriptions was low in rural hospitals, making these molecules effective targets for antimicrobial stewardship.
Urinary tract infection (9/80, 11.3%) was the third most common indication for antibiotic use, but its appropriateness was only 11.1%. This may be due to the unnecessary treatment of asymptomatic bacteriuria, an identified stewardship problem in Newfoundland and Labrador (8).
Only 62.5% of prescriptions had an indication documented, and 88.8% of prescriptions had a stop or review date indicated. Most prescription stop dates were based on an automatic stop policy from pharmacy, not an ordered duration. There is substantial room for improvement because these indicators were lower than the target reliability of health care processes of more than 95% (13).
Limitations
One limitation for this study is the small size of the hospitals. Still, a statistically significant difference in appropriateness compared with urban hospitals was observed. One-day point prevalence methods may be biased if behaviour is different on the day of study compared with other days. Appropriateness is determined by retrospective chart review, which may bias in favour of inappropriateness, based on missing information. Appropriateness of prescription does not have a reference standard.
Conclusion
This study observed high treatment prevalence and low appropriateness in rural hospitals, and it identified areas for quality improvement in antibiotic usage. This research will contribute to the gap that exists in antimicrobial stewardship in rural acute care settings in Canada.
Ethics Approval:
This study received full approval for secondary use of data from the local Health Research Ethics Board (Ref. 2019.062, approved April 15, 2019).
Availability of Data and Material:
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Informed Consent:
N/A
Funding:
None.
Disclosures:
The authors declare that they have no competing interests.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
References
- 1.Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14(1):13. 10.1186/1471-2334-14-13. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System report. 2020. https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-health-products/canadian-antimicrobial-resistance-surveillance-system-2020-report/CARSS-2020-report-2020-eng.pdf (June 15, 2020).
- 3.Schuts EC, Hulscher MEJL, Mouton JW, et al. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):847–56. 10.1016/S1473-3099(16)00065-7. [DOI] [PubMed] [Google Scholar]
- 4.Review on Antimicrobial Resistance. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. https://amr-review.org/sites/default/files/AMRReviewPaper-Tacklingacrisisforthehealthandwealthofnations_1.pdf (June 15, 2020).
- 5.Expert Panel on the Potential Socio-Economic Impacts of Antimicrobial Resistance in Canada. When antibiotics fail. 2019. https://cca-reports.ca/wp-content/uploads/2018/10/When-Antibiotics-Fail-1.pdf (June 15, 2020).
- 6.File TM Jr, Srinivasan A, Bartlett JG. Antimicrobial stewardship: importance for patient and public health. Clin Infect Dis. 2014;59(Suppl 3):S93–6. 10.1093/cid/ciu543. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc. 2011; 86(2):156–67. 10.4065/mcp.2010.0639. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penney CC, Boyd SE, Mansfield A, Dalton J, O’Keefe J, Daley PK. Antimicrobial use and suitability in long-term care facilities: a retrospective cross- sectional study. Off J Assoc Med Microbiol Infect Dis Canada. 2018;3(4):209–16. 10.3138/jammi.2018-0021. [DOI] [Google Scholar]
- 9.Anderson DJ, Watson S, Moehring RW, et al. Feasibility of core antimicrobial stewardship interventions in community hospitals. JAMA Netw Open. 2019;2(8):e199369–e199369. 10.1001/jamanetworkopen.2019.9369. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop JL, Schulz TR, Kong DCM, James R, Buising KL. Similarities and differences in antimicrobial prescribing between major city hospitals and regional and remote hospitals in Australia. Int J Antimicrob Agents. 2019;53(2):171–6. 10.1016/j.ijantimicag.2018.10.009. Medline: [DOI] [PubMed] [Google Scholar]
- 11.Canadian NAPS: a pilot initiative user guide 2018. Melbourne, VIC: National Centre for Antimicrobial Stewardship; 2018. https://www.naps.org.au/ (June 15, 2020). [Google Scholar]
- 12.Bishop JL, Schulz TR, Kong DCM, Buising KL. Qualitative study of the factors impacting antimicrobial stewardship programme delivery in regional and remote hospitals. J Hosp Infect. 2019;101(4):440–6. 10.1016/j.jhin.2018.09.014. Medline: [DOI] [PubMed] [Google Scholar]
- 13.Resar RK. Making noncatastrophic health care processes reliable: learning to walk before running in creating high-reliability organizations. Health Serv Res. 2006. 41(4 Pt 2):1677–89. 10.1111/j.1475-6773.2006.00571.x. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]


