Abstract
A 21-year-old, previously healthy male presented to hospital following 1 week of bilateral asymmetric ascending paralysis, odynophagia, and dysphagia. Initial magnetic resonance imaging (MRI) of the spine revealed an abnormal increased T2 signal with predominant dorsal column involvement and sparing of white matter throughout the cervical cord and extending to T5. The initial presumptive diagnosis was an acute infectious, versus inflammatory, myelitis. On reviewing the history, family members recalled a bat scratch on the left hand, sustained months prior, for which the patient did not seek or receive post-exposure prophylaxis (PEP). Rabies virus (RABV) RNA was detected by quantitative reverse transcription polymerase chain reaction (RT-qPCR) in two saliva samples, while nuchal skin biopsy and cerebrospinal fluid (CSF) were negative. Serum was negative for RABV neutralizing antibody. Sequencing and phylogenetic analyses identified the infecting RABV as a variant associated with silver-haired bats. Following risk assessment of exposure, 67 health care workers and several family members were offered PEP.
Key words: ascending paralysis, encephalitis, post-exposure prophylaxis (PEP), quantitative reverse transcription polymerase chain reaction (RT-qPCR), rabies neutralizing serum antibody, rabies virus
Abstract
Un homme de 21 ans auparavant en santé a consulté à l’hôpital parce qu’il souffrait de paralysie ascendante, asymétrique et bilatérale, d’odynophagie et de dysphagie depuis une semaine. Une première imagerie par résonance magnétique (IRM) du rachis a révélé une augmentation anormale du signal T2 avec atteinte prédominante de la colonne dorsale et épargne de la matière blanche dans toute la colonne cervicale jusqu’à la vertèbre T5. Le diagnostic provisoire en était un de myélite infectieuse, et non inflammatoire. À la prise de l’histoire, les membres de la famille se sont souvenus d’une égratignure de chauve-souris sur la main gauche du patient plusieurs mois auparavant, qui n’a pas été suivie d’une prophylaxie postexposition (PPE). Les chercheurs ont décelé l’ARN du virus de la rage (RABV) par amplification en chaîne par polymérase quantitative de transcription inverse (RT-qPCR) dans deux échantillons de salive, mais constaté un résultat négatif de la biopsie de la peau nucale et du liquide céphalorachidien. Le sérum était négatif à l’anticorps neutralisant du RABV. Les analyses de séquençage et de phylogénétique ont confirmé une contamination par une variante du RABV associée aux chauves-souris argentées. Après une évaluation du risque d’exposition, 67 travailleurs de la santé et plusieurs membres de la famille se sont fait offrir une PPE.
Mots-clés : anticorps neutralisants sériques de la rage, amplification en chaîne par polymérase quantitative de transcription inverse (RT-qPCR), encéphalite, paralysie ascendante, prophylaxie postexposition, virus de la rage
Case Presentation
A 21-year old, previously healthy male presented to hospital following 1 week of bilateral asymmetric ascending paralysis, characterized by left-hand paresthesias, progressing to left-arm hemiplegia, and weakness of the right arm and legs. On presentation to hospital, the patient began to experience odynophagia and dysphagia. Within hours of initial presentation, he developed fever, hypoxemia, and significant upper respiratory secretions requiring intubation.
Initial magnetic resonance imaging (MRI) of the spine revealed an abnormal increased T2 signal with predominant dorsal column involvement and sparing of white matter throughout the cervical cord and extending to T5 (Figure 1). No cerebellar or cerebral abnormalities were visualized on brain MRI. Blood and sputum cultures were negative. Results of cerebrospinal fluid (CSF) analyses are shown in Table 1.
Figure 1:
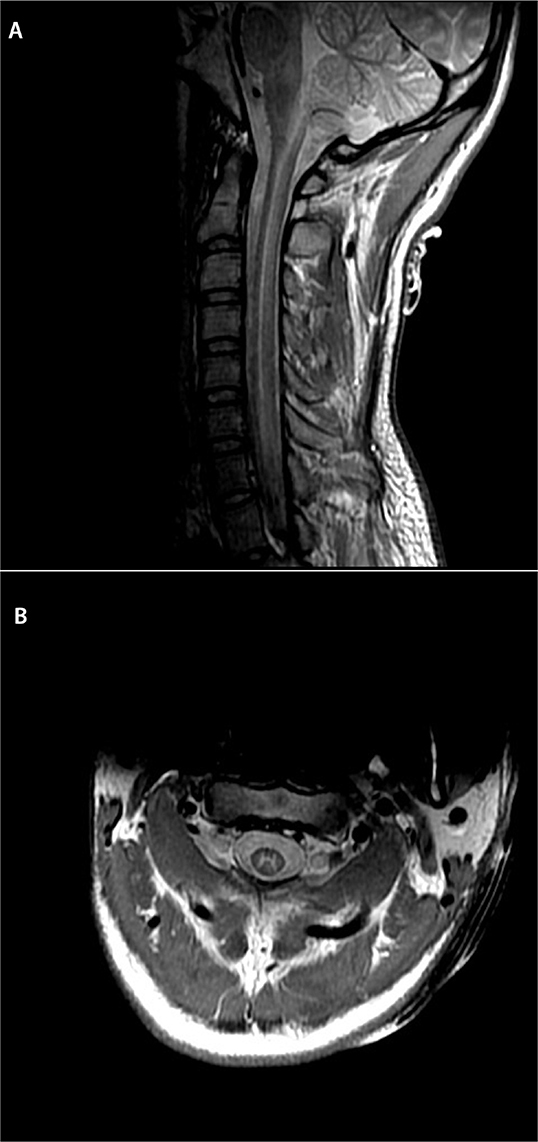
MRI of the spine demonstrating an increase in proton density (A), and T2 signal (B) throughout the spinal cord grey matter, affecting both the anterior and posterior columns from C2 to T5. The white matter is spared; this absence of white matter involvement/cord expansion would be unusual for transverse myelitis or ADEM but can be seen in viral myelitis.
MRI = Magnetic resonance imaging; ADEM = Acute disseminated encephalomyelitis
Table 1:
Cerebrospinal fluid analyses
| Test | Day 2 of presentation | Day 4 of presentation | Normal range |
|---|---|---|---|
| CSF cell count | |||
| Leukocytes (x106 cells/L) | 12 | 46 | 0–5 |
| Neutrophils | 1% | 1% | <1% |
| Lymphocytes | 88% | 94% | 63%–99% |
| Erythrocytes (x106 cells/L) | 5 | 13 | 0–5 |
| CSF Chemistry | |||
| Glucose (mmol/L) | 4.7 | 4.9 | 2.2–4.4 |
| Protein (g/L) | 0.50 | 0.31 | <0.45 |
| Virology (all PCR) | |||
| Cytomegalovirus | negative | negative | |
| Enterovirus | negative | negative | |
| Epstein–Barr virus | negative | negative | |
| Herpes simplex virus | negative | negative | negative |
| Varicella zoster virus | negative | negative | negative |
| West Nile virus | negative | negative | |
| Bacteriology | |||
| Culture and sensitivity | negative | negative | negative |
| VDRL | negative | negative | |
| Mycobacteriology | |||
| Acid fast staining | negative | negative | |
| Mycobacterium tuberculosis PCR | negative | negative | |
| Mycology | |||
| Cryptococcal antigen | negative | negative | |
CSF = Cerebrospinal fluid; PCR = Polymerase chain reaction; VDRL = Venereal disease
The initial presumptive diagnosis was an acute infectious, versus inflammatory, myelitis. The patient was prescribed broad-spectrum antibiotics and antivirals in the form of ceftriaxone, doxycycline, and acyclovir. He was also prescribed intravenous methylprednisolone, cyclophosphamide, and plasma exchange for potential inflammatory/autoimmune causes of myelitis.
On the third day of hospitalization, the patient had further neurologic deterioration with complete paralysis of the arms and legs and inability to trigger the mechanical ventilator. Physical examination revealed absence of corneal, gag, and cough reflexes.
The history was revisited with family members. One recalled the patient mentioning an incident two months prior on Vancouver Island, British Columbia (BC), where during the daytime, a bat flew onto the patient’s leg. When he attempted to remove the bat with his left hand, he received a scratch in the web space between the thumb and forefinger. The patient did not seek medical attention and did not receive post-exposure prophylaxis (PEP) after the incident.
With this information, samples for rabies testing were sent to the Canadian Food Inspection Agency (fluorescent antibody test [FAT] for viral antigen, and quantitative reverse transcription polymerase chain reaction [RT-qPCR] test for viral RNA) and the Public Health Agency of Canada National Microbiology Laboratory (serology for rabies virus [RABV] neutralizing antibody).
Repeat MRI of the brain 5 days after hospitalization now demonstrated restricted diffusion in the cortex at the pre- and post-central gyri bilaterally (Figure 2). This was thought to represent disease progression with cortical involvement. With the patient’s continued neurologic decline, likelihood of RABV infection, and overall prognosis, a decision was made to withdraw care, and the patient died shortly thereafter. An autopsy was not performed.
Figure 2:
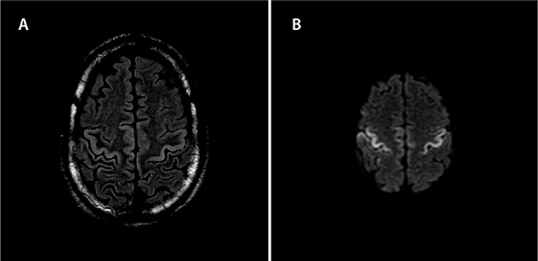
MRI of the brain showing T2 Flair (A), and DWI (B), demonstrating restricted diffusion in the cortex at the pre- and post-central gyri bilaterally seen with rabies encephalitis evolution
MRI = Magnetic resonance imaging; FLAIR = Fluid-attenuated inversion recovery; DWI = Diffusion-weighted imaging
RT-qPCR of saliva samples was the only test to identify the presence of RABV; subsequent sequencing and phylogenetic analysis of the viral N gene amplified from saliva identified this RABV as the variant associated with silver-haired bats (Figure 3).
Figure 3:
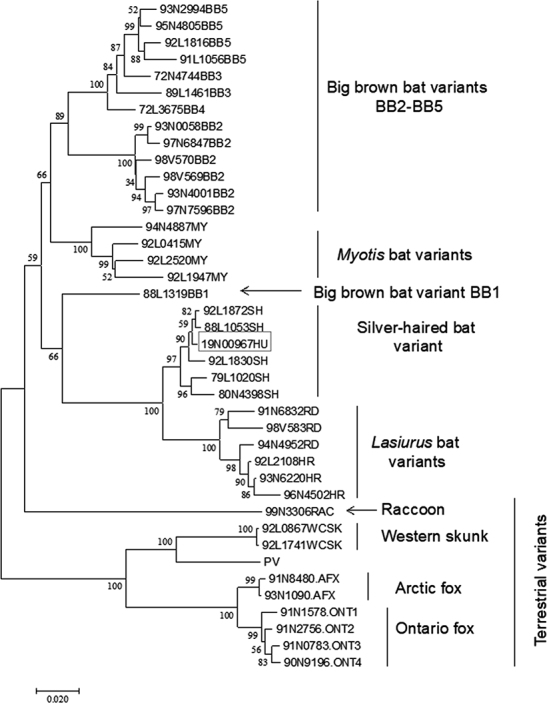
Phylogenetic analysis of the RABV sample (19N00967HU) from the BC human rabies case compared with representative viruses from known RABV variants circulating in Canada
Numbers at all nodes of the tree indicate their respective bootstrap values expressed as a percentage, and branches represent genetic distances according to the scale shown at bottom. Major clades are identified to the right of the tree. RNA extracted from saliva using TRIzol reagent (ThermoFisher) was used as template to amplify the complete RABV N gene as two overlapping nested RT-PCRs according to previously described protocols (1). The amplicons were purified using a GeneJet PCR purification kit (ThermoFisher), sequenced using internal primers with a BDT v3.1 sequencing kit (Applied Biosystems), and run on a 3500xl Genetic Analyzer (Applied Biosystems). Reads were assembled using the SeqManPro software of the DNASTAR Lasergene v 14 package. MEGA v7 was used to align the assembled sequence with a database of N gene sequences representative of the RABV variants that circulate within Canada and to generate the phylogenetic tree using the neighbor-joining method with 1,000 bootstrap replicates.
RABV = Rabies virus; BC = British Columbia
Discussion
Epidemiology
Rabies is distributed worldwide with rare exceptions. While in developing countries rabid dogs account for greater than 90% of rabies cases transmitted to humans, dog-mediated rabies has been eliminated from North America and western Europe, among other regions, where RABV is instead maintained in wildlife reservoirs (2). In Canada, these reservoirs include bats, skunks, foxes, and raccoons; however, bats represent the only known reservoir in BC (3). Approximately 10% of BC bats submitted for rabies testing since 2014 have been positive for the virus, though this likely overestimates the true prevalence of ≤1% described by random bat surveys conducted in other areas of North America (4). In total, 26 cases of rabies in humans have been reported in Canada since 1924, including the present case (3). Since 1968, all indigenously-acquired human rabies cases have been the result of bat RABV variants. In the present case, infection was determined to be caused by a RABV variant associated with silver-haired bats; similar virus variants were responsible for cases in Québec (2000) and Alberta (2007) and are also the most common cause of bat-derived human rabies in the United States (4).
Pathophysiology
RABV enters the body through a break in the skin, via a bite or scratch, or by direct contact with a mucosal surface. The virus may replicate near the inoculation site prior to infecting peripheral nerves, by which it enters the central nervous system (CNS) (2). The incubation period is commonly 3–8 weeks but varies depending on viral inoculum size, density of nerve endings at inoculation site, and proximity of the entry point to the CNS (3). Following abundant replication within brain tissue, centrifugal spread along somatic and autonomic nerves subsequently results in widespread dissemination of the virus to various tissues such as salivary glands, skin, and other organs (2).
Initial prodromal symptoms of rabies are non-specific and include fever, chills, malaise, and myalgias. Early localized symptoms of paresthesias, pain, and pruritis often occur at the inoculation site (5). After several days, further neurological manifestations appear, and classically present as one of two phenotypes—so-called encephalitic, or paralytic rabies. The more common presentation is encephalitic rabies (~80% of cases), wherein signs of CNS irritation predominate, such as agitation, confusion, hydrophobia, aerophobia, and autonomic instability (5). Paralytic rabies presents with a flaccid paralysis and less prominent excitatory features, which was observed in our patient (5). Paralysis is usually ascending, with loss of deep tendon reflexes and sphincter tone. Both forms of the disease invariably progress to coma and death within 7–10 days, secondary to cardiac or respiratory failure, unless intensive care support is provided (2).
Microbiologic diagnosis
For ante-mortem diagnosis of RABV in humans, multiple specimen types should be tested (Table 2) as no single test is sufficient to rule out disease, particularly at very early stages of infection (6,7). Tests for viral antigen and RNA are more sensitive than serologic tests as antibody generally appears >8 days after symptom onset (2). Numerous studies have shown RT-PCR test methods on nuchal biopsy to have a diagnostic sensitivity approaching 100% dependent on the disease presentation, timing of sampling, and gene target amplified (7). The nuchal biopsy is properly sampled at the nape of the neck where viral nucleocapsids are located at nerve endings associated with hair follicles, and positive results may be obtained as early as the first day following symptom onset (7). Post-mortem, FAT, or RT-qPCR on brain stem are considered the gold standard tests and provide a definitive diagnosis (2).
Table 2:
Human specimen types for which ante-mortem rabies virus testing is available in Canada
| Nuchal skin biopsy | Saliva | Cerebrospinal fluid | Serum | ||
|---|---|---|---|---|---|
| Collection | Full thickness biopsy at least 5 mm with several hair follicles | Multiple collections at least 12 hours apart, 2–3 mL/sample | 1–2 mL | 1–2 mL | 2 mL |
| Testing | Fluorescent antibody test, RT-qPCR* | RT-qPCR* | RT-qPCR* | Rabies serum neutralization test† | Rabies serum neutralization test† |
| Detects | Virus | Virus | Virus | Antibody | Antibody |
| Relative diagnostic sensitivity | High | High | Low-average | Low-average | Low-average |
| Notes | Necessary to submit specimen of adequate size | Highest sensitivity is achieved by testing serial samples. Bile or blood may interfere with testing | Requires detection of patient DNA as a control for successful sample extraction and amplification. If absent sample not valid for testing | Antibody generally appears 7–8 d following symptom onset | Antibody generally appears 7–8 d following symptom onset. No diagnostic value if patient has received vaccination for rabies |
Adapted with permission of the Canadian Food Inspection Agency (CFIA). For further details of specimen handling, refer to original document (2).
Note: No single test is sufficient to “rule out” rabies, and early in disease course the predictive value of a negative test is uncertain. It is recommended to submit all samples for ante-mortem testing. Repeat testing may be required, depending on the length of illness.
* Location of testing: Canadian Food Inspection Agency (CFIA), Ottawa, Ontario
† Location of testing: Public Health Agency of Canada (PHAC), Winnipeg, Manitoba
RT-qPCR = Quantitative real time polymerase chain reaction
In the present case, RT-qPCR on saliva, collected on days 3 and 4 post hospitalization were positive on 2/2 samples. Nuchal biopsy, collected on day 4 post hospitalization, was negative, despite a high-quality sample. This result was somewhat unexpected as a multicentre, large cohort study found that in 43 patients with confirmed encephalitic rabies, nuchal biopsy samples had a higher diagnostic sensitivity than saliva (98% versus 70%, respectively). However, 100% sensitivity was achieved when three serial saliva samples were tested (7). In contrast, a study from Thailand found that molecular testing of various samples was less sensitive in cases of paralytic rabies (50%, n = 6) compared with encephalitic rabies (92%, n = 50); however, in four of six paralytic cases, RABV was not detectable in saliva (8), unlike in the present case. Given the limited amount of data, it is unclear whether the lack of virus in the nuchal biopsy of the current patient was a feature related to paralytic rabies, or an atypical diagnostic presentation. The absence of RABV neutralizing antibody was not surprising given the decreased diagnostic sensitivity of serologic tests and an anticipated blunted immune response in the setting of adjunct immunosuppressive therapies administered (corticosteroids, plasma exchange, and cyclophosphamide). The discrepancy in test results in this case highlights that expedient virologic testing of multiple samples is critical to achieve an accurate diagnosis and to guide management of the patient and contacts.
Treatment
Although rabies is a fatal disease in humans, PEP in the form of rabies vaccine and rabies immune globulin (RIG) is very effective when given soon after an exposure (9). Very few survivors of rabies have been reported in the literature, with most receiving PEP prior to onset of clinical symptoms (9). In 2004, a young patient who did not receive PEP was treated with therapeutic coma and N-Methyl-d-aspartate (NMDA) receptor antagonist therapy and survived (10). Since then, adaptations of this initial treatment regimen, called the Milwaukee protocol, have been attempted, with many documented failures (11). Various antiviral combinations have been used in the treatment of rabies, including ribavirin, interferon-alpha, and amantadine with limited studies on their efficacy, significant adverse effects, and disappointing results (11).
Until efficacious therapies are developed for rabies, efforts should focus on education and preventive measures. More aggressive treatment approaches should only be undertaken in exceptional circumstances (2), and the risk of leaving the patient with severe disabling neurologic deficits should be stressed (9).
Post-exposure prophylaxis
While person-to-person transmission of rabies is considered largely theoretical (12), PEP for significant exposures to health care workers (HCW) is recommended by the US Centers for Disease Control and Prevention (13). BC Public Health officials established contact definition criteria for this case and circulated this information to HCW involved in direct patient care. Those considered at risk included individuals with known percutaneous or mucosal exposure to saliva, neural fluid, or neural tissue. In the case of aerosol-generating medical procedures, appropriate droplet precautions including surgical mask or respirator use, and eye protection with either goggles or a face shield were considered protective.
Of the 206 contacts who were assessed, 36 were administered rabies vaccine series, and an additional 31 received vaccine and RIG. Those contacts who received vaccine only were considered very low risk for aerosolized mucosal exposure as they met contact criteria primarily by having managed upper airway secretions without appropriate personal protective equipment. This modified PEP was considered justified based on the low theoretical risk of transmission, short time interval between exposure and intervention, and the need to prioritize a limited RIG supply for those presumed at highest risk of infection.
Several household members and close contacts of the patient were exposed to respiratory secretions during the symptomatic period. These individuals were considered to be at higher risk given that mucosal exposure could not be ruled out, and time elapsed since initial exposure was greatest for this group.
See Box 1 for key points on RABV exposure, presentation, treatment, and prophylaxis.
Box 1:
Key points about rabies virus infection
In Canada, the rabies virus (RABV) reservoirs are skunks, foxes, raccoons, and bats. In British Columbia, bats are the only known reservoir.
Post-exposure prophylaxis (PEP) consisting of thorough wound cleansing followed by administration of rabies immunoglobulin and rabies vaccine series is indicated after direct contact with a confirmed or suspected rabid animal. Direct contact includes exposure to saliva or neural tissue, which is introduced by a bite, scratch, or contamination of mucous membranes.
Rabies presents as two major forms: encephalitic and paralytic. The encephalitic form is characterized by agitation, confusion, aerophobia, hydrophobia, and autonomic instability, whereas the paralytic form presents with an ascending flaccid paralysis, usually beginning at the initial site of inoculation.
To diagnose rabies in humans ante-mortem, immunofluorescence on nuchal biopsy and quantitative reverse transcription polymerase chain reaction (RT-qPCR) on saliva and nuchal biopsy are recommended. In addition, serum and cerebrospinal fluid (CSF) can be tested for RABV neutralizing antibodies. CSF is sometimes also tested by RT-qPCR. Testing of multiple specimen types increases diagnostic sensitivity.
Individuals who do not receive rabies PEP at the time of exposure have near 100% mortality if the disease develops. There continue to be no effective treatment options for RABV infection in humans.
Acknowledgements:
The authors thank Dr Edwin Peramaki from the Department of Radiology, University of British Columbia, for the interpretation and selection of MRI brain and spine images, and M Kimberly Knowles and Allison Hartke for technical assistance in the molecular testing and genetic characterization of the virus.
Funding:
No funding was received for this work.
Disclosures:
The authors have nothing to disclose.
Informed Consent:
Informed consent was obtained from the next of kin.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A.
References
- 1.Nadin-Davis SA. Polymerase chain reaction protocols for rabies virus discrimination. J Virol Methods. 1998;75(1):1–8. 10.1016/s0166-0934(98)00106-2. Medline: [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). WHO expert consultation on rabies: third report. Geneva: WHO; 2018. [cited 2019 Oct 23]. Available from: https://apps.who.int/iris/handle/10665/272364. [Google Scholar]
- 3.BC Centre for Disease Control (BCCDC). Chapter I: Management of specific diseases – rabies. In: Communicable disease control manual. Vancouver: BCCDC; 2019. [cited 2019 Oct 23]. Available from: http://www.bccdc.ca/resource-gallery/Documents/GuidelinesandForms/GuidelinesandManuals/Epid/CDManual/Chapter1-CDC/BCRabiesGuidelines.pdf. [Google Scholar]
- 4.Klug BJ, Turmelle AS, Ellison JA, Baerwald EF, Barclay RM. Rabies prevalence in migratory tree-bats in Alberta and the influence of roosting ecology and sampling method on reported prevalence of rabies in bats. J Wildl Dis. 2011;47(1):64–77. 10.7589/0090-3558-47.1.64. Medline: [DOI] [PubMed] [Google Scholar]
- 5.Jackson AC. Current and future approaches to the therapy of human rabies. Antiviral Res. 2013;99(1): 61–7. 10.1016/j.antiviral.2013.01.003. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Canadian Network for Public Health Intelligence (CNPHI). Guidance for submission of human specimens for rabies testing in Canada—human sample bilingual final 2018-06-27 [Internet]. Ottawa: Government of Canada; 2018. [cited 2019 Oct 6]. Available from: https://cnphi.canada.ca/gts/reference-diagnostic-test/5224?labId=1020&wbdisable=true. [Google Scholar]
- 7.Dacheux L, Reynes JM, Buchy P, et al. A reliable diagnosis of human rabies based on analysis of skin biopsy specimens. Clin Infect Dis. 2008;47(11):1410–7. 10.1086/592969. Medline: [DOI] [PubMed] [Google Scholar]
- 8.Wacharapluesadee S, Hemachudha T. Ante- and post-mortem diagnosis of rabies using nucleic acid-amplification tests. Expert Rev Mol Diagn. 2010;10(2):207–18. 10.1586/erm.09.85. Medline: [DOI] [PubMed] [Google Scholar]
- 9.Jackson AC, Warrell MJ, Rupprecht CE, et al. Management of rabies in humans. Clin Infect Dis. 2003;36(1):60–3. 10.1086/344905. Medline: [DOI] [PubMed] [Google Scholar]
- 10.Willoughby RE, Tieves KS, Hoffman GM, et al. Survival after treatment of rabies with induction of coma. N.Eng.J.Med. 2005;352(24):2508–14. 10.1056/NEJMoa050382. Medline: [DOI] [PubMed] [Google Scholar]
- 11.Appolinario CM, Jackson AC. Antiviral therapy for human rabies. Antivir Ther. 2015;20(1):1–10. 10.3851/IMP2851. Medline: [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO). Rabies fact sheet [Internet]. Geneva: WHO; 2019. [updated 2020 Apr 21; cited 2019 Nov 5]. Available from: https://www.who.int/news-room/fact-sheets/detail/rabies. [Google Scholar]
- 13.Manning SE, Rupprecht CE, Fishbein D, et al. Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008;57(RR-3):1–28. Medline: [PubMed] [Google Scholar]


