Abstract
Background
Cytomegalovirus (CMV) and Epstein–Barr virus (EBV) infections are common, causing significant morbidity in pregnancy (congenital CMV) and transplant recipients (CMV, EBV). Canadian prevalence data are needed to model disease burden and develop strategies for future vaccines. We estimated prevalence using screening data from blood donors and solid organ transplant (SOT) donors and recipients.
Methods
We retrospectively analyzed CMV and EBV serology from Alberta SOT donors (n = 3,016) and recipients (n = 4,614) (1984–2013) and Canadian Blood Services blood donors (n = 1,253,350) (2005–2014), studying associations with age, sex, organ, year, and geographic region.
Results
CMV seroprevalence rises gradually with age. By age 70, CMV seropositivity ranged from 67% (blood donors) to 73% (SOT recipients). Significant proportions of women of child-bearing age were CMV-seronegative (organ donors, 44%; SOT recipients, 43%; blood donors, 61%). Blood donor CMV seroprevalence decreased from 48% in Western Canada to 30% in Eastern Canada. Women were more likely to be CMV-seropositive (ORs = 1.58, 1.45, and 1.11 for organ donors, SOT recipients, and blood donors, respectively) and EBV-seropositive (ORs = 1.87 and 1.46 for organ donors and SOT recipients, respectively). EBV prevalence rises rapidly, and by age 17–29 years, 81% of SOT recipients and 90% of organ donors were seropositive.
Conclusions
Canada has relatively low and perhaps decreasing age-specific EBV and CMV prevalence, making Canadians vulnerable to primary infection-associated morbidity and suggesting benefit from future vaccines. Collection and analysis of routine serology screening data are useful for observing trends.
Keywords: CMV serostatus, cytomegalovirus, EBV serostatus, Epstein-Barr virus, seroprevalence
Abstract
Historique
Les infections à cytomégalovirus (CMV) et au virus d’Epstein–Barr (EBV) sont courantes et responsables d’une morbidité importante pendant la grossesse (infection congénitale à CMV) et les receveurs d’organes (CMV, EBV). Il faut colliger des données de prévalence au Canada pour en modéliser le fardeau et établir des stratégies en vue de futurs vaccins. Les chercheurs en ont évalué la prévalence à l’aide des données de dépistage des donneurs de sang et des donneurs d’organes pleins.
Méthodologie
Les auteurs ont procédé à l’analyse rétrospective de la sérologie du CMV et de l’EBV des donneurs (n = 3 016) et des receveurs (n = 4 614) d’organes pleins de l’Alberta entre 1984 et 2013 et des donneurs de sang de la Société canadienne du sang de 2005 à 2014 (n = 1 253 350) et étudié les associations avec l’âge, le sexe, l’organe, l’année et la région géographique.
Résultats
Le statut sérologique pour le CMV augmente graduellement avec l’âge. À l’âge 70 ans, la séropositivité au CMV se situait entre 67 % (donneurs de sang) et 73 % (receveurs d’organes pleins). Des proportions importantes de femmes en âge de procréer étaient séronégatives au CMV (donneuses d’organes, 44 %; receveuses d’organes pleins, 43 %; donneuses de sang, 61 %). La séroprévalence du CMV chez les donneurs de sang passait de 48 % dans l’Ouest canadien à 30 % dans les Maritimes. Les femmes étaient plus susceptibles d’être séropositives au CMV (RC = 1,58, 1,45, et 1,11 pour les donneuses d’organes, les receveuses d’organes pleins et les donneuses de sang, respectivement) et à l’EBV (RC = 1,87 et 1,46 pour les donneuses d’organes et les receveuses d’organes pleins, respectivement). La prévalence de l’EBV augmente rapidement: entre l’âge de 17 et 29 ans, 81 % des receveurs d’organes pleins et 90 % des donneurs d’organes étaient séropositifs.
Conclusions
Le Canada présente une prévalence relativement faible – et peut-être même à la baisse – d’EBV et de CMV liée à l’âge, ce qui rend les Canadiens vulnérables aux morbidités associées à la primo-infection et laissent croire au caractère avantageux de futurs vaccins. Il est utile d’amasser et d’analyser des données de dépistage pour observer les tendances.
Mots-clés: cytomégalovirus, séroprévalence, statut sérologique pour le CMV, statut sérologique pour l’EBV, virus d’Epstein-Barr
Introduction
Cytomegalovirus (CMV) and Epstein–Barr virus (EBV) infections commonly occur in the general population and persist for life (1,2). Both CMV and EBV can be transmitted by exposure to infected saliva, leukocytes in blood transfusions, and seropositive donor organs or hematopoietic stem cells. Infected secretions such as urine, genital fluids, and breast milk also transmit CMV.
Most CMV infections are asymptomatic, but CMV causes significant morbidity in two settings: congenital infection and transplant recipients. CMV is the most common congenital infection in Canada, occurring in an estimated 0.5% of live births (3). Approximately 32% of primary and 1.4% of non-primary CMV infections during pregnancy result in vertical transmission (4). More than 15% of congenitally infected infants develop permanent sequelae, the most common of which is sensorineural hearing loss (5–8). CMV is the most frequent infectious complication after solid organ transplant (SOT), both directly causing morbidity and indirectly affecting both graft function and other infection risk (9–11). Consequently, the US Institute of Medicine has identified the development of a CMV vaccine as a public health priority (9,12).
Like CMV, most EBV infections are also asymptomatic. However, EBV can cause infectious mononucleosis in healthy adolescents and young adults and has been associated with the pathogenesis of multiple sclerosis (13). However, the greatest EBV-related disease burden is its pathogenic association with hematopoietic and epithelial malignancies in both immunocompetent hosts (non-Hodgkin, Hodgkin, and Burkitt lymphomas; nasopharyngeal and gastric carcinomas) and immunocompromised hosts (post-transplant lymphoproliferative disorder [PTLD], smooth muscle tumours) (2,14–17). EBV vaccine development is also underway (18,19).
In Canada, there is a paucity of data regarding age-specific CMV and EBV prevalence, especially in early childhood. Such data are critical to estimate virus-related disease burden and health resource expenditures and design future vaccine strategies. At our Alberta centre, pre-transplant organ donor and recipient CMV and EBV serology are routinely tested to risk stratify patients for post-transplant prevention strategies targeting CMV- and EBV-associated complications (20). In addition, up until 2017 a subset of Canadian blood donors were screened to identify CMV-seronegative blood products used to reduce risk of transfusion-transmitted CMV infection (21,22).
Our objective was to describe the age- and sex-specific prevalence of CMV and EBV in Canada using data from routine organ donor and recipient and blood donor screening.
Methods
Organ transplant population
Our cross-sectional study retrospectively analyzed all SOT donors and recipients transplanted at the University of Alberta Hospital/Stollery Children’s Hospital, Edmonton, Alberta, between January 1, 1984, and December 31, 2013. Recipients were analyzed using the first transplant event in the study period and re-transplant events for patients initially transplanted before January 1, 1984. Transplant data were collected from Provincial Laboratory for Public Health (ProvLab) paper records (1984–1992), the ProvLab computer database (1993–2013), the University of Alberta Organ Transplant Tracking Registry, and paper records from the Edmonton Human Organ Procurement and Exchange (HOPE) program.
Blood donor population
We analyzed first-time blood donors (aged ≥17 y) donating between January 1, 2005, and May 3, 2014. Collection centres randomly tested a proportion of blood donors for CMV to maintain an inventory of CMV-negative blood products. Blood donor data including CMV serostatus, age, sex, and region were provided by Canadian Blood Services from the National Epidemiology Donor Database with regions grouped as follows: British Columbia and Yukon, Alberta, Saskatchewan and Manitoba, Ontario, and Atlantic (New Brunswick, Newfoundland and Labrador, Prince Edward Island, and Nova Scotia).
Serology testing
Among local transplant donors and recipients, CMV immunoglobulin G (IgG) was assessed using an enzyme immunoassay (Siemens Enzygnost Anti-CMV/IgG, Siemens Healthcare Diagnostics Products, Marburg, Germany). Epstein–Barr viral capsid antigen IgG and Epstein–Barr virus nuclear antigen 1 IgG were assessed via Gull Laboratories, Salt Lake City, Utah (1994–2001), and CaptiaTM Trinity Biotech, Bray, Ireland (2002–2013). EBV serology before 1994 was tested retrospectively on available samples. All other CMV and EBV serology testing was done at the time of collection. The CMV and EBV serostatus of non-local donors were obtained from HOPE records (assay details unknown).
CMV screening of blood donors was performed at time of collection throughout the study period using an Olympus particle agglutination assay (PK 100/200/300) that detects CMV IgG and immunoglobulin M.
Variables
We investigated the associations of age, sex, organ, time period, region, and donation year (for blood donors only) with CMV and EBV seroprevalence. Donor and recipient transplant analyses were stratified by age at transplant or donation (adult, aged ≥17 y; pediatric, aged <17 y). Age was modelled as a continuous variable in regression analysis but is presented as age groups in the tables. Because passive maternal antibody makes positive serology unreliable in infants aged younger than 12 months, these infants were included in the descriptive analyses (Tables 1, 3, and 5 and Supplemental Table S.1) but not in the regression analyses (Tables 2 and 4). Donors (D) and recipients (R) with indeterminate serostatus results and infants aged younger than 12 months with positive or indeterminate serology were reclassified according to the highest risk scenario, that is, D+ or R–, for D/R risk category analysis. In all other analyses, indeterminate serology results were treated as missing. Time trends were analyzed using a continuous year variable for all groups and a binary period variable representing 1984–1998 and 1999–2013 for organ donors and recipients. Women of child-bearing age were defined as women aged 17–45 years.
Table 1:
Adult and pediatric CMV seroprevalence among blood donors, organ donors, and recipients by age and sex
| Blood donors | Organ donors | Recipients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | n | Prevalence, % (95% CI) | POR (95% CI) | n | Prevalence, % (95% CI) | POR (95% CI) | n | Prevalence, % (95% CI) | POR (95% CI) | |
| Overall adult prevalence | 1,253,350 (25, 15) | 42.2bc (42.1 to 42.3) | — | 2,588* (42.6, 23.0) | 53.3ab (51.4 to 55.3) | — | 4,113* (50.9, 19.2) | 62.3ac (60.8 to 63.8) | — | |
| Adult age group | ||||||||||
| 17–29 y | 499,529 | 33.8bc (33.7 to 33.9) | 1.00 (Ref.) | 658 | 43.0ab (39.2 to 46.9) | 1.00 (Ref.) | 445 | 48.3ac (43.6 to 53.1) | 1.00 (Ref.) | |
| 30–39 y | 218,959 | 42.4ab (42.2 to 42.6) | 1.44 (1.43 to 1.46) | 475 | 50.9a (46.4 to 55.5) | 1.38 (1.08 to 1.75) | 599 | 49.6b (45.5 to 53.7) | 1.05 (0.82 to 1.34) | |
| 40–49 y | 264,977 | 46.4bc (46.2 to 46.6) | 1.70 (1.68 to 1.71) | 626 | 55.8ab (51.8 to 59.7) | 1.67 (1.34 to 2.08) | 916 | 59.4ac (56.1 to 62.6) | 1.56 (1.25 to 1.97) | |
| 50–59 y | 205,037 | 51.4bc (51.2 to 51.7) | 2.08 (2.05 to 2.10) | 535 | 57.0ab (52.7 to 61.2) | 1.76 (1.40 to 2.21) | 1,249 | 68.0ac (65.3 to 70.6) | 2.27 (1.82 to 2.83) | |
| 60–69 y | 63,520 | 59.1bc (58.9 to 59.5) | 2.84 (2.79 to 2.89) | 193 | 65.8ab (58.6 to 72.4) | 2.55 (1.83 to 3.58) | 819 | 72.9ac (69.7 to 75.9) | 2.88 (2.26 to 3.67) | |
| >70 y | 1,328 | 66.7 (64.1 to 69.2) | 3.93 (3.51 to 4.41) | 101 | 73.3 (63.5 to 81.5) | 3.63 (2.30 to 5.88) | 85 | 69.4 (58.5 to 79.0) | 2.43 (1.49 to 4.05) | |
| Adult sex | ||||||||||
| Male | 590,675 | 40.8 (40.7 to 40.9) | 1 (Ref.) | 1,308 | 47.7 (45.0 to 50.5) | 1.00 (Ref.) | 2,712 | 59.4 (57.5 to 61.2) | 1.00 (Ref.) | |
| Female | 662,675 | 43.4bc (43.3 to 43.5) | 1.11 (1.10 to 1.12) | 1,280 | 59.1ab (56.3 to 61.8) | 1.58 (1.35 to 1.85) | 1,401 | 67.9ac (65.4 to 70.3) | 1.45 (1.26 to 1.66) | |
| Overall pediatric prevalence for those aged <12 mo | — | — | — | 250* (10.7, 10.5) | 44.4 (38.1 to 50.8) | — | 302* (8.1, 10.1) | 44.4 (38.7 to 50.2) | — | |
| Pediatric age group | <12 mo | — | — | — | 52 | 44.2 (30.5 to 58.7) | — | 112 | 50.0 (40.4 to 59.6) | — |
| 12 mo–2 y | — | — | — | 24 | 29.2 (12.6 to 51.1) | 1.00 (Ref.) | 50 | 34.0 (21.2 to 48.8) | 1.00 (Ref.) | |
| 2–4 y | — | — | — | 42 | 47.6 (32.0 to 63.6) | 2.21 (0.78 to 6.74) | 53 | 45.3 (31.6 to 59.6) | 1.61 (0.73 to 3.60) | |
| 5–9 y | — | — | — | 52 | 38.5 (25.3 to 53.0) | 1.52 (0.55 to 4.52) | 65 | 43.1 (30.8 to 56.0) | 1.47 (0.69 to 3.19) | |
| 10–16 y | — | — | — | 132 | 48.5 (39.7 to 57.3) | 2.29 (0.92 to 6.25) | 134 | 48.5 (39.8 to 57.3) | 1.83 (0.94 to 3.66) | |
| Pediatric sex | ||||||||||
| Male | — | — | — | 147 | 40.8 (32.8 to 49.2) | 1.00 (Ref.) | 148 | 37.8 (30.0 to 46.2) | 1.00 (Ref.) | |
| Female | — | — | — | 103 | 49.5 (39.5 to 59.5) | 1.42 (0.86 to 2.37) | 154 | 50.6 (42.5 to 58.8) | 1.69 (1.07 to 2.67) | |
Notes: Pediatric patients aged younger than 12 mo are included in the table to describe the seroprevalence of this group, but they were excluded from the regression analysis because of the potential presence of passive maternal antibody. Superscript letters indicate statistically significant pairwise comparisons with adjusted p < 0.05. Boldface indicates significant at α = 0.05. Dashes indicate not applicable.
* Exclusions due to missing or indeterminate serology: 35 adult donors (32 indeterminate), 75 adult recipients (49 indeterminate), 8 pediatric donors (8 indeterminate), and 6 pediatric recipients (4 indeterminate)
CMV = Cytomegalovirus; IQR = Interquartile range; CI = Confidence interval; POR = Prevalence odds ratio; Ref. = reference
Table 3:
Adult and pediatric CMV risk categories by organ type
| Age group and organ type | No. (%) of transplants | ||||
|---|---|---|---|---|---|
| D–/R– | D+/R– | D–/R+ | D+/R+ | n | |
| Adult* | |||||
| Kidney | 451 (21.3) | 369 (17.4) | 600 (28.3) | 701 (33.1) | 2,121 |
| Liver | 179 (15.5) | 199 (17.2) | 377 (32.6) | 402 (34.7) | 1,157 |
| Heart | 120 (20.1) | 113 (18.9) | 183 (30.7) | 181 (30.3) | 597 |
| Lung | 117 (20.5) | 116 (20.3) | 135 (23.6) | 203 (35.6) | 571 |
| Total | 867 (19.5) | 797 (17.9) | 1,295 (29.1) | 1,487 (33.4) | 4,446† |
| Pediatric* | |||||
| Kidney | 30 (31.6) | 29 (30.5) | 11 (11.6) | 25 (26.3) | 95 |
| Liver | 85 (39.2) | 74 (34.1) | 31 (14.3) | 27 (12.4) | 217 |
| Heart | 42 (27.8) | 49 (32.5) | 36 (23.8) | 24 (15.9) | 151 |
| Lung | 3 (37.5) | 3 (37.5) | 1 (12.5) | 1 (12.5) | 8 |
| Total | 160 (34.0) | 155 (32.9) | 79 (16.8) | 77 (16.3) | 471† |
Notes: Seropositive and indeterminate donors and recipients aged younger than 12 mo were included in this table and were risk adjusted to be D+ or R–, respectively, to account for the potential effects of passive maternal antibody. Indeterminate donors and recipients aged older than 12 mo were risk adjusted to be D+ or R–, respectively.
* Organs were grouped as follows: kidney–pancreas with kidneys, small bowel and multivisceral with liver, and heart–lung with lung
† Exclusions due to missing serology in adults (n = 34) and pediatrics (n = 4)
CMV = Cytomegalovirus; D– = donor seronegative; R– = recipient seronegative; D+ = donor seropositive; R+ = recipient seropositive
Table 5:
Adult and pediatric EBV risk categories by organ type
| n (%) of transplants within organ type | |||||
|---|---|---|---|---|---|
| Age group and organ type | D–/R– | D+/R– | D–/R+ | D+/R+ | n |
| Adult | |||||
| Kidney* | 12 (0.7) | 91 (5.1) | 142 (8.0) | 1,539 (86.3) | 1,784 |
| Liver | 3 (0.3) | 20 (1.8) | 71 (6.4) | 1,018 (91.5) | 1,112 |
| Heart | 0 (0.0) | 31 (6.0) | 43 (8.3) | 442 (85.7) | 516 |
| Lung | 1 (0.2) | 35 (6.3) | 37 (6.7) | 480 (86.8) | 553 |
| Total | 16 (0.4) | 177 (4.5) | 293 (7.4) | 3,479 (87.7) | 3,965† |
| Pediatric | |||||
| Kidney* | 2 (2.5) | 27 (34.2) | 3 (3.8) | 47 (59.5) | 79 |
| Liver | 17 (8.2) | 106 (51.0) | 21 (10.1) | 64 (30.8) | 208 |
| Heart | 18 (12.7) | 64 (45.1) | 12 (8.5) | 48 (33.8) | 142 |
| Lung | 3 (37.5) | 0 (0.0) | 1 (12.5) | 4 (50.0) | 8 |
| Total | 40 (9.2) | 197 (45.1) | 37 (8.5) | 163 (37.3) | 437† |
Notes: Seropositive and indeterminate donors and recipients aged younger than 12 mo were included in this table and risk adjusted them to be D+ or R–, respectively, to account for the potential effects of passive maternal antibody. Indeterminate donors and recipients aged older than 12 mo were risk adjusted to be D+ or R–, respectively
*Organs were grouped as follows: kidney–pancreas with kidneys, small bowel and multivisceral with liver, and heart–lung with lung
†Exclusions due to missing serology in adults (n = 515) and pediatrics (n = 38). Missing EBV serology results were most common before implementation of routine screening in 1994, accounting for 90% of missing donor and 65% of missing recipient EBV serology
EBV = Epstein-Barr virus; D– = donor seronegative; R– = recipient seronegative; D+ = donor seropositive; D– = donor seronegative
Table 2:
CMV multivariate regression models for blood donors, organ donors, and recipients
| Odds ratio (95% CI) | ||||
|---|---|---|---|---|
| Variable | Blood donor | Adult organ donor | Adult recipient | Pediatric recipient |
| Age | 1.021 (1.021 to 1.022)* | 1.02 (1.01 to 1.03)* | 1.03 (1.03 to 1.04)* | 1.05 (1.00 to 1.10) |
| Sex | ||||
| Male | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | 1 (Ref.) |
| Female | 0.96 (0.94 to 0.98)* | 1.47 (1.26 to 1.73)* | 1.53 (1.33 to 1.76)* | 1.66 (1.04 to 2.66)† |
| Age × Female | 1.0053 (1.0047 to 1.0058)* | — | — | — |
| Organ‡ | ||||
| Kidney | — | — | 1.00 (Ref.) | 1.00 (Ref.) |
| Liver | — | — | 1.38 (1.17 to 1.62)* | 1.31 (0.71 to 2.43) |
| Heart | — | — | 0.95 (0.78 to 1.16) | 2.64 (1.44 to 4.91)§ |
| Lung | — | — | 0.92 (0.75 to 1.12) | 0.54 (0.08 to 2.56) |
| Period | ||||
| 1984–1998 | — | — | 1 (Ref.) | — |
| 1999–2013 | — | — | 0.70 (0.60 to 0.81)† | — |
| Region | ||||
| BC and Yukon | 1.00 (Ref.) | — | — | — |
| Alberta | 0.92 (0.91 to 0.94)† | — | — | — |
| Saskatchewan and Manitoba | 0.90 (0.88 to 0.91)† | — | — | — |
| Ontario | 0.74 (0.74 to 0.75)* | — | — | — |
| Atlantic | 0.46 (0.45 to 0.47)* | — | — | — |
| Donation year | 0.98 (0.98 to 0.98)* | — | — | — |
Notes: An interaction model was used for blood donors. Pediatric patients aged younger than 12 mo at transplant were excluded from regression analysis because of potential passive maternal antibody. No variable was significant in pediatric organ donor regression. Dashes indicate not applicable
* Significant at p < 0.001
§ Significant at p < 0.01
† Significant at p < 0.05
‡Organs were grouped as follows: kidney–pancreas with kidneys, small bowel and multivisceral with liver, and heart–lung with lung CMV = Cytomegalovirus; Ref. = Reference
Table 4.
EBV multivariate regression models for organ donors and recipients
| Variable | Odds ratio (95% CI) | |||
|---|---|---|---|---|
| Adult | Pediatric | |||
| Variable | Organ donor | Recipient | Donor | Recipient |
| Age | 1.04 (1.02 to 1.05)* | 1.05 (1.04 to 1.07)* | 1.08 (1.02 to 1.14)† | 1.16 (1.10 to 1.23)* |
| Sex | ||||
| Male | 1.00 (Ref.) | 1.00 (Ref.) | — | — |
| Female | 1.59 (1.10 to 2.31)* | 1.67 (1.17 to 2.43)† | — | — |
| Organ‡ | ||||
| Kidney | — | 1.00 (Ref.) | — | 1.00 (Ref.) |
| Liver | — | 0.88 (0.19 to 4.51) | — | 2.21 (1.12 to 4.50)§ |
| Heart | — | 2.41 (0.54 to 12.59) | — | 1.60 (0.80 to 3.26) |
| Lung | — | 0.09 (0.02 to 0.35)* | — | 1.06 (0.22 to 5.80) |
| Age × Lung | — | 1.06 (1.02 to 1.10)* | — | — |
| Age × Liver | — | 1.02 (0.98 to 1.06) | — | — |
| Age × Heart | — | 0.98 (0.94 to 1.00) | — | — |
| Period | ||||
| 1984–1998 | 1.00 (Ref.) | — | — | 1.00 (Ref.) |
| 1999–2013 | 1.49 (1.02 to 2.16)§ | — | — | 0.49 (0.26 to 0.88)§ |
Notes: Pediatric patients aged younger than 12 mo at transplant were excluded from the regression analysis because of potential passive maternal antibody. An interaction model was used for adult recipients. Pediatric donor regression revealed only age as a significant predictor. Dashes indicate not applicable
* Significant at p < 0.001
†Significant at p < 0.01
§ Significant at p < 0.05
‡Organs were grouped as follows: kidney–pancreas with kidneys, small bowel and multivisceral with liver, and heart–lung with lung
EBV = Epstein-Barr virus; OR = Odds ratio; CI = Confidence interval; Ref. = reference
Statistical analysis
Categorical variables were compared using χ2 tests for independence or Fisher exact test. Pairwise comparisons were adjusted using the Benjamini–Hochberg procedure. Confidence intervals for proportions were calculated using the binomial exact method. Trends over time were analyzed using linear regression. Prevalence ORs were obtained using purposeful logistic regression model building. Variables were assessed for interaction and confounding. All analyses were performed using R version 3.5.2 (23).
Results
Populations studied
Figure 1 outlines the SOT study groups analyzed. All complete cases, with age, sex, and serology data, were analyzed. Eighty donors were excluded because of missing age. Exclusions because of missing serology are indicated in the Table 1 footnotes.
Figure 1:
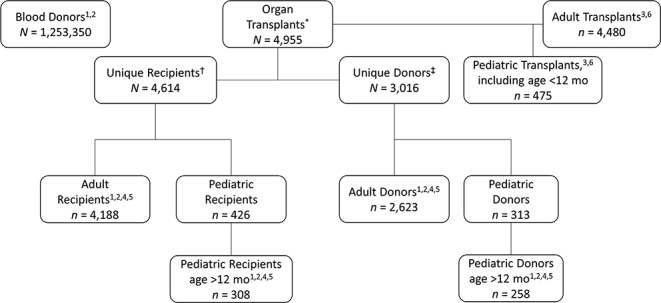
Study population flow chart
Notes: Superscript numerals indicate the table in which these data are analyzed. * 4,548 first-event transplants and 407 total re-transplant events (362 second-event transplants, 42 third-event transplants, 3 fourth-event transplants)
† Includes 4,548 recipients first transplanted during the study period plus 66 recipients first transplanted before the study period
‡ Age was missing for 80 donors; thus, they were not included in the adult or pediatric dataset.
Blood donors
In linear regression of blood donor age versus donation year, average age at donation decreased from 40.0 years in 2005 to 31.1 years in 2014 (p < 0.001). Age and sex distributions of blood donors were not significantly different between provinces.
SOT donors and recipients
Average adult organ donor age decreased from 44.4 years in 1984 to 40.9 years in 2013 (p = 0.003), whereas adult organ recipient age increased from 42.8 years in 1984 to 52.7 years in 2013 (p < 0.001). Excluding infants aged younger than 12 months, the average age of pediatric donors decreased from 12.8 years in 1984 to 8.3 years in 2013 (p = 0.002), whereas it decreased among pediatric recipients from 9.6 years in 1984 to 7 years in 2013 (p = 0.027).
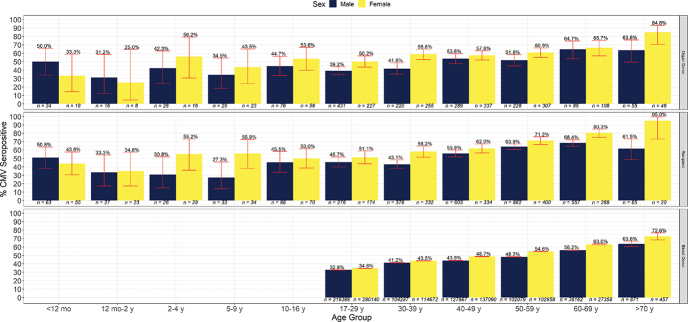
CMV seroprevalence
CMV seroprevalence in blood donors, organ donors, and recipients by age and sex is illustrated in Table 1 and Supplemental Figure S.1. CMV prevalence rose gradually with age; by age 70 years, 67%–73% of the subjects in each group were seropositive. Multivariate regression showed that increasing age and female sex were significantly associated with CMV seropositivity in all three study groups (Table 2).
Blood donor CMV seroprevalence
Among blood donors, seroprevalence decreased with donation year and was region dependent, and an interaction model showed that the effect of age on CMV seroprevalence was stronger in women than in men (Table 2). Between 2005 and 2007, a significant decrease in age-specific prevalence in blood donors was observed over all age groups (Supplemental Figure S.2a). After 2007, the youngest age group (17–29 y) had remarkably stable CMV seroprevalence ranging from 33.2% to 34.0% and had doubled as a proportion of total blood donors, making up 49.3% of the donor pool by 2014 (Supplemental Figure S.2b). Despite this, the general CMV blood donor seroprevalence has remained very stable at about 40.0% since 2007 because of small fluctuations in CMV donor seroprevalence in other age groups as well as their proportional contribution to the total donor pool. Of 1,253,350 blood donors, 38.0% (n = 475,869) were women of child-bearing age. CMV seroprevalence among all female blood donors of child-bearing age was 39.0% (95% CI 38.8% to 39.1%). In Alberta specifically, there were 218,583 first-time blood donors, of whom 40.1% (n = 87,658) were women of child-bearing age. CMV seroprevalence among this group was 43.4% (95% CI 43.1% to 43.8%). Regionally, CMV seroprevalence was highest in British Columbia and Yukon (48.3%) and lowest in the Atlantic provinces (30.8%) (Figure 2). Age- and sex-specific CMV seroprevalence was similar between Alberta blood donors and Alberta organ donors (data not shown).
Figure 2:

CMV prevalence among blood donors across Canada
Notes: Red error bars show 95% confidence intervals for the seroprevalence indicated above the error bar. A trend of decreasing prevalence across Canada is visible. Women’s seroprevalence is higher than men’s seroprevalence in all regions.
CMV = Cytomegalovirus
SOT CMV seroprevalence
Adult SOT CMV seroprevalence
Among recipients, organ type and period were also significant predictors of seropositivity (Table 2): liver recipients were more likely to be seropositive than kidney recipients (adjusted OR [aOR] 1.38; 95% CI 1.17 to 1.62), and recipients transplanted between 1999 and 2013 were more likely to be seronegative (aOR 0.70; 95% CI 0.60 to 0.81) than those transplanted before 1999. CMV seroprevalence was not significantly different between living donors and deceased donors (51.9% versus 53.9%; p = 0.34). The seroprevalence of CMV in women of child-bearing age (n = 689 organ donors, n = 605 organ recipients) was similar among organ donors (56.2%; 95% CI 52.4% to 60.0%) and recipients (56.8%; 95% CI 52.7% to 60.8%).
Pediatric SOT CMV seroprevalence
Among pediatric recipients, multivariate regression (Table 2) indicated that girls were more likely to be seropositive than boys (aOR 1.66; 95% CI 1.04 to 2.66), and heart recipients were more likely to be seropositive than kidney recipients (aOR 2.64; 95% CI 1.44 to 4.91).
D/R SOT CMV disease risk stratification categories
The proportion of transplants in each D/R CMV risk category is shown in Table 3. Nearly 40% of adult recipients were seronegative (R–) at transplant, with 18% being CMV mismatched (D+/R–) and at highest risk for CMV disease. The adult mismatched proportion remained unchanged between periods, but the D–/R– group, at negligible risk for CMV disease, increased significantly from 15.7% to 21.1% (p < 0.001). Among pediatric transplants, two-thirds of transplant recipients were CMV negative pre-transplant, and one-third were mismatched. The proportion of pediatric mismatches significantly increased from 25.5% (1984–1998) to 35.5% (1999–2013) (p = 0.04).
SOT EBV seroprevalence
EBV seroprevalence in adult and pediatric organ donors and recipients is illustrated in Supplemental Table S.1 and Figure 3. As with CMV, EBV seroprevalence increases with age, but it occurs more often in childhood, with 90% of donors and 81% of recipients infected by early adult life (17–29 y) and infection almost universal by ages 40–49 years.
Figure 3:
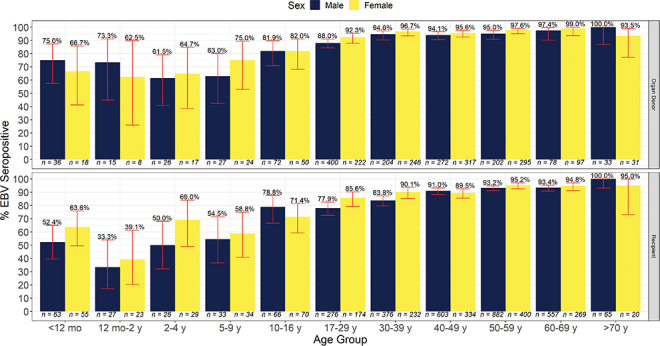
EBV seroprevalence versus age
Notes: Red error bars show 95% confidence intervals for the seroprevalence indicated above the error bar. The rapid rise of EBV seroprevalence is clear among recipients. This pattern is less clear among donors. EBV = Epstein-Barr virus
Adult SOT EBV seroprevalence
Multivariate regression results in Table 4 show that among adult donors, women were more likely to be seropositive than men (aOR 1.59; 95% CI 1.10 to 2.31), and this effect was similar in adult recipients (aOR 1.67; 95% CI 1.17 to 2.43). A significant interaction was found between age and lung recipients because the effect of age on EBV seroprevalence was stronger for lungs than for other organs. EBV seroprevalence was significantly higher in the 1999–2013 period than the 1984–1998 period for adult organ donors (aOR 1.49; 95% CI 1.02 to 2.16) but not for adult recipients. EBV seroprevalence was not significantly different between living donors and deceased donors (94.0% versus 94.5%; p = 0.64).
Pediatric SOT EBV seroprevalence
Among pediatric donors, age was the only significant predictor of EBV seroprevalence, whereas among recipients, organ group, age, and period were important predictors in multivariate analysis (Table 4). Liver recipients were significantly more likely to be seropositive than kidney recipients (aOR 2.21; 95% CI 1.12 to 4.50). Recipients transplanted in 1999–2013 were more likely to be seronegative than those transplanted in 1984–1998 (aOR 0.49; 95% CI 0.26 to 0.88).
D/R EBV SOT risk stratification categories
The proportion of patients in each D/R EBV risk category is shown in Table 5. Forty-five percent of pediatric transplants were EBV mismatched and at highest risk for PTLD. Among adult transplants, EBV mismatches decreased from 5.7% in 1984–1999 to 4.0% in 1999–2013 (p = 0.03). Conversely, among pediatric transplants, EBV mismatches increased from 32.6% in 1984–1999 to 48.1% in 1999–2013 (p = 0.01).
Interpretation
Our study of Canadian age- and sex-specific seroprevalence of CMV and EBV suggests that EBV is acquired throughout childhood, with infection being almost universal by mid-adult life. In contrast, CMV infection is acquired gradually, with 43%–61% of women of child-bearing age in our study being seronegative and at high risk of primary CMV infection during pregnancy and resultant congenital CMV. Similar patterns have been reported in comparable populations in the United States and Western Europe, although our observed seroprevalence is in the lower range of these studies (24–34). Some industrialized countries such as the Scandinavian countries, Australia, Italy, and Spain have significantly higher age-specific seroprevalence than we observed, likely reflecting fertility rates, child care and breast-feeding practices, immigration history, and socio-economic status of these populations (24,35). These trends contrast with those in the Indigenous population of Northern Canada and in less developed countries where infection with these viruses is nearly universal in early childhood (24,36–41).
In our study, we estimated CMV prevalence among women of child-bearing age to be 56% in organ donors and recipients, and this was nearly identical to the 55% reported previously in Edmonton and in two other studies with Canadian pregnant women reporting 55% and 54% (7,36, 42). However, the largest known Canadian study of pregnant women, which was conducted in Quebec, reported a prevalence of 42%, which is closer to the 39% we observed in Canadian blood donors (43). Our higher CMV seroprevalence among women is supported by the results of other studies and may be explained by a propensity for women to be exposed to young children through child care or child-related occupations (24,44).
The true prevalence of CMV in the adult Canadian population is likely between the lower prevalence among blood donors (42%), a highly screened and healthy population with Canadian and US-born individuals overrepresented compared with the general Canadian population (45), and the higher prevalence among organ recipients (62%). Better estimates might be derived by mathematical modelling based on specific information regarding age and sex distribution in the general Canadian population by region with adjustments for other demographic information related to CMV risk factors such as country of birth. A 2006 study of first-time Canadian blood donors suggested that donors born in Canada and the United States were overrepresented (90.2%) relative to the general population (84.3%) (45). Alberta blood donor and organ donor age-specific CMV seroprevalence are similar. Consistent with the results of previous studies, CMV seroprevalence was lower among blood donors from the Atlantic provinces than among those from Western Canada (46,47), likely because of greater immigration from countries with high CMV seroprevalence in Western versus Eastern Canada (48). Similar trends are expected among organ donors because in Western Canada, the site of our study, Caucasian organ donors make up less than 90% of the donor population, whereas in Eastern Canada, they make up more than 90%, and Caucasian donors tend to have much lower CMV prevalence than non-Caucasians donors (49). Organ-specific differences in recipient CMV prevalence likely relate to the indications for transplant. During our study period, 39% of our adult liver transplant recipients had hepatitis B (HBV) or C (HCV) liver disease with or without hepatocellular carcinoma as the indication for transplant. Overrepresentation of immigrants from countries with high HBV and HCV prevalence that also often have high CMV seroprevalence may explain the higher CMV seroprevalence observed in adult liver transplant recipients relative to other organ types (24,50–52).
In addition, Canadian Indigenous populations with higher CMV seroprevalence have a disproportionate burden of diabetes, immune-mediated kidney disease and associated complications of chronic kidney disease, and ischemic heart disease that might result in kidney or heart transplantation (53). This may be an additional factor explaining the higher CMV seroprevalence rates observed in organ transplant recipients compared with Canadian blood donors. In the pediatric SOT recipient population, we noted a higher CMV seroprevalence among heart recipients than among kidney recipients and a higher EBV seroprevalence among liver recipients than among kidney recipients. We hypothesize that these differences may be due to a higher prevalence of passive antibodies resulting from blood transfusion. Pediatric heart transplant recipients have often had prior surgery and frequently receive blood products during periods of mechanical circulatory support before transplant. Pediatric liver recipients are also more likely to be transfused than pediatric kidney recipients because of coagulopathies and recent surgery such as the Kasai procedure for biliary atresia, a common reason for pediatric liver transplantation. Given the relatively small size of the pediatric SOT population, we also need to be cautious about interpreting these statistically significant differences as reflecting real differences.
Our study suggests that CMV seroprevalence among blood donors may have decreased. CMV seroprevalence also decreased over time among adult SOT recipients, in whom average age increased. Decreasing overall and age-specific CMV prevalence has been observed by some investigators but not all (26,30,54,55).
EBV prevalence is strongly dependent on age, and infection tends to be acquired at older ages in developed countries with low population density and high hygiene standards (56). From our study, it is clear that Canadians acquire EBV much later than people in countries such as Thailand, Taiwan, and China, who attain 90% seroprevalence between ages 5 and 8 years, in comparison with Canadians’ aged 30 years (37–39). However, in our transplant population, 39% of recipients were already EBV seropositive by age 2 years. Although donor prevalence was 70% by age 2 years, this is likely an overestimation because donors may have been transfused, and serology could be falsely positive as a result of passive antibodies. Few sources of similar data exist for young children. A Minnesota study reported 31% EBV prevalence among children aged 1–5 years, and a Swedish birth cohort study reported 7% prevalence by age 1 and 18% prevalence by age 2 (32,57). The higher EBV seroprevalence we observed among women differs from the majority of studies, which find no such association, although it has occasionally been reported (31–34,38,58,59,60).
Among our pediatric recipients, we saw a decreasing EBV prevalence trend over time, which has also been observed by others among both healthy children and adults (31,33,61). Declining EBV prevalence in childhood may result in increased rates of infectious mononucleosis, commonly observed when EBV infection is delayed until adolescence or adulthood.
Knowledge of CMV and EBV seroprevalence and trends allows modelling of disease burden and resource needs associated with these viruses in Canada at a population level as well as optimal age targeting of future vaccines. CMV vaccine modelling studies, using American age-specific CMV seroprevalence data, demonstrate tremendous potential reductions in congenital CMV with universal infant immunization (62,63). Even vaccination of female adolescents could realize significant cost savings and gain in quality-adjusted life years for infants born to vaccinated mothers (64). Because Canadian age-specific CMV seroprevalence appears similar, it is reasonable to extrapolate this modelling to Canada. A recent modelling study suggests that an effective EBV vaccine should target infants once they lose their protective maternal antibodies (65). Our data support this recommendation because children aged younger than 2 years were most likely to be seronegative, and infection is acquired rapidly in early childhood.
Seronegative SOT recipients are also targeted for potential CMV and EBV vaccines. Primary infection early after transplant, most often transmitted from a seropositive donor to a seronegative recipient, is the primary risk factor for both CMV disease and EBV-associated PTLD (10,66). CMV and EBV mismatched (D+/R–) recipients use disproportionately greater health care resources, including antiviral prophylaxis and laboratory monitoring to prevent CMV disease and PTLD. Because age-specific CMV prevalence in Canada is relatively low, approximately one-fifth of adult and one-third of pediatric SOT recipients are CMV mismatched and at high risk of CMV disease. Because of the age-specific EBV seroprevalence, pediatric transplant recipients have a much higher PTLD risk than adults, and risk of lymphoma can be as high as 212 times that of children in the general population (66–68). Declining EBV seroprevalence rates among children may increase the future size of the D+/R– subgroup at highest risk of PTLD, especially because most donors for non-thoracic pediatric allograft recipients are adults who are likely to be EBV seropositive. This makes EBV-mismatched organ transplant recipients apt candidates for testing future EBV vaccines with respect to blocking infection and preventing malignancy.
Our study includes data over a 30-year period from a major Canadian transplant centre, but subjects may not be representative of all Canadians because of geographic variations in race, ethnicity, and socio-economic status. These missing variables are known to be associated with CMV and EBV seroprevalence. Moreover, fewer transplants were performed in the early period, so data from that era may be underpowered compared with data from the later period. Transfusion data for organ donors and recipients were unavailable, and the presence of passive antibodies may inflate seroprevalence. Blood donor data do not include data from Quebec.
Conclusion
Use of collated data generated as part of routine screening of transplant recipients and organ and blood donors is an efficient and low-cost means of obtaining CMV and EBV age-specific seroprevalence data that can be used to estimate the current and future disease burden associated with these viruses and the public health impact of future vaccines. Although Canadian Blood Services has recently discontinued large-scale CMV serologic screening in the era of universal blood product leukoreduction, a national effort to collect and collate transplant donor and recipient data may be useful for ongoing surveillance of CMV and EBV seroprevalence trends.
Acknowledgements:
The authors acknowledge Dr Margaret Fearon for her contributions to this work and Dr Sonya Englert for significant assistance with data collection.
Supplemental Appendix
Table S.1:
Adult and pediatric EBV seroprevalence among organ donors and recipients by age and sex
| Characteristics | Organ donors | Recipients | ||||
|---|---|---|---|---|---|---|
| Characteristics | n | Prevalence % (95% CI) | POR (95% CI) | n | Prevalence % (95% CI) | POR (95% CI) |
| Overall adult prevalence | 2,397* (42.1, 22.4) | 94.3 (93.3 to 95.2) | — | 3,964* (51.2, 19) | 95.8 (95.1 to 96.4) | — |
| Adult age group | ||||||
| 17–29 y | 622 | 89.5 (86.9 to 91.8) | 1.00 (Ref.) | 420 | 86.7 (83.0 to 89.8) | 1.00 (Ref.) |
| 30–39 y | 450 | 95.8a (93.5 to 97.4) | 2.65 (1.59 to 4.60) | 566 | 92.6a (90.1 to 94.6) | 1.91 (1.26 to 2.94) |
| 40–49 y | 59 | 94.9 (92.8 to 96.5) | 2.17 (1.40 to 3.45) | 874 | 97.0 (95.7 to 98.0) | 5.02 (3.13 to 8.24) |
| 50–59 y | 497 | 96.6 (94.6 to 98.0) | 3.29 (1.95 to 5.87) | 1,228 | 98.0 (97.0 to 98.7) | 7.40 (4.61 to 1.22) |
| 60–69 y | 175 | 98.3 (95.1 to 99.6) | 6.69 (2.45 to 27.60) | 792 | 97.9 (96.6 to 98.7) | 7.01 (4.11 to 1.26) |
| >70 y | 64 | 96.9 (89.2 to 99.6) | 3.62 (1.10 to 22.37) | 84 | 100 (95.7 to 100) | — |
| Adult sex | ||||||
| Male | 1,189 | 92.7 (91.1 to 94.1) | 1.00 (Ref.) | 2,608 | 95.3 (94.4 to 96.1) | 1.00 (Ref.) |
| Female | 1,208 | 95.9a (94.7 to 97.0) | 1.87 (1.31 to 2.69) | 1,356 | 96.8a (95.7 to 97.6) | 1.46 (1.04 to 2.10) |
| Overall pediatric prevalence | 239* (10.1, 11) | 74.5 (68.5 to 79.9) | — | 292* (8.1, 10) | 65.4 (59.6 to 70.9) | — |
| Pediatric age group | ||||||
| <12 mo | 54 | 72.2 (58.4 to 83.5) | — | 117 | 58.1 (48.6 to 67.2) | — |
| 12 mo–2 y | 23 | 69.6a (47.1 to 86.8) | 1 (Ref.) | 46 | 39.1a (25.9 to 54.6) | 1 (Ref.) |
| 2–4 y | 43 | 62.8 (46.7 to 77.0) | 0.74 (0.24 to 2.14) | 54 | 61.1 (46.9 to 74.1) | 2.44 (1.10 to 5.56) |
| 5–9 y | 51 | 68.6 (54.1 to 80.9) | 0.96 (0.32 to 2.73) | 64 | 59.4 (46.4 to 71.5) | 2.27 (1.06 to 5.00) |
| 10–16 y | 122 | 82.0 (74.0 to 88.3) | 1.99 (0.70 to 5.28) | 128 | 79.7 (71.7 to 86.3) | 6.10 (2.97 to 12.91) |
| Pediatric sex | ||||||
| Male | 140 | 73.6 (65.5 to 80.6) | 1 (Ref.) | 145 | 63.4 (55.1 to 71.3) | 1 (Ref.) |
| Female | 99 | 75.8 (66.1 to 83.8) | 1.12 (0.62 to 2.05) | 147 | 64.6 (56.3 to 72.3) | 1.19 (0.73 to 1.93) |
Notes: Superscript letters indicate statistically significant comparisons with p < 0.05. Pediatric patients aged less than 12 mo are included in the table to describe the seroprevalence of this group, but they were excluded from regression analysis because of the potential presence of passive maternal antibody. Dashes indicate not applicable. Boldface indicates significant at α = 0.05
* Exclusions due to missing or indeterminate serology: 226 adult donors (22 indeterminate), 224 adult recipients (34 indeterminate), 19 pediatric donors (4 indeterminate), and 16 pediatric recipients (4 indeterminate)
EBV = Epstein-Barr virus; IQR = Interquartile range; CI = Confidence interval; POR = Prevalence odds ratio; Ref. = Reference
Figure S.1:
Cytomegalovirus seroprevalence versus age among blood donors, organ donors, and recipients
Notes: Red error bars show 95% confidence intervals for the seroprevalence indicated above the error bar. Seroprevalence in individuals aged younger than 12 mo is inflated by presence of maternal antibodies. Women’s seroprevalence tends to be higher than men’s.
Figure S.2. A:
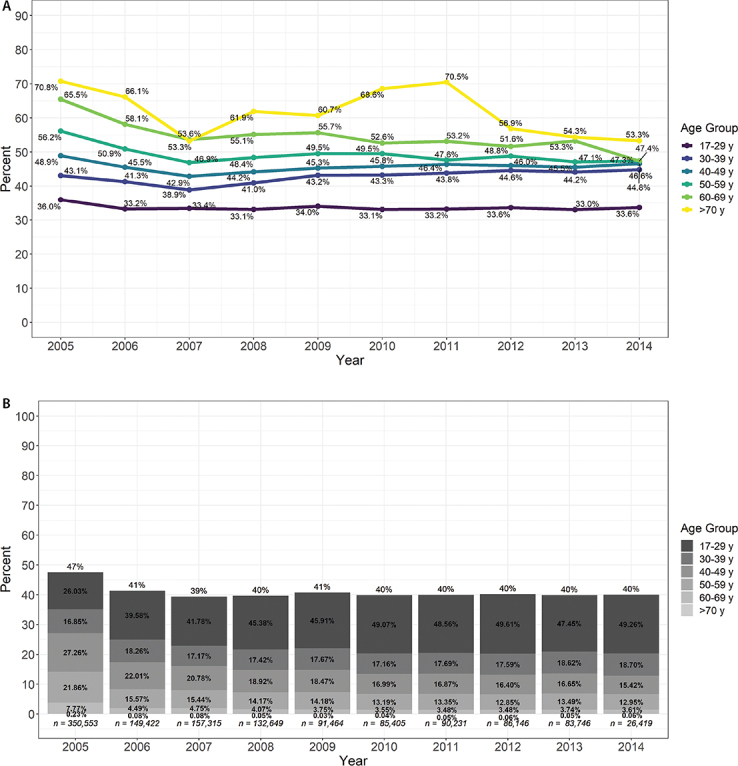
Age-specific CMV seroprevalence in blood donors over time. B: CMV seroprevalence in blood donors by age group over time
Notes: Bar height represents the overall CMV seroprevalence of blood donors each year. Bar proportions represent the age distribution of blood donors each year. CMV = Cytomegalovirus
Funding Statement
This project was funded by Canadian Blood Services grant IG2013-JP.
Ethics Approval:
The study protocol was approved by an ethics committee and the ethics certificate information is available from the authors upon request.
Funding:
This project was funded by Canadian Blood Services grant IG2013-JP.
Disclosures:
The authors report grants from Canadian Blood Services during the conduct of the study.
PEER REVIEW:
This manuscript has been peer reviewed.
ANIMAL STUDIES:
N/A.
References
- 1. Boeckh M, Geballe AP. Science in medicine Cytomegalovirus: pathogen, paradigm, and puzzle. J Clin Invest. 2011;121(5):1673–80. 10.1172/JCI45449. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odumade OA, Hogquist KA, Balfour HH. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin Microbiol Rev. 2011;24(1):193–209. 10.1128/CMR.00044-10. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause PR, Bialek SR, Boppana SB, et al. Priorities for CMV vaccine development. Vaccine. 2013;32(1):4–10. 10.1016/j.vaccine.2013.09.042. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–76. 10.1002/rmv.535. Medline: [DOI] [PubMed] [Google Scholar]
- 5.Fu TM, An Z, Wang D. Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine. 2014;32(22):2525–33. 10.1016/j.vaccine.2014.03.057. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Pembrey L, Raynor P, Griffiths P, Chaytor S, Wright J, Hall AJ. Seroprevalence of cytomegalovirus, Epstein Barr virus and varicella zoster virus among pregnant women in Bradford: a cohort study. PLoS One. 2013; 8(11):e81881. 10.1371/journal.pone.0081881. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaudry W, Rosychuk RJ, Lee BE, Cheung PY, Pang XL, Preiksaitis JK. Congenital cytomegalovirus infection in high-risk Canadian infants: report of a pilot screening study. Can J Infect Dis Med Microbiol. 2010;21:e12–9. 10.1155/2010/942874. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinninti S, Hough-Telford C, Pati S, Boppana S. Cytomegalovirus and Epstein-Barr virus infections. Pediatr Rev. 2016;37(6):223–34. 10.1542/pir.2015-0072. Medline: [DOI] [PubMed] [Google Scholar]
- 9.Blanco-Lobo P, Ngel Bulnes-Ramos Á, Mcconnell MJ, Navarro D, Pé Rez-Romero P. Applying lessons learned from cytomegalovirus infection in transplant patients to vaccine design. Drug Discov Today. 2016;21(4):674–81. 10.1016/j.drudis.2016.03.005. Medline: [DOI] [PubMed] [Google Scholar]
- 10.Lumbreras C, Manuel O, Len O, ten Berge IJM, Sgarabotto D, Hirsch HH. Cytomegalovirus infection in solid organ transplant recipients. Clin Microbiol Infect. 2014;20(Supplement 7):19–26. 10.1111/1469-0691.12594. Medline: [DOI] [PubMed] [Google Scholar]
- 11.Dioverti MV, Razonable RR. Cytomegalovirus. Microbiol Spectr. 2016;4(4):97–125. 10.1128/microbiolspec.DMIH2-0022-2015 [DOI] [PubMed] [Google Scholar]
- 12.Plotkin SA, Boppana SB. Vaccination against the human cytomegalovirus. Vaccine. 2019;37(50):7437–442. 10.1016/j.vaccine.2018.02.089. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pender MP. The essential role of Epstein-Barr virus in the pathogenesis of multiple sclerosis. Neuroscientist. 2011;17(4):351–67. 10.1177/1073858410381531. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shannon-Lowe C, Rickinson AB, Bell AI. Epstein–Barr virus-associated lymphomas. Philos Trans R Soc B Biol Sci. 2017;372(1732):20160271. 10.1098/rstb.2016.0271. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowalk A, Green M. Epstein-Barr virus. Microbiol Spectr. 2016;4(3):127–34. 10.1128/microbiolspec.DMIH2-0011-2015. Medline: [DOI] [PubMed] [Google Scholar]
- 16.Vockerodt M, Yap LF, Shannon-Lowe C, et al. The Epstein-Barr virus and the pathogenesis of lymphoma. J Pathol. 2015;235(2):312–22. 10.1002/path.4459. Medline: [DOI] [PubMed] [Google Scholar]
- 17.Cohen JI, Mocarski ES, Raab-Traub N, Corey L, Nabel GJ. The need and challenges for development of an Epstein-Barr virus vaccine. Vaccine. 2013;31(Supplement 2):B194–6. 10.1016/j.vaccine.2012.09.041. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JI. Vaccine development for Epstein-Barr virus. In: Kawaguch Y, Mori Y, Kimura H (eds.), Advances in experimental and medical biology. Vol. 1045. Human herpesvirus. New York: Wiley; 2018. p. 477–93. 10.1007/978-981-10-7230-7_22. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balfour HH. Progress, prospects, and problems in Epstein-Barr virus vaccine development. Curr Opin Virol. 2014;6:1–5. 10.1016/j.coviro.2014.02.005. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro D, Fernández-Ruiz M, Aguado JM, Sandonís V, Pérez-Romero P. Going beyond serology for stratifying the risk of CMV infection in transplant recipients. Rev Med Virol. 2019;29(1):e2017. 10.1002/rmv.2017. Medline: [DOI] [PubMed] [Google Scholar]
- 21.Nahirniak S, Liebermann L, Preiksaitis J, Wall D. NAC education document: transfusion and cytomegalovirus in the Canadian blood system. https://www.nacblood.ca/resources/guidelines/downloads/NAC_CMV_position_paper.pdf (March 14, 2019).
- 22.Devine D. Changes to the provision of CMV seronegative blood products customer letter #2017-36. http://saskblood.ca/download/canadian-blood-services-customer-letter-2017-36-changes-provision-cmv-seronegative-blood-products/?wpdmdl=1191&ind=0 (July 9, 2019).
- 23.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018. https://www.R-project.org/ (July 28, 2020). [Google Scholar]
- 24.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–13. 10.1002/rmv.655. Medline: [DOI] [PubMed] [Google Scholar]
- 25.Staras SAS, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143–51. 10.1086/508173. Medline: [DOI] [PubMed] [Google Scholar]
- 26.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the National Health and Nutrition Examination Surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–47. 10.1086/652438. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korndewal MJ, Mollema L, Tcherniaeva I, et al. Cytomegalovirus infection in the Netherlands: seroprevalence, risk factors, and implications. J Clin Virol. 2015;63:53–8. 10.1016/j.jcv.2014.11.033. Medline: [DOI] [PubMed] [Google Scholar]
- 28.Lachmann R, Loenenbach A, Waterboer T, et al. Cytomegalovirus (CMV) seroprevalence in the adult population of Germany. PLoS One. 2018;13(7):e0200267. 10.1371/journal.pone.0200267. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecker M, Qiu D, Marquardt K, Bein G, Hackstein H. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang. 2004;86(1):41–4. 10.1111/j.0042-9007.2004.00388.x. Medline: [DOI] [PubMed] [Google Scholar]
- 30.Hassan J, O’Neill D, Honari B, et al. Cytomegalovirus infection in Ireland. Med (United States). 2016;95(6):e2735. 10.1097/MD.0000000000002735. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balfour HH, Sifakis F, Sliman JA, Knight JA, Schmeling DO, Thomas W. Age-specific prevalence of Epstein–Barr virus infection among individuals aged 6–19 years in the United States and factors affecting its acquisition. J Infect Dis. 2013;208(8):1286–93. 10.1093/infdis/jit321. Medline: [DOI] [PubMed] [Google Scholar]
- 32.Condon LM, Cederberg LE, Rabinovitch MD, et al. Age-specific prevalence of Epstein-Barr virus infection among Minnesota children: effects of race/ethnicity and family environment. Clin Infect Dis. 2014;59(4):501–8. 10.1093/cid/ciu342. Medline: [DOI] [PubMed] [Google Scholar]
- 33.Fourcade G, Germi R, Guerber F, et al. Evolution of EBV seroprevalence and primary infection age in a French hospital and a city laboratory network, 2000–2016. PLoS One. 2017;12:1–12. 10.1371/journal.pone.0175574. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris MC, Edmunds WJ, Hesketh LM, et al. Sero-epidemiological patterns of Epstein-Barr and herpes simplex (HSV-1 and HSV-2) viruses in England and Wales. J Med Virol. 2002;67(4):522–7. 10.1002/jmv.10132. Medline: [DOI] [PubMed] [Google Scholar]
- 35.Seale H, MacIntyre CR, Gidding HF, Backhouse JL, Dwyer DE, Gilbert L. National serosurvey of cytomegalovirus in Australia. Clin Vaccine Immunol. 2006;13:1181–4. 10.1128/CVI.00203-06. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preiksaitis JK, Larke RPB, Froese GJ. Comparative seroepidemiology of cytomegalovirus infection in the Canadian Arctic and an urban center. J Med Virol. 1988;24(3):299–307. 10.1002/jmv.1890240307. Medline: [DOI] [PubMed] [Google Scholar]
- 37.Xiong G, Zhang B, Huang MY, et al. Epstein-Barr virus (EBV) infection in Chinese children: a retrospective study of age-specific prevalence. PLoS One. 2014; 9(6):e99857. 10.1371/journal.pone.0099857. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CY, Huang KYA, Shen JH, Tsao KC, Huang YC. A large-scale seroprevalence of Epstein-Barr virus in Taiwan. PLoS One. 2015;10:1–11. 10.1371/journal.pone.0115836. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suntornlohanakul R, Wanlapakorn N, Vongpunsawad S, Thongmee T, Chansaenroj J, Poovorawan Y. Seroprevalence of anti-EBV IgG among various age groups from Khon Kaen Province, Thailand. Asian Pac J Cancer Prev. 2015;16(17):7583–7. 10.7314/APJCP.2015.16.17.7583. Medline: [DOI] [PubMed] [Google Scholar]
- 40.Haque T, Iliadou P, Hossain A, Crawford DH. Seroepidemiological study of Epstein-Barr virus infection in Bangladesh. J Med Virol. 1996;48(1): 17–21. [DOI] [PubMed] [Google Scholar]
- 41.Tuon FF, Wollmann LC, Pegoraro D, et al. Seroprevalence of Toxoplasma gondii, cytomegalovirus and Epstein Barr virus in 578 tissue donors in Brazil. J Infect Public Health. 2019;12(2):289–91. 10.1016/j.jiph.2018.07.001. Medline: [DOI] [PubMed] [Google Scholar]
- 42.Wizman S, Lamarre V, Coic L, et al. Awareness of cytomegalovirus and risk factors for susceptibility among pregnant women, in Montreal, Canada. BMC Pregnancy Childbirth. 2016;16:54 10.1186/s12884-016-0844-9. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamarre V, Gilbert NL, Rousseau C, Gyorkos TW, Fraser WD. Seroconversion for cytomegalovirus infection in a cohort of pregnant women in Québec, 2010–2013. Epidemiol Infect. 2016;144(8):1701–9. 10.1017/S0950268815003167. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol. 2011;21(4):240–55. 10.1002/rmv.695. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien SF, Xi G, Fan W, et al. Epidemiology of hepatitis B in Canadian blood donors. Transfusion. 2008;48(11):2323–30. 10.1111/j.1537-2995.2008.01845.x. Medline: [DOI] [PubMed] [Google Scholar]
- 46.Clair PK, Embil JA, Fahey J. A seroepidemiologic study of cytomegalovirus infection in a Canadian recruit population. Mil Med. 1990;155(10):489–92. 10.1093/milmed/155.10.489. Medline: [DOI] [PubMed] [Google Scholar]
- 47.Embil JA, Haldane E V, MacKenzie RA, van Rooyen CE. Prevalence of cytomegalovirus infection in a normal urban population in Nova Scotia. Can Med Assoc J. 1969;101(12):78–81. [PMC free article] [PubMed] [Google Scholar]
- 48.Statistics Canada. Immigration and ethnocultural diversity: key results from the 2016 Census. https://www150.statcan.gc.ca/n1/daily-quotidien/171025/dq171025b-eng.htm (March 18, 2019).
- 49.Canadian Institute for Health Information. Deceased organ donor potential in Canada. https://www.cihi.ca/sites/default/files/organdonorpotential_2014_en_0.pdf (January 24, 2019).
- 50.Ginzberg D, Wong RJ, Gish R. Global HBV burden: guesstimates and facts. Hepatol Int. 2018;12:315–29. 10.1007/s12072-018-9884-8. Medline: [DOI] [PubMed] [Google Scholar]
- 51.Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22(34):7824–40. 10.3748/wjg.v22.i34.7824. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bayram A, Özkur A, Erkilic S. Prevalence of human cytomegalovirus co-infection in patients with chronic viral hepatitis B and C: a comparison of clinical and histological aspects. J Clin Virol. 2009;45(3):212–7. 10.1016/j.jcv.2009.05.009. Medline: [DOI] [PubMed] [Google Scholar]
- 53.Komenda P, Lavallee B, Ferguson TW, et al. The prevalence of CKD in rural Canadian Indigenous peoples: results From the First Nations Community Based Screening to Improve Kidney Health and Prevent Dialysis (FINISHED) Screen, Triage, and Treat Program. Am J Kidney Dis. 2016;68(4):582–90. 10.1053/j.ajkd.2016.04.014. Medline: [DOI] [PubMed] [Google Scholar]
- 54.Taniguchi K, Watanabe N, Sato A, et al. Changes in cytomegalovirus seroprevalence in pregnant Japanese women—a 10-year single center study. J Clin Virol. 2014;59(3):192–4. 10.1016/j.jcv.2013.12.013. Medline: [DOI] [PubMed] [Google Scholar]
- 55.Enders G, Daiminger A, Lindemann L, et al. Cytomegalovirus (CMV) seroprevalence in pregnant women, bone marrow donors and adolescents in Germany, 1996–2010. Med Microbiol Immunol. 2012;201:303–9. 10.1007/s00430-012-0232-7. Medline: [DOI] [PubMed] [Google Scholar]
- 56.Smatti MK, Al-Sadeq DW, Ali NH, Pintus G, Abou-Saleh H, Nasrallah GK. Epstein–Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol. 2018;8:211. 10.3389/fonc.2018.00211. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hesla HM, Gutzeit C, Stenius F, et al. Herpesvirus infections and allergic sensitization in children of families with anthroposophic and non-anthroposophic lifestyle—the ALADDIN birth cohort. Pediatr Allergy Immunol. 2013;24(1):61–5. 10.1111/pai.12030. Medline: [DOI] [PubMed] [Google Scholar]
- 58.Franci G, Crudele V, Della Rocca MT, et al. Epstein-Barr virus seroprevalence and primary infection at the University Hospital Luigi Vanvitelli of Naples from 2007 to 2017. Intervirology. 2019;62(1):15–22. 10.1159/000496828. Medline: [DOI] [PubMed] [Google Scholar]
- 59.Dowd JB, Palermo T, Brite J, McDade TW, Aiello A. Seroprevalence of Epstein-Barr virus infection in U.S. children ages 6–19, 2003–2010. PLoS One. 2013;8:1–7. 10.1371/journal.pone.0064921. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higgins CDD, Swerdlow AJJ, Macsween KFF, et al. A study of risk factors for acquisition of Epstein‐Barr virus and its subtypes. J Infect Dis. 2007;195(4):474–82. 10.1086/510854. Medline: [DOI] [PubMed] [Google Scholar]
- 61.Takeuchi K, Tanaka-Taya K, Kazuyama Y, et al. Prevalence of Epstein-Barr virus in Japan: trends and future prediction. Pathol Int. 2006;56(3):112–6. 10.1111/j.1440-1827.2006.01936.x. Medline: [DOI] [PubMed] [Google Scholar]
- 62.Lanzieri TM, Bialek SR, Ortega-Sanchez IR, Gambhir M. Modeling the potential impact of vaccination on the epidemiology of congenital cytomegalovirus infection. Vaccine. 2014;32(30):3780–6. 10.1016/j.vaccine.2014.05.014. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogea C, Dieussaert I, Van Effelterre T, Guignard A, Mols J. A dynamic transmission model with age-dependent infectiousness and reactivation for cytomegalovirus in the United States: potential impact of vaccination strategies on congenital infection. Hum Vaccines Immunother. 2015;11(7):1788–802. 10.1080/21645515.2015.1016665. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dempsey AF, Pangborn HM, Prosser LA. Cost- effectiveness of routine vaccination of adolescent females against cytomegalovirus. Vaccine. 2012;30(27):4060–6. 10.1016/j.vaccine.2012.04.011. Medline: [DOI] [PubMed] [Google Scholar]
- 65.Goscé L, Winter JR, Taylor GS, Lewis JEA, Stagg HR. Modelling the dynamics of EBV transmission to inform a vaccine target product profile and future vaccination strategy. Sci Rep. 2019;9:9290. 10.1038/s41598-019-45381-y. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S. Post-transplant lymphoproliferative disorders. Nat Rev Dis Prim. 2016;2:15088. 10.1038/nrdp.2015.88. Medline: [DOI] [PubMed] [Google Scholar]
- 67.Yanik EL, Smith JM, Shiels MS, et al. Cancer risk after pediatric solid organ transplantation. Pediatrics. 2017;139(5):e20163893. 10.1542/peds.2016-3893. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Na R, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, Vajdic CM. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. Am J Transplant. 2013;13(1):174–83. 10.1111/j.1600-6143.2012.04302.x. Medline: [DOI] [PubMed] [Google Scholar]