Abstract
Objective:
To examine associations between statelevel characteristics and state-level preterm birth rates.
Study design:
We conducted a retrospective ecological cross-sectional study using statelevel data from 2013 to 2014 extracted from publicly available sources –the March of Dimes PeriStats database, the U.S. Census Bureau, the US Department of Education, and the US Department of Justice.
Results:
State-level preterm birth rates correlated with the following state characteristics: poverty rate, obesity rate, percentage of non-Hispanic Black women residents, smoking rate, percent of C – section deliveries, percent of births to women <20 years old, pregnancies receiving late/no prenatal care, and violent crimes per capita. Linear regression analysis found that only the percent of non-Hispanic Black women by state remained a significant predictor of state-level preterm birth rates after adjusting for other risk factors.
Conclusions:
States with higher percentages of non-Hispanic Black women had higher rates of preterm birth, even after adjusting for sociodemographic characteristics, prenatal care, and maternal health by state. These findings suggest that public health interventions that target contextual and environmental risk factors affecting non-Hispanic Black women may help to curb rising rates of preterm birth in the United States.
Keywords: Community, socioeconomic, preterm birth, race/ethnicity, health behaviors
Introduction
Preterm birth (PTB), defined as births that occur before 37 weeks’ gestation, represents one of the largest unmet medical challenges worldwide and is the leading cause of death among children under age five (estimates from 2010) [1]. Premature infants who survive have an increased risk for cerebral palsy, cardiovascular disease, respiratory conditions, developmental delays, metabolic disorders, and infection [2]. Medical costs associated with caring for preterm neonates have been estimated at $26 billion annually in the United States [2]. Despite the devastating consequences of PTB to individuals and society, rates of PTB in the United States have increased for the past two years, with 1 in 10 American women delivering prematurely [3]. The United States has one of the highest PTB rates among developed countries [3]. However, between states, there is a great deal of variability. While some states report rates comparable to developing countries such as Pakistan, Indonesia, and Botswana (Mississippi (16.6%), Louisiana (15.1%), and Alabama (15.1%)), other states report PTB rates that are comparable to developed countries such as the Netherlands and the United Kingdom (Vermont (8.1%), California (8.8%), and New Hampshire (9%)) [3,4].
The causes of increasing rates of PTB within the United States are currently unknown. The primary focus of past research has been to identify the confluence of risk factors leading to PTB, including biological, psychosocial, behavioral, and medical that may predispose some women to early delivery. These studies have reported that women at greatest risk for PTB are those with a history of PTB, non – Hispanic Black race/ethnicity, low socioeconomic status, maternal obesity, smoking, infection, and short cervical length [5]. Unfortunately, the identification of these risk factors has failed to reduce national rates of PTB in the United States. Recent efforts to define PTB phenotypes [6–8] and identify genes predisposing women to PTB [9–11] have thus far been insufficient to improve screening and prevention of PTB. The currently available treatments to prevent PTB, including weekly 17 alpha-hydroxyprogesterone caproate (17OHP – C) injections and cervical cerclage, are available only to a small number of pregnant women, who had a prior spontaneous PTB (3–5% of pregnant women) [12] or short cervical length (approximately 2% of pregnant women) [13], reflecting the current lack of specificity in identifying women at risk for PTB and the limited application of effective PTB prevention approaches [14]. Altogether, these results suggest that the focus on within-person risk factors have limited impact on the PTB landscape within the United States. Community-level risk factors may be worthwhile targets for interventions to attenuate the rising rates of PTB in the United States.
A robust literature has identified contextual and environmental risk factors associated with PTB, including neighborhood disadvantage [15], racial residential segregation [16], neighborhood violence [17], neighborhood education attainment [18], high traffic exposure [19], and pollution [20]. Adverse community-level characteristics are believed to trigger early parturition via chronic psychosocial stress and concomitant release of stress hormones, inflammation, and epigenetic processes that may lead to PTB [21,22]. Other factors that may explain the association between community-level risk factors of PTB include behavioral and medical indices such as barriers to receiving prenatal care and proper nutrition [23].
Several studies, both in the United States and abroad, have successfully reduced PTB rates via community-based interventions to improve PTB education and reduce PTB risk factors. Within the United States, the “Healthy African American Families Project” provides an example of a community-based partnership linking community and scientific expertise to address high rates of PTB among African American women [24,25]. A public health campaign in Kentucky, which served as the pilot project for the March of Dimes “Healthy Babies are Worth the Wait” program, involved extensive community education outreach and group prenatal care and was successful at reducing rates of PTB compared to surrounding states [26]. Other public health campaigns have used on-site education and print and social media campaigns to successfully reduce rates of PTB in communities [27]. In Germany, the Thuringia Campaign used vaginal pH self-screening to identify and treat abnormal vaginal flora and demonstrated reductions in PTBs [28].
Results from these campaigns suggest that providing community-level interventions may help to reduce PTB within the United States. Prior to the implementation of public health campaigns, however, it is first necessary to understand factors that explain PTB disparities between states that could serve as high-value targets for PTB interventions. Thus, the aim of this article is to examine state-level characteristics that are associated with PTB rates to inform public policies aimed to appropriately allocate resources to reduce PTB rates in the highest-risk states.
Materials and methods
We conducted a retrospective ecological cross-sectional study to examine risk factors for state-level rates of PTB. State-level data were primarily extracted from the March of Dimes PeriStats database, which is a database-driven website that aggregates data from multiple government agencies and organizations. For state-level statistics not captured by the Peristats database, we sourced information from the U.S. Census Bureau, the U.S. Department of Education, and the Federal Bureau of Justice Statistics. State-level variables were selected to represent community/environmental factors, demographic, psychosocial, behavioral, and medical/obstetric risk factors that have been associated with PTB in the past research (see Figure 1, conceptual model). All data correspond to the years 2013–2014. Puerto Rico was not included in the data. The District of Columbia was included, therefore, the sample size was N = 51. The District of Columbia has been included in the US Census since its inception in 1790. Puerto Rico has been included in the US Census since 1910 [29], however, data from Puerto Rico was not provided by the March of Dimes Peristats database and, therefore, was not included in the current analyses. This study did not need IRB approval as it was an analysis of publicly available datasets.
Figure 1.
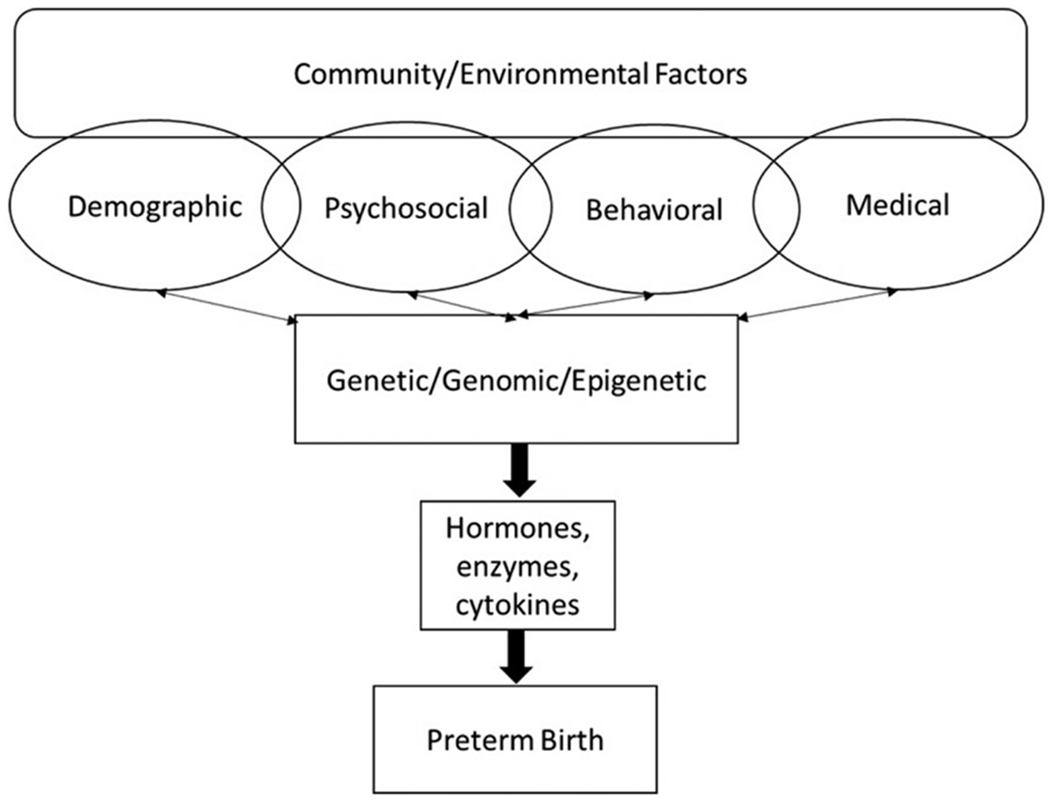
Conceptual framework of the hypothesized relationships among community – level, individual – level, and biological risk factors that may contribute to preterm birth.
Measures
Below we provide a description and source of each variable included in the current study. This information is also presented in Table 1.
TABLE 1.
Variable descriptions and data source.
| Variable | Description | Data source |
|---|---|---|
| Preterm birth | ||
| Preterm birth | Live births before 37 completed weeks of gestation. | March of Dimes Peristats Database |
| Very preterm birth | Live births before 32 completed weeks of gestation | March of Dimes Peristats Database |
| Late preterm birth | Life births between 34–-36 completed weeks of gestation | March of Dimes Peristats Database |
| Demographic/Psychosocial characteristics | ||
| Race/ethnicity | Percent of Non – Hispanic Black women | US Census Bureau |
| Young maternal age | Percent of births to women 20 years old or under | March of Dimes Peristats Database |
| Advanced maternal age | Percent of births to women 40 years old or older | March of Dimes Peristats Database |
| Poverty | Percent of households with annual incomes <$10K | US Census Bureau |
| Medical/Obstetric characteristics | ||
| Delivery mode | Percent of births delivered by Cesarean section | March of Dimes Peristats Database |
| Obesity | Percent of women of childbearing age* with body mass index 30 or greater | March of Dimes Peristats Database |
| Multiple births | The number of multiple births per 1000 births | March of Dimes Peristats Database |
| Late/no prenatal care | Percent of women receiving prenatal care starting in 3rd trimester, or never receiving prenatal care | March of Dimes Peristats Database |
| Behavioral characteristics | ||
| Smoking | Percent of women of childbearing age* who have ever smoked 100 cigarettes in their lifetime and currently smoke every day or some days | March of Dimes Peristats Database |
| Binge drinking | Percent of women of childbearing age who have 5 or more alcoholic drinks on at least one occasion in the past month | March of Dimes Peristats Database |
| Community/Environmental factors | ||
| High school graduation rate | Percent of public high school freshman who graduated within 4 years of starting 9th grade | US Department of Education |
| Violent crime | Number of violent crimes per capita | Bureau of Justice Statistics |
Note:
childbearing age =18–44 years old.
Preterm Birth
We extracted rates of total preterm, late preterm, moderately preterm, and very preterm birth rates by state from the March of Dimes Peristats database. This database includes birth certificate data from the National Center for Health Statistics. The measure of gestational age of the newborn is based on the obstetric estimate of gestation. Preterm births were defined as live births occurring before 37 completed weeks of gestation. We also examined rates of very preterm births (less than 32 completed weeks), moderately preterm births (between 32 and 36 weeks), and late preterm births (between 34 and 36 completed weeks’ gestation) by state as separate variables.
Poverty
Data on the percent of low-income households were gathered by the American Community Survey through the U.S. Census Bureau. The American Community Survey is an ongoing yearly survey conducted by the U.S. Census Bureau of state-level social, economic, housing, and demographic characteristics of communities with populations greater than 65,000. We examined the percent of households by the state with annual incomes less than $10,000. About 3.5 million housing unit addresses are selected annually across all states including the District of Columbia [30]. In 2014, there were approximately 123 million households in the U.S., thus the ACS conducted surveys of approximately 3% of the U.S. households.
Obesity
State-level obesity information was extracted from the March of Dimes Peristats database. Obesity was derived from the Behavioral Risk Factor Surveillance Survey and the Pregnancy Risk Assessment Monitoring System. Calculations were then performed by the March of Dimes Perinatal Data Center. For the current study, we examined the percent of women of childbearing age (18 – 44 years), who have a body mass index of 30 or more.
Race/Ethnicity
Data on race/ethnicity by the state was gathered by the American Community Survey through the U.S. Census Bureau. We examined the percent of non-Hispanic Black women by the state, given that non-Hispanic Black women in the United States are at significantly greater risk for PTB than women of other race/ethnicities [3].
Delivery mode
We examined the percent of births delivered by Cesarean section by state by extracting this data from the March of Dimes Peristats database. Delivery method information was compiled by the March of Dimes from the U.S. Standard Certificate of Live Birth data. The total Cesarean section rate was calculated as the number of births delivered by Cesarean section divided by the total number of live births multiplied by 100. Cesarean section rates by state are important to consider, with respect to PTB, given that elective C-sections are associated with late preterm deliveries [31].
Smoking
Data were extracted from the March of Dimes Peristats database in order to examine the percent of women of childbearing age (18 – 44 years) in each state who smoke. Smoking information was originally collected by the Behavioral Risk Factor Surveillance Survey [32]. Smokers were defined as persons who have ever smoked 100 cigarettes in a lifetime and currently smoke every day or some days.
Binge drinking
Binge alcohol use among women of childbearing age was recorded by the March of Dimes Perinatal Data Center via the Behavioral Risk Factor Surveillance Survey and includes the percent of women of childbearing age (18 – 44 years) who engage in binge alcohol use. Binge alcohol use is defined as having five or more drinks on at least one occasion during the past month.
Young and old maternal age
Maternal age was extracted from the March of Dimes Peristats database. Ages were calculated by the March of Dimes Perinatal Data Center using the difference between the mother’s and infant’s dates of birth as reported on the birth certificate. The percent of births to women <20 years old and ≥40 years old were examined for this study.
Multiple births
The number of multiple births (twins, triplets, etc.) per 1000 births was examined for this study. Multiple birth calculations are shown as a ratio and are multiplied by 1000. These data were extracted from the March of Dimes Peristats database.
Late or no prenatal care
We examined the percent of pregnancies receiving late/no prenatal care, which is care started in the 3rd trimester (7 – 9 months) or no care received. These calculations were based on the Adequacy of Prenatal Care Utilization Index [33]. Calculations are based on the number of live births to mothers receiving late or no prenatal care divided by all live births excluding those missing data on prenatal care, multiplied by 100. These data were extracted from the March of Dimes Peristats database.
High school graduation rate
We examined the percentage of public high school freshman between the years of 2013 and 2014 who graduated with a regular high school diploma within 4 years of starting 9th grade. State education agencies calculate the graduation rate by identifying a cohort of first – time 9th graders in a school year. The cohort is adjusted by adding and subtracting students who leave or join the cohort. These data are collected annually by the U.S. Department of Education [34].
Violent crime
We examined the number of violent crimes per capita by state in the United States in 2014. Violent crimes were defined as murder, rape, robbery, and aggravated assault. These data are collected annually by the FBI among law enforcement agencies serving jurisdictions of populations of 10,000 or more that volunteered to participate in the Uniform Crime Reporting Program. Data are collected in cooperation with the Bureau of Justice Statistics [35].
Statistical analysis
SPSS version 20.0 was used to perform analyses. State-level preterm birth rate data were not skewed (skewness and kurtosis <0.72) and all state-level data were continuous variables; therefore, we performed Pearson correlations to examine associations between PTB rates and state characteristics. A linear regression analysis was then performed to examine the relative contribution of state-level characteristics on rates of PTB. Independent variables did not display multicollinearity (VIF ranged from 1.37 to 7.37).
Results
Descriptive statistics are presented in Table 2. Preterm birth data corresponds to the year 2014. Average rates of PTB in the U.S. were 11.5% (SD =1.73), range: 8.1 – 16.6%. Average rates of late PTB in the U.S. were 6.9% (SD=.78), range: 5.5 – 9.4%. Average rate of very early PTB in the U.S. was 1.6% (SD=.29), range: 1.1 – 2.4%.
TABLE 2.
Preterm birth rates and demographic characteristics by state.
| Demographic characteristic | Mean (SD) | Range |
|---|---|---|
| Preterm Birth (<37 weeks) % | 11.5% (1.73) | 8.1–16.6% |
| Very Early Preterm Birth (<32 weeks) % | 1.6% (0.29) | 1.1–2.4% |
| Moderate Preterm Birth (32–36 weeks) % | 8.0% (0.89) | 6.5–10.5% |
| Late Preterm Birth (34–36 weeks)% | 6.9% (0.78) | 5.5–9.4% |
| Poverty Rate (%) | 14.0% (3.83) | 7.1–27.6% |
| Obesity Rate (%) | 29.3% (3.8) | 20.2–36.2% |
| Non – Hispanic Black Women (%) | 11.0% (11) | 0.0–49.0% |
| Violent crime (% per capita) | 0.004% (0.002) | 0.001 – 0.012% |
| C – section deliveries (%) | 30.8% (4.02) | 22.3–38.3% |
| Smoking (%) | 19.4% (5.20) | 8.3–32.4% |
| Binge drinking (%) | 16.9% (4.28) | 8.4–29.8% |
| Births to women ages <20 (%) | 7.0% (1.96) | 3.9–11.4% |
| Births to women ages >40 (%) | 2.7% (0.95) | 1.4–5.2% |
| Late/No prenatal care (%) | 5.7% (2.04) | 1.4–10.2% |
Results from correlation analyses are listed in Table 3. State-level PTB rates were positively associated with poverty, obesity, race/ethnicity, C-section rate, smoking, young maternal age, late or no prenatal care, and the percent of violent crimes by state. State-level PTB rates were negatively associated with older maternal age and greater rates of binge drinking. The rate of multiple births (r=−0.17, p=.23) and high school graduation rate (r= −0.18, p=.20), were not associated with PTB. Results did not significantly vary by late, moderate, and very PTB rates by state.
TABLE 3.
Correlations among state – level preterm birth rates and state – level characteristics.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PTB | – | ||||||||||||||
| 2. Very PTB | 0.86** | – | |||||||||||||
| 3. Late PTB | 0.88** | 0.74** | – | ||||||||||||
| 4. Poverty | 0.67** | 0.61** | 0.60** | – | |||||||||||
| 5. Obesity | 0.60** | 0.45** | 0.61** | 0.44** | – | ||||||||||
| 6. Race/ethnicity | 0.71** | 0.87** | 0.49** | 0.42** | 0.37** | – | |||||||||
| 7. C – section | 0.60** | 0.72** | 0.58** | 0.45** | 0.24 | 0.62** | – | ||||||||
| 8. Smoking | 0.30* | 0.18 | 0.38** | 0.45** | 0.59** | −0.02 | 0.15 | – | |||||||
| 9. Young age | 0.70** | 0.54** | 0.71** | 0.74** | 0.76** | 0.31* | 0.34* | 0.55** | – | ||||||
| 10. Advanced age | −0.29* | −0.04 | −0.43** | −0.37** | −0.57** | 0.18 | 0.10 | −0.68** | −0.60** | – | |||||
| 11. Late/no care | 0.51** | 0.39** | 0.35* | 0.24 | 0.40** | 0.38** | 0.15 | 0.06 | 0.54** | −0.15 | – | ||||
| 12. Violent crime | 0.39** | 0.36** | 0.18 | 0.42** | −0.002 | 0.35* | 0.21 | −0.04 | 0.29* | 0.004 | 0.46** | – | |||
| 13. Binge drinking | −0.44** | −0.24 | −0.53** | −0.51** | −0.33* | −0.08 | −0.22 | −0.22 | −0.57** | 0.34* | −0.16 | −0.20 | – | ||
| 14. Multiple births | −0.17 | 0.11 | −0.12 | −0.31* | −0.26 | 0.22 | 0.33* | −0.18 | −0.52** | 0.44** | −0.46** | −0.39** | 0.35* | – | |
| 15. HS graduation | −0.18 | −0.08 | −0.02 | −0.33* | 0.08 | −0.08 | 0.12 | 0.14 | −0.16 | −0.03 | −0.23 | −0.57** | 0.23 | 0.49** | – |
p<.001.
p<.05.
A linear regression model was then performed to examine the relative contribution of state-level characteristics on PTB rates. We entered those variables found to be significantly associated with PTB rates as independent variables in the model. PTB rate was entered as the dependent variable. Results revealed that the percent of non-Hispanic Black women by state remained the only significant predictor of state-level PTB rate (β = 0.45, p<.001). See Table 4 and Figure 2.
TABLE 4.
Linear regression analysis of predictors of state-level preterm birth rates.
| Variables | β | Standard error | p–Value |
|---|---|---|---|
| Poverty rate | <0.01 | 0.04 | 0.98 |
| Obesity rate | 0.04 | 0.05 | 0.70 |
| Non-Hispanic Black women | 0.47 | 10.50 | <0.001 |
| C-section deliveries | 0.07 | 0.04 | 0.48 |
| Smoking | 0.09 | 0.03 | 0.39 |
| Binge drinking | −0.15 | 0.04 | 0.12 |
| Births to women ages <20 | 0.23 | 0.16 | 0.22 |
| Late/no prenatal care | 0.08 | 0.08 | 0.43 |
| Violent crime | 0.08 | 0.90 | 0.38 |
Figure 2.
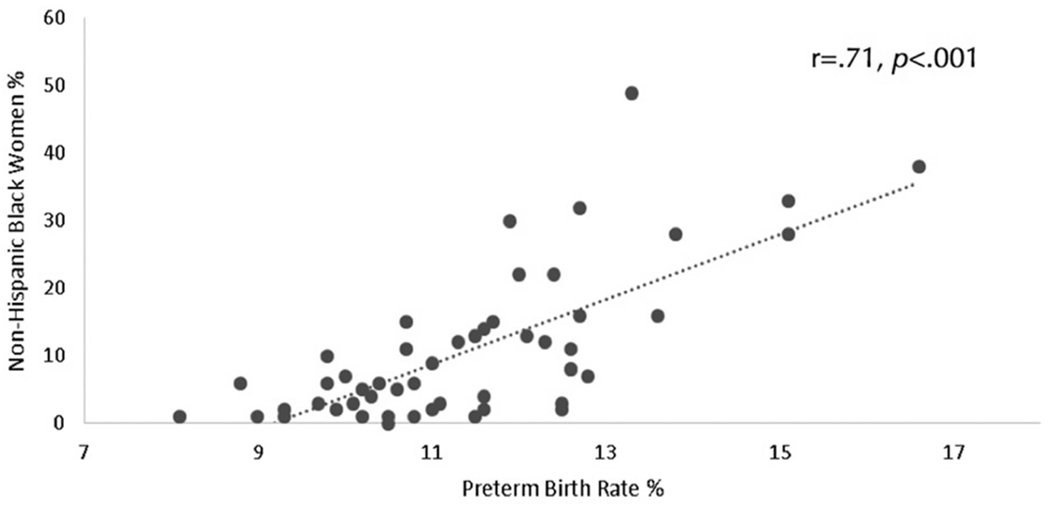
A Pearson correlation was performed to examine the association between the percentage of non – Hispanic Black women by state and state – level preterm birth rate.
Discussion
The goal of this article was to examine state-level characteristics in the United States that predict PTB rates, with the hope that these findings may identify targets for future efforts for public health interventions. We found that state-level poverty, obesity, non-Hispanic Black race/ethnicity, violent crime rates, C-section deliveries, births to women <20 years old, and pregnancies receiving late or no prenatal care were associated with higher state-level rates of PTB. When we examined the relative contribution of each state-level characteristic, we found that race/ethnicity remained the only significant predictor of state-level PTB rates; states with higher percentages of non-Hispanic Black women had higher rates of PTB.
There are several possible explanations for why states with the highest percentage of non-Hispanic Black women also have the highest PTB rates. One explanation is that non-Hispanic Black women are genetically predisposed to deliver preterm. However, this hypothesis has been disputed by intriguing evidence that maternal nativity affects racial disparities in PTB; foreign-born Black women have lower rates of PTB than US-born Black women [36,37]. As well, other westernized countries with a high percentage of citizens of African descent, including France and Italy, have PTB rates that are half of those in the US (6.7 and 6.5%, respectively) [38]. These findings suggest that elevated PTB rates in states with higher percentages of non-Hispanic Black women are unlikely to be driven by genetic factors, as some have proposed [39].
Racial disparities in PTB between states may be driven by other environmental risk factors such as exposure to neighborhood stress, segregation, and discrimination that results in biological “wear and tear” that predisposes Black women to PTB [37]. Indeed, previous studies have reported elevated PTB risk among women who reported racial discrimination in pregnancy [40–43]. The experience of interpersonal and institutional racism, inequality, and discrimination among non-Hispanic Black pregnant women may magnify the impact of additional stressors (e.g. perceived stress, anxiety, and depression), and may diminish women’s ability to cope with stress [44–46].
Another explanation is that non-Hispanic Black women may experience greater obstetric complications and/or receive sub-optimal care for obstetric complications, placing women at increased risk for PTB [47,48]. We were unable to specifically test this hypothesis in the current study. We did include obesity as a proximal measure of pregnancy complications and after accounting for obesity, race/ethnicity remained the only significant predictor of state PTB rate. Future research is needed to determine the degree to which pregnancy complications may explain associations between race/ethnicity and PTB. For example, disparities in obstetric practices and medical management of pregnancy complications may differ by state and may partially account for variability in rates of PTB by maternal race/ethnicity.
We found that states with higher rates of binge drinking had lower rates of PTB. This was surprising and unexpected, given that prior research has reported binge drinking to be associated with increased risk for PTB within individuals [49–51]. However, past research has found that non-Hispanic Black women binge drink significantly less than women of other race/ethnicities [52]. As well, the association between binge drinking and PTB is weaker among non-Hispanic Black women [52]. Therefore, states with a greater number of non-Hispanic Black women may also have less binge drinking (indeed, there was a negative correlation between rate of binge drinking and the percent of non-Hispanic Black women by state, albeit non-significant r=−0.08, p=.56). While binge drinking on an individual level is a well – established risk factor for PTB [49–51], rates of binge drinking by the state may reflect other characteristics of the state that are associated with lower PTB rates, including the racial and ethnic composition of the state.
Results from this study should be interpreted in light of a number of limitations. The sample size for the current analyses was small and thus decreased statistical power in our analyses. Data for this study were extracted from multiple data sources and depended upon the quality of state reporting. For example, a significant limitation included the measure of state-level poverty; the percent of women living below the federal poverty level was calculated based on communities with populations of 65,000 or greater and included only 3% of the US households, thus poverty statistics may not accurately reflect those states with sparsely populated communities. The primary data source, the March of Dimes Peristats Database, was established as a tool for health professionals to make more informed decisions to improve infant health and has not been validated. In addition, when national surveys collect individual self-report measures, such as smoking behaviors, these data would be subject to reporting bias and may have influenced state-level smoking rates. Obesity status was collected categorically, which may have reduced the utility of the data as we were unable to examine obesity ranges as a risk factor for PTB. Variables that were not included in analyses could have served as confounding factors and may have influenced the pattern of results, such as state-level rates of pregnancy complications, rates of assisted reproductive technology, rates of delivery inductions, or elective C-sections, and other factors such as nutrition, exposure to pollutants, and maternal stress. Finally, it is important to highlight the potential for ecological fallacy when interpreting results from this study; results from state-level analysis do not necessarily reflect associations within individuals, and the use of data from states may mask more complicated associations between contextual, environmental, and individual risk factors for PTB
Despite these limitations, results from this study highlight the opportunity for public health interventions that could be developed and delivered to target community-level risk factors in states with the highest rates of PTB. Use of geocoding in future research may further help to identify the highest risk communities that would reap the greatest benefit from public health interventions [53]. Precision public health approaches, which integrate multiple sources of data including genomic, biologic, behavioral, and community characteristics, may allow public health interventions to be delivered to the highest risk populations at the most effective time in the prenatal or preconception periods [54]. There is evidence both in the U.S. and abroad that public health campaigns focused on prenatal care, education, and community outreach have successfully reduced PTB rates [26,27]. As well, a large-scale international randomized trial is currently being conducted that will examine if broad dissemination of low-dose aspirin reduces rates of PTB [56]. If deemed successful and safe, communities in the U.S. with the highest PTB rates could consider adopting prenatal low-dose aspirin administration at a community level.
Findings from this study indicate that disparities in PTB rates between states may be explained by differences in the percentage of non-Hispanic Black women by state. This finding raises questions as to the origin of PTBs and may suggest that community and environmental factors, such as segregation, discrimination, and neighborhood disadvantage are contributing to higher rates of PTB in some regions of the United States. These same risk factors may also help to explain differences in PTB rates between the U.S. and other developed countries [56]. Future studies are needed to understand why specific communities in the U.S. are at higher risk for PTB than others and to develop precision public health interventions that can be utilized to eliminate disparities in PTB rates and to stop the increasing number of PTBs to women in the United States.
Acknowledgements
The authors wish to thank Beth Hott for her help in preparing this manuscript.
Funding
Dr. Bourjeily is funded by NHLBI R01HL – 130702 and NICHD R01HD –078515.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. [DOI] [PubMed] [Google Scholar]
- [2].Institute of Medicine (US). Committee on understanding premature birth and assuring healthy outcomes. Chapter 10. Mortality and acute complications in preterm infants. In: Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington DC: National Academies Press (US); 2007. Available from: https://wwwncbinlmnihgov/books/NBK11385/. [PubMed] [Google Scholar]
- [3].March of Dimes Perinatal Data Center. Premature Birth Report Card; 2016. [cited 2017 June 1]. Available from: https://www.marchofdimes.org/materials/premature–birth–report–card–united–states.pdf.
- [4].March of Dimes. PMNCH, Save the children. Born too soon: The global action report on preterm birth. In: Howson CP, Kinney MV, Lawn JE, editors. Geneva: World Health Organization; 2012. [Google Scholar]
- [5].Koullali B, Oudijk MA, Nijman TA, et al. Risk assessment and management to prevent preterm birth. Semin Fetal Neonatal Med. 2016;21:80–88. [DOI] [PubMed] [Google Scholar]
- [6].Henderson JJ, McWilliam OA, Newnham JP, et al. Preterm birth aetiology 2004–-2008. Maternal factors associated with three phenotypes: spontaneous preterm labour, preterm pre – labour rupture of membranes and medically indicated preterm birth. J Matern Fetal Neonatal Med. 2012;25:642–647. [DOI] [PubMed] [Google Scholar]
- [7].Barros FC, Papageorghiou AT, Victora CG, et al. The distribution of clinical phenotypes of preterm birth syndrome: implications for prevention. JAMA Pediatr. 2015;169:220. [DOI] [PubMed] [Google Scholar]
- [8].Esplin MS, Manuck TA, Varner MW, et al. Cluster analysis of spontaneous preterm birth phenotypes identifies potential associations among preterm birth mechanisms. Am J Obstet Gynecol. 2015;213:e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Frey HA, Stout MJ, Pearson LN, et al. Genetic variation associated with preterm birth in African – American women. Am J Obstet Gynecol. 2016;215:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Strauss JF 3rd, Romero R, Gomez – Lopez N, et al. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstet Gynecol. 2018;218:294–314 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huusko JM, Karjalainen MK, Graham BE, et al. Whole exome sequencing reveals HSPA1L as a genetic risk factor for spontaneous preterm birth. PLoS Genet. 2018;14:e1007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mazaki – Tovi S, Romero R, Kusanovic JP, et al. Recurrent preterm birth. Semin Perinatol. 2007;31:142–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Heath VC, Southall TR, Souka AP, et al. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–317. [DOI] [PubMed] [Google Scholar]
- [14].Petrini JR, Callaghan WM, Klebanoff M, et al. Estimated effect of 17 alpha – hydroxyprogesterone caproate on preterm birth in the United States. Obstet Gynecol. 2005;105:267–272. [DOI] [PubMed] [Google Scholar]
- [15].Ncube CN, Enquobahrie DA, Albert SM, et al. Association of neighborhood context with offspring risk of preterm birth and low birthweight: a systematic review and meta – analysis of population – based studies. Soc Sci Med. 2016;153:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Anthopolos R, Kaufman JS, Messer LC, et al. Racial residential segregation and preterm birth: built environment as a mediator. Epidemiology. 2014;25:397–405. [DOI] [PubMed] [Google Scholar]
- [17].Masho SW, Cha S, Chapman DA, et al. Understanding the role of violence as a social determinant of preterm birth. Am J Obstet Gynecol. 2017;216:e1–83. e7. [DOI] [PubMed] [Google Scholar]
- [18].Auger N, Gamache P, Adam – Smith J, et al. Relative and absolute disparities in preterm birth related to neighborhood education. Ann Epidemiol. 2011;21:481–488. [DOI] [PubMed] [Google Scholar]
- [19].Woods N, Gilliland J, Seabrook JA. The influence of the built environment on adverse birth outcomes. J Neonatal Perinatal Med. 2017;10:233–248. [DOI] [PubMed] [Google Scholar]
- [20].Stieb DM, Chen L, Eshoul M, et al. Ambient air pollution, birth weight and preterm birth: a systematic review and meta – analysis. Environ Res. 2012;117:100–111. [DOI] [PubMed] [Google Scholar]
- [21].Nkansah – Amankra S. Neighborhood contextual factors, maternal smoking, and birth outcomes: multi-level analysis of the South Carolina PRAMS survey, 2000–-2003. J Womens Health (Larchmt). 2010;19:1543–1552. [DOI] [PubMed] [Google Scholar]
- [22].Cha S, Masho SW. Preterm birth and stressful life events. Preterm Birth Offer Erez, IntechOpen. 2013;2018:41–81. [Google Scholar]
- [23].O’Campo P, Xue X, Wang MC, et al. Neighborhood risk factors for low birthweight in Baltimore: a multilevel analysis. Am J Public Health. 1997;87:1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ferre CD, Jones L, Norris KC, et al. The Healthy African American Families (HAAF) project: from community – based participatory research to community – partnered participatory research. Ethn Dis. 2010;20:S2–1-S8. [PMC free article] [PubMed] [Google Scholar]
- [25].Jones L, Wright K, Wright A, et al. The Healthy African American Families’ risk communications initiative: using community partnered participatory research to address preterm birth at the local level. Ethn Dis. 2010;20:S2–30–S5. [PubMed] [Google Scholar]
- [26].McCabe ER, Carrino GE, Russell RB, et al. Fighting for the next generation: US Prematurity in 2030. Pediatrics. 2014;134:1193–1199. [DOI] [PubMed] [Google Scholar]
- [27].Newnham JP, White SW, Meharry S, et al. Reducing preterm birth by a statewide multifaceted program: an implementation study. Am J Obstet Gynecol. 2017;216:434–442. [DOI] [PubMed] [Google Scholar]
- [28].Hoyme UB. Efficient prevention of preterm birth as a primary political task – results of the Thuringia campaign 2017. Arch Gynecol Obstet. 2018;298:461–463. [DOI] [PubMed] [Google Scholar]
- [29].U.S. Census Bureau. History: decennial census. [cited 2018 Oct 22]. Available from: https://www.census.gov/history/www/programs/demographic/decennial_census.html.
- [30].U.S. Census Bureau –- Population Division. Annual estimates of the resident population by sex, race, and Hispanic origin for the United States, States, and Counties: April 1, 2010 to July 1, 2014, Release Date: June 2015. [cited 2017 Sept 27]. Available from: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk
- [31].Fuchs K, Wapner R. Elective cesarean section and induction and their impact on late preterm births. Clin Perinatol. 2006;33:793–801. [DOI] [PubMed] [Google Scholar]
- [32].Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion|Division of Population Health. Behavioral risk factor surveillance system (BRFSS). Available from: https://www.cdc.gov/brfss/.
- [33].Kotelchuck M. The adequacy of prenatal care utilization index: its US distribution and association with low birthweight. Am J Public Health. 1994;84:1486–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].U.S. Department of Education. Consolidated state performance reports. [cited 2018 July 26]. Available from: https://www2.ed.gov/admins/lead/account/consolidated/index.html – sy13–14 (vol 2018). [Google Scholar]
- [35].Federal Bureau of Investigation: Uniform Crime Reporting. Crime in the U.S. 2014: U.S. Department of Justice –-Criminal Justice Information Services Division. [cited 2018 July 26]. Available from: https://ucr.fbi.gov/crime–in–the–u.s/2014/crime–in–the–u.s.–2014 (vol 2018). [Google Scholar]
- [36].Howard DL, Marshall SS, Kaufman JS, et al. Variations in low birth weight and preterm delivery among blacks in relation to ancestry and nativity: New York City, 1998–-2002. Pediatrics. 2006;118:e1399–e1405. [DOI] [PubMed] [Google Scholar]
- [37].Culhane JF, Goldenberg RL. Racial disparities in preterm birth. Semin Perinatol. 2011;35:234–239. [DOI] [PubMed] [Google Scholar]
- [38].March of Dimes. The partnership for maternal newborn and child health, save the children, World Health Organization. Born too soon. The global action report on preterm birth, [cited 2017 Sep 27]. Available from: http://www.marchofdimes.org/mission/global–preterm.aspx.
- [39].Dolan SM. Genetic and environmental contributions to racial disparities in preterm birth. Mt Sinai J Med. 2010;77:160–165. [DOI] [PubMed] [Google Scholar]
- [40].Dole N, Savitz DA, Hertz – Picciotto I, et al. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. [DOI] [PubMed] [Google Scholar]
- [41].Mendez DD, Hogan VK, Culhane JF. Institutional racism, neighborhood factors, stress, and preterm birth. Ethn Health. 2014;19:479–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mustillo S, Krieger N, Gunderson EP, et al. Self – reported experiences of racial discrimination and Black – White differences in preterm and low – birthweight deliveries: the CARDIA Study. Am J Public Health. 2004;94:2125–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rosenberg L, Palmer JR, Wise LA, et al. Perceptions of racial discrimination and the risk of preterm birth. Epidemiology. 2002;13:646–652. [DOI] [PubMed] [Google Scholar]
- [44].Berg CJ, Wilcox LS, d’Almada PJ. The prevalence of socioeconomic and behavioral characteristics and their impact on very low birth weight in black and white infants in Georgia. Matern Child Health J. 2001;5:75–84. [DOI] [PubMed] [Google Scholar]
- [45].Clark R, Anderson NB, Clark VR, et al. Racism as a stressor for African Americans. A biopsychosocial model. Am Psychol. 1999;54:805–816. [DOI] [PubMed] [Google Scholar]
- [46].Finch BK, Frank R, Hummer RA. Racial/ethnic disparities in infant mortality: the role of behavioral factors. Soc Biol. 2000;47:244–263. [DOI] [PubMed] [Google Scholar]
- [47].Samadi AR, Mayberry RM, Zaidi AA, et al. Maternal hypertension and associated pregnancy complications among African – American and other women in the United States. Obstet Gynecol. 1996;87:557–563. [DOI] [PubMed] [Google Scholar]
- [48].James AH, Jamison MG, Brancazio LR, et al. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311–1315. [DOI] [PubMed] [Google Scholar]
- [49].Carr JL, Agnihotri S, Keightley M. Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcohol. Clin Exp Res 2010;34:1022–1032. [DOI] [PubMed] [Google Scholar]
- [50].Sood B, Delaney – Black V, Covington C, et al. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. dose – response effect. Pediatrics. 2001;108:E34. [DOI] [PubMed] [Google Scholar]
- [51].McDonald AD, Armstrong BG, Sloan M. Cigarette, alcohol, and coffee consumption and prematurity. Am J Public Health. 1992;82:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Truong KD, Reifsnider OS, Mayorga ME, et al. Estimated number of preterm births and low birth weight children born in the United States due to maternal binge drinking. Matern Child Health J. 2013;17:677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].MacQuillan EL, Curtis AB, Baker KM, et al. Using GIS mapping to target public health interventions: examining birth outcomes across GIS techniques. J Community Health. 2017;42:633–638. [DOI] [PubMed] [Google Scholar]
- [54].Newnham JP, Kemp MW, White SW, et al. Applying precision public health to prevent preterm birth. Front Public Health. 2017;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hoffman MK, Goudar SS, Kodkany BS, et al. A description of the methods of the aspirin supplementation for pregnancy indicated risk reduction in nulliparas (ASPIRIN) study. BMC Pregnancy Childbirth. 2017;17:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Garn JV, Nagulesapillai T, Metcalfe A, et al. International comparison of common risk factors of preterm birth between the U.S. and Canada, using PRAMS and MES (2005–2006). Matern Child Health J. 2015;19:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]


