Abstract
Background
Engaging in leisure activities was suggested to protect older adults from dementia. However, the association between playing a musical instrument and the risk of dementia is not well-established. This study aimed to investigate this association in older adults using a systematic review and meta-analysis of prospective cohort studies.
Methods
Pooled hazard ratio (HR) and 95% confidence interval (CI) of having dementia for older adults playing a musical instrument were calculated using the random-effects model. We performed the I2 statistic to detect heterogeneity across studies and the test for funnel plot asymmetry to assess publication bias. The risk of bias assessment was conducted using the modified Newcastle–Ottawa Scale.
Results
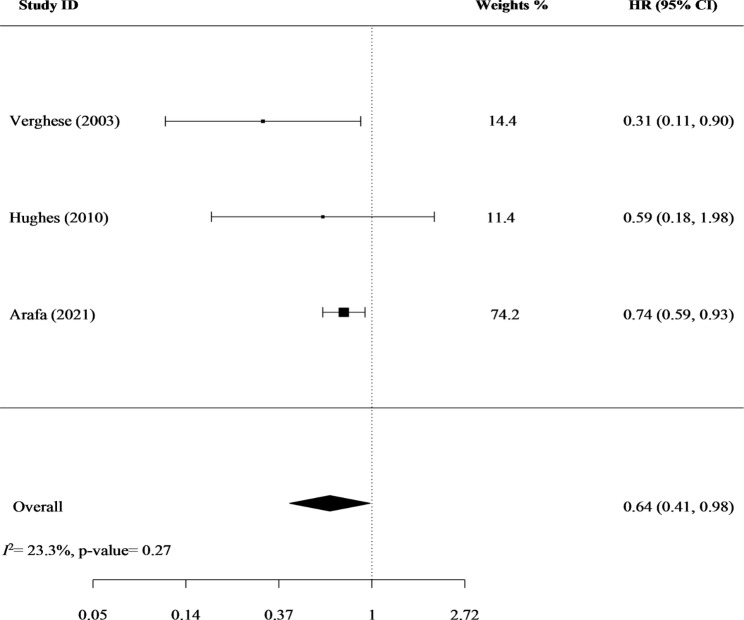
A total of three prospective cohort studies were found eligible: two from the U.S. and one from Japan. Playing a musical instrument, in the meta-analysis, was significantly associated with a decreased risk of dementia (HR = 0.64; 95% CI: 0.41, 0.98) among older adults. No signs of significant heterogeneity across studies (I2 = 23.3% and p-heterogeneity = 0.27) or publication bias (z= -1.3 and p-publication bias = 0.18) were identified.
Conclusion
Playing a musical instrument was associated with a decreased risk of dementia among older adults. Older adults should be encouraged to engage in leisure activities, especially playing musical instruments.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-022-02902-z.
Keywords: Music, Dementia, Older adults, Systematic review, meta-analysis
Introduction
The incidence and prevalence of dementia have been increasing worldwide, especially in the aging societies in North America, Europe, and South-East Asia [1]. It is estimated that the global number of dementia patients will increase from 57.4 million in 2019 to 152.8 million in 2050 [2]. In addition to the physical and psychological pressures affecting patients, families, and healthcare providers, dementia has enormous financial burdens in terms of formal and informal medical and social care costs [3].
However, dementia is not inevitable, and some lifestyle risk factors such as avoiding smoking, refraining from excessive alcohol drinking, and practicing physical activity could reduce its risk [4, 5]. Among these lifestyle factors, leisure activities can increase cognitive reserve, enhance psychological well-being, and improve social relationships; therefore, they were suggested to protect from dementia and delay cognitive decline [6–18].
Playing a musical instrument is a common leisure activity that carries several favorable cognitive effects and improves mental health [19, 20]. However, the relationship between playing a musical instrument and the risk of dementia is not well-established. A few studies investigated the potential effects of musical activities on dementia; however, they were limited by the small number of dementia cases that did not allow for a conclusive association to be drawn and the cross-sectional design of some studies that did not allow for a temporal association to be detected [9–13]. A previous meta-analysis of two studies investigated the same association. However, it included a few dementia cases, and later research was published [21]. We, therefore, conducted an updated systematic review of prospective cohort studies investigating the association between playing a musical instrument and dementia risk before combing the results of eligible studies in a meta-analysis.
Methods
Literature search and study selection
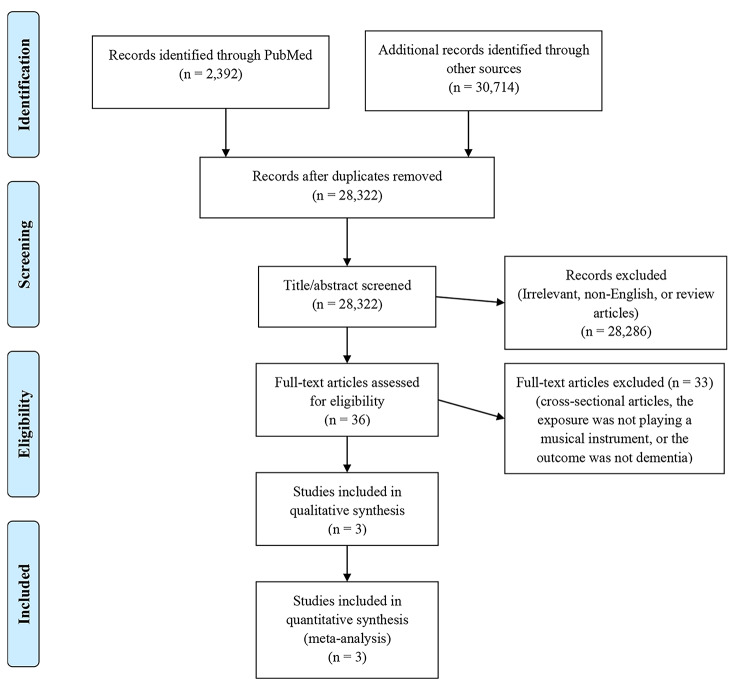
First, we searched PubMed, Scopus, and Google Scholar for potential studies published in English before the 28th of February 2022 using the following terms: (musical instrument OR leisure activities) AND (dementia). A complete search strategy of PubMed was provided (Supplementary file 1). We also manually searched the reference lists of retrieved articles to obtain additional studies. We did not apply limitations regarding the year of publication, but no efforts were made to access unpublished data. We obtained 2,392 studies from PubMed, 2,614 from Scopus, and 28,100 from Google Scholar and the manual search of reference sections. After removing duplicates, we screened the titles and abstracts of 28,322 studies before removing non-English, irrelevant, and review articles, leaving 36 articles for full-text assessment (Fig. 1). Our eligibility criteria included: (1) the exposure was playing a musical instrument, (2) the outcome was dementia, and (3) the study had a prospective cohort design. Of note, our eligibility criteria, which were decided before conducting the systematic review, did not specifically define playing a musical instrument or whether studies investigating specific musical instruments would be only considered. We reported this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist [22]. The following relevant information was extracted from the included studies by the first two authors: study name, publication year, place of study, characteristics of participants, assessment approaches of dementia and playing a musical instrument, follow-up years, number of dementia incident cases, and covariates included in the most adjusted regression models.
Fig. 1.
PRISMA flowchart of the selected articles to be included in the meta-analysis
Later, we conducted another meta-analysis after including studies with cross-sectional design to investigate whether combining the results of cross-sectional studies with those from cohort studies would be different from confining the analysis to cohort studies only.
Statistical analysis
We extracted the hazard ratios (HRs) with 95% confidence intervals (CIs) of dementia for playing a musical instrument in the most adjusted regression models before calculating the pooled HR (95% CI) of the three studies using the random-effects model [23]. We also conducted the I2 statistic to detect heterogeneity across studies [24] and the test for funnel plot asymmetry to assess publication bias [25]. The risk of bias was investigated using the modified Newcastle–Ottawa Scale in terms of studies’ selection, comparability, and outcome [26]. The first two authors assessed the risk of bias, with differences resolved by discussion. The R-3.2.0 statistical package (Metafor: Meta-Analysis Package for R) was used for analysis [27].
Results
A shortlist of five studies was obtained [9–13]. We further excluded two cross-sectional studies [12, 13] and kept three prospective cohort studies for the main meta-analysis [9–11] (Fig. 1). The included studies used data from the Bronx Aging Study [9], the Monongahela Valley Independent Elders Survey (MoVIES) project [10], and the Japan Gerontological Evaluation Study (JAGES) [11]. Total participants, country, minimum age, percentage of women, follow-up period, and incidence of dementia in the included studies were as follows: Bronx: 469 participants, the U.S., 75 years, 64.0%, 5.1 years (median), and 26.4%, MoVIES: 942 participants, the U.S., 65 years, 66.5%, 6.1 years (mean), and 11.8%, and JAGES: 52,601 participants, Japan, 65 years, 53.9%, 5.8 years (median), and 11.0%. Methods of dementia diagnosis differed across the studies: Bronx: The Diagnostic and Statistical Manual of Mental Disorders, third edition or, after 1986, the revised third edition [28, 29], MoVIES: score ≥ one based on the Clinical Dementia Rating [30], and JAGES: level ≥ II according to the Standardized Dementia Scale of the Long-term Care Insurance System [31, 32]. Playing a musical instrument was assessed using an interview in the Bronx study (frequent versus rare) and a self-administered questionnaire in the MoVIES and JAGES studies (yes versus no). However, none of the included studies clarified the type of musical instrument or the duration of musical practice (Table 1).
Table 1.
Summary of the studies included in the meta-analysis
| Study ID | Population | Playing a musical instrument | Follow-up | Dementia | Adjusted variables |
|---|---|---|---|---|---|
| The Bronx Aging Study [9] |
469 participants residing in one community in the US Age: 75–85 years Women: 64.0% |
Interview (frequent or rare) Music players: 3.5% |
5.1 years (median) |
Incidence: 26.4% Diagnosed by the Diagnostic and Statistical Manual of Mental Disorders and the revised edition |
Age, sex, educational level, medical illnesses, the Blessed Information–Memory–Concentration test, and participation in other leisure activities |
| MoVIES project [10] |
942 participants from one rural area in the US Age: ≥65 years Women: 66.5% |
Self-administered questionnaire (yes or no) Music players: 5.0% |
6.1 years (mean) |
Incidence: 11.8% Diagnosed by score ≥ 1 based on the Clinical Dementia Rating |
Age, sex, education, depressive symptoms, physical exercise, functional impairment, self-reported health, medication use, and the recruitment status |
| JAGES [11] |
52,601 participants representing 31 municipalities in 12 Japanese prefectures Age: ≥65 years Women: 53.9% |
Self-administered questionnaire (yes or no musical activities at all) Music players: 2.5% |
5.8 years (median) |
Incidence: 11.0% Diagnosed by level ≥ II according to the Standardized Dementia Scale of the Long-term Care Insurance System |
Age, sex, area, daily walking, mutual assistance, smoking, alcohol intake, marriage, education, annual income, engaging in other leisure cognitive activities, daily activities and meeting friends, body mass index, diabetes, hypertension, hyperlipidemia, stroke, hearing loss, and depression |
In the main meta-analysis that included the three cohort studies, playing a musical instrument was significantly associated with a decreased risk of dementia: pooled HR (95% CI) = 0.64 (0.41, 0.98), and the heterogeneity across studies was minimal (I2 = 23.3% and p-heterogeneity = 0.272) (Fig. 2). Also, no signs of publication bias were identified (z= -1.30 and p-publication bias = 0.182) (Supplementary file 2). Except for the short follow-ups, the included studies carried no major risks of bias (Supplementary file 3).
Fig. 2.
Playing a musical instrument and the risk of dementia
The two cross-sectional studies that were eliminated from the main meta-analysis investigated older adults ≥ 65 years from the Swedish HARMONY Co-twin Study (27 pairs where at least one twin was a musician) and the Japanese National Center for Geriatrics and Gerontology Study for Geriatric Syndromes Study (9,380 community-dwelling participants) [12, 13] (Supplementary file 4). The results did not change after pooling the odds ratios of the two cross-sectional studies [12, 13] with the HRs of the three prospective cohort studies [9–11] in one meta-analysis: Risk estimate (95% CI) = 0.68 (0.50, 0.92), I2 = 34.8%, and p-heterogeneity = 0.189 (Supplementary file 5).
Discussion
This study provided epidemiological evidence from prospective cohort studies indicating an inverse association between playing a musical instrument and the risk of dementia among older adults. No signs of significant heterogeneity across studies or publication bias were detected, and the results did not change after including cross-sectional studies. Our findings came in line with a previous meta-analysis that suggested a protective effect of playing a musical instrument against dementia [21]. Yet, the previous meta-analysis included fewer dementia cases and did not consider pooling the results of prospective cohort studies with those from cross-sectional studies.
The biological explanation of the inverse association between playing a musical instrument and the risk of dementia is not well-understood. However, it could be attributed to the role of music in enriching cognitive reserve, enhancing executive functioning and working memory, stimulating brain plasticity to restore sensorimotor brain networks, reducing stress and depression, and developing social cohesion [33–40]. Cognitive reserve indicates individual differences in susceptibility to dementia-related brain changes. Older people with rich cognitive reserve, attained from leisure activities, can tolerate these changes and maintain cognitive functions [41]. Playing music can also reduce stress [37]. A previous meta-analysis showed that higher perceived stress, stressful events, and neuroticism were significantly associated with a higher risk of dementia [42]. Further, learning music and playing musical instruments in groups can be a chance for more social activities and widen the social network size. A 20-year prospective cohort study showed that engagement in social pursuits was related to delayed cognitive decline [43].
Engaging in other musical activities, such as karaoke, a type of interactive singing with music accompaniment and synchronized lyrics displayed on a video screen, was associated with a reduced risk of dementia in the JAGES [11]. Interestingly, in addition to preventing dementia, a recent systematic review and meta-analysis including 816 dementia patients from eight trials showed that music could be a novel therapy for treating dementia via improving cognitive functions, reducing depression and stress, and improving quality of life [44].
Our study included some limitations that should be considered. First, the outcome in the included studies was all-cause dementia. Risk factors for dementia could differ by dementia type [45]. Second, data on playing a musical instrument was self-reported suggesting reporting bias. However, one study concluded that the risk of cognitive impairment for self-reported playing a musical instrument was similar to the informant-reported risk; therefore, the possibility of reporting bias is not high [12]. Third, the duration of music playing was not collected; thus, a dose-response association could not be assessed. Fourth, the type of musical instrument and genre of practiced music were not assessed. Fifth, the short follow-up period might indicate a possibility of reverse causality. However, the JAGES results remained consistent after censoring the first three years of follow-up [11]. Sixth, we could not stratify our results by sex because only the JAGES calculated the sex-specified risk [11]. This point is specifically important because the protective effect of playing a musical instrument in the JAGES was more obvious among women than men [11]. Seventh, dementia is a complex disease with multiple intersecting genetic, clinical, and sociodemographic etiologies [46]; therefore, residual confounding cannot be excluded given the observational design of the included studies. Eighth, the meta-analysis was limited by the small number of included studies.
Conclusion
In this meta-analysis, playing a musical instrument was associated with a reduced risk of dementia. Our findings support the evidence suggesting that engaging in cognitive leisure activities can reduce the risk of dementia. From a preventive perspective, older adults should be encouraged to engage in musical activities and other cognitive leisure activities. Given that leisure activities, including playing musical instruments, are modifiable lifestyle factors, the findings of our study highlight the importance of targeting leisure activities in risk prevention interventions and health education. Still, more prospective studies that consider the duration of playing music and the type of musical instruments are needed. Further studies to elaborate on the biological explanations of this association are warranted as well.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
List of abbreviations
- CI
Confidence Interval
- HR
Hazard Ratio
- JAGES
Japan Gerontological Evaluation Study
- MoVIES
Monongahela Valley Independent Elders Survey
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
Authors’ contributions
Concept (AA and YK), data collection and formal analysis (AA and YK), resources (YK), supervision (YK), manuscript writing (AA), and revision and editing (MT, SM, YS, SN, QG, HK, RK, CM, and YK). All authors read and approved the final manuscript
Funding
This study was supported by the Intramural Research Fund (20-4-9) for the cardiovascular diseases of the National Cerebral and Cardiovascular Center, the JST Grant Number JPMJPF2018, the Japan Agency for Medical Research and Development (dk0207025), and the Meiji Yasuda Research Institute and Life Insurance Company.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethical considerations
Not applicable.
Consent for publication
Not applicable.
Competing interests
None to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2016 Dementia Collaborators Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Dementia Forecasting Collaborators Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e25. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantarero-Prieto D, Leon PL, Blazquez-Fernandez C, Juan PS, Cobo CS. The economic cost of dementia: A systematic review. Dement (London) 2020;19(8):2637–57. doi: 10.1177/1471301219837776. [DOI] [PubMed] [Google Scholar]
- 4.Korczyn AD. Is dementia preventable? Dialogues Clin Neurosci. 2009;11(2):213–6. doi: 10.31887/DCNS.2009.11.2/adkorczyn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbaraly TN, Portet F, Fustinoni S, Dartigues JF, Artero S, Rouaud O, et al. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73(11):854–61. doi: 10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- 7.Sörman DE, Sundström A, Rönnlund M, Adolfsson R, Nilsson LG. Leisure activity in old age and risk of dementia: a 15-year prospective study. J Gerontol B Psychol Sci Soc Sci. 2014;69(4):493–501. doi: 10.1093/geronb/gbt056. [DOI] [PubMed] [Google Scholar]
- 8.Sommerlad A, Sabia S, Livingston G, Kivimäki M, Lewis G, Singh-Manoux A. Leisure activity participation and risk of dementia: An 18-year follow-up of the Whitehall II Study. Neurology. 2020;95(20):e2803–e15. doi: 10.1212/WNL.0000000000010966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–16. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 10.Hughes TF, Chang CC, Vander Bilt J, Ganguli M. Engagement in reading and hobbies and risk of incident dementia: the MoVIES project. Am J Alzheimers Dis Other Demen. 2010;25(5):432–8. doi: 10.1177/1533317510368399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arafa A, Eshak ES, Shirai K, Iso H, Kondo K. Engaging in musical activities and the risk of dementia in older adults: A longitudinal study from the Japan gerontological evaluation study. Geriatr Gerontol Int. 2021;21(6):451–7. doi: 10.1111/ggi.14152. [DOI] [PubMed] [Google Scholar]
- 12.Balbag MA, Pedersen NL, Gatz M. Playing a musical instrument as a protective factor against dementia and cognitive impairment: a population-based twin study. Int J Alzheimers Dis. 2014;2014:836748. doi: 10.1155/2014/836748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MJ, Tsutsumimoto K, Doi T, Nakakubo S, Kurita S, Makizako H, et al. Relationships between cognitive leisure activities and cognitive function in older adults with depressive symptoms: a cross-sectional study. BMJ Open. 2020;10(2):e032679. doi: 10.1136/bmjopen-2019-032679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–12. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall C, Lipton R, Sliwinski M, Katz M, Derby C, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–61. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez F, Zheng L, Chui H. Aging brain: vasculature, ischemia, and behavior study. Psychometric characteristics of cognitive reserve: how high education might improve certain cognitive abilities in aging. Dement Geriatr Cogn Disord. 2019;47:335–44. doi: 10.1159/000501150. [DOI] [PubMed] [Google Scholar]
- 17.Lee HY, Yu CP, Wu CD, Pan WC. The effect of leisure activity diversity and exercise time on the prevention of depression in the middle-aged and elderly residents of Taiwan. Int J Environ Res Public Health. 2018;15(4):654. doi: 10.3390/ijerph15040654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang PJ, Wray L, Lin Y. Social relationships, leisure activity, and health in older adults. Health Psychol. 2014;33(6):516–23. doi: 10.1037/hea0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dingle GA, Sharman LS, Bauer Z, Beckman E, Broughton M, Bunzli E, et al. How do music activities affect health and well-being? a scoping review of studies examining psychosocial mechanisms. Front Psychol. 2021;12:713818. doi: 10.3389/fpsyg.2021.713818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesseldijk LW, Ullén F, Mosing MA. The effects of playing music on mental health outcomes. Sci Rep. 2019;9(1):12606. doi: 10.1038/s41598-019-49099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh S, Causer R, Brayne C. Does playing a musical instrument reduce the incidence of cognitive impairment and dementia? A systematic review and meta-analysis. Aging Ment Health. 2021;25(4):593–601. doi: 10.1080/13607863.2019.1699019. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available from: www.ohri.ca/programs/clinical_epidemiology\oxford.htm. Accessed on 10 March 2022.
- 27.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36:1–48. [Google Scholar]
- 28.Diagnostic . statistical manual of mental disorders. 3. Washington, D.C.: DSM-III; 1980. [Google Scholar]
- 29.Diagnostic . statistical manual of mental disorders. 3. Washington, D.C.: DSM-III-R.; 1987. [Google Scholar]
- 30.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 31.Tamiya N, Noguchi H, Nishi A, Reich MR, Ikegami N, Hashimoto H, et al. Population ageing and wellbeing: lessons from Japan’s long-term care insurance policy. Lancet. 2011;378(9797):1183–92. doi: 10.1016/S0140-6736(11)61176-8. [DOI] [PubMed] [Google Scholar]
- 32.Arafa A, Eshak ES, Shirai K, Cadar D, Iso H, Tsuji T, et al. Impact of various intensities and frequencies of non-occupational physical activity on the risk of dementia among physically independent older adults: the Japan Gerontological Evaluation Study. Public Health. 2021;196:204–10. doi: 10.1016/j.puhe.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Altenmüller E, Schlaug G. Apollo’s gift: new aspects of neurologic music therapy. Prog Brain Res. 2015;217:237–52. doi: 10.1016/bs.pbr.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Román-Caballero R, Arnedo M, Triviño M, Lupiáñez J. Musical practice as an enhancer of cognitive function in healthy aging - a systematic review and meta-analysis. PLoS ONE. 2018;13:e0207957. doi: 10.1371/journal.pone.0207957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacRitchie J, Breaden M, Milne A, McIntyre S. Cognitive, motor and social factors of music instrument training programs for older adults’ improved wellbeing. Front Psychol. 2020;10:2868. doi: 10.3389/fpsyg.2019.02868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bugos JA, Perlstein WM, McCrae CS, Brophy TS, Bedenbaugh PH. Individualized piano instruction enhances executive functioning and working memory in older adults. Aging Ment Health. 2007;11(4):464–71. doi: 10.1080/13607860601086504. [DOI] [PubMed] [Google Scholar]
- 37.Bugos JA, Kochar S, Maxfield N. Intense piano training on self-efficacy and physiological stress in aging. Psychol Music. 2016;44(4):611–24. doi: 10.1177/0305735615577250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bottiroli S, Rosi A, Russo R, Vecchi T, Cavallini E. The cognitive effects of listening to background music on older adults: processing speed improves with upbeat music, while memory seems to benefit from both upbeat and downbeat music. Front Aging Neurosci. 2014;6:284. doi: 10.3389/fnagi.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miendlarzewska EA, Trost WJ. How musical training affects cognitive development: rhythm, reward and other modulating variables. Front Neurosci. 2014;7:279. doi: 10.3389/fnins.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz Abrahan V, Shifres F, Justel N. Cognitive benefits from a musical activity in older adults. Front Psychol. 2019;10:652. doi: 10.3389/fpsyg.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–12. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franks KH, Bransby L, Saling MM, Pase MP. Association of stress with risk of dementia and mild cognitive impairment: a systematic review and meta-analysis. J Alzheimers Dis. 2021;82(4):1573–90. doi: 10.3233/JAD-210094. [DOI] [PubMed] [Google Scholar]
- 43.Marioni RE, Proust-Lima C, Amieva H, Brayne C, Matthews FE, Dartigues JF, et al. Social activity, cognitive decline and dementia risk: a 20-year prospective cohort study. BMC Public Health. 2015;15:1089. doi: 10.1186/s12889-015-2426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno-Morales C, Calero R, Moreno-Morales P, Pintado C. Music therapy in the treatment of dementia: a systematic review and meta-analysis. Front Med (Lausanne) 2020;7:160. doi: 10.3389/fmed.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen JH, Lin KP, Chen YC. Risk factors for dementia. J Formos Med Assoc. 2009;108(10):754–64. doi: 10.1016/S0929-6646(09)60402-2. [DOI] [PubMed] [Google Scholar]
- 46.Fisher TJ, Schwartz AC, Greenspan HN, Heinrich TW. Dementia: a complex disease with multiple etiologies and multiple treatments. Int J Psychiatry Med. 2016;51(2):171–81. doi: 10.1177/0091217416636579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.