Abstract
The genus Cajanus (Family: Fabaceae) consists of approximately 37 species, and Cajanus cajan (C. cajan) is a significant member of the genus. It is a commercial legume crop widely grown in sub-tropical and semi-arid tropical areas of the world. C. cajan is well known for its folk medicinal uses to treat various disorders, such as toothache, dizziness, diabetes, stomachache, female ailments and chronic infections. These properties have been linked to the presence of several value-added nutritional and bioactive components. Different solvent extracts from C. cajan (leaves, root, stem and seeds) have been evaluated for their phytochemical and biological activities, namely antioxidant, antimicrobial, antidiabetic, neuroprotective, and anti-inflammatory effects. Taken together, and considering the prominent nutraceutical and therapeutic properties of C. cajan, this review article focuses on the important details including ethnomedicinal uses, chemical composition, biological applications and some other medicinal aspects related to C. cajan nutraceutical and pharmacological applications.
Keywords: legumes, Cajanus cajan, bioactive compounds, nutraceuticals, bioactive effects
1. Introduction
India contributes significantly to global grain legume production, accounting for approximately 90% of global production and ranking sixth in terms of production and area cultivated [1]. Most legume species belong to the Fabaceae or Leguminosae families and are depicted due to their fruits generally known as pods. Recognized for their great significance as dietary supplement for humans and animals, these legumes, such as pea, cowpea, chickpea, soybean, mung bean, beans, fava beans, lentils, peanut and pigeon pea, have been increasingly investigated for nutraceutical purposes [2].
Grain legumes are often considered as nature’s treasure offered to mankind and are regarded as “poor man’s meat” because of their high quantity of vitamins, minerals, protein (16–50%) and dietary fiber (10–23%) [3]. Moreover, grain legumes also play a crucial role in ecological services, due to their biological nitrogen fixation capacity [4].
Cajanus cajan (L.) Millsp. is a leguminous annual woody or perennial plant [5], and the genus Cajanus consists of approximately 37 species out of which C. cajan is an extensively used commercial legume crop [6]. It is a native genus from ancient Egypt, Africa, Asia and America, and now it has been widely distributed across the tropical and subtropical regions [7]. Globally, C. cajan has been recognized by various names, like Pigeon pea (Australia); red gram, tur, arhar, dal (India); mu dou (China); guando (Brazil) [8], and gunga pea, congo pea and non-eye pea in some other parts of the world [9]. Asia is the main producer of pigeon pea, and India alone contributes approximately to 77% of the total area and 90% of the total production around the world [10,11]. Despite the high potential of pigeon pea as a crop, the plant as a whole has been shown to be beneficial for use as food, feed and fuel thanks to its high nutritional value. Thus, the need to implement prior information about C. cajan and compile it for convenient access constitutes the main motivation for this work. In this sense, the present study includes all relevant information from the digital platform on the ethnomedicinal uses, bioactive constituents, nutritional value and biological applications of C. cajan, also paying attention to aspects related to its geographical distribution and folk consumption.
2. Botanical Description
2.1. Geographical Distribution and Taxonomy
C. cajan is a perennial drought resistance legume commonly cultivated in the sub-tropical and semi-arid tropical areas of the world [12,13]. India is the prime producer, corresponding to approximately 90% of the total global production. It has also been found since ancient times in Africa, Caribbean, Southeast Asia, and Egypt and has been grown at a wide range of altitudes (up to 3000 m) [8]. C. cajan is from the Genus Cajanus, Family Fabaceae, Order Rosidae, Class Magnoliopsida, and Kingdom Plantae [7].
2.2. Cytology
The cytological analysis of C. cajan showed that it is diploid having 2n = 2x = 22 chromosomes with an average length of 5.73 ± 1.15 µm up to 10.92 ± 2.69 µm and dominantly metacentric in shape, consisting of 14 metacentric and 4 submetacentric chromosomes [14]. C. cajan has a genome of size 858 mega-base pairs [15]. In the comparative genetic characterization of wild and cultivated C. cajan genotypes, the cultivated species present maximum polymorphic loci [6].
2.3. Morphology
From a morphological point of view, C. cajan is a short-lived shrub with erect stems of 1–2 m height [16]. Its roots are finely nodulated, lateral and deep rooted of up to 3 m, possessing a root system having a central taproot with several secondary and lateral branches. The branching pattern in C. cajan is determined based on the habitat, spacing and plant genotype. The leaves are lanceolate to elliptical in shape and size, ranging from 6 to 17 cm in length and are around the same breadth. The flowers are usually, yellow to orange in color, present a long peduncle of 1–8 cm long and terminal or axillary racemes (4–12 cm). Calyx: gamosepalous with 5 lobes, Corolla: zygomorphic and bright yellow, Androecium: 10 stamens (4 with short filaments and 6 with long filaments), Gynoecium: ovary (superior, pubescent, 2–9 ovules and monocarpellary), style (long, filiform and glabrous), stigma (incurved & thickened), Seeds: spherical or lens shaped [8].
3. Traditional Uses
The use of C. cajan for traditional purposes dates since immemorial times, and such information has passed over the generations in order to substantially promote the continuity of knowledge improvement. The diversity and availability in regional flora of plant resources is markedly determined by the use of plant species in folk medicinal practices [17]. Various studies have demonstrated that the leaves, seeds, stems and roots of C. cajan have been used in traditional medicine for the treatment of various ailments, including toothache, diabetes, dizziness, baldness and gastrointestinal discomfort in few domains of India, Bangladesh, China and many other nations. In Oman, C. cajan seeds are used for treating many chronic infections, and native people use the juice from leaves to treat various dermatological conditions [18]. In ancient times, the floral decoction was used for treating pneumonia, coughs, menstrual disorders, dysentery and bronchitis, while leaf decoction was used in Eastern Nigeria for treating measles [19]. A detailed description of the traditional uses of C. cajan is presented in Table 1.
Table 1.
Ethnomedicinal uses of Cajanus cajan from different regions.
| Medicinal Use | Plant Part | Region | References |
|---|---|---|---|
| Gastrointestinal disorders | Seeds (O) | Trinidad and Tobago | [20] |
| Menstrual problems | Seeds (O) | India | [21] |
| Toothache | Stem (T) | China | [22] |
| Sedative | Seeds (O) | India | [21] |
| Wounds | Stem (T) | Nigeria | [23] |
| Diabetes | Seeds, Leaves (O) | Bangladesh, India | [22,24] |
| Laxative | Leaves (O) | India, India, China | [25,26,27] |
| Dizziness | Seeds (O) | India | [20] |
| Poultice | Seeds (T) | India | [28] |
| Wormicide | Seeds, Roots (T) | India | [20] |
| Baldness | Seeds (T) | India | [28] |
| Gingivitis, Stomatitis, Toothbrush | Stem, Seeds, Leaves (T) | India, China, Thailand | [18,23,27] |
| Genital inflammations | Leaves (T) | India | [20] |
| Malaria | Leaves (O) | Nigeria | [23] |
| Ulcers | Leaves (T) | India | [25] |
| Syphilis | Roots (O) | India | [20] |
| Cough | Roots (O) | India | [20] |
| Measles | Seeds (T) | China | [22] |
| Energy stimulant | Seeds (O) | Bangladesh | [24] |
| Induce lactation | Leaves and Seeds (T) | India | [27] |
| Nullify effect of intoxication | Leaves (O) | India | [27] |
(O) = Oral; (T) = Topical.
4. Nutritional Properties
The nutritional profiling of C. cajan, including of its leaves, seeds, roots and stem, has also been investigated by standard methods to determine the proximate, amino acid and mineral composition (Table 2). The maximum fat (15.00 ± 0.090%), moisture (8.20 ± 0.229%), carbohydrate (40.95 ± 0.244%) and nutritive value (333.73 ± 1.500%) were recorded in seeds, however the highest protein content was found in leaves (31.99 ± 0.070%) (Table 2). Results of the proximate composition of protein isolate, full fat flour and defatted flour derived from C. cajan and its comparisons with wheat flour and yellow-pea flour are shown in Table 3.
Table 2.
Proximate composition of Cajanus cajan from different countries.
| Proximate | Seeds (%) [5] (Nigeria) |
Seeds (%) [29] (India) |
Seeds (%) [30] (Taiwan) |
Seeds (%) [31] (India) |
Leaves (%) [32] (Nigeria) |
Leaves (%) [29] (India) |
Leaves (%) [30] (Taiwan) |
Roots (%) [30] (Taiwan) |
Stem (%) [29] (India) |
Seeds (%) [33] (India) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dry matter | 95.89 | 91.80 ± 0.22 | - | - | - | 93.68 ± 0.284 | - | - | 93.88 ± 0.12 | - |
| Protein | 21.03 | 08.62 ± 0.03 | 22.0 ± 0.4 | 25.46 | 22.40 | 31.99 ± 0.070 | 19.4 ± 0.5 | 2.4 ± 0.1 | 21.34 ± 0.56 | 19.53 ± 0.02 |
| Fat | 4.43 | 15.00 ± 0.09 | 5.5 ± 0.3 | 1.65 | 2.74 | 13.00 ± 0.090 | ND | 0.4 ± 0.0 | 14.19 ± 0.26 | 1.64 ± 0.03 |
| Fibre | 7.16 | 05.09 ± 0.08 | - | 6.50 | 7.25 | 21.82 ± 0.238 | - | - | 27.70 ± 0.36 | 4.75 ± 0.02 |
| Ash | 3.76 | 22.11 ± 0.11 | 12.0 ± 0.0 | 3.66 | 8.22 | 20.60 ± 0.114 | 3.6 ± 0.1 | 3.6 ± 0.2 | 23.00 ± 0.22 | 3.23 ± 0.03 |
| Moisture | - | 8.20 ± 0.22 | 14.3 ± 0.1 | 8.50 | 11.20 | 06.31 ± 0.284 | 11.5 ± 0.2 | 3.3 ± 0.1 | 06.11 ± 0.12 | 8.17 ± 0.02 |
| Carbohydrate | - | 40.95 ± 0.24 | 56.2 ± 0.3 | 54.23 | - | 6.269 ± 0.153 | 65.6 ± 0.2 | 90.3 ± 0.1 | 8.131 ± 0.38 | 62.28 ± 0.05 |
| Nutritive value | - | 333.73 ± 1.50 | - | - | - | 236.72 ± 0.591 | - | - | 242.61 ± 1.56 | - |
ND = Not detectable; “-” = Not tested.
Table 3.
Comparison of Cajanus cajan flour proximate composition with generally used flours.
| Proximate | Full Fat Flour (Cajanus cajan) [34] |
Defatted Flour (Cajanus cajan) [34] |
Protein Isolate (Cajanus cajan) [34] |
Wheat Flour (Triticum aestivum) [35] |
Yellow-Pea Flour (Pisum sativum) [35] |
|---|---|---|---|---|---|
| Protein | 24.02 ± 0.016% | 26.30 ± 0.016% | 90.65 ± 0.025% | 12.81 ± 0.06% | 22.33 ± 0.05% |
| Moisture | 6.85 ± 0.012% | 6.76 ± 0.016% | 6.63 ± 0.015% | 12.70 ± 0.0% | 13.35 ± 0.1% |
| Fibre | 1.24 ± 0.016% | 1.56 ± 0.015% | - | 10.08 ± 1.20% | 14.84 ± 0.93% |
| Fat | 2.017 ± 0.062% | - | - | 1.53 ± 0.08% | 1.40 ± 0.04% |
“-” = Not tested.
The study of amino acids content present in C. cajan reveals that leaves (808.8 ± 10.3 mg/100 g) and roots (871.8 ± 11.2 mg/100 g) contain the highest concentration of glutamine, whereas alanine (1547.8 ± 3.9 mg/100 g) and aspartic acid (11.56 g/16 gN) were found in maximum amounts in seeds. The lowest concentration of tryptophan was observed in leaves (2.4 ± 0.4 mg/100 g), roots (1.3 ± 0.4 mg/100 g) and seeds (9.5 ± 0.1 mg/100 g). The detail description of the amino acid composition is mentioned in Table 4.
Table 4.
Amino acid composition of different parts of Cajanus cajan.
| Amino Acids | Seeds (mg/100 g) [30] | Leaves (mg/100 g) [30] | Roots (mg/100 g) [30] |
|---|---|---|---|
| Lysine | 740.8 ± 6.3 | 425.4 ± 10.1 | 297.9 ± 2.0 |
| Histidine | 361.7 ± 3.6 | 266.8 ± 1.3 | 118.4 ± 4.3 |
| Arginine | 279.9 ± 2.6 | 333.4 ± 1.3 | 226.1 ± 5.9 |
| Aspartic acid | 126.4 ± 1.7 | 323.3 ± 5.2 | 112.5 ± 5.2 |
| Threonine | 136.2 ± 5.4 | 406.8 ± 1.3 | 119.9 ± 4.6 |
| Serine | 220.0 ± 8.1 | 494.6 ± 4.8 | 169.2 ± 4.5 |
| Glutamic acid | - | - | - |
| Proline | 72.1 ± 8.2 | 137.9 ± 1.2 | 89.1 ± 8.1 |
| Glycine | 160.7 ± 3.4 | 235.7 ± 2.8 | 139.7 ± 6.9 |
| Alanine | 1547.8 ± 3.9 | 576.5 ± 5.6 | 687.5 ± 12.3 |
| Cystine | ND | ND | ND |
| Valine | 671.4 ± 4.8 | 422.2 ± 3.6 | 381.1 ± 5.6 |
| Methionine | 70.6 ± 1.6 | 86.0 ± 2.3 | 61.1 ± 1.2 |
| Isoleucine | 392.0 ± 3.1 | 314.1 ± 8.3 | 272.7 ± 4.2 |
| Leucine | 679.7 ± 13.5 | 597.8 ± 3.8 | 492.2 ± 4.2 |
| Tyrosine | 186.1 ± 2.0 | 143.9 ± 3.9 | 149.8 ± 5.2 |
| Phenylalanine | 354.7 ± 7.6 | 612.4 ± 3.6 | 262.1 ± 2.5 |
| Tryptophan | 9.5 ± 0.1 | 2.4 ± 0.4 | 1.3 ± 0.4 |
| Glutamine | 648.3 ± 6.3 | 808.8 ± 10.3 | 871.8 ± 11.2 |
ND = Not detectable; “-” = Not tested.
To what concerns to mineral composition, the evaluation of C. cajan revealed higher levels of calcium in leaves (33 ± 4.9 mg/100 g), seeds (581 ± 4.3 mg/100 g) and roots (597 ± 2.5 mg/100 g) and lower levels of zinc (2.1 ± 0.9, 0.7 ± 0.2 and 0.7 ± 0.9 mg/100 g, respectively) (Table 5). Due to the nutritional contribution and health benefits of C. cajan it is regarded as an alternative to produce vegetable meat, with high quality standards and appropriate sensory characteristics that allow consumer acceptance and integration of product in daily diet. Moreover, due to its essential nutrient content, this makes an exquisite preference for vegetarian consumers.
Table 5.
Mineral composition of Cajanus cajan.
| Minerals | Seeds (mg/100 g) [30] (Taiwan) |
Seeds (mg/100 g) [8] (India) |
Seeds (mg/100 g) [33] (India) |
Leaves (mg/100 g) [30] (Taiwan) |
Roots (mg/100 g) [30] (Taiwan) |
|---|---|---|---|---|---|
| Sodium (Na) | 32.5 ± 5.5 | - | - | 19.7 ± 39.0 | 108.0 ± 7.6 |
| Zinc (Zn) | 0.7 ± 0.2 | 2.3 | 0.585 ± 0.04 | 2.1 ± 0.9 | 0.7 ± 0.9 |
| Magnesium (Mg) | 138.8 ± 7.2 | 122.0 | - | 111 ± 9.5 | 130 ± 8.7 |
| Manganese (Mn) | 6.8 ± 5.2 | - | 0.204 ± 0.04 | ND | 0.7 ± 0.2 |
| Iron (Fe) | 51.5 ± 8.7 | 3.9 | 0.335 ± 0.08 | 4.8 ± 2.1 | ND |
| Copper (Cu) | 1.4 ± 0.6 | 1.3 | 0.052 ± 0.03 | ND | 1.0 ± 0.6 |
| Calcium (Ca) | 581 ± 1.3 | 120.8 | - | 33 ± 4.9 | 597 ± 2.5 |
ND = Not detectable; “-” = Not tested.
Scientific investigations of nutraceutical profiling have underlined that C. cajan has relevant nutritional attributes that help in the treatment of different types of human conditions.
5. Chemical Composition
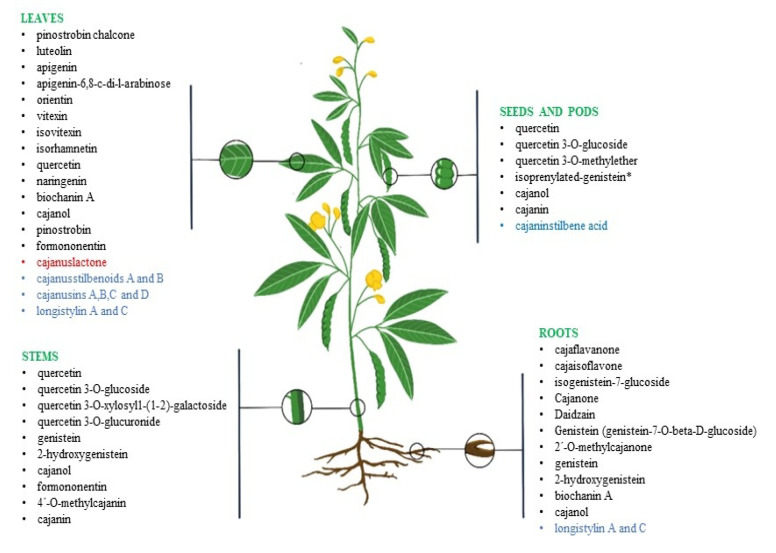
The composition and concentration of active compounds presents in plant matrices largely determines their bioactive effects. In C. cajan the main bioactive compounds identified to date are broadly classified into the flavonoids, phenolics and stilbenes group [36,37]. The literature-based screening of phytochemicals revealed the presence of various phenolic compounds, namely, cajanol, longistylin A and C, genistein and biochanin A [38]. The total phenolic content of C. cajan seeds, root and stem was estimated to be between 4.27–92.00 mg of gallic acid equivalent (GAE) per gram dry weight (DW) (mg GAE/g DW) extract by using different solvent systems (dichloromethane, water and methanol) [38]. The determination of the chemical composition of ethanol leaves extract by high-performance liquid chromatography (HPLC) analysis revealed the presence of seven flavonoids, including pinostrobin, orientin, naringenin, apigenin, apigenin-6,8-di-C-α-L-arabinopyranoside and pinostrobin chalcone, and two stilbenes, namely cajaninstilbene acid and longistyline C [36]. In a study, Zhang and colleagues [39] reported the structure of a novel prenylated flavonone isolated from C. cajan, naringenin-3’-isoprenyl-7-methyl ether 1, by 1D and 2D NMR technology. Other phytochemical studies also indicated the existence of acidic compounds, glycosides, tannins, resins, saponins and reducing sugars [40,41,42]. The description of the bioactive components present in different parts of C. cajan is shown in Figure 1 and Table 6.
Figure 1.
Localization of bioactive components (flavonoids—black, stilbenoids—blue, coumarin—red) in different parts of Cajanus cajan. Note—“*” Isoprenylated-genistein detected in seedlings only.
Table 6.
Bioactive components present in Cajanus cajan from different regions.
| Bioactive Compound | Part Used | Extract | Region | Ref. |
|---|---|---|---|---|
| Cajanuslactone | Leaves | Chloroform | India | [26] |
| Cajanin, Longistylin C, Longistylin A, Betulinic acid, Pinostrobin, Cajaninstilbene acid, Orientin, Vitexin | Leaves | Ethanol | India | [26] |
| Protein fraction Cl-1 | Leaves | Methanol | India | [26] |
| Genistein, Genistin | Roots | Ethanol: Water | India | [26] |
| Cajanol (isoflavonoids) | Roots | Ethanol | India | [26] |
| Phenolics (flavonoids, tannis) | Aerial plants | Hydroalcoholic | China | [43] |
| Cajaninstilbene acid, Vitexin, Orientin, Pinostrobin | Leaves | Ethanol | Bangladesh | [7] |
| Luteolin, Apigenin, Quercitin, Isorhamnetin, Cajaninstilbene acid, Pinostrobin, Cajanin, Longistylin A, Longistylin C | Leaves | - | Bangladesh | [7] |
| Cajanuslactone | Leaves | Chloroform | Bangladesh | [7] |
| Hordenine, Juliflorine, Betulinic acid, Stigmasterol, Beta-sitosterol | Leaves | - | Bangladesh | [7] |
Looking at the essential oil from C. cajan, Ogunbinu et al. [44] identified the presence of 100 constituents in seeds, stem and leaves using gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) analysis. Among all compounds, sesquiterpene hydrocarbons were found in higher amounts in 81.2% (stem), 92.5% (leaves), and 94.3% (seeds). Esters, aldehydes, alcohols, terpenoids and ketones and some other constituents, including α-himachalene, β- himachalene, γ-himachalane, α-humulene and α-copaene were also identified. Qi et al. [45] reported the presence of 27 compounds in the essential oil from C. cajan leaves extracted through solvent-free microwave extraction (SFME) and hydro-distillation (HD) methods. Sesquiterpenes were the most abundant compounds identified, namely α-humulene, α-copaene, α-bisabolene, α-himachalene, β-caryophyllene and alloaromadendrene. The details of other constituents are listed in Table 7.
Table 7.
Essential oil composition of Cajanus cajan.
| Plant Part | Bioactive Compounds | Region | Ref. |
|---|---|---|---|
| Leaves | (E)-2-Hexenal; Benzaldehyde; Nonanal; Decanal; α-Longipinene; Cyclosativene; α-Copaene; β-Longipinene; (Z)-Caryophyllene; Longifolene; α-Gurjunene; trans−α-Bergamotene; α-Guaiene; α-Himachalene; α-Humulene; allo-Aromadendrene; γ-Muurolene; γ-Himachalene; β-Selinene; α-Selinene; β-Himachalene; β-Bisabolene; trans-γ-Cadinene; α-Dehydro-ar-himachalene; δ-Cadinene; trans-Calamenene; γ-Dehydro-ar-himachalene; trans-Cadina-1(2),4-diene; α-Calacorene; trans-Nerolidol; Ledol; Caryophyllenyl alcohol; Himachalene epoxide; Caryophyllene oxide; Globulol; Longiborneol (=juniperol); Humulene oxide II; β-Himachalene oxide; Bisabolol-11-ol; 1-epi-Cubenol; α-Acorenol; τ-Cadinol; τ-Muurolol; α-Muurolol; Himachalol; Selin-11-en-4α-ol; β-Bisabolol; Cadalene; α-Bisabolol; epi-α-Bisabolol; Hexahydrofarnesyl acetone; ar-Himachalene-2-ol; n-Docosane. | Nigeria | [44] |
| Stem | n-Decane; Limonene; Nonanal; Citronellal; 1-Nonanol; Menthol; Methyl salicylate; Decanal; α-Longipinene; Cyclosativene; α-Copaene; n-Tetradecane; Longifolene; Dodecanal; β-Caryophyllene; trans−α-Bergamotene; α-Guaiene; α-Himachalene; α-Humulene; allo-Aromadendrene; γ-Muurolene; γ-Himachalene; β-Selinene; Bicyclosesquiphellandrene (=trans-muurola-4(14),5 diene); α-Selinene; β-Himachalene; β-Bisabolene; trans-γ-Cadinene; α-Dehydro-ar-himachalene; δ-Cadinene; trans-Calamenene; γ-Dehydro-ar-himachalene; α-Cadinene; α-Calacorene; Germacrene B; trans-Nerolidol; Caryophyllenyl alcohol; Himachalene epoxide; Caryophyllene oxide; Longiborneol (=juniperol); Humulene oxide II; β-Himachalene oxide; 1-epi-Cubenol; α-Acorenol; cis-Cadina-4-en-7-ol; τ-Cadinol; Cubenol; α-Muurolol; Himachalol; α-Cadinol; n-Octadecane; Hexahydrofarnesyl acetone; n-Docosane. | Nigeria | [44] |
| Seeds | Benzaldehyde; Nonanal; α-Longipinene; Cyclosativene; α-Copaene; (Z)-Caryophyllene; Longifolene; α-Gurjunene; β-Caryophyllene; β-Cedrene; β-Duprezianene; β-Gurjunene; trans−α-Bergamotene; α-Guaiene; α-Himachalene; α-Humulene; allo-Aromadendrene; γ-Muurolene; γ-Himachalene; β-Selinene; α-Selinene; β-Himachalene; β-Bisabolene; δ-Cadinene; γ-Dehydro-ar-himachalene; trans-Cadina-1(2),4-diene; 10-epi-Cubebol; α-Cadinene; α-Calacorene; trans-Nerolidol; Caryophyllenyl alcohol; Himachalene epoxide; Caryophyllene oxide; Globulol; Viridiflorol; Longiborneol (=juniperol); Humulene oxide II; β-Himachalene oxide; Bisabolol-11-ol; epi-10-γ-Eudesmol; 1-epi-Cubenol; cis-Cadina-4-en-7-ol; τ-Cadinol; τ-Muurolol; α-Muurolol; Himachalol; Selin-11-en-4α-ol; Bulnesol; β-Bisabolol; Cadalene; α-Bisabolol; epi-α-Bisabolol; Hexahydrofarnesyl acetone. | Nigeria | [44] |
| Leaves | 3,6-Dimethyl-octane; Naphthalene; Dodecane; 6-Ethyl-undecane; 4-Methyl-Dodecane; 4-Ethy-undecane; 4,6-Dimethyl-dodecane; 1-Methyl-naphathalene; 2,6,11-Trimethyl-dodecane; α-Longipinene; 2-Methyl-tridecane; (+)-Cyclosativene; α-Copaene; Tetradecane; Longifolene; Caryophyllene; α-Selinene; β-Bergamotene; α-Himachalene; Humulene; Alloaromadendrene; α-Bisabolene; 2,4-Bis(1,1-dimethylethyl)-phenol; Hexadecane; Norphytane. | China | [45] |
6. Biological Applications
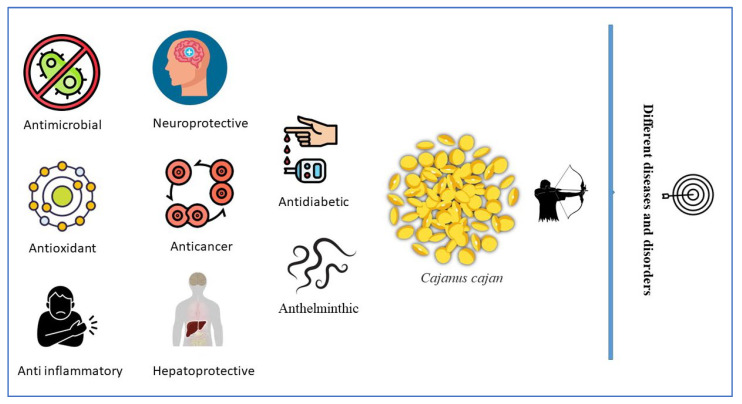
With the growth of world’s economy and enhancement in people’s living standard, several chronic diseases, like neurological, metabolic, inflammatory, cerebrovascular and cardiovascular disorders have increased rapidly [46]. Natural products are widely recognized for their biological or pharmacological potential since ancient times, and recently the interest in their study has re-emerged as upcoming drug candidates. Globally, around 50,000 plants have shown potent therapeutic potentialities [47]. According to pharmacological studies, C. cajan leaves have various bioactivities, including antioxidant, antiplasmodial, anticancer, hypoglycemic, insecticidal, neuroprotective and antimicrobial activities [37,48]. Moreover, the molecular regulatory mechanism of few biological applications/activity are briefly summarized in Table 8. The most relevant therapeutic applications of C. cajan briefly described below and presented in Figure 2.
Table 8.
Molecular regulatory mechanism of few biological activities of Cajanus cajan.
| S. No. | Biological Activity | Isolated Compounds/Extracts | Biological Activity | Reference |
|---|---|---|---|---|
| 1 | Hypocholesterolemic | Methanolic extract | ↑ LDRL; ↓PCSK9 mRNA | [49] |
| 2 | Antidepressant | Cajanin stilbene acid | ↓ Kynurenine pathway | [50] |
| 3 | Neuroprotective | AgNP | ↑ Proline; ↑ Glyoxalase; ↑ Pyrroline-5-carboxylate synthetase gene. | [51] |
| 4 | Antidepressant | Fluoride exposure | ↓ Growth and membrane stability index; ↑ Reactive oxygen species; ↑ Malondialdehyde; ↑ Glutathione; ↑ Lipoxygenase. | [51] |
| 5 | Antioxidant | Hexane extract | ↓ AChE; ↓ BChE; ↓ α-AMYLASE; ↓ α-glucosidase. | [52] |
| 6 | Antidiabetic | Methanolic extract | ↓ Fasting blood sugar | [53] |
| 7 | Anticancer | Betulinic acid, genistin, orientin and vitexin | ↓ Inhibit the Histone deacetylases enzyme | [54] |
| 8 | Antimitotic | - | ↓ Decrease the percentage of Sea urchin embryonic cells | [55] |
| 9 | Anticancer | Cajanin stilbene acid | ↓ Inhibit several human kinases, ↓ serine/threonine-protein kinase WNK3 | [56] |
LDRL = Low density lipoprotein receptor; PCSK9 = Proprotein convertase subtilisin/kesin type 9; AgNP = Silver nanoparticles; AChE = Acetyl cholinesterase; BChE = Butyryl cholinesterase; ↑: Increased; ↓: Decreased.
Figure 2.
Different biological applications of Cajanus cajan.
6.1. Antimicrobial Activity
The antimicrobial activity of plants varies pronouncedly depending on chemical constituents presents, hence it is difficult to classify single antimicrobial mechanisms, as they rely on the phytochemical properties of the plant [57]. Dinore and Farooqui (2022) [58] investigated the antimicrobial activity of C. cajan leaves methanol extract against Escherichia coli and Candida albicans, and the results indicated a remarkable ability to inhibit the growth of the microorganisms, with minimum inhibitory concentrations (MIC) of 50 µg/mL and minimum fungicidal concentrations (MFC) of 250 µg/mL. Cajanuslactone, one of the most abundant phytoconstituents present in C. cajan leaves is expected to be the responsible for the marked antimicrobial properties [22]. The antifungal potential of C. cajan roots were examined by microdilution method to demonstrate the use of plant extract as a novel therapeutic source [59]. The ethanolic extract of the roots showed antifungal activity in terms of MIC (Candida albicans 512 µg/mL, Candida krusei 512 µg/mL and Candida tropicalis 512 µg/mL) [59].
In another study, Qi et al. [45] extracted the essential oil from C. cajan leaves by solvent free microwave extraction and reported antimicrobial properties to the extracted oil. The essential oil revealed an effective antimicrobial potential, addressed through determination of MIC and minimum bactericidal concentration (MBC), against Bacillus subtilis (1.06 and 2.12 mg/mL, respectively), and Propionibacterium acnes (0.13 and 0.26 mg/mL, respectively). Additional studies on antimicrobial potential of C. cajan (different parts) are listed in Table 9 and Table 10.
Table 9.
Antibacterial activity of Cajanus cajan.
| Plant Part | Solvent System | Concentration of Extract | Microorganism | Agar Well Diffusion Method/ Agar Disc Diffusion Method |
Region | Ref. | |
|---|---|---|---|---|---|---|---|
| ZI (mm) | MIC (mg/mL) | ||||||
| Leaves | Methanol | 6.25–200.00 µg/mL |
|
|
|
Nigeria | [60] |
| Leaves | Ethanol | 6.25–200.00 µg/mL |
|
|
|
Nigeria | [60] |
| Leaves | Acetone | 6.25–200.00 µg/mL |
|
|
|
Nigeria | [60] |
| Leaves | Hot water | 6.25–200.00 µg/mL |
|
|
|
Nigeria | [60] |
| Leaves | Cold water | 6.25–200.00 µg/mL |
|
|
|
Nigeria | [60] |
| Leaves | Petroleum ether | 12.5–100 mg/mL |
|
|
|
India | [61] |
| Leaves | Chloroform | 12.5–100 mg/mL |
|
|
|
India | [61] |
| Leaves | Methanol | 12.5–100 mg/mL |
|
|
|
India | [61] |
| Leaves | Ethanol | 12.5–100 mg/mL |
|
|
|
India | [61] |
| Leaves | Aqueous | 12.5–100 mg/mL |
|
|
|
India | [61] |
| Leaves | Methanol | 40 mg/mL |
|
|
|
Nigeria | [62] |
ZI: Zone of inhibition; MIC: Minimum inhibitory concentration; “-”: No zone of inhibition and MIC.
Table 10.
Antifungal activity of Cajanus cajan.
| Plant Part | Solvent System | Extract Concentration (mg/mL) | Microorganism | Agar Well Diffusion Method/ Agar Disc Diffusion Method |
Region | Ref. | |
|---|---|---|---|---|---|---|---|
| ZI (mm) | MIC (mg/mL) | ||||||
| Leaves | Methanol | 3.15–50 |
|
16–17 17–18 |
10–13 10–14 |
Sudan | [63] |
| Leaves | Ethanol | 3.15–50 |
|
- 14–15 |
- - |
Sudan | [63] |
| Leaves | Petroleum ether | 3.15–50 |
|
- - |
- - |
Sudan | [63] |
| Leaves | Ethyl acetate | 3.15–50 |
|
- - |
- - |
Sudan | [63] |
| Leaves | Chloroform | 3.15–50 |
|
- - |
- - |
Sudan | [63] |
| Leaves | Methanol | 12.5–200 |
|
- 2 - |
- 1 - |
Nigeria | [42] |
| Roots | Ethanol | 0.1 |
|
- - - |
0.512 0.512 0.512 |
Brazil | [59] |
ZI: Zone of inhibition; MIC: Minimum inhibitory concentration; “-”: No zone of inhibition and MIC.
6.2. Antioxidant Activity
Different studies also have been performed to assess the antioxidant potential of different parts of C. cajan. Aggarwal et al. (2015) reported the antioxidant potential of C. cajan ethanol seed extract using ferric reducing antioxidant power (FRAP) assay. The results obtained revealed a concentration-dependent antioxidant activity (concentration 25 to 450 µg, 4.4 to 43.0 µM) [64].
The HPLC-FRAP analysis of C. cajan stem bark extract, revealed that it consists of 12 phenolic compounds with notable antioxidant activity [52]. Yang et al. (2020) performed DPPH (2,2-diphenyl-1-picrylhydrazyl), NO (Nitric Oxide) scavenging, ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and FRAP (Ferric reducing antioxidant power) assays for determining the antioxidant potential of leaves, seeds and roots of C. cajan. Among them, C. cajan roots showed high antioxidant efficiency than seeds and leaves [30]. Further data on the antioxidant potential of C. cajan and the respective assays are shown in Table 11.
Table 11.
Antioxidant potential of Cajanus cajan.
| Part Used | Solvent System | Experiment/Assay | Antioxidant Potential | Ref. |
|---|---|---|---|---|
| Leaves | Aqueous |
|
|
[1] |
| Leaves | Ethanol |
|
|
[1] |
| Root | Methanol |
|
|
[65] |
| Leaves | Ethanol |
|
|
[43] |
| Leaves | Aqueous |
|
|
[43] |
| Seeds | Methanol |
|
|
[66] |
| Seeds | Aqueous |
|
|
[66] |
| Seeds | Methanol |
|
|
[67] |
| Root | Hot water |
|
|
[30] |
| Seeds | Hot water |
|
|
[30] |
| Leaves | Hot water |
|
|
[30] |
| Root | Ethanol |
|
|
[30] |
| Seeds | Ethanol |
|
|
[30] |
| Leaves | Ethanol |
|
|
[30] |
| Stem bark | Hexane |
|
|
[52] |
| Stem bark | Ethyl acetate |
|
|
[52] |
| Stem bark | Methanol |
|
|
[52] |
| Stem bark | Infusion |
|
|
[52] |
DPPH = 2,2-diphenyl-1-picrylhydrazyl; ABTS = 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid); AAE = Ascorbic acid equivalent; TE = Trolox equivalent.
6.3. Anti-Diabetic Activity
The antidiabetic potential of C. cajan methanol root extract was addressed by Nahar et al. (2014) in alloxan-induced diabetic Swiss albino mice. The experimental mice were treated with C. cajan extract up to 5 days (200 and 400 mg/kg bw, orally). Glucose tolerance test and hyperglycemic effect studies (involving diabetes induction in mixed sex Swiss albino mice by injection of aqueous alloxan monohydrate, 55 mg/kg, intravenously) were carried out on tested animals, along with determination of the antioxidant activity. The results showed a rapid decline in fasting serum glucose level (p < 0.001) and blood glucose level (p < 0.001) in 5 days. On the basis of these results, the plant extract evidenced potent hypoglycemic and antioxidant properties compared to other species (e.g., Tamarindus indica seeds) [65].
6.4. Tyrosinase Inhibitory Activity
C. cajan root, stems and seeds were also addressed for its ability to inhibit tyrosinase activity, and for that water, dichloromethane and methanol extracts were prepared. The IC50 values of the extracts varied from 3.55–12.43 mg/mL, whereas the maximum inhibitory capacity was reported for methanol root extract (IC50 = 3.55 mg/mL) [38].
6.5. Neuroprotective Activity
A variety of naturally-occurring bioactive compounds are currently being explored for their therapeutic potential in neurodegenerative diseases, but only a few are known to have benefits [68]. The use of plant extracts and their bioactive constituents are one of the promising approaches for the treatment of neurological diseases [69]. C. cajan was also exploited for their neuroprotective abilities. The presence of stilbenoids is able to induce apoptotic neuronal death by Aβ25–35 injection in mice and cause elevation in choline acetyltransferase (ChAT) and superoxide dismutase (SOD) activity in the cortex and hippocampus [70]. In a study with injured larvae of zebrafish, cajanin stilbene acid (CSA) and its derivative were found to decline the migration and production of primitive macrophages and neutrophiles [71], being thus proposed that C. cajan may be a promissory source of biomolecules with neuroprotective abilities.
6.6. Other Bioactivities
In addition to the above listed bioactive effects of C. cajan, other bioactivities, such as hepatoprotective [26,72,73,74,75], anthelminthic [76], anticancer [77], and anti-inflammatory [78] effects have been documented by other researches. Moreover, the C. cajan is also used in paper-making, cosmetic industries and multi-purposely in dietary supplements for human and animals.
7. Conclusions and Future Prospects
Pigeon pea (C. cajan) is one of the most commonly and widely used, tropical and subtropical legume due to its nutrient packed edible seeds, might being effectively used for food and medicinal purposes. However, it is an underutilized/neglected legume species. As yet, several flavonoids, isoflavonoids, tannins, phenolics and proteins have been isolated from various plant parts, and their therapeutic properties have also been confirmed, but many pure and bioactive components were still not taken into consideration. Several studies have identified that the phytochemicals present display excellent bioactive effects for a plethora of human conditions.
A number of extensive research has been done only on extracts rather than isolated fractions and oils, that indicates necessity of further study in this direction. Moreover, majority of studies are limited to in vitro screening, with only a few focusing on in vivo testing. As a result, advanced research is required to explore new phytopharmaceuticals based on C. cajan. Clinical trials should be conducted to assess the toxicity profile of C. cajan in humans in respect of antioxidant activity, antimicrobial activity, anthelminthic activity, anti-inflammatory activity, antidiabetic activity and immunomodulatory aspects. The current review article aims to concentrate attention of researchers as well as pharmaceutical industries on untouched and unexplored aspects related to C. cajan and may serve as a crucial link towards the establishment of C. cajan as a therapeutic drug. Although, as it is a leguminous plant and plays a major role in biological nitrogen fixation, further more relevant knowledge regarding the characteristics of soil, indigenous microbes and plant species-specific responses is required to establish the inoculant for maximum ecological restoration benefits and to support future adoption of this practice.
Acknowledgments
Prabhakar Semwal thanks to the Graphic Era (Deemed to be University), Dehradun, India, for their help and support during this study. Uttarakhand Council for Biotechnology (UCB), Haldi, India is also acknowledged for their financial help (UCB/R&D Project/2022/20).
Author Contributions
Conceptualization, P.S. (Prabhakar Semwal); writing-original draft preparation, B.G., S.B.J.P., P.S. (Pooja Singh), writing-review and editing, B.G., S.B.J.P., S.P., P.S. (Prabhakar Semwal), A.T., N.C.-M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mahitha B., Archana P., Ebrahimzadeh M.H., Srikanth K., Rajinikanth M., Ramaswamy N. In vitro antioxidant and pharmacognostic studies of leaf extracts of Cajanus cajan (I.) millsp. Indian J. Pharm. Sci. 2015;77:170–177. doi: 10.4103/0250-474x.156555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Ron A.M. Grain Legumes. Volume 10 Springer; New York, NY, USA: 2015. [Google Scholar]
- 3.Maphosa Y., Jideani V.A. Functional Food-Improve Health Through Adequate Food. Volume 1. IntechOpen; London, UK: 2017. The role of legumes in human nutrition; p. 13. [Google Scholar]
- 4.Dutta A., Trivedi A., Nath C.P., Gupta D.S., Hazra K.K. A comprehensive review on grain legumes as climate-smart crops: Challenges and prospects. Environ. Chall. 2022;7:100479. doi: 10.1016/j.envc.2022.100479. [DOI] [Google Scholar]
- 5.Akande K., Abubakar M., Adegbola T., Bogoro S., Doma U. Chemical evaluation of the nutritive quality of pigeon pea [Cajanus cajan (L.) Millsp.] Int. J. Poult. Sci. 2010;9:63–65. doi: 10.3923/ijps.2010.63.65. [DOI] [Google Scholar]
- 6.Ganapathy K., Gnanesh B., Byre Gowda M., Venkatesha S., Gomashe S.S., Channamallikarjuna V. AFLP analysis in pigeonpea (Cajanus cajan (L.) Millsp.) revealed close relationship of cultivated genotypes with some of its wild relatives. Genet. Resour. Crop Evol. 2011;58:837–847. doi: 10.1007/s10722-010-9621-1. [DOI] [Google Scholar]
- 7.Orni P.R., Ahmed S.Z., Monefa M., Khan T., Dash P.R. Pharmacological and phytochemical properties of Cajanus cajan (L.) Huth.(Fabaceae): A review. Int. J. Pharm. Sci. Res. 2018;3:27–37. [Google Scholar]
- 8.Sameer Kumar C., Satheesh Naik S., Mohan N., Saxena R.K., Varshney R.K. the Pigeonpea Genome. Springer; Berlin/Heidelberg, Germany: 2017. Botanical description of pigeonpea [Cajanus cajan (L.) Millsp.] pp. 17–29. [Google Scholar]
- 9.Schuster R., Holzer W., Doerfler H., Weckwerth W., Viernstein H., Okonogi S., Mueller M. Cajanus cajan–a source of PPARγ activators leading to anti-inflammatory and cytotoxic effects. Food Funct. 2016;7:3798–3806. doi: 10.1039/C6FO00689B. [DOI] [PubMed] [Google Scholar]
- 10.Jeevarathinam G., Chelladurai V. Pulses. Springer; Berlin/Heidelberg, Germany: 2020. Pigeon pea; pp. 275–296. [Google Scholar]
- 11.Pande S., Sharma M., Mangla U.N., Ghosh R., Sundaresan G. Phytophthora blight of Pigeonpea [Cajanus cajan (L.) Millsp.]: An updating review of biology, pathogenicity and disease management. Crop Prot. 2011;30:951–957. doi: 10.1016/j.cropro.2011.03.031. [DOI] [Google Scholar]
- 12.Abdulkareem K.A., Olayinka B.U., Danzaki M.M., Idris R., Kareem I., Ayinla A., Sagaya A., Mustapha O.T. Genetic variability via protein electrophoresis among some Nigerian accessions of pigeon pea (Cajanus cajan) Rom. J. Biol. Plant Biol. 2021;66:55–64. [Google Scholar]
- 13.Nix A., Paull C.A., Colgrave M. The flavonoid profile of pigeonpea, Cajanus cajan: A review. SpringerPlus. 2015;4:125. doi: 10.1186/s40064-015-0906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuniastuti E., Primanita S.E., Delfianti M. Karyotypic analysis of Pigeon pea (Cajanus cajan L.) IOP Conf. Ser. Earth Environ. Sci. 2021;637:012094. doi: 10.1088/1755-1315/637/1/012094. [DOI] [Google Scholar]
- 15.Ariraman M., Bharathi T., Dhanavel D. Studies on the effects of mutagens on cytotoxicity behaviour in Pigeon pea (Cajanus cajan (L.) Millsp) Var. CO-7. J. Appl. Adv. Res. 2016;1:25–28. doi: 10.21839/jaar.2016.v1i1.10. [DOI] [Google Scholar]
- 16.Fuller D.Q., Murphy C., Kingwell-Banham E., Castillo C.C., Naik S. Cajanus cajan (L.) Millsp. origins and domestication: The South and Southeast Asian archaeobotanical evidence. Genet. Resour. Crop Evol. 2019;66:1175–1188. doi: 10.1007/s10722-019-00774-w. [DOI] [Google Scholar]
- 17.Karpavičienė B. Traditional Uses of Medicinal Plants in South-Western Part of Lithuania. Plants. 2022;11:2093. doi: 10.3390/plants11162093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tungmunnithum D., Hano C. Cosmetic potential of Cajanus cajan (L.) millsp: Botanical data, traditional uses, phytochemistry and biological activities. Cosmetics. 2020;7:84. doi: 10.3390/cosmetics7040084. [DOI] [Google Scholar]
- 19.Nwodo U., Ngene A., Iroegbu C., Onyedikachi O., Chigor V., Okoh A. In vivo evaluation of the antiviral activity of Cajanus cajan on measles virus. Arch. Virol. 2011;156:1551–1557. doi: 10.1007/s00705-011-1032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saxena K.B., Vijaya Kumar R., Sultana R. Quality nutrition through pigeonpea—A review. Health. 2010;2:1335–1344. doi: 10.4236/health.2010.211199. [DOI] [Google Scholar]
- 21.Mula M., Saxena K. Lifting the Level of Awareness on Pigeonpea—A Global Perspective. International Crops Research Institute for the Semi-Arid Tropics; Telangana, India: 2010. [Google Scholar]
- 22.Kong Y., Wei Z.-F., Fu Y.-J., Gu C.-B., Zhao C.-J., Yao X.-H., Efferth T. Negative-pressure cavitation extraction of cajaninstilbene acid and pinostrobin from pigeon pea [Cajanus cajan (L.) Millsp.] leaves and evaluation of antioxidant activity. Food Chem. 2011;128:596–605. doi: 10.1016/j.foodchem.2011.02.079. [DOI] [Google Scholar]
- 23.Oluwole O.B., Nicholas-Okpara V.A.N., Elemo G., Adeyoju O., Ibekwe D., Adegboyega M.O. Medicinal uses, nutraceutical potentials and traditional farm production of Bambara beans and Pigeon pea. Glob. J. Epidemiol. Public Health. 2021;6:41–50. [Google Scholar]
- 24.Pal D., Sarkar A., Gain S., Jana S., Mandal S. CNS depressant activities of roots of Coccos nucifera in mice. Acta Pol. Pharm. 2011;68:249–254. [PubMed] [Google Scholar]
- 25.Aviara N., Lawal A., Atiku A., Haque M. Bambara groundnut processing, storage and utilization in north eastern Nigeria. Cont. J. Eng. Sci. 2013;8:28–36. [Google Scholar]
- 26.Pal D., Mishra P., Sachan N., Ghosh A.K. Biological activities and medicinal properties of Cajanus cajan (L) Millsp. J. Adv. Pharm. Technol. Res. 2011;2:207–214. doi: 10.4103/2231-4040.90874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zu Y.-G., Liu X.-L., Fu Y.-J., Wu N., Kong Y., Wink M. Chemical composition of the SFE-CO2 extracts from Cajanus cajan (L.) Huth and their antimicrobial activity in vitro and in vivo. Phytomedicine. 2010;17:1095–1101. doi: 10.1016/j.phymed.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Upadhyay B., Dhaker A.K., Kumar A. Ethnomedicinal and ethnopharmaco-statistical studies of Eastern Rajasthan, India. J. Ethnopharmacol. 2010;129:64–86. doi: 10.1016/j.jep.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Sahu M., Vermaand D., Harris K. Phytochemicalanalysis of the leaf, stem and seed extracts of Cajanus cajan L (Dicotyledoneae: Fabaceae) J. Pharm. Pharm. Sci. 2014;3:694–733. [Google Scholar]
- 30.Yang S.-E., Vo T.-L.T., Chen C.-L., Yang N.-C., Chen C.-I., Song T.-Y. Nutritional composition, bioactive compounds and functional evaluation of various parts of Cajanus cajan (L.) Millsp. Agriculture. 2020;10:558. doi: 10.3390/agriculture10110558. [DOI] [Google Scholar]
- 31.Shakappa D., Talari A., Naik R. Prebiotic potential and other health benefits of prebiotic mixture of pigeon pea (Cajanus cajan L) in Wistar NIN rats. Res. Sq. 2021 doi: 10.21203/rs.3.rs-414326/v1. [DOI] [Google Scholar]
- 32.Oke D.G. Proximate and phytochemical analysis of Cajanus cajan (Pigeon pea) leaves. Chem. Sci. Trans. 2014;3:1172–1178. [Google Scholar]
- 33.Rizvi Q.U.E.H., Kumar K., Ahmed N., Yadav A.N., Chauhan D., Thakur P., Jan S., Sheikh I. Influence of soaking and germination treatments on the nutritional, anti-nutritional, and bioactive composition of pigeon pea (Cajanus cajan L.) J. Appl. Biol. Biotechnol. 2022;10:127–134. doi: 10.7324/JABB.2022.100317. [DOI] [Google Scholar]
- 34.Olawuni I., Ojukwu M., Eboh B. Comparative study on the physico-chemical properties of pigeon pea (Cajanus cajan) flour and protein isolate. Int. J. Agric. Food Sci. 2012;2:121–126. [Google Scholar]
- 35.Millar K.A., Gallagher E., Burke R., McCarthy S., Barry-Ryan C. Proximate composition and anti-nutritional factors of fava-bean (Vicia faba), green-pea and yellow-pea (Pisum sativum) flour. J. Food Compos. Anal. 2019;82:103233. doi: 10.1016/j.jfca.2019.103233. [DOI] [Google Scholar]
- 36.Wei Z.-F., Jin S., Luo M., Pan Y.-Z., Li T.-T., Qi X.-L., Efferth T., Fu Y.-J., Zu Y.-G. Variation in contents of main active components and antioxidant activity in leaves of different pigeon pea cultivars during growth. J. Agric. Food Chem. 2013;61:10002–10009. doi: 10.1021/jf402455m. [DOI] [PubMed] [Google Scholar]
- 37.Wei Z.-F., Luo M., Zhao C.-J., Li C.-Y., Gu C.-B., Wang W., Zu Y.-G., Efferth T., Fu Y.-J. UV-induced changes of active components and antioxidant activity in postharvest pigeon pea [Cajanus cajan (L.) Millsp.] leaves. J. Agric. Food Chem. 2013;61:1165–1171. doi: 10.1021/jf304973f. [DOI] [PubMed] [Google Scholar]
- 38.Rinthong P.-O., Maneechai S. Total Phenolic Content and Tyrosinase Inhibitory Potential of Extracts from Cajanus cajan (L.) Millsp. Pharmacogn. J. 2018;10:s109–s112. [Google Scholar]
- 39.Zhang N., Huang R., Zhu Y., Fu M., Cai J., Yang J., Xu Z., Hu Y., Qiu S.X. A new isoprenylated flavanone from Cajanus cajan. Chem. Nat. Compd. 2014;50:438–439. doi: 10.1007/s10600-014-0980-2. [DOI] [Google Scholar]
- 40.Abedelgahyoum A.M., Abdelkarim A.M., Shayoub M., Osman Z. Evaluation of the phytochemical characteristics and antimicrobial activity of Cajanus cajan leaves. World J. Pharm. Res. 2016;6:115–128. [Google Scholar]
- 41.Ahomadegbe M., Ladekan E., Assogba F., Agbonon A., Gbenou J. Phytochemical and toxicity studies of the leaves of Mangifera indica, Cajanus cajan and of Piliostigma thonningii, acclimated in Benin, used against diarrheal diseases. J. Pharmacogn. Phytochem. 2018;7:2971–2978. [Google Scholar]
- 42.Obiorah S., Eze E., Obiorah D., Orji N., Umedum C. Phytochemical and antimicrobial studies on the extracts from leaves of Cajanus Cajan and Eucalyptus Globulus; Proceedings of the International Conference on Environment, Chemistry and Biology IPCBEE; Hong Kong, China. 29–30 December 2012; pp. 192–197. [Google Scholar]
- 43.Wu N., Fu K., Fu Y.-J., Zu Y.-G., Chang F.-R., Chen Y.-H., Liu X.-L., Kong Y., Liu W., Gu C.-B. Antioxidant activities of extracts and main components of pigeonpea [Cajanus cajan (L.) Millsp.] leaves. Molecules. 2009;14:1032–1043. doi: 10.3390/molecules14031032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogunbinu A.O., Flamini G., Cioni P.L., Adebayo M.A., Ogunwande I.A. Constituents of Cajanus cajan (L.) Millsp., Moringa oleifera Lam., Heliotropium indicum L. and Bidens pilosa L. from Nigeria. Nat. Prod. Commun. 2009;4:573–578. doi: 10.1177/1934578X0900400427. [DOI] [PubMed] [Google Scholar]
- 45.Qi X.-L., Li T.-T., Wei Z.-F., Guo N., Luo M., Wang W., Zu Y.-G., Fu Y.-J., Peng X. Solvent-free microwave extraction of essential oil from pigeon pea leaves [Cajanus cajan (L.) Millsp.] and evaluation of its antimicrobial activity. Ind. Crops Prod. 2014;58:322–328. doi: 10.1016/j.indcrop.2014.04.038. [DOI] [Google Scholar]
- 46.Wang Y., Zhao Y., Liu X., Li J., Zhang J., Liu D. Chemical constituents and pharmacological activities of medicinal plants from Rosa genus. Chin. Herb. Med. 2022;14:187–209. doi: 10.1016/j.chmed.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira O.O., Cruz J.N., de Moraes Â.A.B., de Jesus Pereira Franco C., Lima R.R., Anjos T.O.d., Siqueira G.M., Nascimento L.D.d., Cascaes M.M., de Oliveira M.S. Essential Oil of the Plants Growing in the Brazilian Amazon: Chemical Composition, Antioxidants, and Biological Applications. Molecules. 2022;27:4373. doi: 10.3390/molecules27144373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashidi J., Houghton P., Hylands P., Efferth T. Ethnobotanical survey and cytotoxicity testing of plants of South-western Nigeria used to treat cancer, with isolation of cytotoxic constituents from Cajanus cajan Millsp. leaves. J. Ethnopharmacol. 2010;128:501–512. doi: 10.1016/j.jep.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Chang H.-Y., Wu J.-R., Gao W.-Y., Lin H.-R., Chen P.-Y., Chen C.-I., Wu M.-J., Yen J.-H. The cholesterol-modulating effect of methanol extract of pigeon pea (Cajanus cajan (L.) Millsp.) leaves on regulating LDLR and PCSK9 expression in HepG2 cells. Molecules. 2019;24:493. doi: 10.3390/molecules24030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang M.-D., Tao X., Pan R.-L., Wang L.-S., Li C.-C., Zhou Y.-F., Liao Y.-H., Chen S.-G., Chang Q., Liu X.-M. Antidepressant-like effects of cajaninstilbene acid and its related mechanisms in mice. Fitoterapia. 2020;141:104450. doi: 10.1016/j.fitote.2019.104450. [DOI] [PubMed] [Google Scholar]
- 51.Yadu B., Chandrakar V., Korram J., Satnami M.L., Kumar M., Keshavkant S. Silver nanoparticle modulates gene expressions, glyoxalase system and oxidative stress markers in fluoride stressed Cajanus cajan L. J. Hazard. Mater. 2018;353:44–52. doi: 10.1016/j.jhazmat.2018.03.061. [DOI] [PubMed] [Google Scholar]
- 52.Sinan K.I., Mahomoodally M.F., Eyupoglu O.E., Etienne O.K., Sadeer N.B., Ak G., Behl T., Zengin G. HPLC-FRAP methodology and biological activities of different stem bark extracts of Cajanus cajan (L.) Millsp. J. Pharm. Biomed. Anal. 2021;192:113678. doi: 10.1016/j.jpba.2020.113678. [DOI] [PubMed] [Google Scholar]
- 53.Ezike A.C., Akah P.A., Okoli C.C., Okpala C.B. Experimental evidence for the antidiabetic activity of Cajanus cajan leaves in rats. J. Basic Clin. Pharm. 2010;1:81–84. [PMC free article] [PubMed] [Google Scholar]
- 54.Adewole K., Ishola A., Olaoye I. In silico profiling of histone deacetylase inhibitory activity of compounds isolated from Cajanus cajan. Beni-Suef Univ. J. Basic Appl. Sci. 2022;11:9. doi: 10.1186/s43088-021-00191-y. [DOI] [Google Scholar]
- 55.Hayati M.H., Kristinawati E., Wiadnya I.B.R. Antimitotic Activity of Pigeon Pea Filtrates (Cajanus cajan) to Sea Urchin (Diadema antillarum) Embryonic Cells. Malays. J. Med. Health Sci. 2021;17:23–26. [Google Scholar]
- 56.Özenver N., Kadioglu O., Fu Y., Efferth T. Kinome-Wide Profiling Identifies Human WNK3 as a Target of Cajanin Stilbene Acid from Cajanus cajan (L.) Millsp. Int. J. Mol. Sci. 2022;23:1506. doi: 10.3390/ijms23031506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foroughi A. A review on medicinal plants; An emphasis on antimicrobial effects. Vet. Res. Biol. Prod. 2022;35:2–17. doi: 10.22092/vj.2021.353171.1809. [DOI] [Google Scholar]
- 58.Dinore J.M., Farooqui M. GC-MS and LC-MS: An integrated approach towards the phytochemical evaluation of methanolic extract of Pigeon Pea [Cajanus cajan (L.) Millsp] leaves. Nat. Prod. Res. 2022;36:2177–2181. doi: 10.1080/14786419.2020.1849197. [DOI] [PubMed] [Google Scholar]
- 59.Brito S.A., Rodrigues F.F., Campos A.R., Da Costa J.G. Evaluation of the antifungal activity and modulation between Cajanus cajan (L.) Millsp. leaves and roots ethanolic extracts and conventional antifungals. Pharmacogn. Mag. 2012;8:103–106. doi: 10.4103/0973-1296.96550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nwachukwu E., Uzoeto H.O. Antimicrobial activities of leaf of Vitex doniana and Cajanus cajan on some bacteria. Researcher. 2010;2:37–47. [Google Scholar]
- 61.Pratima H., Mathad P. Antibacterial activity of various leaf extract of Cajanus cajan L. Bioscan. 2011;6:111–114. [Google Scholar]
- 62.Oyewole O., Owoseniand A., Faboro E. Studies on medicinal and toxicological properties of Cajanus cajan, Ricinus communis and Thymus vulgaris leaf extracts. J. Med. Plants Res. 2010;4:2004–2008. [Google Scholar]
- 63.Okigbo R., Omodamiro O. Antimicrobial effect of leaf extracts of pigeon pea (Cajanus cajan (L.) Millsp.) on some human pathogens. J. Herbs Spices Med. Plants. 2017;12:117–127. doi: 10.1300/J044v12n01_11. [DOI] [Google Scholar]
- 64.Aggarwal A., Nautiyal U., Negi D. Characterization and evaluation of antioxidant activity of Cajanus cajan and Pisum sativum. Int. J. Rec. Adv. Sci. Tech. 2015;2:21–26. doi: 10.30750/ijrast.214. [DOI] [Google Scholar]
- 65.Nahar L., Nasrin F., Zahan R., Haque A., Haque E., Mosaddik A. Comparative study of antidiabetic activity of Cajanus cajan and Tamarindus indica in alloxan-induced diabetic mice with a reference to in vitro antioxidant activity. Pharmacogn. Res. 2014;6:180–187. doi: 10.4103/0974-8490.129043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khanum R., Mazhar F., Jahangir M. Antioxidant evaluations of polar and non-polar fractions of Cajanus cajan seeds. J. Med. Plants Res. 2015;9:193–198. [Google Scholar]
- 67.Sharma S., Singh A., Singh B. Characterization of in vitro antioxidant activity, bioactive components, and nutrient digestibility in pigeon pea (Cajanus cajan) as influenced by germination time and temperature. J. Food Biochem. 2019;43:e12706. doi: 10.1111/jfbc.12706. [DOI] [PubMed] [Google Scholar]
- 68.Semwal P., Kapoor T., Anthwal P., Sati B., Thapliyal A. Herbal extract as potential modulator and drug for synaptic plasticity and neurodegenerative disorders. Int. J. Pharm. Sci. Rev. Res. 2014;25:69–79. [Google Scholar]
- 69.Da Costa I.M., Pedrosa E.C.G.A., de Carvalho Bezerra A.P., Fernandes L.C.B., de Paiva Cavalcanti J.R.L., Freire M.A.M., de Araújo D.P., do Rego A.C.M., Araujo Filho I., Pinheiro F.I. Neuroprotection-New Approaches and Prospects. IntechOpen; London, UK: 2020. Extracts and Essential Oils from Medicinal Plants and Their Neuroprotective Effect. [Google Scholar]
- 70.Kim Y.C. Neuroprotective phenolics in medicinal plants. Arch. Pharm. Res. 2010;33:1611–1632. doi: 10.1007/s12272-010-1011-x. [DOI] [PubMed] [Google Scholar]
- 71.Huang M.-Y., Lin J., Lu K., Xu H.-G., Geng Z.-Z., Sun P.-H., Chen W.-M. Anti-inflammatory effects of cajaninstilbene acid and its derivatives. J. Agric. Food Chem. 2016;64:2893–2900. doi: 10.1021/acs.jafc.6b00227. [DOI] [PubMed] [Google Scholar]
- 72.Akinloye O.A., Olaniyi M.O. Hepatoprotective effect of Cajanus cajan on tissue defense system in D-galactosamine-induced hepatitis in rats. Turk. J. Biochem./Turk Biyokim. Derg. 2011;36:237–241. [Google Scholar]
- 73.Iweala E.E.J., Evbakhavbokun W.O., Maduagwu E.N. Antioxidant and hepatoprotective effect of Cajanus cajan in N-nitrosodiethylamine-induced liver damage. Sci. Pharm. 2019;87:24. doi: 10.3390/scipharm87030024. [DOI] [Google Scholar]
- 74.Singh S., Mehta A., Mehta P. Hepatoprotective activity of Cajanus cajan against carbon tetrachloride induced liver damage. Int. J. Pharm. Pharm. Sci. 2011;3:146–147. [Google Scholar]
- 75.Winifred O., Emeka I.E. Protective Effect of Cajanus cajan in Hepatotoxic Rats; Proceedings of the IOP Conference Series: Earth and Environmental Science; Ogun, Nigeria. 18–20 June 2019; p. 012023. [Google Scholar]
- 76.Adama K., Almamy K., Isidore G.B., Bernadette Y., Amadou T., Aziz T.A., Siaka D., Hamidou T.H., Gaston B. Seed germination and anthelmintic activity of Cajanus cajan on sheep. J. Chem. Pharm. Res. 2016;8:403–410. [Google Scholar]
- 77.Zhao J., Li C., Wang W., Zhao C., Luo M., Mu F., Fu Y., Zu Y., Yao M. Hypocrea lixii, novel endophytic fungi producing anticancer agent cajanol, isolated from pigeon pea (Cajanus cajan [L.] M illsp.) J. Appl. Microbiol. 2013;115:102–113. doi: 10.1111/jam.12195. [DOI] [PubMed] [Google Scholar]
- 78.Patel N.K., Bhutani K.K. Pinostrobin and Cajanus lactone isolated from Cajanus cajan (L.) leaves inhibits TNF-α and IL-1β production: In vitro and in vivo experimentation. Phytomedicine. 2014;21:946–953. doi: 10.1016/j.phymed.2014.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.