Abstract
Since current studies indicate the possible involvement of Chlamydia pneumoniae in the pathogenesis of multiple sclerosis (MS), demonstration of C. pneumoniae in the cerebrospinal fluid (CSF) of patients with MS is highly desirable. However, there is controversy concerning the detection of C. pneumoniae in CSFs from MS patients due to the lack of a standard protocol for extraction and detection of C. pneumoniae DNA. In this regard, we attempted to establish a highly effective extraction protocol for C. pneumoniae DNA from CSFs utilizing a commercial kit and a PCR detection method. The extraction and PCR detection protocol established in this study succeeded in detecting as few as 20 C. pneumoniae organisms in 200 μl of mock CSF. The use of this protocol to detect C. pneumoniae DNA in CSFs revealed that 68% of CSF samples obtained from patients with MS were positive (11 out of 16 samples) for chlamydia DNA. Thus, the protocol established here is sensitive enough to detect chlamydia DNA from CSFs and can be used by other laboratories for evaluation of the presence of chlamydiae in CSFs because the protocol is based on the use of a commercial kit.
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system (CNS) characterized by focal areas of demyelination. Although the exact etiology of MS is unknown, it is generally accepted that autoimmunity is involved and that the autoantigen(s) probably resides in CNS myelin, the target of the immune response (1). In this regard, current studies argue for an infectious agent as an initiating or enhancing factor for MS with immunological mechanisms (5). To identify a specific causative agent for MS, many groups have attempted to detect microbes in cerebrospinal fluid (CSF) as well as in CNS lesions obtained from MS patients. However, no consistent results have been obtained with any given pathogen. Recent studies conducted by Sriram et al. (12) highlighted the possible involvement of a bacterium in MS, with the finding of Chlamydia pneumoniae in the CSF of almost all patients with MS but in only a small proportion of CSF samples from control subjects without MS. That study has shown the highest association of any organism with MS to date. However, other research groups either could not detect C. pneumoniae in CSFs from MS patients or detected it only in a small proportion of specimens (2, 8, 14). This may be due to the lack of a standard method for C. pneumoniae detection in CSFs. For study of the involvement of C. pneumoniae in the pathogenesis of MS, a reliable standard evaluation protocol for C. pneumoniae in clinical specimens is essential. Therefore, in the present study, we attempted to establish an efficient extraction protocol for C. pneumoniae DNA in CSFs by use of a commercial kit followed by PCR specific for C. pneumoniae. Furthermore, the extraction and detection system established for C. pneumoniae DNA was applied to demonstration of the presence of C. pneumoniae in CSFs obtained from patients with MS. The results indicate that the protocol established was sufficient to detect C. pneumoniae DNA in CSFs of patients with MS.
MATERIALS AND METHODS
CSF.
Sixteen CSF samples from nine patients with MS were collected at the University Hospital of St. Marianna University School of Medicine, Kawasaki, Japan, and stored at −80°C until they were used for an assessment. All MS patients were diagnosed clinically, with six diagnosed, as probable MS patients and three as definite MS patients. The study protocol was approved by the University Ethics Committee.
Bacterial DNA.
Formalin-fixed C. pneumoniae (strain TWAR) organisms were obtained from the Washington Research Foundation, Seattle, Wash. The chlamydia organisms were spiked into phosphate-buffered saline (PBS) with 2% fetal calf serum (FCS) at concentrations from 106 to 101 bacterial particles/ml.
DNA extraction.
Bacterial DNA was extracted from 200 μl of PBS–2% FCS spiked with C. pneumoniae or from CSFs from MS patients using either a QIAmp Blood Mini Kit (QIAGEN Inc., Valencia, Calif.) or a QIAmp DNA Mini Kit with a bacterial DNA extraction protocol. When the QIAmp DNA Mini Kit with bacterial DNA extraction was used, 200 μl of sample was centrifuged for 30 min at 20,000 × g. The pellet was resuspended in 180 μl of buffer ATL (QIAGEN) with 20 μl of proteinase K and then incubated at 56°C with occasional vortexing until the pellet was completely lysed, which usually took 30 min. After lysis of the sample, 200 μl of buffer AL was added to the sample and the mixture was incubated for 10 min at 70°C. The mixture was then combined with 200 μl of absolute ethanol and mixed by pulse-vortexing for 15 s. The mixture was applied to a spin column, which holds a silica gel membrane, and spun for 1 min at 6,000 × g. The spin column was washed with 500 μl of buffer AW2 by centrifugation at 20,000 × g for 3 min. The DNA bound on a membrane was eluted by centrifugation with 50 μl of buffer AE after a 5-min incubation at room temperature. The resulting DNA extracts were stored at −20°C until PCR assessment. When the QIAmp Blood Mini Kit was used, 200 μl of sample was combined with 20 μl of protease and 200 μl of buffer AL and then incubated at 56°C for 10 min. After incubation, the mixture was combined with 200 μl of absolute ethanol and mixed by pulse-vortexing for 15 s; then the protocol described, for the QIAmp DNA Mini Kit was followed. All reagents and spin columns were supplied in the kit (QIAGEN).
PCR.
The extracted DNAs were subjected to PCR with primers specific for C. pneumoniae omp1 (7) or the 16S rRNA gene (4). In brief, 2 μl of DNA extracts was processed in a 25-μl reaction volume containing PCR buffer (10 mM Tris [pH 9.0], 50 mM KCl, 0.01% gelatin), 200 μM deoxynucleoside triphosphates, 3.5 mM MgCl2, 0.5 μM each primer, and 1 U of Taq polymerase (Promega, Madison, Wis.). The sequences of the primers are shown in Table 1. Amplifications were carried out in a Minicycler (MJ Research, Watertown, Mass.). The first cycle, consisting of a 5-min denaturation at 94°C, was followed by 50 cycles each of 30 s at 94°C, 45 s at 50°C, and 1 min, 30 s, at 72°C, with a final extension for 10 min at 72°C. In the case of the temperature gradient experiment, a range of annealing temperatures from 45 to 58°C was carried out in a Mastercycler gradient (Eppendorf Scientific Inc., Westbury, N.Y.).
TABLE 1.
Primer sequences used for PCR
| Primer | Sequence | Size of fragment (bp) |
|---|---|---|
| omp1 | ||
| Sense | 5′-TTA TTA ATT GAT GGT ACA ATA-3′ | 207 |
| Antisense | 5′-ATC TAC GGC AGT AGT ATA GTT-3′ | |
| 16S rRNA | ||
| Sense | 5′-TCT AAC GAG ACT GCC TGG GT-3′ | 231 |
| Antisense | 5′-GTA TTC ACG GCG TTA TGG CT-3′ |
Optimized conditions for PCR, such as pH and concentration of Mg2+, were determined using a PCR Optimizer Kit (Invitrogen, Carlsbad, Calif.). The PCR products were visualized in 2% agarose gels containing 0.5 μg of ethidium bromide/ml. The specificity of the PCR products for omp1 was confirmed by Southern blot analysis with a probe of omp1 cDNA provided by S. Sriram, Vanderbilt Stallworth Rehabilitation Hospital, Nashville, Tenn.
RESULTS
C. pneumoniae DNA extraction.
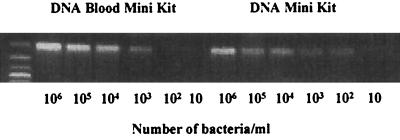
In order to determine whether commercial DNA extraction kits can be used for extraction of C. pneumoniae DNA from CSF, two commercial kits, the QIAmp Blood Mini Kit and the QIAmp DNA Mini Kit, were used for this purpose. The QIAmp Blood Mini Kit is designed to extract mammalian DNA from biological specimens, including whole blood and body fluids, and has been utilized for extraction of C. pneumoniae DNA from CSF (2, 8). The QIAmp DNA Mini Kit is designed for extraction of DNA from solid tissues and bacteria. The C. pneumoniae-spiked PBS-FCS was used as a mock CSF with bacteria for evaluation of the efficacies of extraction of C. pneumoniae DNA by the two kits. Two hundred microliters of the mock CSF, which contained various concentrations of C. pneumoniae, 106 to 101 organisms/ml was processed with each of the two kits, and the resulting DNA was dissolved into 50 μl of the buffer. The extracted DNA was further subjected to PCR with primers specific for C. pneumoniae omp1. The results are shown in Fig. 1. The QIAmp DNA Mini Kit with a bacterial DNA extraction protocol showed high efficiency in extracting C. pneumoniae DNA from mock CSF compared with the QIAmp Blood Mini Kit. As few as 20 bacteria in 200 μl of mock CSF (100 bacteria per ml of sample) were consistently detected in the experiments by the protocol of the DNA Mini Kit, whereas the lower limit of detection for the Blood Mini Kit was 200 bacteria.
FIG. 1.
Comparison of extraction efficacy of C. pneumoniae DNA from mock CSFs using two DNA extraction kits. The mock CSFs (200 μl) spiked with serially diluted bacteria were extracted either with the QIAmp DNA Mini Kit with a bacterial DNA extraction protocol or with the QIAmp DNA Blood Mini Kit. Two microliters of the extracted DNA (50 μl) was subjected to PCR with primers for omp1. Results are representative of three experiments.
PCR conditions for C. pneumoniae DNA.
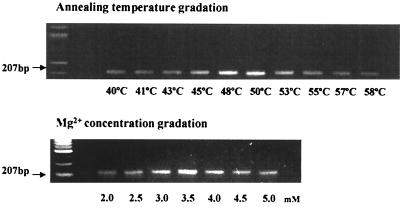
Since PCR conditions, such as the annealing temperature, concentrations of Mg2+, and pH, are known to affect the final products of PCR, the optimized PCR conditions for primers specific for C. pneumoniae omp1 and the 16S rRNA gene were determined. As shown in Fig. 2, the optimized annealing temperature and the optimized concentration of Mg2+ for PCR with primers for omp1 were 50°C and 3.5 mM, respectively. The optimized pH for the PCR was 9.0. The optimized PCR conditions for the 16S rRNA gene were also determined. It was found that the conditions for 16S rRNA gene PCR were the same as those for the omp1 PCR (data not shown).
FIG. 2.
Optimization of annealing temperature and Mg2+ concentration for PCR with primers for omp1. The annealing temperature was optimized in a Mastercycler gradient (Eppendorf). The Mg concentration for PCR specific for omp1 was optimized with a PCR optimization kit (Invitrogen). The target DNA for PCR was extracted from C. pneumoniae with the QIAmp DNA Mini Kit.
Sensitivity of detection of C. pneumoniae DNA by PCR.
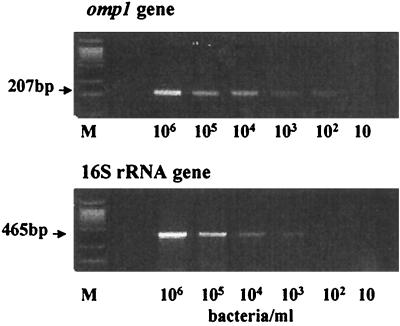
Since it is known that the sensitivity of PCR for detecting chlamydia antigen in clinical specimens is dependent on the primers used (9), we examined the sensitivities of two PCRs, one with primers for omp1 and one with primers for the 16S rRNA gene. As shown in Fig. 3, the PCR specific for omp1 was at least 10 times more sensitive than the PCR for the 16S rRNA gene. The DNA obtained from more than 0.8 chlamydia organism in 2 μl was detected by the PCR for omp1. In contrast, the PCR for the 16S rRNA gene detected DNA extracted from more than 8 chlamydia organisms.
FIG. 3.
Detection sensitivity of PCR for omp1 versus the 16S rRNA gene. Two hundred microliters of the mock CSF containing a specific number of C. pneumoniae organisms (as indicated), was used for DNA extraction with the QIAmp DNA Mini Kit. A 2-μl portion of the 50-μl volume of DNA extracts was subjected to PCR. The optimized PCRs for omp1 and 16S rRNA gene were conducted (see Materials and Methods).
Detection of C. pneumoniae DNA in CSFs.
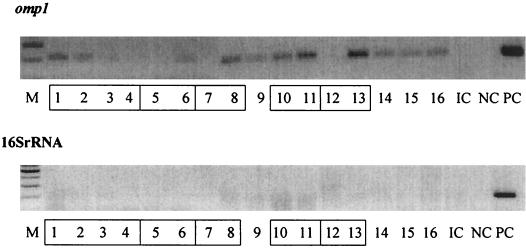
In order to determine how the established system is sensitive enough to detect C. pneumoniae DNA in CSFs, CSFs obtained from patients with MS were utilized. The DNAs extracted from 16 CSFs from nine MS patients were evaluated by PCR with primers specific for either omp1 or the 16S rRNA gene. Figure 4 shows a representative PCR result of three experiments, which indicates clearly that the system utilizing the PCR with primers specific for omp1 was sensitive enough to detect C. pneumoniae DNA in the CSF of patients. The proportion of CSFs positive for C. pneumoniae DNA was 68% (11 out of 16 CSF samples), but the proportion of MS patients among the CSF donors was 100% (9 out of 9 patients). It is noteworthy that CSF samples obtained at different clinical stages from the same patients were not all PCR positive. In contrast with the PCR for omp1, when the PCR with primers for the 16S rRNA gene was utilized for detection of C. pneumoniae DNA in CSFs, no positive result was obtained, even though positive controls showed a strong band for PCR-specific product, indicating that the PCR worked correctly.
FIG. 4.
Detection of C. pneumoniae DNA by PCR in CSFs obtained from patients with MS. Two hundred microliters of CSFs obtained from MS patients was processed for extraction of DNA utilizing the QIAmp DNA Mini Kit, and 2 μl of the resulting 50-μl DNA solution was subjected to PCR with primers for either omp1 or the 16S rRNA gene. Data are representative of three PCR experiments. Boxed numbers indicate CSFs obtained from the same patient at different time points. Each number is the sample number. M, molecular marker; PC, positive control for PCR; NC, negative control for PCR; IC, negative control for DNA extraction.
DISCUSSION
Since bacteria have a rigid cell wall, which may resist an ordinary digestion protocol for DNA extraction, the extraction protocol for bacterial DNA in clinical specimens should be considered. C. pneumoniae is a gram-negative bacterium and has peptidoglycan, lipopolysaccharide, and other outer membrane components in its cell wall (6, 11), which contribute to osmotic stability as well as to rigidness, particularly of elementary bodies, an infectious form that resists physical and chemical pressures in the extracellular environment. Therefore, the procedure for extraction of C. pneumoniae DNA from clinical specimens must be designed for bacterial DNA extraction, particularly for specimens which may have few bacteria, such as CSF of MS patients. In fact, when two extraction protocols, one designed for extraction of mammalian DNA from blood samples and one designed for extraction of bacterial DNA, were examined in this study, the extraction protocol for bacterial DNA (QIAmp DNA Mini Kit with a bacterial DNA extraction protocol) extracted C. pneumoniae DNA more efficiently.
The PCR protocol and selection of target genes for PCR are also critical for the overall sensitivity of detection by PCR. In the present study, we attempted to establish the optimized PCR conditions for two different chlamydia target genes, i.e., species-specific omp1 and the 16S rRNA gene. Even though the sequences of the primers for omp1 and the 16S rRNA gene were different, the optimized PCR conditions, such as annealing temperature, concentration of Mg2+, and pH, were the same for the two PCRs. The detection sensitivities of the two PCRs with omp1 versus 16S rRNA gene primers were compared under the same PCR conditions, which were optimal for both primers. It was found that the PCR for omp1 was at least 10 times more sensitive than that for the 16S rRNA gene. Furthermore, when both PCRs were used for detection of C. pneumoniae in CSFs obtained from MS patients, the PCR for the 16S rRNA gene could not detect any C. pneumoniae DNA, even though the PCR for omp1 detected C. pneumoniae DNA in the same CSF samples. These results indicate that the number of C. pneumoniae organisms in CSFs from MS patients was low and was not sufficient to be detected by the PCR for the 16S rRNA gene. Therefore, the sensitivity of detection by PCR as well as the DNA extraction efficacy is critical for detection of C. pneumoniae in clinical specimens of patients with neurological disease, such as MS.
The recent study by Mahony et al. showed similar findings, i.e., there are differences in sensitivity among PCRs with three different primers for C. pneumoniae DNA (9). The reason for these differences in sensitivity is not known. The target copy numbers of the two genes in C. pneumoniae are not much different, since only a single omp1 gene and only two rRNA operons are present in the C. pneumoniae genome (13). Therefore, differences in sensitivity between the primers specific for omp1 and those specific for the 16S rRNA gene may be due to the nature of each primer sequence, which may affect the affinity of the primer for the target DNA.
The sensitivity of the omp1 PCR (DNA obtained from more than 0.8 C. pneumoniae organism per PCR) was not comparable with previous reports of C. pneumoniae DNA detection by PCR, which showed a sensitivity as low as 0.004 to 0.4 inclusion-forming unit (IFU; activity of chlamydial inclusion formation in epithelial cells) of C. pneumoniae (3, 4, 9). However, these reports used sequentially diluted DNAs as test samples, which are different from the individually extracted DNAs from different concentrations of C. pneumoniae. Furthermore, the number of IFU utilized in previous studies may contain more bacterial particles due to the counting of infectious bacteria only.
The application of the extraction and detection protocol established in this study to clinical specimens, such as CSFs obtained from patients with MS, succeeded in detecting C. pneumoniae DNA. The proportion of PCR-positive CSFs among those tested was as high as 68% (11 out of 16 samples). In previous studies by other investigators, the percentage of CSFs from MS patients that tested positive for C. pneumoniae DNA by PCR was variable, from 97 to 0% (2, 8, 12). Sriram et al. employed their own DNA extraction protocol for isolation of C. pneumoniae DNA from CSF and obtained a high positive rate for CSFs from MS patients (12). However, their DNA extraction protocol requires many sensitive steps with house reagents and a tiny DNA precipitate, which necessitated guesswork. In contrast, both Boman et al. (2) and Layh-Schmitt et al. (8) utilized a commercial kit (QIAmp Blood Mini Kit) for isolation of C. pneumoniae DNA, but they failed to detect bacterial DNA in CSFs of MS patients or found only a low positive rate. As shown in this study, the QIAmp Blood Mini Kit is not designed for isolation of bacterial DNA and was less effective for extracting bacterial DNA from CSFs, particularly when specimens contained few bacteria. The reasons for the variation in the C. pneumoniae DNA positive rate between the reports are not clear. However, from the findings in this study, it can be speculated that the extraction protocol used may be one of the reasons.
The percentage of CSFs in this study that tested positive for C. pneumoniae DNA does not permit any conclusion regarding the possible involvement of C. pneumoniae in the pathogenesis of MS, since only a limited number of CSFs were tested, and these included no control CSFs obtained from non-MS patients. Although the aim of this study was not to evaluate a possible involvement of C. pneumoniae in MS, it is noteworthy that both positive and negative PCR results were observed in CSFs obtained from the same patients at different clinical stages. These results may indicate a possible relation between a recrudescence of C. pneumoniae in the CSF and clinical symptoms or treatments, because these patients were treated with steroids for a certain period between CSF samplings (data not shown). Nevertheless, the extraction and detection protocol established for C. pneumoniae DNA was demonstrated to be sufficiently sensitive to detect only a few C. pneumoniae organisms in CSFs. Since the protocol established was based on a commercial kit, this can be used by other laboratories to assess the presence of C. pneumoniae in CSFs.
ACKNOWLEDGMENT
We thank Subramaniam Sriram, Vanderbilt Stallworth Rehabilitation Hospital, for kindly supplying omp1 cDNA.
REFERENCES
- 1.Amor S, Baker D, Layward L, McCormack K, van Noort J M. Multiple sclerosis: variations on a theme. Immunol Today. 1997;8:368–371. doi: 10.1016/s0167-5699(97)01100-6. [DOI] [PubMed] [Google Scholar]
- 2.Boman J, Roblin P M, Sundström P, Sundström M, Hammerschlag M R. Failure to detect Chlamydia pneumoniae in the central nervous system of patients with MS. Neurology. 2000;54:265. doi: 10.1212/wnl.54.1.265. [DOI] [PubMed] [Google Scholar]
- 3.Campbell L A, Melgosa M P, Hamilton D J, Kuo C-C, Grayston J T. Detection of Chlamydia pneumoniae by polymerase chain reaction. J Clin Microbiol. 1992;30:434–439. doi: 10.1128/jcm.30.2.434-439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaydos C A, Quinn T C, Eiden J J. Identification of Chlamydia pneumoniae by DNA amplification of the 16S rRNA gene. J Clin Microbiol. 1992;30:796–800. doi: 10.1128/jcm.30.4.796-800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilden D H. Chlamydia: a role for multiple sclerosis or more confusion? Ann Neurol. 1999;46:4–5. doi: 10.1002/1531-8249(199907)46:1<4::aid-ana3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Hatch T P. Developmental biology. In: Stephens R S, editor. Chlamydia: intracellular biology, pathogenesis, and immunity. Washington, D.C.: American Society for Microbiology; 1999. pp. 29–67. [Google Scholar]
- 7.Jantos C A, Roggendorf R, Wuppermann F N, Hegemann J H. Rapid detection of Chlamydia pneumoniae by PCR-enzyme immunoassay. J Clin Microbiol. 1998;36:1890–1894. doi: 10.1128/jcm.36.7.1890-1894.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Layh-Schmitt G, Bendl C, Hidt U, Dong-Si T, Jüttler E, Schnitzler P, Grond-Ginsbach C, Grau A J. Evidence for infection with Chlamydia pneumoniae in a subgroup of patients with multiple sclerosis. Ann Neurol. 2000;47:652–655. [PubMed] [Google Scholar]
- 9.Mahony J B, Chong S, Coombes B K, Smieja M, Petrich A. Analytical sensitivity, reproducibility of results, and clinical performance of five PCR assays for detecting Chlamydia pneumoniae DNA in peripheral blood mononuclear cells. J Clin Microbiol. 2000;38:2622–2627. doi: 10.1128/jcm.38.7.2622-2627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newhall W J, Batteiger B, Jones R B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982;38:1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson E M, de la Maza L M, Brade L, Brade H. Characterization of a neutralizing monoclonal antibody directed at the lipopolysaccharide of Chlamydia pneumoniae. Infect Immun. 1998;66:3848–3855. doi: 10.1128/iai.66.8.3848-3855.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sriram S, Stratton C W, Yao S, Tharp A, Ding L, Bannan J D, Mitchell W M. Chlamydia pneumoniae infection of the central nervous system in multiple sclerosis. Ann Neurol. 1999;46:6–14. [PubMed] [Google Scholar]
- 13.Stephens R S, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov R L, Zhao Q, Koonin E V, Davis R W. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 14.Treib J, Haaß A, Stille W, Maass M, Stephan C, Holzer G, Morgenthaler M, Woessner R, Grauer M T. Multiple sclerosis and Chlamydia pneumoniae. Ann Neurol. 2000;47:408. doi: 10.1002/1531-8249(200003)47:3<408::aid-ana24>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]