Abstract

Mold growth, especially Aspergillus spp. and Penicillium spp., deteriorates the quality of bakery products. Essential oils (EOs) have been categorized as good natural antimicrobials. Hereby, this study aimed to evaluate the antifungal activity of six EOs, ginger, cumin, cinnamon, black pepper, origanum, and clove, and their volatile compounds against fungal strains isolated from bread: Penicillium carneum DDS4, Aspergillus flavus DDS6, and Aspergillus niger DDS7 by disc diffusion and disc volatilization methods, respectively. Among EOs, cumin, cinnamon, origanum, and clove were found to be effective against fungal strains, and their minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) were determined. The observed lowest MIC value of EOs was obtained at 1000 μg/mL concentration, and the lowest MFC value was obtained from the results of clove at a concentration of 1000 μg/mL. Based on the MIC and MFC values, clove and cinnamon EOs were found to be more effective at lower concentrations. Electrospun nanofiber films of clove and cinnamon were produced with 6% poly(vinyl alcohol) (PVA), 2% β-cyclodextrin (β-CD), and 2% EO to overcome the unfavorable sensory impact of EOs on food products. The inhibitory activity of cinnamon EO film (2.64–2.51 log(CFU/mg)) was considerably lower than clove EO film (3.18–3.24 log(CFU/mg)) against P. carneum DDS4 and A. niger DDS7. Furthermore, these nanofiber films prevented fungal growth on bread samples visibly and were shown to be an alternative application for active food packaging.
1. Introduction
Food safety is a common public health issue due to the increase in food-borne diseases. Approximately 600 million people fall ill from consuming contaminated food, and 90% of recorded cases result from only diarrheal disease, causing 230,000 deaths every year.1 In recent years, there has been an observable effort to increase food products’ quality and safety by using natural antimicrobials by virtue of their inhibition ability of bacterial and fungal growth.2,3 Plants, animals, bacteria, algae, and fungi are the main sources of natural antimicrobials, and the antimicrobial effectiveness of plant-based products, including EOs, was shown in many studies.4−7 EOs are secondary metabolites of plants, and the presence of several components in their composition has been demonstrated as the main reason for their antimicrobial activity.8,9 In addition to their antimicrobial effects, EOs may also exhibit different activities such as antiviral,10,11 antimycotic,12 antiparasitic,13,14 and insecticidal.15,16 However, these activities, which are mainly dependent on the composition affected by various factors, including extraction method,17,18 harvesting time,19,20 geographical origin, and plant parts,21,22 differ even for the same plant.
The antimicrobial activity of EOs has been reported in several studies.23−26 Although most of the studies have focused on the antibacterial activities of EOs, their antifungal activity has been a subject of recent studies.27−30 Although there are studies showing the in vivo antifungal effect of EOs for several foods,31,32 it is also necessary to focus on studies showing the antifungal effect of EOs without changing the food composition or direct EO treatment. There are some generally mentioned mechanisms of action, including the destruction of the cell wall, damage to protein and membrane, changes in permeability, and inhibition of synthesis pathways.33−35
Bread is one of the most consumed products in the bakery industry. However, it has a short shelf life, and some physicochemical changes occur during storage. As a result, bread quality deteriorates gradually, and that loss is associated with microbial spoilage in general.36Penicillium spp. and Aspergillus spp. are the most common spoilage molds for bread.37,38 Therefore, recent technologies have been getting attention to inhibit spoilage and increase shelf life without any quality loss.
Food packaging is an important part of the food industry both to ensure safety and to reduce food waste.39,40 EOs are classified as generally recognized as safe, and they have been incorporated into packaging material as antimicrobial agent.41 However, their usage is often limited due to their strong flavors, and above a certain dosage they may not be acceptable organoleptically. Nowadays, new approaches provide admissible solutions for the utilization of EOs as food packaging components. Therefore, encapsulation of EOs is important to mask the undesirable flavor of EOs, to distribute EOs in the food matrix, and increase both the solubility and antimicrobial activity.42−45 In addition, the fact that EO encapsulation is stated as a green solution in terms of food safety has led researchers to focus on this issue.46 Cyclodextrins are among the agents used for encapsulation,47 and β-CD is a widely used natural CD type due to its cavity size, cost, and ability to encapsulate hydrophobic compounds.42,48 However, a limited number of studies demonstrated the antifungal activity of encapsulated EOs.49−51
On the other hand, the release and effect mechanism of EOs may change according to the type of packaging.52 Nanoencapsulation of antimicrobial agents with polymers is one of the proposed methods for encapsulation. The used polymers can be synthetic or biobased like lipids, polysaccharides, and protein. The edible and nontoxic properties of biobased polymers are their major advantages, and they also provide the ability to control shelf life. PVA is a nontoxic and biocompatible polymer and is commonly selected for electrospinning solution preparation of antimicrobial agents.53,54 Many strategies have been developed to produce nanomaterial, and electrospinning is a well-referenced and attractive method.55 The electrospun nanofibers provide several advantages, including a large surface-to-volume ratio, high porosity, and favorable structure characteristics.56,57
In this respect, the aim of this study is to investigate and compare the antifungal activity of essential oils and their electrospun nanofibers against molds isolated from bread: Penicillium carneum, Aspergillus flavus, and Aspergillus niger. Hence, this study differentiates from other studies by using isolated and identified target microorganisms, examining the antifungal activity, and encapsulating essential oils using β-CD. Additionally, it is thought that this study will increase the understanding of active packaging by applying produced electrospun nanofibers to bread.
2. Results and Discussion
2.1. Identification of Fungal Cultures Isolated from Bread
A total of 12 different fungal cultures were isolated from selected bread samples. After the microscopic observation, five selected fungal cultures were molecularly identified (Table 1).
Table 1. Identification of Fungal Cultures Isolated from Bread.
Among them, three fungal cultures showing a homology of 99% to Penicillium carneum DDS4, Aspergillus flavus DDS6, and Aspergillus niger DDS7 were selected for further experiments. They also were mentioned as the most common bread spoilage fungi in refs (37) and (38).
2.2. Antifungal Properties of Essential Oils
2.2.1. Disc Diffusion and Disc Volatilization Method
The assessment of the antifungal activity of the EOs was carried out by both diffusion and vapor assays. As shown in Table 2, except ginger EO (GEO), all remaining EOs exhibited antifungal activity. While origanum EO (OEO) exhibited greater inhibition zones against all tested fungal isolates, black pepper EO (BPEO) showed slight inhibition against only P. carneum DDS4. However, Li et al.58 determined the notable antifungal activity of black and white pepper; it can be interpreted that this result may be due to the type of tested fungal culture and compositional differences of EOs. Figure S1 shows images of disc diffusion plates conducted to determine the antifungal activity of EOs.
Table 2. Inhibition Zones (Diameter, cm) of Essential Oils (EOs), and Volatile Components of Essential Oils (VEO) (Mean ± SD).
|
Penicillium carneum DDS4 |
Aspergillus flavus DDS6 |
Aspergillus niger DDS7 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EO |
VEO |
EO |
VEO |
EO |
VEO |
|||||||
| EOs§ | 3rd day | 6th day | 3rd day | 6th day | 3rd day | 6th day | 3rd day | 6th day | 3rd day | 6th day | 3rd day | 6th day |
| BPEO | 4.7 ± 0.3c | NEe | 2.3 ± 0.3c | 1.6 ± 0.6c | NEe,* | NEd | NEd | NEc | 0.9 ± 0.1d | 0.8 ± 0.14d | NEc,# | NEc |
| CEO | 6.8 ± 0.1b | 7.0 ± 0.4b | 4.2 ± 1.0b | 3.0 ± 0.4b | 3.9 ± 0.1c | 3.9 ± 0.1b | 2.2 ± 0.5c | 1.7 ± 0.3b | 3.8 ± 0.4c | 2.7 ± 0.2c | 1.6 ± 0.3b | 0.7 ± 0.2b |
| CLEO | 7.3 ± 0.1b | 7.3 ± 0.1b | 3.9 ± 0.0b | 3.0 ± 0.6b | 4.3 ± 0.5c | 4.6 ± 0.6b | 2.5 ± 0.0c | 2.0 ± 0.2b | 4.1 ± 0.1c | 3.8 ± 0.1b | 2.1 ± 0.0b | 0.9 ± 0.0b |
| CUEO | >9a,# | 3.7 ± 1.06c | >9a | >9a | >9a3 | 1.6 ± 0.7c | >9a | NEc | 6.6 ± 0.3b | 1.0 ± 0.3d | NEc | NEc |
| GEO | 1.7 ± 0.5d | 1.2 ± 0.3d | NEd | NEd | NEd | NEd | NEd | NEc | NEd | NEe | NEc | NEc |
| OEO | >9a | >9a | >9a | >9a | 7.5 ± 0.4b | >9a | 5.3 ± 0.6b | 4.7 ± 0.6a | >9a | >9a | 6.3 ± 0.6a | 6.2 ± 0.8a |
NE: Noninhibitory effect.
>9: Strong inhibition effect. No growth has been detected.
Values within the same column with different superscript small letters are significantly different (p < 0.05).
BPEO: black pepper essential oil, CEO: cinnamon essential oil, CLEO: clove essential oil, CUEO: cumin essential oil, GEO: ginger essential oil, OEO: origanum essential oil.
Moreover, the results indicated that fungal cultures showed varied sensitivity to EOs. In the meantime, the sensitivity of a culture to different EOs demonstrated variation as parallel to the study of Davari and Ezazi.59 Here, P. carneum DDS4 was found to be less resistant against EOs among studied fungal cultures, while remarkable growth of A. flavus DDS6 and A. niger DDS7 were stated under the same conditions. Until now, few studies have compared the susceptibility of strains to EOs. While it was found that there were susceptibility differences between Penicillium spp.,60,61 the difference between various genera should be found to be acceptable.
Generally, the increases in the incubation period decreased the inhibitory effect of EOs in both diffusion and vapor assays. As can be seen from Table 2, it was possible to conclude that OEO had a prolonged inhibitory effect among four EOs.
On the other hand, the disc volatilization method provided knowledge about the antifungal ability of volatile components in the composition of EOs due to the absence of direct contact with fungal culture. This is an important feature to provide biocontrol with the help EOs. A study by Perumal et al.62 showed that EO vapor had a non-negligible effect on inoculated mango, and its effectiveness increased with increasing concentration, as expected. Though volatile components possessed antifungal activity, it was seen that EOs in the liquid form exhibited stronger antifungal activity than their vapor phase. The study carried out to investigate the antibacterial activity of EOs against several pathogens also found that the volatile component showed a remarkable but usually lower antibacterial effect than the liquid form.63 It is known that EOs show their antimicrobial effect through various mechanisms.64 The less effective volatile components may be due to the difference in their mechanism of action.
In light of these findings, cinnamon EO (CEO), clove EO (CLEO), cumin EO (CUEO), and OEO were chosen for further study.
2.2.2. Poisoned Culture Method
After the initial screening of the antifungal activity of EOs, the MIC and MFC values of selected EOs were determined. As seen in Table 3, the MIC and MFC values of EOs ranged from 1000 to 3000 μg/mL. Except for CUEO, the MFC values of the remaining EOs were generally higher than their MIC value. In addition, the MFC values of CEO were higher than the MIC values against all tested fungal strains.
Table 3. Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) Values of Four Essential Oils (EOs) for Identified Fungal Cultures.
| MIC (μg/mL) |
MFC (μg/mL) |
|||||
|---|---|---|---|---|---|---|
| EOsb | P. carneuma DDS4 | A. flavusa DDS6 | A. nigera DDS7 | P. carneuma DDS4 | A. flavusa DDS6 | A. nigera DDS7 |
| CEO | 1000 | 1500 | 1000 | 1500 | 2000 | 1500 |
| CLEO | 1000 | 1000 | 1500 | 1000 | 1500 | 1500 |
| CUEO | 3000 | 2000 | 1500 | 3000 | 2000 | 1500 |
| OEO | 2000 | 2000 | 1500 | 3000 | 3000 | 1500 |
P. carneum, Penicillium carneum; A. flavus, Aspergillus flavus; A. niger, Aspergillus niger.
CEO: cinnamon essential oil, CLEO: clove essential oil, CUEO: cumin essential oil, OEO: origanum essential oil.
CLEO exhibited high antifungal activity with a MIC value of 1000 μg/mL against P. carneum DDS4 and A. flavus DDS6, and its highest MIC value was 1500 μg/mL for A. niger DDS7. Pinto et al.65 reported MIC values of clove EO for Aspergillus spp. between 0.32 and 0.64 μL/mL, and MFC values were determined as 1.25 μL/mL. Purkait et al.66 determined the MFC values of clove and cinnamon EOs against Aspergillus niger. Compared to the current study, the MFC values of EOs were found to be lower (65–73 μg/mL). This observed difference may be related the use of different Aspergillus species and differences in EO composition. Similar to this situation, Melo et al.67 found that the MIC value of Ocimum gratissimum L. EO against S. aureus changed based on the its strains (1000 μg/mL for ATCC 6538 and 2000 μg/mL for 5B). Another study performed by 12 different strains of E. coli showed that the MIC value of the same EO changed between 50 and 250 ppm.68 Raybaudi-Massilia et al.69 also concluded that different MIC/MBC values of EOs are available in the literature, and not only the specific strain but also the method applied were effective. They also commented that these parameters make it difficult to compare with the literature.
When the MIC and MFC values of the citrus-derived EOs were analyzed against Penicillium spp. and Aspergillus spp., these values were found to be the same, or the MFC value was higher. The MIC and MFC values determined for Aspergillus species were found to be in the range of 560–1130 and 560–2250 μg/mL, respectively, and it has been observed that there are similarities with the results of some EOs in the current study.70 OEO exhibited the same MIC (2000 μg/mL) and MFC (3000 μg/mL) values for P. carneum DDS4 and A. flavus DDS6. Manso et al.71 reported the MIC values of cinnamon and origanum EOs against A. flavus as 100 and 800 ppm, respectively, which were lower than the current study. On the other hand, Vitoratos et al.61 determined MIC value of origanum as 0.02 μL/mL (20 μg/mL for density: 1 g/mL) for Botrytis cinerea, Kocić-Tanackov et al.72 found 2 μL/mL (2000 μg/mL for density: 1 g/mL) for Aspergillus versicolor. These different results seen in the literature may be due to the difference in EO composition. For example, while carvone was the major compound (18.05%) of origanum in the study mentioned above,72 the isomerized form of carvone, carvacrol (57.22%), was found to be noticeably different in the composition of used OEO in the current study.
In another study, the MIC values of cinnamon and clove EOs for Aspergillus parasiticus were found to be 1000 and 1500 ppm, respectively, and were similar to the obtained findings. However, the MIC value for the EOs of cumin, black pepper, and ginger could not be determined in the studied concentration range of 500–2500 ppm.27 Apart from the differences that may arise from the composition of EOs, it has been interpreted that the reason for the detection of high MIC values by in vitro studies may be the interaction of phenolic components with the food matrix.
Although OEO and CUEO were found to be highly effective against fungal strains by the disc diffusion method, MIC and MFC values showed that the required concentration of OEO and CUEO to inhibit fungal cultures were higher than CEO and CLEO. Similar situations have been reported in the literature. In the study examining the antibacterial effect of Lippia grandis EO, although the Escherichia coli ATCC 35218 inhibition zone (29.3 mm) was higher than that for Enterococcus faecalis ATCC 29212 (13 mm), the MIC value for E. faecalis (0.57 mg/mL) was found to be lower than that for E. coli (1.15 mg/mL).73 In addition, Melo et al.67 reported that EOs with high inhibition zones in their study gave a high MIC value result.
2.3. Nanofiber Film Characterization
It was found that PVA, the polymer used in this study, formed a higher-quality fiber, and the parameters were optimized74 as in the publication of Wen et al.42 The nanofiber films were developed by incorporating PVA (6%), EO (2%), and β-CD (1%). From the operation conditions, the voltage was adjusted to 14–16 kV, the distance was 12 cm, and the flow rate was 0.4 mL/h. In this study, the EO concentration was determined on the basis of studies about nanofiber production with the most optimum properties. While Seydim and Sarikus75 could see no antimicrobial effect with 1% EO, the study of Wen et al.42 used a cinnamon EO concentration in the range of 1–3% and decided to use 2% for an antimicrobial effect. PVA and β-CD concentrations were also determined as the most optimum according to the viscosity and conductivity of the electrospinning solution.
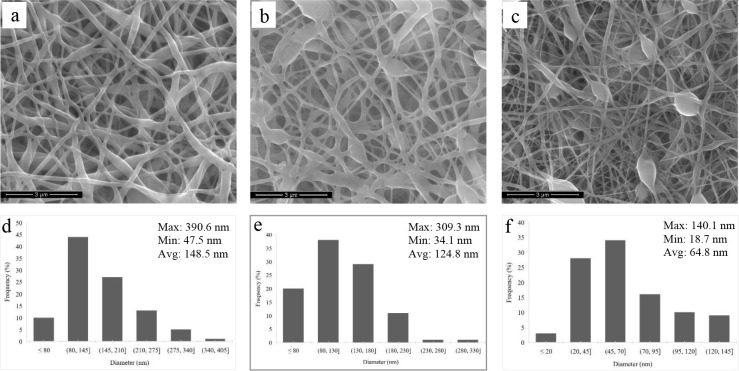
The SEM images of nanofiber films of PVA, CEO, and CLEO are represented in Figure 1. After the incorporation of β-CD and EO with PVA, some bead formation was presented, unlike PVA nanofiber film (Figure 1a). The possible reason for this situation was expressed as a relationship between polymer concentration and the viscosity of the polymer solution.76 Munhuweyi et al.77 also observed beads on the nanofibers’ SEM images, including β-CD, PVA, chitosan, and EO. The diameter distributions of all produced nanofibers are presented in Figure 1d–f. As seen here, the average diameter of the PVA nanofiber (148.5 nm) was close to the average diameter of PVA/CEO/β-CD nanofiber (124.8 nm). However, when the diameters of 100 different fibers belonging to the PVA/CLEO/β-CD nanofiber were examined, the average diameter was observed to be half of the average diameter of PVA and PVA/CEO/β-CD nanofibers. In the study of Tavassoli-Kafrani et al.,78 while the average fiber diameter of the gelatin nanofiber film obtained by electrospinning was 83.5 nm, they concluded that the fiber diameter slightly increased by incorporation of the EO into nanofiber contrary to the current study. As Lin et al.53 indicated, the characteristic differences of the electrospinning solution may be the reason for this result.
Figure 1.
SEM images of (a) poly(vinyl alcohol) (PVA), (b) PVA/cinnamon essential/β-cyclodextrin (β-CD), and (c) PVA/clove essential oil/β-CD nanofibers.
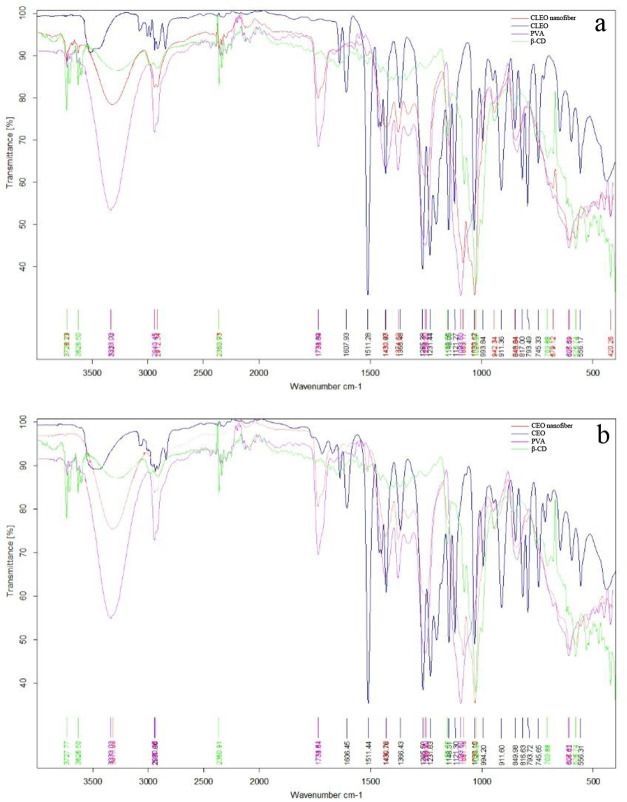
The FTIR spectra of EO nanofiber film and its components, PVA, β-CD, and EO, were interlaced (Figure 2). It is seen that the majority of characteristic absorption peaks appeared between 1700 and 500 cm–1 and around 3300 cm–1. As the control group of the current study, PVA nanofiber film (purple line), bands were observed at 3333 and 2940 cm–1 representing O–H and C–H bonds,79 respectively. The study of Wen et al.42 also showed peaks at almost the same points. In additon, the important bands at 1424 and 1085 cm–1 were correlated with bands seen at 1432 and 1093 cm–1, representing the stretching of C–H and C–O in the C–O–H bond, respectively.
Figure 2.
FTIR spectra of poly(vinyl alcohol), β-cyclodextrin, (a) clove essential oil (CLEO) nanofiber and CLEO, and (b) cinnamon essential oil (CEO) nanofiber and CEO.
The study of Pan et al.80 with the same results of PVA spectra and Wen et al.42 detected specific bands of β-CD between 1030 and 1080 cm–1 (coupling stretching of C–O and C–C) and at 3300–3500 cm–1 (stretching of C–H) in the analogy of the current study.
As shown in Figure 2a,b, the spectra of the EO nanofiber film exhibited some characteristics of the absorption bands from EO, PVA, and β-CD. The band seen in CEO nanofiber film spectra at 1030 cm–1 was specific to β-CD, which proved the presence of β-CD in the film composition (Figure 2b). On the other hand, the bands at 2930, 1430, and 600 cm–1 were highly specific to both film and CEO. Similar to the FTIR spectra of the CEO nanofiber film, common peaks were observed for the CLEO nanofiber film (Figure 2a). Most of the nanofiber film’s specific bands belong to either PVA or β-CD. The bands at 1430 cm–1 represented the C–H bond, and the bands at 942 cm–1 indicated the presence of an α-1,4 bond.
According to these two spectra, CEO and CLEO had similarities; however, some of the observable specific peaks of both CEO and CLEO disappeared on nanofiber film spectra. It was explained by Lin et al.53 and Wen et al.42 as a result of encapsulation with β-CD. Therefore, this situation demonstrates the sign of a successfully produced EO and β-CD complex.
2.4. Antifungal Activity of Nanofiber Films
According to the antifungal activity of the essential oils, the most resistant fungal culture, A. niger DDS7, was followed by A. flavus DDS6 and P. carneum DDS4. Therefore, the antifungal activity of produced CEO and CLEO nanofiber films was tested against the most resistant and most sensitive cultures, A. niger DDS7 and P. carneum DDS4. As seen in Table 4, none of the nanofiber films showed complete inhibition of tested fungi. CLEO nanofiber film was insufficient, contrary to CLEO nanofiber film against A. niger DDS7, and was similar to PVA nanofiber film. On the other hand, CLEO nanofiber film was found to be effective against P. carneum DDS4 as compared to control film PVA. CEO nanofiber film demonstrated 0.6 log and 1.06 log reduction in regard to PVA for A. niger DDS7 and P. carneum DDS4, respectively. Therefore, the observed colonies’ size was smaller than colonies counted for PVA and CLEO nanofiber film. Similar to results obtained by screening the antifungal activity of EO, P. carneum DDS4 was detected as the most sensitive fungal culture against EO nanofiber films. Differences in the results of antimicrobial effects were observed when the EO was integrated into the nanofiber film, as the case of CLEO nanofiber film. The most important parameter that enables EO-integrated nanofilms to show antimicrobial effect is the release of EO from the film. According to studies, it has been seen that the release ability changes based on the components in the EO composition.81,82 Although both cinnamon and clove EO contain high levels of eugenol, the difference in their minor constituents may have caused variation in their release ability and a change in the antimicrobial effect of their nanofiber films.
Table 4. Antifungal Activity of Nanofiber Films (log(CFU/mg)) against A. niger and P. carneum.
| nanofiber filmsa | A. niger DDS7 | P. carneum DDS4 |
|---|---|---|
| PVA | 3.24 | 3.57 |
| PVA/CLEO/β-CD | 3.24 | 3.18 |
| PVA/CEO/β-CD | 2.64 | 2.51 |
PVA: Poly(vinyl alcohol), β-CD: β-cyclodextrin, CLEO: clove essential oil, CEO: cinnamon essential oil.
It is seen in the literature that there are studies using different polymers or nanocomposite components to test their effect on food model as packaging material.83 Bodbodak et al.84 also produced nanofibers by electrospinning, using polylactic acid and hydroxypropyl methylcellulose as polymer, and examined its antibacterial activity by including pomegranate peel extract as an antimicrobial component. Its antifungal activity and application as food packaging are not included, but its effectiveness against Staphylococcus aureus and Escherichia coli has been demonstrated with the addition of extract. Besides the plant extracts, although there are a certain number of studies on nanofibers prepared with PVA and β-CD together with EO, similar to the current study, the majority of the studies were carried out to examine their antibacterial effect. In a study examining the antifungal activity, the nanofiber film composed of PVA, chitosan, β-CD, and essential oil showed 47–54% inhibition of Botrytis sp. Cinnamon EO nanofiber film had a higher antifungal effect than oregano, and because of this, it has been commented that cinnamon had better entrapment into the β-CD cavity. Therefore, EO components may release better, and their solubility may be improved.77 It has been interpreted that this may be one of the reasons for the antifungal difference observed between the clove and cinnamon nanofibers examined in this study. In order to observe the high antifungal effect of EO in nanofiber, the EO concentration may be increased, as seen in the study of Yilmaz et al.85 They showed the activity of chitosan nanoparticle samples loaded with EOs against Alternaria alternata and observed inhibition of mycelial growth. Although the effectiveness increased by increasing the concentration of loaded EO, the implication of higher EO concentration into nanofiber film should be unfeasible due to the affected nanofiber film and solutions’ characteristics.
While the usability of the produced films as food packaging is interpreted in the studies, the changes observed in packed food products are not seen in each study. Guo et al.86 have been investigated the effect of polylactic acid packaging films from an antibacterial point of view for meat products. On the other hand, Aman Mohammadi et al.87 indicated that zein, polylactic acid, and hydroxypropyl methylcellulose nanofibers incorporated with Zenian EO could be evaluated as a potential food packaging material by increased antibacterial activity against Escherichia coli and Staphylococcus aureus after addition of EO. Although several studies about food packaging materials with antimicrobial activity are available, antifungal packaging materials have received less attention.88 The in vivo antifungal effects of CLEO and CEO nanofiber films were tested with organic bread samples. The visible fungal growth on packed and nonpacked bread samples stored under sterile conditions at 25 °C was checked during a 6-day storage period. In addition to studies stating that EOs are considered as GRAS, there are also studies indicating that cinnamon and clove EOs are considered as GRAS.89−91 Besides, eugenol, the major component of the cinnamon (70.80%) and clove (81.93%) EOs used in this study, is accepted as GRAS92,93 and used as a food preservative in many countries and regions.94 As stated by Munetaka et al..90 having no toxic effects to mammals as a result of in vivo studies also makes these EOs available for use in food packaging.
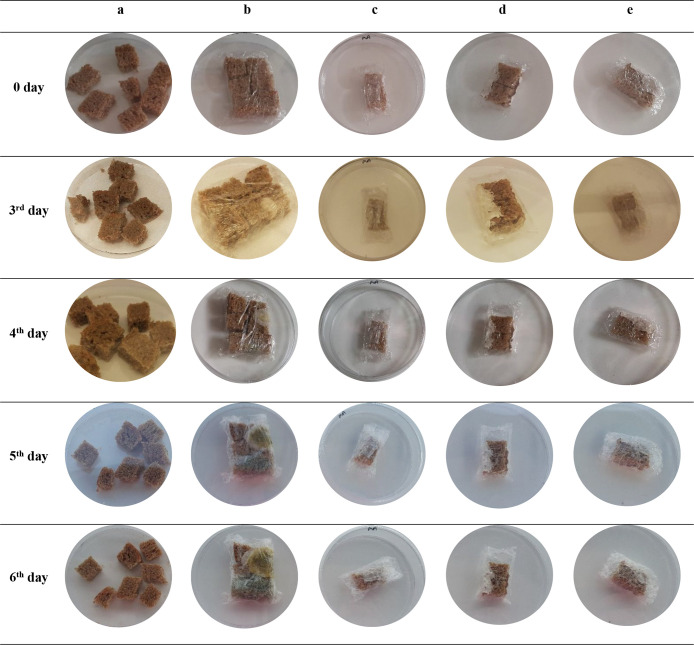
The fungal growth started on the third day of storage on bread packed with cling wrap, as seen in Figure 3, and the observed fungal growth increased day by day during storage. However, there was no fungal growth on bread without cling wrap due to loss of moisture, which is an important criterion for fungal growth. When the bread group packaged with cling wrap was examined, it was concluded that packaging with PVA also slowed down the fungal growth but it was not as effective as the film containing EO as the storage time increased. In other words, slices of bread packed with either CLEO or CEO nanofiber films showed no sign of any fungal growth.
Figure 3.
Appearance changes of different bread samples stored at 25 °C: (a) unpacked bread; (b) packed bread with cling wrap; (c) packed bread with poly(vinyl alcohol) (PVA) nanofiber film; (d) packed bread with PVA/clove essential oil/β-cyclodextrin (β-CD) nanofiber film; (e) packed bread with PVA/cinnamon essential oil/β-CD nanofiber film.
In the study of Wen et al.,42 cinnamon EO nanofiber film, including PVA and β-CD as similar to the current study, was used for packaging of strawberries to check its efficiency on strawberries’ shelf life and quality. During the storage period at 4 °C, strawberries conserved their weight and firmness and besides shelf life was found to be prolonged contrary to samples either left unpacked or packed with fresh-keeping film. Both shelf life and sensory properties may be positively affected by the use of nanofiber films.
Overall, this phenomenon is an area still requiring development; therefore, research has been continuing. In spite of examining quality parameters during storage80 and antibacterial effecst,53 nanofiber film usage potential for the antifungal activity should be a concern of scientists as well, considering food security.
3. Conclusion
The antifungal activities of several EOs were tested against P. carneum DDS4, A. flavus DDS6, and A. niger DDS7. CLEO and CEO exhibited high inhibition efficiency among tested EOs. The PVA/CLEO/β-CD and PVA/CEO/β-CD nanofiber films were produced successfully, and it has been found that they prevent fungal growth during bread storage. The results of this study shed light on the use of nanofiber films for food packaging. It would provide the opportunity to develop packaging material consisting of EOs with antimicrobial activity as a bioactive agent. Besides, toxicity and sensory analysis tests should be done as a further study, and sustainability should be ensured with food products with extended shelf life.
4. Materials and Methods
4.1. Materials
Packaged bread products (organic, whole wheat, and sliced bread) close to the expiry date were collected from the local market. Black pepper essential oil (0.876 g/mL, containing 23.57% β-caryophyllene, 15.80% limonene D, 12.41% δ-3-carene+myrecene β, and 12.17% α-pinene), cinnamon leaf essential oil (1.027 g/mL, containing 70.80% eugenol), clove bud essential oil (1.004 g/mL, containing 81.93% eugenol and 12.28% β-caryophyllene), cumin seed essential oil (0.945 g/mL, containing 38.10% cumin aldehyde, 15.13% p-mentha-1,4-dien-7-al, and 12.03% p-cymene), ginger essential oil (0.815 g/mL, containing 30.55% zingiberene α and 18.84% α-curcumene + β-sesquiphellandrene), and origanum essential oil (0.944 g/mL, containing 57.22% carvacrol and 15.59% p-cymene) were kindly provided by Aromsa Besin Aroma ve Katkı Maddeleri Sanayi Ticaret A.Ş. (Kocaeli, Turkey). They were stored in the dark at 4 °C until analysis.
PVA (purity: 95.40%, 87.16% hydrolysis) was purchased from ZAG (Istanbul, Turkey), and β-CD (≥97%) was obtained from Sigma-Aldrich (Saint Louis, MO). Other chemicals used in the study were purchased from Merck (Darmstadt, Germany).
4.2. Isolation and Identification of Fungal Cultures from Bread
The isolation of fungal cultures from collected bread samples was performed by spreading the direct plating method using Dichloran Rose Bengal Chloramphenicol agar (Merck, Darmstadt, Germany) and Dichloran 18% Glycerol agar (Merck, Darmstadt, Germany). Purification was performed with malt extract agar, including 20 g/L glucose (Merck, Darmstadt, Germany). For their molecular identifications, DNA was extracted with Biospeedy Fungal DNA kit according to the instructions of the manufacturer. Molecular identification was carried out by amplifying the internal transcribed spacer region (ITS) using ITS1-5.8S rRNA and ITS2 (5′TCCTCCGCTTATTGATATGC3′) as forward and (5′GGAAGTAAAAGTCGTAACAAGG3′) as reverse primers for real-time polymerase chain reaction (QPCR). QPCR amplification was performed under the following conditions: initial denaturation at 95 °C for 10 min, 45 cycles at 95 °C for 15 s, 53 °C for 20 s, and final extension at 98 °C for 40 s. QPCR products were purified using the PCR Purification Kit following the manufacturer’s instructions. Sanger Dideoxy Sequence Termination Method using ABI Prism 377 DNA Sequencing Analyzer (Applied Biosystems, ABD) was used to analyze DNA sequences. Sequences for the 18S and ITS region were compared with the sequences available in National Center for Biotechnology Information (NCBI) using the online BLAST tool.94
4.3. Antifungal Properties of Essential Oils
4.3.1. Disc Diffusion and Disc Volatilization Method
The antifungal activities of EOs against Penicillium carneum DDS4, Aspergillus flavus DDS6, and Aspergillus niger DDS7 were evaluated using disc diffusion and disc volatilization methods with a slight modification of Wen et al.42 Briefly, 100 μL of a prepared spore suspension (peptone water including 1 g/1 L Tween 80) containing approximately 105 spores/mL was inoculated to Czapek dox agar (CZ) plates (Ø = 90 mm) individually. Inoculated plates were allowed to dry for 20 min at room temperature (CZ: 10 mL czapek concentrate, 1 g of dipotassium phosphate (K2HPO4), 30 g of sucrose, 17.5 g of agar in 1 L of distilled water, and czapek concentrate: 30 g of sodium nitrate (NaNO3), 5 g of potassium chloride (KCl), 5 g of magnesium sulfate heptahydrate (MgSO4·7H2O), 0.1 g of ferrous sulfate heptahydrate (FeSO4·7H2O), 0.1 g of zinc sulfate heptahydrate (ZnSO4·7H2O), and 0.05 g of copper sulfate pentahydrate (CuSO4·5H2O) in 100 mL of distilled water).
The sterilized filter disc (Ø = 6 mm) was placed on the inoculated CZ plate’s surface for the disc diffusion method and the inside surface of another plate’s medium-free cover for the disc volatilization method. Then, 5 μL of pure EO was added to the disc. These plates were sealed with parafilm to eliminate EO vapor leakage and incubated at 25 °C for 6 days. The diameter of the inhibition zone was measured after 3 days and 6 days of incubation.
4.3.2. Poisoned Culture Method
The determination of essential oils’ antifungal activity was carried out by using the method described by Kocić-Tanackov et al.72 CZ 1% Tween 20 medium was cooled to 45 °C after sterilization, and CEO, CLEO, CUEO, and OEG in different concentrations (500, 750, 1000, 1500, 2000, 3000, and 5000 μg/mL) were added separately. After EO was dispersed in the medium, it was poured into a Petri plate (Ø = 60 mm). The plates, including medium without essential oil, were indicated as control. The sterilized filter paper disc (Ø = 6 mm) was placed at the center of the medium, and 1 μL of spore suspension, including 105 spores/mL, was inoculated on a disc. The plates were sealed with parafilm and left for incubation at 25 °C for 14 days. The daily radial colony growth diameter was measured.72
MIC was determined as the minimum concentration that prevents fungal growth at the end of 14 days. Then the parafilm was removed from plates in which there was no colony growth, and they were left for a further 16 days of incubation. The filter paper discs without fungal growth after 30 days were transferred to a CZ medium free from EO, and they were incubated at 25 °C for 5 days to determine the MFC.
4.4. Electrospinning Process
The preparation of electrospinning solutions and performed processes was adapted from Wen et al.42 First, a 6% PVA solution was prepared by dissolving in distilled water under constant stirring by using a magnetic stirrer (IKA, RCT classic) at 700 rpm and 80 °C for 3 h. Then, 2% β-CD for CEO and CLEO was added and dissolved in PVA solution at 700 rpm and 55 °C for 1 h. After that, 2% EO was put inside the mixture, and the solution was stirred at 55 °C and 700 rpm for 2.5 h. As a control, a pure PVA solution was prepared.
The electrospinning procedure was driven with a 20-gauge steel needle at a distance of 12 cm, 0.4 mL/s flow rate, and 14–16 kV voltage. The nanofiber film was collected on a collecting plate covered by aluminum foil. The characterization of EO nanofilms was examined by Fourier transform infrared spectrophotometry (FTIR, Bruker Tensor II) in the range of scanning spectra from 500 to 4000 cm–1 and scanning electron microscopy (SEM, FEI Quanta FEG 250) at 20 kV and 10000× magnification. The distribution of fiber diameter was determined by analyzing 100 random fibers from SEM image. The produced nanofiber films were stored at 4 °C in a closed container.
4.5. Antifungal Activity of Nanofiber Films
The experiment of in vitro antifungal activity of nanofiber films was performed by the method adapted from Shi et al.95 First, 10 mg of each nanofiber film (CEO, CLEO, and PVA) was weighed and placed into 1.5 mL tubes as duplicates. The 1 mL of spore suspension (peptone water including 1 g/1 L Tween 80) containing 103 spores/mL was added into the tubes. The tube, including PVA was selected as negative control against EO nanofiber films. They were incubated for 24 h at 25 °C. Then, the spread plate method with DRBC agar was performed, and plates were left 2 days of incubation at 25 °C to count fungal colonies.
To evaluate the effectiveness of CEO and CLEO nanofiber films as antimicrobial food packaging, they were applied to organic bread samples. The collected bread samples were sliced into small pieces and placed into Petri dishes as packed inside cling wrap to inhibit moisture loss either with or without nanofiber films (CEO, CLEO, and PVA) and as nonpackaged. They were stored at around 25 °C until the sixth day under sterile conditions.
4.6. Statistical Analysis
All the experiments were performed at least in triplicate. Excel 2016 and SPSS software (Version 16.0) were used to analyze the resulting data. One-way analysis of variance (ANOVA), Tukey’s range test, and Duncan’s new multiple range test were applied at 0.05 significance level. Results were reported as mean value ± standard deviations.
Acknowledgments
There is no funding declaration.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c05105.
Additional experimental details; disc diffusion assay plates for antifungal activity of EOs against fungal cultures (PDF)
Author Contributions
D.D., F.K.G., and M.T. investigated and designed the study. D.D. performed experiments and data analysis and wrote–original draft preparation. F.K.G. and M.T. supervised, wrote—review, and editing. F.K.G. was responsible for funding acquisition. All authors have read and agreed to the published version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- World Health Organization . WHO estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007–2015, World Health Organization, 2015. [Google Scholar]
- Arshad M. S.; Batool S. A.. Natural antimicrobials, their sources and food safety. In Food Additives; Karunaratne D. N., Pamunuwa G., Eds.; InTech: Rijeka, 2017; Vol. 87 ( (1), ). [Google Scholar]
- Mutlu-Ingok A.; Devecioglu D.; Dikmetas D. N.; Karbancioglu-Guler F.; Capanoglu E. Antibacterial, Antifungal, Antimycotoxigenic, and Antioxidant Activities of Essential Oils: An Updated Review. Molecules 2020, 25 (20), 4711. 10.3390/molecules25204711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarlo A.; Zeng T.; Dosoky N. S.; Satyal P.; Setzer W. N. The composition and antimicrobial activity of Liquidambar formosana oleoresin. Plants 2020, 9 (7), 822. 10.3390/plants9070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouhan S.; Sharma K.; Guleria S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines 2017, 4 (3), 58. 10.3390/medicines4030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshafie H. S.; Aliberti L.; Amato M.; De Feo V.; Camele I. Chemical composition and antimicrobial activity of chia (Salvia hispanica L.) essential oil. Eur. Food Res. Technol. 2018, 244 (9), 1675–1682. 10.1007/s00217-018-3080-x. [DOI] [Google Scholar]
- Gyawali R.; Ibrahim S. A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- Hammami S.; Debbabi H.; Jlassi I.; Joshi R. K.; El Mokni R. Chemical composition and antimicrobial activity of essential oil from the aerial parts of Plantago afra L. (Plantaginaceae) growing wild in Tunisia. S. Afr. J. Bot. 2020, 132, 410–414. 10.1016/j.sajb.2020.05.012. [DOI] [Google Scholar]
- Borugă O.; Jianu C.; Mişcă C.; Goleţ I.; Gruia A. T.; Horhat F. G. Thymus vulgaris essential oil: chemical composition and antimicrobial activity. J. Med. Life 2014, 7 (Spec Iss 3), 56. [PMC free article] [PubMed] [Google Scholar]
- Tseliou M.; Pirintsos S. A.; Lionis C.; Castanas E.; Sourvinos G. Antiviral effect of an essential oil combination derived from three aromatic plants (Coridothymus capitatus (L.) Rchb. f., Origanum dictamnus L. and Salvia fruticosa Mill.) against viruses causing infections of the upper respiratory tract. J. Herb. Med. 2019, 17, 100288. 10.1016/j.hermed.2019.100288. [DOI] [Google Scholar]
- Snene A.; El Mokni R.; Jmii H.; Jlassi I.; Jaïdane H.; Falconieri D.; Piras A.; Dhaouadi H.; Porcedda S.; Hammami S. In vitro antimicrobial, antioxidant and antiviral activities of the essential oil and various extracts of wild (Daucus virgatus (Poir.) Maire) from Tunisia. Ind. Crops Prod. 2017, 109, 109–115. 10.1016/j.indcrop.2017.08.015. [DOI] [Google Scholar]
- Dudoiu R.; Petrisor C.; Fatu V.; Lupu C.; Cristea S. Antimycotic activity of Thymus vulgaris essential oil against cereals storage moulds. J. Biotechnol. 2017, 256, S70. 10.1016/j.jbiotec.2017.06.1038. [DOI] [Google Scholar]
- Ferreira L. C.; da Cruz M. G.; Lima T. B. C.; Serra B. N. V.; Chaves F. C. M.; Chagas E. C.; Ventura A. S.; Jerônimo G. T. Antiparasitic activity of Mentha piperita (Lamiaceae) essential oil against Piscinoodinium pillulare and its physiological effects on Colossoma macropomum (Cuvier, 1818). Aquaculture 2019, 512, 734343. 10.1016/j.aquaculture.2019.734343. [DOI] [Google Scholar]
- Soares B. V.; Neves L. R.; Oliveira M. S. B.; Chaves F. C. M.; Dias M. K. R.; Chagas E. C.; Tavares-Dias M. Antiparasitic activity of the essential oil of Lippia alba on ectoparasites of Colossoma macropomum (tambaqui) and its physiological and histopathological effects. Aquaculture 2016, 452, 107–114. 10.1016/j.aquaculture.2015.10.029. [DOI] [Google Scholar]
- dos Santos A. T. B.; Junior J. S. Z.; Parreira L. A.; de Abreu K. M. P.; de Oliveira Bernardes C.; de Carvalho J. R.; Menini L. Chemical identification and insecticidal effect of Tephrosia vogelii essential oil against Cerosipha forbesi in strawberry crop. Crop Prot. 2020, 105405. 10.1016/j.cropro.2020.105405. [DOI] [Google Scholar]
- Oyedeji A. O.; Okunowo W. O.; Osuntoki A. A.; Olabode T. B.; Ayo-Folorunso F. Insecticidal and biochemical activity of essential oil from Citrus sinensis peel and constituents on Callosobrunchus maculatus and Sitophilus zeamais. Pestic. Biochem. Physiol. 2020, 168, 104643. 10.1016/j.pestbp.2020.104643. [DOI] [PubMed] [Google Scholar]
- Taban A.; Saharkhiz M. J.; Niakousari M. Sweet bay (Laurus nobilis L.) essential oil and its chemical composition, antioxidant activity and leaf micromorphology under different extraction methods. Sustainable Chem. Pharm. 2018, 9, 12–18. 10.1016/j.scp.2018.05.001. [DOI] [Google Scholar]
- Sadeh D.; Nitzan N.; Chaimovitsh D.; Shachter A.; Ghanim M.; Dudai N. Interactive effects of genotype, seasonality and extraction method on chemical compositions and yield of essential oil from rosemary (Rosmarinus officinalis L.). Ind. Crops Prod. 2019, 138, 111419. 10.1016/j.indcrop.2019.05.068. [DOI] [Google Scholar]
- Božović M.; Garzoli S.; Baldisserotto A.; Romagnoli C.; Pepi F.; Cesa S.; Vertuani S.; Manfredini S.; Ragno R. Melissa officinalis L. subsp. altissima (Sibth. & Sm.) Arcang. essential oil: Chemical composition and preliminary antimicrobial investigation of samples obtained at different harvesting periods and by fractionated extractions. Ind. Crops Prod. 2018, 117, 317–321. 10.1016/j.indcrop.2018.03.018. [DOI] [Google Scholar]
- Mabizela G. S.; Muller M.; de Beer D.; van der Rijst M.; Slabbert M. M.; Joubert E.; Bester C. Effect of genotype and harvest season on quality characteristics of Cyclopia subternata: Phenolic content and sensory profile. S. Afr. J. Bot. 2020, 132, 491–501. 10.1016/j.sajb.2020.06.010. [DOI] [Google Scholar]
- Zribi I.; Bleton J.; Moussa F.; Abderrabba M. GC-MS analysis of the volatile profile and the essential oil compositions of Tunisian Borago Officinalis L.: Regional locality and organ dependency. Ind. Crops Prod. 2019, 129, 290–298. 10.1016/j.indcrop.2018.12.021. [DOI] [Google Scholar]
- Dodoš T.; Rajčević N.; Janaćković P.; Vujisić L.; Marin P. D. Essential oil profile in relation to geographic origin and plant organ of Satureja kitaibelii Wierzb. ex Heuff. Ind. Crops Prod. 2019, 139, 111549. 10.1016/j.indcrop.2019.111549. [DOI] [Google Scholar]
- Mutlu-Ingok A.; Tasir S.; Seven A.; Akgun N.; Karbancioglu-Guler F. Evaluation of the single and combined antibacterial efficiency of essential oils for controlling Campylobacter coli, Campylobacter jejuni, Escherichia coli, Staphylococcus aureus, and mixed cultures. Flavour Fragr. J. 2019, 34 (4), 280–287. 10.1002/ffj.3501. [DOI] [Google Scholar]
- Akarchariya N.; Sirilun S.; Julsrigival J.; Chansakaowa S. Chemical profiling and antimicrobial activity of essential oil from Curcuma aeruginosa Roxb., Curcuma glans K. Larsen & J. Mood and Curcuma cf. xanthorrhiza Roxb. collected in Thailand. Asian Pacific J. Trop. Biomed. 2017, 7 (10), 881–885. 10.1016/j.apjtb.2017.09.009. [DOI] [Google Scholar]
- Ghavam M.; Manca M. L.; Manconi M.; Bacchetta G. Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea DC. ex Benth. Sci. Rep. 2020, 10 (1), 1–10. 10.1038/s41598-020-73193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournomiti M.; Kimbaris A.; Mantzourani I.; Plessas S.; Theodoridou I.; Papaemmanouil V.; Kapsiotis I.; Panopoulou M.; Stavropoulou E.; Bezirtzoglou E. E.; Alexopoulos A. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health. Dis. 2015, 26 (1), 23289. 10.3402/mehd.v26.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yooussef M. M.; Pham Q.; Achar P. N.; Sreenivasa M. Y. Antifungal activity of essential oils on Aspergillus parasiticus isolated from peanuts. J. Plant Prot. Res. 2016, 56 (2), 139–142. 10.1515/jppr-2016-0021. [DOI] [Google Scholar]
- Hu F.; Tu X. F.; Thakur K.; Hu F.; Li X. L.; Zhang Y. S.; Zhang J. G.; Wei Z. J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. 10.1016/j.fct.2019.110821. [DOI] [PubMed] [Google Scholar]
- Pizzolitto R. P.; Jacquat A. G.; Usseglio V. L.; Achimón F.; Cuello A. E.; Zygadlo J. A.; Dambolena J. S. Quantitative-structure-activity relationship study to predict the antifungal activity of essential oils against Fusarium verticillioides. Food Control 2020, 108, 106836. 10.1016/j.foodcont.2019.106836. [DOI] [Google Scholar]
- Xie Y.; Wang Z.; Huang Q.; Zhang D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crops Prod. 2017, 108, 278–285. 10.1016/j.indcrop.2017.06.041. [DOI] [Google Scholar]
- da Silva W. M. F.; Kringel D. H.; de Souza E. J. D.; da Rosa Zavareze E.; Dias A. R. G. Basil essential oil: Methods of extraction, chemical composition, biological activities, and food applications. Food Bioprocess Technol. 2021, 1–27. 10.1007/s11947-021-02690-3. [DOI] [Google Scholar]
- Saggiorato A. G.; Gaio I.; Treichel H.; de Oliveira D.; Cichoski A. J.; Cansian R. L. Antifungal activity of basil essential oil (Ocimum basilicum L.): evaluation in vitro and on an Italian-type sausage surface. Food Bioprocess Technol. 2012, 5 (1), 378–384. 10.1007/s11947-009-0310-z. [DOI] [Google Scholar]
- da Cruz Cabral L.; Pinto V. F.; Patriarca A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166 (1), 1–14. 10.1016/j.ijfoodmicro.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Khorshidian N.; Yousefi M.; Khanniri E.; Mortazavian A. M. Potential application of essential oils as antimicrobial preservatives in cheese. Innovative Food Sci. Emerg. Technol. 2018, 45, 62–72. 10.1016/j.ifset.2017.09.020. [DOI] [Google Scholar]
- Mutlu-Ingok A.; Karbancioglu-Guler F. Cardamom, Cumin, and Dill Weed Essential Oils: Chemical Compositions, Antimicrobial Activities, and Mechanisms of Action against Campylobacter spp. Molecules 2017, 22 (7), 1191. 10.3390/molecules22071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafarga T.; Gallagher E.; Walsh D.; Valverde J.; Hayes M. Chitosan-containing bread made using marine shellfishery byproducts: Functional, bioactive, and quality assessment of the end product. J. Agric. Food. Chem. 2013, 61 (37), 8790–8796. 10.1021/jf402248a. [DOI] [PubMed] [Google Scholar]
- Legan J. D. Mould spoilage of bread: the problem and some solutions. Int. Biodeterior. Biodegrad. 1993, 32 (1–3), 33–53. 10.1016/0964-8305(93)90038-4. [DOI] [Google Scholar]
- Pateras I. M.Bread spoilage and staling. In Technology of breadmaking; Springer: Boston, MA, 2007; pp 275–298. [Google Scholar]
- Lau O. W.; Wong S. K. Contamination in food from packaging material. J. Chromatogr. A 2000, 882 (1–2), 255–270. 10.1016/S0021-9673(00)00356-3. [DOI] [PubMed] [Google Scholar]
- Williams H.; Wikström F.; Otterbring T.; Löfgren M.; Gustafsson A. Reasons for household food waste with special attention to packaging. J. Cleaner Prod. 2012, 24, 141–148. 10.1016/j.jclepro.2011.11.044. [DOI] [Google Scholar]
- Kabara J. J. Phenols and chelators. Food Preservatives 1991, 200, 214. [Google Scholar]
- Wen P.; Zhu D. H.; Wu H.; Zong M. H.; Jing Y. R.; Han S. Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. 10.1016/j.foodcont.2015.06.005. [DOI] [Google Scholar]
- Simionato I.; Domingues F. C.; Nerín C.; Silva F. Encapsulation of cinnamon oil in cyclodextrin nanosponges and their potential use for antimicrobial food packaging. Food Chem. Toxicol. 2019, 132, 110647. 10.1016/j.fct.2019.110647. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Santos R.; Andrade M.; Sanches-Silva A. Application of encapsulated essential oils as antimicrobial agents in food packaging. Curr. Opin. Food Sci. 2017, 14, 78–84. 10.1016/j.cofs.2017.01.012. [DOI] [Google Scholar]
- da Cunha J. A.; de Ávila Scheeren C.; Fausto V. P.; de Melo L. D. W.; Henneman B.; Frizzo C. P.; de Almeida Vaucher R.; de Vargas A. C.; Baldisserotto B. The antibacterial and physiological effects of pure and nanoencapsulated Origanum majorana essential oil on fish infected with Aeromonas hydrophila. Microb. Pathogen. 2018, 124, 116–121. 10.1016/j.micpath.2018.08.040. [DOI] [PubMed] [Google Scholar]
- Plati F.; Paraskevopoulou A. Micro-and Nano-encapsulation as Tools for Essential Oils Advantages’ Exploitation in Food Applications: the Case of Oregano Essential Oil. Food Bioprocess Technol. 2022, 1–29. 10.1007/s11947-021-02746-4. [DOI] [Google Scholar]
- Abarca R. L.; Rodríguez F. J.; Guarda A.; Galotto M. J.; Bruna J. E.; Fávaro Perez M. A.; Padula M. Application of β-cyclodextrin/2-nonanone inclusion complex as active agent to design of antimicrobial packaging films for control of Botrytis cinerea. Food Bioprocess Technol. 2017, 10 (9), 1585–1594. 10.1007/s11947-017-1926-z. [DOI] [Google Scholar]
- Rakmai J.; Cheirsilp B.; Mejuto J. C.; Torrado-Agrasar A.; Simal-Gándara J. Physico-chemical characterization and evaluation of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Food Hydrocolloids 2017, 65, 157–164. 10.1016/j.foodhyd.2016.11.014. [DOI] [Google Scholar]
- Hasheminejad N.; Khodaiyan F.; Safari M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019, 275, 113–122. 10.1016/j.foodchem.2018.09.085. [DOI] [PubMed] [Google Scholar]
- Kalagatur N. K.; Nirmal Ghosh O. S.; Sundararaj N.; Mudili V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018, 9, 610. 10.3389/fphar.2018.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya-Serna C. M.; Dacanal G. C.; Fernandes A. M.; Pinho S. C. Antifungal activity of nanoemulsions encapsulating oregano (Origanum vulgare) essential oil: in vitro study and application in Minas Padrão cheese. Braz. J. Microbiol. 2018, 49 (4), 929–935. 10.1016/j.bjm.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbo S.; Khaneghah A. M.. Active packaging of foods and its combination with electron beam processing. In Electron Beam Pasteurization and Complementary Food Processing Technologies; Pillai S. D., Shayanfar S., Eds.; Woodhead Publishing: Cambridge, 2015; pp 195–217. [Google Scholar]
- Lin L.; Zhu Y.; Cui H. Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT 2018, 97, 711–718. 10.1016/j.lwt.2018.08.015. [DOI] [Google Scholar]
- Wang H.; Zhang R.; Zhang H.; Jiang S.; Liu H.; Sun M.; Jiang S. Kinetics and functional effectiveness of nisin loaded antimicrobial packaging film based on chitosan/poly (vinyl alcohol). Carbohydr. Polym. 2015, 127, 64–71. 10.1016/j.carbpol.2015.03.058. [DOI] [PubMed] [Google Scholar]
- Xue J.; Wu T.; Dai Y.; Xia Y. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem. Rev. 2019, 119 (8), 5298–5415. 10.1021/acs.chemrev.8b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Li Y.; Wang P.; Zhang H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19 (2), 479–502. 10.1111/1541-4337.12536. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Duan G.; Zhang G.; Yang H.; He S.; Jiang S. Electrospun functional materials toward food packaging applications: A review. Nanomaterials 2020, 10 (1), 150. 10.3390/nano10010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. X.; Zhang C.; Pan S.; Chen L.; Liu M.; Yang K.; Zeng X.; Tian J. Analysis of chemical components and biological activities of essential oils from black and white pepper (Piper nigrum L.) in five provinces of southern China. LWT 2020, 117, 108644. 10.1016/j.lwt.2019.108644. [DOI] [Google Scholar]
- Davari M.; Ezazi R. Chemical composition and antifungal activity of the essential oil of Zhumeria majdae, Heracleum persicum and Eucalyptus sp. against some important phytopathogenic fungi. J. Mycol. Med. 2017, 27 (4), 463–468. 10.1016/j.mycmed.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Caccioni D. R.; Guizzardi M.; Biondi D. M.; Renda A.; Ruberto G. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. Int. J. Food Microbiol. 1998, 43 (1–2), 73–79. 10.1016/S0168-1605(98)00099-3. [DOI] [PubMed] [Google Scholar]
- Vitoratos A.; Bilalis D.; Karkanis A.; Efthimiadou A. Antifungal activity of plant essential oils against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41 (1), 86–92. 10.15835/nbha4118931. [DOI] [Google Scholar]
- Perumal A. B.; Sellamuthu P. S.; Nambiar R. B.; Sadiku E. R. Effects of essential oil vapour treatment on the postharvest disease control and different defence responses in two mango (Mangifera indica L.) cultivars. Food Bioprocess Technol. 2017, 10 (6), 1131–1141. 10.1007/s11947-017-1891-6. [DOI] [Google Scholar]
- Ács K.; Balázs V. L.; Kocsis B.; Bencsik T.; Böszörményi A.; Horváth G. Antibacterial activity evaluation of selected essential oils in liquid and vapor phase on respiratory tract pathogens. BMC Complement Altern. Med. 2018, 18 (1), 1–9. 10.1186/s12906-018-2291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.; Lin Y.; Chai X.; Duan X.; Zhao X.; Chun C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019, 7 (8), 2546–2555. 10.1002/fsn3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto E.; Vale-Silva L.; Cavaleiro C.; Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58 (11), 1454–1462. 10.1099/jmm.0.010538-0. [DOI] [PubMed] [Google Scholar]
- Purkait S.; Bhattacharya A.; Bag A.; Chattopadhyay R. R. (2020). Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch. Microbiol. 2020, 202 (6), 1439–1448. 10.1007/s00203-020-01858-3. [DOI] [PubMed] [Google Scholar]
- Melo R. S.; Albuquerque Azevedo Á. M.; Gomes Pereira A. M.; Rocha R. R.; Bastos Cavalcante R. M.; Carneiro Matos M. N.; Carneiro V. A. Chemical composition and antimicrobial effectiveness of Ocimum gratissimum L. essential oil against multidrug-resistant isolates of Staphylococcus aureus and Escherichia coli. Molecules 2019, 24 (21), 3864. 10.3390/molecules24213864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokaeian M.; Shiri Y.; Bazi S.; Saeidi S.; Sahi Z.. Antibacterial activities of Cuminum cyminum Linn. Essential oil against multi-drug resistant Escherichia coli. Int. J. Infect. 2014, 1( (1), ). 10.17795/iji-18739 [DOI] [Google Scholar]
- Raybaudi-Massilia R. M.; Mosqueda-Melgar J.; Martin-Belloso O. Antimicrobial activity of essential oils on Salmonella enteritidis, Escherichia coli, and Listeria innocua in fruit juices. J. Food Prot. 2006, 69 (7), 1579–1586. 10.4315/0362-028X-69.7.1579. [DOI] [PubMed] [Google Scholar]
- Rammanee K.; Hongpattarakere T. Effects of tropical citrus essential oils on growth, aflatoxin production, and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Bioprocess Technol. 2011, 4 (6), 1050–1059. 10.1007/s11947-010-0507-1. [DOI] [Google Scholar]
- Manso S.; Nerín C.; Gómez-Lus R.. Antifungal activity of the essential oil of cinnamon (cinnamomum zeylanicum), oregano (origanum vulgare) and lauramide argine ethyl ester (lae) against the mold aspergillus flavus cect 2949. Ital. J. Food Sci. 2011.23151–156. [Google Scholar]
- Kocić-Tanackov S.; Dimić G.; Tanackov I.; Pejin D.; Mojović L.; Pejin J. The inhibitory effect of oregano extract on the growth of Aspergillus spp. and on sterigmatocystin biosynthesis. LWT-Food Sci. Technol. 2012, 49 (1), 14–20. 10.1016/j.lwt.2012.04.013. [DOI] [Google Scholar]
- Sarrazin S. L. F.; Oliveira R. B.; Barata L. E. S.; Mourão R. H. V. Chemical composition and antimicrobial activity of the essential oil of Lippia grandis Schauer (Verbenaceae) from the western Amazon. Food Chem. 2012, 134 (3), 1474–1478. 10.1016/j.foodchem.2012.03.058. [DOI] [PubMed] [Google Scholar]
- Gundewadi G.; Rudra S. G.; Gogoi R.; Banerjee T.; Singh S. K.; Dhakate S.; Gupta A. Electrospun Essential oil encapsulated nanofibers for the management of anthracnose disease in Sapota. Ind. Crops Prod. 2021, 170, 113727. 10.1016/j.indcrop.2021.113727. [DOI] [Google Scholar]
- Seydim A. C.; Sarikus G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39 (5), 639–644. 10.1016/j.foodres.2006.01.013. [DOI] [Google Scholar]
- Lamarra J.; Calienni M. N.; Rivero S.; Pinotti A. Electrospun nanofibers of poly (vinyl alcohol) and chitosan-based emulsions functionalized with cabreuva essential oil. Int. J. Biol. Macromol. 2020, 160, 307–318. 10.1016/j.ijbiomac.2020.05.096. [DOI] [PubMed] [Google Scholar]
- Munhuweyi K.; Caleb O. J.; van Reenen A. J.; Opara U. L. Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT 2018, 87, 413–422. 10.1016/j.lwt.2017.09.012. [DOI] [Google Scholar]
- Tavassoli-Kafrani E.; Goli S. A. H.; Fathi M. Encapsulation of orange essential oil using cross-linked electrospun gelatin nanofibers. Food Bioprocess Technol. 2018, 11 (2), 427–434. 10.1007/s11947-017-2026-9. [DOI] [Google Scholar]
- Aktürk A.; Taygun M. E.; Güler F. K.; Goller G.; Küçükbayrak S. Fabrication of antibacterial polyvinylalcohol nanocomposite mats with soluble starch coated silver nanoparticles. Colloids Surf. A Physicochem. Eng. 2019, 562, 255–262. 10.1016/j.colsurfa.2018.11.034. [DOI] [Google Scholar]
- Pan J.; Ai F.; Shao P.; Chen H.; Gao H. Development of polyvinyl alcohol/β-cyclodextrin antimicrobial nanofibers for fresh mushroom packaging. Food Chem. 2019, 300, 125249. 10.1016/j.foodchem.2019.125249. [DOI] [PubMed] [Google Scholar]
- Hammoud Z.; Gharib R.; Fourmentin S.; Elaissari A.; Greige-Gerges H. Drug-in-hydroxypropyl-β-cyclodextrin-in-lipoid S100/cholesterol liposomes: Effect of the characteristics of essential oil components on their encapsulation and release. Int. J. Pharm. 2020, 579, 119151. 10.1016/j.ijpharm.2020.119151. [DOI] [PubMed] [Google Scholar]
- Kfoury M.; Auezova L.; Greige-Gerges H.; Fourmentin S. Encapsulation in cyclodextrins to widen the applications of essential oils. Environ. Chem. Lett. 2019, 17 (1), 129–143. 10.1007/s10311-018-0783-y. [DOI] [Google Scholar]
- Amjadi S.; Emaminia S.; Nazari M.; Davudian S. H.; Roufegarinejad L.; Hamishehkar H. Application of reinforced ZnO nanoparticle-incorporated gelatin bionanocomposite film with chitosan nanofiber for packaging of chicken fillet and cheese as food models. Food Bioprocess Technol. 2019, 12 (7), 1205–1219. 10.1007/s11947-019-02286-y. [DOI] [Google Scholar]
- Bodbodak S.; Shahabi N.; Mohammadi M.; Ghorbani M.; Pezeshki A. Development of a novel antimicrobial electrospun nanofiber based on polylactic acid/hydroxypropyl methylcellulose containing pomegranate peel extract for active food packaging. Food Bioprocess Technol. 2021, 14 (12), 2260–2272. 10.1007/s11947-021-02722-y. [DOI] [Google Scholar]
- Yilmaz M. T.; Yilmaz A.; Akman P. K.; Bozkurt F.; Dertli E.; Basahel A.; Al-Sasi B.; Taylan O.; Sagdic O. Electrospraying method for fabrication of essential oil loaded-chitosan nanoparticle delivery systems characterized by molecular, thermal, morphological and antifungal properties. Innovative Food Sci. Emerg. Technol. 2019, 52, 166–178. 10.1016/j.ifset.2018.12.005. [DOI] [Google Scholar]
- Guo M.; Jin T. Z.; Yang R. Antimicrobial polylactic acid packaging films against Listeria and Salmonella in culture medium and on ready-to-eat meat. Food Bioprocess Technol. 2014, 7 (11), 3293–3307. 10.1007/s11947-014-1322-x. [DOI] [Google Scholar]
- Aman Mohammadi M.; Ramezani S.; Hosseini H.; Mortazavian A. M.; Hosseini S. M.; Ghorbani M. Electrospun antibacterial and antioxidant zein/polylactic acid/hydroxypropyl methylcellulose nanofibers as an active food packaging system. Food Bioprocess Technol. 2021, 14 (8), 1529–1541. 10.1007/s11947-021-02654-7. [DOI] [Google Scholar]
- Topuz F.; Uyar T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. 10.1016/j.foodres.2019.108927. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Pacheco M. M.; Ortega-Ramirez L. A.; Cruz-Valenzuela M. R.; Silva-Espinoza B. A.; Gonzalez-Aguilar G. A.; Ayala-Zavala J. F. Combinational Approaches for Antimicrobial Packaging: Pectin and Cinnamon Leaf Oil. Antimicrobial food packaging 2016, 609–617. 10.1016/B978-0-12-800723-5.00050-4. [DOI] [Google Scholar]
- Munekata P. E.; Pateiro M.; Rodríguez-Lázaro D.; Domínguez R.; Zhong J.; Lorenzo J. M. The role of essential oils against pathogenic Escherichia coli in food products. Microorganisms 2020, 8 (6), 924. 10.3390/microorganisms8060924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D.; Xu Y.; Jiang Q.; Xia W. Effects of chitosan coating combined with essential oils on quality and antioxidant enzyme activities of grass carp (Ctenopharyngodon idellus) fillets stored at 4 C. Int. J. Food Sci. 2017, 52 (2), 404–412. 10.1111/ijfs.13295. [DOI] [Google Scholar]
- Barakat H. Composition, antioxidant, antibacterial activities and mode of action of clove (Syzygium aromaticum L.) buds essential oil. Br. J. Appl. Sci. 2014, 4 (13), 1934. 10.9734/BJAST/2014/8902. [DOI] [Google Scholar]
- Hu Q.; Zhou M.; Wei S. Progress on the antimicrobial activity research of clove oil and eugenol in the food antisepsis field. J. Food Sci. 2018, 83 (6), 1476–1483. 10.1111/1750-3841.14180. [DOI] [PubMed] [Google Scholar]
- GenBank. National Center for Biotechnology Information (NCBI) US National Library of Medicine, 2021; Retrieved in September, 15, 2021 from http://www.ncbi.nlm.nih.gov/.
- Shi Q.; Vitchuli N.; Nowak J.; Caldwell J. M.; Breidt F.; Bourham M.; Zhang X.; McCord M. Durable antibacterial Ag/polyacrylonitrile (Ag/PAN) hybrid nanofibers prepared by atmospheric plasma treatment and electrospinning. Eur. Polym. J. 2011, 47 (7), 1402–1409. 10.1016/j.eurpolymj.2011.04.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.