Abstract
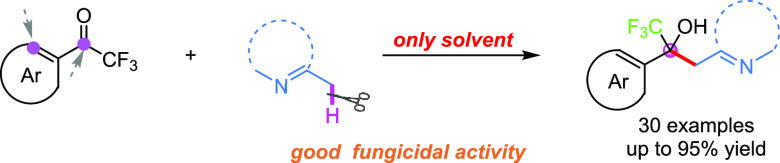
A highly chemoselective reaction between α,β-unsaturated trifluoromethyl ketones with azaarenes under metal-free conditions was carried out, affording a range of valuable azaarene-equipped CF3-tertiary alcohols in moderate to excellent yields (up to 95% yield) with good tolerance of functional groups, and their structures were confirmed by NMR, HRMS, and X-ray diffraction for validation. This method features simple reaction conditions (only solvent), high atom- and step-economy, and broad substrate scope. Moreover, most of the target products exhibited promising fungicidal activities, and compound 3al exhibited 91.65% fungicidal activity against R. solani, with an EC50 value of 0.18 mg/mL.
Introduction
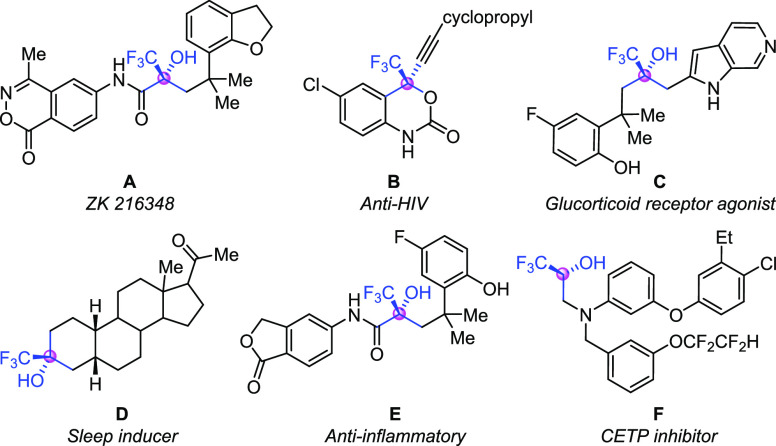
Fluorine-containing molecules are pervasive motifs, and they exhibit broad applications in the field of medicines, agrochemicals, polymers, functional materials, and other chemical industries, owing to the specific characteristics of the fluorine atom, such as high electronegativity, high lipophilicity, good hydrophobicity, metabolic stability, and bioavailability.1 Particularly, trifluoromethyl-substituted tertiary alcohols are embedded in a range of pharmaceuticals.2,3 For example, compounds A and C are glucorticoid receptor agonists,4 compound B is a reverse transcriptase inhibitor of HIV,5 compound D is a sleep inducer,6 compound E is an anti-inflammatory reagent,7 and compound F is a cholesteryl ester transfer protein inhibitor8 (Scheme 1). Therefore, the synthesis of these skeletons is highly attractive and a plethora of practical protocols for rapid assembly of CF3-substituted tertiary alcohols have been developed in recent years.
Scheme 1. Representative Examples of Drug Molecules with CF3-Tertiary Alcohol Motifs.
On the other hand, the direct functionalization of ubiquitous inert C–H bonds has been rapidly developed in past decades, owing to the advantages in terms of atom- and step-economy.9 In this regard, C(sp3)–H functionalization of azaarenes has emerged as a powerful method for the rapid assembly of functional N-heterocycle molecules, which are pervasive motifs found in a series of pharmaceuticals, natural products, and materials.10 However, C(sp3)–H functionalization of methylquinoline and its derivatives coupled with trifluoromethyl ketones to construct CF3-tertiary alcohols have been less explored, and Lewis acid is often required (Scheme 2a).11,12 Thus, further development of new efficient functional transformations from the point of more sustainable and environmental chemistry is extremely valuable.13
Scheme 2. Strategies for the Synthesis of the Azaarene-Equipped CF3-Tertiary Alcohols.
In this context, with our ongoing interest in C–H activation and CF3-functionalization chemistry,14 we present a novel CF3-tertiary alcohol synthesis protocol of trifluoromethyl ketones with azaarenes under metal-free conditions, thus resulting in various ubiquitous CF3-tertiary alcohols, which are frequently found in pharmaceutical chemistry (Scheme 2b).2
Results and Discussion
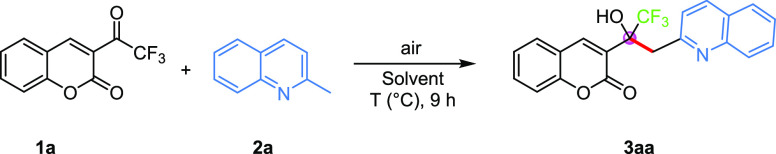
To validate our hypothesis, we began our studies by employing β-(trifluoroacetyl)coumarin 1a and 2-methylquinoline 2a as model substrates to explore the reaction conditions, and the results are summarized in Table 1. Initially, a comprehensive screening of reaction solvents was carried out (entries 1–11). To our delight, all the cases such as DMF, DMSO, EtOH, acetone, EA, DCE, CH3CN, 1,4-dioxane, toluene, THF, and n-hexane were feasible to perform the titled conversion, as shown in Scheme 2, and the CH3CN solvent was identified as the best choice, affording anticipated product 3aa in 82% yield (entry 7). Further adjusting the reaction temperature, neither increase nor decrease had a positive effect (entries 12–13). Gratifyingly, it was found that the ratio of two substrates could affect the reaction performance dramatically (entries 14–15), improving the yield of 3aa to 93% when altering the ratio of 1a:2a to 1:2 (entry 15). Additionally, the potential 1,4-addition byproduct was not discovered during the whole optimization reaction conditions. Accordingly, the optimized reaction conditions were determined as follows: 1a (0.2 mmol), 2a (0.4 mmol), and CH3CN (2.0 mL) at 90 °C under an air atmosphere for 9 h.
Table 1. Optimization of the Reaction Conditionsa.
| entry | solvent | 2a (mmol) | T (°C) | yieldb (%) |
|---|---|---|---|---|
| 1 | DMF | 0.2 | 90 | 55 |
| 2 | DMSO | 0.2 | 90 | 35 |
| 3 | EtOH | 0.2 | 90 | 27 |
| 4 | Acetone | 0.2 | 90 | 49 |
| 5 | EA | 0.2 | 90 | 31 |
| 6 | DCE | 0.2 | 90 | 24 |
| 7 | CH3CN | 0.2 | 90 | 82 |
| 8 | 1,4-dioxane | 0.2 | 90 | 57 |
| 9 | Toluene | 0.2 | 90 | 63 |
| 10 | THF | 0.2 | 90 | 50 |
| 11 | n-hexane | 0.2 | 90 | 48 |
| 12 | CH3CN | 0.2 | 70 | 68 |
| 13 | CH3CN | 0.2 | 110 | 81 |
| 14 | CH3CN | 0.3 | 90 | 86 |
| 15 | CH3CN | 0.4 | 90 | 93 |
All reactions were performed with 1a (0.2 mmol) and 2a in solvent (2.0 mL) under an air atmosphere at 90 °C for 9 h.
Isolated yield. DMF = N,N-dimethylformamide, DMSO = dimethylsulfoxide, EA = ethyl acetate, DCE = 1,2-dichloroethane, and THF = tetrahydrofuran.
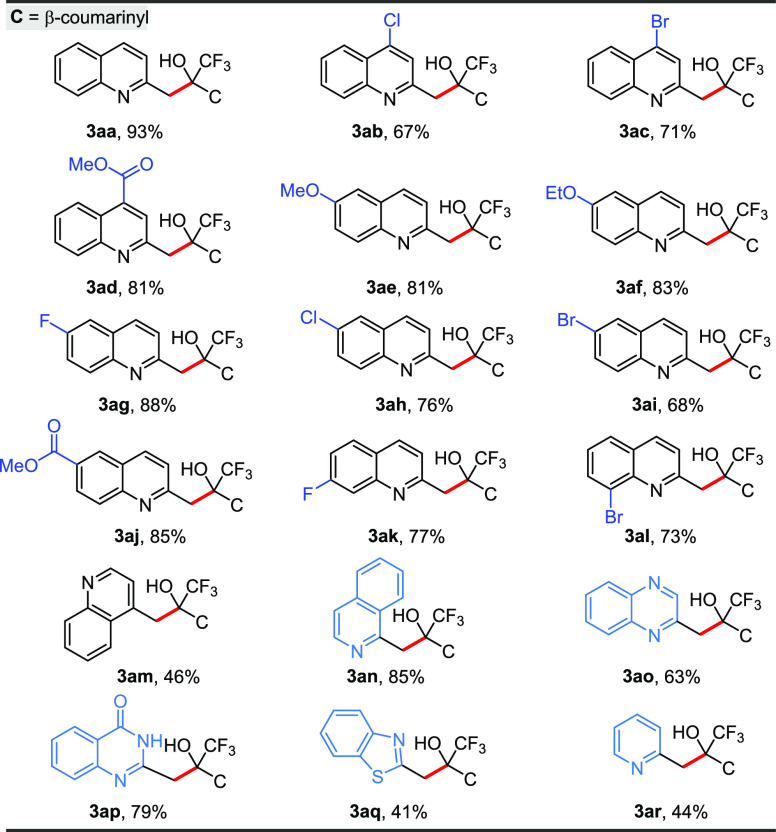
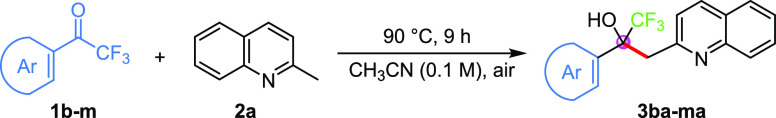
With efficient protocols in hand, the scope with respect to the azaarenes was first examined. The results indicated that various azaarenes are well tolerated, and the reaction of azaarenes 2b-r with 1a underwent the envisioned pathways to deliver the CF3-tertiary alcohols 3ab-ar in 41–93% yields (Table 2). For instance, diversified functionalization of the 4-, 6-, 7-, and 8-positions of 2a with electron-donating groups (EDGs) such as methoxy (2e) and ethoxy (2f) and electron-withdrawing groups (EWGs) such as fluoro (2g, 2k), chloro (2b, 2h), bromo (2c, 2i, 2l), and ester (2d, 2j) is tolerated with good yields. Moreover, methylquinoline-bearing methyl at C4 also proved to be feasible and afforded the anticipated CF3-tertiary alcohol product 3am in 46% yield. Notably, the established reaction conditions also led to anticipated product 3an when 1-methylisoquinoline (2n) was used as a coupling partner. After examining the tolerance of methylquinoline under the optimized conditions as shown in Table 1, we turned our attention on the various methyl N-heterocycles (2o–q) with 1a. To our delight, various methyl N-heterocycles such as quinoxaline (2o), quinazolinone (2p), and benzothiazole (2q) were reacted with 1a smoothly, which produced various 1-(β-coumarinyl)-1-(β-heterocyclyl)trifluoroethanols in moderate to good yields. Furthermore, 2-methylpyridine (2r) was found to participate in the established reaction, giving 3ar in 44% yield.
Table 2. Scope of Azaarenesa.
All reactions were performed with 1a (0.2 mmol) and 2a–r (0.4 mmol) in 2.0 mL CH3CN under an air atmosphere at 90 °C for 9 h; Isolated yields are given.
To further investigate the scope of this reaction, diversified trifluoromethyl ketones were evaluated (Table 3). Overall, a series of trifluoroacetylcoumarins or trifluoroacetylbenzene equipped with EDGs and EWGs are well tolerated, affording the anticipated trifluoromethyl tertiary alcohols 3ba–ma in 52–95% yields. Specifically, C6 sites with methyl (1b)-, fluoro (1c)-, chloro (1d)-, and bromo (1e)-modified CF3-coumarin ketones reacted smoothly with 2a, delivering desired products with moderate to excellent yields (67–95%). Particularly, a sensitive but versatile iodine group (1f) was also well compatible, which is often used in transition metal-catalyzed cross coupling transformations. Next, C7 and C8 sites with strong EDGs such as methoxy (1g, 1h) and ethoxy (1i) are well tolerated, giving rise to anticipated 3ga–ia in 83–88% yields. Notably, disubstituted substrates (1j) and (1k) were well compatible and delivered the corresponding adduct products in 84 and 89% yields, respectively. Meanwhile, the structure of 3ja was also determined by analogy on the basis of X-ray (CCDC 2179504). Gratifyingly, the fused-ring system 1l also reacted smoothly, generating tertiary alcohol product 3la in 89% yield. Finally, it should be mentioned that trifluoroacetophenone (1m) could be subjected as the electrophilic reagent to produce 3ma with moderate yield (52%) in an established metal-free system, which is quite different from previous reports that often rely on Lewis acid catalysis, such as iron salt, indium salt, and so on.11,12
Table 3. Scope of Trifluoromethyl Ketonesa.
All reactions were performed with 1b–m (0.2 mmol) and 2a (0.4 mmol) in 2.0 mL CH3CN under an air atmosphere at 90 °C for 9 h. Isolated yields are given.
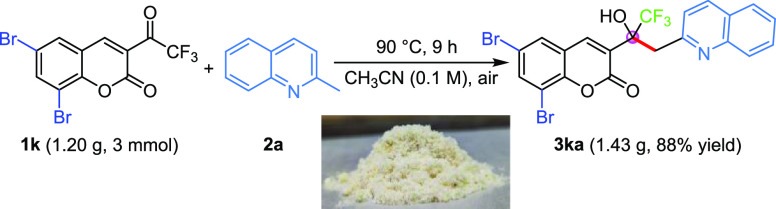
To further showcase the potential utility of this newly established method, a larger-scale (3 mmol) experiment was carried out between 1k and 2a (Scheme 3), which delivered the desired product 3ka with comparable yield (1.43 g, 88% yield).
Scheme 3. Gram-Scale Reaction.
Based on the abovementioned results and previous reports,11 a plausible reaction pathway for the conversion of trifluoromethyl ketone and azaarene to CF3-tertiary alcohols under metal-free conditions is outlined in Scheme 4. Initially, 2-methylquinoline 2a was converted to its enamine species G, and then, the enamide species G underwent nucleophilic addition to β-(trifluoroacetyl)coumarin 1a; further protonation could provide the expected azaarene-equipped CF3-tertiary alcohol 3aa.
Scheme 4. Proposed Mechanism.
To evaluate the biological activity of the newly prepared azaarene-equipped CF3-tertiary alcohols, preliminary antifungal activity against F. oxysporum, F. graminearum, P. nicotianae, F. moniliforme, and R. solani with target compounds 3aa–ma was performed based on the early reference,15 and the results are summarized in Figure 1. Overall, most of the desired CF3-tertiary alcohol products 3aa–ma showed fungicidal activities against the abovementioned five fungi. First, the inhibitor rate of the model product 3aa against F. oxysporum was 29.84% at 0.5 mg/mL. The inhibitor rate was improved to 44.32 and 45.11% when compounds 3aq and 3ao were used, respectively. Next, compounds 3aa–ma against F. graminearum, P. nicotianae, F. moniliforme, and R. solani were tested. Gratifyingly, the antifungal activity against R. solani was obviously better compared to other three fungi, with a moderate to good inhibitor rate. Particularly, compound 3al displayed good (91.65%) in vitro fungicidal activity, with the median effective concentration (EC50) value of 0.18 mg/mL.
Figure 1.
Antifungal activities of the CF3-tertiary alcohols 3aa–3ma.
Conclusions
In conclusion, we have developed a novel reaction for the synthesis of azaarene-equipped CF3-tertiary alcohols through addition of azaarenes to CF3-ketones under metal-free conditions. The azaarenes, including quinolones, isoquinoline, quinoxaline, quinazolinone, and benzothiazole, were used as coupling partners. The corresponding CF3-tertiary alcohol products were obtained in excellent yields (up to 95% yield) with high chemoselectivity (only 1,2-addition). Compared to previous reports, this established reaction features simple reaction conditions (only solvent), high atom- and step-economy, and broad substrate scope. Moreover, most of the synthesized compounds displayed promising fungicidal activities, and compound 3al exhibited 91.65% fungicidal activity against R. solani, with an EC50 value of 0.18 mg/mL. Further studies on the synthesis of novel CF3-tertiary alcohol scaffolds with green methods and their fungicidal activities are ongoing in our laboratories.
Experimental Section
General Information
All reactions were carried out under an air atmosphere. All reagents were used as received unless otherwise noted. Analytical thin-layer chromatography was performed with 0.25 mm-coated commercial silica gel plates (TLC Silica Gel 60 F254); the developed chromatogram was visualized using fluorescence. Flash chromatography was performed with silica gel (200–300 mesh). Proton nuclear magnetic resonance (1H NMR) data were acquired at 400 MHz on a Bruker Ascend spectrometer. Chemical shifts are reported in delta (δ) units, in parts per million (ppm) downfield from tetramethylsilane. Splitting patterns are designated as s, singlet; d, doublet; t, triplet; and m, multiplet. Coupling constants J are quoted in Hz. Carbon-13 nuclear magnetic resonance (13C NMR) data were acquired at 100 MHz on a Bruker Ascend 400 spectrometer. Chemical shifts are reported in ppm relative to the center line of a triplet at 77.0 ppm for chloroform-d and the center line of a septet at 44.0 ppm for DMSO-d6. Fluorine nuclear magnetic resonance (19F NMR) data were acquired at 376 MHz on a Bruker Ascend 400 spectrometer, and chemical shifts are reported relative to interstandard CFCl3 at 0.0 ppm. Mass spectra were acquired on a Bruker Daltonics MicroTof-Q II mass spectrometer.
General Procedure for the Preparation of Azaarene-Equipped CF3-Tertiary Alcohols
A 10.0 mL vial equipped with a stirring bar was charged with trifluoromethyl ketones 1 (0.2 mmol, 1.0 equiv), azaarenes 2 (0.4 mmol, 2.0 equiv), and CH3CN (2.0 mL). The vial was sealed with a Teflon screw cap, and the reaction mixture was heated at 90 °C for 9 h under pressure. After the reaction vessel was cooled to room temperature, the crude reaction mixture was filtered with celite and washed with DCM. The solvent was removed under reduced pressure. Then, the residue was purified by silica gel column chromatography to afford the desired product 3.
3-(1,1,1-Trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3aa)
(PE:EA = 5:1, Rf = 0.37, white solid, mp = 91.7–92.3 °C, 93% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.64 (s, 1H), 8.47 (s, 1H), 8.10 (d, J = 8.4 Hz, 1H), 7.93 (d, J = 8.5 Hz, 1H), 7.76 (d, J = 8.2 Hz, 1H), 7.67 (t, J = 7.7 Hz, 1H), 7.51–7.44 (m, 4H), 7.23–7.19 (m, 2H), 4.56 (d, J = 14.7 Hz, 1H), 3.73 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.2, 158.3, 154.0, 146.1, 137.8, 132.4, 130.2, 128.7, 128.2, 127.8, 127.1, 126.7, 126.5, 124.8, 124.6 (q, J = 284.0 Hz), 124.5, 123.1, 118.7, 116.2, 76.8 (q, J = 30.0 Hz), 37.4. 19F NMR (376 MHz, Chloroform-d) δ −79.98. IR (KBr): 3421, 2837, 2810, 1653, 1586, 1373, 1357, 783, 770 cm–1. HRMS (ESI) m/z calculated for C21H15F3NO3 [M + H]+ 386.1004, found 386.1007.
3-(3-(4-Chloroquinolin-2-yl)-1,1,1-trifluoro-2-hydroxypropan-2-yl)-2H-chromen-2-one (3ab)
(PE:EA = 5:1, Rf = 0.35, white solid, mp = 140.8–141.2 °C, 65% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.48 (s, 1H), 8.30 (s, 1H), 8.16 (d, J = 8.5 Hz, 1H), 7.93 (d, J = 8.4 Hz, 1H), 7.72 (t, J = 8.4 Hz, 1H), 7.60 (t, J = 8.3 Hz, 2H), 7.51–7.46 (m, 2H), 7.25–7.21 (m, 2H), 4.52 (d, J = 14.7 Hz, 1H), 3.70 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.3, 158.0, 154.0, 147.0, 146.2, 144.2, 132.5, 131.0, 128.7, 128.5, 127.6, 125.9, 125.4, 124.6, 124.5 (q, J = 284.0 Hz), 124.3, 123.0, 118.6, 116.3, 76.7 (q, J = 30.1 Hz), 37.5. 19F NMR (376 MHz, Chloroform-d) δ −80.06. IR (KBr): 3436, 2849, 2820, 2722, 1743, 1673, 1641, 1597, 1391, 1378, 1346, 784, 768 cm–1. HRMS (ESI) m/z calculated for C21H14ClF3NO3 [M + H]+ 420.0614, found 420.0602.
3-(3-(4-Bromoquinolin-2-yl)-1,1,1-trifluoro-2-hydroxypropan-2-yl)-2H-chromen-2-one (3ac)
(PE:EA = 3:1, Rf = 0.45, white solid, mp = 138.7–139.5 °C, 71% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.47 (s, 1H), 8.29 (s, 1H), 8.11 (d, J = 8.5 Hz, 1H), 7.91 (d, J = 8.4 Hz, 1H), 7.79 (s, 1H), 7.73–7.69 (m, 1H), 7.59 (d, J = 5.6 Hz, 1H), 7.48 (dd, J = 10.5, 7.8 Hz, 2H), 7.25–7.19 (m, 2H), 4.50 (d, J = 14.7 Hz, 1H), 3.70 (d, J = 14.8 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.3, 157.9, 154.0, 146.7, 146.2, 135.7, 132.5, 131.0, 128.7, 128.6, 127.9, 126.9, 126.8, 126.6, 124.6, 124.5 (q, J = 286.1 Hz), 124.3, 118.6, 116.3, 76.7 (q, J = 30.0 Hz), 37.3. IR (KBr): 3444, 2847, 2816, 2735, 1631, 1595, 1391, 1352, 1269, 1183, 791, 763, 739 cm–1. 19F NMR (376 MHz, Chloroform-d) δ −80.05. HRMS (ESI) m/z calculated for C21H14BrF3NO3 [M + H]+ 464.0109, found 464.0108.
Methyl 2-(3,3,3-Trifluoro-2-hydroxy-2-(2-oxo-2H-chromen-3-yl)propyl)quinoline-4-carboxylate (3ad)
(PE:EA = 5:1, Rf = 0.33, white solid, mp = 185.3–186.5 °C, 81% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.69 (d, J = 8.7 Hz, 1H), 8.48 (s, 1H), 7.98 (s, 2H), 7.70 (d, J = 7.0 Hz, 1H), 7.61–7.57 (m, 1H), 7.48 (dd, J = 14.3, 8.0 Hz, 2H), 7.22 (t, J = 7.4 Hz, 3H), 4.58 (d, J = 14.9 Hz, 1H), 4.02 (s, 3H), 3.77 (d, J = 14.8 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 166.0, 159.3, 157.7, 153.9, 147.2, 146.2, 136.2, 132.5, 130.4, 128.7, 128.6, 128.2, 125.7, 124.6, 124.5 (q, J = 286.2 Hz), 124.2, 124.1, 118.6, 116.2, 76.5 (q, J = 29.9 Hz), 52.9, 37.7. 19F NMR (376 MHz, Chloroform-d) δ −80.04. IR (KBr): 3425, 2844, 2810, 2755, 1650, 1623, 1583, 1388, 1349, 1265, 789, 756 cm–1. HRMS (ESI) m/z calculated for C23H17F3NO5 [M + H]+ 444.1059, found 444.1017.
3-(1,1,1-Trifluoro-2-hydroxy-3-(6-methoxyquinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3ae)
(PE:EA = 5:1, Rf = 0.31, white solid, mp = 185.5–186.7 °C, 81% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.65 (s, 1H), 8.60 (s, 1H), 8.45 (s, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.81 (d, J = 9.2 Hz, 1H), 7.46 (t, J = 7.9 Hz, 2H), 7.41 (d, J = 8.4 Hz, 1H), 7.31 (dd, J = 9.2, 2.7 Hz, 1H), 7.21 (ddt, J = 7.6, 4.1, 2.0 Hz, 2H), 7.00 (d, J = 2.8 Hz, 1H), 4.51 (d, J = 14.6 Hz, 1H), 3.88 (s, 3H), 3.68 (d, J = 14.6 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.2, 157.8, 155.4, 154.0, 146.0, 142.3, 136.4, 132.3, 129.5, 128.6, 128.2, 124.9, 124.6 (q, J = 286.2 Hz), 124.4, 123.3, 123.0, 118.7, 116.1, 105.0, 76.8 (q, J = 29.9 Hz), 55.6, 37.1. 19F NMR (376 MHz, Chloroform-d) δ −79.86. IR (KBr): 3437, 2847, 2735, 2701, 1673, 1650, 1603, 1391, 1347, 797, 768, 750 cm–1. HRMS (ESI) m/z calculated for C22H17F3NO4 [M + H]+ 416.1110, found 416.1128.
3-(3-(6-Ethoxyquinolin-2-yl)-1,1,1-trifluoro-2-hydroxypropan-2-yl)-2H-chromen-2-one (3af)
(PE:EA = 5:1, Rf = 0.38, white solid, mp = 190.9–191.6 °C, 83% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.60 (s, 1H), 8.44 (s, 1H), 7.96 (d, J = 8.4 Hz, 1H), 7.80 (d, J = 9.2 Hz, 1H), 7.50–7.44 (m, 2H), 7.40 (d, J = 8.4 Hz, 1H), 7.31 (dd, J = 9.2, 2.7 Hz, 1H), 7.25–7.17 (m, 2H), 6.98 (d, J = 2.7 Hz, 1H), 4.50 (d, J = 14.6 Hz, 1H), 4.10 (dd, J = 7.0, 2.9 Hz, 2H), 3.67 (d, J = 14.6 Hz, 1H), 1.45 (t, J = 7.0 Hz, 3H). 13C NMR (100 MHz, Chloroform-d) δ 159.2, 157.2, 155.2, 154.0, 146.0, 142.2, 136.4, 132.3, 129.5, 128.6, 128.2, 126.0, 124.9, 124.6 (q, J = 286.1 Hz), 124.4, 123.2, 118.7, 116.1, 105.7, 76.8 (q, J = 29.3 Hz), 63.9, 37.1, 14.7. 19F NMR (376 MHz, Chloroform-d) δ −79.91. IR (KBr): 3450, 2852, 2806, 2732, 1657, 1597, 1383, 1352, 1269, 786, 763 cm–1. HRMS (ESI) m/z calculated for C23H19F3NO4 [M + H]+ 430.1266, found 430.1288.
3-(1,1,1-Trifluoro-3-(6-fluoroquinolin-2-yl)-2-hydroxypropan-2-yl)-2H-chromen-2-one (3ag)
(PE:EA = 5:1, Rf = 0.30, white solid, mp = 121.9–122.8 °C, 63% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.46 (s, 1H), 8.34 (s, 1H), 8.06 (d, J = 8.5 Hz, 1H), 7.91 (s, 1H), 7.46 (dt, J = 14.5, 7.4 Hz, 4H), 7.38 (dd, J = 8.7, 2.8 Hz, 1H), 7.22 (t, J = 7.1 Hz, 2H), 4.55 (d, J = 14.6 Hz, 1H), 3.71 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 161.7, 159.3, 157.6, 154.0, 146.2, 137.1, 132.5, 130.7, 128.7, 127.8, 127.7, 124.6, 124.5 (q, J = 285.8 Hz), 123.9, 120.3, 118.6, 116.2, 111.0, 110.8, 76.7 (q, J = 30.0 Hz), 37.5. 19F NMR (376 MHz, Chloroform-d) δ −79.99, −112.81. IR (KBr): 3444, 2848, 2821, 2724, 1743, 1673, 1639, 1605, 1089, 1378, 1347, 786, 769 cm–1. HRMS (ESI) m/z calculated for C21H14F4NO3 [M + H]+ 404.0910, found 404.0924.
3-(3-(6-Chloroquinolin-2-yl)-1,1,1-trifluoro-2-hydroxypropan-2-yl)-2H-chromen-2-one (3ah)
(PE:EA = 5:1, Rf = 0.39, white solid, mp = 161.2–162.7 °C, 76% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.45 (s, 1H), 8.27 (s, 1H), 8.02 (d, J = 8.5 Hz, 1H), 7.86 (d, J = 9.0 Hz, 1H), 7.74 (d, J = 2.3 Hz, 1H), 7.60 (dd, J = 9.0, 2.3 Hz, 1H), 7.54–7.46 (m, 3H), 7.22 (t, J = 7.3 Hz, 2H), 4.54 (d, J = 14.7 Hz, 1H), 3.72 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.2, 158.6, 154.0, 146.1, 144.6, 136.7, 132.4, 131.0, 129.8, 128.7, 127.7, 126.4, 125.9, 124.6, 124.5 (q, J = 286.6 Hz), 124.0, 123.1, 118.6, 116.2, 76.7 (q, J = 30.0 Hz), 37.6. 19F NMR (376 MHz, Chloroform-d) δ −80.01. IR (KBr): 3436, 2849, 2813, 1633, 1594, 1589, 1378, 1349, 781, 770 cm–1. HRMS (ESI) m/z calculated for C21H14ClF3NO3 [M + H]+ 420.0614, found 420.0598.
3-(3-(6-Bromoquinolin-2-yl)-1,1,1-trifluoro-2-hydroxypropan-2-yl)-2H-chromen-2-one (3ai)
(PE:EA = 5:1, Rf = 0.37, white solid, mp = 180.3–180.9 °C, 68% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.45 (s, 1H), 8.24 (s, 1H), 8.02 (d, J = 8.5 Hz, 1H), 7.92 (s, 1H), 7.79 (d, J = 9.2 Hz, 1H), 7.73 (d, J = 6.9 Hz, 1H), 7.53–7.46 (m, 3H), 7.23 (t, J = 7.4 Hz, 2H), 4.54 (d, J = 14.7 Hz, 1H), 3.70 (d, J = 14.6 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.4, 158.9, 154.1, 146.7, 144.2, 137.4, 133.8, 132.7, 130.6, 128.9, 128.4, 124.8, 124.7, 124.6 (q, J = 286.2 Hz), 124.2, 120.7, 118.7, 116.4, 76.5 (q, J = 30.3 Hz), 37.8. 19F NMR (376 MHz, Chloroform-d) δ −79.37. IR (KBr): 3431, 2852, 2800, 1631, 1615, 1589, 1390, 1363, 1348, 780, 765, 736 cm–1. HRMS (ESI) m/z calculated for C21H14BrF3NO3 [M + H]+ 464.0109, found 464.0101.
Methyl-2-(3,3,3-trifluoro-2-hydroxy-2-(2-oxo-2H-chromen-3-yl)propyl)quinoline-6-carboxylate (3aj)
(PE:EA = 5:1, Rf = 0.25, white solid, mp = 198.1–199.8 °C, 85% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.52 (d, J = 1.9 Hz, 1H), 8.47 (s, 1H), 8.25 (dd, J = 8.9, 1.9 Hz, 1H), 8.21 (d, J = 8.5 Hz, 1H), 7.96 (d, J = 8.8 Hz, 1H), 7.55 (d, J = 8.5 Hz, 1H), 7.48 (dd, J = 12.7, 7.3 Hz, 2H), 7.22 (t, J = 7.4 Hz, 8H), 4.58 (d, J = 14.8 Hz, 1H), 3.99 (s, 1H), 3.96 (s, 3H), 3.75 (d, J = 14.8 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 166.3, 160.7, 159.2, 154.0, 148.0, 146.2, 138.9, 132.5, 130.9, 129.7, 128.7, 128.5, 128.2, 126.3, 124.6, 124.5 (q, J = 285.9 Hz), 124.4, 124.0118.6, 116.2, 76.7 (q, J = 30.0 Hz), 52.5, 37.9. 19F NMR (376 MHz, Chloroform-d) δ −80.07. IR (KBr): 3429, 2850, 2808, 2735, 1667, 1631, 1592, 1389, 1350, 1269, 794, 747 cm–1. HRMS (ESI) m/z calculated for C23H17F3NO5 [M + H]+ 444.1059, found 444.1073.
3-(1,1,1-Trifluoro-3-(7-fluoroquinolin-2-yl)-2-hydroxypropan-2-yl)-2H-chromen-2-one (3ak)
(PE:DCM = 1:1, Rf = 0.25, white solid, mp = 141.6–142.2 °C, 62% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.47 (s, 1H), 8.33 (s, 1H), 8.10 (d, J = 8.4 Hz, 1H), 7.76 (dd, J = 9.0, 6.0 Hz, 1H), 7.55 (d, J = 8.2 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.46 (dd, J = 8.2, 5.0 Hz, 2H), 7.29 (dd, J = 8.6, 2.6 Hz, 1H), 7.27–7.18 (m, 3H), 4.55 (d, J = 14.6 Hz, 1H), 3.72 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 164.6, 162.1, 159.4, 159.2, 154.0, 146.1, 137.7, 132.4, 130.0, 129.9, 128.7, 124.6, 124.5 (q, J = 286.1 Hz,), 124.2, 122.5, 118.6, 117.4, 116.2, 112.0, 76.7 (q, J = 30.1 Hz), 37.6. 19F NMR (376 MHz, Chloroform-d) δ −80.00, −87.20. IR (KBr): 3429, 2850, 2810, 2743, 1634, 1605, 1383, 1350, 1263, 789, 768 cm–1. HRMS (ESI) m/z calculated for C21H14F4NO3 [M + H]+ 404.0910, found 404.0927.
3-(3-(8-Bromoquinolin-2-yl)-1,1,1-trifluoro-2-hydroxypropan-2-yl)-2H-chromen-2-one (3al)
(PE:EA = 3:1, Rf = 0.45, white solid, mp = 155.6–156.4 °C, 73% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.61 (s, 1H), 8.11 (d, J = 8.4 Hz, 1H), 7.95 (d, J = 6.3 Hz, 1H), 7.72 (d, J = 8.1 Hz, 1H), 7.55–7.49 (m, 2H), 7.48–7.43 (m, 1H), 7.34 (t, J = 7.8 Hz, 1H), 7.22 (t, J = 7.6 Hz, 2H), 7.18 (d, J = 8.3 Hz, 1H), 4.66 (d, J = 15.2 Hz, 1H), 3.75 (d, J = 15.2 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.3, 159.2, 153.9, 146.5, 143.2, 138.1, 133.4, 132.3, 128.7, 128.3, 127.6, 127.1, 124.7, 124.5 (q, J = 285.6 Hz), 124.4, 123.8, 123.6, 118.7, 116.1, 76.8 (q, J = 30.2 Hz), 37.6. 19F NMR (376 MHz, Chloroform-d) δ −80.01. IR (KBr): 3416, 3090, 3048, 3032, 2659, 1717, 1634, 1597, 1378, 1357, 1196, 1120, 945, 768, 729 cm–1. HRMS (ESI) m/z calculated for C21H14BrF3NO3 [M + H]+ 464.0109, found 464.0108.
3-(1,1,1-Trifluoro-2-hydroxy-3-(quinolin-4-yl)propan-2-yl)-2H-chromen-2-one (3am)
(PE:EA = 3:1, Rf = 0.45, white solid, mp = 183.8–184.6 °C, 46% yield). 1H NMR (400 MHz, DMSO-d6) δ 8.68 (d, J = 4.5 Hz, 1H), 8.51 (s, 1H), 8.26 (d, J = 7.2 Hz, 1H), 7.97 (d, J = 9.7 Hz, 1H), 7.84 (d, J = 7.9 Hz, 1H), 7.74 (d, J = 6.9 Hz, 1H), 7.65–7.60 (m, 2H), 7.56 (s, 1H), 7.50 (d, J = 4.6 Hz, 1H), 7.38–7.33 (m, 2H), 4.60 (d, J = 15.7 Hz, 1H), 3.80 (d, J = 15.8 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 158.6, 153.7, 150.1, 148.2, 145.8, 141.8, 133.4, 130.0, 129.8, 129.5, 128.2, 126.9, 125.8 (q, J = 289.5 Hz), 125.3, 124.8, 123.6, 121.9, 118.5, 116.3, 76.3 (q, J = 28.6 Hz), 33.0. 19F NMR (376 MHz, DMSO-d6) δ −80.12. IR (KBr): 3437, 2805, 2737, 1631, 1603, 1389, 1350, 1269, 786, 768 cm–1. HRMS (ESI) m/z calculated for C21H15F3NO3 [M + H]+ 386.1004, found 386.1017.
3-(1,1,1-Trifluoro-2-hydroxy-3-(isoquinolin-1-yl)propan-2-yl)-2H-chromen-2-one (3an)
(PE:EA = 5:1, Rf = 0.35, white solid, mp = 140.8–141.2, 85% yield). 1H NMR (400 MHz, Chloroform-d) δ 9.27 (s, 1H), 8.56 (s, 1H), 8.40 (d, J = 7.0 Hz, 1H), 8.23 (d, J = 5.8 Hz, 1H), 7.79–7.76 (m, 1H), 7.73–7.66 (m, 2H), 7.53 (t, J = 6.9 Hz, 2H), 7.48–7.42 (m, 1H), 7.23 (t, J = 7.6 Hz, 1H), 7.16 (d, J = 8.3 Hz, 1H), 5.14 (d, J = 15.7 Hz, 1H), 3.83 (d, J = 15.6 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.1, 158.1, 153.9, 145.9, 139.2, 136.6, 132.3, 131.1, 128.7, 128.2, 127.5, 127.3, 125.8, 125.3, 124.70 (q, J = 284.5 Hz), 124.5, 120.6, 118.7, 116.2, 76.9 (q, J = 29.7 Hz). 31.9. 19F NMR (376 MHz, Chloroform-d) δ −79.65. IR (KBr): 3421, 2844, 2820, 2732, 1676, 1644, 1605, 1391, 1376, 1350, 760, 742 cm–1. HRMS (ESI) m/z calculated for C21H15F3NO3 [M + H]+ 386.1004, found 386.1022.
3-(1,1,1-Trifluoro-2-hydroxy-3-(quinoxalin-2-yl)propan-2-yl)-2H-chromen-2-one (3ao)
(PE:DCM = 1:2, Rf = 0.22, white solid, mp = 178.9–179.7 °C, 63% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.95 (s, 1H), 8.45 (s, 1H), 8.07 (s, 1H), 7.92 (s, 1H), 7.75–7.70 (m, 2H), 7.54–7.48 (m, 2H), 7.40 (s, 1H), 7.24 (d, J = 8.3 Hz, 3H), 4.55 (d, J = 14.8 Hz, 1H), 3.77 (d, J = 14.9 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.6, 153.9, 152.4, 146.9, 146.4, 141.9, 140.4, 132.8, 130.6, 130.0, 129.6, 128.7, 128.3, 124.7, 124.4 (q, J = 285.6 Hz), 123.3, 118.4, 116.4, 76.8 (q, J = 27.3 Hz), 36.1. 19F NMR (376 MHz, Chloroform-d) δ −80.26. IR (KBr): 3426, 2839, 2805, 2735, 1733, 1634, 1584, 1433, 1386, 1350, 1266, 1159, 1057, 1013, 765, 744, 705 cm–1. HRMS (ESI) m/z calculated for C20H14F3N2O3 [M + H]+ 387.0957, found 387.0974.
2-(3,3,3-Trifluoro-2-hydroxy-2-(2-oxo-2H-chromen-3-yl)propyl)quinazolin-4(3H)-one (3ap)
(PE:DCM = 1:2, Rf = 0.22, white solid, mp = 190.1–190.9 °C, 79% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.47 (s, 1H), 8.33 (s, 1H), 8.10 (d, J = 8.4 Hz, 1H), 7.79–7.73 (m, 1H), 7.58–7.43 (m, 4H), 7.29 (dd, J = 8.6, 2.6 Hz, 1H), 7.27–7.18 (m, 3H), 4.55 (d, J = 14.7 Hz, 1H), 3.72 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 159.7, 156.9, 151.6, 151.0, 146.0, 141.5, 132.8, 130.9, 127.4, 124.7, 124.0, 123.2 (q, J = 287.0 Hz), 123.1, 123.0, 119.3, 116.7, 116.4, 114.1, 73.2 (q, J = 29.0 Hz), 34.1. 19F NMR (376 MHz, DMSO-d6) δ −80.91. IR (KBr): 3442, 2846, 2815, 2724, 1636, 1592, 1389, 1355, 1266, 789, 768 cm–1. HRMS (ESI) m/z calculated for C20H14F3N2O4 [M + H]+ 403.0906, found 403.0928.
3-(3-(Benzo[d]thiazol-2-yl)-1,1,1-trifluoro-2-hydroxypropan-2-yl)-2H-chromen-2-one (3aq)
(PE:EA = 3:1, Rf = 0.32, yellow solid, mp = 141.5–142.8 °C, 41% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.34 (s, 1H), 7.91 (d, J = 7.3 Hz, 1H), 7.81 (d, J = 8.1 Hz, 1H), 7.53 (t, J = 7.6 Hz, 2H), 7.45–7.40 (m, 1H), 7.37–7.33 (m, 1H), 7.28 (d, J = 7.6 Hz, 4H), 7.16 (s, 1H), 4.60 (d, J = 15.3 Hz, 1H), 3.85 (d, J = 15.3 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 165.9, 160.2, 153.8, 152.0, 146.1, 134.8, 132.9, 128.9, 127.3 (q, J = 285.0 Hz), 126.2, 125.4, 124.9, 122.5, 122.3, 121.7, 118.4, 116.4, 76.4 (q, J = 30.7 Hz), 35.9. 19F NMR (376 MHz, Chloroform-d) δ −80.21. IR (KBr): 3442, 2846, 2807, 1632, 1587, 1435, 1388, 1350, 1164, 782, 759, 730 cm–1. HRMS (ESI) m/z calculated for C19H13F3NO3S [M + H]+ 392.0568, found 392.0571.
3-(1,1,1-Trifluoro-2-hydroxy-3-(pyridin-2-yl)propan-2-yl)-2H-chromen-2-one (3ar)
(PE:EA = 5:1, Rf = 0.42, white solid, mp = 156.1–157.7 °C, 44% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.43 (s, 1H), 8.36 (d, J = 5.0 Hz, 1H), 7.64 (t, J = 7.7 Hz, 1H), 7.51 (t, J = 8.2 Hz, 2H), 7.36 (d, J = 7.8 Hz, 1H), 7.27 (s, 3H), 7.25 (d, J = 7.6 Hz, 2H), 7.21–7.10 (m, 1H), 4.35 (d, J = 14.6 Hz, 1H), 3.57 (d, J = 14.5 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.1, 157.0, 154.0, 147.4, 146.0, 138.0, 132.4, 128.7, 125.4, 124.7, 124.5 (q, J = 286.4 Hz), 124.4, 122.5, 118.6, 116.2, 76.7 (q, J = 30.0 Hz), 36.9. 19F NMR (376 MHz, Chloroform-d) δ −79.84. IR (KBr): 3441, 2849, 2810, 2734, 1633, 1584, 1383, 1349, 796, 765 cm–1. HRMS (ESI) m/z calculated for C17H13F3NO3 [M + H]+ 336.0848, found 336.0861.
6-Methyl-3-(1,1,1-trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3ba)
(PE:EA = 5:1, Rf = 0.32, white solid, mp = 136.4–137.9 °C, 85% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.64 (s, 1H), 8.43 (s, 1H), 8.11 (d, J = 8.4 Hz, 1H), 7.94 (d, J = 8.4 Hz, 1H), 7.77 (d, J = 8.1 Hz, 1H), 7.68 (t, J = 7.7 Hz, 1H), 7.53–7.46 (m, 2H), 7.27 (d, J = 6.9 Hz, 3H), 7.11 (d, J = 8.9 Hz, 1H), 4.59 (d, J = 14.6 Hz, 1H), 3.73 (d, J = 14.6 Hz, 1H), 2.34 (s, 3H). 13C NMR (100 MHz, Chloroform-d) δ 159.5, 158.3, 152.1, 146.1, 137.8, 134.2, 133.4, 130.1, 128.4, 128.2, 127.8, 127.1, 126.7, 124.6 (q, J = 286.0 Hz), 124.5, 123.1, 120.3, 118.4, 115.9, 76.7 (q, J = 29.9 Hz), 37.4, 20.7. 19F NMR (376 MHz, Chloroform-d) δ −80.01. IR (KBr): 3439, 2842, 2808, 2735, 1631, 1603, 1592, 1389, 1350, 1266, 1190, 797, 744, 705 cm–1. HRMS (ESI) m/z calculated for C22H17F3NO3 [M + H]+ 400.1161, found 400.1120.
6-Fluoro-3-(1,1,1-trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3ca)
(PE:EA = 5:1, Rf = 0.29, white solid, mp = 155.1–156.3 °C, 93% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.69 (s, 1H), 8.42 (s, 1H), 8.11 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 8.4 Hz, 1H), 7.77 (d, J = 8.1 Hz, 1H), 7.71–7.66 (m, 1H), 7.50 (t, J = 7.0 Hz, 1H), 7.46 (d, J = 8.4 Hz, 1H), 7.21–7.13 (m, 3H), 4.54 (d, J = 14.7 Hz, 1H), 3.73 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.9, 158.1, 150.1, 146.1, 145.1, 137.8, 130.2, 128.1, 127.8, 127.3 (q, J = 285.8 Hz), 127.1, 126.7, 126.2, 119.9, 119.7, 119.2, 117.8, 113.9, 113.6, 76.8 (q, J = 30.1 Hz), 37.3. 19F NMR (376 MHz, Chloroform-d) δ −79.85, −117.27. IR (KBr): 3431, 2810, 2735, 2657, 1634, 1605, 1389, 1350, 1193, 1170, 765, 741 cm–1. HRMS (ESI) m/z calculated for C21H13F4NO3 [M + H]+ 404.0910, found 404.0922.
6-Chloro-3-(1,1,1-trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3da)
(PE:EA = 5:1, Rf = 0.28, white solid, mp = 168.4–169.8 °C, 95% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.71 (s, 1H), 8.40 (s, 1H), 8.11 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 8.5 Hz, 1H), 7.76 (d, J = 8.2 Hz, 1H), 7.68 (t, J = 7.7 Hz, 1H), 7.50 (t, J = 6.9 Hz, 1H), 7.46–7.43 (m, 2H), 7.39 (dd, J = 8.8, 2.5 Hz, 1H), 7.14 (d, J = 8.8 Hz, 1H), 4.54 (d, J = 14.9 Hz, 1H), 3.72 (d, J = 14.8 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 158.5, 158.1, 152.3, 146.1, 144.9, 137.9, 132.2, 130.2, 129.7, 128.1, 127.8, 127.1, 126.7, 126.3, 125.8, 124.4 (q, J = 284.0 Hz), 123.0, 119.6, 117.6, 76.8 (q, J = 30.3 Hz), 37.2. 19F NMR (376 MHz, Chloroform-d) δ −79.81. IR (KBr): 3433, 2846, 2721, 2700, 1670, 1639, 1594, 1373, 1346, 783, 767 cm–1. HRMS (ESI) m/z calculated for C21H14ClF3NO3 [M + H]+ 420.0614, found 420.0636.
6-Bromo-3-(1,1,1-trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3ea)
(PE:EA = 5:1, Rf = 0.25, white solid, mp = 177.3–178.6 °C, 77% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.70 (s, 1H), 8.40 (s, 1H), 8.11 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 8.5 Hz, 1H), 7.76 (d, J = 8.1 Hz, 1H), 7.69 (t, J = 7.7 Hz, 1H), 7.60 (d, J = 2.3 Hz, 1H), 7.54–7.48 (m, 2H), 7.45 (d, J = 8.4 Hz, 1H), 7.08 (d, J = 8.8 Hz, 1H), 4.54 (d, J = 14.7 Hz, 1H), 3.72 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 158.5, 158.1, 152.8, 146.1, 144.9, 137.9, 135.1, 130.9, 130.3, 128.1, 127.8, 127.7 (q, J = 286.0 Hz), 127.1, 126.8, 126.3, 123.0, 120.1, 117.9, 117.0, 76.8 (q, J = 29.9 Hz), 36.3. 19F NMR (376 MHz, Chloroform-d) δ −79.87. IR (KBr): 3446, 2844, 2820, 2802, 2726, 2703, 1675, 1641, 1602, 1391, 1373, 1344, 1049, 791, 767 cm–1. HRMS (ESI) m/z calculated for C21H14BrF3NO3 [M + H]+ 464.0109, found 464.0133.
6-Iodo-3-(1,1,1-trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3fa)
(PE:EA = 5:1, Rf = 0.27, white solid, mp = 173.9–174.4 °C, 67% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.67 (s, 1H), 8.37 (s, 1H), 8.11 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 8.5 Hz, 1H), 7.81–7.74 (m, 2H), 7.73–7.65 (m, 2H), 7.50 (t, J = 7.5 Hz, 1H), 7.44 (d, J = 8.4 Hz, 1H), 6.95 (d, J = 8.7 Hz, 1H), 4.53 (d, J = 14.7 Hz, 1H), 3.72 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 158.4, 158.1, 153.5, 146.1, 144.7, 140.7, 137.8, 137.0, 130.2, 128.1, 127.8, 127.1, 126.7, 126.1, 124.4 (q, J = 284.0 Hz), 123.0, 120.7, 118.1, 87.7, 76.7 (q, J = 30.3 Hz), 37.3. 19F NMR (376 MHz, Chloroform-d) δ −79.83. IR (KBr): 3439, 2847, 2813, 2740, 1634, 1600, 1386, 1350, 1266, 786, 768 cm–1. HRMS (ESI) m/z calculated for C21H14F3INO3 [M + H]+ 511.9970, found 511.9993.
7-Methoxy-3-(1,1,1-trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3ga)
(PE:EA = 5:1, Rf = 0.37, white solid, mp = 84.8–85.4 °C, 86% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.56 (s, 1H), 8.39 (s, 1H), 8.10 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 8.3 Hz, 1H), 7.76 (d, J = 9.6 Hz, 1H), 7.67 (t, 1H), 7.50 (d, J = 7.9 Hz, 2H), 7.36 (d, J = 8.7 Hz, 1H), 6.77 (dd, J = 8.7, 2.4 Hz, 1H), 6.68 (d, J = 2.4 Hz, 1H), 4.55 (d, J = 14.6 Hz, 1H), 3.80 (s, 3H), 3.70 (d, J = 14.6 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 163.4, 159.6, 158.4, 155.9, 146.2, 146.1, 137.7, 130.1, 129.7, 128.2, 127.8, 127.1, 126.6, 124.7 (q, J = 285.7 Hz), 123.2, 120.8, 112.9, 112.4, 100.0, 76.6 (q, J = 29.4 Hz), 55.8, 37.4. 19F NMR (376 MHz, Chloroform-d) δ −80.20. IR (KBr): 3439, 2855, 2800, 2659, 1633, 1616, 1593, 1391, 1372, 1346, 778, 736 cm–1. HRMS (ESI) m/z calculated for C22H17F3NO4 [M + H]+ 416.1110, found 416.1129.
8-Methoxy-3-(1,1,1-trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3ha)
(PE:EA = 5:1, Rf = 0.32, white solid, mp = 176.1–177.2 °C, 88% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.59 (s, 1H), 8.43 (s, 1H), 8.09 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 8.5 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H), 7.66 (t, 1H), 7.52–7.44 (m, 2H), 7.12 (t, J = 7.9 Hz, 1H), 7.03 (d, J = 6.4 Hz, 1H), 6.98 (d, J = 8.0 Hz, 1H), 4.59 (d, J = 14.6 Hz, 1H), 3.88 (s, 3H), 3.70 (d, J = 14.4 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 158.6, 158.2, 146.7, 146.3, 143.6, 137.8, 130.1, 128.1, 127.8, 127.1, 126.7, 125.0, 124.5 (q, J = 284.0 Hz), 124.3, 123.2, 120.3, 119.9, 119.2, 113.9, 76.4 (q, J = 29.9 Hz), 56.2, 37.3. 19F NMR (376 MHz, Chloroform-d) δ −79.97. IR (KBr): 3462, 2852, 2821, 1741, 1625, 1617, 1609, 1597, 1586, 1579, 1369, 1360, 1281, 1106, 945, 830, 734 cm–1. HRMS (ESI) m/z calculated for C22H17F3NO4 [M + H]+ 416.1110, found 416.1090.
8-Ethoxy-3-(1,1,1-trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3ia)
(PE:EA = 5:1, Rf = 0.42, white solid, mp = 147.2–147.9 °C, 83% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.59 (s, 1H), 8.43 (s, 1H), 8.09 (d, J = 8.4 Hz, 1H), 7.91 (d, J = 8.5 Hz, 1H), 7.75 (d, J = 9.5 Hz, 1H), 7.69–7.64 (m, 1H), 7.48 (t, J = 9.0 Hz, 2H), 7.10 (t, J = 7.9 Hz, 1H), 7.03 (d, J = 7.9 Hz, 1H), 6.98 (d, J = 8.0 Hz, 1H), 4.60 (d, J = 14.7 Hz, 1H), 4.09 (q, J = 7.0 Hz, 2H), 3.70 (d, J = 14.6 Hz, 1H), 1.44 (t, J = 7.0 Hz, 3H). 13C NMR (100 MHz, Chloroform-d) δ 158.8, 158.3, 146.3, 146.2, 146.1, 143.8, 137.7, 130.1, 128.1, 127.8, 127.1, 126.6, 124.9, 124.5 (q, J = 284.0 Hz), 124.3, 123.2, 119.9, 119.4, 115.1, 76.7 (q, J = 29.9 Hz), 64.9, 37.4, 14.7. 19F NMR (376 MHz, Chloroform-d) δ −80.05. IR (KBr): 3454, 2852, 2807, 2732, 1657, 1597, 1383, 1352, 1269, 786, 763 cm–1. HRMS (ESI) m/z calculated for C23H18F3NO4[M + H]+ 430.1266, found 430.1262.
6,8-Dichloro-3-(1,1,1-trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3ja)
(PE:EA = 5:1, Rf = 0.32, white solid, mp = 195.1–196.1 °C, 84% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.74 (s, 1H), 8.39 (s, 1H), 8.13 (d, J = 8.3 Hz, 1H), 7.92 (d, J = 8.5 Hz, 1H), 7.78 (d, J = 8.2 Hz, 1H), 7.69 (t, J = 7.7 Hz, 1H), 7.52 (d, J = 7.5 Hz, 1H), 7.49 (d, J = 2.3 Hz, 1H), 7.45 (d, J = 8.4 Hz, 1H), 7.37 (s, 1H), 4.53 (d, J = 14.7 Hz, 1H), 3.72 (d, J = 15.0 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 158.0, 157.5, 148.4, 146.1, 144.8, 138.1, 132.2, 130.3, 129.6, 128.1, 127.9, 127.4, 127.2, 126.9, 126.4, 125.7, 124.3 (q, J = 285.8 Hz), 123.0, 122.9, 122.1, 76.5 (q, J = 29.9 Hz), 37.9. 19F NMR (376 MHz, Chloroform-d) δ −79.86. IR (KBr): 3442, 2842, 2803, 2737, 1673, 1634, 1587, 1394, 1370, 1350, 1263, 1190, 780, 746 cm–1. HRMS (ESI) m/z calculated for C21H13Cl2F3NO3 [M + H]+ 454.0237, found 454.0237.
6,8-Dibromo-3-(1,1,1-trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-2H-chromen-2-one (3ka)
(PE:EA = 5:1, Rf = 0.43, white solid, mp = 189.1–189.6 °C, 89% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.28 (s, 1H), 8.05 (d, J = 8.4 Hz, 1H), 7.83 (d, J = 8.5 Hz, 1H), 7.72–7.66 (m, 2H), 7.63–7.58 (m, 1H), 7.47–7.40 (m, 2H), 7.36 (d, J = 8.4 Hz, 1H), 4.45 (d, J = 14.8 Hz, 1H), 3.64 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 157.9, 157.4, 149.8, 146.0, 144.6, 138.0, 137.6, 130.3, 130.1, 128.0, 127.8, 127.3, 127.1, 126.8, 124.3 (q, J = 285.8 Hz), 123.0, 120.8, 116.9, 110.6, 76.8 (q, J = 29.9 Hz), 37.1. 19F NMR (376 MHz, Chloroform-d) δ −79.80. IR (KBr): 3431, 2844, 2805, 2732, 1736, 1673, 1634, 1590, 1389, 1347, 1269, 1193, 1154, 783, 763, 737 cm–1. HRMS (ESI) m/z calculated for C21H13Br2F3NO3 [M + H]+ 543.9194, found 543.9181.
2-(1,1,1-Trifluoro-2-hydroxy-3-(quinolin-2-yl)propan-2-yl)-3H-benzo[f]chromen-3-one (3la)
(PE:DCM = 1:1, Rf = 0.33, white solid, mp = 146.9–147.3 °C, 91% yield). 1H NMR (400 MHz, Chloroform-d) δ 9.26 (s, 1H), 8.71 (s, 1H), 8.35 (d, J = 8.4 Hz, 1H), 8.11 (d, J = 8.4 Hz, 1H), 7.92 (d, J = 9.2 Hz, 2H), 7.84 (d, J = 8.2 Hz, 1H), 7.74 (d, J = 7.9 Hz, 1H), 7.69 (t, 1H), 7.63 (t, 1H), 7.56–7.50 (m, 2H), 7.46 (t, J = 7.5 Hz, 1H), 7.34 (d, J = 9.0 Hz, 1H), 4.62 (d, J = 14.6 Hz, 1H), 3.78 (d, J = 14.7 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 159.4, 158.3, 153.9, 146.2, 141.8, 137.7, 133.8, 130.2, 129.2, 128.9, 128.4, 128.2, 127.8, 127.1, 126.6, 126.1, 125.4 (q, J = 283.1 Hz), 123.4, 123.3, 123.1, 121.8, 116.3, 112.9, 76.9 (q, J = 26.3 Hz), 37.4. 19F NMR (376 MHz, Chloroform-d) δ −79.96. IR (KBr): 3420, 2843, 2805, 2732, 1673, 1644, 1584, 1389, 1376, 1347, 1268, 794, 768, 739 cm–1. HRMS (ESI) m/z calculated for C25H16F3NO3 [M + H]+ 436.1161, found 436.1171.
1,1,1-Trifluoro-2-phenyl-3-(quinolin-2-yl)propan-2-ol (3ma)
(PE:EA = 3:1, Rf = 0.45, white solid, mp = 97.9–98.8 °C, 52% yield). 1H NMR (400 MHz, Chloroform-d) δ 8.52 (s, 1H), 8.07 (d, J = 8.3 Hz, 1H), 7.98 (d, J = 8.5 Hz, 1H), 7.76 (d, J = 8.2 Hz, 1H), 7.70 (d, J = 7.9 Hz, 3H), 7.51 (t, J = 8.1 Hz, 1H), 7.31 (t, J = 7.4 Hz, 2H), 7.25 (dd, J = 7.8, 4.7 Hz, 3H), 3.80 (d, J = 15.1 Hz, 1H), 3.69 (d, J = 15.1 Hz, 1H). 13C NMR (100 MHz, Chloroform-d) δ 157.8, 146.3, 138.5, 137.6, 130.2, 129.5, 128.4, 128.2, 128.1, 127.7, 126.8, 126.7, 126.6, 125.3 (q, J = 282.8 Hz), 123.8, 122.5, 77.4 (q, J = 29.7 Hz), 40.2. 19F NMR (376 MHz, Chloroform-d) δ −79.28. IR (KBr): 3428, 2810, 2732, 2658, 1654, 1633, 1592, 1386, 1349, 1266, 1180, 796, 741, 700 cm–1. HRMS (ESI) m/z calculated for C18H14F3NO [M + H]+ 318.1106, found 318.1114.
General Procedure for the Evaluation of Fungicidal Activities
16.6 mg of the test sample was taken and dissolved in 0.66 mL of DMSO, and then, an aqueous solution containing 1% Tween 80 was added to it to make 5 mg/mL of the original drug. An appropriate amount of the test drug was pipetted into a conical flask under aseptic conditions and shaken well, and then the same amount was poured into three petri dishes with a diameter of 9 cm to make a 500 μg/mL drug-containing plate. In the abovementioned experiments, the treatment without the drug was set as a blank control, and each treatment was repeated three times. The cultured pathogenic bacteria were cut along the edge of the colony with a hole punch with a diameter of 6.5 mm under aseptic conditions, and the bacterial cake was inoculated in the center of the drug-containing plate with an inoculator. The culture dish was cultured in a constant temperature incubator at 25 °C. When the diameter of the control colony expanded to more than 6 cm, the colony diameter was measured by the cross method, the average value was taken, and the bacteriostatic rate was calculated at the end of the culture.
Acknowledgments
We thank the Natural Science Foundation of Henan Province (222300420188, 222300420456, and 222300420459), the Science and Technology Project of Henan Province (192102110054), the special fund for topnotch talents in Henan Agricultural University (30500925), the Technology Innovation Project of Pingdingshan Tobacco Company (PYKJ 202210), the Key Scientific Research Projects in Henan Colleges and Universities (21A150021), the Natural Science Foundation of Shaanxi Province (2020JQ-348), and the Fundamental Research Funds for the Central Universities, CHD (300102121304) for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c05855.
1H NMR, 19F NMR, 13C NMR, and HRMS spectra for all new compounds (PDF)
Author Contributions
B.Z. and G.Y. performed the experiments and analyzed the data. C.W. and L.S. helped with characterizing some new compounds. L.L. performed the X-ray crystal structure analysis. Z.P., H.Z., and L.W. provided useful and valuable advice. X.J. and C.X. conceived and directed the project. L.F. directed the project and wrote the paper.
The authors declare no competing financial interest.
Supplementary Material
References
- a Liu Q.; Ni C.; Hu J. China’s flourishing synthetic organofluorine chemistry: innovations in the new millennium. Natl. Sci. Rev. 2017, 4, 303–325. 10.1093/nsr/nwx058. [DOI] [Google Scholar]; b Marsh E. N. Fluorinated proteins: from design and synthesis to structure and stability. Acc. Chem. Res. 2014, 47, 2878–2886. 10.1021/ar500125m. [DOI] [PubMed] [Google Scholar]; c Kirk K. L. Fluorination in Medicinal Chemistry: Methods, Strategies, and Recent Developments. Org. Process Res. Dev. 2008, 12, 305–321. 10.1021/op700134j. [DOI] [Google Scholar]; d Yoder N. C.; Kumar K. Fluorinated amino acids in protein design and engineering. Chem. Soc. Rev. 2002, 31, 335–341. 10.1039/b201097f. [DOI] [PubMed] [Google Scholar]; e Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]; f Hagmann W. K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]; g Tsui G. C.; Hu J. Organofluorine Chemistry. Asian J. Org. Chem. 2019, 8, 566–567. 10.1002/ajoc.201900271. [DOI] [Google Scholar]
- a Nie J.; Guo H.-C.; Cahard D.; Ma J.-A. Asymmetric Construction of Stereogenic Carbon Centers Featuring a Trifluoromethyl Group from Prochiral Trifluoromethylated Substrates. Chem. Rev. 2011, 111, 455–529. 10.1021/cr100166a. [DOI] [PubMed] [Google Scholar]; b Alonso C.; Martinez de Marigorta E.; Rubiales G.; Palacios F. Carbon trifluoromethylation reactions of hydrocarbon derivatives and heteroarenes. Chem. Rev. 2015, 115, 1847–1935. 10.1021/cr500368h. [DOI] [PubMed] [Google Scholar]
- a Xiao H.; Zhang Z.; Fang Y.; Zhu L.; Li C. Radical trifluoromethylation. Chem. Soc. Rev. 2021, 50, 6308–6319. 10.1039/D1CS00200G. [DOI] [PubMed] [Google Scholar]; b Begue J. P.; Bonnet-Delpon D.; Crousse B.; Legros J. The chemistry of trifluoromethyl imines and related acetals derived from fluoral. Chem. Soc. Rev. 2005, 34, 562–572. 10.1039/b401707m. [DOI] [PubMed] [Google Scholar]; c Li S.; Ma J. A. Core-structure-inspired asymmetric addition reactions: enantioselective synthesis of dihydrobenzoxazinone- and dihydroquinazolinone-based anti-HIV agents. Chem. Soc. Rev. 2015, 44, 7439–7448. 10.1039/C5CS00342C. [DOI] [PubMed] [Google Scholar]
- a Reuter K. C.; Grunwitz C. R.; Kaminski B. M.; Steinhilber D.; Radeke H. H.; Stein J. Selective glucocorticoid receptor agonists for the treatment of inflammatory bowel disease: studies in mice with acute trinitrobenzene sulfonic acid colitis. J. Pharmacol. Exp. Ther. 2012, 341, 68–80. 10.1124/jpet.111.183947. [DOI] [PubMed] [Google Scholar]; b Riether D.; Harcken C.; Razavi H.; Kuzmich D.; Gilmore T.; Bentzien J.; Pack E. J.; Souza D.; Nelson R. M.; Kukulka A.; Fadra T. N.; Zuvela-Jelaska L.; Pelletier J.; Dinallo R.; Panzenbeck M.; Torcellini C.; Nabozny G. H.; Thomson D. S. Nonsteroidal dissociated glucocorticoid agonists containing azaindoles as steroid A-ring mimetics. J. Med. Chem. 2010, 53, 6681–6698. 10.1021/jm100751q. [DOI] [PubMed] [Google Scholar]
- Pikuleva I. A. Efavirenz as a cholesterol-targeting drug for AD. Alzheimer’s Dementia 2020, 16, e040603 10.1002/alz.040603. [DOI] [Google Scholar]
- Bégué J.-P.; Bonnet-Delpon D. Recent advances (1995–2005) in fluorinated pharmaceuticals based on natural products. J. Fluorine Chem. 2006, 127, 992–1012. 10.1016/j.jfluchem.2006.05.006. [DOI] [Google Scholar]
- Isanbor C.; O’Hagan D. Fluorine in medicinal chemistry: A review of anti-cancer agents. J. Fluorine Chem. 2006, 127, 303–319. 10.1016/j.jfluchem.2006.01.011. [DOI] [Google Scholar]
- a Hegele R. A. CETP Inhibitors - A New Inning?. N. Engl. J. Med. 2017, 377, 1284–1285. 10.1056/NEJMe1711407. [DOI] [PubMed] [Google Scholar]; b Mullard A. CETP inhibitors stumble on. Nat. Rev. Drug Discovery 2017, 16, 669. 10.1038/nrd.2017.198. [DOI] [PubMed] [Google Scholar]
- a Yeung C. S.; Dong V. M. Catalytic dehydrogenative cross-coupling: forming carbon-carbon bonds by oxidizing two carbon-hydrogen bonds. Chem. Rev. 2011, 111, 1215–1292. 10.1021/cr100280d. [DOI] [PubMed] [Google Scholar]; b Dino A.; Scott M. E.; Mark L. Aryl-Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev. 2007, 107, 174–238. 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]; c Gensch T.; Hopkinson M. N.; Glorius F.; Wencel-Delord J. Mild metal-catalyzed C-H activation: examples and concepts. Chem. Soc. Rev. 2016, 45, 2900–2936. 10.1039/C6CS00075D. [DOI] [PubMed] [Google Scholar]; d Seregin I. V.; Gevorgyan V. Direct transition metal-catalyzed functionalization of heteroaromatic compounds. Chem. Soc. Rev. 2007, 36, 1173–1193. 10.1039/b606984n. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Mandal R.; Garai B.; Sundararaju B. Weak-Coordination in C-H Bond Functionalizations Catalyzed by 3d Metals. ACS Catal. 2022, 12, 3452–3506. 10.1021/acscatal.1c05267. [DOI] [Google Scholar]
- a Campeau L.-C.; Schipper D. J.; Fagnou K. Site-Selective sp2 and Benzylic sp3 Palladium-Catalyzed Direct Arylation. J. Am. Chem. Soc. 2008, 130, 3266–3267. 10.1021/ja710451s. [DOI] [PubMed] [Google Scholar]; b Luo Y.; Teng H.-L.; Nishiura M.; Hou Z. Asymmetric Yttrium-Catalyzed C(sp3)-H Addition of 2-Methyl Azaarenes to Cyclopropenes. Angew. Chem., Int. Ed. 2017, 56, 9207–9210. 10.1002/anie.201705431. [DOI] [PubMed] [Google Scholar]; c Liu X. J.; Zhang W. Y.; Zheng C.; You S.-L. Iridium-Catalyzed Asymmetric Allylic Substitution of Methyl Azaarenes. Angew. Chem., Int. Ed. 2022, 61, e202200164 10.1002/anie.202200164. [DOI] [PubMed] [Google Scholar]; d Sun C. L.; Li B. J.; Shi Z. J. Direct C-H transformation via iron catalysis. Org. Lett. 2011, 111, 1293–1314. 10.1021/cr100198w. [DOI] [PubMed] [Google Scholar]; e Gao X.; Zhang F.; Deng G.; Yang L. Bronsted acid catalyzed benzylic C-H bond functionalization of azaarenes: nucleophilic addition to nitroso compounds. Org. Lett. 2014, 16, 3664–3667. 10.1021/ol501422k. [DOI] [PubMed] [Google Scholar]; f Xu L.; Shao Z.; Wang L.; Xiao J. Tandem sp3 C-H functionlization/decarboxylation of 2-alkylazaarenes with coumarin-3-carboxylic acids. Org. Lett. 2014, 16, 796–799. 10.1021/ol403541g. [DOI] [PubMed] [Google Scholar]; g Yan Y.; Xu K.; Fang Y.; Wang Z. A catalyst-free benzylic C-H bond olefination of azaarenes for direct Mannich-like reactions. J. Org. Chem. 2011, 76, 6849–6855. 10.1021/jo2008934. [DOI] [PubMed] [Google Scholar]; h Qian B.; Guo S.; Shao J.; Zhu Q.; Yang L.; Xia C.; Huang H. Palladium-Catalyzed Benzylic Addition of 2-Methyl Azaarenes to N-Sulfonyl Aldimines via C-H Bond Activation. J. Am. Chem. Soc. 2010, 132, 3650–3651. 10.1021/ja910104n. [DOI] [PubMed] [Google Scholar]
- a Talwar D.; Gonzalez-de-Castro A.; Li H. Y.; Xiao J. Regioselective acceptorless dehydrogenative coupling of N-heterocycles toward functionalized quinolines, phenanthrolines, and indoles. Angew. Chem., Int. Ed. 2015, 54, 5223–5227. 10.1002/anie.201500346. [DOI] [PubMed] [Google Scholar]; b Lim J. A.; Teo Y.-C. Iron-catalyzed benzylic addition of 2-methyl azaarenes to substituted trifluoromethyl ketones. Synth. Commun. 2021, 51, 1076–1084. 10.1080/00397911.2020.1867178. [DOI] [Google Scholar]; c Jamal Z.; Teo Y.-C. Indium-catalyzed C(sp3)-H functionalization of 2-methylazaarenes through direct benzylic addition to trifluoromethyl ketones. RSC Adv. 2015, 5, 26949–26953. 10.1039/C4RA17182A. [DOI] [Google Scholar]
- a Sittaramane V.; Padgett J.; Salter P.; Williams A.; Luke S.; McCall R.; Arambula J. F.; Graves V. B.; Blocker M.; Van Leuven D.; et al. Discovery of Quinoline-Derived Trifluoromethyl Alcohols, Determination of Their in vivo Toxicity and Anticancer Activity in a Zebrafish Embryo Model. ChemMedChem 2015, 10, 1802–1807. 10.1002/cmdc.201500341. [DOI] [PubMed] [Google Scholar]; b Zhang X.-J.; Cao J.-K.; Ren J.-J.; Hong L.; Liang R.-J.; Hao K.-Y.; Wei K.-L.; Mi B.-J.; Liu Y.; Zhu Y.-P. Generation of azaarene nitrile oxides from methyl azaarenes and t-BuONO enabling the synthesis of furoxans and 1,2,4-oxadiazoles. Org. Chem. Front. 2022, 9, 1121–1126. 10.1039/D1QO01872H. [DOI] [Google Scholar]; c Pi D.; Jiang K.; Zhou H.; Sui Y.; Uozumi Y.; Zou K. Iron-catalyzed C(sp3)-H functionalization of methyl azaarenes: a green approach to azaarene-substituted α- or β-hydroxy carboxylic derivatives and 2-alkenylazaarenes. RSC Adv. 2014, 4, 57875–57884. 10.1039/C4RA10939B. [DOI] [Google Scholar]; d Jiang K.; Pi D.; Zhou H.; Liu S.; Zou K. Iron-catalyzed C(sp3)-H functionalization of methyl azaarenes with α-oxoesters: a facile approach to lactic acid derivatives. Tetrahedron 2014, 70, 3056–3060. 10.1016/j.tet.2014.02.069. [DOI] [Google Scholar]; e Graves V. B.; Shaikh A. Lewis acid-catalyzed Csp3-H functionalization of methyl azaarenes with α-trifluoromethyl carbonyl compounds. Tetrahedron Lett. 2013, 54, 695–698. 10.1016/j.tetlet.2012.12.013. [DOI] [Google Scholar]
- a Zimmerman J. B.; Anastas P. T.; Erythropel H. C.; Leitner W. Designing for a green chemistry future. Science 2020, 367, 397–400. 10.1126/science.aay3060. [DOI] [PubMed] [Google Scholar]; b Meng Y.; Wang M.; Jiang X. Transition-Metal-Free Reductive Cross-Coupling Employing Metabisulfite as a Connector: General Construction of Alkyl-Alkyl Sulfones. CCS Chem. 2021, 3, 17–24. 10.31635/ccschem.021.202000638. [DOI] [Google Scholar]
- a Fan L.; Hao J.; Yu J.; Ma X.; Liu J.; Luan X. Hydroxylamines As Bifunctional Single-Nitrogen Sources for the Rapid Assembly of Diverse Tricyclic Indole Scaffolds. J. Am. Chem. Soc. 2020, 142, 6698–6707. 10.1021/jacs.0c00403. [DOI] [PubMed] [Google Scholar]; b Fan L.; Liu J.; Bai L.; Wang Y.; Luan X. Rapid Assembly of Diversely Functionalized Spiroindenes by a Three-Component Palladium-Catalyzed C-H Amination/Phenol Dearomatization Domino Reaction. Angew. Chem., Int. Ed. 2017, 56, 14257–14261. 10.1002/anie.201708310. [DOI] [PubMed] [Google Scholar]; c Zhang J. J.; Zhang M.; Lu M.; He Y.; Li S.; Fan L.; Zhang X.; Wu J. K.; Yang X. Synthesis of spiropyrans via the Rh(III)-catalyzed annulation of 3-aryl-2H-benzo[b][1,4]oxazines with diazo ketoesters. Chem. Commun. 2022, 58, 5144–5147. 10.1039/D2CC00916A. [DOI] [PubMed] [Google Scholar]; d Yang G.; Shi L.; Pan Z.; Wu L.; Fan L.; Wang C.; Xu C.; Liang J. The synthesis of coumarin thiazoles containing a trifluoromethyl group and their antifungal activities. Arab. J. Chem. 2021, 14, 102880. 10.1016/j.arabjc.2020.10.027. [DOI] [Google Scholar]; e Wu L.; Yuan X.; Yang G.; Xu C.; Pan Z.; Shi L.; Wang C.; Fan L. An eco-friendly procedure for the synthesis of new phosphates using KF/Al2O3 under solventless conditions and their antifungal properties. J. Saudi Chem. Soc. 2021, 25, 101273 10.1016/j.jscs.2021.101273. [DOI] [Google Scholar]; f Bao J. P.; Xu C. L.; Yang G. Y.; Wang C. X.; Zheng X.; Yuan X. X. Novel 6a,12b-Dihydro-6H,7H-chromeno[3,4-c] chromen-6-ones: Synthesis, Structure and Antifungal Activity. Molecules 2019, 24, 1745. 10.3390/molecules24091745. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Shi L.; Liu Y.; Wang C.; Yuan X.; Liu X.; Wu L.; Pan Z.; Yu Q.; Xu C.; Yang G. Synthesis of 1-(β-coumarinyl)-1-(β-indolyl)trifluoroethanols through regioselective Friedel–Crafts alkylation of indoles with β-(trifluoroacetyl)coumarins catalyzed by Sc(OTf)3. RSC Adv. 2020, 10, 13929–13935. 10.1039/D0RA01237H. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Yuan X.; Wu L.; Xu C.; Pan Z.; Shi L.; Yang G.; Wang C.; Fan S. A consecutive one-pot two-step approach to novel trifluoromethyl-substituted bis(indolyl)methane derivatives promoted by Sc(OTf)3 and p-TSA. Tetrahedron Lett. 2019, 60, 151329 10.1016/j.tetlet.2019.151329. [DOI] [Google Scholar]; i Yang G.; Wang C.; Fan S.; Xie P.; Jin Q.; Xu C. Microwave Assisted Solvent-Free Synthesis of 3-(Trifluoroacetyl)coumarins. Chin. J. Org. Chem. 2015, 35, 1173–1178. 10.6023/cjoc201411012. [DOI] [Google Scholar]; j Yang G.; Jin Q.; Xu C.; Fan S.; Wang C.; Xie P. Synthesis, characterization and antifungal activity of coumarin-functionalized chitosan derivatives. Int. J. Biol. Macromol. 2018, 106, 179–184. 10.1016/j.ijbiomac.2017.08.009. [DOI] [PubMed] [Google Scholar]; k Yang G.-Y.; Yang J.-T.; Wang C.-X.; Fan S.-F.; Xie P.-H.; Xu C.-L. Microwave-assisted TsOH/SiO2-catalyzed one-pot synthesis of novel fluoro-substituted coumarin hydrazones under solvent-free conditions. J. Fluorine Chem. 2014, 168, 1–8. 10.1016/j.jfluchem.2014.07.004. [DOI] [Google Scholar]
- Tan W.; Li Q.; Dong F.; Zhang J.; Luan F.; Wei L.; Chen Y.; Guo Z. Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: Synthesis, characterization, and antifungal property. Carbohydr. Polym. 2018, 182, 180–187. 10.1016/j.carbpol.2017.11.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.