Abstract

In this paper, silver-loaded phosphorous and carbon co-doped boron nitride quantum dot (Ag@CP-BNQD) nanocomposites were synthesized using a co-precipitation method followed by a hydrothermal approach. The nanocomposites of Ag@CP-BNQDs were characterized by scanning electron microscopy, energy-dispersive spectroscopy, Fourier transform infrared spectroscopy, X-ray diffraction, and ultraviolet–visible spectrophotometry. The as-prepared Ag@CP-BNQDs were used for photocatalytic degradation of 10 common organic pollutants, including dyes, pharmaceuticals, and pesticides in aqueous solution under visible light irradiation. The high-performance photocatalysis of Ag@CP-BNQDs proved that Ag@CP-BNQDs is plasmonic and the n–p junction photocatalyst. Theoretical calculations were done to measure the crystals and electronic structures of Ag@CP-BNQDs. Theoretical results showed that loading of Ag behaves as plasmonic sensitizers and co-catalysts and provides extra bands, which make electron movement easier between valance and conduction bands. The mechanism of the charge separation enhancement was postulated. Our findings might deepen our understanding of how sensitizer surface modification works in photodegradation applications.
1. Introduction
In recent years, much emphasis has been paid to the use of photocatalysts to remove undesirable organic and/or inorganic contaminants. Aromatic structures of most drugs, pesticides, and dyes are highly hazardous, carcinogenic, and mutagenic.1,2 Photocatalytic degradation is one the methods for solving this issue, in which the photocatalyst absorbs electromagnetic radiation greater than or equal to the semiconductor’s band gap and excites electrons from the valance band to the conduction band. Thus, creating holes in the valence band (h+) and electrons in the conduction band, and as a result, reactive oxygen species (ROS), primarily hydroxyl radicals (•OH) and superoxide radical anions (O2•–), are formed at valance and conduction bands, respectively.3−6
Many materials have been used for photocatalysis, such as inorganic metal oxide nanoparticles,7 organic compounds, polymers,8 carbon nanomaterials,9−11 and nanocomposites.12−14
Several attempts have been made to improve the activity of the photocatalyst, including loading of other atoms.15,16 Noble metals, such as Ag, have been widely employed to improve photocatalytic activity in visible light.15,16 Here, the interaction between the electrons of Ag and incident photons occurs (plasmonic effect) and, hence, enhances the visible light harvesting properties of the photocatalyst.17,18 Thus, the addition of silver atoms is to increase light absorption in the visible region, delay recombination time, and provide extra orbitals (bands), which facilitate the movement of electrons between electron–hole (exciton) and traps the fast recombination.19,20 It is clear from the literature that a hybrid photocatalyst suppresses the movement of photogenerated exciton pairs in heterojunctions, that is, it is possible to reduce the recombination of electron–hole pairs, extend their lifetime, and considerably improve photocatalytic performance.15,21
Another advantage of the hybrid junction photocatalyst is providing an n–p junction.15,22,23 When the heterojunction photocatalyst is irradiated by photons with energy higher than or equal to the gap energy of both photocatalysts, the electrons can move to the conduction band of the n-type photocatalyst and the holes move to the valence band of the p-type photocatalyst due to the formation of the inner electric field.15,22,23 As a result, it can reduce the recombination rate of photogenerated exciton pairs and enhance photodegradation performance.15,24 The reason behind this is that an internal electrostatic field in the region of p–n junction is formed, which separates and moves e– and h+ in the opposite directions and this reduces the rate of recombination. It also accelerates the separation rate of electrons between the valance band minimum (VBM) and conduction band maximum (CBM) and, hence, the rate of hydroxyl (OH•) and super oxide (O2•–) radical production increases.22,23
Boron nitride quantum dots (BNQDs) are a class of zero-dimension nanoparticles. BNQDs appear in a variety of sizes ranging from 5 to 10 nm.25,26 Jung et al. measured the average thickness of the BNQDs to be 3.2 nm, indicating that the BNQDs had a few-layered structure.27 The ability to exhibit quantum confinement, the functional edge effect, and high water dispersibility provide BNQDs with photoluminescence properties.28,29
The band gap of BNQDs ranges from 4.5 to 6 eV, is large, and not suitable for visible light applications.30–32 On the other hand, Angizi et al. found that functionalized boron nitride has a band gap, for example, below 4.5 eV, which mainly depends on the type of functional group; for instance, hydroxyl-functionalized BNQDs gives, for example, 2.3–3.6 eV, methyl functionalized is 3.2–4.2 eV, and amine functionalized is 3.1–4 eV. Thus, functionalization of BNQDs makes them have potential use in the photocatalysis research.33
In our previous work, we co-doped phosphorous and carbon into boron nitride quantum dots (CP-BNQDs).2 Then, we applied photodegradation of toluidine blue using UV light (274 nm) as a light source. The rate constant was competitive, this is may be due to the fast recombination of excitons. Additionally, gap energy of the nanocomposite was 4.2 eV, which is a wide band gap and cannot harvest in the visible region.
In the present work, we report loading Ag on CP-BNQDs using a hydrothermal method (Figure 1). Ag@CP-BNQDs are used to improve photocatalytic activity via enhancing the p–n heterojunction. Eight pollutants were tested, such as pharmaceuticals, dyes, and pesticides. Theoretical calculations were performed to find out the physicochemical properties of Ag@CP-BNQD nanocomposite. A mechanism for the photodegradation catalysis was suggested.
Figure 1.

Schematic diagram demonstrates preparation and application of Ag@CP-BNQD.
2. Experimental Part
2.1. Instrumentation
Surface morphology, chemical composition, crystal quality, and structural properties of synthesized Ag@CP-BNQDs have been characterized and studied utilizing field-emission scanning electron microscopy (FE-SEM) (SEM 4500-Quanta, FEI, USA) and the chemical composition of synthesized nanostructures was measured by energy-dispersive X-ray spectroscopy (EDX) performed in FE-SEM. X-ray powder diffraction (XRD) was used to measure the crystal structure using X’Pert PRO (PANalytical, Netherlands) using Kα for copper element (Cu Kα = 1.5406 A° at 40 kV, 30 mA) in the 2θ range of (20–70°) with a rate of scanning of 1°/min, respectively. For monitoring the photodegradation process, absorbance spectra were recorded using a Lambda 25 Perkin-Elmer UV–vis spectrophotometer (Perkin-Elmer, USA). FTIR spectra were taken using an FTIR spectrometer (Thermo Scientific, USA).
2.2. Chemicals
All chemicals used were of analytical grades and used as purchased without further treatments. Silver nitride was purchased from Alpha Aesar GmbH (Karlsruhe, Germany). Boric acid, sodium oxalate, potassium bromates, 80% phosphoric acid, and congo red dye were obtained from CARL ROTH GmbH (Karlsruhe, Germany). Isopropyl alcohol was purchased from Fluka AG in Sigma Aldrich (Steinheim, Germany). Ascorbic acid was bought from Scharlau Chemicals (Barcelona, Spain) and folic acid was purchased from UNI-CHEM (China). Paracetamol was brought from PiONEERco (Sulaymaniyah—Iraq). Toluidine blue, urea, and methyl orange were bought from labPak chemicals Ltd. (UK). The hydrochlorides of tetracycline were purchased from Sigma–Aldrich, St. Louis, MO, USA. 99% purity of amlodipine besylate was purchased from Awa medical (Hawler, Iraq). Chlorothalonil pesticide was supplied by Syngenta (Switzerland).
2.3. Synthesis of CP-BNQDs
For the fabrication of CP-BNQDs, 1.5 mg of urea in deionized water was mixed with 1.5 mg boric acid drop-by-drop in aqueous solution, along with 1 μL 1.0 M H3PO4. The solution was mixed for 30 min with a magnetic stirrer before being placed in a 250 mL Teflon-lined autoclave and heated to 200 °C for 24 h. The resulting solution was transparent and emitted a blue glow when illuminated by fluorescent lights. Next, the solvent was removed, and the solid was dried at 200 C for 2 h until a white powder was obtained.
2.4. Synthesis of Ag@CP-BNQDs
To synthesize Ag@CP-BNQDs, 1:1 mole ratio was mixed from Ag+ along with CP-BNQDs; in brief, 0.5 g of CP-BNQDs was mixed with 0.1 M of silver nitrate drop-by-drop, the yellow-like turbid solution of Ag@CP-BNQDs was mixed using a magnetic stirrer for half-hour. The resulting colloid solution was hydrothermally heated for 24 h 200 °C. Finally, the powder was purified and washed several times with deionized water, dried, and used in the photodegradation application. Figure 1 shows the scheme for the preparation of the nanostructure of Ag@CP-BNQDs.
2.5. Computational Study
The ab-initio computations were carried out utilizing the plane-wave basis and pseudopotential methods in the context of DFT. The CASTEP and Dmol3 codes have been used for all computations. We employed hybrid functionals, such as Becke-3 Parameter-Lee-Yang-Parr (B3LYP)34 and Heyd–Scuseria–Ernzerhof exchange–correlation functional (HSE06). The kinetic energy cutoff for each exchange–correlation (XC) functional was 400 eV at norm conserving pseudopotentials. A 5 × 5 × 5 k-point Monkhorst–Pack grid was used to integrate the Brillouin zone, as well as the self-consistent field (SCF) tolerance was 2 × 10–6 (eV/atom).
2.6. Photocatalysis Experiments
Photocatalytic activities of Ag@BNQDs were evaluated by the degradation of tetracycline and nine other molecules tabulated in Table 1, under 5 W of blue LED (Light Bulb LED GU10 5W 400Lm Grow, GREENICE company, Madrid, Spain). Prior to illumination, a total of 25 mg of photocatalyst was dispersed into 25 mL of the solution containing the test compound and then magnetically stirred in the dark for 30 min to establish an adsorption–desorption equilibrium. At predetermined intervals, 3 mL of suspension was taken and centrifuged at 8000 rpm for 6 min to remove the photocatalyst, Then, the absorbance spectra of the tetracycline in the supernatants were analyzed by a UV–vis spectrophotometer at 367 nm.
Table 1. Photocatalytic Degradation of Different Organic Waste on Ag@CP-BNQDs at 25 °C.
| organic waste | conc. ppm | rate constant/min–1 | rate constant/mol–1.L.min–1 |
|---|---|---|---|
| amlodipine | 10 | 0.047 | |
| caffeine | 25 | 0.0002 | |
| chlorothalonile | 25 | 0.0053 | |
| congo-red | 15 | 0.0033 | |
| eosin yellowish | 25 | 0.011 | |
| folic acid | 5 | 0.138 | |
| methyl orange | 10 | 0.0072 | |
| paracetamol | 10 | 0.0071 | |
| toluidine blue | 5 | 0.0106 | |
| tetracycline | 10 | 0.207 |
To determine the ROS, isopropyl alcohol (IPA) was used as hydroxyl radical scavenger, ascorbic acid as O2–• trapping species, sodium oxalate and potassium bromate was added as a scavenger for h+ and e– accordingly.2,35−38
3. Results and Discussion
3.1. Characterizations of Ag@CP-BNQDs
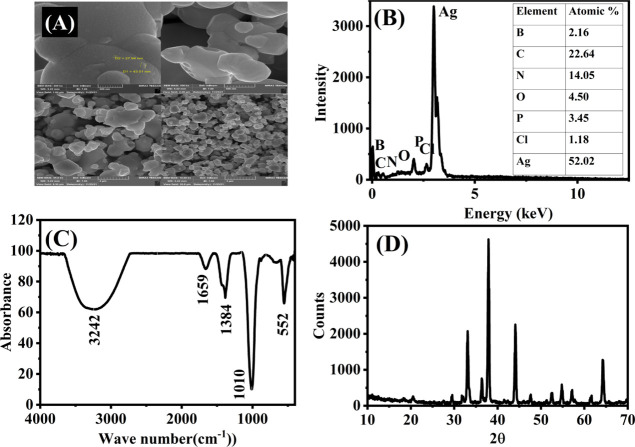
As shown in Figure 2A, the morphology of Ag@CP-BNQDs was imaged using SEM. It can be observed that a sheet-like morphology is synthesized, and the size according to the SEM image is roughly 27 nm. The SEM results were in a good agreement with the data obtained by XRD using Scherrer and Willimson–Hall equations. It was found that the average particle size of Ag@CP-BNQDs is roughly equal to 21 nm according to the Scherrer equation and 29 nm according to the W–H equation (see Supporting Information). Figure 2B and S1 shows images corresponding to EDX spectra and mapping of Ag@CP-BNQD nanocomposite that shows the detection of silver, carbon, phosphorous, nitrogen, and boron.31,40,41,45−47,55
Figure 2.
(A) SEM images of Ag@CP-BNQDs, (B) EDX spectrum of Ag@CP-BNQDs, (C) FTIR spectra of Ag@CP-BNQDs, and (D) XRD pattern of fabricated photocatalyst for Ag@CP-BNQDs.
FTIR spectra were characterized to investigate the formation of Ag@CP-BNQDs. Figure 2C shows the FTIR spectra of Ag@CP-BNQDs. The signals at 552 cm–1 could be detected for BNQDs and symmetric stretching vibration mode of P–O–P.39–43 Another peak was observed at 1010 cm–1 due to the B–N–O vibration mode and it is also similar to the related results of BNQDs elsewhere, it may also be due to the P–O–Ag vibration mode.,3944–48 Moreover, the signal at 1384 cm–1 resulted from the stretching vibration of C–O or due to the bending mode of B–OH and stretching mode of B–N.,4950 In addition, the peak at 1659 cm–1 resulted from the vibration mode of the Ag–N bond Another peak was observed at 3242 cm–1 due to the Ag–N vibration mode as well.425152 The two peaks between 1300 and 1700 cm–1 are assigned for the heterocyclic C–N stretching vibration mode as well.53
The crystal structure of Ag@CP-BNQDs was analyzed utilizing XRD spectra. Figure 2D shows the XRD pattern of Ag@CP-BNQDs with the diffraction peaks of 2θ equal to 29.45, 33, 36.2, 37.9, 44, 47.5,52.6, 54.8, 57.1, 61.4, 64.3, 71.7, and 77.3° with the corresponding crystal planes indexed to 020, 201, 121, 111, 202, 200, 222, 253, 411, 412, and 242 that match with the JCPDS cards (No. 96-153-0490, 96-406-5374, and 96-901-6253)54–.56
In terms of how Ag atoms were formed on the surface of CP-BNQDs, a yellow colloid solution was first created by mixing Ag+ ions and CP-BNQDs. Due to loading of Ag+ ions to the lone pair of N in the CP-BNQD nanostructure, the Ag + ions could be reduced to Ag atoms. This redox reaction may have been triggered by the presence of functional groups OH-and NH257,58 that are present on the surface of CP-BNQDs.2,27,28,33,59 An illustration of the procedures followed in the synthesis of Ag@CP-BNQDs is provided in Figure 1.
3.2. Photocatalytic Degradation
3.2.1. Organic Waste Removal
Ag@CP-BNQDs as a photocatalyst was applied to remove different pollutants, including pesticides, dyes, and pharmaceuticals. All experiments were performed at room temperature 25 °C. Ag@CP-BNQDs could photodegrade some pharmaceuticals, dyes, and other common molecules with different rate constants, as illustrated in Table 1 and Figure 3. The difference of rate constants is attributed to ROS, which is the product of photocatalysis, as different molecules react differently with ROS and as a result, they have different mechanisms and so different kinetics.60−63
Figure 3.
Change of UV–vis spectra as a function of time (A) tetracycline, (C) amlodipine, (E) eosin yellowish, and (G) chlorothalonile. Integral kinetic plot of (B) second-order tetracycline, (D) first-order Amlodipine, (F) first-order Eosin yellowish, and (H) first order chlorothalonil at 25 °C and LED blue light.
As illustrated in Figure 3A, typical UV–vis spectra and integral kinetic model for the removal of amlodipine, chlorothalonil, eosin yellowish, and tetracycline each with the degradation pathway were monitored by a spectrophotometer. Tetracycline was chosen for further study in this research as it shows good degradation and has a clear spectrum among all other pollutants.
3.2.2. Effect of Temperature and pH
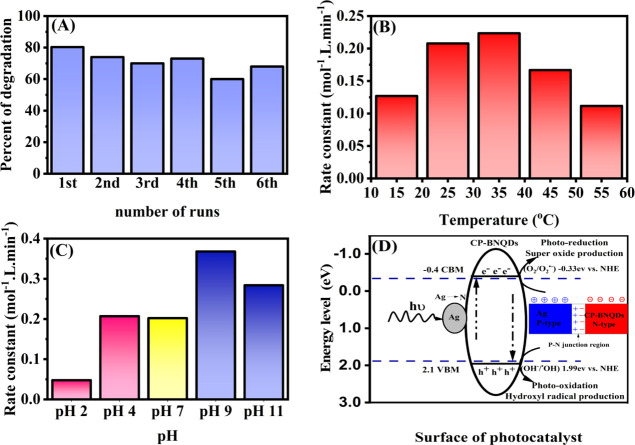
Photodegradation of tetracycline was studied as a function of temperature. The effect of temperature on the apparent rate of photodegradation is shown in Figure 4B. It can be observed that the optimum temperature is 35 °C and the rate constant drops as the temperature rises. When operating above 35 °C (like 45 and 55 °C), the desorption of the produced product restricts the photocatalytic process because it reduces surface degradation and reactant adsorption.64−67 It also reduces dissolved oxygen as well as the decreased saturation value of oxygen, which is important because it maintains the photocatalytic mechanism by trapping the photogenerated electrons. However, at lower temperatures, adsorption of pollutants onto the photocatalyst surface is the restriction stage.64−67Iin addition, the photocatalytic activity decreases at elevated temperatures because the electron–hole recombination rate increased.68
Figure 4.
(A) Time course of photocatalytic degradation cycles of tetracycline over Ag@CP-BNQDs. (B) Effect of temperature on photocatalytic degradation of tetracycline over Ag@CP-BNQDs. (C) Effect of initial pH on photocatalytic degradation of Ag@CP-BNQDs. (D) Schematic diagram illustrates the principal mechanism of Ag@CP-BNQDs.
Figure 4C shows the effect of pH on the degradation efficiency. Based on our results, relatively a range of pHs from 4 to 11 was good for degradation of tetracycline, while the best one was pH 9. We can conclude that the isoelectric point (the p2oint of zero charge pHpzc) is just above pH 9 and at this point Ag@CP-BNQDs is positively charged, since tetracycline is negatively charged in aqueous solution, therefor at pH 9 maximum concentration of tetracycline is adsorbed at the surface of photocatalyst and maximum rate of degradation is obtained.14,69−73
3.2.3. Effect of Concentration and Kinetics of Degradation
To study the kinetics of photocatalytic degradation, the tetracycline molecule was chosen first, and then the experiment was carried out under the same conditions and with the same radiation source. The rate of tetracycline degradation was estimated by examining the order of degradation at peak 375 nm. For this reason, the log–log graphical approach, as well as integral and deferential rate laws, have been used. The validity of the kinetic order can also be assessed using the correlation coefficient (R2) obtained from fitting curves, especially when R2 is close to unity.4,5,67 It shows that the degradation of tetracycline 375 nm is the second order with a R2 value of 0.99 as shown in Figure 3B.
As indicated in Table 2, the influence of initial concentration on the rate constant, the rate constant of waste removal does not remain constant when the initial concentration is changed. The reason behind this is due to secondary photochemical processes that occur during photocatalytic degradation and also a change caused by the quenching process due to excimer formation, in which some intermediates absorb light and re-emit it as heat without initiating semiconductor photocatalysts, resulting in photons not reaching the photocatalyst’s surface.4,5,67,74,75
Table 2. Rate Constants (k) of Tetracycline Degradation at Different Concentrations, Temperature oC Kept Constant at 25, and 20 min.
| conc. ppm | rate constant/mol–1.L.min–1 |
|---|---|
| 10 | 0.207 |
| 25 | 0.0854 |
| 50 | 0.033 |
| 75 | 0.016 |
3.2.4. Effect of Light Source and Efficiency of Degradation
Various light sources available in our laboratories were studies to find the best photocatalytic performance. Our aim is toward a visible light source and avoid using UV light. Table 3 illustrates the degradation rate constant as a function of the light source. With regard to degradation efficiency, the sample was irradiated 30 min each time and the cycle was repeated six times, as shown in Figure 4 It can be concluded that the efficiency was close to 80% in the first cycle of irradiation and then fluctuates between above and just below 70%. In an efficiency experiment, 10 ppm of tetracycline was irradiated using 5 watt blue LED light and the temperature was kept constant 25 °C. It is worth mentioning that the photocatalyst Ag@CP-BNQDs is applied in a one-pot degradation and no further activation is performed. Prior to the photocatalytic experiment. It is worthy to mention that the solution is kept in the dark to gain adsorption–desorption equilibrium to minimize the adsorption effect on the photocatalytic experiments.
Table 3. Influence of Wavelength in a Rate Constant of 10 ppm Tetracycline after Irradiation with Different Light Sources at 25 °C.
| light source | intensity | rate constant/mol–1.L.min–1 |
|---|---|---|
| fluorescent 380 nm | 4 (mW/cm2) | 0.01 |
| blue (420–480) nm | 5 (W/cm2) | 0.207 |
| white (520–560) nm | 4 (W/cm2) | 0.08 |
| yellow (560–590) nm | 4 (W/cm2) | 0.0529 |
| red (625–750) nm | 5 (W/cm2) | 0.018 |
3.3. Photocatalytic Mechanism and Scavenger Trapping Experiments
Scavenger trapping analyses were conducted using a procedure equivalent to photodegradation in order to verify the main active species that was involved in the photodegradation process as well as the photocatalytic mechanism. As illustrated in Table 4, the rate constant of tetracycline degradation was influenced by all trapping agents that means all active species are involved in the photocatalytic process. However, the degradation was more significantly affected by the addition of KBrO3, as it traps e– movement; as a result, the recombination rate of electrons between the VBM and CBM slowed down.
Table 4. Scavengers Trapping Experiments as a Function of Rate Constant Using LED Light 25 °C, 10 ppm Tetracycline and 20 min Time Interval.
| trapping agent | rate constant/mol–1.L.min–1 | degradation % | active species |
|---|---|---|---|
| IPA | 0.12 | 49 | ·OH |
| Na2C2O4 | 0.106 | 50 | h+ |
| KBrO3 | 0.305 | 85.1 | e– |
| ascorbic acid | 0.065 | 34 | O2–· |
| no trapping agent | 0.207 |
With respect to the band structure of Ag@CP-BNQDs, the Tauc plot was plotted from the data obtained from UV–vis absorption spectra.2,76,77 As shown in Figure S2B,D, for example, Ag@CP-BNQDs was evaluated by extrapolation of the linear part of the curves measured by plotting (αhυ)2 versus hυ. Accordingly, for example, it was determined to be 2.29 eV in water and 2.53 eV when Ag@CP-BNQDs were dispersed in DMF. From the UV–vis absorption spectra, as shown in Figure S2A,C, we found that the Ag@CP-BNQDs show photon absorption more extended to the visible region that indicates more visible energy harvesting. Thus, Ag loading contributes to decreasing the band gap of the photocatalyst.
Photoredox potentials using the Mulliken electronegativity rules were utilized for the calculation of energy levels (band edges) of Ag@CP-BNQDs, as shown in Figure 4B. The band edge energy of valance band (EVB) was calculated to be 2.1 eV and the band edge energy of conduction band (ECB) is −0.4 eV. The Mulliken electronegativity rules were utilized in this calculation.2,36,78−83
As illustrated in Figure 4D, the level of energy of the band edge at EVB of Ag@CP-BNQDs was estimated 2.1 eV, which is more positive than that of (OH–/·OH) (1.99 eV vs NHE); so that, h+ at VBM is able to oxidize OH– to produce ·OH. At the same time, the CBM potential is measured to be −0.4 eV versus NHE, which is more negative than that of O2/O2·– (−0.33 eV vs NHE),37,84 so the electrons at the CBM band edge can be injected to O2 to form O2·–; these results are in good agreement with the scavenger trapping experiments, which strongly indicate that all active species are involved in the photodegradation of tetracycline over Ag@CP-BNQDs. Hence, the loading of silver atoms provides extra orbitals (bands), which facilitate the movement of electrons and they provide a p–n junction, which slows down the recombination rate. The applied trapping agents with their rate constants are tabulated in Table 4.
3.4. Computational Simulation
To have a better understanding of the fabricated nanoparticles, theoretical simulations were carried out using B3LYP hybrid functionals. In these calculations, we supposed silver is bound to CP-BNQDs via a nitrogen atom. The band gap of the Ag@CP-BNQDs has been observed to decrease after loading with Ag, this is because of the n–p junction of outer electrons between Ag p-type and n-type of CP-BNQDs.85,86Figure 5A shows a band structure and total density of state (TDOS) of Ag bound to CP-BNQDs via a nitrogen atom.
Figure 5.
(A) Band structure and TDOS Ag@CP-BNQD (Ag–N position) photocatalyst and (B) PDOS of the Ag@CP-BNQDs (Ag–N position) utilizing DFT/B3LYP/norm conserving.
The partial density of state (PDOS) of electronic levels of Ag@CP-BNQDs is plotted in Figure 5B. It can be noted that the Fermi level bisects the VBM and CBM that means the energy gap from CP-BNQDs is predominant in photocatalysis. With regard to Ag atoms, the 4d-orbital showed a high DOS covering the Fermi level at the valance band, which appears as multiple peaks in the range of −12.8–0.67 eV, explaining that most of the DOS of Ag atoms is located at 4d-orbitals. The bottom of the conduction band consists of a mixed 4p and 5s orbitals with medium intensity at 1.02 eV up to 10.7 eV, as shown in Figure 5B. With respect to nitrogen atoms, most of the DOS is concentrated at 2p orbitals, broad multiple peaks near at the VBM −12.5 to 0.9 eV at the CBM have been appeared, the DOS intensity for 2s orbitals are very low compared to 2p-orbitals. As it is found in Figure 5A, the intensity of s orbitals for both Ag and N atoms is low, so that they have a minimum contribution to the DOS at the VBM and the CBM. As shown in Table 5, the optimized lattice parameter and bond length for Ag@CP-BNQDs was calculated using the PBE exchange functional.2,87,88 From theoretical calculations, we found that the band edges of CP-BNQDs have a maximum contribution in the photocatalytic process and Ag provides extra bands, which facilitate the electron movement. According to DFT calculations in the literature, boron nitride is considered an n-type semiconductor;8586 therefore, there is an n–p junction, which enhances and delay recombination of excited electrons.
Table 5. Calculated Lattice Parameters of Ag@CP-BNGDs.
| lattice parameter (primitive centered) | bond length (Ao) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| photocatalyst | a(Å) | b(Å) | c(Å) | V(Å3) | alpha (α) | beta (β) | gamma (γ) | B–N | 1.56534 |
| B–B | 2.55619 | ||||||||
| Ag@CP-BNQDs | 3.61 | 3.61 | 7.23 | 94.48 | 90° | 90° | 90° | N–Ag | 1.56534 |
| B–Ag | 2.55619 | ||||||||
4. Conclusions
A novel photocatalyst nanocomposite was synthesized via loading of silver atoms on CP-BNQDs. Photocatalytic degradations were dramatically enhanced and can harvest a blue weak LED lamp for the degradation of 10 common compounds, including dyes, pesticides, and pharmaceuticals. Experimental and theoretical results showed that loading Ag atoms can decrease the energy gap of the nanocomposite and enhance the charge separation through the n–p junction and plasmonic effect. Theoretical simulation using the DFT calculation proved that Ag atoms share bands with CP-BNQDs, which enhances the charge separation.
Acknowledgments
The authors would like to thank the Ministry of Higher Education and Scientific research in Kurdistan and the University of Zakho for their support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04480.
EDX mapping of sample Ag@CP-BNQDs, UV–vis. absorption spectra, Tauc plot of Ag@CP-BNQDs in DMF and H2O, respectively, and band edge calculations (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Fard N. E.; Fazaeli R. A Novel Kinetic Approach for Photocatalytic Degradation of Azo Dye with CdS and Ag/CdS Nanoparticles Fixed on a Cement Bed in a Continuous-Flow Photoreactor. Int. J. Chem. Kinet. 2016, 48, 691–701. 10.1002/kin.21025. [DOI] [Google Scholar]
- Idrees S. A.; Jamil L. A.; Omer K. M. Fabrication Of Novel Metal-Free Phosphorous Doped Boron Nitride As UV . Active Photo-Catalyst. Iran. J. Catal. 2021, 11, 405–416. [Google Scholar]
- Pelaez M.; Falaras P.; Likodimos V.; O’Shea K.; de la Cruz A. A.; Dunlop P. S. M.; Byrne J. A.; Dionysiou D. D. Use of selected scavengers for the determination of NF-TiO2 reactive oxygen species during the degradation of microcystin-LR under visible light irradiation. J. Mol. Catal. A Chem. 2016, 425, 183–189. 10.1016/j.molcata.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla-Babaker M. M.; Idreesb S. A. Degradation of Congo Red Dye Using Homogeneous Photo Fenton Catalyst Coupled with Oxygen Kinetics and Statistical Analysis. Asian J. Appl. Chem. Res. 2020, 6, 1–9. 10.9734/ajacr/2020/v6i130147. [DOI] [Google Scholar]
- Idrees S. A.; Salih R. N.; Bashir K.; Hamasaeed A. A. Kinetic Study of Congo-Red Photo-Catalytic Degradation in Aqueous Media Using Zinc Oxide as Photo Catalyst Under Led Light. Sci. J. Univ. Zakho 2021, 9, 20–24. 10.25271/sjuoz.2021.9.1.777. [DOI] [Google Scholar]
- Hoffmann M. R.; Martin S. T.; Choi W.; Bahnemann D. W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. 10.1021/cr00033a004. [DOI] [Google Scholar]
- Hama Aziz K. H. H.; Omer K. M.; Mahyar A.; Miessner H.; Mueller S.; Moeller D.. Application of photocatalytic falling film reactor to elucidate the degradation pathways of pharmaceutical diclofenac and ibuprofen in aqueous solutions. Coatings 2019, 9–8. 10.3390/coatings9080465. [DOI] [Google Scholar]
- Guo M.; Hu Y.; Wang R.; Yu H.; Sun L. Molecularly imprinted polymer-based photocatalyst for highly selective degradation of methylene blue. Environ. Res. 2021, 194, 110684. 10.1016/j.envres.2020.110684. [DOI] [PubMed] [Google Scholar]
- Omer K. M.; Ku S.-Y.; Wong K.-T.; Bard A. J. Green electrogenerated chemiluminescence of highly fluorescent benzothiadiazole and fluorene derivatives. J. Am. Chem. Soc. 2009, 131, 904135. 10.1021/ja904135y. [DOI] [PubMed] [Google Scholar]
- Omer K. M.; Ku S.-Y.; Cheng J.-Z.; Chou S.-H.; Wong K.-T.; Bard A. J. Electrochemistry and electrogenerated chemiluminescence of a spirobifluorene-based donor (Triphenylamine)-acceptor (2,1,3-Benzothiadiazole) molecule and its organic nanoparticles. J. Am. Chem. Soc. 2011, 133, 5499. 10.1021/ja2000825. [DOI] [PubMed] [Google Scholar]
- Omer K. M.; Bard A. J.. Electrogenerated chemiluminescence of aromatic hydrocarbon nanoparticles in an aqueous solution. J. Phys. Chem. C 2009, 113–11578. 10.1021/jp901038h. [DOI] [Google Scholar]
- Lee K. M.; Lai C. W.; Ngai K. S.; Juan J. C.. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review; Elsevier Ltd, 2016; Vol. 88.. [DOI] [PubMed] [Google Scholar]
- Khodami Z.; Nezamzadeh-Ejhieh A. Investigation of photocatalytic effect of ZnO-SnO2/nano clinoptilolite system in the photodegradation of aqueous mixture of 4-methylbenzoic acid/2-chloro-5-nitrobenzoic acid. J. Mol. Catal. A Chem. 2015, 409, 59–68. 10.1016/j.molcata.2015.08.013. [DOI] [Google Scholar]
- Ghattavi S.; Nezamzadeh-Ejhieh A. A brief study on the boosted photocatalytic activity of AgI/WO3/ZnO in the degradation of methylene blue under visible light irradiation. Desalin. Water Treat. 2019, 166, 92–104. 10.5004/dwt.2019.24638. [DOI] [Google Scholar]
- Wang M.; Hu Y.; Han J.; Guo R.; Xiong H.; Yin Y. TiO2/NiO hybrid shells: P-n junction photocatalysts with enhanced activity under visible light. J. Mater. Chem. A 2015, 3, 20727–20735. 10.1039/c5ta05839b. [DOI] [Google Scholar]
- Hou X.; Huang M.; Wu X.; Liu A. Preparation and studies of photocatalytic silver-loaded TiO 2 films by hybrid sol – gel method. Chem. Eng. J. 2009, 146, 42–48. 10.1016/j.cej.2008.05.041. [DOI] [Google Scholar]
- Belessiotis G.; Kontos A. G. Plasmonic silver (Ag)-based photocatalysts for H2 production and CO2 conversion: Review, analysis and perspectives. Renewable Energy 2022, 195, 497–515. 10.1016/j.renene.2022.06.044. [DOI] [Google Scholar]
- Cheng H.; Wang P.; Wang Z.; Liu Y.; Huang B.. Silver-based visible light-responsive photocatalysts Interface Science and Technology; Elsevier, 2020; Vol. 31. [Google Scholar]
- Liu S. X.; Qu Z. P.; Han X. W.; Sun C. L. A mechanism for enhanced photocatalytic activity of silver-loaded titanium dioxide. Catal. Today 2004, 93-95, 877–884. 10.1016/j.cattod.2004.06.097. [DOI] [Google Scholar]
- Wang H.; Baek S.; Lee J.; Lim S. High photocatalytic activity of silver-loaded ZnO-SnO 2 coupled catalysts. Chem. Eng. J. J. 2009, 146, 355–361. 10.1016/j.cej.2008.06.016. [DOI] [Google Scholar]
- Wang C.; Shao C.; Zhang X.; Liu Y. SnO2 nanostructures-tio2 nanofibers heterostructures: Controlled fabrication and high photocatalytic properties. Inorg. Chem. 2009, 48, 7261–7268. 10.1021/ic9005983. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Crittenden S.; Hackney L.; Sutter D. W.; Hand D. W. Preparation of a Novel TiO 2 -Based p - n Junction Nanotube Photocatalyst. Environ. Sci. Technol. 2005, 39, 1201–1208. 10.1021/es049252g. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Jin Y.; Gao C. H.; Gu W.; Jin Z. M.; Lei Y. L.; Liao L. S. A simple method for fabricating p-n junction photocatalyst CuFe 2O4/Bi4Ti3O12 and its photocatalytic activity. Mater. Chem. Phys. 2014, 143, 952–962. 10.1016/j.matchemphys.2013.10.026. [DOI] [Google Scholar]
- Sabri M.; Habibi-Yangjeh A.; Khataee A. Nanoarchitecturing TiO2/NiCr2O4 p-n heterojunction photocatalysts for visible-light-induced activation of persulfate to remove tetracycline hydrochloride. Chemosphere 2022, 300, 134594. 10.1016/j.chemosphere.2022.134594. [DOI] [PubMed] [Google Scholar]
- Liu B.; Yan S.; Song Z.; Liu M.; Ji X.; Yang W.; Liu J. One-Step Synthesis of Boron Nitride Quantum Dots: Simple Chemistry Meets Delicate Nanotechnology. Chem. - A Eur. J. 201672016, 22, 18899. 10.1002/chem.201603935. [DOI] [PubMed] [Google Scholar]
- Acharya A.; Sharma S.; Liu X.; Zhang D.; Yap Y. K. A Review on van der Waals Boron Nitride Quantum Dots. C 2021, 7, 35. 10.3390/c7020035. [DOI] [Google Scholar]
- Jung J. H.; Kotal M.; Jang M. H.; Lee J.; Cho Y. H.; Kim W. J.; Oh I. K. Defect engineering route to boron nitride quantum dots and edge-hydroxylated functionalization for bio-imaging. RSC Adv. 2016, 6, 73939–73946. 10.1039/c6ra12455k. [DOI] [Google Scholar]
- Zhan Y.; Yang J.; Guo L.; Luo F.; Qiu B.; Hong G.; Lin Z. Targets regulated formation of boron nitride quantum dots – Gold nanoparticles nanocomposites for ultrasensitive detection of acetylcholinesterase activity and its inhibitors. Sensors Actuators, B Chem. 2019, 279, 61–68. 10.1016/j.snb.2018.09.097. [DOI] [Google Scholar]
- Krepel D.; Kalikhman-Razvozov L.; Hod O. Edge Chemistry Effects on the Structural, Electronic, and Electric Response Properties of Boron Nitride Quantum Dots. J. Phys. Chem. C 2014, 118, 21110. 10.1021/jp5038766. [DOI] [Google Scholar]
- He Z.; Kim C.; Lin L.; Jeon T. H.; Lin S.; Wang X.; Choi W. Formation of heterostructures via direct growth CN on h-BN porous nanosheets for metal-free photocatalysis. Nano Energy 2017, 42, 58–68. 10.1016/j.nanoen.2017.10.043. [DOI] [Google Scholar]
- Yu J.; Qin L.; Hao Y.; Kuang S.; Bai X.; Chong Y. M.; Zhang W.; Wang E. Vertically aligned boron nitride nanosheets: Chemical vapor synthesis, ultraviolet light emission, and superhydrophobicity. ACS Nano 2010, 4, 414–422. 10.1021/nn901204c. [DOI] [PubMed] [Google Scholar]
- Zhou D.; Lai M.; Zhang L.; Zeng W.; Huang D.; Cheng M.; Hu L.; Xiong W.; Chen M.; Wang J.; Yang Y.; Jiang L.. Semiconductor/boron nitride composites: synthesis, properties, and photocatalysis applications. Applied Catal. B, Environ. 2018, 238. 10.1016/j.apcatb.2018.07.011. [DOI] [Google Scholar]
- Angizi S.; Shayeganfar F.; Azar M. H.; Simchi A. Surface/edge functionalized boron nitride quantum dots: Spectroscopic fingerprint of bandgap modification by chemical functionalization. Ceram. Int. 2020, 46, 978–985. 10.1016/j.ceramint.2019.09.061. [DOI] [Google Scholar]
- Lee R. G. P.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- Ju P.; Wang P.; Li B.; Fan H.; Ai S.; Zhang D.; Wang Y. A novel calcined Bi2WO6/BiVO4 heterojunction photocatalyst with highly enhanced photocatalytic activity. Chem. Eng. J. 2014, 236, 430–437. 10.1016/j.cej.2013.10.001. [DOI] [Google Scholar]
- Pirhashemi M.; Habibi-Yangjeh A.. Ultrasonic-assisted preparation of plasmonic ZnO/Ag/Ag2WO4 nanocomposites with high visible-light photocatalytic performance for degradation of organic pollutants. J. Colloid Interface Sci. 2017, 491. 10.1016/j.jcis.2016.12.044. [DOI] [PubMed] [Google Scholar]
- Du J.; Zhang J.; Yang T.; Liu R.; Li Z.; Wang D.; Zhou T.; Liu Y.; Liu C.; Che G. The Research on the Construction and the Photocatalytic Performance of BiOI/NH2-MIL-125(Ti) Composite. Catalysts 2021, 125, 1–15. [Google Scholar]
- Cao F.; Wang J.; Wang Y.; Zhou J.; Li S.; Qin G.; Fan W. An in situ Bi-decorated BiOBr photocatalyst for synchronously treating multiple antibiotics in water. Nanoscale Adv 2019, 1, 1124–1129. 10.1039/c8na00197a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY C. H. G. R. Normal Modes in Hexagonal Boron. PH YSI CAL Rev 1966, 146, 543–547. [Google Scholar]
- Bayón B.; Cacicedo M. L.; Álvarez V. A.; Castro G. R. Self-Assembly Stereo-Specific Synthesis of Silver Phosphate Microparticles on Bacterial Cellulose Membrane Surface For Antimicrobial Applications. Colloids Interface Sci. Commun. 2018, 26, 7–13. 10.1016/j.colcom.2018.07.002. [DOI] [Google Scholar]
- Kabi S.; Ghosh A. Structural investigation on silver phosphate glasses embedded with nanoparticles. J. Alloys Compd. 2012, 520, 238–243. 10.1016/j.jallcom.2012.01.031. [DOI] [Google Scholar]
- Upadhyay P.; Mishra S. K.; Purohit S.; Dubey G. P.; Singh Chauhan B.; Srikrishna S. Antioxidant, antimicrobial and cytotoxic potential of silver nanoparticles synthesized using flavonoid rich alcoholic leaves extract of Reinwardtia indica. Drug Chem. Toxicol. 2019, 42, 65–75. 10.1080/01480545.2018.1488859. [DOI] [PubMed] [Google Scholar]
- Zhu C.; Zheng J.; Fang L.; Hu P.; Liu Y.; Cao X.; Wu M. Advanced visible-light driven photocatalyst with enhanced charge separation fabricated by facile deposition of Ag3PO4 nanoparticles on graphene-like h-BN nanosheets. J. Mol. Catal. A Chem. 2016, 424, 135–144. 10.1016/j.molcata.2016.08.028. [DOI] [Google Scholar]
- Zhai L.; Liu Z.; Li C.; Qu X.; Zhang Q.; Li G.; Zhang X.; Abdel-Magid B. Cyanate ester resin based composites with high toughness and thermal conductivity. RSC Adv. 2019, 9, 5722–5730. 10.1039/c8ra10244a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester D. J.; Ailey K. S.; Davis R. F.; More N.; More K. L. Phase evolution in boron nitride thin films. Mater. Res. Soc. 1993, 8, 1213–1216. 10.1557/jmr.1993.1213. [DOI] [Google Scholar]
- Tang B. C.; Bando Y.; Huang Y.; Zhi C.; Golberg D. Synthetic Routes and Formation Mechanisms of Spherical Boron Nitride Nanoparticles. Adv. Funct. Mater. 2008, 18, 3653–3661. 10.1002/adfm.200800493. [DOI] [Google Scholar]
- Luo M. M.; Zhang Y.; Zandén C.; Cao Yu.; Ye L.; Liu J. Novel thermal interface materials: boron nitride nanofiber and indium composites for electronics heat dissipation applications. Mater Sci. Mater Electron 2014, 25, 2333–2338. 10.1007/s10854-014-1880-8. [DOI] [Google Scholar]
- Govarthanan M.; Thangasamy T.; Koildhasan K.; Radhika R.; Shanthi K.; Lee K. J.; Cho M.; Seralathan S.; Byung-Taek B. T. Biosynthesis and characterization of silver Nanoparticles using Panchakavya, an Indian traditional farming formulating agent. Int. J. Nanomedicine 2014, 9, 1593–1599. 10.2147/IJN.S58932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T.; Liu S.; Yan W.; Wang J. Highly efficient preparation of hexagonal boron nitride by direct microwave heating for dye removal. J. Mater. Sci. 2019, 54, 8852–8859. 10.1007/s10853-019-03514-8. [DOI] [Google Scholar]
- Singh B.; Kaur G.; Singh P.; Singh K.; Kumar B.; Vij A.; Kumar M.; Bala R.; Meena R.; Singh A.; Thakur A.; Kumar A. Nanostructured Boron Nitride With High Water Dispersibility For Boron Neutron Capture Therapy. Nat. Sci. Rep. 2016, 6, 35535. 10.1038/srep35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat D.; Katariya M.; Patil C.; Deshmukh S.; Shisodia S.; Pandule S.; Pawar R. Yttrium oxide : A highly efficient catalyst for the synthesis of pyrano [2 ,3- d] pyrimidine derivatives in aqueous methanol media. Eur. Chem. Bull. 2015, 4, 450–453. 10.17628/ecb.2015.4.450-453. [DOI] [Google Scholar]
- Narasimha K. M.; Janardhan G.; Alzohairy M.; Khadri H. Extracellular synthesis , characterization and antibacterial activity of Silver nanoparticles by Actinomycetes isolative. Int. J. Nano Dimens. 2013, 4, 77–83. [Google Scholar]
- Yang Y.; Zhang C.; Huang D.; Zeng G.; Huang J.; Lai C.; Zhou C.; Wang W.; Guo H.; Xue W.; Deng R.; Cheng M.; Xiong W. Applied Catalysis B : Environmental Boron nitride quantum dots decorated ultrathin porous g-C 3 N 4 : Intensi fi ed exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B Environ. 2019, 245, 87–99. 10.1016/j.apcatb.2018.12.049. [DOI] [Google Scholar]
- Wiench D. M.; Jansen M. Na3PO4, Über Versuche zur Reindarstellung, Kristallstruktur der Hochtemperaturform [1]. ZAAC - J. Inorg. Gen. Chem. 1980, 461, 101–108. 10.1002/zaac.19804610116. [DOI] [Google Scholar]
- Fortman G. C.; Slawin A. M. Z.; Nolan S. P. Highly active iridium(III)-NHC system for the catalytic B-N bond activation and subsequent solvolysis of ammonia-borane. Organometallics 2011, 30, 5487–5492. 10.1021/om2007437. [DOI] [Google Scholar]
- Novgorodova M. I.; Gorshkov A. I.; Mokhov A. V. Native Silver and Its New Structural Modifications. Int. Geol. Rev. 1981, 23, 485–494. 10.1080/00206818109455083. [DOI] [Google Scholar]
- Weng Q.; Wang X.; Wang X.; Bando Y.; Golberg D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. 10.1039/c5cs00869g. [DOI] [PubMed] [Google Scholar]
- Shi X.; Wang K.; Tian J.; Yin X.; Guo B.; Xi G.; Wang W.; Wu W. Few-Layer Hydroxyl-Functionalized Boron Nitride Nanosheets for Nanoscale Thermal Management. ACS Appl. Nano Mater. 2020, 3, 2310–2321. 10.1021/acsanm.9b02427. [DOI] [Google Scholar]
- Kim D.; Nakajima S.; Sawada T.; Iwasaki M.; Kawauchi S.; Zhi C.; Bando Y.; Golberg D.; Serizawa T. Sonication-assisted alcoholysis of boron nitride nanotubes for their sidewalls chemical peeling. Chem. Commun. 2015, 51, 7104–7107. 10.1039/c5cc00388a. [DOI] [PubMed] [Google Scholar]
- Dai K.; Chen H.; Peng T.; Ke D.; Yi H. Photocatalytic degradation of methyl orange in aqueous suspension of mesoporous titania nanoparticles. Chemosphere 2007, 69, 1361–1367. 10.1016/j.chemosphere.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Zhu X. D.; Wang Y. J.; Sun R. J.; Zhou D. M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. 10.1016/j.chemosphere.2013.02.066. [DOI] [PubMed] [Google Scholar]
- Wang M.; Shi H.; Shao S.; Lu K.; Wang H.; Yang Y.; Gong Z.; Zuo Y.; Gao S. Montmorillonite promoted photodegradation of amlodipine in natural water via formation of surface complexes. Chemosphere 2022, 286, 131641. 10.1016/j.chemosphere.2021.131641. [DOI] [PubMed] [Google Scholar]
- Chuang L. C.; Luo C. H.; Huang S. W.; Wu Y. C.; Huang Y. C. Photocatalytic degradation mechanism and kinetics of caffeine in aqueous suspension of nano-TiO2. Adv. Mater. Res. 2011, 214, 97–102. 10.4028/www.scientific.net/AMR.214.97. [DOI] [Google Scholar]
- Soares E. T.; Lansarin M. A.; Moro C. C. A study of process variables for the photocatalytic degradation of rhodamine B. Brazilian J. Chem. Eng. 2007, 24, 29–36. 10.1590/S0104-66322007000100003. [DOI] [Google Scholar]
- Herrmann J.-M. Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. 10.1016/s0920-5861(99)00107-8. [DOI] [Google Scholar]
- Reza K. M.; Kurny A.; Gulshan F. Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl. Water Sci. 2017, 7, 1569–1578. 10.1007/s13201-015-0367-y. [DOI] [Google Scholar]
- Idrees S. A.; Naman S. A.; Shorachi A.. Kinetic and thermodynamic study of Trifluralin photo-degradation by ultra violet light. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454(). 10.1088/1757-899X/454/1/012045. [DOI] [Google Scholar]
- Chen Y. W.; Hsu Y. H.. Effects of reaction temperature on the photocatalytic activity of TiO2 with Pd and Cu cocatalysts. Catalysts 2021, 11–8. 10.3390/catal11080966. [DOI] [Google Scholar]
- Ahile U. J.; Wuana R. A.; Itodo A. U.; Sha’Ato R.; Dantas R. F. Stability of iron chelates during photo-Fenton process: The role of pH, hydroxyl radical attack and temperature. J. Water Process Eng. 2020, 36, 101320. 10.1016/j.jwpe.2020.101320. [DOI] [Google Scholar]
- Nadjia L.; Abdelkader E.; Ahmed B. Photodegradation study of Congo Red in Aqueous Solution using ZnO/ UV-A: Effect of pH And Band Gap of other Semiconductor Groups. J. Chem. Eng. Process Technol. 2011, 02, 1–7. 10.4172/2157-7048.1000108. [DOI] [Google Scholar]
- Konstantinou I. K.; Albanis T. A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. 10.1016/j.apcatb.2003.11.010. [DOI] [Google Scholar]
- Tang W. Z.; Zhang Z.; An H.; Quintana M. O.; Torres D. F. Tio2/uv photodegradation of azo dyes in aqueous solutions. Environ. Technol. (United Kingdom) 1997, 18, 1–12. 10.1080/09593330.1997.9618466. [DOI] [Google Scholar]
- Simonsen M. E. Heterogeneous Photocatalysis. Chem. Adv. Environ. Purif. Process. Water Fundam. Appl. 2014, 135–170. 10.1016/B978-0-444-53178-0.00004-3. [DOI] [Google Scholar]
- Idrees S. A.; Khalil Ibrahim M. K.. Optimization of Congo-Red Photo-Catalytic Degradation by Central Composite Design International Conference on Advanced Science and Engineering; ICOASE, 2018, pp 389–393.
- Mohammad Salim H. A. M.; Ahmed Idrees S. A.; Rashid R. A.; Ali Mohammed A. A.; Simo S. M.; Salim Khalo I. S. Photo-catalytic degradation of Toluidine Blue Dye in Aqueous Medium under Fluorescent Light. ICOASE 2018 - Int. Conf. Adv. Sci. Eng. 2018, 384–388. 10.1109/ICOASE.2018.8548935. [DOI] [Google Scholar]
- Mohammed R.; Ahmed S.; Abdulrahman A.; Hamad S. Synthesis and Characterizations of ZnO Thin Films Grown by Physical Vapor Deposition Technique. J. Appl. Sci. Technol. Trends 2020, 1, 135–139. 10.38094/jastt1456.. [DOI] [Google Scholar]
- Abdulrahman A. F.; Ahmed S. M.; Almessiere M. A. Effect of the growth time on the optical properties of ZnO nanorods grown by low temperature method. Dig. J. Nanomater. Biostructures 2017, 12, 1001–1009. [Google Scholar]
- Habibi-Yangjeh A.; Shekofteh-Gohari M. Novel magnetic Fe 3 O 4 / ZnO / NiWO 4 nanocomposites : Enhanced visible-light photocatalytic performance through p-n heterojunctions. Sep. Purif. Technol. 2017, 184, 334–346. 10.1016/j.seppur.2017.05.007. [DOI] [Google Scholar]
- Mousavi M.; Habibi-Yangjeh A.; Abitorabi M. Journal of Colloid and Interface Science Fabrication of novel magnetically separfable nanocomposites using graphitic carbon nitride , silver phosphate and silver chloride and their applications in photocatalytic removal of different pollutants using visibl. J. Colloid Interface Sci. 2016, 480, 218–231. 10.1016/j.jcis.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Dong H.; Chen G.; Sun J.; Li C.; Hu Y.; Lv C. An advanced Ag-based photocatalyst Ag2Ta4O11 with outstanding activity. Phys. Chem. Chem. Phys. 2014, 16, 23915–23921. [DOI] [PubMed] [Google Scholar]
- Brook S. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar]
- Di L.; Yang H.; Xian T.; Chen X. Enhanced Photocatalytic Degradation Activity of BiFeO3 Microspheres by Decoration with g-C3 N4 Nanoparticles. Materials Research. 2018, 21, e20180081 10.1590/1980-5373-mr-2018-0081. [DOI] [Google Scholar]
- Shenoy S.; Tarafder K. Enhanced photocatalytic efficiency of layered CdS/CdSe heterostructures: Insights from first principles electronic structure calculations. J. Phys. Condens. Matter 2020, 32, 275501–14. 10.1088/1361-648X/ab7b1c. [DOI] [PubMed] [Google Scholar]
- Ruan X.; Hu H.; Che H.; Jiang E.; Zhang X.; Liu C.; Che G. A visible-light-driven Z-scheme CdS/Bi 12 GeO 20 heterostructure with enhanced photocatalytic degradation of various organics and the reduction of aqueous Cr(VI). J. Colloid Interface Sci. 2019, 543, 317–327. 10.1016/j.jcis.2019.02.052. [DOI] [PubMed] [Google Scholar]
- Lu S.; Shen P.; Zhang H.; Liu G.; Guo B.; Cai Y.; Chen H.; Xu F.; Zheng T.; Xu F.; Chen X.; et al. Towards n-type conductivity in hexagonal boron nitride. Nat. Commun. 2022, 13, 1–10. 10.1038/s41467-022-30762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H. J.; Yang J.; Hou J. G.; Zhu Q. Are fluorinated boron nitride nanotubes n -type semiconductors?. Appl. Phys. Lett. 2005, 87, 243113–3. 10.1063/1.2142290. [DOI] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Abdel-Sattar M. K.; Taha M.. Electronic structures and optoelectronic properties of ATiOPO4 (A = H, Li, Na, K, Rb, Cs, Fr, NH4, Ag) compounds and their applications in water splitting, CO2 reduction, and photo-degradation. Mater. Res. Express 2020, 7(). 10.1088/2053-1591/ab8338. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.