Abstract
Aim: The effect of dietary salt intake on the risk of gastric cancer is not clear. A meta-analysis was performed to estimate the association between dietary salt intake and the risk of gastric cancer. Methods: Three major databases were searched to retrieve case-control studies published in English before 1 July 2022. Random effects model analysis was used to obtain the pooled odds ratios (ORs) and 95% confidence intervals (CIs) of the association between dietary salt intake and risk of gastric cancer. Subgroup analyses were used to identify possible sources of heterogeneity. Results: Thirty-eight case-control studies were included in this meta-analysis (total population: n = 37,225). The pooled ORs showed a significantly positive association between high salt intake and gastric cancer compared with low salt intake (OR = 1.55, 95% CI (1.45, 1.64); p < 0.001). In subgroup meta-analysis for geographic region, estimation method for dietary salt intake and the source of controls, this association was not changed. Conclusion: Higher dietary salt intake increased the risk of gastric cancer. This study has implications for the prevention of gastric cancer.
Keywords: salt, gastric cancer, case-control study, meta-analysis, prevention
1. Introduction
Gastric cancer has long been a major public health issue [1]. Although the incidence and mortality rates of gastric cancer have declined in recent decades, it remains one of the most common cancers and the leading cause of cancer deaths [1,2]. According to GLOBOCAN estimates of cancer incidence and mortality, there were more than 1 million gastric cancer cases in 2020, resulting in more than 768,793 deaths [2]. The rise of gastric cancer as a leading cause of death has sparked concern. A prominent strategy is to prevent or delay the onset of gastric cancer.
The World Cancer Research Fund (WCRF), London, UK and its affiliates, including the American Institute for Cancer Research (AICR), Washington, DC, USA, have suggested cancer-prevention behaviors such as a healthy diet [3]. Lifestyle factors, including diet, may have an impact on cancer risk over a lifetime [3,4]. High salt consumption is one of the leading risk factors for a variety of non-communicable diseases, including gastric cancer [5]. Furthermore, one study founded that a high salt intake may be a risk factor for the development of gastric adenocarcinoma [6]. The association may be explained by two important factors. (1) Salt irritates the stomach wall and strongly enhances and promotes chemical gastric carcinogenesis [6,7]. (2) Excess salt may promote gastric Helicobacter pylori (H. pylori) colonization in the stomach, which is a known risk factor for gastric cancer [8,9]. High dietary salt intake is also contributing to the global burden of gastric cancer [10,11]. High sodium intake accounts for many the gastric cancer cases [10]. A healthy diet and lifestyle are required. By implementing the optimal lifestyle for all populations, half of all gastric cancer events could be prevented by the year 2031 [3,12]. If action is taken as early as possible, better effects can be achieved.
Among previous studies, the association between high dietary salt intake and gastric cancer was investigated, but the conclusion was inconsistent [13,14,15,16,17,18]. This is partly caused by the absence of reliable methods for estimating dietary salt intake. Taste preference, a food frequency questionnaire (FFQ), dietary behaviors, and other methods are used to estimate dietary salt intake. Inconsistent results may be due to the inconsistency of the estimation methods.
Given this, we performed a meta-analysis based on current published case-control studies to provide scientific and theoretical evidence for gastric cancer prevention. The focus should be on modifiable factors addressed as early as possible, which could show high effectiveness in preventing gastric cancer at a low cost.
2. Methods
The design, implementation, analysis, and reporting of our meta-analysis were reported in accordance with the PRISMA statement.
2.1. Data Sources and Search Strategy
We systematically searched three literature databases, including PubMed, Web of Science, and Cochrane Library, for studies published up to 1 July 2022 in English. The following Mesh terms and combinations of words were used for the literature search: (‘stomach neoplasms’ [Mesh] OR ‘gastric neoplasms’ OR ‘stomach cancer’ OR ‘gastric cancer’) AND (‘sodium, dietary’ [Mesh] OR ‘salt-heavy diet’ OR ‘high salt diet’ OR ‘salty food’). The searches were unlimited by time up to 1 July 2022, but were limited to human studies.
2.2. Selection Criteria and Exclusion Criteria
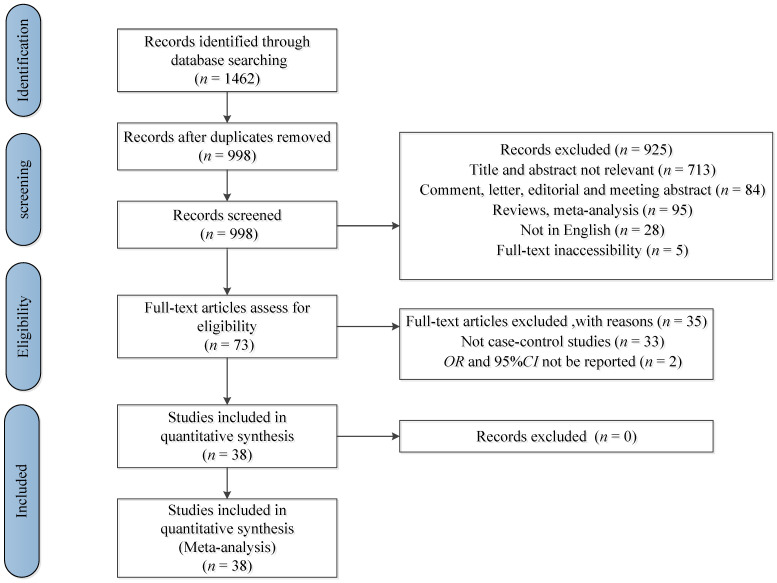
The studies were selected if they met all of the following criteria: (1) being a case-control study; (2) total sample size over 100; (3) assessment of salty food intake, preference of salty food, use of table salt and relevant indexes as exposure; (4) the authors reported odds ratio (OR) estimates, including 95% confidence intervals (CIs), for different salt intake categories. The studies were excluded if they met any of the following criteria: (1) being duplicate publications; (2) not being relevant; (3) being systematic reviews, meta-analyses, meeting abstracts, letters, and dissertations without the relevant information; (4) not being case-control studies; (5) OR and 95% CI not be reported. Studies with larger sample sizes was chosen among duplicate publications from the same case-control study. Exclusion criteria were applied sequentially by first screening the titles and abstracts, and then the full text. Duplicate records were excluded before screening began. The flow chart of the selection of studies is shown in Figure 1.
Figure 1.
Flow chart of selection of studies for the meta-analysis.
2.3. Data Extraction and Quality Assessment
Two investigators (Xiaomin Wu and Liling Chen) independently conducted the literature search, reviewed the retrieved articles, and extracted detailed information from included articles.
Any disagreement about whether a study met the inclusion criteria was resolved by group discussions with the third investigator (Junxia Cheng). The following characteristics of the identified studies and respective populations were recorded: first author, year of publication, country, region, gender, age (years) (mean/range), sample size of participants, match or not, the source of controls, estimation methods for dietary salt intake, comparisons, and adjustment variables for each study. The estimation methods for dietary salt intake in the different studies were provided in terms of total dietary salt intake or in terms of preference for salty food, or both. For our analysis, we used the outcome provided for total dietary salt intake whenever possible. Furthermore, we extracted OR estimates with the greatest adjustment.
Quality assessment was performed according to the Newcastle–Ottawa scale for observational studies [19]. This scale assigns a maximum of nine points to each study: four for selection of participants, two for comparability between both groups, and three for assessment of exposure. A greater score was considered to be an indicator of better quality on a scale of 9.
2.4. Statistical Analysis
STATA/SE 16 for Windows was used to analyze the data. The ORs and 95% CIs were considered as the effect size for all studies in this meta-analysis. The value from each study and the corresponding standard error were transformed into their natural logarithms to stabilize the variances and normalize their distribution. The pooled OR with corresponding 95% CI was estimated using a random effect model, weighting for the inverse of the variance. Heterogeneity among the studies was estimated using the I2 statistic, with values of 25%, 50%, and 75% representing low, moderate, and high degrees of heterogeneity, respectively, with a p value < 0.10 deemed to be significant. A forest plot was used to visualize the ORs and 95% CIs of the included studies. A funnel plot was used to visualize a potential publication bias and Egger’s linear regression test was used to measure the asymmetry of the funnel plot, with a p value < 0.10 deemed to be significant. The influence of a single study was examined by sensitivity analysis. Subgroup analyses were used to identify associations between the risk of gastric cancer and relevant study characteristics (region and estimation method for dietary salt intake) as possible sources of heterogeneity. All tests were two-tailed and statistical significance was defined as p < 0.05.
3. Results
3.1. Literature Search and Study Characteristics
The study selection process and results from the literature search are shown in Figure 1. Of a total of 1462 publications retrieved, 38 studies were identified that met the inclusion criteria. The relevant characteristics of the 38 studies included in the meta-analysis are reported in Table 1. Overall, the meta-analysis involved 37,225 participants from 20 countries (11 studies from China; 4 from Korea; 4 from Italy; 2 from Iran; 2 from Turkey; 1 from France, England, Spain, Japan; Puerto Rico, Sweden, Mexico, Thailand, Uruguay, Colombia, Portugal, Serbia, Canada, Ecuador, and Poland). In all studies; the dietary salt intake was estimated by FFQ or relevant tests. The estimation methods for dietary salt intake are shown in Table 2. The Newcastle–Ottawa Scale was used to assess the quality of included articles. The results of the quality scoring are shown in Table 1. A summary of the characteristics and quality assessment of the included studies is listed in Table 1 and Table 2. The information on the adjustment variables for each study is shown in Table S2.
Table 1.
Characteristics of the case-control studies included in the meta-analysis.
| First Author | Year | Country | Region | Male (n) | Age (Years) Mean/Range |
Sample Size | Quality Score | |
|---|---|---|---|---|---|---|---|---|
| Case | Control | |||||||
| Tuyns [20] | 1988 | France | Europe | 1597 | — | — | 4061 | 5 |
| Buiatti [21] | 1989 | Italy | Europe | 1345 | ≤75 | ≤75 | 2175 | 5 |
| Negri [22] | 1990 | England | Europe | 219 | 61 | 64 | 282 | 8 |
| Demirer [23] | 1990 | Turkey | Asia | 131 | 55 | 52 | 200 | 7 |
| Hoshiyama [24] | 1992 | Japan | Asia | 699 | — | — | 699 | 6 |
| Ramón [25] | 1993 | Spain | Europe | 297 | 62 | 61 | 351 | 8 |
| Nazario [26] | 1993 | Puerto Rico | America | — | ≥30 | ≥30 | 271 | 8 |
| Hansson [27] | 1993 | Sweden | Europe | 662 | 67.7 | 67.0 | 1017 | 8 |
| Lee [28] | 1995 | Korea | Asia | 264 | >25 | >25 | 426 | 7 |
| Vecchia [29] | 1997 | Italy | Europe | 1662 | 61 | 55 | 2799 | 5 |
| Ye [15] | 1998 | China | Asia | 699 | 30–78 | 30–78 | 816 | 8 |
| Ji [30] | 1998 | China | Asia | 1589 | 61 | 59 | 2575 | 8 |
| Ward [31] | 1999 | Mexico | America | — | ≥20 | ≥20 | 972 | 8 |
| Palli [32] | 2001 | Italy | Europe | 567 | — | — | 943 | 6 |
| Sriamporn [33] | 2002 | Thailand | Asia | 254 | — | — | 393 | 7 |
| Kim [34] | 2002 | Korea | Asia | 186 | — | — | 314 | 7 |
| Sun [35] | 2002 | China | Asia | 568 | 59.8 | 59.5 | 840 | 8 |
| Lee [36] | 2003 | Korea | Asia | 166 | — | — | 268 | 7 |
| Stefani [37] | 2004 | Uruguay | America | 840 | 30–89 | 30–89 | 1200 | 7 |
| Lissowska [38] | 2004 | Poland | Europe | 479 | — | — | 737 | 8 |
| Qiu [39] | 2005 | China | Asia | 176 | 63 | 60 | 176 | 6 |
| Campos [40] | 2006 | Colombia | America | 407 | — | — | 647 | 7 |
| Hsu [41] | 2008 | China | Asia | 131 | 66.0 | 51.8 | 349 | 8 |
| Pelucchi [42] | 2009 | Italy | Europe | 429 | 63 | 63 | 777 | 7 |
| Pourfarzi [43] | 2009 | Iran | Asia | 416 | 65.4 | 64.3 | 611 | 8 |
| Wen [44] | 2010 | China | Asia | 642 | 58.9 | 57.7 | 900 | 7 |
| Peleteiro [16] | 2011 | Portugal | Europe | 503 | 18–92 | 18–92 | 1071 | 8 |
| Yang [45] | 2011 | China | Asia | 642 | 52.1 | 52.4 | 900 | 7 |
| Lazarević [46] | 2011 | Serbia | Europe | — | 65.8 | 65.8 | 306 | 7 |
| Zhang [47] | 2011 | China | Asia | 424 | 53.3 | 52.8 | 645 | 6 |
| Hu [48] | 2011 | Canada | America | 1528 | 57.1 | 60.1 | 6221 | 6 |
| Pakseresht [49] | 2011 | Iran | Asia | 427 | 66.3 | 62.9 | 590 | 6 |
| Yassıbaş [50] | 2012 | Turkey | Asia | 132 | 57.4 | 57.9 | 212 | 7 |
| Chen [9] | 2012 | China | Asia | 390 | 53.1 | 52.8 | 617 | 6 |
| Epplein [51] | 2014 | China | Asia | 677 | 62.6 | 63.6 | 677 | 8 |
| Lin [52] | 2014 | China | Asia | 241 | 59.1 | 56.5 | 316 | 6 |
| Salvador [17] | 2015 | Ecuador | America | 95 | 62.0 | 55.5 | 257 | 7 |
| Kwak [18] | 2021 | Korea | Asia | 412 | 56.9 | 56.2 | 614 | 7 |
Note: “—” not reported or not acquired.
Table 2.
Detailed exposure of the studies.
| First Author | Publication Year | Match | Source of Controls | Estimation Method for Dietary Salt Intake | Comparisons |
|---|---|---|---|---|---|
| Tuyns [20] | 1988 | Non-matched | Population-based | Addition of salt | Never |
| Buiatti [21] | 1989 | Non-matched | Population-based | Add salt | Never/seldom |
| Negri [22] | 1990 | Matched | Population-based | Levels of salt intake | Low |
| Demirer [23] | 1990 | Matched | Hospital-based | Consumption frequency of salted foods | No consumption/“rare” consumption/ Once or twice a month |
| Hoshiyama [24] | 1992 | Non-matched | Population-based | Preference for salty foods | Low |
| Ramón [25] | 1993 | Matched | Population-based | Salt intake | <1.96 (g/day) |
| Nazario [26] | 1993 | Non-matched | Population-based | Salt index | <6.979 (g/week) |
| Hansson [27] | 1993 | Matched | Population-based | Salted fish | Low |
| Lee [28] | 1995 | Non-matched | Hospital-based | Salt preference | Low |
| Vecchia [29] | 1997 | Non-matched | Hospital-based | Salt preference | Low |
| Ye [15] | 1998 | Matched | Population-based | Salt | ≤0.25 kg/month |
| Ji [30] | 1998 | Matched | Population-based | Consume salted foods | Occasionally |
| Ward [31] | 1999 | Non-matched | Population-based | Salty snacks/crackers | Never |
| Palli [32] | 2001 | Non-matched | Population-based | Sodium intake | Low tertile |
| Sriamporn [33] | 2002 | Matched | Hospital-based | Salted food | Low |
| Kim [34] | 2002 | Matched | Hospital-based | Salted food | Low |
| Sun [35] | 2002 | Matched | Population-based | Salt preference | Moderate |
| Lee [36] | 2003 | Non-matched | Hospital-based | Salt fermented fish | <1/month |
| Stefani [37] | 2004 | Matched | Hospital-based | Salted meat consumption | Low |
| Lissowska [38] | 2004 | Matched | Population-based | Weekly frequency of salt consumption | Low |
| Qiu [39] | 2005 | Non-matched | Population-based | Daily intake of sodium | Low |
| Campos [40] | 2006 | Matched | Hospital-based | Salting meals before tasting | No |
| Hsu [41] | 2008 | Non-matched | Population-based | Salty food intake | Low |
| Pelucchi [42] | 2009 | Matched | Hospital-based | Intake of sodium | Low |
| Pourfarzi [43] | 2009 | Matched | Population-based | Salt preference | Not salty |
| Wen [44] | 2010 | Matched | Hospital-based | STST ≥ 5 | STST < 5 |
| Peleteiro [16] | 2011 | Non-matched | Population-based | Use of table salt (salt consumption by visual analogical scale) | <35 (mm) |
| Yang [45] | 2011 | Matched | Hospital-based | Salt taste preference | Not salty |
| Lazarević [46] | 2011 | Matched | Hospital-based | Intake of salt | Low |
| Zhang [47] | 2011 | Non-matched | Population-based | Salt taste preference * | 0.9 (g/L) |
| Hu [48] | 2011 | Non-matched | Population-based | Added salt at table | Never |
| Pakseresht [49] | 2011 | Non-matched | Population-based | Salt | Per g |
| Yassıbaş [50] | 2012 | Matched | Hospital-based | Salt status of dishes | Salt-free |
| Chen [9] | 2012 | Non-matched | Population-based | Salt taste preference * | <1.8 |
| Epplein [51] | 2014 | Matched | Population-based | Intake of sodium | Low |
| Lin [52] | 2014 | Matched | Hospital-based | Salt taste preference | Not salty |
| Salvador [17] | 2015 | Non-matched | Hospital-based | Adding salt >50% of meals | No |
| Kwak [18] | 2021 | Matched | Hospital-based | Salt taste preference | No opinion |
Note: STST: Salt taste sensitivity threshold, *: the salt preference was assessed by threshold level of salty taste.
3.2. Effects of Dietary Salt Intake on Risk of Gastric Cancer
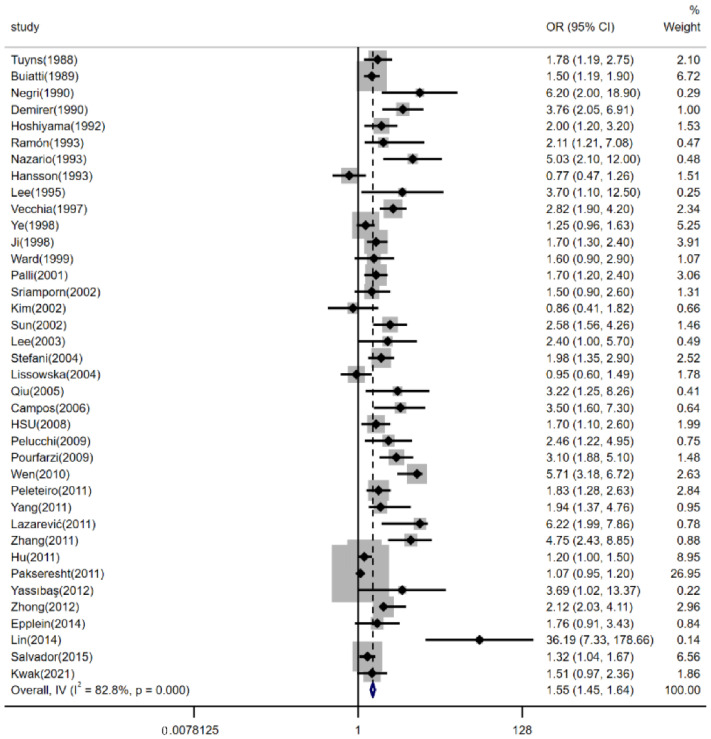
There were 38 case-control studies to evaluate the risk of dietary salt intake with gastric cancer. The form of a forest plot is used to show the results of the pooled analyses in Figure 2 (pooled OR: 1.55, 95% CI: 1.45–1.64). There was significant heterogeneity between studies (p < 0.001, I2 = 82.8%). There was publication bias detected in the meta-analysis (p < 0.001) (The funnel plot is shown in the Supplementary Materials). Additional analyses were performed to check for potential sources of heterogeneity that might explain the association between high dietary salt intake and gastric cancer events. Subgroup analyses were performed.
Figure 2.
Forest plot of associations between high dietary salt intake and gastric cancer risk. References [9,15,16,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] are cited in the Figure.
3.3. Sensitivity Analysis
We explored the effect of a single study on the pooled OR with the sensitivity analysis. The result indicated that the Pakseresht et al. study influenced the pooled OR [49]. If this study was omitted, the pooled OR would be 1.77 (95% CI: 1.65–1.90) (shown in Table 3). The results showed that high dietary salt intake was significantly associated with a greater risk of gastric cancer compared with low salt intake after omitting single studies one by one.
Table 3.
OR estimates and 95% CI after omitting studies one by one.
| Study Omitted | OR | 95% CI |
|---|---|---|
| Tuyns (1988) [20] | 1.54 | (1.45, 1.64) |
| Buiatti (1989) [21] | 1.55 | (1.45, 1.65) |
| Negri (1990) [22] | 1.54 | (1.45, 1.64) |
| Demirer (1990) [23] | 1.53 | (1.44, 1.63) |
| Hoshiyama (1992) [24] | 1.54 | (1.45, 1.64) |
| Ramón (1993) [25] | 1.54 | (1.45, 1.64) |
| Nazario (1993) [26] | 1.54 | (1.45, 1.63) |
| Hansson (1993) [27] | 1.56 | (1.47, 1.66) |
| Lee (1995) [28] | 1.54 | (1.45, 1.64) |
| Vecchia (1997) [29] | 1.52 | (1.43, 1.62) |
| Ye (1998) [15] | 1.56 | (1.47, 1.66) |
| Ji (1998) [30] | 1.54 | (1.45, 1.64) |
| Ward (1999) [31] | 1.54 | (1.45, 1.64) |
| Palli (2001) [32] | 1.54 | (1.45, 1.64) |
| Sriamporn (2002) [33] | 1.55 | (1.45, 1.64) |
| Kim (2002) [34] | 1.55 | (1.46, 1.65) |
| Sun (2002) [35] | 1.53 | (1.44, 1.63) |
| Lee (2003) [36] | 1.54 | (1.45, 1.64) |
| Stefani (2004) [37] | 1.54 | (1.44, 1.63) |
| Lissowska (2004) [38] | 1.56 | (1.47, 1.66) |
| Qiu (2005) [39] | 1.54 | (1.45, 1.64) |
| Campos (2006) [40] | 1.54 | (1.45, 1.63) |
| Hsu (2008) [41] | 1.54 | (1.45, 1.64) |
| Pelucchi (2009) [42] | 1.54 | (1.45, 1.64) |
| Pourfarzi(2009) [43] | 1.53 | (1.44, 1.63) |
| Wen (2010) [44] | 1.50 | (1.40, 1.59) |
| Peleteiro (2011) [16] | 1.54 | (1.45, 1.64) |
| Yang (2011) [45] | 1.54 | (1.45, 1.64) |
| Lazarević (2011) [46] | 1.53 | (1.44, 1.62) |
| Zhang (2011) [47] | 1.53 | (1.44, 1.63) |
| Hu (2011) [48] | 1.58 | (1.49, 1.69) |
| Pakseresht (2011) [49] | 1.77 | (1.65, 1.90) |
| Yassıbaş (2012) [50] | 1.54 | (1.45, 1.64) |
| Chen (2012) [9] | 1.53 | (1.44, 1.63) |
| Epplein (2014) [51] | 1.54 | (1.45, 1.64) |
| Lin (2014) [52] | 1.54 | (1.45, 1.63) |
| Salvador (2015) [17] | 1.56 | (1.47, 1.66) |
| Kwak (2021) [18] | 1.55 | (1.45, 1.64) |
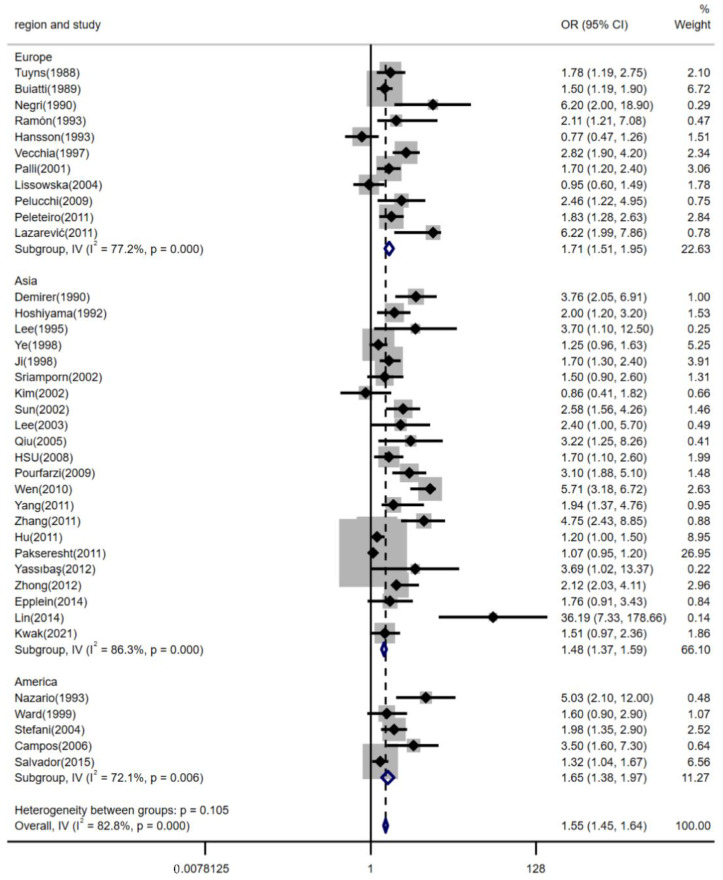
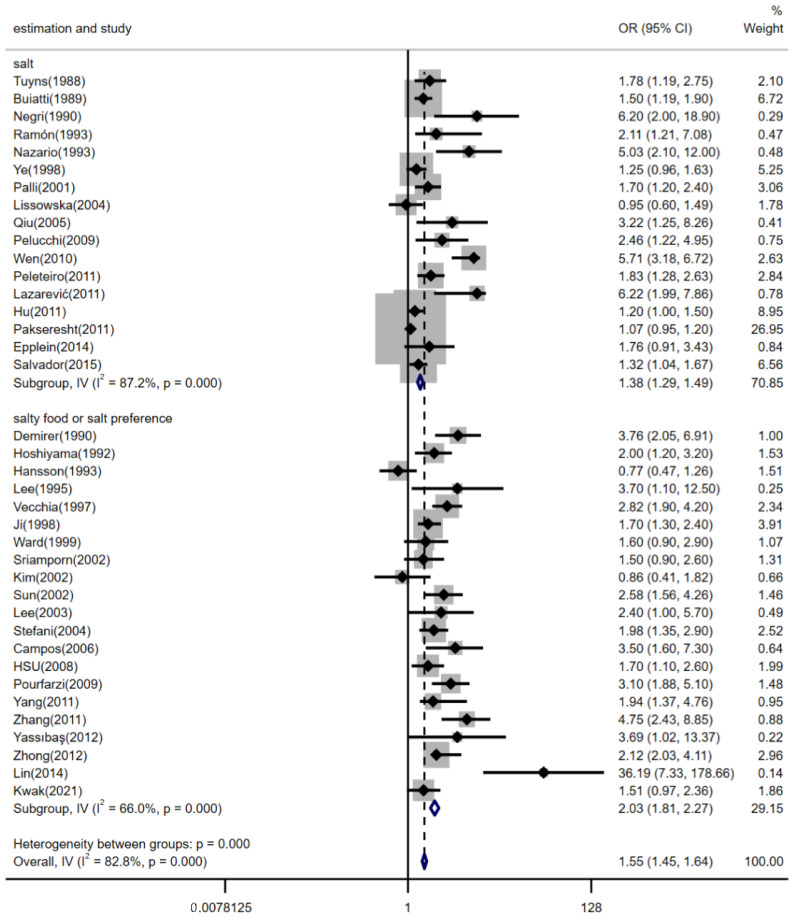
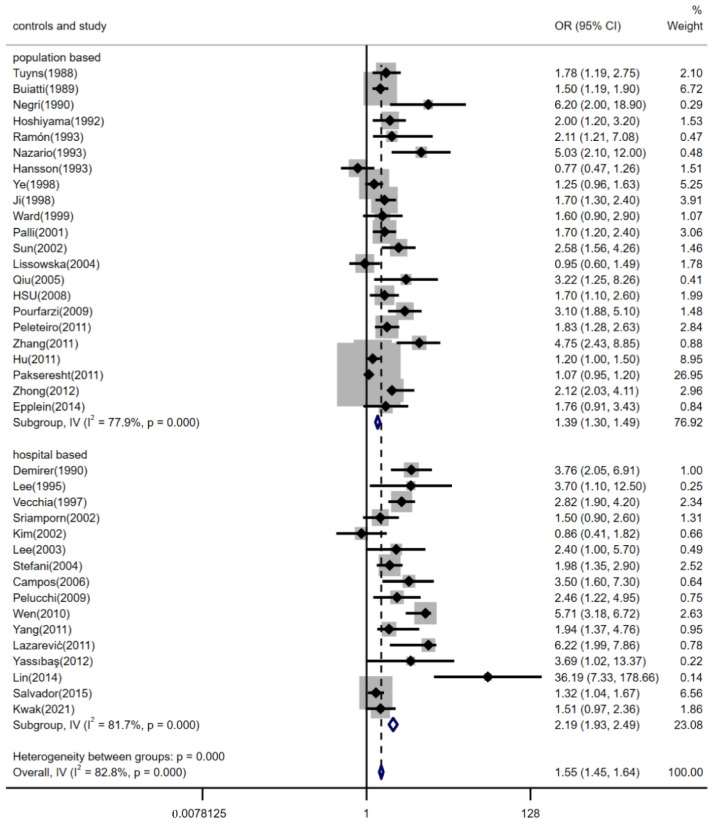
3.4. Subgroup Analyses by Region, Estimation Methods for Dietary Salt Intake and the Source of Controls
The relationship between high dietary salt intake and risk of gastric cancer was not significantly different between geographic regions, estimation methods of dietary salt intake, and the source of controls (shown in Figure 3, Figure 4 and Figure 5). The pooled ORs were changed after stratifying by geographic region. The pooled ORs of gastric cancer for the salt intake were 1.71 (95% CI, [1.51, 1.95]) for studies conducted in Europe, 1.48 (95% CI, [1.37, 1.59]) for studies conducted in Asia, and 1.65 (95% CI, [1.38, 1.97]) for studies conducted in America; there was statistically significant heterogeneity among studies of salt intake in Europe (p < 0.001 and I2 = 77.2%), Asia (p < 0.001 and I2 = 86.3%), and America (p = 0.006 and I2 = 72.1%) (shown in Figure 3). Furthermore, stratifying by estimation method for dietary salt intake, the pooled ORs of gastric cancer for salt intake were 1.38 (95% CI, [1.29, 1.49]) for studies that estimated salt addition and 2.03 (95% CI, [1.81, 2.27]) for studies that estimated consumption of salty foods or salt preference; there was statistically significant heterogeneity among studies that estimated salt addition (p < 0.001 and I2 = 87.2%) and there was statistically medium heterogeneity among studies that estimated consumption of salty foods or salt preference (p < 0.001 and I2 = 66.0%) (shown in Figure 4). The pooled ORs of gastric cancer for salt intake were 1.39 (95% CI, [1.30, 1.49]) for studies with controls from the community and 2.19 (95% CI, [1.93, 2.49]) for studies with controls from hospitals; there was statistically significant heterogeneity in studies (I2 = 77.9% for population-based studies and I2 = 81.7% for hospital-based studies, p < 0.001) (shown in Figure 5).
Figure 3.
Forest plot of associations between high dietary salt intake and gastric cancer risk among different regions. References [9,15,16,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] are cited in the Figure.
Figure 4.
Forest plot of associations between high dietary salt intake and gastric cancer risk among different estimation methods for salt intake. References [9,15,16,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] are cited in the Figure.
Figure 5.
Forest plot of associations between high dietary salt intake and gastric cancer risk among the source of controls. References [9,15,16,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] are cited in the Figure.
4. Discussion
In this meta-analysis, it was found that (1) compared with low dietary salt intake, high dietary salt intake could increase the gastric cancer risk (overall OR = 1.55, 95% CI [1.45, 1.64]; p < 0.001). (2) In subgroup analyses by geographical region and estimation method for salt intake, the significantly positive association was not changed.
Our findings suggest that a high salt intake is associated with gastric cancer, which is consistent with the findings of other studies [13,14]. In addition, this association was not confirmed in some studies [15,34,38,49]. The different results may be explained by several important factors. (1) The frequency of salty food consumption was used to estimate salt intake in some studies, but the definition of salty foods was different. Soy sauce, which has been shown to have a protective effect against gastric cancer in other studies, was classified as a salty food in the Hyun Ja Kim et al. study [34]. (2) One study conducted in Poland estimated dietary salt intake through weekly frequency of salt consumption from food. However, foods that are of high salt content are universally consumed in Poland, so it was difficult to detect any differences [38]. (3) One study used a salt-added increment to estimate the association between high dietary salt intake and gastric cancer, but the incremented unit was 1 g [49]. The estimation method may underestimate the actual association [49]. (4) Researchers used the lowest intake as the reference group in their studies. However, in one study, the no opinion group was chosen as the reference group; this choice may not detect the actual effect of high dietary salt intake on gastric cancer [18]. (5) Confounding factors, comorbidities, and observational bias could all have an impact on the actual association.
Similar to other studies, our study also found that dietary salt intake is associated with gastric cancer. There are several mechanisms to explain this association: (1) The gastric mucosa could be damaged by high salt concentration directly, which leads to hyperplasia of the gastric pit epithelium and increases the probability of endogenous mutations [13,14]. Additionally, the damage to gastric mucosa could increase DNA damage and glandular atrophy [14]. (2) High salt intake could accelerate the procedure of intestinal metaplasia, which could develop into early gastric cancer [14]. (3) Salty foods that have too much nitrate and nitrite could contribute to the formation of N-nitroso compounds [53]. The carcinogenic effect of nitroso compounds may be promoted or enhanced by high salt intake [4]. Additionally, high salt intake may also promote or enhance the effect of other carcinogens [4]. (4) High salt intake increases H. pylori colonization in the stomach. H. pylori is one of the main predisposing factors for gastric cancer [4,13,14]. The cag pathogenicity island is one of the H. pylori virulence determinants, which could increase gastric cancer risk [54]. More severe gastric injury in the stomach was induced by cag-positive strains compared with cag-negative strains, and cag-positive strains further augment the risk for gastric cancer [54]. Elevated salt concentrations caused an upregulation of the cagA gene in some strains, enhancing cagA’s ability to translocate into gastric epithelial cells [54,55]. This indicates that high dietary salt intake could enhance the carcinogenic effects of cagA+ H. pylori strains [14]. (5) High salt intake could alter the viscosity of the protective mucous barrier, disrupt immune homeostasis, and increase susceptibility to H. pylori infection [11,56,57]. These factors would result in chronic inflammation, such as atrophic gastritis and gastric ulcers both of which are common precancerous diseases [13,14,56,58].
There was significant heterogeneity among the included studies. This situation was also observed in other comparable studies [13,14]. The potential sources of heterogeneity were checked with further subgroup analyses, which might explain the association between dietary salt intake and gastric cancer events. Among the studies that estimated salt intake by consumption of salty foods or salt preference, the heterogeneity was decreased. This indicates that estimation methods for dietary salt intake may be a source of heterogeneity. It is difficult to quantify the intake of sodium, which is the main component of salt. The FFQ was used to estimate dietary salt intake in most studies. The actual intake of salt could not be estimated through the FFQ, and recall bias is inevitable. Cases tend to overestimate their exposure to risk factors; possibly, this may lead to a spurious association between risk factors and disease [59].
Publication bias existed in our meta-analysis. Negative results were not be reported, especially in studies published in the 1990s, which is the main source of publication bias.
Limitations
There exist several potential limitations in this study. First, we only included studies published in English and we did not search grey literatures. The actual total number of eligible studies may be larger than the currently included studies. Second, confounding risk factors such as H. pylori, smoking, and other relevant risk factors were not able to be considered in this meta-analysis. Third, given the observational nature of the included studies, our study lacked evidence to clarify causation. Fourth, the estimation methods for dietary salt intake, which contributed to the heterogeneity of this study, were not classified in more detail.
5. Conclusions
In conclusion, it was indicated that higher dietary salt intake increased the risk of gastric cancer. Participants who prefer salty foods need to receive dietary education and diet management for the prevention of gastric cancer. This finding has important public health implications. Societies and individuals may succeed in lowering their risk for gastric cancer by reducing dietary salt intake. An additional meta-analysis that includes more cohort studies is needed.
Acknowledgments
We express deep thanks to the authors of the original studies who provided additional data about their case-control studies, which was critical to this meta-analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14204260/s1, Figure S1: Funnel plots for identifying publication bias in the meta-analysis of observational studies; Table S1: The PRISMA checklist; Table S2: Adjustment variables of the case-control studies included in the meta-analysis; Table S3: The study quality scores of the studies included in meta-analysis. References [9,15,16,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] are cited in the supplementary materials.
Author Contributions
Conception, design (selection criteria), writing, and corrections: X.W., L.C., J.C., J.Q., Z.F. and J.W.; Search strategy, data extraction, and analysis: X.W. and L.C.; Statistical expertise: X.W. and J.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All reported data are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (grant number: 82273676), the National Key Research and Development Program of China (grant numbers: 2021YFA1301200, 2021YFA1301202) and Liaoning province scientific and technological project (2021JH2/10300039).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mukkamalla S., Recio-Boiles A., Babiker H.M. Gastric Cancer. StatPearls Publishing; Treasure Island, FL, USA: 2022. [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Clinton S.K., Giovannucci E.L., Hursting S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020;150:663–671. doi: 10.1093/jn/nxz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norat T., Scoccianti C., Boutron-Ruault M.C., Anderson A., Berrino F., Cecchini M., Espina C., Key T., Leitzmann M., Powers H., et al. European Code against Cancer 4th Edition: Diet and cancer. Cancer Epidemiol. 2015;39:S56–S66. doi: 10.1016/j.canep.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Azadnajafabad S., Ebrahimi N., Mohammadi E., Ghasemi E., Moghaddam S.S., Aminorroaya A., Rezaei N., Ghanbari A., Masinaei M., Fateh S.M., et al. Disparities and spatial variations of high salt intake in Iran: A subnational study of districts based on the small area estimation method. Public Health Nutr. 2021;24:6281–6291. doi: 10.1017/S1368980021002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loh J.T., Torres V.J., Cover T.L. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709–4715. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]
- 7.Ngoan L.T., Yoshimura T. Work, salt intake and the development of stomach cancer. Med. Hypotheses. 2003;60:552–556. doi: 10.1016/S0306-9877(03)00014-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhu L.Q., Huang Y., Li H., Shao S.H. Helicobacter pylori promotes gastric cancer progression through the tumor microenvironment. Appl. Microbiol. Biotechnol. 2022;106:4375–4385. doi: 10.1007/s00253-022-12011-z. [DOI] [PubMed] [Google Scholar]
- 9.Zhong C., Li K.N., Bi J.W., Wang B.C. Sodium intake, salt taste and gastric cancer risk according to Helicobacter pylori infection, smoking, histological type and tumor site in China. Asian Pac. J. Cancer Prev. 2012;13:2481–2484. doi: 10.7314/APJCP.2012.13.6.2481. [DOI] [PubMed] [Google Scholar]
- 10.Peleteiro B., Barros S., Castro C., Ferro A., Morais S., Lunet N. Worldwide burden of gastric cancer in 2010 attributable to high sodium intake in 1990 and predicted attributable burden for 2030 based on exposures in 2010. Br. J. Nutr. 2016;116:728–733. doi: 10.1017/S0007114516002518. [DOI] [PubMed] [Google Scholar]
- 11.Ning F.L., Lyu J., Pei J.P., Gu W.J., Zhang N.N., Cao S.Y., Zeng Y.J., Abe M., Nishiyama K., Zhang C.D. The burden and trend of gastric cancer and possible risk factors in five Asian countries from 1990 to 2019. Sci. Rep. 2022;12:5980. doi: 10.1038/s41598-022-10014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y., Li Y.P., Giovannucci E. Potential Impact of Time Trend of Lifestyle Risk Factors on Burden of Major Gastrointestinal Cancers in China. Gastroenterology. 2021;161:1830–1841. doi: 10.1053/j.gastro.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 13.D’Elia L., Rossi G., Ippolito R., Cappuccio F.P., Strazzullo P. Habitual salt intake and risk of gastric cancer: A meta-analysis of prospective studies. Clin. Nutr. 2012;31:489–498. doi: 10.1016/j.clnu.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Ge S., Feng X., Shen L., Wei Z., Zhu Q., Sun J. Association between Habitual Dietary Salt Intake and Risk of Gastric Cancer: A Systematic Review of Observational Studies. Gastroenterol. Res. Pract. 2012;2012:808120. doi: 10.1155/2012/808120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye W.M., Yi Y.N., Luo R.X., Zhou T.S., Lin R.T., Chen G.D. Diet and gastric cancer: A casecontrol study in Fujian Province, China. World J. Gastroenterol. 1998;4:516–518. doi: 10.3748/wjg.v4.i6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peleteiro B., Lopes C., Figueiredo C., Lunet N. Salt intake and gastric cancer risk according to Helicobacter pylori infection, smoking, tumour site and histological type. Br. J. Cancer. 2011;104:198–207. doi: 10.1038/sj.bjc.6605993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvador I., Mercado A., Bravo G.L., Baldeón M., Fornasini M. Risk and Protective Factors for Gastric Metaplasia and Cancer: A Hospital-Based Case-Control Study in Ecuador. Nutr. Hosp. 2015;32:1193–1199. doi: 10.3305/nh.2015.32.3.9257. [DOI] [PubMed] [Google Scholar]
- 18.Kwak J.H., Eun C.S., Han D.S., Kim Y.S., Song K.S., Choi B.Y., Kim H.J. Gastric Cancer and the Daily Intake of the Major Dish Groups Contributing to Sodium Intake: A Case-Control Study in Korea. Nutrients. 2021;13:1365. doi: 10.3390/nu13041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Tuyns A.J. Salt and gastrointestinal cancer. Nutr. Cancer. 1988;11:229–232. doi: 10.1080/01635588809513992. [DOI] [PubMed] [Google Scholar]
- 21.Buiatti E., Palli D., Decarli A., Amadori D., Avellini C., Bianchi S., Biserni R., Cipriani F., Cocco P., Giacosa A. A case-control study of gastric cancer and diet in Italy. Int. J. Cancer. 1989;44:611–616. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- 22.Negri E., Vecchia C.L., D’Avanzo B., Gentile A., Boyle P., Franceschi S. Salt preference and the risk of gastrointestinal cancers. Nutr. Cancer. 1990;14:227–232. doi: 10.1080/01635589009514097. [DOI] [PubMed] [Google Scholar]
- 23.Demirer T., Icli F., Uzunalimoglu O., Kucuk O. Diet and stomach cancer incidence. A case-control study in Turkey. Cancer. 1990;65:2344–2348. doi: 10.1002/1097-0142(19900515)65:10<2344::AID-CNCR2820651030>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 24.Hoshiyama Y., Sasaba T. A case-control study of single and multiple stomach cancers in Saitama Prefecture, Japan. Jpn. J. Cancer Res. 1992;83:937–943. doi: 10.1111/j.1349-7006.1992.tb02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramon J.M., Serra L., Cerdo C., Oromí J. Dietary factors and gastric cancer risk. A case-control study in Spain. Cancer. 1993;71:1731–1735. doi: 10.1002/1097-0142(19930301)71:5<1731::AID-CNCR2820710505>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 26.Nazario C.M., Szklo M., Diamond E., Román-Franco A., Climent C., Suarez E., Conde J.G. Salt and gastric cancer: A case-control study in Puerto Rico. Int. J. Epidemiol. 1993;22:790–797. doi: 10.1093/ije/22.5.790. [DOI] [PubMed] [Google Scholar]
- 27.Hansson L.E., Nyren O., Bergstrom R., Wolk A., Lindgren A., Baron J., Adami H.O. Diet and risk of gastric cancer. A population-based case-control study in Sweden. Int. J. Cancer. 1993;55:181–189. doi: 10.1002/ijc.2910550203. [DOI] [PubMed] [Google Scholar]
- 28.Lee J.K., Park B.J., Yoo K.Y., Ahn Y.O. Dietary factors and stomach cancer: A case-control study in Korea. Int. J. Epidemiol. 1995;24:33–41. doi: 10.1093/ije/24.1.33. [DOI] [PubMed] [Google Scholar]
- 29.La Vecchia C., Negri E., Franceschi S., Decarli A. Case-control study on influence of methionine, nitrite, and salt on gastric carcinogenesis in northern Italy. Nutr. Cancer. 1997;27:65–68. doi: 10.1080/01635589709514503. [DOI] [PubMed] [Google Scholar]
- 30.Ji B.T., Chow W.H., Yang G., McLaughlin J.K., Zheng W., Shu X.O., Jin F., Gao R.N., Gao Y.T., Fraumeni J.F., Jr. Dietary habits and stomach cancer in Shanghai, China. Int. J. Cancer. 1998;76:659–664. doi: 10.1002/(SICI)1097-0215(19980529)76:5<659::AID-IJC8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 31.Ward M.H., Lopez-Carrillo L. Dietary factors and the risk of gastric cancer in Mexico City. Am. J. Epidemiol. 1999;149:925–932. doi: 10.1093/oxfordjournals.aje.a009736. [DOI] [PubMed] [Google Scholar]
- 32.Palli D., Russo A., Decarli A. Dietary patterns, nutrient intake and gastric cancer in a high-risk area of Italy. Cancer Causes Control. 2001;12:163–172. doi: 10.1023/A:1008970310963. [DOI] [PubMed] [Google Scholar]
- 33.Sriamporn S., Setiawan V., Pisani P., Suwanrungruang K., Sirijaichingkul S., Mairiang P., Parkin D.M. Gastric Cancer: The Roles of Diet, Alcohol Drinking, Smoking and Helicobacter pylori in Northeastern Thailand. Asian Pac. J. Cancer Prev. 2002;3:345–352. [PubMed] [Google Scholar]
- 34.Kim H.J., Chang W.K., Kim M.K., Lee S.S., Choi B.Y. Dietary factors and gastric cancer in Korea: A case-control study. Int. J. Cancer. 2002;97:531–535. doi: 10.1002/ijc.10111. [DOI] [PubMed] [Google Scholar]
- 35.Sun X.B., Moller H., Evans H.S., Dai X.D., Duan W.J., Lu J.B. Residential Environment, Diet and Risk of Stomach Cancer: A Case-control Study in Linzhou, China. Asian Pac. J. Cancer Prev. 2002;3:167–172. [PubMed] [Google Scholar]
- 36.Lee S.A., Kang D., Shim K.N., Choe J.W., Hong W.S., Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J. Epidemiol. 2003;13:162–168. doi: 10.2188/jea.13.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Stefani E., Correa P., Boffetta P., Deneo-Pellegrini H., Ronco A.L., Mendilaharsu M. Dietary patterns and risk of gastric cancer: A case-control study in Uruguay. Gastric Cancer. 2004;7:211–220. doi: 10.1007/s10120-004-0295-2. [DOI] [PubMed] [Google Scholar]
- 38.Lissowska J., Gail M.H., Pee D., Groves F.D., Sobin L.H., Nasierowska-Guttmejer A., Sygnowska E., Zatonski W., Blot W.J., Chow W.H. Diet and stomach cancer risk in Warsaw, Poland. Nutr. Cancer. 2004;48:149–159. doi: 10.1207/s15327914nc4802_4. [DOI] [PubMed] [Google Scholar]
- 39.Qiu J.L., Chen K., Zheng J.N., Wang J.Y., Zhang L.J., Sui L.M. Nutritional factors and gastric cancer in Zhoushan Islands, China. World J. Gastroenterol. 2005;11:4311–4316. doi: 10.3748/wjg.v11.i28.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campos F., Carrasquilla G., Koriyama C., Serra M., Carrascal E., Itoh T., Nomoto M., Akiba S. Risk factors of gastric cancer specific for tumor location and histology in Cali, Colombia. World J. Gastroenterol. 2006;12:5772–5779. doi: 10.3748/wjg.v12.i36.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsu P.I., Jwo J.J., Yang C.L., Hsu P.N., Yang H.B., Lai K.H., Chen I.S., Chuah S.K., Wu D.C., Chen A. Association of the myeloperoxidase polymorphism with the risk of gastric cancer. Anticancer Res. 2008;28:1317–1323. [PubMed] [Google Scholar]
- 42.Pelucchi C., Tramacere I., Bertuccio P., Tavani A., Negri E., La Vecchia C. Dietary intake of selected micronutrients and gastric cancer risk: An Italian case-control study. Ann. Oncol. 2009;20:160–165. doi: 10.1093/annonc/mdn536. [DOI] [PubMed] [Google Scholar]
- 43.Pourfarzi F., Whelan A., Kaldor J., Malekzadeh R. The role of diet and other environmental factors in the causation of gastric cancer in Iran--a population based study. Int. J. Cancer. 2009;125:1953–1960. doi: 10.1002/ijc.24499. [DOI] [PubMed] [Google Scholar]
- 44.Wen X.Y. Salt taste sensitivity, physical activity and gastric cancer. Asian Pac. J. Cancer Prev. 2010;11:1473–1477. [PubMed] [Google Scholar]
- 45.Yang W.G., Chen C.B., Wang Z.X., Liu Y.P., Wen X.Y., Zhang S.F., Sun T.W. A case-control study on the relationship between salt intake and salty taste and risk of gastric cancer. World J. Gastroenterol. 2011;17:2049–2053. doi: 10.3748/wjg.v17.i15.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarevic K., Nagorni A., Bogdanovic D., Rancic N., Stosic L., Milutinovic S. Dietary micronutrients and gastric cancer: Hospital based study. Cent. Eur. J. Med. 2011;6:783–787. doi: 10.2478/s11536-011-0079-0. [DOI] [Google Scholar]
- 47.Zhang Z., Zhang X. Salt taste preference, sodium intake and gastric cancer in China. Asian Pac. J. Cancer Prev. 2011;12:1207–1210. [PubMed] [Google Scholar]
- 48.Hu J., La Vecchia C., Morrison H., Negri E., Mery L. Canadian Cancer Registries Epidemiology Research Group. Salt, processed meat and the risk of cancer. Eur. J. Cancer Prev. 2011;20:132–139. doi: 10.1097/CEJ.0b013e3283429e32. [DOI] [PubMed] [Google Scholar]
- 49.Pakseresht M., Forman D., Malekzadeh R., Yazdanbod A., West R.M., Greenwood D.C., Crabtree J.E., Cade J.E. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control. 2011;22:725–736. doi: 10.1007/s10552-011-9744-5. [DOI] [PubMed] [Google Scholar]
- 50.Yassibas E., Arslan P., Yalcin S. Evaluation of dietary and life-style habits of patients with gastric cancer: A case-control study in Turkey. Asian Pac. J. Cancer Prev. 2012;13:2291–2297. doi: 10.7314/APJCP.2012.13.5.2291. [DOI] [PubMed] [Google Scholar]
- 51.Epplein M., Zheng W., Li H.L., Peek R.M., Jr., Correa P., Gao J., Michel A., Pawlita M., Cai Q.Y., Xiang Y.B., et al. Diet, Helicobacter pylori strain-specific infection, and gastric cancer risk among Chinese men. Nutr. Cancer. 2014;66:550–557. doi: 10.1080/01635581.2014.894096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin S.H., Li Y.H., Leung K., Huang C.Y., Wang X.R. Salt processed food and gastric cancer in a Chinese population. Asian Pac. J. Cancer Prev. 2014;15:5293–5298. doi: 10.7314/APJCP.2014.15.13.5293. [DOI] [PubMed] [Google Scholar]
- 53.Fang X.X., Wei J.Y., He X.Y., An P., Wang H., Jiang L., Shao D.D., Liang H., Li Y., Wang F.D., et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Cancer. 2015;51:2820–2832. doi: 10.1016/j.ejca.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Noto J.M., Chopra A., Loh J.T., Romero-Gallo J., Piazuelo M.B., Watson M., Leary S., Beckett A.C., Wilson K.T., Cover T.L., et al. Pan-genomic analyses identify key Helicobacter pylori pathogenic loci modified by carcinogenic host microenvironments. Gut. 2018;67:1793–1804. doi: 10.1136/gutjnl-2017-313863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raei N., Behrouz B., Zahri S., Latifi-Navid S. Helicobacter pylori Infection and Dietary Factors Act Synergistically to Promote Gastric Cancer. Asian Pac. J. Cancer Prev. 2016;17:917–921. doi: 10.7314/APJCP.2016.17.3.917. [DOI] [PubMed] [Google Scholar]
- 56.Li J.Y., Sun F., Guo Y.C., Fan H. High-Salt Diet Gets Involved in Gastrointestinal Diseases through the Reshaping of Gastroenterological Milieu. Digestion. 2019;99:267–274. doi: 10.1159/000493096. [DOI] [PubMed] [Google Scholar]
- 57.Bouras E., Tsilidis K.K., Triggi M., Siargkas A., Chourdakis M., Haidich A.-B. Diet and Risk of Gastric Cancer: An Umbrella Review. Nutrients. 2022;14:1764. doi: 10.3390/nu14091764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen X., Hu Y., Xie Y., Wang Y. High salt diet can down-regulate TFF2 expression level in gastric mucosa of MGs after H. pylori infection. Microb. Pathog. 2018;118:316–321. doi: 10.1016/j.micpath.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 59.Barry D., Livingstone V. The investigation and correction of recall bias for an ordinal response in a case-control study. Stat. Med. 2006;25:965–975. doi: 10.1002/sim.2238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All reported data are available in the manuscript.