Abstract
Soft tissue sarcomas (STS) are heterogeneous mesenchymal tumors with limited therapeutic options in the advanced setting. Immune checkpoint inhibitors have been shown to have significant clinical activity in inflamed STS which are characterized by the presence of tertiary lymphoid structures (TLS). New strategies are needed to sensitize TLS-negative STS to immunotherapy. Engagement of the toll-Like Receptor 4 (TLR4) signal pathway contributes to the development of a favorable tumor microenvironment in solid tumors. G100 is a highly potent toll-like receptor 4 (TLR4) agonist. We hypothesized that intra-tumoral G100 would induce a robust local and potentially systemic anti-tumor immune response in the microenvironment of TLS-negative sarcoma, leading to improved response to PD1 inhibition. Twenty metastatic STS patients who had a superficial injectable lesion were treated with 50 mg of cyclophosphamide (CP) orally twice daily (1 week on and 1 week off), 200 mg of pembrolizumab intravenously on day 8 of a planned 21-day cycle and G100 20 µg one weekly intra-tumoral injection for at least 6 weeks and for a maximum of 12 weeks (1st injection one week before CP administration, ie. Day -7). Biopsies and blood were collected pre and post treatment. Of the 17 patients assessable for efficacy analysis, 2 were progression-free at 6 months, and the 6-month non-progression rate was 11.8% (95% CI: 1.5–36.4), indicating that the first endpoint of the study was not reached. In 8 patients, there was an increase in T-cell infiltration into tumor after treatment. The ratio CD8/Fox-P3 + CD4 on treatment decreased in 11 cases out of 14 suggesting a predominant induction of Treg. Soluble PDL1 levels at baseline were also with adverse outcome. G100 appears to modulate the tumor microenvironment with significant infiltration of T cells. However, clinical activity in combination with PD1 inhibition was limited and no clear correlation was observed between tumor shrinkage and increased inflammation. TLR4 stimulation might have both antitumor and pro-tumor consequences.
Trial registration: This study was registered with ClinicalTrial.gov, number NCT02406781.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-022-01377-2.
To the editor
We report here the results of the cohort of the PEMBROSARC study [1] aiming to assess the safety, efficacy, and immunologic effects of the TLR4 agonist G100 administered intra-tumorally (IT) combined with pembrolizumab in patients with advanced soft-tissue sarcomas (STS).
Patients received 50 mg of cyclophosphamide (CP) orally twice daily (1 week on and 1 week off), 200 mg of pembrolizumab intravenously on day 8 of a planned 21-day cycle and G100 20 µg one weekly intra-tumoral injection for at least 6 weeks and for a maximum of 12 weeks (1st injection one week before CP administration, i.e., Day -7). Eligibility criteria and methods are detailed in Additional file 1: Methods.
Between February 8, 2019 and December 3, 2020, 20 patients were included in the study (Additional file 1: Figure S1). Seventeen patients were assessable for the primary efficacy endpoint, which was the 6-month non-progression rate (Additional file 1: Figure S1) Baseline patient characteristics are listed in Additional file 1: Table S1.
After a median follow-up of 15.6 months [95% confidence interval (CI): 13.6–17.9], one patient was (13.3%) still receiving treatment. Discontinuation was related to disease progression in 18 cases (73%) and investigator decision for one patient (15.5%) (Additional file 1: Figure S1).
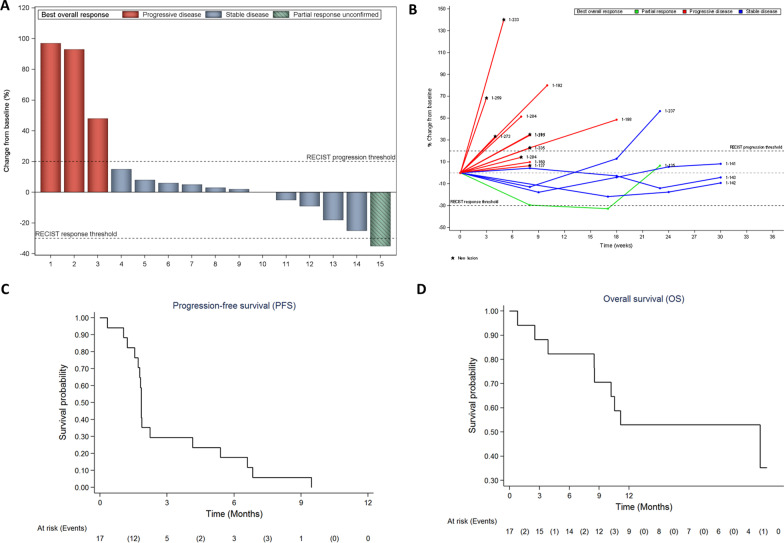
Two patients were progression-free at 6 months, and the 6-month non-progression rate was 11.8% (95% CI: 1.5–36.4), indicating that the first endpoint of the study was not reached. Of the 17 patients eligible and assessable for efficacy, 5 (29.4%) had tumor shrinkage resulting in partial response in 1 case (5.9%) and stable disease in 4 cases (23.5%) (Fig. 1A). The best response was partial response for one patient [1 undifferentiated pleomorphic sarcoma], stable disease for 4 patients [1 epithelioid sarcoma, 1 leiomyosarcoma, 2 dedifferentiated liposarcomas] and progressive disease for 12 patients [1 angiosarcoma, 2 solitary fibrous tumor, 8 leiomyosarcoma, 1 pleomorphic rhabdomyosarcoma].
Fig. 1.
Waterfall plots of tumor response A spider plots B and Kaplan–Meier curves of progression-free C and overall survival D Only patients with available tumor assessments after central review at data cutoff are shown. Changes in tumor size were centrally assessed by blinded independent review according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Maximum change in sum of diameters from baseline is shown on the waterfall plots (A); time on treatment is shown on spider plots (B)
The median follow-up time was 11.2 months (95% CI: 7.6–11.7 months). Median PFS was 1.8 months (95% CI: 1.5–5.0 months) (Fig. 1C). The 6-month and 1-year PFS rates were 11.8% (95% CI: 2–31.2%) and 0%, respectively. Additionally, the median overall survival (OS) was 10.6 months (95% CI: 8.5 months – NA) (Fig. 1D). After treatment discontinuation, 18 patients received additional lines of systemic therapies (median: n = 1, range 1–4). The most frequent regimens were gemcitabine combined with dacarbazine (n = 7) and gemcitabine (n = 4). The safety analysis was reported in Additional file 1: Table S2.
We have recently demonstrated that a subset of STS is characterized by the presence of tertiary lymphoid structures (TLS) and that TLS status represent a reliable biomarker to select patients with advanced STS for treatment with immune checkpoint inhibitors [2, 3]. All the patients enrolled in this study had negative TLS-status and can be considered as “cold” STS [2]. We have evaluated the ability of G100 to alter the tumor microenvironment of STS in sequential tumor samples obtained at baseline and at cycle 2 Day 1 treatment in 14 patients. Multiplex immunofluorescence (IF) analysis demonstrated increase in intra-tumoral CD8 + T and CD4 T-cells infiltration post-G100 therapy in 7 (50%) and 8 patients (57%), respectively (Additional file 1: Figure S2). While these data suggest that G100 was able to increase intra-tumoral inflammation in a subset of patients, there was no clear correlation between baseline or dynamic expression status and responses in this cohort. Since Treg represent an important subset of CD4 + T cells and a major obstacle for the elimination of tumors by immune cells, we investigated the ratio of CD8 + /Fox-P3 + CD4 T cells. Strikingly, the ratio CD8/Fox-P3 + CD4 on treatment decrease in 11 cases out of 14 suggesting a predominant induction of Treg.
We also found that high circulating soluble programmed death-1 ligand (sPD-L1) was the sole protein significantly associated with worse PFS and OS (Additional file 1: Tables S3 and S4).
This study represents the first investigation of the TLR4 agonist G100 administered IT in combination with intravenous PD1 antagonist in patients with advanced cold STS. Analysis of the sequential biopsies revealed that IT G100 resulted in CD4 and CD8 T cell increase in more than 50% of patients and particularly for the CD4. These results are in line with those of previous study which investigated the effect of intratumorally injection of G100 in 15 metastatic STS patients with superficial lesions and which showed that G100 pushed the tumor microenvironment into a more inflammatory state, driven largely by an increase in T cell infiltration [4]. However, as observed in our study this increase in lymphocytic infiltration did not translate into substance clinical benefit. Moreover, no clear correlation was observed between tumor shrinkage and increased inflammation. One potential explanation for this observation may be related to the fact that TLR4 stimulation might have both antitumor and pro-tumor consequences. For instance, it has been shown that TLR4 activation can result in enhanced regulatory T-cell proliferation and suppressor function favoring tumor development [5]. Although we observed an increased density of CD8 + and FoxP3 + CD4T post-treatment in a significant proportion of the patients included in our study, the CD8/Foxp3 CD4 T cell decreased in all patients but 3 suggesting a predominant induction of Treg by G100 that may explain the limited clinical activity.
Overall, our results demonstrate that TLR4 agonist may have significant impact of tumor microenvironment of TLS-negative STS. However, our findings showing an impact of G100 on Treg-cell tumor infiltration indicate that further studies are needed to clarify the role of TLR4 agonist on tumor microenvironment.
Supplementary Information
Additional file 1: Supplementary Methods, Tables and Figures.
Acknowledgements
None.
Abbreviations
- CP
Cyclophosphamide
- PFS
Progression-free survival
- Soluble programmed death-1 ligand
Soluble programmed death-1 ligand
- STS
Soft-tissue sarcoma
- TK
Thymidine kinase
- OS
Overall survival
Author contributions
Concept and design: AI; Acquisition, analysis, or interpretation of data: All authors; Drafting of the manuscript: AI; Critical revision of the manuscript for important intellectual content: all authors; Statistical analysis: FB, MP Administrative, technical, or material support: AI; Validation: all authors; Supervision: AI. All authors read and approved the final manuscript.
Funding
This research did received funding from MSD and from The French Ministry of Health (Programme Hospitalier de Recherche Clinique), Institut National du Cancer, from the Association pour la Recherche contre le Cancer and from the Agence Nationale de la Recherche (RHU CONDOR). G100 was supplied by ImmuneDesign.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are not publicly available due to the clinical and confidential nature of the material but can be made available from the corresponding author on reasonable request.
Declarations
Ethics in approval and consent to participate
This study was approved by the Comité de Protection des Personnes (CPP) Sud-Ouest et Outre Mer III (Bordeaux, France) according to good clinical practices and applicable laws and regulations. All methods were performed in accordance with the relevant guidelines and regulations. All patients provided written informed consent.
Consent for publication
The article does not contain any individual person’s data.
Competing interests
AI: Research grant (AstraZeneca, Bayer, BMS, Merck, MSD, Pharmamar). The other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Toulmonde M, Penel N, Adam J, Chevreau C, Blay JY, Le Cesne A, Bompas E, Piperno-Neumann S, Cousin S, Grellety T, Ryckewaert T, Bessede A, Ghiringhelli F, Pulido M, Italiano A. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas: a phase 2 clinical trial. JAMA Oncol. 2018;4(1):93–97. doi: 10.1001/jamaoncol.2017.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petitprez F, de Reyniès A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougoüin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang WL, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautès-Fridman C, Tawbi HA, Fridman WH. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 3.Italiano A, Bessede A, Pulido M, Bompas E, Piperno-Neumann S, Chevreau C, Penel N, Bertucci F, Toulmonde M, Bellera C, Guegan JP, Rey C, Sautès-Fridman C, Bougoüin A, Cantarel C, Kind M, Spalato M, Dadone-Montaudie B, Le Loarer F, Blay JY, Fridman WH. Pembrolizumab in soft-tissue sarcomas with tertiary lymphoid structures: a phase 2 PEMBROSARC trial cohort. Nat Med. 2022;8(6):1199–1206. doi: 10.1038/s41591-022-01821-3. [DOI] [PubMed] [Google Scholar]
- 4.Seo YD, Kim EY, Conrad EU, O'Malley RB, Cooper S, Donahue B, Cranmer LD, Lu H, Hsu F, Loggers ET, Hain T, Davidson DJ, Bonham L, Pillarisetty VG, Kane GM, Riddell SR, Jones RL, Pollack SM. Intratumoral injection of the toll-like receptor 4 agonist G100 induces a T-cell response in the soft tissue sarcoma microenvironment [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1–5; Washington, DC. Philadelphia (PA): AACR; Cancer Res. 2017;77(13 Suppl):947. 10.1158/1538-7445.AM2017-2947
- 5.Oblak A, Jerala R. Toll-like receptor 4 activation in cancer progression and therapy. Clin Dev Immunol. 2011;2011:609579. doi: 10.1155/2011/609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Methods, Tables and Figures.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the clinical and confidential nature of the material but can be made available from the corresponding author on reasonable request.